Objective:
To evaluate the impact of MSA on lower esophageal sphincter (LES) and esophageal body using high resolution impedance manometry.
Background:
MSA is an effective treatment in patients with gastroesophageal reflux disease, but there is limited data on its impact on esophageal functional physiology.
Methods:
Patients who underwent MSA were approached 1-year after surgery for objective foregut testing consists of upper endoscopy, esophagram, high resolution impedance manometry, and esophageal pH-monitoring. Postoperative data were then compared to the preoperative measurements.
Results:
A total of 100 patients were included in this study. At a mean follow up of 14.9(10.1) months, 72% had normalization of esophageal acid exposure. MSA resulted in an increase in mean LES resting pressure [29.3(12.9) vs 25(12.3), P < 0.001]. This was also true for LES overall length [2.9(0.6) vs 2.6(0.6), P = 0.02] and intra-abdominal length [1.2(0.7) vs 0.8(0.8), P < 0.001]. Outflow resistance at the EGJ increased after MSA as demonstrated by elevation in intrabolus pressure (19.6 vs 13.5 mmHg, P < 0.001) and integrated relaxation pressure (13.5 vs 7.2, P < 0.001). MSA was also associated with an increase in distal esophageal body contraction amplitude [103.8(45.4) vs 94.1(39.1), P = 0.015] and distal contractile integral [2647.1(2064.4) vs 2099.7(1656.1), P < 0.001]. The percent peristalsis and incomplete bolus clearance remained unchanged (P = 0.47 and 0.08, respectively).
Conclusions:
MSA results in improvement in the LES manometric characteristics. Although the device results in an increased outflow resistance at the EGJ, the compensatory increase in the force of esophageal contraction will result in unaltered esophageal peristaltic progression and bolus clearance.
Keywords: esophageal motility, gastroesophageal reflux disease, high-resolution manometry, magnetic sphincter augmentation
Gastroesophageal reflux disease (GERD) is very prevalent in the U.S. population and greatly impacts quality of life in up to 20% of the general population. 1,2 The majority of GERD patients are treated with antisecretory medications; however, up to 40% have breakthrough symptoms such as regurgitation. 3 The large-scale adoption of laparoscopic fundoplication has not transpired because of issues related to heterogeneity in outcome, durability and procedure-related side effects. 4 In response to these gaps in therapy, magnetic sphincter augmentation (MSA) was developed as a reproducible, outpatient procedure that addresses the etiology of GERD using a ring of magnetic beads that serves to augment a defective lower esophageal sphincter (LES) and theoretically arrest disease progression. 5 The device is placed around the esophagogastric junction (EGJ) and it is incumbent upon esophageal peristalsis to generate adequate force to overcome the magnetic attraction of the beads leading to separation and thus passage of food and fluid into the stomach. 6,7
Historically, the impact of prior circumferential devices placed around or immediately distal to the EGJ has not been favorable, with reports of pseudoachalasia, device migration and erosion occurring at unacceptable rates. 8,9 Unlike MSA, the Angel-chik prosthesis, popularized for the treatment of reflux in the 1980 to 1990s, possessed a fixed and rigid diameter that did not accommodate for variations in bolus size or texture driven by esophageal peristalsis; this device ultimately led to chronic EGJ outlet obstruction with resultant peristaltic failure in many patients. 9 Similarly, the chronic contact associated with the esophageal and gastric walls by the noncompliant devices such as the Angelchik and gastric band may have led to the higher rates of transmural erosion. 10 Erosion has been a rare event in patients having undergone MSA with a reported rate of 0.3% in a study of 9453 cases performed over a decade. 11
The paucity of data on the effect of MSA on esophageal motility has led some clinicians to express concerns about placement of a foreign body around the EGJ with potential interference with esophageal motor function. To address this concern, we designed the present study to evaluate the impact of MSA on the LES characteristics, esophageal body peristalsis and bolus clearance using high resolution impedance manometry (HRIM).
Methods
This study is based on a retrospective review of prospectively collected data of all patients who underwent MSA for the treatment of GERD at our institution between 03, 2012 and 03, 2018. These patients were approached for objective esophageal physiology testing and upper endoscopy at 1-year post procedure regardless of outcome. All patients who completed follow up examinations within the stated time interval were included. Because the primary aim of this study was to evaluate the impact of MSA on esophageal body function and LES characteristics, all pre- and post-operative manometry studies were reanalyzed by 1 of the authors to eliminate the interobserver variability which may be associated with multiple examiners. This study was evaluated and approved by the local Institutional Review Board of Allegheny Health Network (IRB 2018-161).
Study Population
Medical history, GERD symptom complex, medication use, and quality of life questionnaires were collected on all patients at baseline, 6 months and yearly after MSA. Patients were considered to be a candidate for MSA if they had symptoms of GERD, abnormal distal esophageal acid exposure or impedance testing or dependency on antisecretory medication. Patients who had complete GERD symptom control on antisecretory medication but who wished to discontinue this treatment and met physiology criteria were also candidates for MSA. Patients with hiatal hernia (any size or type) were considered candidates for MSA provided they met the above criteria. The present study includes the first 100 patients in our experience who underwent MSA and completed 1-year follow-up including HRIM, esophageal pH monitoring, upper endoscopy and standardized questionnaires.
Device and Surgical Technique
The LINX reflux management system (Ethicon, Johnson & Johnson; Shoreview, MN), utilized for MSA, consists of interlinked magnetic titanium beats and features a Roman Arch design assuring non-compressing device closure. It is dynamic design ensures that the esophageal range of motion is not limited. 5 The procedure is performed laparoscopically and consists of complete posterior mediastinal esoph-ageal mobilization with restoration of intra-abdominal esophageal length (≥ 3 cm), posterior crural closure and device placement at the level of the EGJ with the posterior vagus nerve trunk located on the outside of the magnetic ring. Intraoperative esophagogastroscopy is performed to assist in identifying the anatomic EGJ and to assess device position.
Assessment of Outcome and Quality of Life Measures
During postoperative clinic visits at 6 months and then annually, patients were asked to complete standardized questionnaires including the GERD Health-related Quality of Life (GERD-HRQL)
and Reflux Symptom Index (RSI). 12,13 Further, postoperative gastrointestinal symptoms and proton pump inhibitor intake were assessed. The frequency and severity of persistent postoperative dysphagia was reported based on the RSI “difficulty swallowing” item and significant dysphagia was defined as a score ≥3.
Esophageal pH or Impedance-pH Monitoring
These tests were performed selectively using either Bravo pH monitoring (Medtronics, Shoreview, MN) or multichannel intraluminal impedance pH monitoring (Diversatek, Milwaukee, WI). Before pH testing proton pump inhibitors were discontinued for 10 days. A DeMeester score > 14.7 was considered as abnormal distal esophageal acid exposure. Impedance-pH testing was used in patients with predominate symptoms of laryngopharyngeal reflux with or without typical reflux symptoms using previously described criteria.
HRIM Protocol
All patients underwent pre- and post-operative HRIM with a trans-nasally placed 4.2 mm solid-state HRIM catheter with 36 pressure transducers spaced at 1 cm intervals (Medtronic Inc, Minneapolis, MN). After calibration of the transducer, the procedures were performed in Fowler's position. Our standardized protocol consists of a baseline swallow-free recording of at least 3 consecutive respiratory cycles followed by ten consecutive liquid swallows.
HRIM Data Analysis and Assessment of LES and Esophageal Body
All pre- and post-operative HRIM studies were re-analyzed for this study by 1 of the authors. This was performed to eliminate the inter-observer variability in analysis of the studies. Manoview version 3.3 software (Covidien/Medtronic, Duluth, GA) was used for analysis of all studies.
The LES resting pressure was referenced to the intragastric pressure. Deglutitive EGJ relaxation was evaluated by calculating the median integrated relaxation pressure (IRP). This was defined as the mean of the 4 seconds (contiguous or non-contiguous) of maximal LES relaxation in the 10 seconds window beginning at deglutitive UES relaxation. The IRP was also referenced to gastric pressure. The intrabolous pressure (iBP) was measured for each swallow 2 cm proximal to the LES during the emptying phase of esophageal peristaltic topography. 14 Because the recent version of the Manoview software does not allows measurement of the iBP, this value was only available for a subgroup of 43 patients.
Esophageal body metrics included distal contractile integral (DCI) and distal contraction amplitude. 15 HRIM catheter is also incorporated with multiple impedance sensors in addition to circumferential pressure sensors. They allow visualization of the bolus as it travels down the esophagus.
Data Analysis and Statistical Methods
Data are expressed as median (interquartile range) or mean (standard deviation). Categorical variables were assessed using the Fisher exact test and continuous data using Wilcoxon signed rank test and Kruskal-Wallis tests as appropriate. The correlation analyses were performed using Spearman test and expressed as the correlation coefficient R with 95% confidence intervals (CI).
To evaluate which LES manometric characteristic is affected the most by MSA, percentage change from baseline measurement for each LES parameter was calculated using the following formula:
[(post-op value = baseline value)/baseline value] × 100%
The proportion of the patients with ≥ 50% increase in baseline value was then compared across LES characteristics. Statistical significance was defined as a P value < 0.05 for all analyses. All statistical analyses were performed using Statistical Analysis System (SAS) software (version 9.4, SAS Institute, Cary, NC).
Results
The study population consisted of 100 patients who underwent MSA and completed objective foregut testing at 1 year after surgery. Baseline demographic and clinical findings of these patients are presented in Table 1. The most prevalent reflux symptoms before surgery were heartburn (77%) and regurgitation (68%), and 28% of the patients experienced preoperative dysphagia.
Table 1.
Demographic Data and Preoperative Clinical Characteristics
| Baseline Characteristics | n (%) |
|---|---|
| Age, mean (range) | 55 (23–84) |
| Sex: male (%); female (%) | 38 (38%); 62 (62%) |
| BMI, mean (SD) | 28.9 (4.5) |
| Hiatal hernia present | 83 (83) |
| Hiatal hernia size | |
| Small (≤3 cm) | 55 (66.3%) |
| Large (>3 cm) | 23 (27.7%) |
| PEH | 5 (6%) |
| Esophagitis present | 42 (42%) |
| LA grade A | 14 (33.3%) |
| LA grade B | 20 (47.6%) |
| LA grade C | 5 (12%) |
| LA grade D | 3 (7.1%) |
| DeMeester score, mean (SD) | 31.1 (26.8) |
| GERD-HRQL total score, mean (SD) | 33.4 (18.4) |
BMI indicates body mass index; LA, Los Angeles; PEH, paraesophageal hernia; SD, standard deviation.
At a mean follow-up of 14.9 (10.1) months, there was significant improvement in GERD-HRQL total scores compared to baseline values [33.4 (18.4) to 10.6 (12.8), P < 0.001]. Heartburn and regurgitation were eliminated in 85.7% and 88.2% of the patients, respectively, and 92% of the patients were free from use of antisecretory medications.
LES Characteristics and Esophageal Body Function
MSA resulted in an increase in mean LES resting pressure when compared to preoperative values [29.3 (12.9) vs 25 (12.3) cm, P < 0.001]. This was also true for LES overall length [2.9 (0.6) vs 2.6 (0.6) cm, P = 0.02] and LES intra-abdominal length [1.2 (0.7) vs 0.8 (0.8) cm, P < 0.001] (Fig. 1). Thoracic length did not change significantly after MSA [1.7(0.7) vs 1.6(0.6) cm, P = 0.27].
Figure 1.
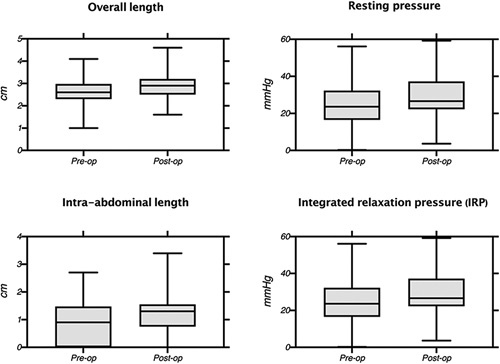
Comparison of the pre- and post-operative LES characteristic.
Patients with 50% or more increase in the postoperative measurements for LES characteristics are compared in Table 2. This analysis shows which LES manometric component is affected by MSA the most. More than half of the patients had > 50% increase in the IRP after MSA; this was only 13% for LES overall length.
Table 2.
Percentage of Patients With >50% Increase for Different LES Parameters After LES *
| % of Patients With ≥ 50% Increase in Baseline Value | |
|---|---|
| LES overall length (cm) | 13% |
| LES intraabdominal length (cm) | 28% |
| LES resting pressure, (mmHg) | 31% |
| Integrated relaxation pressure | 55% |
| (IRP), (mmHg) | |
| P value | <0.0001 |
[(post-op value - baseline value) baseline value] x 100% > 50%.
Outflow resistance at the EGJ increased after MSA evidenced by elevation in LES IRP [13.3 (6) vs 8.1 (5.8) mmHg, P < 0.001] and the iBP [19.6 (5.9) vs 13.5 (4.7) mmHg, P < 0.001].
MSA was also associated with a significant increase in distal contraction amplitude [103.8 (45.4) vs 94.1 (39.1) mmHg, P = 0.015] and DCI [2647.1 (2064.4) vs 2099.7 (1656.1) mmHg-s-cm, P < 0.001]. The percent peristalsis and incomplete bolus clearance remained unchanged after MSA (P = 0.868 and 0.500, respectively) (Table 3).
Table 3.
Comparison of Baseline and Postoperative Esoph-ageal Body Motor Functions
| Baseline Median (IQR) | Post-op Median (IQR) | P Value | |
|---|---|---|---|
| Distal contraction amplitude, | 94.1 (39.1) | 103.8 (45.4) | 0.015 |
| mmHg | |||
| Distal contractile integral | 2099.7 (1656.1) | 2647.1 (2064.4) | <0.001 |
| (DCI), (mmHg-s-cm) | |||
| Incomplete bolus clearance | 21 (30.1) | 19.9 (30.2) | 0.500 |
| (%) | |||
| Intact peristalsis (%) | 91.8 (15.9) | 91.3 (18.2) | 0.868 |
IQR indicates interquartile range.
There was a direct correlation between postoperative and preoperative measurements for both DCI [Spearman R: 0.69 (95% CI: 0.56–0.78), P < 0.0001] and distal contraction amplitude [Spearman R: 0.75 (95% CI: 0.63–0.83), P < 0.0001] (Fig. 2).
Figure 2.
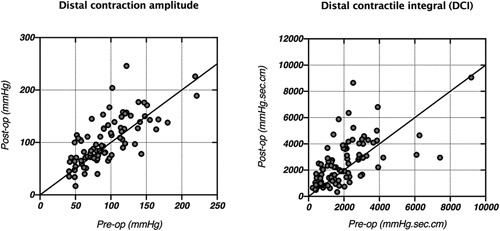
Correlation between pre- and post-operative distal contraction amplitude [Spearman R: 0.75 (95% CI: 0.63-0.83), P < 0.0001] and distal contractile integral (DCI) [Spearman R: 0.69 (95% CI: 0.56–0.78), P < 0.0001].
The percentage of swallows with complete bolus clearance after surgery was directly correlated with postoperative DCI [Spearman R: 0.35 (95% CI: 0.16–0.52), P = 0.0004] and postoperative distal contraction amplitude [Spearman R: 0.54 (95% CI: 0.37–0.67), P < 0.0001].
The Impact of Device Size
The median size of the LINX device used was 14 (interquartile range: 13–15) bead system. The postoperative manometric characteristics of patients with a smaller sized device (≤ 14 magnetic beads) are compared to those with a larger device (≥15 magnetic beads) in Table 4. There was a trend toward higher rate of pH normalization in patients with a smaller-size device (≤14 magnetic beads) compared to those with a larger device (82% vs 66%, P = 0.11). Patients with a smaller device, however, had a higher rate of postoperative dysphagia (19.6% vs 7.7%, P = 0.10).
Table 4.
Comparison of Postoperative Manometric Characteristics of Patients With a Smaller Sized LINX Device to Those With a Larger Device
| Smaller Size Devices (<15 Magnetic Beads) | Larger Size Devices (≥ 15 Magnetic Beads) | P Value | |
|---|---|---|---|
| LES overall length (cm) | 2.9 (2.6–3.1) | 2.7 (2.3-3.5) | 0.851 |
| LES intraabdominal length (cm) | 1.3 (1.0, 1.5) | 1.2 (0.3-1.9) | 0.585 |
| LES resting pressure, (mmHg) | 31.6 (23.7–38.3) | 25.9 (18.4–34.7) | 0.209 |
| Integrated relaxation pressure (IRP), (mmHg) | 14.7 (11.6–18.1) | 12.0 (8.9–14.2) | 0.005 |
| Distal contraction amplitude, (mmHg) | 106.6 (78.4–144.3) | 77.6 (59.1–109.2) | 0.003 |
| Distal contractile integral (DCI), (mmHg s cm) | 2757 (1466–3894) | 1720 (948–2929.0) | 0.023 |
| Intact peristalsis (%) | 100 (90–100) | 100 (80–100) | 0.239 |
| Incomplete bolus clearance (%) | 0 (0–20) | 10 (0–50) | 0.146 |
Postoperative pH Normalization and Manometric Characteristics
There was a significant decrease in DeMeester score after MSA [31.1 (26.8) vs 14.7 (30.5), P <.001]. Seventy-two patients (72%) had normalization of distal esophageal acid exposure after surgery. These patients had a significantly higher IRP on their postoperative manometry compared to those with an abnormal DeMeester score after MSA. Other manometric parameters were not significantly different between these 2 groups. There was an inverse correlation between postoperative DeMeester score and postoperative IRP [Spearman R: –0.28 (95% CI: –0.47 to –0.07), P = 0.008].
Postoperative Dysphagia and Manometric Characteristics
Fifteen patients (15%) reported persistent dysphagia (RSI difficulty swallowing score ≥3) after surgery. There was a trend toward higher IRP on the postoperative manometry of patients with dysphagia compared to those without dysphagia [16.7 (13.9–19.2) vs 13.2 (9.9–16.3), P = 0.061]. The results of comparison of other postoperative manometry parameters were comparable between the 2 groups. Of note, patients with postoperative dysphagia had a higher IRP on their preoperative manometry compared to those with no postoperative dysphagia [10.3 (5.6–16.7) vs 6.9 (4.0–11.1), P = 0.026].
Four patients (4%) in this cohort required device removal secondary to persistent dysphagia or chest pain not relived by up to 3 endoscopic dilations. There was a trend toward higher IRP in these patients compared to the rest of patients. There was no device erosion in this group of patients.
Discussion
The increasing utilization of MSA in the treatment of GERD has raised concerns whether the device may ultimately impair esophageal physiology. The primary aim of this study was to address these concerns by comparing HRIM findings in the preoperative and postoperative settings. Earlier work has identified LES pressure, LES intraabdominal length and LES overall length as crucial components of restoring the anti-reflux barrier. 16–18 In this study we found that MSA increased LES parameters including resting pressure, overall length, and intraabdominal length (Fig. 1); this positive impact on the mano-metric LES characteristics translates into successful clinical outcomes as evidenced by significant reductions in GERD-HRQL scores, anti-secretory medication use, and distal esophageal acid exposure.
We found that the increase in the overall length of the LES associated with MSA was primarily the result of an increase in the intraabdominal segment of the LES. This is supported by our finding that sphincter thoracic length remained unchanged after surgery. The LINX device is designed to prevent the effacement of the sphincter during gastric distention 5 ;this effect will result in restoration of the length of the sphincter exposed to the increased intraabdominal pressure.
MSA was initially adopted as a minimal hiatal dissection procedure and was first utilized in patients with small or no preop-erative hiatal hernia. 19 As the procedure has evolved, MSA has been expanded to patients with more severe reflux disease and complex anatomy including the presence of paraesophageal hernia. 20 Previous studies have demonstrated the important relationship between LES position, pressure and length, suggesting that all components factor into EGJ competency. 21 It has been shown that selective augmentation of the high-pressure zone (ie, not actively restoring intraabdominal length) has limited potential benefit because the augmented intrinsic tone cannot become effective without being exposed to the positive abdominal pressure. 21 Thus, all anti-reflux procedures should aim to repair a hernia or widened crural opening, which will ultimately improve LES competency and the effectiveness of its intrinsic muscular tone.
Another major finding of this study was that increased postoperative esophageal outflow resistance after MSA did not result in impaired esophageal peristalsis but instead caused a compensatory increase in contractility (Fig. 3). We found a strong correlation between pre- and post-operative DCI measurements, which indicates that the esophagus not only maintains preservation of peristaltic sequencing and latency but accommodates with increased force of contraction in response to the device, whereas eliminating symptomatic exposure to gastric fluid. This is further highlighted by the observation of a direct correlation between esophageal outflow resistance and postoperative DCI. Esophageal peristalsis and bolus clearance remained unchanged after MSA. These results match the findings of the 2 other studies that investigated the changes of esophageal peristalsis after MSA. 22,23 We further observed improved postoperative motility in 4 of 6 ineffective esophageal motility (IEM) patients, which were similar to findings published by Riva and colleagues who reported a 36% resolution rate of IEM after MSA. They interpreted this phenomenon as a response of the esophageal body to increased LES resistance. 22 These observations of unchanged (or potentially improved esophageal peristalsis in selected cases) after MSA are of relevance as they differentiate the effects of MSA from those of other anti-reflux procedures such as fundoplication or the other historically unsuccessful fixed prosthesis.
Figure 3.
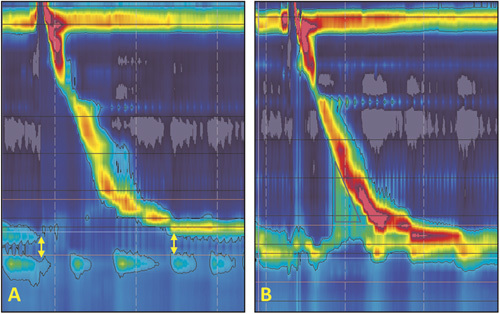
Comparison of LES characteristics of a GERD patient before and after magnetic sphincter augmentation (MSA): (A) HRIM topographic plot of the patient before surgery shows a defective LES with a low resting pressure, no intra-abdominal length and short overall length. The separation between LES and crura diaphragm (CD), indicates presence of an axial hiatal hernia (yellow arrows); (B) HRIM topographic plot of the same patient after surgery shows elimination of separation between LES and CD and augmentation of the LES resting pressure and abdominal length. Note the elevated intra-bolus pressure (iBP) marked with black triangle.
The effects of fundoplication on esophageal motility are described as complex with studies reporting conflicting results. Fibbe et al 24 described that esophageal motor function remains unchanged after laparoscopic Nissen and Toupet fundoplication. They reported a significant improvement of primary peristalsis, only in patients with preoperative dysmotility and only after Toupet fundoplication. Some authors have reported more vigorous contractions after Nissen compared to partial fundoplication, whereas other studies did not detect significant changes in distal esophageal contraction amplitude after Nissen fundoplication. 25,26 Scheffer and colleagues 27 reported that nadir EGJ relaxation, iBP and contractile activity in the distal esophagus increase after Nissen fundoplication. 30 They hypothesized that increased esophageal contractility might be necessary to overcome the increased resistance at the EGJ after surgery.
As the term “augmentation” implies, MSA serves to add an additional resistive component to the native EGJ. Past experiences with circumferential devices placed around EGJ have been associated with high rates of complications. It has to be noted, however, that the dynamic design of the Linx device significantly differs from the rigid nature of such former implants. 5 The MSA device is a ring comprised of 5 mm magnetic beads connected via titanium wires, with each bead capable of independent motion around the EGJ. The magnetic attraction between beads in the closed position is approximately 40 g. In the open position, the inner diameter of the ring approximately doubles, and the inter-bead attractive force is roughly 7g. 5,28 In order for substances to pass through the augmented EGJ, the pressure has to exceed not only the native LES resistance but also overcome the magnetic forces between beads. Another resistance required to overcome is the fibrous capsule which forms and matures around the device in the weeks to months after placement. 29 This collagen-rich capsule restricts the achievable cross-sectional area, but interestingly does not limit motion between individual beads. 29 Restriction from the capsule is thought to be a variable, but significant component of late dysphagia in MSA patients.
In this series of MSA cases, postoperative HRIM characteristics of patients with and without postoperative dysphagia were comparable. However, a trend towards a higher postoperative IRP was detected in patients with dysphagia. Based on the findings of this study, persistent dysphagia after MSA is the result of a complex interplay between increased esophageal outflow resistance and esophageal peristalsis and contraction forces. In contrast to our findings, Bonavina and co-authors 22 reported no association between postoperative HRM parameters and the development of dysphagia. The presence of preoperative dysphagia was reported as the only factor associated with the development of postoperative dysphagia in that study.
In this cohort, 4 patients (4%) required device removal secondary to persistent dysphagia or chest pain not relived by up to 3 endoscopic dilations. The small number of events limit our ability to perform a detailed analysis on factors associated with the risk of device explanation. However, we found a trend toward higher IRP on the postoperative manometry of these patients, indicating a higher degree of resistance at the EGJ compared to the rest of population. It is likely that the compensatory response in esophageal contractility after MSA may have not been sufficient in these patients to facilitate bolus transit across the augmented EGJ.
Mechanical outflow obstruction has also been proposed as the likely mechanism for the development of dysphagia after laparo-scopic Nissen fundoplication. LES relaxation and focal inhibition of the crura during inspiration ensure reduction in intraluminal pressure at the EGJ which can be impaired after anti-reflux surgery. These changes impact esophageal peristalsis and are associated with postoperative dysphagia. 30 Some investigators have hypothesized that esophageal contraction amplitude needs to overcome outflow resistance imposed by anti-reflux procedures to avoid postoperative dysphagia. 31 A previous study has characterized the outflow resistance, reflected by iBP and IRP, after Nissen fundoplication and identified an iBP of 20 as threshold that should not be overstepped to avoid postoperative dysphagia. 32 A recent study has measured the iBP after MSA and found 30 mmHg as the threshold that needs to be overcome by esophageal contractions. 33 The higher values for iBP after MSA supports prior notions that the LINX device imposes more resistance at the EGJ compared to Nissen fundoplication and emphasizes on the need to develop novel manometric criteria when selecting patients for MSA. In a recent study, DCI < 750 mmHg-cm-s, distal wave amplitude < 43 mmHg and less than 80% peristaltic contractions on preoperative manometry were found to be risk factors for postoperative dysphagia. 34 These are the manometric criteria that we currently use in our practice during patient selection and preoperative counseling when offering MSA to a patient.
Patients with a smaller size LINX were found to have a higher rate of pH normalization. This is explained by the fact that smaller devices impose a higher resistance at the EGJ evidenced by higher postoperative IRP in these patients. Esophageal smooth muscle in turn responds to the stress of higher EGJ resistance by generating higher contractility evidenced by higher postoperative DCI in patients with smaller device. The higher pH normalization in patients with smaller device did not necessarily result in a better outcome as it came at the cost of higher dysphagia rate in these patients. These findings emphasize the delicate balance between achieving adequate reflux control and preventing postoperative dysphagia. 35
Although our study did show improved manometric LES structure and function, with preservation of peristaltic function, the durability of these effects remains unknown. Further, the relatively small sample sizes might have limited some subgroup analyses from reaching statistical significance. In addition, iBP data was only available for a small subgroup of patients; this limited our ability to include this useful surgical metric in our analysis. Future studies with larger sample sizes and longer follow-up time are necessary to confirm our findings and further investigate physiological consequences of MSA on the esophagus and their clinical implications.
Conclusions
MSA represents an effective treatment option for GERD resulting in significant symptom relief and high rates of pH normalization. Postoperative manometric assessment after MSA shows restored competence of the EGJ with significantly increased LES resting pressure, and overall and intra-abdominal LES length. Esophageal peristaltic progression and bolus clearance remain unchanged despite postoperative increased esophageal outflow resistance due to a compensatory increase in the force of esophageal contraction after MSA. The effects of MSA on esophageal motility and EGJ pressure profile differ significantly from those observed after utilization of rigid circumferential devices used in the past. Due to the dynamic design of the LINX device, rates of significant postoperative esoph-ageal outflow obstruction, impaired esophageal motility and complications such as erosion are rare after MSA.
Footnotes
This research received no specific grant or funding from any agency.
The authors report no conflicts of interest.
REFERENCES
- 1. El-Serag HB, Petersen NJ, Carter J, et al. Gastroesophageal reflux among different racial groups in the United States. Gastroenterology. 2004;126:1692–1699. [DOI] [PubMed] [Google Scholar]
- 2. Shaheen NJ, Hansen RA, Morgan DR, et al. The burden of gastrointestinal and liver diseases. Am J Gastroenterol. 2006;101:2128–2138. [DOI] [PubMed] [Google Scholar]
- 3. Fass R, Shapiro M, Dekel R, et al. Systematic review: proton-pump inhibitor failure in gastro-oesophageal reflux disease-where next? Aliment Pharmacol Ther. 2005;22:79–94. [DOI] [PubMed] [Google Scholar]
- 4. Richter JE, Dempsey DT. Laparoscopic antireflux surgery: key to success in the community setting. Am J Gastroenterol. 2008;103:289–291. [DOI] [PubMed] [Google Scholar]
- 5. Bonavina L, Saino G, Lipham JC, et al. LENX (®) reflux management system in chronic gastroesophageal reflux: a novel effective technology for restoring the natural barrier to reflux. Therap Adv Gastroenterol. 2013;6:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ayazi S, Zheng P, Zaidi AH, et al. Magnetic sphincter augmentation and postoperative dysphagia: characterization, clinical risk factors, and management. J Gastrointest Surg. 2020;24:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bonavina L, DeMeester TR, Ganz RA. LINX (®) reflux management system: magnetic sphincter augmentation in the treatment of gastroesophageal reflux disease. Expert Rev Gastroenterol Hepatol. 2012;6:667–674. [DOI] [PubMed] [Google Scholar]
- 8. Maddern GJ, Myers JC, McIntosh N, et al. The effect of the Angelchik prosthesis on esophageal and gastric function. Arch Surg. 1991;126:1418–1422. [DOI] [PubMed] [Google Scholar]
- 9. Crookes PF, DeMeester TR. The Angelchik prosthesis: what have we learned in fifteen years? Ann Thorac Surg. 1994;57:1385–1386. [DOI] [PubMed] [Google Scholar]
- 10. Suter M, Giusti V, Heraief E, et al. Band erosion after laparoscopic gastric banding: occurrence and results after conversion to Roux-en-Y gastric bypass. Obes Surg. 2004;14:381–386. [DOI] [PubMed] [Google Scholar]
- 11. Alicuben ET, Bell RCW, Jobe BA, et al. Worldwide experience with erosion of the magnetic sphincter augmentation device. J Gastrointest Surg. 2018;22:1442–1447. [DOI] [PubMed] [Google Scholar]
- 12. Velanovich V, Vallance SR, Gusz JR, et al. Quality of life scale for gastroesophageal reflux disease. J Am Coll Surg. 1996;183:217–224. [PubMed] [Google Scholar]
- 13. Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI). J Voice. 2002;16:274–277. [DOI] [PubMed] [Google Scholar]
- 14. Roman S, Gyawali CP, Xiao Y, et al. The Chicago classification of motility disorders: an update. Gastrointest Endosc Clin North Am. 2014;24:545–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27:160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zaninotto G, DeMeester TR, Schwizer W, et al. The lower esophageal sphincter in health and disease. Am J Surg. 1988;155:104–111. [DOI] [PubMed] [Google Scholar]
- 17. O’Sullivan GC, DeMeester TR, Joelsson BE, et al. Interaction of lower esophageal sphincter pressure and length of sphincter in the abdomen as determinants of gastroesophageal competence. Am J Surg. 1982;143:40–47. [DOI] [PubMed] [Google Scholar]
- 18. Ghosh SK1, Kahrilas PJ, Brasseur JG. Liquid in the gastroesophageal segment promotes reflux, but compliance does not: a mathematical modeling study. Am J Physiol Gastrointest Liver Physiol. 2008;295:G920–G933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bonavina L, Saino GI, Bona D, et al. Magnetic augmentation of the lower esophageal sphincter: results of a feasibility clinical trial. J Gastrointest Surg. 2008;12:2133–2140. [DOI] [PubMed] [Google Scholar]
- 20. Ayazi S, Chowdhury N, Zaidi AH, et al. Magnetic sphincter augmentation (MSA) in patients with hiatal hernia: clinical outcome and patterns of recurrence. Surg Endosc. 2020;34:1835–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DeMeester TR, Wernly JA, Bryant GH, et al. Clinical and in vitro analysis of determinants of gastroesophageal competence. A study of the principles of antireflux surgery. Am J Surg. 1979;137:39–46. [DOI] [PubMed] [Google Scholar]
- 22. Riva CG, Siboni S, Sozzi M, et al. High-resolution manometry findings after Linx procedure for gastro-esophageal reflux disease. Neurogastroenterol Motil. 2020;32:e13750. [DOI] [PubMed] [Google Scholar]
- 23. Warren HF, Louie BE, Farivar AS, et al. Manometric changes to the lower esophageal sphincter after magnetic sphincter augmentation in patients with chronic gastroesophageal reflux disease. Ann Surg. 2017;266:99–104. [DOI] [PubMed] [Google Scholar]
- 24. Fibbe C, Layer P, Keller J, et al. Esophageal motility in reflux disease before and after fundoplication: a prospective, randomized, clinical, and manometric study. Gastroenterology. 2001;121:5–14. [DOI] [PubMed] [Google Scholar]
- 25. Draaisma WA, Rijnhart-de Jong HG, Broeders IA, et al. Five-year subjective and objective results of laparoscopic and conventional Nissen fundoplication: a randomized trial. Ann Surg. 2006;244:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weijenborg PW, Savarino E, Kessing BF, et al. Normal values of esophageal motility after antireflux surgery; a study using high-resolution manometry. Neurogastroenterol Motil. 2015;27:929–935. [DOI] [PubMed] [Google Scholar]
- 27. Scheffer RC, Samsom M, Frakking TG, et al. Long-term effect of fundoplication on motility of the oesophagus and oesophagogastric junction. Br J Surg. 2004;91:1466–1472. [DOI] [PubMed] [Google Scholar]
- 28. Ganz RA. A modern magnetic implant for gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2017;15:1326–1337. [DOI] [PubMed] [Google Scholar]
- 29. Ganz RA, Gostout CJ, Grudem J, et al. Use of a magnetic sphincter for the treatment of GERD: a feasibility study. Gastrointest Endosc. 2008;67:287–294. [DOI] [PubMed] [Google Scholar]
- 30. Myers JC, Jamieson GG, Sullivan T, et al. Dysphagia and gastroesophageal junction resistance to flow following partial and total fundoplication. J Gastrointest Surg. 2012;16:475–485. [DOI] [PubMed] [Google Scholar]
- 31. Marjoux S, Roman S, Juget-Pietu F, et al. Impaired postoperative EGJ relaxation as a determinant of post laparoscopic fundoplication dysphagia: a study with high-resolution manometry before and after surgery. Surg Endosc. 2012;26:3642–3649. [DOI] [PubMed] [Google Scholar]
- 32. Ayazi S, DeMeester SR, Hagen JA, et al. Clinical significance of esophageal outflow resistance imposed by a Nissen fundoplication. J Am Coll Surg. 2019;229:210–216. [DOI] [PubMed] [Google Scholar]
- 33. Ayazi S, Grubic AD, Zheng P, et al. Measurement of outflow resistance imposed by magnetic sphincter augmentation: defining normal values and clinical implication. Surg Endosc. 2021;35:5787–5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ayazi S, Schwameis S, Zheng P, et al. Establishing preoperative risk factors and development of a predictive nomogram for dysphagia after magnetic sphincter augmentation. J Am Coll Surg. 2020;231:E1–E2.32805402 [Google Scholar]
- 35. Ayazi S, Zheng P, Zaidi AH, et al. Clinical outcomes and predictors of favorable result after laparoscopic magnetic sphincter augmentation: single-institution experience with more than 500 patients. J Am Coll Surg. 2020;230:733–743. [DOI] [PubMed] [Google Scholar]


