Objective:
The aim of this study was to analyze outcomes of open lobectomy (OL), VATS, and robotic-assisted lobectomy (RL).
Summary Background Data:
Robotic-assisted lobectomy has seen increasing adoption for treatment of early-stage lung cancer. Comparative data regarding these approaches is largely from single-institution case series or administrative datasets.
Methods:
Retrospective data was collected from 21 institutions from 2013 to 2019. All consecutive cases performed for clinical stage IA-IIIA lung cancer were included. Neoadjuvant cases were excluded. Propensity-score matching (1:1) was based on age, sex, race, smoking-status, FEV1%, Zubrod score, American Society of Anesthesiologists score, tumor size, and clinical T and N stage.
Results:
A total of 2391 RL, 2174 VATS, and 1156 OL cases were included. After propensity-score matching there were 885 pairs of RL vs OL, 1,711 pairs of RL vs VATS, and 952 pairs of VATS vs OL. Operative time for RL was shorter than VATS (P < 0.0001) and OL (P = 0.0004). Compared to OL, RL and VATS had less overall postoperative complications, shorter hospital stay (LOS), and lower transfusion rates (all P<0.02). Compared to VATS, RL had lower conversion rate (P<0.0001), shorter hospital stay (P<0.0001) and a lower postoperative transfusion rate (P =0.01). RL and VATS cohorts had comparable postoperative complication rates. In-hospital mortality was comparable between all groups.
Conclusions:
RL and VATS approaches were associated with favorable perioperative outcomes compared to OL. Robotic-assisted lobectomy was also associated with a reduced length of stay and decreased conversion rate when compared to VATS.
Keywords: lobectomy, lung cancer, robotics
Pulmonary lobectomy is a procedure which can be performed by 3 competing approaches – video-assisted, robotic-assisted and open thoracotomy. There is no single approach that is performed in a majority of cases in the United States. 1,2 Open lobectomy (OL) continues to be routinely performed despite publications suggesting VATS lobectomy is associated with reduced complications compared to OL, and guidelines recommending VATS as the preferred approach for early-stage lung cancer. 3–5 Robotic-assisted lobectomy (RL) is a newer modality, and its adoption has grown rapidly from less than 1% of lobectomies in 2008 to 18% by 2015. 2,6 Debate persists on whether there is an optimal approach.
Comparative data on the efficacy of these approaches have largely come from single institutions or administrative datasets. 1,2,6–11 While databases may reflect real-world data, there is no ideal database, and none contain all of the information of interest to clinicians. For instance, The Society of Thoracic Surgery database has excellent perioperative data but no information on cancer-related outcomes. In contrast, the National Cancer Database has granular oncologic data but little information on perioperative outcomes.
Administrative databases such as the National Inpatient Sample and Premier are based on ICD coding with their attendant limitations.
The present study was designed to be a large, multi-center retrospective study to assess outcomes for all 3 lobectomy techniques from experienced surgeons. The aim was to compare the perioperative outcomes at a large scale with sufficient granular data that could not be accomplished by single-institution studies or databases. We hypothesized that a minimally invasive approach to lobectomy would be associated with a reduction in length of stay and overall complication rate compared to open thoracotomy.
Methods
Data Sources
Retrospective data was collected from 21 centers in the United States (Supplemental Table 1, http://links.lww.com/SLA/D312). Centers with specific expertise in RL, VATS and/or OL participated. Three surgeons with significant experience in RL (MSK), VATS (MH) and OL (EV) were designated as co-chairs to conduct this study.
To ensure adequate experience, participating surgeons were required to have performed at least 50 total lobectomies before case submission. Data from all consecutive lobectomies for clinical stage IA-IIIA lung cancer from January 2013 to 30-days before institutional review board (IRB) approval at each center were included. Chart review and data collection were performed in a reverse chronological order in accordance with the IRB guidelines. Deidentified data was retrieved using standardized electronic data collection forms to ensure uniformity. A study-specific informed consent waiver for retrospective data collection was obtained from each institution’s IRB. Information from all patients was maintained confidential and managed according to the requirements of the Health Insurance Portability and Accountability Act of 1996. Data collection closed for all centers on June 21, 2019.
Emergency cases, indications other than lung cancer, bilobectomies, and sleeve lobectomies were excluded from analysis. Data was collected on demographics, clinical and pathologic staging, induction therapy, operative details including conversions, perioperative complications as well as overall and cancer-specific survival. Data on periperative mortality and complications was collected from the in-hospital stay as well as the last follow-up within 90 days. Operative time was inclusive of docking time for robotic cases. Data regarding induction therapy, pathologic staging, and survival will be examined in future analyses.
Data analysis was performed on an intent-to-treat basis. Consequently, conversions were analyzed under the initial operative approach, regardless of the reason for conversion. Conversions were categorized as elective due to failure to progress or emergent due to life-threatening hemorrhage. Conversions in the RL cohort included conversions to VATS as well as to thoracotomy. All major complications and conversions were independently reviewed by the site’s principal investigator to ensure data integrity. Operative and pathology reports and discharge summaries were also randomly audited from 40% of cases to verify accuracy.
Statistical Analysis
Standard univariate and bivariate techniques were used to describe the clinical results. Continuous variables were defined as means (and standard deviations), median, first and third quartiles. Discrete variables (i.e., conversions, complications) were described as rates and proportions of the totals. All statistical analyses were performed by an independent statistician.
A propensity-score model for adjustment of baseline variables was implemented to decrease bias between groups. Comparisons were made for RL versus OL, VATS versus OL, and RL versus VATS. Propensity score-adjusted comparisons were calculated by fitting a multivariable logistic regression analysis. A one-to-one nearest neighbor matching algorithm was applied using a caliper of 0.10. Controls were not re-used during matching. Covariates used for matching were age, sex, race, smoking status, American Society of Anesthesiologists grade, Zubrod score, FEV1%, tumor size category (≤ 3 cm, 3–7cm and >7 cm), clinical T stage, and clinical N stage. Standardized differences and histograms of propensity score before and after matched were used to evaluate effectiveness of the matching procedure to balance the populations on the covariates.
RL, VATS, and OL cohorts were compared using standard statistical tests appropriate for paired comparisons. McNemar test was used for categorical variables, paired t-test for continuous approximately normally distributed variables, and Wilcoxon signed rank test for ordinal and significantly non-normally distributed continuous variables. The Kaplan-Meier method was used to calculate survival rates at 30 days to account for subjects censored before 30 days. The stratified log rank test was used to compare the survival through 30 days and to account for the paired nature of the data. A P-value of less than 0.05 was considered statistically significant.
All analyses, with the exception of PSM, were performed in SAS version 9.4. The PSM was performed in R version 3.5.2 using the Matchit package.
Results
Baseline Characteristics
A total of 6,216 lobectomy patients (2557 RL, 2324 VATS, and 1335 OL) were reviewed. Patients who underwent induction therapy were excluded, leaving 2391 RL, 2174 VATS, and 1156 OL cases. PSM allowed 3 pairwise comparisons: RL vs OL (n = 885 each), VATS vs OL (n =952 each), and RL vs VATS (n =1711 each) (Fig. 1). Summary statistics for the 3 groups at baseline prior to matching are shown in Supplemental Table 2, http://links.lww.com/SLA/D312. After matching, there were no significant differences between baseline characteristics between cohorts, with the exception of Zubrod score in some comparisons (Supplemental Table 3, http://links.lww.com/SLA/D312). Standardized difference plots and mirror histograms also demonstrated balanced groups after propensity matching (Supplemental Figures 1–6, http://links.lww.com/SLA/D313). While mean tumor size was also statistically different among some groups by up to 3 mm, T stage, N stage, and TNM stage were comparable across all groups.
Figure 1.
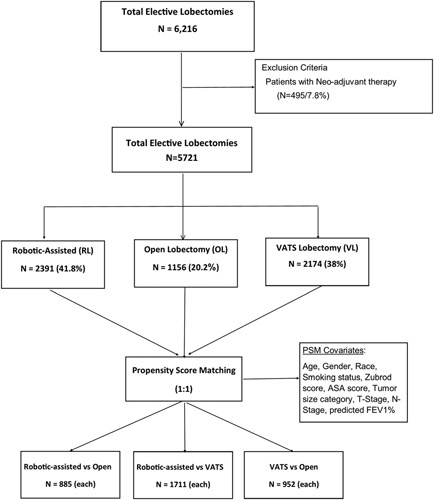
Flowchart for propensity-matched analysis.
Intraoperative Outcomes: Propensity-Matched Analysis
Pairwise comparisons of intraoperative characteristics are presented in Supplemental Table 4, http://links.lww.com/SLA/D312. Mean operating-room (OR) time (wheels-in to wheels-out) was analyzed by excluding cases that included a concomitant procedure such as a mediastinoscopy or additional lung resection. In cases with no concomitant procedures, there was a statistically shorter OR time for the RL cohort compared to both OL and VATS cohorts. OR time for the VATS cohort was 20 minutes longer than for the OL cohort (P = 0.01).
The conversion rate was also lower in RL cases compared to VATS (Fig. 2A). To adjust for tumor characteristics that could influence conversion to thoracotomy, conversion was analyzed based on stage. RL was associated with a lower conversion rate than VATS for all clinical stages (Fig. 2B). The majority of conversions were non-emergent in both groups (Fig. 2C).
Figure 2.
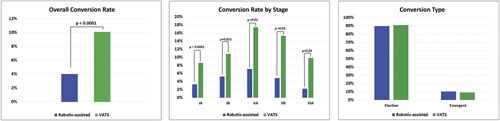
Conversions from minimally invasive to open lobectomy. Panel A: Overall conversion rate by approach. Panel B: Conversion rate by clinical stage. Panel C: Reason for conversion.
Intraoperative estimated blood loss was statistically lower for both minimally invasive approaches compared to OL. The intraoperative transfusion rate was also statistically lower for both MIS approaches compared to open. Between the 2 MIS approaches, RL was associated with less EBL compared to VATS but there was no statistical difference in transfusion rates.
Post-operative Outcomes: Propensity-Matched Analysis
Both MIS approaches were associated with a lower rate of inhospital and postoperative complications compared to OL (Table 1). The most frequent complication in all cohorts was pulmonary (including prolonged air leak, pneumonia and need for bronchoscopy). The overall rate of pulmonary complications was significantly lower for both MIS approaches when compared to open. No difference in pulmonary or overall morbidity was observed between the robotic and VATS cohorts.
Table 1.
Propensity-Matched Pairwise Comparisons of Postoperative Details Before Patient Discharge Outcomes for RL, VATS, and OL Cases
| Variable | RL versus OL | VATS versus OL | RL versus VATS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RL (n = 885) | OL (n = 885) | P-value | VATS (n = 952) | OL (n = 952) | P-value | RL (n = 1711) | VATS (n = 1711) | P-value | |
| Complications, n (%) | 237 (26.8) | 315 (35.6) | <0.0001 | 266 (27.9) | 339 (35.6) | 0.001 | 463 (27.1) | 511 (29.9) | 0.07 |
| Pulmonary | 156 (17.6) | 198 (22.4) | 0.01 | 170 (17.9) | 214 (22.5) | 0.01 | 304 (17.8) | 333 (19.5) | 0.20 |
| Cardiac | 83 (9.4) | 125 (14.1) | 0.002 | 102 (10.7) | 141 (14.8) | 0.03 | 169 (9.9) | 187 (10.9) | 0.32 |
| Gastrointestinal | 8 (0.9) | 6 (0.7) | 0.59 | 11 (1.2) | 8 (0.8) | 0.35 | 13 (0.8) | 20 (1.2) | 0.22 |
| Neurological | 12 (1.4) | 17 (1.9) | 0.34 | 15 (1.6) | 18 (1.9) | 0.72 | 24 (1.4) | 25 (1.5) | 0.88 |
| Wound | 1 (0.1) | 2 (0.2) | 0.56 | 2 (0.2) | 3 (0.3) | 1.00 | 5 (0.3) | 4 (0.2) | 0.74 |
| Genitourinary | 31 (3.5) | 15 (1.7) | 0.02 | 33 (3.5) | 17 (1.8) | 0.01 | 66 (3.9) | 77 (4.5) | 0.35 |
| Unexpected return to operating rooma, n (%) | 25 (2.9) | 27 (4.9) | 0.15 | 37 (4.3) | 31 (5.3) | 0.32 | 50 (3.0) | 66 (4.2) | 0.14 |
| Postoperative blood transfusion, n (%) | 13 (1.5) | 67 (7.6) | <0.0001 | 24 (2.5) | 77 (8.1) | <0.0001 | 22 (1.3) | 42 (2.5) | 0.01 |
| Chest tube durationa, d (±SD) | 3.8 ± 5.2 | 5.2 ± 5.2 | <0.0001 | 4.3 ± 4.7 | 5.3 ± 5.3 | <0.0001 | 4.0 ± 5.5 | 4.4 ± 5.1 | <0.0001 |
| Length of hospital stay, Mean d (±SD) | 4.2 ± 4.9 | 6.1 ± 4.9 | <0.0001 | 5.1 ± 4.4 | 6.1 ± 6.4 | <0.0001 | 4.1 ± 4.4 | 5.2 ± 4.6 | <0.0001 |
| Median d | 3 | 5 | 4 | 5 | 3 | 4 | |||
| Prolonged length of hospital stay (>7 d), | 77 (8.7) | 157 (18.2) | <0.0001 | 151 (15.9) | 169 (18.2) | 0.29 | 150 (8.8) | 275 (16.1) | <0.0001 |
| d (±SD) | |||||||||
| In-hospital mortality, n (%) b,c | 3 (0.3) | 7 (0.8) | 0.21 | 4 (0.4) | 7 (0.7) | 0.37 | 8 (0.5) | 7 (0.4) | 0.80 |
OL indicates open lobectomy; RL, robotic-assisted lobectomy; VATS, video-assisted thoracoscopic lobectomy.
Unexpected returns to the OR were similar among all operative approaches. Postoperative transfusions were less frequent with RL versus OL and RL versus VATS. Median LOS was also significantly different among groups. Prolonged LOS (>7 days) was also significantly lower among the RL group compared to both OL and VATS groups. Similarly, significant differences in chest tube duration were also observed among the 3 groups. No significant differences in in-hospital mortality were observed among the 3 operative approaches. Mortality at 30-days was below 1% and was equivalent among groups.
Stratification of the propensity-matched data by tumor location did not impact any perioperative outcomes such as conversion rate, length of stay, overall morbidity or mortality.
Discussion
In this study, a minimally invasive approach to lobectomy was associated with significant reductions in peri-operative morbidity, lower transfusion rate, and lower LOS compared to OL. These findings are concordant with several previous publications, which have documented a reduction in morbidity and length of stay with both VATS and RL compared to OL. 2,4,6,12,13
A direct comparison of outcomes between VATS and RL is of particular interest given current clinical practice trends. Previous publications based on national databases have reported conflicting results. For instance, in a review of The Society of Thoracic Surgeons database from 2009–2013, morbidity, mortality, and length of stay were comparable between both approaches. 14 Similar findings were also observed in a recent analysis of the Surveillance, Epidemiology and End-Results Medicare database. 9 In contrast, other recent publications have suggested that RL is associated with improvements in clinically relevant outcome measures compared to VATS. For instance, 2 publications analyzing the Premiere database observed a reduced conversion rate, overall complication rate, and shorter LOS with RL. 2,15 Furthermore, a recent meta-analysis of 14 studies demonstrated statistically significant reductions in conversion and mortality rates with RL compared to VATS 16
In the current study, we observed differences in outcomes between the 2 minimally invasive approaches. RL was associated with a shorter operative time, suggesting that with experienced teams, robotic cases are not inherently longer than VATS. In the postoperative period, RL was associated with shorter chest tube duration and LOS compared to VATS. There were also fewer patients in the RL cohort with a prolonged LOS (>7 days). However, there was no difference in overall complications or mortality between RL and VATS.
The difference in conversion rates between RL and VATS was unexpected given that both groups had similar patient and stage characteristics after matching. The higher conversion rate in VATS was due to failure to progress during the case, as opposed to hemorrhage. It is possible that surgeons in the RL group only scheduled cases they were confident could be done robotically since the robotic system had to be scheduled in advance, whereas VATS surgeons may have considered starting any case thoracoscopically as a relatively low-risk approach. However, the observation that the conversion rate was lower in the RL group for every clinical stage suggests that there could be advantages with the robotic system during more difficult dissection.
A strength of this study is that we analyzed cases performed up to 2019, in contrast to previous database studies which did not include cases beyond 2015. 1,2,9 The inclusion of more recent cases is important for 2 reasons: 1) surgeons collectively will have greater experience with the robotic procedure and 2) the latest generation robotic platform was introduced in 2014 and has several technological advancements over the previous platform. We believe the inclusion of more recent cases allows a suitable comparison to the well-established alternatives of VATS and open lobectomy. We also mitigated possible bias by including only surgeons who were beyond their learning curve for each particular surgical approach.
However, there are important limitations to the present data. Although we performed a propensity-matched and intention-to-treat analysis, there are selection biases in a retrospective study that cannot be entirely overcome with statistical methods. For example, the higher transfusion rate in the OL group may reflect differences in tumor characteristics not accounted for by propensity score matching. Furthermore, variability between institutions, such as training of operating room personnel and the use of enhanced recovery after surgery programs may have an impact on operative times and LOS, independent of the operative approach utilized. 17 Every center had different postoperative patient management protocols, making LOS difficult to interpret. In addition, participation of trainees in the case may also have had an impact on operative time.
Other limitations in this multicenter retrospective study should also be acknowledged. For example, factors such as the cumulative lobectomy volume of individual surgeons and institutions, as well as the specific case volume on each platform would be expected to have an impact on outcomes. However, this data was not collected and consequently was not considered in the propensity-matched analysis. In addition, we should note that we only reported in-hospital complication rates, rather than the gold-standard 30 and 90-day rates. In part this was due to the variable follow-up of patients across surgeons and institutions, and thus patients were not consistently assessed at 30 and 90 days. In addition, we found that centers did not always distinguish between complications which occurred in-hospital and persisted after discharge versus new complications which developed after discharge. For both of these reasons we therefore reported in-hospital complication rates only in this analysis.
Importantly, the sponsorship of this study by industry and the potential for reporting bias should be discussed. We acknowledge that the concern for potential bias can never be completely mitigated. However, we feel that it is important to emphasize the measures undertaken to reduce such bias and maintain data integrity, and to place this study in the larger context of other studies performed on this subject.
To reduce bias, sites with recognized expertise in VATS and open lobectomy were invited to participate in this study, and cochairs from these institutions were selected to provide oversight on data integrity. Furthermore, institutions were required to submit data on all consecutive lobectomy cases during the study period, regardless of operative approach. Consequently, it is not the case that an institution with a high volume of robotic cases only submitted data on those cases while excluding open and VATS procedures.
Measures were also instituted to maintain integrity of the dataset. Nearly half of the cases were audited and compared to imaging and pathology reports to ensure accuracy. Similarly, all conversions to open thoracotomy were reviewed to document that the conversion was unplanned and to verify the reason for conversion. Additionally, outcome measures were analyzed based on the initial operative approach regardless of the reason for conversion. Furthermore, statistical analysis was performed by an independent biostatistician. Importantly, we should also emphasize that collection of data was independently undertaken by investigators at each institution. Although this does not eliminate bias, it is not the case that outcome data was submitted and extracted by industry representatives. The investigators at each site, regardless their personal experience in robotic, VATS or open lobectomy were responsible for data accuracy and submission.
Finally, while acknowledging the potential for bias we should also note that the findings in this study are concordant with previous publications. We observed no difference in mortality or morbidity between the VATS and robotic cohorts, and the reduction in length of stay and conversion rates in the robotic cohort has been observed in prior studies. Certainly, a randomized controlled trial comparing VATS, robotic-assisted and open lobectomy would provide more clarity on this topic. However, such a study would be very difficult to undertake in the United States. Issues of patient preference notwithstanding, many surgeons with minimally invasive experience are not equally skilled in both VATS and robotic-assisted procedures. As such it would be difficult to randomize patients to 1 approach over the other while ensuring the highest quality surgical care. In addition, financial considerations for such a study would pose a significant barrier outside of industry sponsorship. Given these limitations, we feel that the present design of a large-scale, multicenter retrospective study may provide the best source of information to guide clinical decision making at present.
In summary, in this retrospective multi-institutional data analysis, both RL and VATS lobectomy were associated with improved peri-operative outcomes compared to OL. RL was associated with additional differences compared to VATS, such as a reduced length of stay and conversion rate. The specific etiology of these differences requires further investigation. As additional cases are collected, more granular analysis of conversions, costmodeling, and cancer outcomes are planned.
Acknowledgments
The authors thank Intuitive Surgical, Inc. (Sunnyvale, CA USA) for funding this study in association with the PORTaL Consortium under a supportive clinical trial agreement, and we especially thank the project managers Madhu Gorrepati, MD and Clifford Lavarias, MS. We also acknowledge Daniel Oh, MD, Tami Crabtree, MS and Mimi Wainwright, respectively, for statistical analysis and editorial support.
Footnotes
Funding Source- Intuitive Surgical, Inc.; Abbas E. Abbas- Consultant, Intuitive Surgical; Robert J. Cerfolio- Consultant, Intuitive Surgical; Mark R. Dylewski- Consultant for Intuitive Surgical and Verb Surgical, Clinical Education Ethicon; Luis J. Herrera- Consultant, Intuitive Surgical; Kimble G. Jett- Consultant, Intuitive Surgical; Michael S. Kent- Speaker, Intuitive Surgical; Rishindra M. Reddy- Consultant for Intuitive Surgical and Auris Health, Advisory Board Medtronic; Patrick Ross- Consultant, Intuitive Surgical; Inderpal S.Sarkaria- Consultant, Intuitive Surgical; Lana Y. Schumacher- Proctor, Intuitive Surgical; William B. Tisol- Consultant, Intuitive Surgical.
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.annalsofsurgery.com.
References
- 1. Subramanian MP, Liu J, Chapman WC, et al. Utilization trends, outcomes, and cost in minimally invasive lobectomy. Ann Thorac Surg. 2019;108:1648–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oh DS, Reddy RM, Gorrepati ML, et al. Robotic-assisted, video-assisted VATS and open lobectomy: propensity-matched analysis of recent Premier data. Ann Thorac Surg. 2017;104:1733–1740. [DOI] [PubMed] [Google Scholar]
- 3. Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted VATS surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg. 2008;86:2008–2016. [DOI] [PubMed] [Google Scholar]
- 4. Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol. 2009;27:2553–2562. [DOI] [PubMed] [Google Scholar]
- 5. Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II nonsmall cell lung cancer: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e278S–e313S. [DOI] [PubMed] [Google Scholar]
- 6. Kent MS, Wang T, Whyte R, et al. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg. 2014;97:236–242. [DOI] [PubMed] [Google Scholar]
- 7. Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg. 2011;142:740–746. [DOI] [PubMed] [Google Scholar]
- 8. Casiraghi M, Galetta D, Borri A, et al. Ten years’ experience in robotic- assisted thoracic surgery for early stage lung cancer. J Thorac Cardiovasc Surg. 2019;67:564–572. [DOI] [PubMed] [Google Scholar]
- 9. Veluswamy R, Whittaker Brown SA, Mhango G, et al. Comparative effectiveness of robotic-assisted surgery for resectable lung cancer in older patients. Chest. 2020;157:1313–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feczko AF, Wang H, Nishimura K, et al. Proficiency of robotic lobectomy based on prior surgical technique in The Society of Thoracic Surgeons General Thoracic Database. Ann Thorac Surg. 2019;108:1013–1020. [DOI] [PubMed] [Google Scholar]
- 11. Paul S, Jalbert J, Isaacs AJ, et al. Comparative effectiveness of robotic-assisted vs VATS lobectomy. Chest. 2014;146:1505–1512. [DOI] [PubMed] [Google Scholar]
- 12. Cajipe MD, Chu D, Bakaeen FG, et al. Video-assisted VATS lobectomy is associated with better perioperative outcomes than open lobectomy in a veteran population. Am J Surg. 2012;204:607–612. [DOI] [PubMed] [Google Scholar]
- 13. Swanson SJ, Meyers BF, Gunnarsson CL, et al. Video-assisted VATS lobectomy is less costly and morbid than open lobectomy: a retrospective multi- institutional database analysis. Ann Thorac Surg. 2012;93:1027–1032. [DOI] [PubMed] [Google Scholar]
- 14. Louie B, Wilson JL, Kim S, et al. Comparison of video-assisted thoracoscopic surgery and robotic approaches for clinical stage I and stage II non-small cell lung cancer using The Society of Thoracic Surgeons database. Ann Thorac Surg. 2016;102:917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reddy RM, Gorepatti ML, Oh DS, et al. Robotic-assisted versus thoracoscopic lobectomy outcomes from high-volume thoracic surgeons. Ann Thorac Surg. 2018;106:902–908. [DOI] [PubMed] [Google Scholar]
- 16. Liang H, Liang W, Zhao L, et al. Robotic versus video-assisted lobectomy/segmentectomy for lung cancer: a meta-analysis. Ann Surg. 2018;268:254–259. [DOI] [PubMed] [Google Scholar]
- 17. Rogers LJ, Bleetman D, Messenger DE, et al. The impact of enhanced recovery after surgery (ERAS) protocol compliance on morbidity from resection for primary lung cancer. J Thorac Cardiovasc Surg. 2018;155:1843–1852. [DOI] [PubMed] [Google Scholar]


