Objective:
To investigate postoperative functional connectivity (FC) alterations across impaired cognitive domains and their causal relationships with systemic inflammation.
Background:
Postoperative cognitive dysfunction commonly occurs after cardiac surgery, and both systemic and neuroinflammation may trigger its development. Whether FC alterations underlying deficits in specific cognitive domains after cardiac surgery are affected by inflammation remains unclear.
Methods:
Seventeen patients, who underwent cardiac valve replacement, completed a neuropsychological test battery and brain MRI scan before surgery and on days 7 and 30 after surgery compared to age-matched healthy controls. Blood samples were taken for tumor necrosis factor-a and interleukin-6 measurements. Seed-to-voxel FC of the left dorsolateral prefrontal cortex (DLPFC) was examined. Bivariate correlation and linear regression models were used to determine the relationships among cognitive function, FC alterations, and cytokines.
Results:
Executive function was significantly impaired after cardiac surgery. At day 7 follow-up, the surgical patients, compared to the controls, demonstrated significantly decreased DLPFC FC with the superior parietal lobe and attenuated negative connectivity in the default mode network, including the angular gyrus and posterior cingulate cortex. The left DLPFC enhanced the connectivity in the right DLPFC and posterior cingulate cortex, all of which were related to the increased tumor necrosis factor-a and decreased executive function up to day 7 after cardiac surgery.
Conclusions:
The decreased FC of executive control network and its anticorrelation with the default mode network may contribute to executive function deficits after cardiac surgery. Systemic inflammation may trigger these transient FC changes and executive function impairments.
Keywords: cardiac surgery, executive function network, fMRI, postoperative cognitive dysfunction, systemic inflammation
Postoperative cognitive dysfunction (POCD) is a common complication after surgery and is associated with prolonged hospitalization, greater risk of withdrawal from work, and increased use of healthcare resources. 1 The incidence of POCD varies widely depending on the type of surgery and the criteria used to define POCD. Previous studies have reported that up to 46% of patients might experience POCD after cardiac surgery, which is much higher than other types of surgery. 2 POCD after cardiac surgery was once attributed to cerebral particulate and gaseous emboli from cardiopulmonary bypass (CPB). 3 Several randomized studies have suggested, however, that there are no significant or lingering associations between perioperative emboli and cognition. 4 Mounting evidence shows that systemic inflammatory responses induced by the surgery itself and the use of CPB may be responsible. 5
Surgical trauma causes the release of damage-associated molecular patterns which activate the innate immune response stimulating cytokine release. These, in turn, trigger a systemic inflammatory response and neuroinflammation; all of which contribute to the POCD development. 6–8 CPB can cause a more widespread inflammatory response by activating the immune system after contact between blood and artificial materials of the bypass circuit in cardiac surgery. In addition, ischemia-reperfusion injury after CPB may increase bloodbrain barrier permeability, which further enhances neuroinflammation and disrupts postoperative cognition. 9 An animal study showed that POCD after cardiac surgery, compared to noncardiac surgery, affects more cognitive domains and was associated with more widespread neuroinflammation in the brain. 10 However, to date, research is limited to explore the causal relationship among inflammation, brain functional connectivity (FC), and cognitive decline after surgery in humans.
Functional magnetic resonance imaging (fMRI) is a noninva- sive and powerful tool that can examine brain FC and alterations. A resting-state fMRI (rsfMRI) study suggested that systemic inflammation induced by lipopolysaccharide affects brain FC in large-scale networks, 11 such as disrupting connectivity within the default mode network (DMN), which is known to play a role in working memory and other aspects of cognition. 12 Browndyke et al reported that the activity and connectivity of the posterior cingulate cortex (PCC), a critical region in the DMN, was positively correlated with global cognitive changes 6 weeks after cardiac surgery. 13,14 Huang et al found that more than 25% of older patients showed significant functional network decline within 48 hours of total knee arthro- plasty. 15 However, it remains elusive whether and how systemic inflammation induced by cardiac surgery affects the connectivity between functional networks.
In this study, we comprehensively assessed the cognitive outcomes of patients who underwent cardiac surgery and age-matched nonsurgical controls and examined postoperative alterations in corresponding brain networks according to specific cognitive decline. Inflammatory cytokines in blood were also measured for correlation analyses with neuropsychological performance and FC alterations over the perioperative period. We hypothesized that cardiac-surgical patients with higher levels of plasma inflammatory cytokines would show a decline in some cognitive domains, accompanied by abnormalities in corresponding brain networks.
Methods
Participants and Study Design
This study was approved by the Ethics Committee of Xuzhou Central Hospital, Jiangsu, China (Code: XZXY-LJ-20170818), and registered on the Chinese clinical trial registration (ChiCTR-OOC- 17012542). The study was conducted in accordance with principles of the Declaration of Helsinki. After obtained written informed consent, 17 patients scheduled to undergo valve replacement surgery under CPB and general anesthesia were enrolled into this cohort study. The healthy controls of 18 volunteers matched by age, education, and sex were also recruited. All eligible participants met the following inclusion criteria: aged between 50 and 70, more than sixth grade education, and had a Mini-Mental State Examination score ≥23. Patients and healthy controls with a history of craniocerebral surgery, cerebrovascular disease, hepatorenal failure, psychiatric illness, alcoholism, illiteracy, left handedness, or metal implants unsafe for MRI were excluded. All participants completed neuropsychological tests, brain MRI scans, and blood sample harvesting. Participants undergoing surgery were scanned preoperatively and at the day 7 and day 30 after cardiac surgery, while controls had an MRI scan once.
Perioperative Management
All patients received general anesthesia according to our standardized practice. Anesthesia was induced with midazolam (0.05 mg/kg), cisatracurium (0.3 mg/kg), etomidate (0.3 mg/kg), and sufentanil (5 µg/kg) and maintained with remifentanil, sevoflurane, and propofol maintaining bispectral index between 40 and 60. Heart rate, arterial pressure, respiratory rate, body temperature, PETCO2, and SpO2 were continuously monitored. All patients received surgery under the standard CPB. The nasopharyngeal or rectal temperature during CPB was maintained under mild hypothermia (32°C). Intraoperative blood salvage and a-stat pH management were used. Perfusion pressure was kept at 60–80 mm Hg using norepinephrine and the pump flow was maintained at a rate of 2.0–2.5 L/min/m2. The hematocrit was maintained at above 21% during CPB and above 25% during the remaining perioperative period. The rate of body rewarm was maintained at around 0.25°C per minute. Postoperative delirium was assessed using the confusion assessment method for intensive care units on day 1 to 3 after cardiac surgery. All patients received standardized postoperative pain relief with hydromorphone. Immediately after arrival at the intensive care unit, patients received hydromorphone as target-controlled infusion with a plasma target concentration of 2 ng/mL until extubation, and then intravenous hydromorphone patient-controlled analgesia was initiated and infused at a rate of 0.3 µg/kg/min, and a 0.5 mg bolus per hour when needed via the pump (CP-E200 PCA device, Zhejiang Sujia Medical Device Co., Ltd, Jiaxing, China) for the first 2 days after surgery.
Neuropsychological Assessment
Patients’ cognitive function was assessed with neuropsychological battery tests before surgery and on days 7 and 30 after cardiac surgery. The controls received the same neuropsychological tests at an identical time interval. The neuropsychological tests include 5 cognitive domains assessments: attention, language, memory, executive function, and visuospatial function. The tests included digit span (forward and backward), Corsi block, verbal fluency test, paired associate verbal learning, digit symbol test, trail-making test, and grooved pegboard test (favored hand). All assessment measures were carried out by a neurologist.
Inflammatory Cytokine Measurement
Blood samples were collected before surgery and on day 2, 1 week, and 1 month after surgery. Blood samples from controls were collected only once. Blood samples were centrifuged at 4000 rpm for 10 minutes and plasma samples were kept at –80°C. The samples were then used to measure tumor necrosis factor-a (TNF-a) and interleukin-6 (IL-6) with enzyme-linked immunosorbent assay kits (Abclonal Biotechnology Co., Ltd., Woburn, Massachusetts, United States).
Neuroimaging Procedures and Data Acquisition
Patients underwent MRI scans (Siemens Skyra 3 Tesla scanner with a 20-channel head coil) before surgery and on days 7 and 30 after cardiac surgery. The controls were only scanned once. High- resolution sagittal three-dimensional magnetization-prepared rapid acquisition with gradient echo structural images were acquired with the following parameters: matrix, 256 × 256; field-of-view, 256 mm × 224 mm; 192 one-millimeter-thick slices; echo time, 2.98 ms; repetition time, 2530 ms. Axial T2-weighted imaging images were acquired with the following parameters: matrix, 320 × 320; field-of-view, 230 mm × 230 mm; 18 six-millimeter-thick slices; echo time, 99 ms; repetition time, 6000 ms. Rs-fMRI was acquired with the following parameters: matrix, 64 × 64; field-of-view, 220 mm × 220 mm; 35 three-millimeter-thick slices; echo time, 30 ms; repetition time, 2000 ms.
fMRI Data Preprocessing
Rs-fMRI data were preprocessed using SPM12 (Statistical Parametric Mapping, Wellcome Department of Imaging Neuroscience, London, UK), and seed-to-voxel correlation analysis was carried out by the FC (CONN) toolbox v19c. 16 The first 10 time points were removed to avoid magnetic coil saturation. The remaining images were corrected for slice timing and then realigned. Next, the T1 images were co-registered to the realigned images and segmented into grey matter, white matter, and cerebrospinal fluid. Then, the functional images were spatially normalized into Montreal Neurological Institute space using transformations from segmentation, resampled to 3 × 3 × 3 mm 3 images, and smoothed with a 6-mm full-width at half-maximum isotropic Gaussian kernel. After preprocessing, the processes of bandpass filtering (0.008–0.09 Hz), detrending, and regression of 6 motion parameters and their first- order derivatives, and signals of white matter and cerebrospinal fluid (using CompCor strategy 17 ) were further performed. Hypothesis- driven seed-to-voxel correlation analysis was employed. The seed regions of the left DLPFC were defined by WFU_PickAtlas (http://fmri.wfubmc.edu/cms/software). The correlation coefficients between the seed voxels and all other brain voxels were computed to generate correlation maps. For group analyses, the correlation coefficients were converted to z-values using Fisher r-to-z transformation to improve normality. 18
Statistical Analyses
Sample size is normally not applicable for human fMRI study. The sample size in our current study was chosen based on previous studies reported in this field. 13,14,19 Clinical parameters and neuropsychological measurements were expressed as dot plot, mean ± standard deviation or number (percentage) and then statistically analyzed with analysis of variance followed by post hoc Norman- Keuls test for comparison or unpaired t-test where appropriate (SPSS, Chicago, IL).
General linear models were performed on FC maps using a voxel-wise comparison across the whole brain. An absolute grey matter threshold of 0.2 was used to avoid possible edge effects around the border between grey matter and white matter. Paired t- tests were conducted to examine the alterations between visit times within the surgery group. Unpaired t-tests were performed to explore dysconnectivity in the surgery group compared to the control group. FC results were reported based on an uncorrected voxel-wise height threshold of P < 0.001 combined with a false discovery rate- corrected cluster-wise threshold of P < 0.05.
Regions with significant alterations in patients were further defined as regions of interest (ROIs). To explore the relationships among FC values in these ROIs, performance on neuropsychological assessments, and levels of inflammation, correlation analyses were first performed. Multiple linear regression was then used to explore the relationships between potential correlates and cognitive performance. A 2-sided P value < 0.05 was considered to be of statistical significance.
Results
Demographics and Surgical Variables
Forty patients and 25 healthy age-matched volunteers were screened for this study recruitment. Six patients and 3 controls were excluded because of low Mini-Mental State Examination scores. Sixteen patients and 4 controls were further excluded because of cerebral infarction confirmed by MRI. In addition, 1 patient was excluded due to excessive head motion. Seventeen patients and 18 controls were finally recruited and completed the study. There were no significant differences of the demographics and clinical characteristics between the 2 groups (Table 1). In addition, 4 (23.5%) of 17 patients had postoperative delirium occurred up to the postoperative day 2 (Table 1) and they all recovered on the postsurgical day 3. There was no regional cerebral ischemia or stroke in our patients after cardiac surgery from their MRI scan.
Table 1.
Demographics, Clinical and Surgical Characteristics
| Patients (n = 17) | Controls (n = 18) | P value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (yr) | 57.9 ± 7.8 | 57.1 ± 6.9 | 0.741 |
| Sex (F/M) | 14/3 | 14/4 | 0.534 |
| BMI (kg/m2) | 25.1 ± 2.0 | 25.8 ± 2.4 | 0.347 |
| Education (yr) | 9.3 ± 2.0 | 9.56 ± 2.0 | 0.706 |
| Clinical characteristics | |||
| History of hypertension | 2 (11.8%) | 3 (16.7%) | 0.528 |
| Diabetes mellitus | 2 (11.8%) | 1 (5.6%) | 0.478 |
| MMSE scores | 25.4 ± 1.6 | 25.6 ± 1.5 | 0.696 |
| LVEF % | 56.7 ± 3.0 | 57.4 ± 2.8 | 0.494 |
| Postoperative delirium | 4 (23.5%) | ||
| Surgical characteristics | |||
| Duration of procedure (min) | 207.5 ± 21.4 | ||
| Duration of pump CPB (min) | 115.4 ± 14.6 | ||
| Duration of aortic cross clamp (min) | 80.1 ± 14.7 |
Data are presented as mean ± SD or number (percentage). Qualitative data were analyzed with Chi-square test, and other data were analyzed with 2-sample t-tests. BMI indicates body mass index; CPB, cardiopulmonary bypass; LVEF, left ventricular ejection fraction; MMSE, Mini-Mental State Examination; SD, standard deviation.
Baseline and Postoperative Cognitive Outcomes
There were no statistically significant differences between the groups in their baseline cognitive performance. Significant intrasubject differences were detected for the Corsi block, digit symbol, trail-making, pegboard (favored hand), and paired association learning tests in the patients but not in the controls (Fig. 1). These neurocognitive battery variables showed executive function deficits on day 7 up to 30 days after cardiac surgery. Significant differences were found between the 2 groups (intersubject differences) in the scores of the Corsi block, digit symbol, trail-making, pegboard (favored hand), and paired association learning tests on day 7 after cardiac surgery. On day 30 after cardiac surgery, there were no significant differences between the 2 groups in the scores on the trailmaking test and pegboard test (Supplemental Table 1, http://links.lww.com/SLA/D243).
Figure 1.
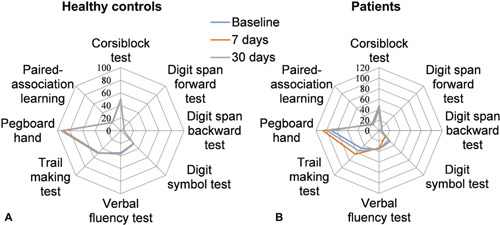
Neuropsychological assessment scores at baseline and at days 7 and 30 follow-ups in the 2 groups. A, The spider map shows that healthy controls did not have any significant changes in cognitive performance over a month. B, Surgery patients showed poor performance on the Corsi block, digit symbol, trail-making, pegboard (favored hand), and paired association learning tests on day 7 after cardiac surgery. These deficits were in remission on day 30 after cardiac surgery. For the trail-making test and pegboard test, the lower the scores are, the better the performance.
FC within the Group
The above findings suggested that executive function was significantly impaired among several cognitive domains. Therefore, we further investigated the FC of the executive control networks (ECNs) with other brain regions. The left dorsolateral prefrontal cortex (DLPFC), a critical region of the ECN, was selected as a seed region. The activity in the left DLPFC was positively correlated with that in the right DLPFC, paracingulate gyrus, frontal pole, intraparietal sulcus, and inferior temporal gyrus and negatively correlated with that in the medial frontal cortex and PCC in the 2 groups at baseline. In the surgery group, the anticorrelation between the DLPFC and PCC/medial frontal cortex disappeared on day 7 after cardiac surgery and reappeared on day 30 after cardiac surgery (Fig. 2; Supplemental Table 2, http://links.lww.com/SLA/D243).
Figure 2.
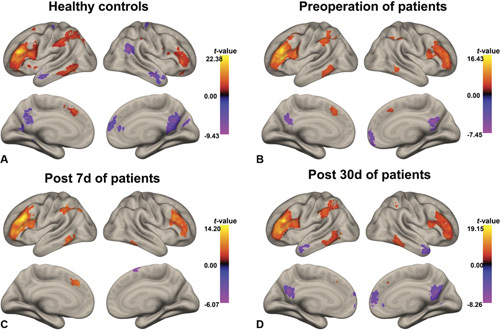
Positive and negative functional connectivity (FC) maps of the left DLPFC in healthy controls and surgery patients. A, Healthy controls. B, Surgery patients at baseline. C, Surgery patients on day 7 after cardiac surgery. D, Surgery patients on day 30 after cardiac surgery. The statistical threshold was P < 0.05. Colour bar indicates the t-value. DLPFC indicates dorsolateral prefrontal cortex.
FC between Groups and Longitudinal Changes within the Surgery Group
Compared with the controls, surgical patients demonstrated significant DLPFC FC changes with increased connections in the contralateral DLPFC (Fig. 3A and D) and decreased negative connections in the DMN, including with the angular gyrus (AG) and PCC, on day 7 after cardiac surgery (AG, t = 4.97, P < 0.001; PCC, t = 3.46, P < 0.005 uncorrected) (Fig. 3A, B, E, and F). The decreased negative connections between the left DLPFC and PCC on day 7 after cardiac surgery were alleviated at day 30 after cardiac surgery. Surgical patients also specifically exhibited decreased DLPFC FC in the superior parietal lobe (SPL) at day 7 after cardiac surgery compared to the preoperative assessment (t = –6.83, P < 0.001), and this decreased connectivity was alleviated at day 30 after cardiac surgery (Fig. 3C and G; Supplemental Table 3, http://links.lww.com/SLA/D243).
Figure 3.
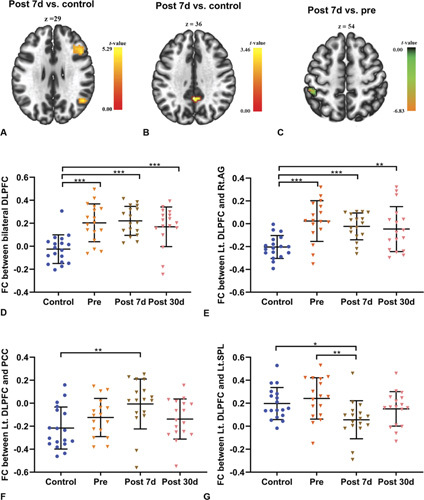
Functional connectivity between groups and longitudinal changes within the surgery group. A, Axial view of significant clusters in the right DLPFC and AG. B, Axial view of significant clusters in the PCC. C, Axial view of significant clusters in the left SPL. D, The Lt. DLPFC showed increased connectivity with the right DLPFC from baseline to day 30 after cardiac surgery. E, The Lt.DLPFC showed decreased connectivity with the right Ag from baseline to day 30 after cardiac surgery. F, The Lt.DLPFC showed decreased negative connectivity with the PCC in the patients on day 7 after cardiac surgery compared with the healthy controls. G, The Lt.DLPFC showed decreased connectivity with the left SPL on day 7 after cardiac surgery compared to baseline. Data are presented as mean ± SD (control, n = 18; patients, n = 17). *P < 0.05, **P < 0.01, ***P < 0.001. AG indicates angular gyrus; Control, healthy controls; Lt.DLPFC, left dorsolateral prefrontal cortex; Post 7 d, day 7 follow-up after the operation; Post 30 d, day 30 follow-up after the operation; Pre, preoperation; SD, standard deviation.
The Relationship of Executive Function Deficits and DLPFC Connectivity Changes after Cardiac Surgery
To elucidate the relationships between executive function deficits and DLPFC connectivity changes after cardiac surgery, we first conducted correlation analyses. The reduced negative connectivity between the left DLPFC and PCC/AG was associated with higher scores on the trail-making test (higher scores indicated worse cognitive performance). The increased connectivity between the bilateral DLPFC was associated with higher scores on the digit symbol test. The increased connectivity between the left DLPFC and SPL were associated with higher scores on the digit symbol test, and lower scores on the pegboard (favored hand) test (higher scores indicated worse cognitive performance) (Fig. 4; Supplemental Table 4, http://links.lww.com/SLA/D243).
Figure 4.

Association between DLPFC connectivity and executive function in surgery patients on day 7 after surgery. A, The reduced negative connectivity between the Lt.DLPFC and AG was associated with higher scores on the trail-making test. B, The reduced negative connectivity between the Lt.DLPFC and PCC was associated with higher scores on the trail-making test. C, The increased connectivity between the bilateral DLPFC was associated with higher scores on the digit symbol test. D, The increased connectivity between the Lt.DLPFC and SPL was associated with lower scores on the pegboard (favored hand) test. For the pegboard and trail-making tests, higher scores indicate worse cognitive performance. AG indicates angular gyrus; Lt.DLPFC, left dorsolateral prefrontal; PCC, posterior cingulate; SPL, superior parietal lobule.
We conducted multivariable linear regression models to further analyze the relationships between executive function and correlated connectivity. Performance on the digit symbol test was predicted by connectivity between the bilateral DLPFC [ß = 25.66, 95% confidence interval (CI): 10.25, 41.06] and by connectivity between the left DLPFC and SPL (ß = 14.22, 95% CI: 0.3233, 28.12). Performance on the trail-making test was predicted by connectivity between the left DLPFC and PCC (ß = 18.25, 95% CI: 0.33, 36.17). Performance on the pegboard (favored hand) test was predicted by connectivity between the left DLPFC and SPL (ß = –68.28, 95% CI: –133.8, –2.74). More details are presented in Supplemental Table 5, http://links.lww.com/SLA/D243.
Cytokine Changes and its Relationship with Connectivity Alterations and Executive Function
Enzyme-linked immunosorbent assay data showed that the plasma levels of TNF-α and IL-6 in the surgery patients were higher than those in the controls at baseline (TNF-α, F = 34.67, P < 0.001; IL-6, F = 90.09, P < 0.001]). However, the levels of TNF-α and IL-6 showed further increases on day 7 (TNF-α, F = 237.58, P < 0.001; IL-6, F = 1121.74, P < 0.001) after cardiac surgery and returned to near baseline by day 30 after cardiac surgery (Fig. 5). We first conducted bivariate correlation analyses to elucidate the relationships between cytokines and executive function deficits and the relationships between cytokines and DLPFC connectivity changes after cardiac surgery (Supplemental Table 4, http://links.lww.com/SLA/D243). The results of multiple linear regression indicated that performance on the trail-making test was predicted by the levels of TNF-a (ß = 2.41, 95% CI: 0.91, 3.91) and IL-6 (ß = 2.46, 95% CI: 0.69, 4.23). The connectivity between the left DLPFC and PCC (ß = 0.079, 95% CI: 0.022, 0.137) and connectivity between the bilateral DLPFC (ß = 0.061, 95% CI: 0.017, 0.104) were predicted by the level of TNF-α. These results suggested that a close relationship exists between executive function, DLPFC connectivity, and systemic inflammation, especially TNF-a levels, and between left DLPFC-PCC connectivity and the trail-making test (Fig. 6; Supplemental Table 6, http://links.lww.com/SLA/D243).
Figure 5.
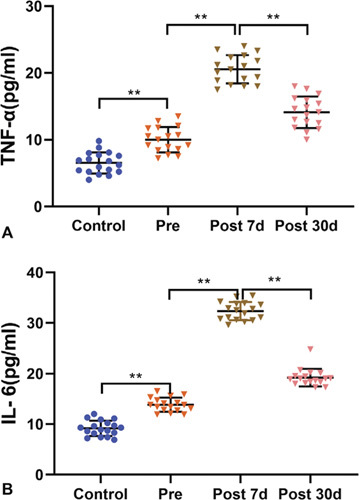
Plasma levels of TNF-α and IL-6 before and after surgery in surgery patients and healthy controls. A, Plasma levels of TNF-α in surgery patients were higher than those in healthy controls at baseline and further increased on day 7 after cardiac surgery before returning to near baseline on day 30 after cardiac surgery. B, The changes in the levels of IL-6 were similar to those of TNF-α in surgery patients. Data are presented as mean ± SD (control, n = 18; patients, n = 17). **P < 0.01. Control indicates healthy controls; IL-6, interleukin-6; Post 7 d, day 7 follow-up after the operation; Post 30 d, day 30 follow-up after the operation; Pre, preoperation; TNF-α, tumor necrosis factor-α; SD, standard deviation.
Figure 6.
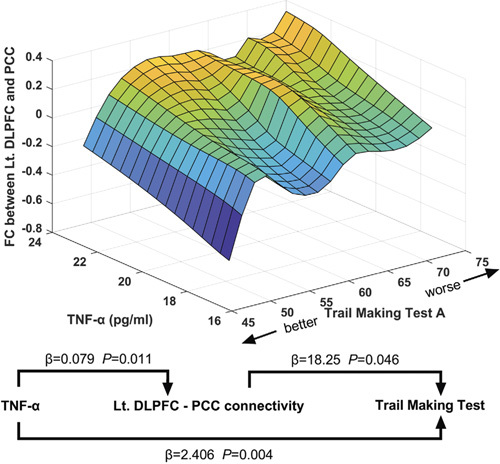
The relationships between TNF-α, Lt.DLPFC-PCC connectivity and executive function in 3 dimensions, based on multivariable linear regression models. Prediction of executive function by Lt.DLPFC-PCC connectivity and plasma levels of TNF-αon day 7 after surgery. Prediction of Lt.DLPFC-PCC connectivity by plasma levels of TNF-α on day 7 after surgery. Lt.DLPFC indicates left dorsolateral prefrontal; TNF- α, tumor necrosis factor-α.
Discussion
The current study examined the potential relationships/interaction between postoperative cognitive function, DLPFC connectivity, and systemic inflammation. The results revealed several aspects of neurocognition that were disrupted largely in executive function on day 7 after cardiac surgery. Compared to the controls, the surgical patients exhibited both preoperative and postoperative changes in DLPFC connectivity. In particular, the changes in the bilateral DLPFC, left DLPFC-SPL, and left DLPFC-AG/PCC connectivity predicted the executive function in the trail-making, digit symbol, and pegboard tests. The levels of TNF-α and IL-6 in the surgery patients were remarkably increased on day 7 after cardiac surgery. Importantly, the connectivity changes in the left DLPFC-PCC and the bilateral DLPFC were associated with the change in TNF-α levels. In addition, the data in the trail-making test was related to the levels of TNF-α and IL-6. Together, our findings suggest that systemic inflammation may cause the changes of DLPFC connectivity which, in turn, may be responsible for the POCD development after surgery.
POCD is a common complication after major surgeries, in particular, cardiac surgery, and is characterized with the impairments of several cognitive domains including executive function, memory, attention, and psychomotor speed. We found that surgery patients showed executive function deficits (Corsi block, trail-making, and digit symbol tests). The results are in agreement with other previous studies showing that executive function declined significantly in several cognitive domains after surgery. 2,20 The executive function has been broadly described as higher-order cognitive processes, such as working memory, task switching, fluency, and inhibitory control. 21 As an advanced cognition, the brain regions that govern executive function are vulnerable to various pathophysiological factors including neuroinflammation. Furthermore, in line with our findings, several studies have demonstrated poorer performance of the executive function during preclinical disease stages. 22 Intact executive function is critical for the ability to adapt environmental changes, and deficits in executive function lead to disproportionate cognitive impairment and daily living activities. 23 This may explain why a previous study showed that patients with POCD had an increased risk of leaving the labor market. 24
Several brain regions involved in executive function are collectively called the ECN. 25 This network is associated with information maintenance and manipulation (working memory), goal-directed attentional control, and other high-order cognitive control. 26 Much of the research has shown that executive function declines in mild cognitive impairment and Alzheimer’s disease are strongly linked to the activity changes in the ECN. 27,28 In the present study, the left DLPFC, a critical region in the ECN, was selected as a ROI. The FC in the left DLPFC showed decreased negative connections with the AG throughout the entire perioperative period and decreased negative connections with the PCC up to day 7 after cardiac surgery. One recent study suggested that other networks may also play an important role in executive function, especially the DMN. 29,30 The PCC and AG are the main elements of the DMN. The activity in the DMN is anticorrelated with the brain networks associated with executive function. 31 At the resting state, the ECN is deactivated, and the DMN is active. In contrast, during the demanding cognitive tasks, the ECN is active, and the activity of the DMN is reduced. 32 The DMN is known to be responsible for internally focused thought processes, such as experience of the self and autobiographical memory. 33 The DMN needs to be deactivated to maintain attention and focus on externally directed tasks. Inadequate DMN suppression during externally directed tasks inevitably interferes with executive function performance, and has been shown to be associated with a number of diseases involving cognitive impairment. 29,34 Our results indicate that the decreased negative connectivity between the ECN and DMN very likely contributed to the poorer executive function performance on day 7 after cardiac surgery.
FC changes within the ECN during the perioperative period have attracted much attention. We found that positive connectivity between the bilateral DLPFC was higher at the baseline in the surgery patients. Previous studies found similar increased functional brain activity within the ECN in elderly individuals. 35 The increased connectivity within the ECN can be interpreted as its connectivity inefficiency or as compensation for the decreased negative connectivity between the ECN and DMN. 36 Interestingly, we also found that the positive connectivity between the left DLPFC and SPL was decreased on day 7 after cardiac surgery. The SPL is a component of the ECN and plays an important role in the attentional guidance of saccades. 37 The decreased connectivity between the left DLPFC and SPL showed that functional brain activity within the ECN declined after surgery. Coincidentally, a recent study showed that left DLPFC connectivity with the parietal operculum was negatively correlated with postoperative executive function together with reduced connectivity of the right superior parietal lobule with the precentral gyrus. 19 These similar results indicate that the ECN may fail in attempts at compensation when the negative connectivity between the ECN and DMN is further weakened postoperatively in surgical patients.
Evidence suggests that neuroinflammation after surgery is an important aetiological mechanism of POCD development. 38,39 The peripheral proinflammatory cytokines generated by surgical tissue damage enter the central nervous system causing neuroinflammation. 40 Neuroinflammation may impair neurogenesis, precipitate neuronal apoptosis and synaptic remodeling, and disrupt the integrity of the blood-brain barrier. 41 However, it remains elusive whether and how systemic inflammation towards neuroinflammation induced by surgery affects the connectivity of brain functional networks. Previous work conducted on surgical patients suggests that changes in the brain network connectivity and slow-wave activity (SWA) were likely responsible for delirium or even POCD development after surgery. 42 Interestingly, inflammation-driven SWA was also reported previously in mice. 43 In the present study, we found a significant association between the circulating inflammatory cytokines, DLPFC connectivity, and executive function performance. The higher levels of TNF-α predicted a decreased negative connectivity between the left DLPFC and PCC and an increased connectivity between the bilateral DLPFC on day 7 after cardiac surgery. In line with our data, recent studies also showed that higher peripheral inflammation was associated with FC changes within the ECN and DMN. 44 We also noted that compared with healthy controls, slight changes in the DLPFC connectivity and slightly higher levels of TNF-α and IL-6 were detected before surgery, which may support the notion that low- grade inflammation is associated with altered connectivity within large-scale cognitive networks and may confer as a risk for subsequent adverse neurocognitive outcomes. 45 Undoubtedly, surgery exacerbates the magnitude of inflammation in patients with valvul- opathy, which further weakened the negative connectivity between the ECN and DMN, leading to executive function decline after surgery.
The current study has several potential limitations. First, the present study had a relatively small sample size, which may have been underpowered to detect the effects of other brain functional connections on executive function. However, fMRI is a powerful tool to detect even a marginal change in the brain. The results of several independent studies with similar sample size, including our data, showed the perioperative change in FC is considerably reproducible. 14,19 Second, while peripheral inflammation predicted DLPFC connectivity and DLPFC connectivity, in turn, predicted executive function performance, causality, and mediating effects cannot be directly inferred from the present results. It might be possible for other unmeasured variables to be responsible for these relationships and therefore, this warrants further study. Third, the current study focused on 2 inflammatory cytokines (TNF-α and IL-6) that have been previously implicated in POCD pathogenesis. 46,47 Whether other inflammatory mediators including damage-associated molecular patterns play any role on the POCD development remains unknown. Although circulating inflammatory cytokines are directly related to neuroinflammation, 48 we still do not know the impact of the severity of neuroinflammation on regional brain function and the development of pathology. Using ligands that bind to activated microglia and imaging with positron emission tomography may be a good approach to study the distribution and/or severity of neuroinflammation and brain network, connectivity, and functional changes after surgery. 49 Fourth, postoperative delirium often occurs in patients who received major surgery; indeed, there is a high prevalence in patients after cardiac surgery. 50 Four (23.5%) of 17 patients had delirium on postoperative day 1 or 2 in our study which is in line with the incident rate in elderly patients after noncardiac surgery documented previously. 51 A previous elegant study demonstrated that neural connectivity changes and brain SWA may be very likely responsible for its development although other mechanisms including systemic inflammation and executive functional network changes found in the current study can be not excluded. However, one can argue that because in our study the MRI scan was only done on postoperative day 7 due to patients’ safety considerations, the causal relationship of delirium incidence and network changes reported here warrants further study. Fifth, postoperative cerebral regional ischemia or stroke including perioperative covert stroke has previously been shown to contribute to the development of postoperative delirium and/or POCD. 52 No such incidence was found in our patients albeit small sample size but the use of magnetic resonance diffusion-weighted technique in future study is warranted to further our understanding. Lastly, cluster-wise correction benefits from its high sensitivity to weak and disperse signals, while it provides low spatial specificity of the statistical significance of specific voxel within the clusters. To reduce the potential false-positive clusters, Woo et al. recommended uncorrected P < 0.001 as the primary voxel-wise threshold for cluster-forming. 53 However, to further improve the confidence degree of statistical inferences in specific anatomical regions, voxel-wise correction is required in the future.
In summary, the current study provides evidence that attenuated anticorrelation between the DMN and ECN together with decreased FC within the ECN likely contribute to executive function deficits after cardiac surgery. Furthermore, systemic inflammation before and after surgery may trigger the brain functional structure changes and, in turn, result in neurocognitive impairments but the exact mechanisms and causes of these changes warrant further study.
Supplementary Material
Acknowledgments
The authors thank Dr Lei Ping for the neuropsychological assessment and Dr Rui Li for instructions on the statistical analyses.
Footnotes
Y.Z., M.Z., and X. J. contributed equally to the study.
Author Contributions: D.M., Q.Y., L.W. contributed to conception and design of the studies. Y.Z., M.Z., X.J., W.Z., Y.S., S.B., S.R., M.P.V., K.W., and C.W. contributed to acquisition or analysis of data. Y.Z., M.Z., and X.J. contributed to drafting the text or preparing the figures. All authors wrote the manuscript and approved the submission.
This work was supported by grants from the National Natural Science Foundation of P.R. China (No. 82071903, No. 81870857, No. 81801332, No. 82025018, and No. 62076169); this work was also supported in part by the Science and Technology Planning Project of Xuzhou (KC20071) and the Medical Research Project of Jiangsu Commission of Health (No. 201848).
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.annalsofsurgery.com.
References
- 1. Steinmetz J, Christensen KB, Lund T, et al. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110:548–555. [DOI] [PubMed] [Google Scholar]
- 2. Relander K, Hietanen M, Rantanen K, et al. Postoperative cognitive change after cardiac surgery predicts long-term cognitive outcome. Brain Behav. 2020;10:e01750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abu-Omar Y, Cader S, Guerrieri Wolf L, et al. Short-term changes in cerebral activity in on-pump and off-pump cardiac surgery defined by functional magnetic resonance imaging and their relationship to microembolization. J Thorac Cardiovasc Surg. 2006;132:1119–1125. [DOI] [PubMed] [Google Scholar]
- 4. Scott DA, Evered LA, Gerraty RP, et al. Cognitive dysfunction follows left heart catheterisation but is not related to microembolic count. Int J Cardiol. 2014;175:67–71. [DOI] [PubMed] [Google Scholar]
- 5. Jongman RM, Zijlstra JG, Kok WF, et al. Off-pump CABG surgery reduces systemic inflammation compared with on-pump surgery but does not change systemic endothelial responses: a prospective randomized study. Shock. 2014;42:121–128. [DOI] [PubMed] [Google Scholar]
- 6. Alam A, Hana Z, Jin Z, et al. Surgery, neuroinflammation and cognitive impairment. EBioMedicine. 2018;37:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vizcaychipi MP, Watts HR, O’Dea KP, et al. The therapeutic potential of atorvastatin in a mouse model of postoperative cognitive decline. Ann Surg. 2014;259:1235–1244. [DOI] [PubMed] [Google Scholar]
- 8. Wan Y, Xu J, Meng F, et al. Cognitive decline following major surgery is associated with gliosis, ß-amyloid accumulation, and t phosphorylation in old mice. Crit Care Med. 2010;38:2190–2198. [DOI] [PubMed] [Google Scholar]
- 9. Ma D, Yang H, Lynch J, et al. Xenon attenuates cardiopulmonary bypass- induced neurologic and neurocognitive dysfunction in the rat. Anesthesiology. 2003;98:690–698. [DOI] [PubMed] [Google Scholar]
- 10. Hovens IB, van Leeuwen BL, Mariani MA, et al. Postoperative cognitive dysfunction and neuroinflammation; cardiac surgery and abdominal surgery are not the same. Brain Behav Immun. 2016;54:178–193. [DOI] [PubMed] [Google Scholar]
- 11. Labrenz F, Wrede K, Forsting M, et al. Alterations in functional connectivity of resting state networks during experimental endotoxemia–an exploratory study in healthy men. Brain Behav Immun. 2016;54:17–26. [DOI] [PubMed] [Google Scholar]
- 12. Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38:433–447. [DOI] [PubMed] [Google Scholar]
- 13. Browndyke JN, Berger M, Harshbarger TB, et al. Resting-state functional connectivity and cognition: after major cardiac surgery in older adults without preoperative cognitive impairment: preliminary findings. J Am Geriatr Soc. 2017;65:e6–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Browndyke JN, Berger M, Smith PJ, et al. Task-related changes in degree centrality and local coherence of the posterior cingulate cortex after major cardiac surgery in older adults. Hum Brain Mapp. 2018;39:985–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang H, Tanner J, Parvataneni H, et al. Impact of total knee arthroplasty with general anesthesia on brain networks: cognitive efficiency and ventricular volume predict functional connectivity decline in older adults. J Alzheimers Dis. 2018;62:319–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. [DOI] [PubMed] [Google Scholar]
- 17. Behzadi Y, Restom K, Liau J, et al. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. NeuroImage. 1998;7:119–132. [DOI] [PubMed] [Google Scholar]
- 19. Mohanty R, Lindroth H, Twadell S, et al. A pilot study of neural correlates of perioperative executive function associated with noncardiac surgery in the elderly. Br J Anaesth. 2019;123:e517–e518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Price CC, Garvan CW, Monk TG. Type and severity of cognitive decline in older adults after noncardiac surgery. Anesthesiology. 2008;108:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Munro BA, Weyandt LL, Hall LE, et al. Physiological substrates of executive functioning: a systematic review of the literature. Atten Defìc Hyperact Disord. 2018;10:1–20. [DOI] [PubMed] [Google Scholar]
- 22. Kirova AM, Bays RB, Lagalwar S. Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer’s disease. Biomed Res Int. 2015;2015:748212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roy S, Ficarro S, Duberstein P, et al. Executive function and personality predict instrumental activities of daily living in alzheimer disease. Am J Geriatr Psychiatry. 2016;24:1074–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Funder KS, Steinmetz J, Rasmussen LS. Cognitive dysfunction after cardiovascular surgery. Minerva Anestesiol. 2009;75:329–332. [PubMed] [Google Scholar]
- 25. Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Uddin LQ, Supekar KS, Ryali S, et al. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J Neurosci. 2011;31:18578–18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chand GB, Wu J, Hajjar I, et al. Interactions of the salience network and its subsystems with the default-mode and the central-executive networks in normal aging and mild cognitive impairment. Brain Connect. 2017;7:401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li C, Li Y, Zheng L, et al. Abnormal brain network connectivity in a triplenetwork model of Alzheimer’s disease. J Alzheimers Dis. 2019;69:237–252. [DOI] [PubMed] [Google Scholar]
- 29. Syan SK, Owens MM, Goodman B, et al. Deficits in executive function and suppression of default mode network in obesity. Neuroimage Clin. 2019;24:102015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brown CA, Schmitt FA, Smith CD, et al. Distinct patterns of default mode and executive control network circuitry contribute to present and future executive function in older adults. NeuroImage. 2019;195:320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Andrews-Hanna JR, Reidler JS, Huang C, et al. Evidence for the default network’s role in spontaneous cognition. J Neurophysiol. 2010;104:322–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barber AD, Caffo BS, Pekar JJ, et al. Decoupling of reaction time-related default mode network activity with cognitive demand. Brain Imaging Behav. 2017;11:666–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. [DOI] [PubMed] [Google Scholar]
- 34. Fassbender C, Zhang H, Buzy WM, et al. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res. 2009;1273:114–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fernandez NB, Hars M, Trombetti A, et al. Age-related changes in attention control and their relationship with gait performance in older adults with high risk of falls. NeuroImage. 2019;189:551–559. [DOI] [PubMed] [Google Scholar]
- 36. Grady C. The cognitive neuroscience of ageing. Nat Rev Neurosci. 2012;13:491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Molenberghs P, Mesulam MM, Peeters R, et al. Remapping attentional priorities: differential contribution of superior parietal lobule and intraparietal sulcus. Cereb Cortex. 2007;17:2703–2712. [DOI] [PubMed] [Google Scholar]
- 38. Jin Z, Hu J, Ma D. Postoperative delirium: perioperative assessment, risk reduction, and management. Br J Anaesth. 2020;125:492–504. [DOI] [PubMed] [Google Scholar]
- 39. Fung A, Vizcaychipi M, Lloyd D, et al. Central nervous system inflammation in disease related conditions: mechanistic prospects. Brain Res. 2012;1446:144–155. [DOI] [PubMed] [Google Scholar]
- 40. Fidalgo AR, Cibelli M, White JP, et al. Systemic inflammation enhances surgery-induced cognitive dysfunction in mice. Neurosci Lett. 2011;498:63–66. [DOI] [PubMed] [Google Scholar]
- 41. Miller AA, Spencer SJ. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav Immun. 2014;42:10–21. [DOI] [PubMed] [Google Scholar]
- 42. Tanabe S, Mohanty R, Lindroth H, et al. Cohort study into the neural correlates of postoperative delirium: the role of connectivity and slow-wave activity. Br J Anaesth. 2020;125:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sultan ZW, Jaeckel ER, Krause BM, et al. Electrophysiological signatures of acute systemic lipopolysaccharide-induced inflammation: potential implications for delirium science. Br J Anaesth. 2021;126:996–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marsland AL, Kuan DC, Sheu LK, et al. Systemic inflammation and resting state connectivity of the default mode network. Brain Behav Immun. 2017;62:162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cervellati C, Trentini A, Bosi C, et al. Low-grade systemic inflammation is associated with functional disability in elderly people affected by dementia. GeroScience. 2018;40:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Terrando N, Monaco C, Ma D, et al. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci USA. 2010;107:20518–20522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hu J, Feng X, Valdearcos M, et al. Interleukin-6 is both necessary and sufficient to produce perioperative neurocognitive disorder in mice. Br J Anaesth. 2018;120:537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reinsfelt B, Westerlind A, Blennow K, et al. Open-heart surgery increases cerebrospinal fluid levels of Alzheimer-associated amyloid ß. Acta Anaes- thesiol Scand. 2013;57:82–88. [DOI] [PubMed] [Google Scholar]
- 49. Forsberg A, Cervenka S, Jonsson Fagerlund M, et al. The immune response of the human brain to abdominal surgery. Ann Neurol. 2017;81:572–582. [DOI] [PubMed] [Google Scholar]
- 50. Sanders RD, Pandharipande PP, Davidson AJ, et al. Anticipating and managing postoperative delirium and cognitive decline in adults. BMJ. 2011;343:d4331. [DOI] [PubMed] [Google Scholar]
- 51. Su X, Meng ZT, Wu XH, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;388:1893–1902. [DOI] [PubMed] [Google Scholar]
- 52. Mrkobrada M, Chan MTV, Cowan D, et al. Perioperative covert stroke in patients undergoing non-cardiac surgery (NeuroVISION): a prospective cohort study. Lancet. 2019;394:1022–1029. [DOI] [PubMed] [Google Scholar]
- 53. Woo CW, Krishnan A, Wager TD. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage. 2014;91:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]


