Background:
According to the World Health Organization, the global burden of nosocomial infections is poorly characterized as surveillance systems are lacking. Nosocomial infections occur at higher rates in low- and lower-middle-income countries (LMICs) than in high-income countries (HICs). Current global RSV burden estimates are largely based on community-acquired infection. We aimed to characterize children with nosocomial RSV-related mortality and to understand the potential impact of RSV immunization strategies.
Materials:
RSV GOLD is a global registry of children younger than 5 years who died with laboratory-confirmed RSV infection. We compared clinical and demographic characteristics of children with nosocomial and community-acquired RSV in-hospital mortality.
Results:
We included 231 nosocomial and 931 community-acquired RSV-related in-hospital from deaths from 65 countries. Age at death was similar for both groups (5.4 vs. 6 months). A higher proportion of nosocomial deaths had comorbidities (87% vs. 57%; P < 0.001) or was born preterm (46% vs. 24%; P < 0.001) than community-acquired deaths. The proportion of nosocomial deaths among all RSV deaths was lower in LMICs than in upper-middle-income countries (UMICs) and HICs (12% vs. 18% and 26%, respectively).
Conclusions:
This is the first global case series of children dying with nosocomial RSV infection. Future infant-targeted immunization strategies could prevent the majority of nosocomial RSV-related deaths. Although nosocomial RSV deaths are expected to occur at highest rates in LMICs, the number of reported nosocomial RSV deaths was low in these countries. Hospital-based surveillance is needed to capture the full burden of nosocomial RSV mortality in LMICs.
Keywords: respiratory syncytial virus, nosocomial infection, community-acquired infection, child mortality, global health
INTRODUCTION
Respiratory syncytial virus (RSV) is a major cause of lower respiratory tract infection (LRTI) and LRTI-related death in young children.1 In 2015, the overall RSV mortality burden was estimated at 118,200 annual deaths in children younger than 5 years of age worldwide.2 These global burden estimates are largely based on community-acquired RSV-related deaths because of the paucity of data on healthcare-associated or nosocomial RSV-related mortality. According to the World Health Organization (WHO), the burden of nosocomial infections is poorly characterized in LMICs as surveillance systems are lacking.3 Nevertheless, available data show that the burden of nosocomial infections is significantly higher in low- and middle-income countries (LMICs) compared with high-income countries (HICs).4 Furthermore, RSV has been reported as the most important viral cause of nosocomial LRTI in children.5–8
The proportional contribution of nosocomial RSV mortality to the global RSV mortality burden is expected to be substantial, as nosocomial RSV infection is generally more severe than community-acquired RSV infection.9–11 Currently, several RSV vaccine candidates and monoclonal antibodies are in late phase clinical development.12 To identify vaccine target populations and to estimate the potential impact of these RSV prevention strategies, healthcare policy makers require information on the characteristics of children with life-threatening RSV infection including those with nosocomial RSV infection. However, previous reports of pediatric nosocomial RSV-related deaths are from single centers and not large enough (number of deaths ranging between 1 and 21)13 to understand the demographic and clinical characteristics of children dying with nosocomial RSV infection globally.
Through our global RSV mortality registry, we aimed to describe demographic and clinical characteristics of children younger than 5 years of age dying in the hospital with laboratory-confirmed nosocomial RSV infection.
MATERIALS AND METHODS
Study Design and Patients
The RSV GOLD study is an ongoing global online mortality registry that includes individual patient data of children younger than 5 years of age who died with RSV infection. The first study results, published in 2017, included only community-acquired, in-hospital deaths.14 Since then, the registry has been extended to also include nosocomial and out-of-hospital pediatric RSV-related deaths from January 1, 1995, onwards.15 To obtain nosocomial RSV-related mortality patient data, we performed a systematic search in PubMed using the following search terms: “RSV” or “bronchiolitis” combined with “healthcare-associated pneumonia,” “nosocomial,” “community-acquired,” “in-hospital,” “hospital-borne,” or “cross-infection” (See Table, Supplemental Digital Content 1, http://links.lww.com/INF/E850). We invited authors to share patient data until July 31, 2021, using a link to our electronic data capture platform Research Online.16 We included published and unpublished data of children 0–59 months old who had died with laboratory-confirmed RSV infection in the hospital between January 1, 1995, and July 31, 2021. We excluded children for whom it was unknown where RSV infection had been acquired (in-community or in-hospital [n = 489]). We collected demographic and clinical characteristics using an online questionnaire within Research Online.
Definitions
We categorized country of origin as LMIC (LIC and LMIC combined), upper-middle-income country (UMIC), or HIC according to the World Bank classifications for 2021.17 We defined nosocomial RSV infection as a positive laboratory-confirmed RSV test in combination with a ≥48-hour timeframe between hospital admission and onset of respiratory symptoms, and as reported by the RSV GOLD collaborator. Laboratory-confirmed RSV infection was defined as the presence of RSV in a patient’s sample detected through PCR, immunofluorescence, enzyme immunoassay, culture, or serology. Length of hospital stay was defined as the duration of the hospital admission in days starting from the beginning of respiratory symptoms. Comorbidity was defined as the presence of at least 1 underlying disease. Prematurity was defined as gestational age less than 37 weeks. If data for comorbidities or prematurity were not reported (n = 17), we assumed that the children were otherwise healthy and born at term. If data for location of death were missing (n = 3), we assumed that the death had occurred in hospital.
Nosocomial RSV Infection and Community-acquired RSV-related Mortality
We compared the demographic and clinical characteristics of children with nosocomial RSV-related in-hospital death and those with community-acquired RSV-related in-hospital death, including previously published deaths.14,18 We analyzed the data separately for different income groups and calculated for each income group the odds ratios and 95% confidence interval (CI) for the presence of demographic and clinical characteristics in children with hospital-acquired RSV relative to children with community-acquired RSV.
Sensitivity Analyses
We excluded deaths with missing data for comorbidities or prematurity and compared demographic and clinical characteristics. We also analyzed the age distribution for nosocomial RSV deaths from LMICs after excluding deaths collected from a postmortem study conducted in Zambia in which only children <6 months of age were included (n = 12).19
Ethical Approval
The institutional research board of the University Medical Centre Utrecht waived the requirement for parental consent as only deidentified secondary patient data are used in the RSV GOLD study. Collaborators sharing data were encouraged to adhere to local site regulations for ethics approval. Where required, local ethical approval was obtained.
Statistical Analysis
We present descriptive statistics for all variables. For continuous variables we report the median with interquartile range (IQR); for categorical variables we report the frequency and proportion. We used the Fisher’s exact test to determine statistical significance between groups for dichotomous parameters and the Mann-Whitney U test for all continuous parameters. We applied the Bonferroni correction for multiple testing between World Bank Income groups. All statistical analyses were performed with SPSS (version 21.0; IBM Corp, Armonk, NY). The forest plot with odds ratios and 95% CIs was made in R version 4.0.3.
RESULTS
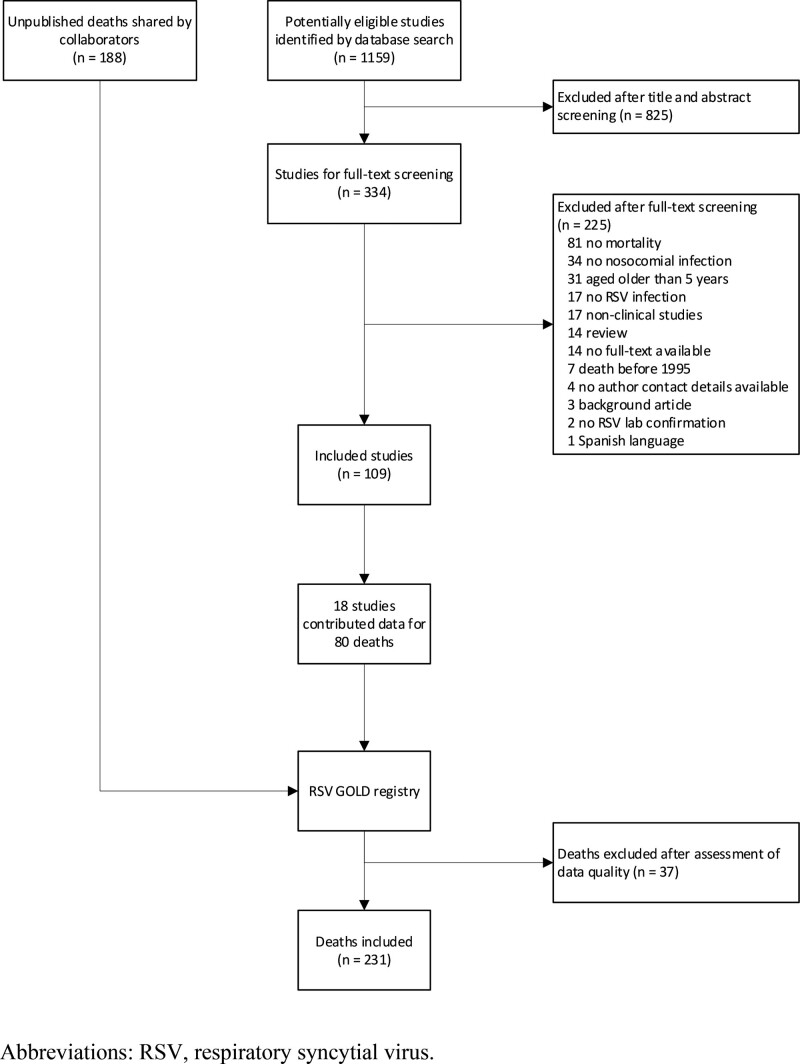
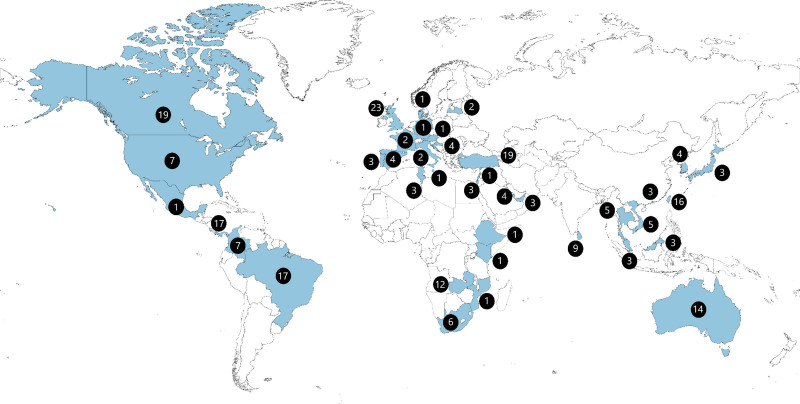
We included a total of 231 nosocomial RSV-related deaths from 38 countries (Figs. 1 and 2, see Table, Supplemental Digital Content 3, http://links.lww.com/INF/E850) of which 80 deaths were identified through the literature search (see Tables, Supplemental Digital Content 1–2, http://links.lww.com/INF/E850) and 188 comprised unpublished reports shared by collaborators. The 231 deaths accounted for 20% of all deaths reported to the RSV GOLD registry with available data on where RSV infection was acquired. This proportion was even higher (31%) when only taking into account countries from where both nosocomial as well as community-acquired in-hospital deaths were contributed. The majority (n = 123; 53%) of reported deaths were from HICs. The proportion of reported nosocomial deaths among all RSV-related in-hospital deaths was substantially lower in LMICs (12%) than in UMICs (18%, p=0.03) or HICs (26%, P < 0.001).
FIGURE 1.
Flowchart of included nosocomial RSV deaths.
FIGURE 2.
Location of death for children younger than 5 yrs with nosocomial RSV-related mortality included in the analysis.
Clinical and Demographic Characteristics of Children With Nosocomial RSV-related Mortality
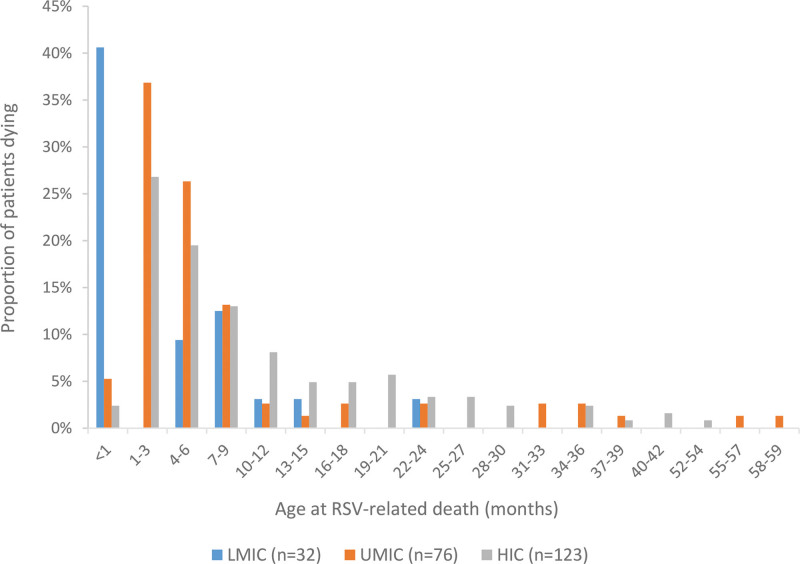
The vast majority of children with nosocomial RSV-related death had 1 or more comorbidities (n = 202, 87.4%) as expected. Congenital heart disease was the most prevalent comorbidity occurring in almost half (n = 96, 41.6%) of all nosocomial deaths. Of these, at least 7 (7%) acquired RSV infection following cardiac surgery. In total, 106 (45.9%) children had been born prematurely. Median age at time of death was 5.4 (IQR: 2.5–12.1) months. Half of the deaths (51.9%) occurred within the first 6 months of life (Table 1, Fig. 3). All healthy term or preterm children without comorbidities died at an age younger than 10 months, whereas children with comorbidities also died at an older age (see Figure, Supplemental Digital Content 10, http://links.lww.com/INF/E850).
TABLE 1.
Characteristics of Children Younger Than 5 Yrs With Nosocomial and Community-acquired RSV-related In-hospital Death
| Nosocomial (n = 231) | Community-acquired (n = 931) | P | |
|---|---|---|---|
| Male sex | 107 (46.3) | 500 (53.7) | 0.05 |
| Age at death (mo) | 5.4 (2.5-12.1) | 6.0 (2.6-13.0) | 0.24 |
| Neonatal death | 18 (7.8) | 38 (4.1) | 0.03 |
| <3 mo at death | 66 (28.6) | 244 (26.2) | 0.51 |
| <6 mo at death | 120 (51.9) | 452 (48.5) | 0.38 |
| Year of death | 2012 (2006-2016) | 2011 (2006-2015) | 0.12 |
| LMIC | 32 (13.9) | 236 (25.3) | <0.001 |
| UMIC | 76 (32.9) | 338 (36.3) | 0.36 |
| HIC | 123 (53.2) | 357 (38.3) | <0.001 |
| Comorbidity* | 202 (87.4) | 527 (56.6) | <0.001 |
| Congenital heart disease | 96 (41.6) | 209 (22.4) | <0.001 |
| Chronic lung disease | 42 (18.2) | 112 (12.0) | 0.02 |
| Genetic disease | 48 (20.8) | 134 (14.4) | 0.02 |
| Down syndrome | 18 (7.8) | 47 (5.0) | 0.11 |
| Neurological disease | 39 (16.9) | 135 (14.5) | 0.36 |
| Immune disorder | 15 (6.5) | 31 (3.3) | 0.04 |
| Other | 68 (29.4) | 139 (14.9) | <0.001 |
| Prematurity* | 106 (45.9) | 225 (24.2) | <0.001 |
| Gestational age (weeks) | 36.0 (32.0–38.3); n = 174 | 38.0 (34.0–39.0); n = 496 | 0.004 |
| Birth weight (kgs) | 2.5 (1.7–3.1); n = 168 | 2.7 (2.0–3.2); n = 449 | 0.02 |
| Breastfeeding until age ≤4 mo | 36/139 (25.9) | 161/404 (39.9) | 0.003 |
| Length of hospital stay (d) | 23.0 (9.8–53.3); n = 230 | 9.0 (4.0–20.0); n = 901 | <0.001 |
| Intensive care unit (ICU) admission | 209/216 (96.8) | 641/868 (73.8) | <0.001 |
| Length of ICU stay (d) | 14.0 (7.0–30.0); n = 194 | 10.0 (4.0–21.0); n = 601 | <0.001 |
| Respiratory support | 210/210 (100) | 685/686 (99.9) | 1.00 |
| Mechanical ventilation | 186/230 (80.9) | 611/902 (67.7) | <0.001 |
| Duration of respiratory support (d) | 14.0 (7.0–29.0); n = 195 | 10.0 (4.0–20.0); n = 588 | <0.001 |
Data are presented as n (%), n/N (%), or median (IQR).
Considered absent when missing.
HIC indicates high-income country; LMIC, low-income and lower-middle-income country; UMIC, upper-middle-income country.
FIGURE 3.
Age distribution at time of RSV-related nosocomial in-hospital death for children younger than 5 yrs from LMIC, UMIC and HIC. HIC indicates high-income countries; LMIC, low- and middle-income countries; UMIC, upper-middle-income countries.
Nosocomial RSV-related Mortality Analyzed by World Bank Income Group
Children from LMICs were younger at time of death than children from UMICs (1.5 vs. 5 months, P = 0.003) and HICs (1.5 vs. 7 months, P < 0.001). More children from LMICs died during the neonatal period (40.6%) than children from UMICs (3.9%) and HICs (1.6%), P < 0.001. Furthermore, compared with HICs, children from LMICs were more often born prematurely (68.8% vs. 43.1%, p=0.01), had a shorter hospital stay (7 days vs. 37 days, P < 0.001) and were less often mechanically ventilated (46.9% vs. 85.2%, P < 0.001). See Table, Supplemental Digital Content 4, http://links.lww.com/INF/E850, for additional characteristics for children analyzed by income group are included in.
Nosocomial Versus Community-acquired RSV-related Mortality
The proportion of preterm children was higher among children with nosocomial RSV infection compared with children with community-acquired RSV infection (45.9% vs. 24.2%, P < 0.001). Likewise, a higher proportion of children with nosocomial RSV infection had at least 1 comorbidity (87.4% vs. 56.6%, P < 0.001). Age at death was similar for both groups. We compared both nosocomial and community-acquired deaths for each income group separately to rule out sampling bias by underrepresentation of deaths from LMICs. In this analysis, children from LMICs with nosocomial RSV-related death were younger than children with community-acquired death (1.5 vs. 5.0 months, P = 0.002). For children from UMICs and HICs, age at death was similar (see Tables, Supplemental Digital Content 6–8, http://links.lww.com/INF/E850). The odds ratios and 95% CIs for the presence of demographic and clinical characteristics in children with nosocomial versus community-acquired RSV-related mortality in all included children and stratified by income group are presented in Figure (Supplemental Digital Content 5, http://links.lww.com/INF/E850).
Sensitivity Analyses
When analyzing the data after excluding children with missing data for comorbidity or prematurity (n = 19 for nosocomial RSV deaths; n = 231 for community-acquired RSV deaths), our findings did not change (see Table, Supplemental Digital Content 9, http://links.lww.com/INF/E850). After excluding deaths collected from the postmortem study in Zambia (n = 12), median age increased to 5 months for nosocomial deaths from LMICs.
DISCUSSION
Globally, RSV is a major cause of child mortality. Current RSV mortality burden estimates are largely based on community-acquired RSV infections. We report the first global case series characterizing children younger than 5 years who died with nosocomial RSV infection. We observed that nosocomial RSV-related deaths accounted for 20% of all in-hospital RSV deaths reported to the RSV GOLD registry. This proportion indicates that nosocomial RSV-related deaths substantially contribute to the global burden of RSV-related child mortality.
While literature indicates that nosocomial infections generally occur at higher rates in LMICs than in HICs,4 the small number of nosocomial deaths from LMICs suggests these cases are underreported in LMICs. For example, data from the Zambia Pertussis Infant Mortality Estimation Study (ZPRIME) showed that 32% of RSV deaths in a tertiary referral hospital in Lusaka, Zambia, were caused by nosocomial RSV infection.19 The low number of LMIC nosocomial deaths in the RSV GOLD registry may be explained by suboptimal testing capacity and a lack of active RSV surveillance systems as well as general underreporting of nosocomial deaths because of limited time and resources. Furthermore, ascertainment bias could play a role as data from LMICs mainly result from RSV studies that usually do not include nosocomial RSV deaths, whereas data from HICs may also result from routine testing.
We found that age at death was similar for children with nosocomial and community-acquired RSV-related death. To our knowledge, there are no other studies available reporting on age in deceased pediatric patients with nosocomial and community-acquired infection. Two prospective studies from Vietnam and Germany report a younger age in pediatric patients with nosocomial RSV infection,9,20 while studies from Brazil and the UK report an older age for nosocomial RSV patients (although the age difference was no longer significant when comparing comorbidity-matched controls in the UK study).13,21 More than half of reported nosocomial deaths were in children younger than 6 months at time of death. This age distribution indicates that infant-targeted RSV immunization strategies will prevent a large part of nosocomial RSV-related mortality globally.
Most children with nosocomial RSV-related mortality had, as expected, at least 1 comorbidity. Most frequently reported comorbidities were congenital heart disease, certain genetic disorders such as trisomy 21 (Down syndrome), and neurological disease. These findings are in line with findings from other studies that identified congenital heart disease, Down syndrome, immunodeficiency,7 and prematurity9 as risk factors for acquiring RSV infection in the hospital. In a Canadian study, children with nosocomial RSV infection were more likely to have pre-existing risk factors for severe disease including prematurity, immunodeficiency, lung disease, or heart disease, and stayed longer in the hospital than children with community-acquired RSV infection, which is comparable to our results.10 In our registry, there were more premature infants with RSV-related nosocomial death from LMICs (69%) compared with UMICs (41%) or HICs (43%) and less children with comorbidity (69%) compared with children from HICs (96%). Possibly, in LMICs, children with comorbidities may die as a result from these comorbidities and do not survive long enough to be exposed to RSV. Furthermore, children who are born preterm in the hospital may acquire nosocomial RSV infection and subsequently die at a younger age before comorbidities are identified. This contrasts with HICs, where children who die with RSV generally have comorbidities and are susceptible to severe nosocomial infection at an older age from repeated exposure to RSV through multiple hospital admissions. Taken together, differences in clinical characteristics may partially be explained by differences in quality of healthcare.
This study has several limitations inherent to retrospective data collection. First, the majority of the reported deaths were from UMICs and HICs while LMICs represent more than 75% of the global pediatric population and have a higher prevalence of nosocomial infections.22 Second, since not all authors responded or shared data, we missed at least 24 deaths reported in the literature. All but 3 missed published deaths were from UMICs or HICs and median age at death was 1 month (information on age available for 7 deaths). Third, authors employed variable definitions for nosocomial infection (time interval between admission and start of respiratory symptoms ranging from 48 hours to 7 days), which may have over- or underestimated our results. Although we did not collect these definitions, we checked for each hospital-acquired RSV mortality case whether the time interval between admission and symptoms was at least 48 hours. Furthermore, there were no studies that involved surveillance on nosocomial postdischarge deaths. The actual burden of nosocomial RSV-related mortality may therefore be higher than reported in the literature. Reporting bias could also lead to an underestimation of nosocomial RSV-related mortality as RSV outbreaks may not always be reported or detected by hospitals. Fourth, we did not collect information on the reason for hospital admission before the child acquired RSV, nor on location where RSV infection was acquired (ward or intensive care unit). Furthermore, nearly half of nosocomial RSV deaths had pre-existing cardiac disease. We have limited data on whether these children acquired RSV infection during admission for cardiac surgery or during admission for other reasons as this was not part of our questionnaire. Fifth, 35% of the cases identified in the literature resulted from an RSV outbreak. This may have created a bias in terms of some of the factors associated with nosocomial RSV death such as age or ICU admission. Last, retrospective data vary in quality and completeness, although we attempted to limit the impact of this methodological weakness with data quality checks and direct verification with collaborators.
Our study has clinical implications. RSV is one of the major causes of nosocomial outbreaks in pediatric wards as it is highly transmissible, with a basic reproduction number—the number of secondary infections caused by 1 infected child—estimated between 1 and 9.23 RSV is spread through direct contact or large droplet transmission but not through aerosol transmission.24 Nosocomial RSV infections are associated with increased hospital costs because of prolonged hospitalization and extra interventions, and with high morbidity and mortality.25,26 Our results show that nosocomial RSV-related deaths may substantially contribute to the global burden of RSV-related child mortality. More than half of reported deaths were younger than 6 months and may have been prevented by infant-targeted RSV immunization strategies such as long-acting monoclonal antibodies. Future modeling studies should consider nosocomial RSV-related deaths as a target group when calculating potential vaccine impact. While the global community awaits market-approval and implementation of maternal vaccination and introduction of nirsevimab, a highly potent long-acting antibody that recently met the primary endpoint in a phase-III trial,12 infection-control measures remain of major importance to prevent nosocomial infections. Although high-quality evidence is lacking, multicomponent control strategies appear broadly successful.27 Strict infection-control measures including hand hygiene and the use of protective equipment (goggles, gloves, gowns, masks), can reduce nosocomial infection rates by 39–76%.28
In conclusion, this study provides insight into characteristics of global nosocomial RSV-related mortality in young children. Prospective studies and hospital-based surveillance are needed to demonstrate the magnitude of the nosocomial RSV burden in LMICs. Infection-control measures, in particular hand hygiene, remain of significant importance to prevent nosocomial infections in pediatric wards worldwide.
ACKNOWLEDGMENTS
We are grateful to the collaborators of the RSV GOLD study group for sharing data with the RSV GOLD registry. We thank Dr. Sheikh Wasik Rahman, Mr. Shuborno Islam, Ms. Naito Kanon, Prof. Nawshad Ahmed, Dr. Rubana Sultana Aflatun and Dr. Sohel Mahmud of the Child Health Research Foundation for their intellectual, laboratory, and technical assistance with compiling the RSV dataset of Bangladesh. We thank Prachi Vora and Padmini Srikantiah for their scientific advice. Furthermore, we thank Femke Vernooij from the RSV GOLD student operations team for her help with data analyses. RSV GOLD study group collaborators are listed at Supplemental Digital Content 11, http://links.lww.com/INF/E895.
Supplementary Material
Footnotes
This work was supported by the Bill & Melinda Gates Foundation [OPP1148988].
L.J.B. has regular interaction with pharmaceutical and other industrial partners. He has not received personal fees or other personal benefits. UMCU has received major funding (>€100,000 per industrial partner) for investigator-initiated studies from AbbVie, MedImmune, Janssen, the Bill and Melinda Gates Foundation, Nutricia (Danone) and MeMed Diagnostics. UMCU has received major cash or in kind funding as part of the public private partnership IMI-funded RESCEU project from GSK, Novavax, Janssen, AstraZeneca, Pfizer and Sanofi. UMCU has received major funding by Julius Clinical for participating in the INFORM study sponsored by MedImmune. UMCU has received minor funding for participation in trials by Regeneron and Janssen from 2015-2017 (total annual estimate less than €20,000). UMCU received minor funding for consultation and invited lectures by AbbVie, MedImmune, Ablynx, Bavaria Nordic, MabXience, Novavax, Pfizer, Janssen (total annual estimate less than €20,000). L.J.B. is the founding chairman of the ReSViNET Foundation. H.N. has received grants from Innovative medicines initiative and Pfizer. He has received honorarium from Abbvie, Sanofi, ReViral, Janssen and Novavax. For the remaining authors none were declared.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
Contributor Information
Yvette N. Löwensteyn, Email: y.n.loewensteyn@umcutrecht.nl.
Joukje E. Willemsen, Email: J.E.Willemsen-9@umcutrecht.nl.
Natalie I. Mazur, Email: N.I.Mazur@umcutrecht.nl.
Nienke M. Scheltema, Email: nmscheltema@gmail.com.
Nynke C. J. van Haastregt, Email: C.J.vanHaastregt-2@umcutrecht.nl.
Amber A. A. ten Buuren, Email: a.a.a.tenbuuren@students.uu.nl.
Ichelle van Roessel, Email: I.M.A.vanRoessel-2@umcutrecht.nl.
Dunja Scheepmaker, Email: d.scheepmaker@students.uu.nl.
Harish Nair, Email: Harish.Nair@ed.ac.uk.
Peter M. van de Ven, Email: P.M.vandeVen-3@umcutrecht.nl.
Collaborators: Josep Estrada, Angela Gentile, Maria Florencia Lucion, Marcela Echavarria, Noelia Reyes, Fernando P. Polack, Mauricio T. Caballero, Annette Alafaci, Nigel Crawford, Jenny Thompson, Warwick Butt, Nusrat Homaira, Adam Jaffe, Gemma Saravanos, Philip Britton, Christoph Binder, Angelika Berger, Bernhard Resch, Fahmida Chowdhury, Md. Ariful Islam, Senjuti Saha, Samir Saha, José Gareca Perales, Felipe Cotrim de Carvalho, Sergio de Andrade Nishioka, Maria Tereza da Costa Oliveira, Carla Cecília de Freitas Lázaro Emediato, Heloisa Giamberardino, Jane Melissa Webler, Regina GrigolliCesar, Daniel Jarovsky, Daniela Gregória Bomfim Prado da Silva, Patricia Gomes de Matos Bezerra, Maria do Carmo Menezes Bezerra Duarte, Fernanda de-Paris, Márcia Rosane Pires, Sonia M. Raboni, Tani Sagna, Serge Diagbouga, Daniel Garros, Michael Hawkes, Joanne M. Langley, Jill Mutch, Kirk Leifso, Shaun K. Morris, Waison Wong, Bosco A. Paes, Jesse Papenburg, Franco Diaz, Rodrigo A. Fasce, Olga Lopez, Ting F. Leung, Wei Su, Chiang Chun Yuan, Zhengde Xie, Junhong Ai, Evelyn Obando Belalcazar, Jaime Fernández-Sarmiento, Ledys Izquierdo, Rubén Lasso, Rosalba Pardo-Carrero, Pablo Vasquez, Eliana Zemanate, Srđan Roglić, Thea K. Fischer, Sune Rubak, Mette Holm, Domenica de Mora, Alfredo Bruno, Jenny Ojeda, Lida Zamora, Erica Dueger, Terho Heikkinen, Gilles Cambonie, Jean-Christophe Dubus, Melina Messaoudi, Juliet Bryant, Haoua Tall, Bradford D. Gessner, Dominique Ploin, Come Horvat, Nour Hanna, Christian Vogelberg, Christoph Härtel, Andrea Streng, Johannes Liese, Barbara A. Rath, Jürgen Seidenberg, Geeske Stelljes, Evangeline Obodai, John Kofi Odoom, Irini Eleftheriou, Maria Tsolia, Vassiliki Papaevangelou, Elpiniki Kartsiouni, Tapan Dhole, Sheetal Verma, Rashmi Ranjan Das, Ashish Satav, Agustinus Sutanto, Dario Prais, Orli Megged, Francesca Tortora, Fabrizio Chiusolo, Giovanni Gabutti, Kazuki Iio, Satoshi Kusuda, Hitoshi Oshitani, Hisato Ito, Najwa Khuri-Bulos, Loai Saadah, Iman Basheti, Sandra S. Chaves, Gideon Emukule, Patrick K. Munywoki, D. James Nokes, Grieven Paul Otieno, Ieva Silina, Abdulla Alfraij, Mohammad Alghounaim, Ghassan Dbaibo, Rima Hanna-Wakim, Yoke-Fun Chan, Jamal I-Ching Sam, Teck-Hock Toh, Jeffrey Soon-Yit Lee, David Pace, Socorro P Lupisan, Marilla G. Lucero, Daniel E. Noyola, Andreu ComasGarcía, Túfaria Mussá, Brigitte Buiteman, Diederick E. Grobbee, Rosalie Linssen, Namrata Prasad, José F. Sánchez, Uzma Bashir Aamir, Qalab Abbas, Abdul Momin Kazi, Sidra Asif Khan, Rodrigo DeAntonio, Xavier Saez-Llorens, Juana del Valle-Mendoza, Wojciech Feleszko, Karolina Dumycz, Sara Cristina de Tavares Ferreira, Ana Beatriz de Sousa Pereira Luzio Vaz, Mohammed Ahmed Abdullah Qashnon, Khalid Alansari, Eun Hwa Choi, Yae-Jean Kim, Eun Lee, Eun-Ae Yang, Hyun Mi Kang, Kirill Stolyarov, Thoon Koh Cheng, Kee Thai Yeo, Chee Fu Yung, Stefan Grosek, Marko Pokorn, Cheryl Cohen, Shabir A. Madhi, Michelle J. Groome, Jocelyn Moyes, Marietjie Venter, Adele Visser, Cristina O’Callaghan-Gordo, Quique Bassat, Cristina Calvo, Xavier Carbonell-Estrany, Francisco J. Elola, Manuel Sánchez Luna, Rosa Rodriguez Fernandez, Cristian Launes, Carmen Muñoz-Almagro, J.A.A. Sampath Jayaweera, Martin W. Weber, Joachim Luthander, Ulrich Heininger, Daniel Trachsel, Claudia E. Kuehni, Cristina Ardura-Garcia, Chia-Yu Chi, Hsin Chi, Shuenn-Nan Chiu, Jou-Kou Wang, Yhu-Chering Huang, Piyarat Suntarattiwong, Somsak Thamthitiwat, Nasamon Wanlapakorn, Aida Borgi, Ahmed Ayari, Imen Bel Hadj, Khadija Boussetta, Benan Bayrakci, Esra Koçkuzu, Muhterem Duyu, Sule Gökçe, Aykut Eşki, Tanil Kendirli, Emrah Gün, Edward A. Goka, Simon Nadel, Marwa Ghazaly, Kentigern Thorburn, Paul S. McNamara, Soledad Menta, Nicolás Monteverde, Sebastián González-Dambrauskas, Dianna M. Blau, Katherine Horton, Robert F. Breiman, Andrea Buchwald, Helen Chu, Leah Forman, Christopher J. Gill, Lawrence Mwananyanda, Aubree Gordon, Natasha Halasa, Danielle Hessong, Diego Raul Hijano, Kim J. Allison, Matthew S. Kelly, Veena Kumar, Asuncion Mejias, Octavio Ramilo, Katherine O’Brien, Saad B. Omer, Thyyar Ravindranath, Steven L. Shein, Ananya Parlapalli, Eric A F Simões, Michael C Spaeder, Pham Thi Minh Hong, Tran Anh Tuan, Mohammed Al Amad, and Abdul Wahed Al Serouri
REFERENCES
- 1.Troeger C, Blacker B, Khalil IA, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18:1191–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390:946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Report on the burden of endemic health-care associated infection worldwide. https://apps.who.int/iris/bitstream/handle/10665/80135/9789241501507_eng.pdf. Accessed May 22, 2022.
- 4.Allegranzi B, Nejad SB, Combescure C, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet. 2011;377:228–241. [DOI] [PubMed] [Google Scholar]
- 5.Hall CB. Nosocomial respiratory syncytial virus infections: the “Cold War” Has Not Ended. Clin Infect Dis. 2000;31:590–596. [DOI] [PubMed] [Google Scholar]
- 6.Spaeder MC, Fackler JC. Hospital-acquired viral infection increases mortality in children with severe viral respiratory infection. Pediatr Crit Care Med. 2011;12:e317–e321. [DOI] [PubMed] [Google Scholar]
- 7.Sampath Jayaweera JAA, Reyes M. Childhood nosocomial viral acute respiratory tract infections in teaching hospital Anuradhapura, Sri Lanka. BMC Res Notes. 2019;12:581. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Ford-Jones EL, Mindorff CM, Langley JM, et al. Epidemiologic study of 4684 hospital-acquired infections in pediatric patients. Pediatr Infect Dis J. 1989;8:668–675. [DOI] [PubMed] [Google Scholar]
- 9.Simon A, Muller A, Khurana K, et al. Nosocomial infection: a risk factor for a complicated course in children with respiratory syncytial virus infection--results from a prospective multicenter German surveillance study. Int J Hyg Environ Health. 2008;211:241–250. [DOI] [PubMed] [Google Scholar]
- 10.Langley JML JC, Wang EEL, Law BJ, et al. Nosocomial respiratory syncytial virus infection in Canadian pediatric hospitals: a pediatric investigators collaborative network on infections in Canada study. Pediatrics. 1997;100:943–946. [DOI] [PubMed] [Google Scholar]
- 11.Lee YI, Peng CC, Chiu NC, et al. Risk factors associated with death in patients with severe respiratory syncytial virus infection. J Microbiol Immunol Infect. 2016;49:737–742. [DOI] [PubMed] [Google Scholar]
- 12.Nirsevimab MELODY Phase III trial met primary endpoint of reducing RSV lower respiratory tract infections in healthy infants. https://www.astrazeneca.com/media-centre/press-releases/2021/nirsevimab-phase-iii-trial-met-primary-endpoint.html. Accessed May 22, 2022.
- 13.Thorburn K, Eisenhut M, Riordan A. Mortality and morbidity of nosocomial respiratory syncytial virus (RSV) infection in ventilated children – a ten year perspective. Minerva Anestesiol. 2012;78:782–789. [PubMed] [Google Scholar]
- 14.Scheltema NM, Gentile A, Lucion F, et al. Global respiratory syncytial virus-associated mortality in young children (RSV GOLD): a retrospective case series. Lancet Global Health. 2017;5:e984–e991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazur NI, Löwensteyn YN, Willemsen JE, et al. Global respiratory syncytial virus–related infant community deaths. Clin Infect Dis. 2021;73(Supplement_3):S229–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Research Online. https://www.researchonline.info/en-us/. Accessed May 22, 2022.
- 17.The World Bank. World Bank Country and Lending Groups. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed May 22, 2022.
- 18.Löwensteyn YN, Phijffer E, Simons JVL, et al. respiratory syncytial virus-related death in children with down syndrome: the RSV GOLD Study. Pediatr Infect Dis J. 2020;39:665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forman LS, Macleod W, Mwananyanda L, et al. Association of respiratory syncytial virus infection and underlying risk factors for death among young infants who died at University Teaching Hospital, Lusaka Zambia. Clin Infect Dis. 2021;73(Supplement_3):S180–S186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuan TA, Thanh TT, Hai N, et al. Characterization of hospital and community-acquired respiratory syncytial virus in children with severe lower respiratory tract infections in Ho Chi Minh City, Vietnam, 2010. Influenza Other Respir Viruses. 2015;9:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De-Paris FB C, de Souza Nunes L, Mombach Pinheiro Machado AB, et al. Evaluation of RSV group A and B genotypes among nosocomial and community-acquired pediatric infections in southern Brazil. Virol J. 2014;11:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vilar-Compte D, Camacho-Ortiz A, Ponce-de-Leon S. Infection control in limited resources countries: challenges and priorities. Curr Infect Dis Rep. 2017;19:20. [DOI] [PubMed] [Google Scholar]
- 23.Reis J, Shaman J. Simulation of four respiratory viruses and inference of epidemiological parameters. Infect Dis Model. 2018;3:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bont L. Nosocomial RSV infection control and outbreak management. Paediatr Respir Rev. 2009;10:16–17. [DOI] [PubMed] [Google Scholar]
- 25.Comas-Garcia A, Aguilera-Martinez JI, Escalante-Padron FJ, et al. Clinical impact and direct costs of nosocomial respiratory syncytial virus infections in the neonatal intensive care unit. Am J Infect Control. 2020;48:982–986. [DOI] [PubMed] [Google Scholar]
- 26.Mosalli R, Alqarni SA, Khayyat WW, et al. Respiratory syncytial virus nosocomial outbreak in neonatal intensive care: a review of the incidence, management, and outcomes. Am J Infect Control. 2021:801–808. [DOI] [PubMed] [Google Scholar]
- 27.French CE, McKenzie BC, Coope C, et al. Risk of nosocomial respiratory syncytial virus infection and effectiveness of control measures to prevent transmission events: a systematic review. Influenza Other Respir Viruses. 2016;10:268–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drysdale SB, Green CA, Sande CJ. Best practice in the prevention and management of paediatric respiratory syncytial virus infection. Ther Adv Infect Dis. 2016;3:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.