Abstract
Symptomatic nonstenotic carotid artery disease has been increasingly recognized as a thromboembolic source in patients who would otherwise be classified as having embolic stroke of undetermined source. Evidence suggests that certain plaque features seen on sonography, CT, and MR imaging in nonstenotic carotid artery disease may predispose to recurrent stroke in patients with embolic stroke of undetermined source. We performed a focused literature review to further study plaque features in the context of embolic stroke of undetermined source and to determine which plaque features may be associated with ipsilateral ischemic events in such patients. Plaque thickness as seen on both ultrasound and CT appears to have a consistent association with ipsilateral stroke in patients with embolic stroke of undetermined source across multiple studies. Intraplaque hemorrhage as seen on MR imaging is now understood to have a strong association with ipsilateral stroke in patients with embolic stroke of undetermined source. Continued study of various plaque features as seen on different modalities is warranted to uncover other potential associations.
Up to one-third of strokes have no established mechanism and are considered to be cryptogenic.1 In 2014, the term “embolic stroke of undetermined source” (ESUS) was established as a clinical entity in patients with nonlacunar cryptogenic stroke in which an embolic source was thought to be most likely, despite an appropriate diagnostic evaluation with negative findings (Fig 1).2 This term (along with “cryptogenic stroke”) has also been used to define patients who may have multiple, competing stroke etiologies in which a definitive source cannot be determined. Patients with ESUS have been estimated to have a >4% risk of recurrent stroke per annum despite being on antiplatelet medication.1 Subsequent thought was directed to the idea that anticoagulation may be beneficial in such patients, given the likelihood that emboli originated from various nonevident atheroembolic sources.3 This premise provided the impetus for 2 large clinical trials that compared anticoagulation with aspirin alone in patients with ESUS.4,5 Despite this plausible theory, no benefit of anticoagulation was observed. These results spurred continued interest in additional diagnostics in order identify potential embolic sources in patients with ESUS.
FIG 1.
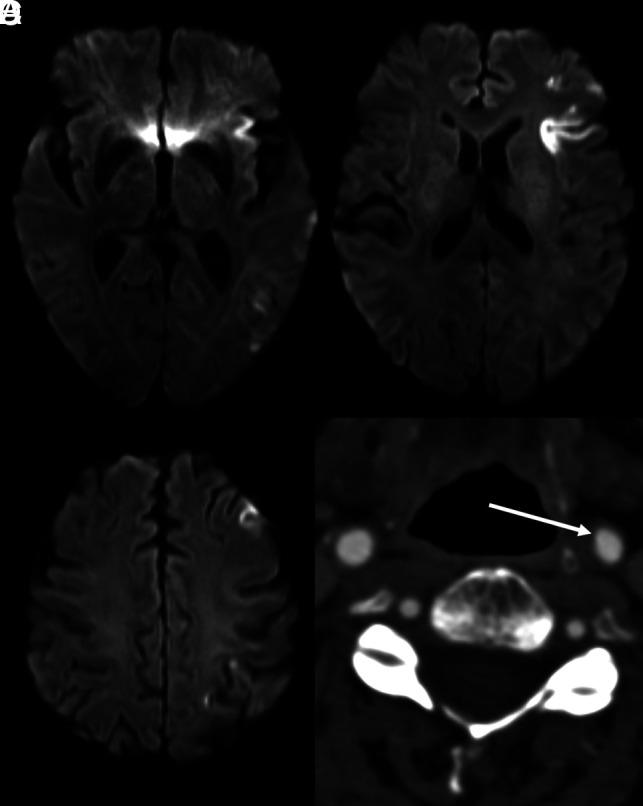
Sample case of ESUS. A–C, Axial diffusion-weighted MR images demonstrate multiple foci of restricted diffusion throughout the left MCA territory, consistent with an embolic stroke. Subsequent work-up for a cardioembolic source was negative. In addition, axial CTA of the cervical carotid artery demonstrates likely atherosclerotic disease resulting in <50% luminal stenosis (D, arrow). Given the diagnostic evaluation with negative findings and a likely embolic source, this patient meets the criteria for ESUS, despite a minimal degree of luminal carotid stenosis.
One such avenue has been the use of adjunctive and advanced imaging techniques, as well as alternative imaging features on traditional studies to identify potential embolic sources. Of particular interest has been a deeper interrogation of carotid artery plaque features beyond the degree of luminal stenosis. Current guidelines suggest that large-artery atherosclerosis may be the cause of an ischemic stroke in cases in which the degree of ipsilateral stenosis is >50% as calculated by the NASCET criteria.6,7 This rule is based on data from trials performed in the 1970s and 80s that could assess only the degree of stenosis and luminal morphology. Therefore, a biomarker was produced according to the only threshold that could be assessed at that time. Currently, however, advanced imaging modalities have enabled the assessment of the plaque itself and not only its effects, such as the stenosis, along with the detection of various carotid plaque features that have been highly associated with the presence of ipsilateral ischemic stroke, even in cases of <50% ipsilateral stenosis.8-10
Even in the case of traditional imaging modalities such as ultrasound (US) and CTA, several studies have indicated that the presence of certain carotid features may be associated with ipsilateral ischemic stroke in patients who would otherwise have been classified as having ESUS. Given the multitude of such studies showing an increased risk of recurrent stroke in patients with ESUS with nonstenotic carotid artery disease with certain radiographic features, Goyal et al8 have proposed that symptomatic nonstenotic carotid artery disease (SyNC) is a plausible stroke etiology in patients with ESUS. These patients may, therefore, benefit from a targeted therapeutic approach.11,12
Although the SyNC criteria remain in their infancy and will likely evolve, an important feature of the SyNC criteria is the presence of carotid plaque with high-risk features. An understanding of which radiographic features have been associated with ipsilateral ischemic stroke in patients with ESUS is crucial to determine whether a patient likely meets the criteria for SyNC and may, therefore, benefit from targeted therapy. To date however, a review of such features that may be associated with ipsilateral ischemic events in patients with ESUS specifically is absent from the literature. We therefore aimed to provide such a review of carotid artery features as seen on US, CT, and MR imaging in the context of ESUS.
MATERIALS AND METHODS
We performed a focused literature search related to radiographic carotid plaque features specifically in patients with ESUS. This was not a systematic review or meta-analysis and did not, therefore, use rigorous search criteria or inclusion/exclusion criteria. PubMed was queried using words such as “carotid,” “plaque,” “nonstenotic,” “ESUS,” “stroke,” “ischemia,” and “cryptogenic.” Titles and abstracts were reviewed for relevance. Full articles were accessed when titles/abstracts suggested that a study consisted of evaluation of carotid plaque features in patients with ESUS or in patients with cryptogenic stroke. Articles were fully reviewed, and the references of each article were scrutinized for additional studies of interest.
The strength of associations between plaque features and ipsilateral stroke in patients with ESUS was graded in a subjective fashion as follows: If all or nearly all studies of a particular feature demonstrated such an association, the evidence for that feature being associated with ipsilateral stroke in ESUS was considered “strong.” If most studies demonstrated an association, that feature was considered as having “moderate” evidence. If roughly half of studies demonstrated a relationship while roughly the other half demonstrated no such relationship, the evidence for that feature was graded as “conflicting.” If a feature did not demonstrate a relationship across all or nearly all studies or had only been minimally studied, the evidence was graded as “weak.”
Ultrasound
Ultrasound has historically been the most-commonly used imaging technique to evaluate carotid artery disease because of its ease of use and lack of radiation exposure. The results of NASCET provided further impetus for the use of US for assessment of suspected carotid artery disease.6 However, the use of US has limited consideration for operative interventions to the degree of stenosis alone without consideration of other US features that may have been associated with ischemic events. In fact, some studies performed in the 1980s before the completion of NASCET suggested that specific plaque features on US were likely associated with ipsilateral ischemic stroke.13,14 While the degree of luminal stenosis remains a critical feature, advances in US technology have likely led to identification of other US plaque features that require consideration as a culprit lesion in patients with stroke.15,16 Unfortunately, a paucity of data specific to patients with ESUS exists.
Several studies (some of which were performed pre-NASCET) have indicated that US features such as fibrous cap ulceration, the presence of thrombus, plaque length, plaque volume, and hypoechoic/heterogeneous plaque likely indicate vulnerable plaques that may be culprit lesions in patients with stroke.17 Recently, Buon et al18 evaluated 22 patients with carotid atherosclerosis with <50% stenosis. Despite a relatively small sample size, the presence of plaque echolucency was more prevalent in carotid arteries ipsilateral to the presenting ischemic symptoms versus contralateral carotid arteries (100% versus 63%, respectively), though statistical significance was not reached. Most intriguing, the difference in plaque ulceration and thrombus did not reach statistical significance. However, carotid arteries on the symptomatic side had a higher median plaque thickness, length, and volume index.
In a similar study, Komatsu et al19 found that carotid plaques with >1.5 mm intimal medial thickness were present in the carotid artery ipsilateral to symptoms in 59% of patients with ESUS compared with 42% on the contralateral asymptomatic side, a finding that was statistically significant. Hypoechoic plaques were present on the ipsilateral side in 9% of patients versus 4% on the contralateral side, though this difference did not reach statistical significance. Together, data from these 2 studies suggest that features related to plaque size and thickness may be more important US markers of culprit lesions in patients with ESUS compared with other features. Patients with these lesions may, therefore, be more strongly considered for intervention to reduce the risk of recurrent stroke. However, these studies assess an association between features and the presence of ipsilateral ESUS in a cross-sectional manner. It is therefore uncertain whether these features place patients at risk for additional, recurrent ischemic events. An example case of a patient with ESUS and US findings suggestive of carotid plaque as a culprit lesion is demonstrated in Fig 2.
FIG 2.
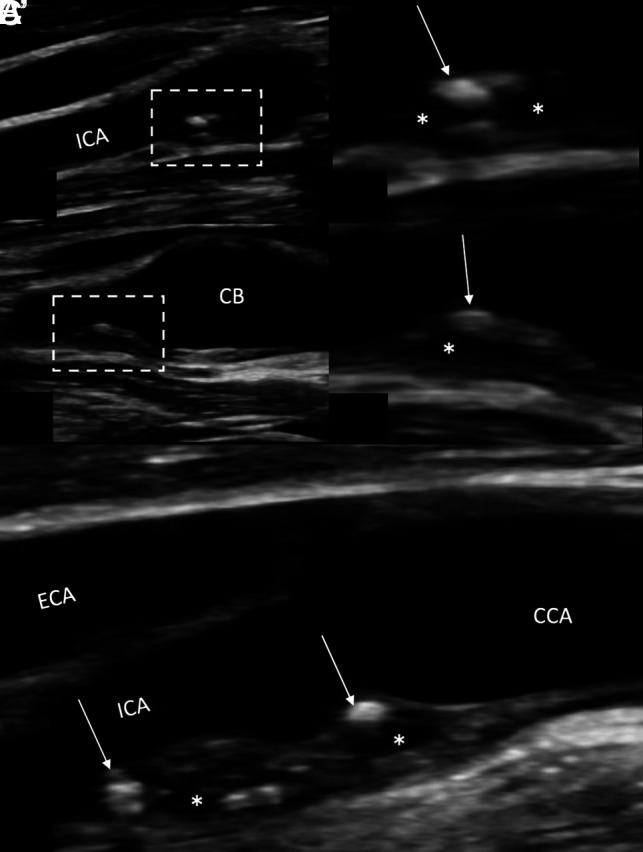
Carotid sonography demonstrating large (≥3 mm), heterogeneous, nonstenotic carotid plaque in a 66-year-old man who presented with acute-onset vision loss in his right eye, suspicious for retinal ischemia. The patient underwent bilateral carotid US. A, B-mode long axis view of the right carotid vasculature demonstrates the presence of a moderately-sized (∼3 mm) complicated plaque in the proximal ICA. A’, Close-up of the plaque demonstrates heterogeneous plaque with areas both hyperechoic (arrow) and echolucent (asterisk). B and B’, Long view of the proximal ICA and carotid bulb (CB) demonstrates heterogeneous plaque as seen in A and A’. C, The left proximal ICA also demonstrates the presence of large (>3mm) heterogenous plaque with hyperechoic (arrow) and echolucent (asterisk) areas. The right-sided plaque may be smaller than the left secondary to recent dislodgment and embolization of plaque material. The patient underwent an appropriate work-up to identify a potential embolic source, which was negative. This patient was diagnosed with ESUS despite the presence of moderately-sized, heterogeneous plaque on the right resulting in <50% luminal stenosis. Although more data are needed relating to plaque US in the context of ESUS, plaque thickness appears to have a consistent association with ipsilateral stroke across the existing literature. To date, plaque echolucency has not been found to have such an association, which is significant. ECA indicates external carotid artery; CCA, common carotid artery.
To our knowledge, these are the only studies in the literature evaluating plaque features on US in the context of ESUS. Given the ease, availability, low cost, and lack of radiation in using US, additional studies evaluating potential US biomarkers of ipsilateral stroke in patients with ESUS are warranted.
CTA
Like US, CTA represents a quick and easily accessible method of assessing carotid artery disease in patients with stroke. Technical advances in CT technology (ie, such as high-speed multidetector hardware and 3D reformatting software) have enabled detection of carotid artery disease on the scale of millimeters.16 Because CTA is performed as a part of most institutional acute stroke algorithms, any features of nonstenotic plaques associated with ipsilateral stroke would be easily assessed in the clinical setting. Thus, CTA may, therefore, be the most practical technique to assess nonstenotic plaque features.16 Unlike US however, more data exist regarding which plaque features on CTA are associated with ipsilateral ischemic events in nonstenotic carotid arteries in patients with ESUS specifically. Unfortunately, conflicting evidence has been reported for multiple plaque features up to this point.
Coutinho et al20 were the first to hypothesize that large, nonstenotic plaques were more prevalent in carotid arteries ipsilateral to cryptogenic stroke. In this original study that included a cohort of 85 patients with ESUS, 2 primary findings were evident. First, large-but-nonstenotic plaques >3mm in thickness were found to be associated with ipsilateral cryptogenic strokes. Second, there was no difference in the percentage of stenosis in ipsilateral-versus-contralateral carotid arteries.
Following this original study by Coutinho et al,20 Ospel et al21 analyzed patients with ESUS from the Identifying New Approaches to Optimize Thrombus Characterization for Predicting Early Recanalization and Reperfusion with IV Alteplase and Other Treatments Using Serial CT Angiography (INTERRSeCT) study22 to determine the prevalence of nonstenotic carotid plaques ipsilateral to the side of the stroke. The authors found that in patients with <50% stenosis, plaques were more prevalent within the carotid artery ipsilateral to the side of the stroke, suggesting that nonstenotic plaques may be culprit lesions in patients with ESUS. Most interesting, several plaque features (irregularity, ulceration, hypoattenuation, and so forth) had no significant association with ipsilateral stroke, though plaque thickness (>3 mm) was more prevalent on the ipsilateral side but did not reach statistical significance.
More recently, Singh et al23 analyzed the Systematic Evaluation of Patients Treated with Neurothrombectomy Devices for Acute Ischemic Stroke (STRATIS) registry to retrospectively evaluate plaque features on CTA in 141 patients diagnosed with cryptogenic stroke. In essence, plaque surface irregularity, plaque hypodensity, and increasing plaque thickness were all associated with ipsilateral stroke in patients diagnosed as having cryptogenic stroke. Notably, predominately calcified and ulcerated plaques had no association with ipsilateral cryptogenic stroke.
The Plaque at Risk (PARISK) study was a recently published multicenter, prospective analysis of carotid plaque CT and MR imaging biomarkers and recurrent stroke risk in patients with carotid stenosis of <70% by the NASCET criteria.24 Most interesting, plaque calcifications and ulcerations were not found to be statistically significant risk factors for recurrent ipsilateral stroke at a mean follow-up of 5 years. In terms of CT plaque features, these findings suggest that ulceration and proportional calcification are not likely to be risk factors for recurrent ischemic events. Whether a longer follow-up interval changes these findings remains unclear.
In contrast, a recent retrospective study of 152 patients with ESUS by Jumah et al25 found that plaque ulceration as seen on CTA was more prevalent in ipsilateral carotid arteries versus contralateral ones. In addition, plaque thickness (>3mm), plaque length (>1 cm), and hypodense plaque were significantly associated with ipsilateral stroke, whereas calcification was not.
Certainly, these studies further support the theory that the presence of carotid plaque (regardless of the degree of stenosis) may be a culprit lesion in patients with ESUS without another identifiable source. Furthermore, it also appears that plaque thickness of >3 mm has been most consistently reported across studies associated with ipsilateral ischemic events in patients with ESUS (Fig 3). Although certain CT features of carotid plaques such as surface irregularity, ulceration, hypodensity, and calcification are thought to represent features of vulnerable plaque, their association with ipsilateral stroke in patients with ESUS remains unclear in the context of conflicting reports (Fig 3).10,23,25,26 Given the high clinical utility of CT and CTA in the acute stroke setting, continued exploration of the potential association between these features and ischemic events in patients with ESUS is likely worthwhile to clarify these discrepancies.
FIG 3.

Example of “vulnerable” plaque features as seen on CTA in 3 different patients. A, Ulcerated plaque within the proximal ICA (dashed box) seen on CTA. Inset in A demonstrates an area of slight luminal stenosis with plaque surface irregularity suspicious for ulceration (arrow). B, CTA from a different patient demonstrates hypodense (“soft”) plaque (solid arrow) with a thin band of peripheral calcifications (arrowhead), resulting in luminal narrowing (dashed arrow). Similar findings are seen in C apart from a much thicker peripheral band of calcification. Hypodense plaques may represent the present of LRNC or IPH, though it can be difficult to distinguish between the 2 on CTA. Plaque hypodensity has been reported to be associated with ipsilateral ischemia in patients with ESUS in most studies. Conflicting evidence exists regarding the relationship between surface irregularity, ulceration, and ipsilateral stroke. In contrast, calcification appears to be unrelated to ipsilateral stroke in patients with ESUS.
MR Imaging
Without doubt, advances in MR imaging technology have enhanced detection and interrogation of carotid plaque features.16,27 Not the least of these is carotid vessel wall imaging, which uses flow-suppression techniques to null signal from adjacent flowing blood. This enables enhanced detection of carotid plaque features beyond the degree of luminal stenosis or plaque size. MR imaging of plaque is, therefore, considered as the criterion standard for carotid wall analysis, particularly in patients with ESUS.16
Several MR imaging plaque biomarkers have been proposed to be associated with ipsilateral ischemic events, specifically in patients with ESUS.10 Of these, intraplaque hemorrhage (IPH) has been established as being associated with cerebral ischemia (Fig 4). Pathologically, IPH involves extravasation of hematogenous constituents from fragile neocapillaries within an atheromatous plaque.28,29 The presence of this inflammatory milieu within the plaque can result in thrombus formation, plaque growth, plaque rupture, and distal embolization of thrombotic material.29 Most commonly, IPH can be visualized on heavily T1-weighted sequences such as MPRAGE. On such sequences, IPH appears as a region of hyperintensity in a carotid plaque that carries a signal intensity of 50% of the adjacent sternocleidomastoid muscle.30
FIG 4.
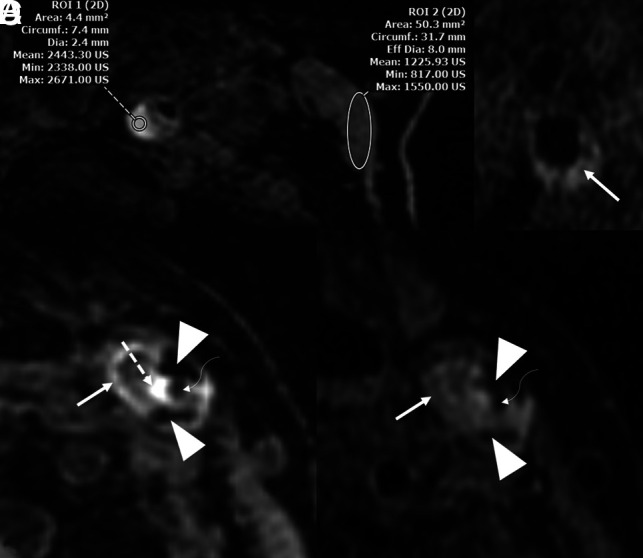
Representative MR imaging plaque features that may have an association with ipsilateral stroke in patients with ESUS. A, MPRAGE of a patient showing an area of hyperintensity within the proximal ICA (left circle), which has a mean intensity value of 200× that of the adjacent sternocleidomastoid muscle (right circle). These findings suggest the presence of IPH. This patient had >50% luminal stenosis. B, T1 Cube (GE Healthcare) imaging of a different patient shows a LRNC. C, MPRAGE in another patient demonstrates multiple features including hypointense areas suggestive of peripheral calcifications (arrowheads), a hyperintense area (dashed arrow) adjacent to the narrowed carotid lumen (curved arrow), and a peripheral area of LRNC (solid arrow). D, The same patient/artery as in C with T1 Cube imaging demonstrating the narrowed lumen (curved arrow) along with peripheral calcifications (arrowheads) and a LRNC (solid arrow). IPH is not as apparent on TI Cube imaging as on MPRAGE sequences. This patient also had >50% luminal stenosis. Strong evidence exists suggesting that IPH is associated with ipsilateral stroke in ESUS. A LRNC as seen on MR imaging has also been consistently reported to have such an association, albeit not to the same degree as IPH.
Despite a suspicion that IPH is associated with distal events, the association of IPH in ESUS has been uncertain until recently.31 Of all MR imaging biomarkers of plaque vulnerability, IPH has been most strongly associated with ipsilateral ischemia in patients with ESUS, even in cases of minimal luminal stenosis.9,21,32-36 In fact, our group has demonstrated that patients with ESUS with ipsilateral IPH had an annual recurrence rate of 9.5% compared with 2.5% in patients without IPH.9 Perhaps the most convincing evidence to date comes from the aforementioned PARISK study, in which patients with MR imaging evidence of IPH were at higher risk of developing recurrent ischemic events at 5-year follow-up.24
With these multiple sources of evidence suggesting an independent association between IPH and ipsilateral stroke, the presence of IPH on vessel wall MR imaging in patients without an otherwise-defined thromboembolic source should be strongly considered as a culprit lesion. Some studies have even suggested that patients with ischemic stroke and ipsilateral IPH in minimal carotid stenosis may benefit from carotid endarterectomy.11,12
Nevertheless, several questions remain regarding IPH. For example, IPH may persist within a plaque for years without any obvious progression or distal ischemic events.37 This finding has led some to postulate that the mere presence of IPH may not be adequate in determining whether a patient is at risk. In turn, more specific features of the IPH have been assessed, including the signal intensity within the hemorrhage and the size of the IPH.30,38
Beyond IPH, the presence of a lipid-rich necrotic core (LRNC) within a carotid plaque has also been suggested as a potential culprit in patients with ESUS.16,36,39,40 For example, the Carotid Plaque Imaging in Acute Stroke (CAPIAS) study demonstrated that patients with cryptogenic strokes had larger-volume LRNC ipsilateral to the stroke compared with patients with known cardioembolic or small-vessel stroke. Other MR imaging features such as ulceration and thinning/rupture of the fibrous cap have been identified as vulnerable features but have not been studied in patients with ESUS specfically.41,42
Summary and Future Directions
Our understanding of symptomatic, nonstenotic carotid disease as a cause of ESUS continues to evolve. Several plaque features are important to consider in this context (Online Supplemental Data). On US and CT, volumetric features including plaque volume and plaque thickness have been demonstrated to have an association with ipsilateral stroke in nonstenotic carotid disease. Regarding CT specifically, hypodense plaque has also had such an association, albeit in a smaller number of studies. Additional investigation is required to identify other potential US and CT plaque features as markers of culprit lesions in nonstenotic disease. Intraplaque hemorrhage, as identified on vessel wall MR imaging, has been most strongly associated with ipsilateral stroke in patients with ESUS. Therefore, the presence of IPH in patients without an otherwise identifiable source should raise strong suspicion for a culprit lesion. However, additional work is required to identify more specific features of IPH that indicate an active lesion. Other MR imaging features of plaque vulnerability have been suggested as being associated with ipsilateral stroke (ie, LRNC, thinning/rupture of the fibrous cap, and ulceration). Besides LRNC, these features have yet to be studied in patients with ESUS. Further work is, therefore, necessary to examine the role of these features in the context of ESUS.
Although these radiographic features are important to evaluate in patients with ESUS, their presence should not preclude evaluation of other potential causes of embolic stroke. Grosse et al43 demonstrated that the presence of nonstenosing carotid artery plaques was associated with markers of left atrial disease and atrial fibrillation. Although the reasoning for this association is unclear, the importance of a thorough evaluation for potential cardiac causes of stroke in patients with ESUS is emphasized. Moreover, recent evidence has also suggested that a high proportion of patients with ESUS have multiple, potential embolic sources, further stressing the importance of thorough evaluation beyond plaque features.44
Of course, many patients with suspected ESUS will have carotid imaging performed by >1 technique. This may be particularly true with a more widespread understanding of SyNC. For example, a patient with US findings suggestive of thick plaque may undergo an MR imaging or CT study of the plaque to further characterize additional features such as hypodensity or IPH, which may, in turn, guide further management. To our knowledge, the combinatorial benefit of imaging modalities for assessment of plaque in patients with ESUS has not been directly addressed. Such information would be beneficial in improving the detection of plaque features and determining which patients would benefit from advanced imaging. Further study in this regard is therefore warranted.
The use of molecular-based imaging modalities has been of recent interest to detect inflammatory activity in carotid plaques. In the clinical realm, plaque uptake on FDG-PET studies is used as a surrogate marker for plaque inflammation and has, indeed, been correlated with inflammatory markers.45,10 Most intriguing, a systematic review and meta-analysis performed by Chaker et al46 demonstrated that recent ipsilateral cerebral ischemic events may be associated with an increased plaque uptake of [18F] FDG on PET imaging, independent of the degree of luminal stenosis. While detection of more specific molecular markers of inflammation and plaque vulnerability remains largely within the research realm, the potential role of molecular plaque imaging in the context of ESUS is an exciting avenue of discovery.
Certainly, the primary objective in detecting carotid plaque as a potential source of embolism in patients with ESUS is to ultimately provide a targeted form of therapy to reduce the risk of future ischemic events. This objective contrasts with general stroke prevention, without an identifiable source. In theory, patients meeting the criteria for SyNC may benefit from carotid endarterectomy. Early data have suggested that endarterectomy for patients with SyNC is safe and may have a benefit in preventing recurrent ischemia.11,12 However, well-designed clinical studies are needed to draw more robust conclusions regarding the efficacy of endarterectomy in preventing future ischemic events in patients meeting criteria for SyNC.
ABBREVIATIONS:
- ESUS
embolic stroke of undetermined source
- IPH
intraplaque hemorrhage
- LRNC
lipid-rich necrotic core
- SyNC
symptomatic nonstenotic carotid artery disease
- US
ultrasound
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.Hart RG, Catanese L, Perera KS, et al. Embolic stroke of undetermined source: a systematic review and clinical update. Stroke 2017;48:867–72 10.1161/STROKEAHA.116.016414 [DOI] [PubMed] [Google Scholar]
- 2.Hart RG, Diener HC, Coutts SB, et al. ; Cryptogenic Stroke/ESUS International Working Group. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol 2014;13:429–38 10.1016/S1474-4422(13)70310-7 [DOI] [PubMed] [Google Scholar]
- 3.Navi BB, Kasner SE, Elkind MS, et al. Cancer and embolic stroke of undetermined source. Stroke 2021;52:1121–30 10.1161/STROKEAHA.120.032002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hart RG, Connolly SJ, Mundl H; NAVIGATE ESUS Investigators. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med 2018;379:987–2201 10.1056/NEJMoa1802686 [DOI] [PubMed] [Google Scholar]
- 5.Diener HC, Sacco RL, Easton JD, et al. ; RE-SPECT ESUS Steering Committee and Investigators. Dabigatran for prevention of stroke after embolic stroke of undetermined source. N Engl J Med 2019;380:1906–17 10.1056/NEJMoa1813959 [DOI] [PubMed] [Google Scholar]
- 6.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445–53 10.1056/NEJM199108153250701 [DOI] [PubMed] [Google Scholar]
- 7.Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial. TOAST—Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 8.Goyal M, Singh N, Marko M, et al. Embolic stroke of undetermined source and symptomatic nonstenotic carotid disease. Stroke 2020;51:1321–25 10.1161/STROKEAHA.119.028853 [DOI] [PubMed] [Google Scholar]
- 9.Larson AS, Nasr DM, Rizvi A, et al. Embolic stroke of undetermined source: the association with carotid intraplaque hemorrhage. JACC Cardiovasc Imaging 2021;14:506–08 10.1016/j.jcmg.2020.08.007 [DOI] [PubMed] [Google Scholar]
- 10.Saba L, Agarwal N, Cau R, et al. Review of imaging biomarkers for the vulnerable carotid plaque. JVS Vasc Sci 2021;2:149–58 10.1016/j.jvssci.2021.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larson A, Nardi V, Brinjikji W, et al. Endarterectomy for symptomatic non-stenotic carotids: a systematic review and descriptive analysis. Stroke Vasc Neurol 2022;7:6–12 10.1136/svn-2021-001122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nardi V, Benson JC, Larson AS, et al. Carotid artery endarterectomy in patients with symptomatic non-stenotic carotid artery disease. Stroke Vasc Neurol 2022;7:251–57 10.1136/svn-2021-000939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray-Weale AC, Graham JC, Burnett JR, et al. Carotid artery atheroma: comparison of preoperative B-mode ultrasound appearance with carotid endarterectomy specimen pathology. J Cardiovasc Surg (Torino) 1988;29:676–81 [PubMed] [Google Scholar]
- 14.Steffen C, Gray‐Wealed A, Byrne K, et al. Carotid artery atheroma: ultrasound appearance in symptomatic and asymptomatic vessels. Aust N Z J Surg 1989;59:529 534 10.1111/j.1445-2197.1989.tb01625.x [DOI] [PubMed] [Google Scholar]
- 15.Scoutt LM, Gunabushanam G. Carotid ultrasound. Radiol Clin North Am 2019;57:501–18 10.1016/j.rcl.2019.01.008 [DOI] [PubMed] [Google Scholar]
- 16.Saba L, Antignani PL, Gupta A, et al. International Union of Angiology (IUA) consensus paper on imaging strategies in atherosclerotic carotid artery imaging: from basic strategies to advanced approaches. Atherosclerosis 2022;354:23–40 10.1016/j.atherosclerosis.2022.06.1014 [DOI] [PubMed] [Google Scholar]
- 17.Bluth E, Kay D, Merritt C, et al. Sonographic characterization of carotid plaque: detection of hemorrhage. AJNR Am J Neuroradiol 1986;146:1061–65 10.2214/ajr.146.5.1061 [DOI] [PubMed] [Google Scholar]
- 18.Buon R, Guidolin B, Jaffre A, et al. Carotid ultrasound for assessment of nonobstructive carotid atherosclerosis in young adults with cryptogenic stroke. J Stroke Cerebrovasc Dis 2018;27:1212–16 10.1016/j.jstrokecerebrovasdis.2017.11.043 [DOI] [PubMed] [Google Scholar]
- 19.Komatsu T, Iguchi Y, Arai A, et al. Large but nonstenotic carotid artery plaque in patients with a history of embolic stroke of undetermined source. Stroke 2018;49:3054–56 10.1161/STROKEAHA.118.022986 [DOI] [PubMed] [Google Scholar]
- 20.Coutinho JM, Derkatch S, Potvin AR, et al. Nonstenotic carotid plaque on CT angiography in patients with cryptogenic stroke. Neurology 2016;87:665–72 10.1212/WNL.0000000000002978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ospel JM, Singh N, Marko M, et al. Prevalence of ipsilateral nonstenotic carotid plaques on computed tomography angiography in embolic stroke of undetermined source. Stroke 2020;51:1743–49 10.1161/STROKEAHA.120.029404 [DOI] [PubMed] [Google Scholar]
- 22.Lau HL, Gardener H, Coutts SB, et al. Radiographic characteristics of mild ischemic stroke patients with visible intracranial occlusion: the INTERRSeCT study. Stroke 2022;53:913–20 10.1161/STROKEAHA.120.030380 [DOI] [PubMed] [Google Scholar]
- 23.Singh N, Ospel J, Mayank A, et al. ; STRATIS Investigators. Nonstenotic carotid plaques in ischemic stroke: analysis of the STRATIS Registry. AJNR Am J Neuroradiol 2021;42:1645–52 10.3174/ajnr.A7218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Dam-Nolen DH, Truijman MT, van der Kolk AG, et al. Carotid plaque characteristics predict recurrent ischemic stroke and TIA: the PARISK (Plaque At Risk) study. JACC: Cardiovasc Imaging 2022;15:1715–26 10.1016/j.jcmg.2022.04.003 [DOI] [PubMed] [Google Scholar]
- 25.Jumah A, Aboul Nour H, Intikhab O, et al. Non-stenosing carotid artery plaques in embolic stroke of undetermined source: a retrospective analysis. Neurol Sci 2022. Sept 27. [Epub ahead of print] 10.1007/s10072-022-06425-w [DOI] [PubMed] [Google Scholar]
- 26.Baradaran H, Al-Dasuqi K, Knight-Greenfield A, et al. Association between carotid plaque features on CTA and cerebrovascular ischemia: a systematic review and meta-analysis. AJNR Am J Neuroradiol 2017;38:2321–26 10.3174/ajnr.A5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saba L, Yuan C, Hatsukami T, et al. ; Vessel Wall Imaging Study Group of the American Society of Neuroradiology. Carotid artery wall imaging: perspective and guidelines from the ASNR vessel wall imaging study group and expert consensus recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol 2018;39:E9–31 10.3174/ajnr.A5488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mura M, Della Schiava N, Long A, et al. Carotid intraplaque haemorrhage: pathogenesis, histological classification, imaging methods and clinical value. Ann Transl Med 2020;8:1273–73 10.21037/atm-20-1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holmes DR Jr, Alkhouli MA, Klaas JP, et al. Change of heart: the underexplored role of plaque hemorrhage in the evaluation of stroke of undetermined etiology. J Am Heart Assoc 2022;11:e025323 10.1161/JAHA.122.025323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larson AS, Brinjikji W, Kroll NJ, et al. Normalized intraplaque hemorrhage signal on MP-RAGE as a marker for acute ischemic neurological events. Neuroradiol J 2022;35:112–18 10.1177/19714009211029263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takaya N, Yuan C, Chu B, et al. Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques: a high-resolution magnetic resonance imaging study. Circulation 2005;111:2768–75 10.1161/CIRCULATIONAHA.104.504167 [DOI] [PubMed] [Google Scholar]
- 32.Larson A, Brinjikji W, Savastano L, et al. Carotid intraplaque hemorrhage and stenosis: at what stage of plaque progression does intraplaque hemorrhage occur, and when is it most likely to be associated with symptoms? AJNR Am J Neuroradiol 2021;42:1285–90 10.3174/ajnr.A7133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nardi V, Benson J, Bois MC, et al. Carotid plaques from symptomatic patients with mild stenosis is associated with intraplaque hemorrhage. Hypertension 2022;79:271–82 10.1161/HYPERTENSIONAHA.121.18128 [DOI] [PubMed] [Google Scholar]
- 34.Singh N, Moody AR, Panzov V, et al. Carotid intraplaque hemorrhage in patients with embolic stroke of undetermined source. J Stroke Cerebrovasc Dis 2018;27:1956–59 10.1016/j.jstrokecerebrovasdis.2018.02.042 [DOI] [PubMed] [Google Scholar]
- 35.Tao L, Li XQ, Hou XW, et al. Intracranial atherosclerotic plaque as a potential cause of embolic stroke of undetermined source. J Am Coll Cardiol 2021;77:680–91 10.1016/j.jacc.2020.12.015 [DOI] [PubMed] [Google Scholar]
- 36.Kopczak A, Schindler A, Bayer-Karpinska A, et al. Complicated carotid artery plaques as a cause of cryptogenic stroke. J Am Coll Cardiol 2020;76:2212–22 10.1016/j.jacc.2020.09.532 [DOI] [PubMed] [Google Scholar]
- 37.Sun J, Underhill HR, Hippe DS, et al. Sustained acceleration in carotid atherosclerotic plaque progression with intraplaque hemorrhage: a long-term time course study. JACC Cardiovasc Imaging 2012;5:798–804 10.1016/j.jcmg.2012.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Wang M, Zhang B, et al. Size of carotid artery intraplaque hemorrhage and acute ischemic stroke: a cardiovascular magnetic resonance Chinese atherosclerosis risk evaluation study. J Cardiovasc Magn Reson 2019;21:9 10.1186/s12968-018-0515-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kashiwazaki D, Shiraishi K, Yamamoto S, et al. Efficacy of carotid endarterectomy for mild (<50%) symptomatic carotid stenosis with unstable plaque. World Neurosurg 2019;121:e60–69 10.1016/j.wneu.2018.09.013 [DOI] [PubMed] [Google Scholar]
- 40.Gupta A, Baradaran H, Schweitzer AD, et al. Carotid plaque MRI and stroke risk: a systematic review and meta-analysis. Stroke 2013;44:3071–77 10.1161/STROKEAHA.113.002551 [DOI] [PubMed] [Google Scholar]
- 41.Rafailidis V, Chryssogonidis I, Tegos T, et al. Imaging of the ulcerated carotid atherosclerotic plaque: a review of the literature. Insights Imaging 2017;8:213–25 10.1007/s13244-017-0543-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benson JC, Cheek H, Aubry MC, et al. Cervical carotid plaque MRI. Clin Neuroradiol 2021;31:295–306 10.1007/s00062-020-00987-y [DOI] [PubMed] [Google Scholar]
- 43.Grosse GM, Sieweke JT, Biber S, et al. Nonstenotic carotid plaque in embolic stroke of undetermined source: interplay of arterial and atrial disease. Stroke 2020;51:3737–41 10.1161/STROKEAHA.120.030537 [DOI] [PubMed] [Google Scholar]
- 44.Ntaios G, Pearce LA, Veltkamp R. et al. ; NAVIGATE ESUS Investigators. Potential embolic sources and outcomes in embolic stroke of undetermined source in the NAVIGATE-ESUS trial. Stroke 2020;51:1797–1804 10.1161/STROKEAHA.119.028669 [DOI] [PubMed] [Google Scholar]
- 45.Kerwin W, Alessio A, Ferguson M, et al. High-resolution [18] fluorodeoxyglucose-positron emission tomography and coregistered magnetic resonance imaging of atherosclerotic plaque from a patient undergoing carotid endarterectomy. Circ Cardiovasc Imaging 2012;5:683–84 10.1161/CIRCIMAGING.112.975144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaker S, Al-Dasuqi K, Baradaran H, et al. Carotid plaque positron emission tomography imaging and cerebral ischemic disease: a systematic review and meta-analysis. Stroke 2019;50:2072– 79 10.1161/STROKEAHA.118.023987 [DOI] [PMC free article] [PubMed] [Google Scholar]


