To the Editor: Since its emergence in November 2021, the B.1.1.529 (omicron) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has continued to evolve into sublineages.1 To mitigate the omicron pandemic, in late August and early September 2022, the Food and Drug Administration and the European Medicines Agency authorized emergency use of the BNT162b2 bivalent vaccine (Pfizer–BioNTech) that targets both the omicron BA.4–BA.5 spike (BA.4 and BA.5 encode an identical spike protein) and the ancestral wild-type (D614G) spike of SARS-CoV-2. The vaccine was subsequently authorized for use in many countries worldwide.
New omicron sublineages, including those that have descended from BA.2 and BA.4–BA.5, have emerged. These sublineages include BA.4.6, BA.2.75.2, BQ.1.1, and XBB.1. Although early epidemiologic data suggest these new sublineages have not led to increased disease severity, they have accumulated additional spike mutations that could further evade vaccine-elicited antibody neutralization, infection-elicited antibody neutralization, or both.2-4 Here, we compare the neutralization activity against these omicron sublineages in persons who had received three doses of BNT162b2 vaccine and then received a fourth dose of either the original BNT162b2 vaccine or the bivalent BA.4–BA.5 booster.
Participants were older than 55 years of age and had received three 30-μg doses of BNT162b2 vaccine and either a fourth monovalent booster dose of BNT162b2 vaccine (30 μg) approximately 6.6 months after the third dose (in the C4591031 clinical trial5) or the bivalent vaccine (15 μg of messenger RNA [mRNA] directed against the ancestral strain of SARS-CoV-2 and 15 μg of mRNA directed against BA.4–BA.5) approximately 11 months after the third dose (in the C4591044 clinical trial; ClinicalTrials.gov number, NCT05472038). The protocols are available with the full text of this letter at NEJM.org. All the participants provided written informed consent.
Serum samples were obtained on the day of the fourth dose and 1 month after the fourth dose was administered. Viral nucleocapsid antibody testing and a reverse-transcriptase–polymerase-chain-reaction assay to detect SARS-CoV-2 infection were performed in all the participants. A subgroup of participants from both vaccine groups, with an equal distribution of participants with or without evidence of infection at baseline (according to serum samples obtained on the day of the fourth dose), was selected for the neutralization analysis. This study was not a single randomized trial; therefore, unmeasured variables could have differed between the two groups.
The complete spike gene from omicron BA.4–BA.5, BA.4.6, BA.2.75.2, BQ.1.1, or XBB.1 was engineered into the backbone of the reporter USA-WA1/2020 SARS-CoV-2 (a strain isolated in January 2020) with the use of a live-virus focus reduction neutralization mNeonGreen (mNG) test.6 The resulting wild-type (WT) spike, BA.4–BA.5 spike, BA.4.6 spike, BA.2.75.2 spike, BQ.1.1 spike, and XBB.1 spike mNG USA-WA1/2020 viruses were used to measure titers on a 50% fluorescence-basedt focus reduction neutralization test (FRNT50) (the reciprocal dilution of serum that neutralizes 50% of the input virus). Tables S2 through S4 in the Supplementary Appendix (available at NEJM.org) summarize the data from serum samples and their values as measured with fluorescence-based FRNT50.
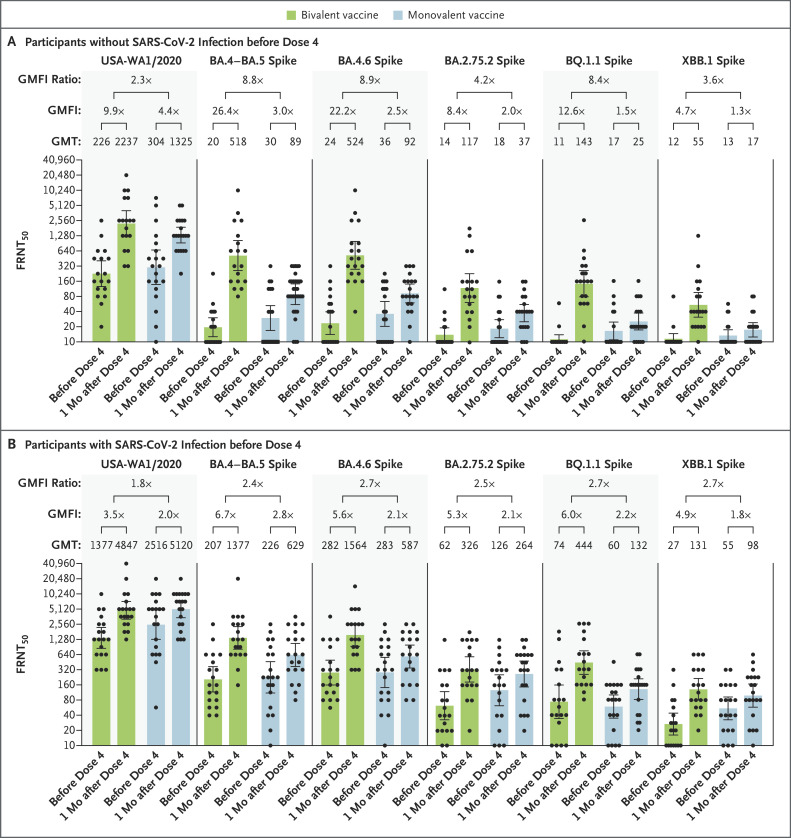
Among all the participants, the fourth dose of monovalent BNT162b2 vaccine induced a geometric mean factor increase (i.e., an increase from the day of the fourth dose to 1 month after the fourth dose) in titers of 3.0 against WT; 2.9 against BA.4–BA.5; 2.3 against BA.4.6; 2.1 against BA.2.75.2; 1.8 against BQ.1.1; and 1.5 against XBB.1; the bivalent vaccine induced neutralizing geometric mean factor increases of 5.8, 13.0, 11.1, 6.7, 8.7, and 4.8, respectively (Fig. S1). In the participants without previous SARS-CoV-2 infection, the monovalent BNT162b2 vaccine induced neutralizing geometric mean factor increases of 4.4 against WT; 3.0 against BA.4–BA.5; 2.5 against BA.4.6; 2.0 against BA.2.75.2; 1.5 against BQ.1.1; and 1.3 against XBB.1; the bivalent vaccine induced neutralizing geometric mean factor increases of 9.9, 26.4, 22.2, 8.4, 12.6, and 4.7, respectively (Figure 1A). In the participants with previous SARS-CoV-2 infection, the monovalent BNT162b2 vaccine induced neutralizing geometric mean factor increases of 2.0 against WT; 2.8 against BA.4–BA.5; 2.1 against BA.4.6; 2.1 against BA.2.75.2; 2.2 against BQ.1.1; and 1.8 against XBB.1; the bivalent vaccine induced neutralizing geometric mean factor increases of 3.5, 6.7, 5.6, 5.3, 6.0, and 4.9, respectively (Figure 1B). Despite different intervals from dose 3 to dose 4, the neutralizing titers before the fourth dose were similar in the monovalent-vaccine group and the bivalent-vaccine group in all the participants, regardless of history of SARS-CoV-2 infection.
Figure 1. Neutralizing Responses with Bivalent BA.4–BA.5 Vaccine or Monovalent BNT162b2 Booster Vaccine.
Shown is the neutralizing activity against the USA-WA1/2020 strain of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the omicron sublineages BA.4–BA.5, BA.4.6, BA.2.75.2, BQ.1.1, and XBB.1 in all participants who did not have evidence of SARS-CoV-2 infection before the fourth dose of vaccine (Panel A) and in those who had evidence of SARS-CoV-2 infection before the fourth dose of vaccine (Panel B). The heights of the bars and the numbers immediately above the bars indicate the geometric mean titers (GMTs) of neutralizing antibodies. The 𝙸 bars indicate 95% confidence intervals. The results of the fluorescence-based focus reduction neutralization test (FRNT50 [the reciprocal dilution of serum that neutralizes 50% of the input virus]) against USA-WA1/2020, BA.4–BA.5 spike, BA.4.6 spike, BA.2.75.2 spike, BQ.1.1 spike, and XBB.1 spike are shown. Each circle in the figure represents an individual participant. The geometric mean factor increases (GMFI [the increase in neutralizing titers in serum samples from the day of the fourth dose to 1 month after the fourth dose]) and GMFI ratios between GMFIs of bivalent vaccine and GMFIs of monovalent vaccine are shown. In both panels, the lower boundaries of the two-sided 95% confidence intervals for the GMFI of bivalent or monovalent booster against USA-WA1/2020, BA.4–BA.5, BA.4.6, BA.2.75.2, BQ.1.1, and XBB.1 are all greater than 1.
Our results support three conclusions. First, the bivalent BA.4–BA.5 vaccine consistently elicited higher neutralizing responses against BA.5-derived sublineages (BA.4.6, BQ.1.1, and XBB.1) and the BA.2-derived sublineage (BA.2.75.2) than the original BNT162b2 vaccine when administered as a fourth booster dose, regardless of the participants’ history of SARS-CoV-2 infection. Second, after the fourth dose, higher neutralizing titers developed in participants with a history of SARS-CoV-2 infection than in those without a history of infection. Third, for each tested omicron sublineage, the difference between the original and bivalent neutralizing geometric mean factor increase was greater in the serum samples obtained from participants without previous infection than in those obtained from participants with previous infection.
Among all omicron sublineages, BA.2.75.2, BQ.1.1, and XBB.1 had the lowest vaccine-elicited neutralization; however, neutralizing titers after a bivalent booster were several times as high as those after the original BNT162b2 vaccine. These data suggest that the bivalent vaccine is more immunogenic than the original vaccine, with greater breadth of responses against circulating omicron sublineages. These findings support the use of the current bivalent vaccine and underscore the importance of monitoring real-world effectiveness.
Protocol
Supplementary Appendix
Disclosure Forms
This letter was published on January 25, 2023, at NEJM.org.
Footnotes
Supported by BioNTech and Pfizer.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Cele S, Jackson L, Khoury DS, et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 2022;602:654-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurhade C, Zou J, Xia H, et al. Neutralization of omicron sublineages and deltacron SARS-CoV-2 by three doses of BNT162b2 vaccine or BA.1 infection. Emerg Microbes Infect 2022;11:1828-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Z, VanBlargan LA, Bloyet LM, et al. Identification of SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization. Cell Host Microbe 2021;29(3):477-488.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis-Gardner ME, Lai L, Wali B, et al. mRNA bivalent booster enhances neutralization against BA.2.75.2 and BQ.1.1. November 1, 2022. (https://www.biorxiv.org/content/10.1101/2022.10.31.514636v1). preprint. [DOI] [PMC free article] [PubMed]
- 5.Winokur P, Gayed J, Fitz-Patrick D, et al. Bivalent omicron BA.1–adapted BNT162b2 booster in adults older than 55 years. New Engl J Med 2023;388:214-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muruato AE, Fontes-Garfias CR, Ren P, et al. A high-throughput neutralizing antibody assay for COVID-19 diagnosis and vaccine evaluation. Nat Commun 2020;11:4059-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.