Abstract
Objectives
To evaluate the efficacy of guselkumab for the treatment of active PsA utilizing composite indices.
Methods
Data were pooled from the phase 3 DISCOVER-1 (n = 381) and DISCOVER-2 (n = 739) studies. In both studies, patients were randomized 1:1:1 to subcutaneous guselkumab 100 mg every 4 weeks (Q4W); guselkumab 100 mg at week 0, week 4, then Q8W; or placebo Q4W with crossover to guselkumab 100 mg Q4W at week 24. Composite indices used to assess efficacy through week 52 included Disease Activity Index for Psoriatic Arthritis (DAPSA), Psoriatic Arthritis Disease Activity Score (PASDAS), minimal disease activity (MDA), and very low disease activity (VLDA). Through week 24, treatment failure rules were applied. Through week 52, non-responder imputation was used for missing data.
Results
Greater proportions of guselkumab- than placebo-treated patients achieved DAPSA low disease activity (LDA) and remission, PASDAS LDA and VLDA, MDA, and VLDA at week 24 vs placebo (all unadjusted P < 0.05). At week 52, in the guselkumab Q4W and Q8W groups, respectively, response rates were as follows: DAPSA LDA, 54.2% and 52.5%; DAPSA remission, 18.2% and 17.6%; PASDAS LDA, 45.3% and 41.9%; PASDAS VLDA, 16.9% and 19.5%; MDA, 35.9% and 30.7%; and VLDA, 13.1% and 14.4%. In the placebo-crossover-to-guselkumab group, response rates for all composite indices increased after patients switched to guselkumab, from week 24 through week 52.
Conclusion
Treatment with guselkumab provided robust and sustained benefits across multiple PsA domains through 1 year, indicating that guselkumab is an effective therapy for the diverse manifestations of PsA.
Trial registration
Keywords: guselkumab, PsA, composite indices, remission, skin clearance, joint disease
Rheumatology key messages.
Composite indices combine physician-assessed and patient-reported measures to comprehensively evaluate outcomes across PsA domains.
Assessments of guselkumab efficacy using composite indices showed significant, sustained achievement of low disease activity/remission.
These results suggest that guselkumab is an effective treatment for diverse manifestations of PsA.
Introduction
PsA is a chronic inflammatory disease characterized by several distinct clinical manifestations, including peripheral arthritis, axial disease, dactylitis, enthesitis, skin disease, and nail disease [1, 2]. The initial presentation and disease course of PsA is remarkably heterogeneous, making diagnosis and treatment challenging. Most patients develop a range of both musculoskeletal and extra-articular disease manifestations, which respond differently to available therapies [2, 3].
As a result of the multifaceted, systemic nature of PsA development and progression, treatment guidelines evolved to recommend a ‘treat-to-target’ approach, with the goal of achieving remission or low disease activity (LDA) across all domains of disease [2, 4, 5]. The need to define thresholds for achievement of remission and LDA resulted in the development of several multidimensional indices [6, 7], including the Disease Activity in Psoriatic Arthritis (DAPSA) [8], the Psoriatic Arthritis Disease Activity Score (PASDAS) [9, 10], and minimal disease activity (MDA)/very low disease activity (VLDA) criteria [11, 12]. These composite indices combine various physician-assessed and patient-reported outcome measures applicable to both PsA clinical trials and real-world clinical practice to comprehensively evaluate outcomes across PsA domains [6, 7, 13–16].
Guselkumab is a fully human monoclonal antibody that selectively and specifically binds and inhibits the p19 subunit of IL-23. Guselkumab is approved for the treatment of adults with moderate to severe plaque psoriasis and active PsA [17]. The safety and efficacy of guselkumab 100 mg administered every 4 weeks (Q4W) or every 8 weeks (Q8W) were evaluated in the DISCOVER-1 and DISCOVER-2 randomized, placebo-controlled, phase 3 studies in patients with active PsA. In these studies, guselkumab Q4W and Q8W significantly improved signs and symptoms of PsA, including psoriasis-related skin symptoms, enthesitis, dactylitis, physical function and health-related quality of life, compared with placebo at week 24 [18, 19], with sustained improvements through 1 year [20, 21]. In the current analyses, data were pooled from the DISCOVER-1 and DISCOVER-2 phase 3 studies to assess guselkumab efficacy through 1 year utilizing various composite indices of PsA disease activity.
Methods
Patients and study designs
DISCOVER-1 (NCT03162796) [18] and DISCOVER-2 (NCT03158285) [19] were similarly designed, randomized, placebo-controlled studies of guselkumab in patients with active PsA, diagnosed according to Classification Criteria for Psoriatic Arthritis (CASPAR) [22], who had an inadequate response or intolerance to non-biologic DMARDs, apremilast, and/or NSAIDs. In DISCOVER-1, active PsA was defined as ≥3 tender and ≥3 swollen joints and serum CRP concentration ≥0.3 mg/dl. In DISCOVER-2, the definition of active PsA was more stringent, requiring ≥5 tender and ≥5 swollen joints and serum CRP concentration ≥0.6 mg/dl. In DISCOVER-1, prior treatment with one or two TNF-ɑ inhibitors (TNFi) was permitted but was limited to ∼30% of the study population [18], while all patients in DISCOVER-2 were biologic-naïve [19].
In both studies, patients were randomized in a 1:1:1 ratio to receive subcutaneous guselkumab 100 mg Q4W; guselkumab 100 mg at week 0, week 4, then Q8W; or placebo Q4W with crossover to guselkumab 100 mg Q4W at week 24. Patients could continue stable baseline use of selected non-biologic DMARDs, including methotrexate ≤25 mg/week, sulfasalazine ≤3 g/day, hydroxychloroquine ≤400 mg/day, or leflunomide ≤20 mg/day; oral corticosteroids (≤10 mg/day of prednisone or equivalent); and NSAIDs or other analgesics. At week 16, patients with <5% improvement from baseline in both swollen joint count (SJC) and tender joint count (TJC) were eligible for early escape and had the option to initiate or increase their dose of one selected non-biologic DMARD, oral corticosteroids, NSAIDs, or other analgesics.
DISCOVER-1 and DISCOVER-2 were conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. The study protocols were approved by ethics committees at each site (Sterling institutional review board numbers [US sites]: 5959C and 5910C). All participants provided written informed consent.
Assessments
The proportions of patients achieving DAPSA LDA, DAPSA remission, PASDAS LDA, PASDAS VLDA, MDA, and VLDA by visit were prespecified efficacy assessments in the DISCOVER-1 and DISCOVER-2 studies. The proportions of patients achieving clinical DAPSA (cDAPSA) LDA and remission were determined post hoc for both studies. For the current analyses, data for these outcomes were pooled across the two studies and are also presented separately for each study.
DAPSA scores are calculated as the sum of TJC (range, 0–68), SJC (range, 0–66), patient pain assessment (0–10 cm visual analogue scale [VAS]), patient global assessment (PtGA) of arthritis activity (0–10 cm VAS), and CRP (mg/dl) [3, 8]. cDAPSA is a modification of the DAPSA that excludes CRP [23]. Higher DAPSA and cDAPSA scores represent more severe disease activity. DAPSA LDA and remission are defined as scores of ≤14 and ≤4, respectively, and cDAPSA LDA and remission are defined as scores of ≤13 and ≤4, respectively [23]. Based on the measurement schedule for the individual assessments included, DAPSA and cDAPSA scores were determined at weeks 4, 8, 12, 16, 20, 24, 28, 36, 44, and 52.
PASDAS scores are calculated based on a formula that includes physician global assessment of skin and joints (0–100 mm VAS), PtGA of skin and joints (0–100 mm VAS), 36-item Short Form Health Survey physical component summary score (range, 0–100), SJC (0–66), TJC (0–68), Leeds Enthesitis Index (LEI) score (range, 0–6) [24], tender dactylitis count (range, 0–20), and CRP (mg/l). Total PASDAS scores range from 0 to 10, with higher scores representing more severe disease activity [9, 10, 12]. PASDAS LDA is defined as a score of ≤3.2, and PASDAS VLDA is defined as a score of ≤1.9 [12]. Based on the measurement schedule for the individual assessments included, PASDAS scores were calculated at weeks 8, 16, 24, and 52.
Patients are considered to meet MDA or VLDA criteria, respectively, if they achieve five (MDA) or seven (VLDA) of the following seven outcomes: TJC ≤1, SJC ≤1, LEI ≤1, Psoriasis Area and Severity Index (PASI) score ≤1, patient pain VAS ≤15 mm (range, 0–100), PtGA (arthritis and psoriasis) VAS ≤20 mm (range, 0–100), and HAQ-Disability Index (HAQ-DI) score ≤0.5 (range, 0–3) [3, 11, 12]. The proportions of patients achievieng MDA and VLDA were determined at weeks 16, 24, and 52.
In DISCOVER-2, single radiographs of the hands (posteroanterior) and feet (anteroposterior) were obtained at weeks 0, 24, and 52 (or at discontinuation if between weeks 24 and 52; Reading Session 2) and scored by blinded central primary readers using the modified van der Heijde–Sharp score for PsA [25]. Mean changes in PsA-modified van der Heijde-Sharp score from week 0 to week 52 were compared for patients who did and did not achieve PASDAS LDA, DAPSA LDA, and MDA responses at week 24 (all using observed data).
Statistical analyses
Through week 24, data were imputed as non-response for patients meeting treatment failure criteria (i.e. discontinued study treatment, terminated study participation, initiated or increased their dose of non-biologic DMARDs or oral corticosteroids, or initiated any protocol-prohibited PsA treatments) and for patients with missing data (non-responder imputation). After week 24, patients with missing data were considered to be non-responders (non-responder imputation), and treatment failure rules were not applied.
Comparisons between the guselkumab groups and the placebo group through week 24 were performed using a Cochran–Mantel–Haenszel test stratified by randomization stratification factors (baseline use of non-biologic DMARDs [yes/no] and prior exposure to TNFi [yes/no] in DISCOVER-1, and baseline use of non-biologic DMARDs [yes/no] and most recent CRP value prior to randomization [<2.0 or ≥2.0 mg/dl] in DISCOVER-2). P-values were not adjusted for multiplicity. No treatment group comparisons were performed after week 24.
Results
Patient disposition and baseline characteristics
In these pooled analyses, 1120 patients were randomized and treated in the DISCOVER-1 (guselkumab Q4W, n = 128; guselkumab Q8W, n = 127; placebo, n = 126) and DISCOVER-2 (guselkumab Q4W, n = 245; guselkumab Q8W, n = 248; placebo, n = 246) studies. A total of 118 patients in DISCOVER-1 (10.5% of the pooled population) had prior exposure to one or two TNFi. In this pooled population, 351 (94%) patients randomized to guselkumab Q4W, 350 (93%) patients randomized to guselkumab Q8W, and 335 (90%) patients randomized to placebo who crossed over to guselkumab at week 24 received study treatment through 1 year.
At baseline, pooled patient demographic and PsA disease characteristics were comparable across randomized treatment groups (Table 1). On average, patients had PsA for >5 years, with active skin disease (mean PASI score of 8.8–10.4), joint inflammation (mean SJC of 11.4–11.5, mean TJC of 19.9–21.0), and impaired physical function (mean HAQ-DI score of 1.2–1.3); >60% of patients presented with enthesitis, and >40% of patients presented with dactylitis. Mean DAPSA (45.9–46.9), PASDAS (6.4–6.5), patient assessment of pain (6.1–6.2), and PtGA of disease activity (6.3–6.5) scores, and mean CRP concentrations (1.6–1.9 mg/dl) were consistent with highly active PsA.
Table 1.
Baseline characteristics for randomized and treated patients in DISCOVER-1 and DISCOVER-2
| Characteristic | Guselkumab Q4W | Guselkumab Q8W | Placebo |
|---|---|---|---|
| Randomized and treated patients, n | 373 | 375 | 372 |
| Age, mean (s.d.), years | 46.5 (11.5) | 46.2 (11.9) | 47.2 (11.5) |
| Male, n (%) | 208 (55.8) | 197 (52.5) | 178 (47.8) |
| BMI, mean (s.d.), kg/m2 | 29.4 (5.8) | 29.1 (6.3) | 29.2 (6.1) |
| PsA duration, mean (s.d.), years | 5.9 (6.1) | 5.6 (5.7) | 6.2 (6.4) |
| Swollen joint count (0–66), mean (s.d.) | 11.4 (7.5) | 11.4 (7.7) | 11.5 (7.0) |
| Tender joint count (0–68), mean (s.d.) | 20.8 (13.6) | 19.9 (12.8) | 21.0 (13.5) |
| Patients with enthesitis (LEI > 0), n (%)a | 243 (65.1) | 230 (61.5) | 255 (68.7) |
| LEI score (1–6), mean (s.d.) | 3.0 (1.6) | 2.7 (1.5) | 2.8 (1.6) |
| Patients with dactylitis, n (%)a | 159 (42.6) | 160 (42.8) | 154 (41.5) |
| Dactylitis score (1–60), mean (s.d.)b | 8.8 (10.3) | 8.1 (9.7) | 7.7 (8.7) |
| Patient assessment of pain (VAS 0–10 cm), mean (s.d.) | 6.1 (2.0) | 6.2 (2.0) | 6.1 (2.0) |
| Patient global assessment of disease activity (arthritis, VAS 0–10 cm), mean (s.d.) | 6.3 (2.0) | 6.5 (2.0) | 6.4 (2.0) |
| Physician global assessment of disease activity (VAS 0–10 cm), mean (s.d.) | 6.5 (1.6) | 6.4 (1.6) | 6.5 (1.6) |
| CRP, mean (s.d.), mg/dl | 1.6 (2.0) | 1.9 (2.4) | 1.9 (2.4) |
| PASI (0–72), mean (s.d.)c | 10.4 (11.2) | 9.2 (11.1) | 8.8 (9.5) |
| HAQ-DI (0–3), mean (s.d.) | 1.2 (0.6) | 1.3 (0.6) | 1.3 (0.6) |
| DAPSA score (remission ≤4, high disease activity >28), mean (s.d.) | 46.2 (20.7) | 45.9 (20.9) | 46.9 (20.2) |
| cDAPSA score (remission ≤4, high disease activity >27), mean (s.d.) | 44.6 (20.3) | 44.1 (20.4) | 45.0 (19.9) |
| PASDAS score (low disease activity ≤3.2, high disease activity >5.4), mean (s.d.)d | 6.4 (1.1) | 6.5 (1.1) | 6.5 (1.0) |
| Patients receiving methotrexate, n (%) | 218 (58.4) | 209 (55.7) | 227 (61.0) |
| Dose, mean (s.d.), mg/week | 15.6 (4.7) | 15.8 (5.3) | 15.4 (4.6) |
| Patients receiving oral corticosteroids, n (%) | 62 (16.6) | 68 (18.1) | 69 (18.5) |
| Dose, mean (s.d.), mg/day | 6.9 (2.4) | 6.6 (2.4) | 7.4 (2.6) |
Guselkumab Q4W, n = 373; guselkumab Q8W, n = 374; placebo, n = 371.
Each of 20 digits is scored from 0 (no dactylitis) to 3 (severe dactylitis), resulting in a total score range of 0–60; mean scores shown here are for patients with dactylitis (score ≥1) at baseline.
Guselkumab Q4W, n = 373; guselkumab Q8W, n = 375; placebo, n = 371.
Guselkumab Q4W, n = 369; guselkumab Q8W, n = 372; placebo, n = 367. cDAPSA: clinical DAPSA (excludes CRP); DAPSA: Disease Activity Index for Psoriatic Arthritis; HAQ-DI: HAQ-Disability Index; LEI: Leeds Enthesitis Index; PASDAS: Psoriatic Arthritis Disease Activity Score; PASI: Psoriasis Area and Severity Index; Q4W: every 4 weeks; Q8W: every 8 weeks; VAS: visual analogue scale.
Placebo-controlled period through week 24
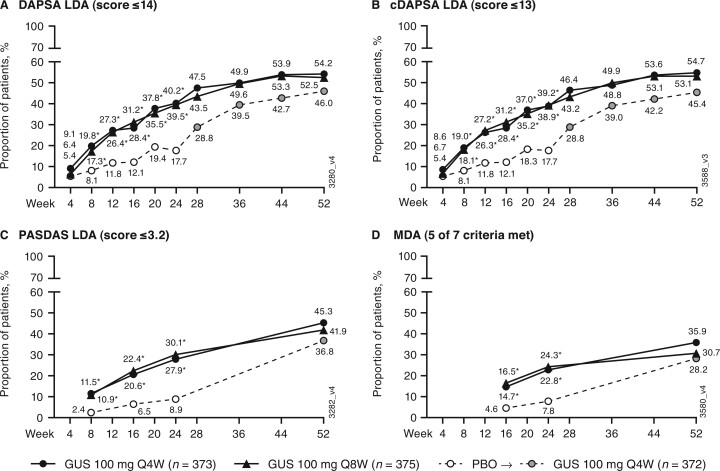
At week 24, greater proportions of guselkumab- than placebo-treated patients achieved low levels of disease activity when assessed with DAPSA LDA (≤14), cDAPSA LDA (≤13), PASDAS LDA (≤3.2), and MDA (five of seven components) composite indices (Fig. 1). Separation from placebo was observed in the guselkumab groups as early as week 8 for achievement of DAPSA LDA, cDAPSA LDA, and PASDAS LDA, and at week 16 (earliest time point assessed) for MDA.
Fig. 1.
Achievement of DAPSA LDA, cDAPSA LDA, PASDAS LDA, and MDA
(A) DAPSA LDA; (B) cDAPSA LDA; (C); PASDAS LDA; (D) MDA. Through week 24, patients meeting treatment failure criteria or with missing data were considered non-responders. Treatment group comparisons through week 24 were not adjusted for multiplicity of testing. After week 24 and through week 52, patients with missing data were considered non-responders. *P < 0.001, vs PBO (nominal). cDAPSA: clinical DAPSA (excludes CRP); DAPSA: Disease Activity in Psoriatic Arthritis; GUS: guselkumab; LDA: low disease activity; MDA: minimal disease activity; PASDAS: Psoriatic Arthritis Disease Activity Score; PBO: placebo; Q4W: every 4 weeks; Q8W: every 8 weeks.
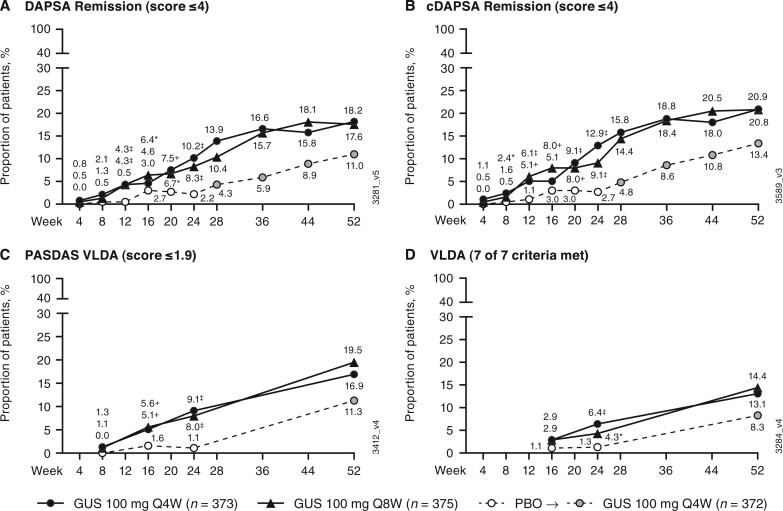
At week 24, greater proportions of guselkumab- than placebo-treated patients achieved near or complete remission when assessed with DAPSA (≤4) and cDAPSA (≤4) remission, PASDAS VLDA (≤1.9), and VLDA (seven of seven components) composite indices (Fig. 2). Guselkumab-treated patients separated from the placebo group as early as week 12 for DAPSA and cDAPSA remission, at week 16 for PASDAS VLDA, and at week 24 for VLDA.
Fig. 2.
Achievement of DAPSA remission, cDAPSA remission, PASDAS VLDA, and VLDA
(A) DAPSA remission; (B) cDAPSA remission; (C) PASDAS VLDA; (D) VLDA. Through week 24, patients meeting treatment failure criteria or with missing data were considered non-responders. Treatment group comparisons through week 24 were not adjusted for multiplicity of testing. After week 24 and through week 52, patients with missing data were considered non-responders. *P < 0.05, +P < 0.01, ‡P < 0.001, vs PBO (nominal). cDAPSA: clinical DAPSA (excludes CRP); DAPSA: Disease Activity in Psoriatic Arthritis; GUS: guselkumab; PASDAS: Psoriatic Arthritis Disease Activity Score; PBO: placebo; Q4W: every 4 weeks; Q8W: every 8 weeks; VLDA: very low disease activity.
In the pooled results, no notable differences in response rates were observed between the guselkumab Q4W and Q8W dosing regimens. Response trends were generally consistent in the individual DISCOVER-1 (biologic-naïve and TNFi-experienced patients) and DISCOVER-2 (biologic-naïve patients) studies (Supplementary Figs S1 and S2, available at Rheumatology online). However, response rates were higher in DISCOVER-1 than in DISCOVER-2 at week 24 for both guselkumab dosing regimens (Q4W and Q8W) for DAPSA LDA, cDAPSA LDA, and PASDAS LDA, and for the guselkumab Q4W regimen for DAPSA remission, cDAPSA remission, PASDAS VLDA, and VLDA.
Active treatment period after week 24 through week 52
Among the guselkumab-randomized patients, composite response rates increased post-week 24 through week 52, with indications of the potential for higher response rates beyond week 52 (i.e. no plateau was reached by week 52 for PASDAS LDA/VLDA or MDA/VLDA). At week 52, 54% (Q4W) and 52% (Q8W) of guselkumab-randomized patients achieved DAPSA LDA; 55% (Q4W) and 53% (Q8W) achieved cDAPSA LDA; 45% (Q4W) and 42% (Q8W) achieved PASDAS LDA; and 36% (Q4W) and 31% (Q8W) achieved MDA (Fig. 1). For placebo-randomized patients who crossed over to guselkumab Q4W at week 24, response rates for DAPSA LDA, cDAPSA LDA, PASDAS LDA, and MDA at week 52 approached those achieved by guselkumab-randomized patients.
For the more stringent remission-related endpoints at week 52, 18% (Q4W and Q8W) achieved DAPSA remission; 21% (Q4W and Q8W) achieved cDAPSA remission; 17% (Q4W) and 20% (Q8W) achieved PASDAS VLDA; and 13% (Q4W) and 14% (Q8W) achieved VLDA (Fig. 2). Notably, VLDA was more difficult to attain than DAPSA remission and PASDAS remission when assessing longer-term treatment effect via these composite indices. In patients who crossed over from placebo to guselkumab Q4W at week 24, substantial increases in composite response rates were seen by week 52.
Across all composite indices, no notable differences in response rates were observed between the guselkumab Q4W and Q8W dosing regimens. Results were generally consistent in the individual DISCOVER-1 and DISCOVER-2 studies (Supplementary Figs S1 and S2, available at Rheumatology online). Differences in response rates between DISCOVER-1 and DISCOVER-2 were generally smaller at week 52 than at week 24, although DISCOVER-1 response rates remained notably higher in patients randomized to guselkumab Q4W for DAPSA and cDAPSA LDA and remission, PASDAS LDA, and MDA (Supplementary Figs S1 and S2, available at Rheumatology online).
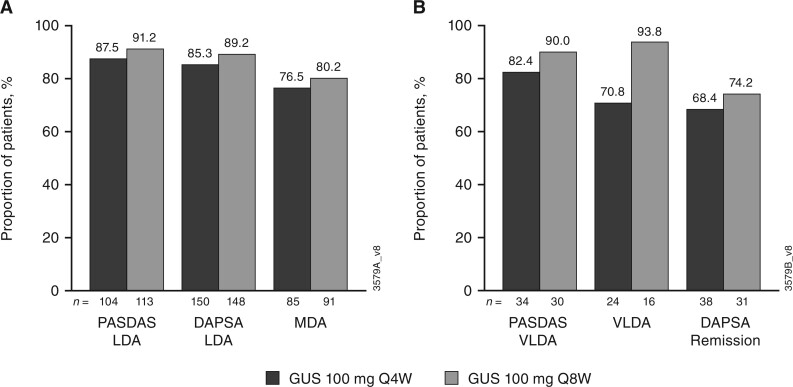
Maintenance of response
As shown in Fig. 3, among guselkumab-treated patients who achieved low levels of disease activity at week 24, the majority maintained responses at week 52 [PASDAS LDA: 88% (Q4W) and 91% (Q8W); DAPSA LDA: 85% (Q4W) and 89% (Q8W); MDA: 76% (Q4W) and 80% (Q8W)]. Similarly, among guselkumab-treated patients who achieved remission/VLDA at week 24, the majority maintained response at week 52 [PASDAS VLDA: 82% (Q4W) and 90% (Q8W); VLDA: 71% (Q4W) and 94% (Q8W); DAPSA remission: 68% (Q4W) and 74% (Q8W)].
Fig. 3.
Maintenance of LDA (A) and remission (B) at week 52 among week-24 responders (guselkumab-randomized patients)
n represents the total number of patients with response at week 24. Through week 24, patients meeting treatment failure criteria or with missing data were considered non-responders. After week 24 and through week 52, patients with missing data were considered non-responders. DAPSA: Disease Activity Index for Psoriatic Arthritis; GUS: guselkumab; LDA: low disease activity; MDA: minimal disease activity; PASDAS: Psoriatic Arthritis Disease Activity Score; Q4W: every 4 weeks; Q8W: every 8 weeks; VLDA: very low disease activity.
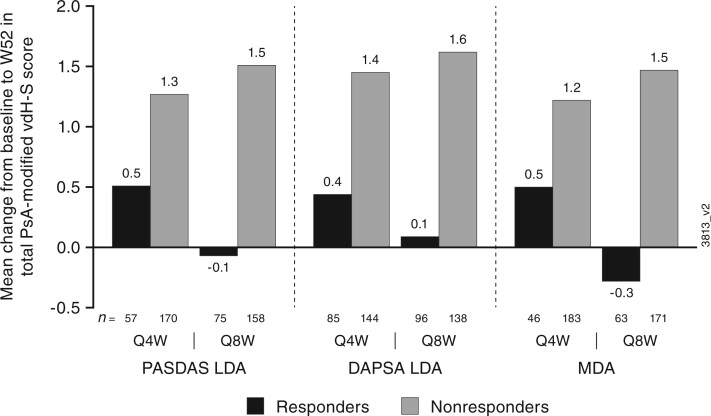
Association of radiographic progression at 1 year with composite efficacy responses at week 24
In DISCOVER-2, patients who achieved PASDAS LDA, DAPSA LDA, and MDA at week 24 had smaller mean changes in total PsA-modified van der Heijde–Sharp scores from week 0 to week 52 compared with patients who did not achieve these responses (Fig. 4).
Fig. 4.
Mean vdH-S score changes by week 24 PASDAS LDA, DAPSA LDA, and MDA response (DISCOVER-2)
Evaluable patients had an observed change from baseline to week 52 in PsA-modified vdH-S score and observed PASDAS, DAPSA, or MDA status, respectively, at week 24. DAPSA: Disease Activity Index for Psoriatic Arthritis; LDA: low disease activity; MDA: minimal disease activity; PASDAS: Psoriatic Arthritis Disease Activity Score; Q4W: every 4 weeks; Q8W: every 8 weeks; vdH-S: van der Heijde–Sharp.
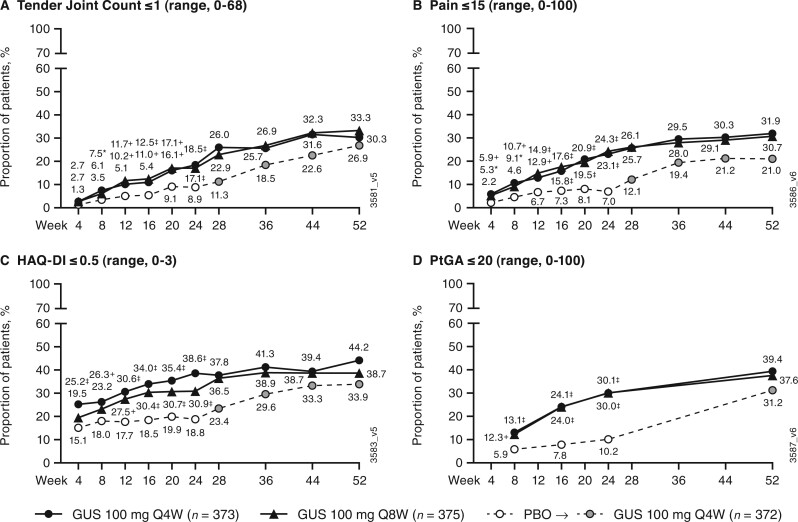
Assessment of residual disease based on components of MDA and VLDA
At week 52, among guselkumab-randomized patients, a majority achieved ≤1 tender entheseal point [79% (Q4W) and 77% (Q8W)], PASI score ≤1 [79% (Q4W) and 73% (Q8W)], and SJC ≤1 [66% (Q4W) and 61% (Q8W)] (Supplementary Fig. S3, available at Rheumatology online). However, most patients continued to show signs of potential residual disease activity, as evidenced by lower rates for achieving normalized physical function [HAQ-DI ≤0.5; 44% (Q4W) and 39% (Q8W)], PtGA ≤20 [39% (Q4W) and 38% (Q8W)], TJC ≤1 [30% (Q4W) and 33% (Q8W)], and pain ≤15 [32% (Q4W) and 31% (Q8W)] (Fig. 5).
Fig. 5.
Achievement of ≤1 tender joint, patient assessment of pain VAS ≤15, HAQ-DI score ≤0.5, and PtGA ≤20
(A) ≤1 tender joint; (B) VAS ≤15; (C) HAQ-DI ≤0.5; (D) PtGA ≤20. Through week 24, patients meeting treatment failure criteria or with missing data were considered non-responders. Treatment group comparisons through week 24 were not adjusted for multiplicity of testing. After week 24 and through week 52, patients with missing data were considered non-responders. *P < 0.05, +P < 0.01, ‡P < 0.001, vs PBO (nominal). GUS: guselkumab; HAQ-DI: HAQ-Disability Index; PBO: placebo; PtGA: patient global assessment of disease activity (arthritis and psoriasis); Q4W: every 4 weeks; Q8W: every 8 weeks; VAS: visual analogue scale.
Discussion
Results of these pooled post hoc analyses from DISCOVER-1 and DISCOVER-2 showed that patients with active PsA who received guselkumab Q4W or Q8W achieved consistent and robust responses over placebo based on achievement of numerous composite measures of disease activity. Separation of guselkumab from placebo was observed as early as week 8 for DAPSA LDA and PASDAS LDA. Among guselkumab-randomized patients, response rates for all composite indices continued to increase from baseline through week 52. Furthermore, the majority of guselkumab-randomized patients who achieved responses at week 24 had maintained or increased levels of response at week 52, and in many cases slopes of the response curves suggest the potential for further improvement over time. Placebo-randomized patients who crossed over to guselkumab Q4W at week 24 showed steady improvements in all composite indices, with response rates approaching those observed for guselkumab-randomized patients at week 52.
Response trends were generally consistent when results from DISCOVER-1 and DISCOVER-2 were considered separately. However, across most composite indices, week 24 response rates were higher in DISCOVER-1 than in DISCOVER-2, despite the fact that ∼30% of patients in DISCOVER-1 had prior exposure to TNFi, and these TNFi-experienced patients had numerically lower MDA response rates compared with TNFi-naïve patients at week 24 (17–26% vs 32–34%) [20]. The observed differences between studies may be related to differences in eligibility criteria requiring patients in DISCOVER-2 to have more severe disease (SJC and TJC ≥5, and CRP ≥0.6 mg/dl) at baseline than in DISCOVER-1 (SJC and TJC ≥3, and CRP ≥0.3 mg/dl).
In DISCOVER-2, composite measure responses at week 24 were associated with reduced long-term radiographic progression based on smaller mean changes in total PsA-modified van der Heijde–Sharp scores at week 52. This finding suggests that early treatment with guselkumab may reduce the risk for permanent joint damage.
Pooled results showed that by week 52, approximately one-third of all guselkumab-treated patients achieved the widely accepted treatment target of MDA. Evaluation of the proportions of patients achieving the individual MDA components showed particularly high and consistent response rates for objective measures, including resolution of enthesitis, swollen joints, and skin disease. Through week 52, pain and TJC >1—which would be expected to heavily influence the patient-reported outcomes of physical function (HAQ-DI) and PtGA—appeared to be the symptoms limiting achievement of MDA for many patients. However, the trajectory of responses for achievement of TJC ≤1, pain ≤15, and PtGA ≤20 suggests a possibility of increased response rates with continued treatment with guselkumab.
Our findings that disease activity based on TJC, pain, and PtGA measurements often limit the achievement of composite outcomes are generally consistent with published studies identifying pain, PtGA, skin disease, and tender joints as the most common unmet criteria in patients failing to achieve MDA/VLDA treatment targets [16, 26, 27]. However, in contrast to other studies [3, 16, 26], achievement of the skin disease criterion was not a limiting factor in attaining MDA/VLDA for most patients in DISCOVER-1 and DISCOVER-2. This finding is likely related to better efficacy of guselkumab (an IL-23 inhibitor) for the treatment of psoriasis compared with biologic DMARDs with different mechanisms of action (i.e. TNF, IL-17, or IL-12/23 inhibition) [28–33]. Specifically, in the DISCOVER studies, ∼70% of guselkumab-randomized patients achieved PASI ≤1 at week 24, and response rates increased to up to 79% by week 52; in the placebo to guselkumab Q4W crossover group, the PASI ≤1 response rate increased from 27% at week 24 to 74% at week 52. These results are particularly noteworthy since these analyses used the more stringent of two MDA definitions of skin response (i.e. PASI score ≤1 instead of body surface area of psoriasis ≤3%) [3, 11, 12].
Similarly, for DAPSA, high pain and PtGA parameters often limit the achievement of remission, even in patients without active joint inflammation [16, 34]. For patients with established structural damage to affected joints before initiating treatment with guselkumab, achieving low levels of pain and satisfactory physical function and PtGA may not have been possible. Furthermore, recent studies indicate that substantial proportions of patients with PsA experience persistent, widespread pain despite effective control of inflammation with conventional or biologic DMARDs [34–36]. In one model of chronic pain, peripheral neurological hypersensitivity develops following pathological changes in the central nervous system [35]. This altered pain perception in patients with PsA may lead to overestimation of joint tenderness and inflammatory activity [35]. Thus, achievement of low levels of pain may be especially difficult for some patients with PsA, and adjustment of treatment targets may be warranted (e.g. based on disease duration, amount of structural damage, or altered pain perception). Alternatively, centralized pain may develop in association with prevalent comorbidities, such as obstructive sleep apnoea, anxiety and/or depression, and deconditioned muscles and joints.
These analyses of the pooled DISCOVER-1 and DISCOVER-2 dataset provide a unique opportunity to demonstrate consistent and robust response rates with guselkumab vs placebo for several different composite measures, each with emphasis on different clinical outcomes. The DAPSA, which focuses mainly on measures of peripheral articular disease, does not include specific assessments of dactylitis, enthesitis or skin disease, and requires laboratory testing to measure CRP levels [3, 22, 37–40]. In contrast to the DAPSA, the PASDAS and MDA assess multiple domains of articular and extra-articular disease activity [37, 38]; therefore, these measures are more likely to be relevant as PsA treatment targets. In routine clinical practice, MDA is used more often than the PASDAS because PASDAS scoring is complicated and not intuitive, limiting its use in real-world settings [7, 13]. Overall, increased use of composite indices is important to promote recommended treat-to-target approaches, which may reduce the undertreatment of PsA and improve patient outcomes [38].
As with all controlled clinical trials, patients enrolled in the DISCOVER-1 and DISCOVER-2 studies were required to meet predefined selection criteria based on previous treatment and medical history. Additionally, while DAPSA LDA and remission, PASDAS LDA and VLDA, and MDA were prespecified secondary efficacy endpoints of the individual DISCOVER-1 and DISCOVER-2 studies, pooling the results across both studies was not prespecified and was performed post hoc to increase the sample size. Therefore, results from the current analyses may not be generalizable to all patients with PsA. However, consistent with results of these analyses, in a recent small real-world observational study in 24 patients with early PsA and severe skin disease, 75% (18/24) of patients achieved DAPSA LDA or remission after 6 months of guselkumab treatment; 17 of these patients completed 1 year of treatment, and all achieved DAPSA LDA (65% [11/17]) or remission (35% [6/17]) [41].
Comparison of the results from these analyses with other published studies is inherently limited. To date, few clinical studies have reported composite index results, and among published results, there are often differences in baseline disease and demographic characteristics, sample sizes, analysis methods (i.e. observed vs imputed data), and the composite endpoints reported [37, 42–47]. However, results of analyses published to date suggest that composite indices can provide more comprehensive assessments of the range of PsA disease manifestations than conventional peripheral joint-based outcome measures (e.g. ACR20), and that treatment targets such as MDA, DAPSA LDA/remission, and PASDAS LDA/VLDA are achievable for many patients using currently available biologic or targeted synthetic therapies [37, 42–47]. Thus, composite indices may be useful in routine clinical practice and as key endpoints in future PsA clinical trials.
Taken together, results of these post hoc analyses indicate that patients with active PsA who receive treatment with guselkumab Q4W or Q8W can achieve robust and sustained LDA or remission, based on various composite indices that measure responses across domains including joint pain, swelling, stiffness, inflammation and tenderness, and skin clearance. As such, guselkumab is an important treatment option for the diverse manifestations of this disease.
Supplementary Material
Acknowledgements
Medical writing support was provided by Cherie Koch, PhD, of Janssen Scientific Affairs, LLC, under the direction of the authors in accordance with Good Publication Practice guidelines (Ann Intern Med 2015; 163:461-4).
Funding: These studies were funded by Janssen Research & Development, LLC.
Disclosure statement: L.C.C.: consultant fees from AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer and UCB; grant/research support from AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Novartis, Pfizer and UCB; speaker fees from AbbVie, Amgen, Biogen, Celgene, Eli Lilly, Galapagos, Gilead, Janssen, Medac, Novartis, Pfizer and UCB. L.C.C. is funded by a National Institute for Health Research (NIHR) Clinician Scientist award. The research was supported by the NIHR Oxford Biomedical Research Centre (BRC). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
C.T.R.: grant/research support from AbbVie, Amgen and UCB, and consulting fees from AbbVie, Amgen, Eli Lilly, Gilead, Janssen, Novartis, Pfizer and UCB.
L.G.: research grants from Amgen, Galapagos, Lilly, Pfizer, Sandoz; consulting fees from AbbVie, Amgen, Bristol Myers Squibb, Galapagos, Gilead, GlaxoSmithKline, Janssen, Lilly, Novartis, Pfizer, Samsung Bioepis, Sanofi-Aventis and UCB.
P.S.H.: consulting fees from Eli Lilly; fees for educational services from AbbVie, Amgen, Janssen and Novartis.
P.R.: consulting fees from AbbVie, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Merck, Novartis, Pfizer and UCB (less than $10 000 each); meeting attendance/travel support from Janssen; and research grants from Janssen and Novartis.
A.P.K.: employee of Janssen Research & Development; owns stock in Johnson & Johnson.
X.L.X.: employee of Janssen Research & Development; owns stock in Johnson & Johnson.
M.S.: employee of Immunology Global Medical Affairs, Janssen Pharmaceutical Companies of Johnson & Johnson; owns stock in Johnson & Johnson.
C.S.K.: employee of Janssen Research & Development; owns stock in Johnson & Johnson.
C.C.: former employee of Janssen Cilag; owns stock in Johnson & Johnson; current employee of Chiesi, France.
W.N.: employee of Janssen Scientific Affairs; owns stock in Johnson & Johnson.
S.S.: employee of Janssen Research & Development; owns stock in Johnson & Johnson.
Y.W.: consultant employed by IQVIA, Inc. and funded by Janssen to provide statistical support.
S.X.: employee of Janssen Research & Development; owns stock in Johnson & Johnson.
P.J.M.: research support, consulting fees and/or speaker bureau support from AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, SUN Pharma and UCB.
Contributor Information
Laura C Coates, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK.
Christopher T Ritchlin, Department of Medicine, Allergy/Immunology and Rheumatology, University of Rochester Medical Center, Rochester, NY, USA.
Laure Gossec, Department of Rheumatology, Institut Pierre Louis d'Epidémiologie et de Santé Publique, INSERM, Sorbonne Université, Paris; Rheumatology Department, Pitié-Salpêtrière Hospital, AP-HP, Paris, France.
Philip S Helliwell, Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, Leeds, UK.
Proton Rahman, Discipline of Medicine, Division of Rheumatology, Craig L Dobbin Genetics Research Centre, Memorial University of Newfoundland, St Johns, NL, Canada.
Alexa P Kollmeier, Department of Immunology, Janssen Research & Development, LLC, San Diego, CA.
Xie L Xu, Department of Immunology, Janssen Research & Development, LLC, San Diego, CA.
May Shawi, Immunology, Rheumatology Global Medical Affairs, Janssen Pharmaceutical Companies of Johnson & Johnson, Horsham.
Chetan S Karyekar, Department of Immunology, Janssen Research & Development, LLC, Spring House, PA, USA.
Christine Contré, Janssen Cilag, Issy-les-Moulineaux, Île-de-France, France.
Wim Noël, Department of Immunology, Janssen Scientific Affairs, LLC, Brussels, Belgium.
Shihong Sheng, Department of Statistics and Decision Sciences, Immunology, Janssen Research & Development, LLC, Spring House, PA.
Yanli Wang, Department of Statistics and Decision Sciences, Immunology, Janssen Research & Development, LLC, Spring House, PA.
Stephen Xu, Department of Statistics and Decision Sciences, Immunology, Janssen Research & Development, LLC, Spring House, PA.
Philip J Mease, Department of Rheumatology Research, Swedish Medical Center/Providence St. Joseph Health; University of Washington, Rheumatology Research, Seattle, WA, USA.
Data availability statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA).
Project site at http://yoda.yale.edu.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Ritchlin CT, Colbert RA, Gladman DD.. Psoriatic arthritis. N Engl J Med 2017;376:957–70. [DOI] [PubMed] [Google Scholar]
- 2. Coates LC, Kavanaugh A, Mease PJ. et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol 2016;68:1060–71. [DOI] [PubMed] [Google Scholar]
- 3. Coates LC, Gottlieb AB, Merola JF. et al. Comparison of different remission and low disease definitions in psoriatic arthritis and evaluation of their prognostic value. J Rheumatol 2019;46:160–5. [DOI] [PubMed] [Google Scholar]
- 4. Smolen JS, Schöls M, Braun J. et al. Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann Rheum Dis 2018;77:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singh JA, Guyatt G, Ogdie A. et al. Special Article: 2018 American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol 2019;71:5–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lubrano E, Scriffignano S, Perrotta FM.. Residual disease activity and associated factors in psoriatic arthritis. J Rheumatol 2020;47:1490–5. [DOI] [PubMed] [Google Scholar]
- 7. Mease PJ, Coates LC.. Considerations for the definition of remission criteria in psoriatic arthritis. Semin Arthritis Rheum 2018;47:786–96. [DOI] [PubMed] [Google Scholar]
- 8. Schoels M, Aletaha D, Funovits J. et al. Application of the DAREA/DAPSA score for assessment of disease activity in psoriatic arthritis. Ann Rheum Dis 2010;69:1441–7. [DOI] [PubMed] [Google Scholar]
- 9. Helliwell PS, FitzGerald O, Fransen J. et al. The development of candidate composite disease activity and responder indices for psoriatic arthritis (GRACE project). Ann Rheum Dis 2013;72:986–91. [DOI] [PubMed] [Google Scholar]
- 10. Helliwell PS, FitzGerald O, Fransen J.. Composite disease activity and responder indices for psoriatic arthritis: a report from the GRAPPA 2013 meeting on development of cutoffs for both disease activity states and response. J Rheumatol 2014;41:1212–7. [DOI] [PubMed] [Google Scholar]
- 11. Coates LC, Fransen J, Helliwell PS.. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis 2010;69:48–53. [DOI] [PubMed] [Google Scholar]
- 12. Coates LC, Helliwell PS.. Defining low disease activity states in psoriatic arthritis using novel composite disease instruments. J Rheumatol 2016;43:371–5. [DOI] [PubMed] [Google Scholar]
- 13. Orbai AM. Content validity of psoriatic arthritis composite indices: anchoring with the patient perspective and the core domain set. Rheumatology (Oxford) 2020;59:1–4. [DOI] [PubMed] [Google Scholar]
- 14. Coates LC, Strand V, Wilson H. et al. Measurement properties of the minimal disease activity criteria for psoriatic arthritis. RMD Open 2019;5:e001002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gossec L, McGonagle D, Korotaeva T. et al. Minimal disease activity as a treatment target in psoriatic arthritis: a review of the literature. J Rheumatol 2018;45:6–13. [DOI] [PubMed] [Google Scholar]
- 16. Gezer HH, Duruöz MT, Nas K. et al. Inconsistencies of the disease activity assessment tools for psoriatic arthritis: challenges to rheumatologists. Joint Bone Spine 2022;89:105296. [DOI] [PubMed] [Google Scholar]
- 17. TREMFYA ®[package insert] Horsham, PA: Janssen Biotech, Inc., 2020.
- 18. Deodhar A, Helliwell PS, Boehncke WH. et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet 2020;395:1115–25. [DOI] [PubMed] [Google Scholar]
- 19. Mease PJ, Rahman P, Gottlieb AB. et al. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet 2020;395:1126–36. [DOI] [PubMed] [Google Scholar]
- 20. Ritchlin CT, Helliwell PS, Boehncke WH. et al. Guselkumab, an inhibitor of the IL-23p19 subunit, provides sustained improvement in signs and symptoms of active psoriatic arthritis: 1 year results of a phase III randomised study of patients who were biologic-naïve or TNFα inhibitor-experienced. RMD Open 2021;7:e001457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McInnes IB, Rahman P, Gottlieb AB. et al. Efficacy and safety of guselkumab, an interleukin-23p19-specific monoclonal antibody, through one year in biologic-naive patients with psoriatic arthritis. Arthritis Rheumatol 2021;73:604–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taylor W, Gladman D, Helliwell P. et al. ; CASPAR Study Group. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73. [DOI] [PubMed] [Google Scholar]
- 23. Schoels MM, Aletaha D, Alasti F, Smolen JS.. Disease activity in psoriatic arthritis (PsA): defining remission and treatment success using the DAPSA score. Ann Rheum Dis 2016;75:811–8. [DOI] [PubMed] [Google Scholar]
- 24. Healy PJ, Helliwell PS.. Measuring clinical enthesitis in psoriatic arthritis: assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheum 2008;59:686–91. [DOI] [PubMed] [Google Scholar]
- 25. van der Heijde D, Sharp J, Wassenberg S, Gladman DD.. Psoriatic arthritis imaging: a review of scoring methods. Ann Rheum Dis 2005;64(Suppl 2):ii61–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marin J, Acosta Felquer ML, Ferreyra Garrot L. et al. Patients with psoriatic arthritis fulfilling the minimal disease activity criteria do not have swollen and tender joints, but have active skin. J Rheumatol 2016;43:907–10. [DOI] [PubMed] [Google Scholar]
- 27. Lubrano E, Scriffignano S, Perrotta FM.. The "climb" towards minimal disease activity in psoriatic arthritis. Rheumatol Ther 2021;8:1443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blauvelt A, Papp KA, Griffiths CEM. et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol 2017;76:405–17. [DOI] [PubMed] [Google Scholar]
- 29. Reich K, Armstrong AW, Foley P. et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol 2017;76:418–31. [DOI] [PubMed] [Google Scholar]
- 30. Reich K, Armstrong AW, Langley RG. et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet 2019;394:831–9. [DOI] [PubMed] [Google Scholar]
- 31. Langley RG, Tsai TF, Flavin S. et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol 2018;178:114–23. [DOI] [PubMed] [Google Scholar]
- 32. Mease PJ, McInnes IB, Tam LS. et al. Comparative effectiveness of guselkumab in psoriatic arthritis: results from systematic literature review and network meta-analysis. Rheumatology (Oxford) 2021;60:2109–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Armstrong AW, Puig L, Joshi A. et al. Comparison of biologics and oral treatments for plaque psoriasis: a meta-analysis. JAMA Dermatol 2020;156:258–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Højgaard P, Ellegaard K, Nielsen SM. et al. Pain mechanisms and ultrasonic inflammatory activity as prognostic factors in patients with psoriatic arthritis: a prospective cohort study. Arthritis Care Res (Hoboken) 2019;71:798–810. [DOI] [PubMed] [Google Scholar]
- 35. Rifbjerg-Madsen S, Christensen AW, Christensen R. et al. Pain and pain mechanisms in patients with inflammatory arthritis: a Danish nationwide cross-sectional DANBIO registry survey. PLoS One 2017;12:e0180014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lampa J. Pain without inflammation in rheumatic diseases. Best Pract Res Clin Rheumatol 2019;33:101439. [DOI] [PubMed] [Google Scholar]
- 37. Coates LC, Nash P, Kvien TK. et al. Comparison of remission and low disease activity states with DAPSA, MDA and VLDA in a clinical trial setting in psoriatic arthritis patients: 2-year results from the FUTURE 2 study. Semin Arthritis Rheum 2020;50:709–18. [DOI] [PubMed] [Google Scholar]
- 38. Dures E, Shepperd S, Mukherjee S. et al. Treat-to-target in PsA: methods and necessity. RMD Open 2020;6:e001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gonçalves RSG, de Almeida Martins LM, de Ataide Mariz H, Tavares Dantas A, Pinto Duarte ALB.. DAPSA versus cDAPSA: do we need to use CRP? Ann Rheum Dis 2020;79:e142. [DOI] [PubMed] [Google Scholar]
- 40. van Mens LJJ, van de Sande MGH, van Kuijk AWR, Baeten D, Coates LC.. Ideal target for psoriatic arthritis? Comparison of remission and low disease activity states in a real-life cohort. Ann Rheum Dis 2018;77:251–7. [DOI] [PubMed] [Google Scholar]
- 41. Pantano I, Mauro D, Romano F. et al. Real-life efficacy of guselkumab in patients with early psoriatic arthritis. Rheumatology (Oxford) 2022;61:1217–21. [DOI] [PubMed] [Google Scholar]
- 42. Wervers K, Vis M, Tchetveriko I. et al. Burden of psoriatic arthritis according to different definitions of disease activity: comparing minimal disease activity and the disease activity index for psoriatic arthritis. Arthritis Care Res (Hoboken) 2018;70:1764–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Helliwell P, Coates LC, FitzGerald O. et al. Disease-specific composite measures for psoriatic arthritis are highly responsive to a Janus kinase inhibitor treatment that targets multiple domains of disease. Arthritis Res Ther 2018;20:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mease P, Husni ME, Kafka S. et al. Inhibition of radiographic progression across levels of composite index-defined disease activity in patients with active psoriatic arthritis treated with intravenous golimumab: results from a phase-3, double-blind, placebo-controlled trial. Arthritis Res Ther 2020;22:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Perrotta FM, Delle Sedie A, Scriffignano S. et al. Remission, low disease activity and improvement of pain and function in psoriatic arthritis patients treated with IL-12/23 and IL-17 inhibitors. A multicenter prospective study. Reumatismo 2020;72:52–9. [DOI] [PubMed] [Google Scholar]
- 46. McInnes IB, Kato K, Magrey M. et al. Upadacitinib in patients with psoriatic arthritis and an inadequate response to non-biological therapy: 56-week data from the phase 3 SELECT-PsA 1 study. RMD Open 2021;7:e001838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mease PJ, Lertratanakul A, Anderson JK. et al. Upadacitinib for psoriatic arthritis refractory to biologics: SELECT-PsA 2. Ann Rheum Dis 2020;80:312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA).
Project site at http://yoda.yale.edu.