Abstract
Objective
IL-17A and TNF act in synergy to induce proinflammatory mediators in synovial fibroblasts thus contributing to diseases associated with chronic arthritis. Many of these factors are regulated by transcription factor E74-like factor-3 (ELF3). Therefore, we sought to investigate ELF3 as a downstream target of IL-17A and TNF signalling and to characterize its role in the molecular mechanism of synergy between IL-17A and TNF.
Methods
Regulation of ELF3 expression by IL-17A and TNF was studied in synovial fibroblasts of RA and OA patients and RA synovial explants. Signalling leading to ELF3 mRNA induction and the impact of ELF3 on the response to IL-17A and TNF were studied using siRNA, transient overexpression and signalling inhibitors in synovial fibroblasts and HEK293 cells.
Results
ELF3 was marginally affected by IL-17A or TNF alone, but their combination resulted in high and sustained expression. ELF3 expression was regulated by the nuclear factor-κB (NF-κB) pathway and CCAAT/enhancer-binding protein β (C/EBPβ), but its induction required synthesis of the NF-κB co-factor IκB (inhibitor of NF-κB) ζ. siRNA-mediated depletion of ELF3 attenuated the induction of cytokines and matrix metalloproteinases by the combination of IL-17A and TNF. Overexpression of ELF3 or IκBζ showed synergistic effect with TNF in upregulating expression of chemokine (C-C motif) ligand 8 (CCL8), and depletion of ELF3 abrogated CCL8 mRNA induction by the combination of IκBζ overexpression and TNF.
Conclusion
Altogether, our results establish ELF3 as an important mediator of the synergistic effect of IL-17A and TNF in synovial fibroblasts. The findings provide novel information of the pathogenic mechanisms of IL-17A in chronic arthritis and implicate ELF3 as a potential therapeutic target.
Keywords: fibroblasts, synovial, inflammation, IL-17A, TNF, ELF3
Graphical Abstract
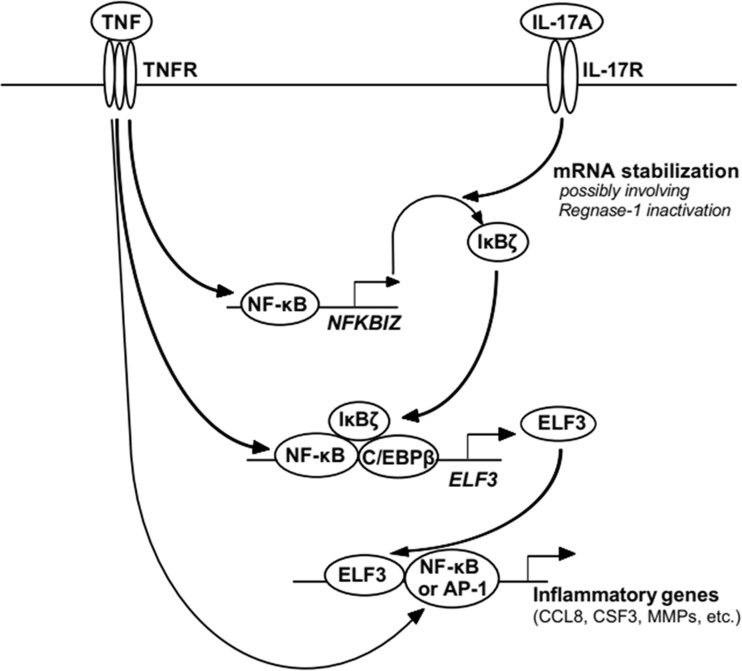
Overview of the signalling leading to induction of ELF3 and subsequent upregulation of expression of proinflammatory factors. First, TNF stimulation leads to NF-κB-mediated transcriptional activation of NFKBIZ. Robust expression of IκBζ protein requires additionally stabilization of NFKBIZ mRNA, which occurs following binding of IL-17A to its cell surface receptor. Secondly, IκBζ protein associates with DNA-bound NF-κB and C/EBPβ transcription factors to induce expression of secondary response genes, including ELF3. Third, ELF3 induces the expression of a variety of proinflammatory factors by functioning in collaboration with NF-κB and AP-1. The model is simplified to show only main signalling events. AP-1: activator protein-1; C/EBPβ: CCAAT/enhancer-binding protein-β; ELF3: E74-like factor-3; IκBζ: NF-κB inhibitor-ζ; NF-κB: nuclear factor-κB; NFKBIZ: gene encoding IκBζ.
Rheumatology key messages.
Transcription factor ELF3 mediates the synergistic proinflammatory effects of TNF and IL-17A in synovial fibroblasts.
ELF3 is regulated by the NF-κB pathway, but the induction requires synthesis of the NF-κB co-factor, IκBζ.
Introduction
Synovial fibroblasts actively contribute to inflammation and tissue destruction in RA by producing large variety of cytokines and extracellular matrix degrading enzymes [1]. Increasing attention has also been given to synovitis in OA, wherein synovial fibroblasts may play a prominent role [2]. Although the aggressive phenotype of synovial fibroblasts in inflamed joints is partly imprinted and maintained in the absence of external stimulation, it is strongly promoted by the cytokine milieu [1]. Of the proinflammatory cytokines, Tumor necrosis factor α (TNF) has a central role in the pathogenesis of RA and several other inflammatory diseases, such as AS and PsA [3], as well as to a lesser degree in OA [2]. Synovial fibroblasts are one of the target cells of TNF, and in TNF-driven disease models of arthritis they are sufficient for driving the full inflammatory and destructive disease process [4].
IL-17A is another cytokine with a clear role in murine models of inflammatory arthritides [5] and OA [6]. Proinflammatory effects of IL-17A have also been shown in synovial fibroblast isolated from RA [7–9] and OA [10] patients. Increased production of IL-17A has been described in synovium, synovial fluid and serum of RA, OA, AS and PsA patients [11–14]. However, compared with other inflammatory cytokines the effects of IL-17A alone on target cells are modest [15]. One explanation for the critical contribution of IL-17A in vivo entails the capability of IL-17A to significantly augment TNF-induced proinflammatory responses in target cells, including synovial fibroblasts and chondrocytes [15–17].
E74-like factor-3 (ELF3) is a member of the E26 transformation-specific (ETS) family of transcription factors. Under physiological conditions ELF3 shows mostly an epithelially restricted expression pattern [18], but inflammatory stimuli, including IL-1β, TNF and lipopolysaccharide, can induce its expression in various cell types [19, 20]. ELF3 is highly expressed in RA synovial membrane, wherein it mainly localizes into the intimal lining [20]. ELF3 contributes to the inflammatory response by regulating the expression of genes including NOS2 (nitric-oxide synthase 2) and PTGS2 (cyclooxygenase 2) [19, 20]. By mediating IL-1β-induced MMP13 expression in chondrocytes through regulation of activator protein-1 (AP-1) activity, ELF3 is directly implicated in tissue destruction in chronic arthritis [21].
As the ELF3-regulated genes NOS2, PTGS2 and MMP13 are all known targets for synergistic regulation by TNF and IL-17A, we hypothesized that ELF3 might be directly involved in the molecular mechanism mediating this synergy. Thus, our aim was to investigate the mechanisms that regulate ELF3 expression in response to IL-17A and/or TNF, and to characterize the downstream inflammatory response promoted by ELF3 in synovial fibroblasts.
Methods
Subjects
The study was approved by the ethical committee of the Helsinki University Central Hospital. Guidelines of the Declaration of Helsinki were followed. Written informed consent was received from patients participating in the study. RA patients fulfilled the 2010 ACR–EULAR classification criteria of RA [22].
Cell cultures
Primary fibroblast cultures from RA and OA tissues were established as previously described [23]. Cells were used in passages 4–6. Cells were cultured in RPMI-1640 (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS) and GlutaMAX (Thermo Fisher Scientific). For stimulations, cells were seeded at 8 × 104 cells/ml 2 days prior to experiments. Medium was changed to RPMI-1640 containing 1% FBS 16 h prior to the experiment. Considering the comparable induction of ELF3, as well as NF-κB inhibitor-ζ (IκBζ), by the combination of IL-17A and TNF in both RA and OA synovial fibroblast in the preliminary experiments, and the smaller interindividual variation in the response to TNF in the latter cell type, we chose to primarily use OA synovial fibroblasts for studying the molecular mechanisms of induction of ELF3. HEK293 cells were cultured in DMEM (Thermo Fisher Scientific) containing 10% FBS. IL-17A (50 ng/ml), TNF (10 ng/ml) and IL-1β (10 ng/ml; all from R&D Systems, Minneapolis, MN, USA) were used for stimulations and cycloheximide (5 μg/ml), actinomycin D (5 μg/ml), IMD-0354 (2 μM), SP600125 (10 μM), U0126 (10 μM), or SB202190 (10 μM) were used for inhibiting transcription, translation or signalling by nuclear factor-κB (NF-κB), c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK) and p38 (all from Sigma-Aldrich, St Louis, USA).
Synovial tissue culture
Synovial explants were cut into 1–2 mm3 pieces and randomly distributed into treatment groups. Tissue pieces were cultured in DMEM with or without 50 ng/ml IL-17A and/or 10 ng/ml TNF (R&D Systems).
RNA isolation, cDNA synthesis and qRT-PCR
RNeasy Mini Kit (Qiagen, Hilden, Germany) was used for RNA isolation. Prior to RNA extraction, tissues were homogenized with FastPrep FP120 homogenizer in lysing matrix D tubes (MP Biomedicals, Irvine, CA, USA), and treated with proteinase K for 10 min at 55°C. cDNA synthesis and qRT-PCR were performed as previously described [24] with gene specific primers (Supplementary Table S1, available at Rheumatology online). PBGD was used as reference gene in fibroblast experiments and RPLP0 in HEK293 cells. Relative gene expression of a sample was calculated as 2−ΔCt.
Immunofluorescence
After fixation in 4% paraformaldehyde, permeabilization with 0.1% Triton X‐100 and blocking in 1% BSA/PBS, cells were stained with 1 μg/ml rabbit anti-human ELF3 (Abcam, Cambridge, UK), 1 μg/ml anti-human IκBζ (Atlas Antibodies, Stockholm, Sweden) or 1 μg/ml non-immune rabbit IgG (Thermo Fisher Scientific) overnight at 4°C. Alexa Fluor 568-conjugated secondary antibody (Thermo Fisher Scientific) was used for immunodetection and 4′,6-diamidino-2-phenylindole for counterstaining.
Lactate dehydrogenase release assay
Lactate dehydrogenase activity in cell culture medium was assessed by Cytotoxicity Detection Kit (Roche, Basel, Switzerland) according to the manufacturer’s recommendations.
Cloning and plasmids
ELF3 and IκBζ expression construct and ELF3 reporter construct cloning are described in Supplementary Data S1, available at Rheumatology online.
Transfection and nucleofection
HEK293 cells were transfected using Fugene HD as previously described [24]. Synovial fibroblasts were nucleofected using Amaxa Nucleofector II and Human Dermal Fibroblast Nucleofector Kit (Lonza, Basel, Switzerland), using 4 × 105 cells, 3 μg DNA and transfection program U023. Expression of target protein and variability in transfection were evaluated by immunoblotting and qRT-PCR, respectively. Synovial fibroblasts showed lower transfection efficiency (35%) compared with HEK293 (85%), as evaluated by transfection of pmaxGFP plasmid (Lonza) and microscopy, and higher variability in transfection efficiency (Supplementary Fig. S1, available at Rheumatology online). For siRNA, cells were seeded on 24-well plates at a density of 4 × 104 cells per well 24 h prior to transfection. Controls and gene-specific siRNAs (Supplementary Table S2, available at Rheumatology online) were transfected using RNAiMAX reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions. The efficiency of gene silencing was assessed by qRT-PCR (Supplementary Fig. S2, available at Rheumatology online).
Luciferase reporter assay
HEK293 cells were transfected as described using 500 ng IκBζ expression plasmid or empty plasmid, 10 ng reporter plasmid with the 1.0 kb part of ELF3 promoter and 1 ng Renilla luciferase plasmid. A luciferase assay was performed using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) according to the manufacturer’s instructions.
Western blotting
Cells were lysed in Cell Lysis Buffer (Cell Signaling Technology, Danvers, MA, USA), supplemented with protease inhibitor cocktail (Roche). Immunoblotting was performed as previously described [25] with overnight incubation at +4°C using antibodies against human ELF3 (1:250, Atlas Antibodies) or IκBζ (1:500, Origene, Rockville, MD, USA) and detection with horseradish peroxidase-conjugated anti-rabbit and anti-mouse antibodies.
Multiplex immunoassay
IL-8, chemokine (C-X-C motif) ligand-5 (CXCL5; ENA-78), chemokine (C-C motif) ligand 8 (CCL8; MCP-2), colony-stimulating factor-3 (CSF3; G-CSF), MMP3 and MMP12 concentrations were determined with ProcartaPlex® Multiplex Immunoassay (Thermo Fisher Scientific) using Bio-Plex 200 (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer’s instructions.
Statistical analysis
Statistical significance was analysed with blocked one-way ANOVA and Tukey’s post hoc test, except for siRNA experiments for which the Holm–Šidák post hoc test was used. Analysis was done with IBM SPSS Statistics 25 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 9 (GraphPad Software Inc., San Diego, CA, USA).
Results
Co-stimulation with IL-17A and TNF induces ELF3 expression
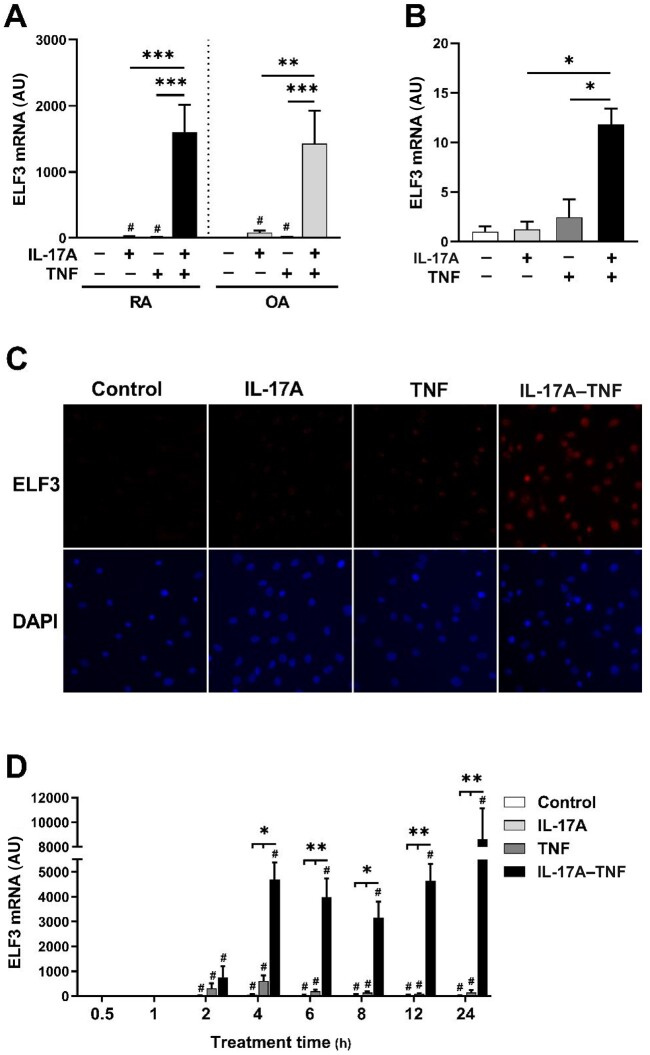
In synovial fibroblasts IL-1β but not TNF induces ELF3 expression [20]. To study whether IL-17A alone or in combination with TNF (IL-17A–TNF) induces ELF3 expression, we stimulated RA and OA synovial fibroblasts for 24 h with IL-17A and/or TNF (Fig. 1A). IL-17A or TNF alone had only a modest effect on ELF3 mRNA expression. However, stimulation with the combination of IL-17A and TNF resulted in strong upregulation of ELF3 mRNA in fibroblasts derived from patients with RA or OA. Also, in accordance with Grall et al. [20], IL-1β stimulation strongly induced ELF3 mRNA expression (Supplementary Fig. S3, available at Rheumatology online). Validating the results at the level of tissue, we analysed the response of RA synovial explants to simulation with IL-17A and/or TNF. The stimulation with IL-17A–TNF resulted in strong upregulation of ELF3 mRNA in contrast to IL-17A or TNF alone (Fig. 1B). ELF3 protein level of synovial fibroblasts examined by immunofluorescence staining showed little to no change by IL-17A or TNF alone, but the combination of IL-17A and TNF resulted in a substantial increase in the amount of ELF3 (Fig. 1C).
Fig. 1.
ELF3 expression is induced in synovial fibroblasts and synovial tissue by co-stimulation with IL-17A and TNF
Human primary synovial fibroblasts from RA and OA patients or synovial tissue explants of RA patients were stimulated with IL-17A (50 ng/ml) and/or TNF (10 ng/ml) or left untreated. (A) ELF3 mRNA expression in RA and OA synovial fibroblasts stimulated for 24 h. (B) ELF3 mRNA expression in RA synovial tissue explants stimulated for 16 h. (C) ELF3 immunofluorescence in OA synovial fibroblasts stimulated for 24 h. (D) Time course of ELF3 mRNA expression in OA synovial fibroblasts. Data represent means ± SEM of three (A, D) or four (B) individual experiments carried out with cells and synovial explants from different donors. *P < 0.05, **P < 0.01, and ***P < 0.001 for indicated comparisons. #P < 0.05 compared with control. In time series experiment groups were compared separately at each time point. DAPI: 4′,6-diamidino-2-phenylindole; ELF3: E74-like factor-3.
To study the kinetics of ELF3 expression, we stimulated synovial fibroblasts with IL-17A and/or TNF for different times (Fig. 1D). ELF3 mRNA was induced relatively rapidly, leading to several hundredfold induction already at 2 h, both in TNF- and in IL-17A–TNF-stimulated samples. However, TNF-induced expression was always less compared with IL-17A–TNF treatment. After peaking at 4 h, ELF3 expression rapidly declined in TNF treated samples, whereas in IL17A–TNF-treated cells ELF3 mRNA remained at high level.
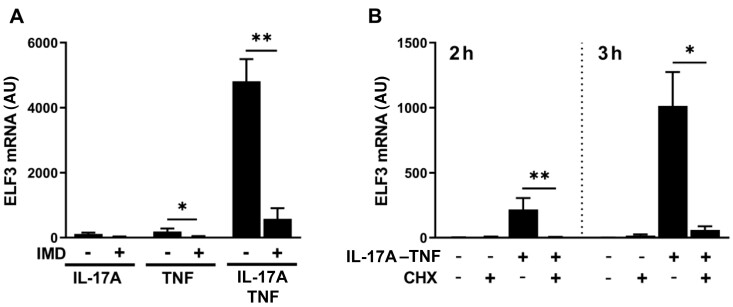
ELF3 mRNA upregulation in response to IL-17A and TNF co-stimulation is mediated by NF-κB pathway and requires de novo protein synthesis
Mutations of the most proximal NF-κB binding site at the ELF3 promoter eradicate the activating effect of IL-1β on the promoter [20]. To find out whether the canonical NF-κB pathway is involved in the response to IL-17A–TNF, we studied induction of ELF3 mRNA in cells pre-treated with selective IkappaB kinase β (IKKβ) inhibitor (IMD-0354). The upregulation of ELF3 mRNA by IL-17A–TNF was abrogated by inhibition of the canonical NF-κB pathway (Fig. 2A). NFKBIA, encoding NF-κB inhibitor-α (IκBα), is a primary response gene and a target of canonical NF-κB signalling. IκBα mRNA expression showed strong induction by TNF that was not enhanced by the addition of IL-17A (Supplementary Fig. S4, available at Rheumatology online), indicating that overall NF-κB activity is comparable in cells treated with TNF and IL-17A–TNF. Furthermore, regulation of TNF receptor expression did not have a role in controlling ELF3 induction, as TNF receptor-2 mRNA upregulation by IL-17A–TNF showed delayed kinetics compared with ELF3 (Supplementary Fig. S4, available at Rheumatology online) and TNF receptor-1 mRNA level remained unchanged by all treatments (data not shown).
Fig. 2.
ELF3 mRNA upregulation by IL-17A and TNF co-stimulation is mediated by NF-κB pathway and requires protein synthesis
Human OA primary synovial fibroblasts were pretreated with inhibitor or vehicle for 1 h followed by stimulation with IL-17A (50 ng/ml) and/or TNF (10 ng/ml). (A) The effect of NF-κB inhibitor IMD-0354 (IMD; 2 μM) on ELF3 mRNA expression induced by IL-17A and/or TNF for 8 h. (B) The effect of cycloheximide (CHX; 5 μg/ml) on ELF3 mRNA expression induced by IL-17A–TNF for 2 or 3 h. Data represent means ± SEM of four (A) or three (B) individual experiments with cells from different donors. *P < 0.05, **P < 0.01 for indicated comparisons. ELF3: E74-like factor-3; NF-κB: nuclear factor-κB.
NF-κB directly induces primary response genes, but the induction of secondary response genes requires in addition new protein synthesis and chromatin remodelling. To determine whether ELF3 mRNA induction requires protein synthesis, we treated cells with cycloheximide prior to and during stimulation with IL-17A–TNF. ELF3 mRNA upregulation by IL-17A–TNF was completely abrogated by cycloheximide (Fig. 2B). Neither IMD-0354 nor cycloheximide affected cell viability (Supplementary Fig. S5, available at Rheumatology online).
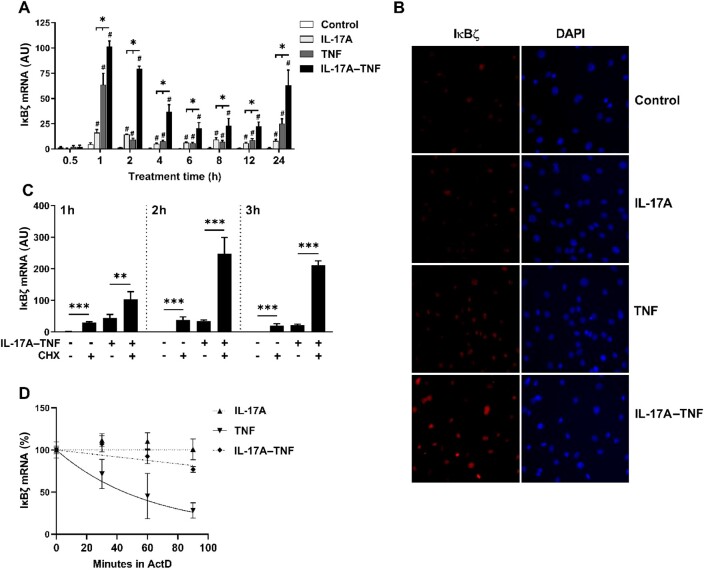
IκBζ is synergistically induced by co-stimulation with IL-17A and TNF and regulates ELF3 gene expression
Requirement for de novo protein synthesis for ELF3 mRNA upregulation indicates involvement of a transcriptional coactivator that is rapidly induced by IL-17A–TNF. The atypical IκB protein IκBζ, encoded by gene NFKBIZ, is a transcriptional coactivator of secondary response genes and is involved in the synergistic regulation by IL-17A and TNF in human primary keratinocytes and A549 cells [26–28]. Analysis of the kinetics of IκBζ mRNA expression in OA synovial fibroblasts showed a rapid induction by IL-17A or TNF alone, and a stronger synergistic induction in response to their combination (Fig. 3A). The peak of IκBζ mRNA expression occurred already at 1 h post-stimulation, thereby clearly preceding that of ELF3. After the initial peak, IκBζ mRNA declined rapidly in TNF stimulated cells but showed slower decay in cells treated with IL-17A or IL-17A–TNF. Immunofluorescence studies showed increased IκBζ nuclear staining in response to IL-17A–TNF at 3 h (Fig. 3B), well before the peak of ELF3 mRNA expression at 4 h. Analysis of synovial fibroblasts from RA patients indicated similar regulation of IκBζ expression by IL-17A and TNF (Supplementary Fig. S6, available at Rheumatology online).
Fig. 3.
IκBζ is synergistically induced by co-stimulation with IL-17A and TNF
(A) Time course of IκBζ mRNA expression in OA synovial fibroblasts stimulated with IL-17A (50 ng/ml) and/or TNF (10 ng/ml). (B) IκBζ immunofluorescence of OA synovial fibroblasts stimulated for 3 h with IL-17A (50 ng/ml) and/or TNF (10 ng/ml). (C) OA synovial fibroblasts were pretreated with cycloheximide (CHX; 5 μg/ml) or dimethyl sulfoxide (vehicle) for 1 h followed by stimulation with IL-17A (50 ng/ml) and TNF (10 ng/ml) for 1–3 h. IκBζ mRNA expression was analysed by qRT-PCR. (D) OA synovial fibroblasts were stimulated with IL-17A (50 ng/ml) and/or TNF (10 ng/ml) for 40 min, after which actinomycin D (ActD; 5 μg/ml) was added. IκBζ mRNA remaining at each time point after addition of actinomycin D was analysed by qRT-PCR. Data represent means ± SEM of three (A, C, D) individual experiments with cells from different donors. *P < 0.05, **P < 0.01, ***P < 0.001 for indicated comparisons. #P < 0.05 compared to control. DAPI: 4′,6-diamidino-2-phenylindole; IκBζ: NF-κB inhibitor-ζ.
In line with rapid induction typical for primary response genes, cycloheximide did not prevent IκBζ mRNA induction. Instead, IκBζ mRNA expression was increased by cycloheximide (Fig. 3C). In addition to transcriptional regulation by NF-κB, IκBζ expression requires post-transcriptional mRNA stabilization [29]. As TNF only activates the transcription, the resulting expression of IκBζ is minor compared with the effect of IL-1β or Toll-like receptor ligands [30, 31]. IL-17A induces the stabilization of IκBζ mRNA in primary murine hepatocytes and NIH3T3 cells [29, 32], and therefore we studied whether IκBζ mRNA stabilization plays a role in synovial fibroblasts. The cells were activated with IL-17A and/or TNF, followed by treatment with actinomycin D to prevent further transcription. Increased IκBζ transcript stability was observed in cells treated with IL-17A or IL-17A–TNF compared with TNF alone (Fig. 3D). Actinomycin D had a slight, statistically significant effect on viability, irrespective of stimulation (Supplementary Fig. S5, available at Rheumatology online).
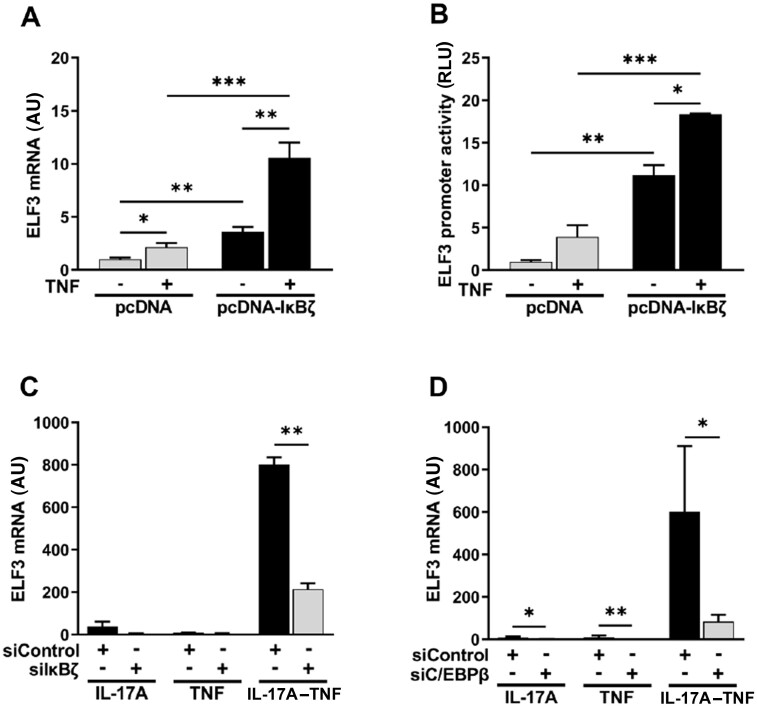
Overexpression was used to examine the involvement of IκBζ in the transcriptional regulation of ELF3. Due to low efficiency and high variability of synovial fibroblast transfections, overexpression experiments, including IκBζ, were mainly carried out in HEK293 cells. TNF stimulation was included in the experiment to provide additional signalling, as IκBζ is known to function by associating with DNA-bound NF-κB subunit p50 [28]. Overexpression of IκBζ alone was sufficient for induction of ELF3 mRNA, but the expression was highly increased upon addition of TNF stimulation (Fig. 4A). To further characterize the effects of IκBζ in regulation of ELF3 expression, we analysed whether IκBζ affected ELF3 promoter activation. IκBζ overexpression resulted in activation of ELF3 promoter, which was further increased by the presence of TNF (Fig. 4B).
Fig. 4.
IκBζ mediates regulation of ELF3 gene expression
(A) HEK293 cells were transfected with plasmid coding for IκBζ or with empty vector and after 48 h the cells were stimulated for 24 h with TNF (10 ng/ml) or left untreated. ELF3 mRNA expression was analysed by qRT-PCR. (B) HEK293 cells were transfected with plasmid coding for IκBζ or with empty vector in combination with ELF3 reporter plasmid. After 48 h the cells were stimulated for 24 h with TNF (10 ng/ml) or left untreated. ELF3 promoter activity was determined with dual-luciferase assay. (C and D) OA synovial fibroblasts were transfected with targeting siRNA or with negative control siRNA. After 48 h cells were stimulated for 24 h with IL-17A (50 ng/ml) and/or TNF (10 ng/ml). (C) The effect of IκBζ silencing on ELF3 mRNA expression. (D) The effect of C/EBPβ silencing on ELF3 mRNA expression. Data in (A, B) represent means ± SEM of five (A) and three (B) individual experiments. Data in (C, D) represent means ± SEM of four (C) and three (D) individual experiments with cells from different donors. *P < 0.05, **P < 0.01, ***P < 0.001. C/EBPβ: CCAAT/enhancer-binding protein β; ELF3: E74-like factor-3; IκBζ: NF-κB inhibitor-ζ; RLU: relative light units.
In order to confirm these observations, we silenced the expression of IκBζ with siRNA in synovial fibroblasts prior to stimulation with IL-17A–TNF. The induction of ELF3 mRNA by IL-17A–TNF was significantly attenuated by IκBζ silencing (Fig. 4C).
Activation of hBD-2 and NGAL promoters by transfection of IκBζ and NF-κB subunits requires C/EBP-binding sites in the promoters, and depletion of CCAAT/enhancer-binding protein β (C/EBPβ) suppresses the activation of NGAL promoter [33]. LASAGNA-Search [34] identified four potential C/EBPβ-binding sites in ELF3 proximal promoter, with one supported also by the chromatin immunoprecipitation data from the ENCODE project [35]. Analysis of C/EBPβ mRNA expression in synovial fibroblasts revealed induction by TNF and by IL-17A–TNF, with a similar magnitude (Supplementary Fig. S4, available at Rheumatology online). Silencing C/EBPβ in synovial fibroblasts resulted in lower induction of ELF3 mRNA in response to IL-17A–TNF compared with cells treated with control siRNA (Fig. 4D). However, C/EBPβ silencing also affected ELF3 mRNA expression in cells treated with IL-17A or TNF alone.
ELF3 is a regulator of several proinflammatory and catabolic mediators
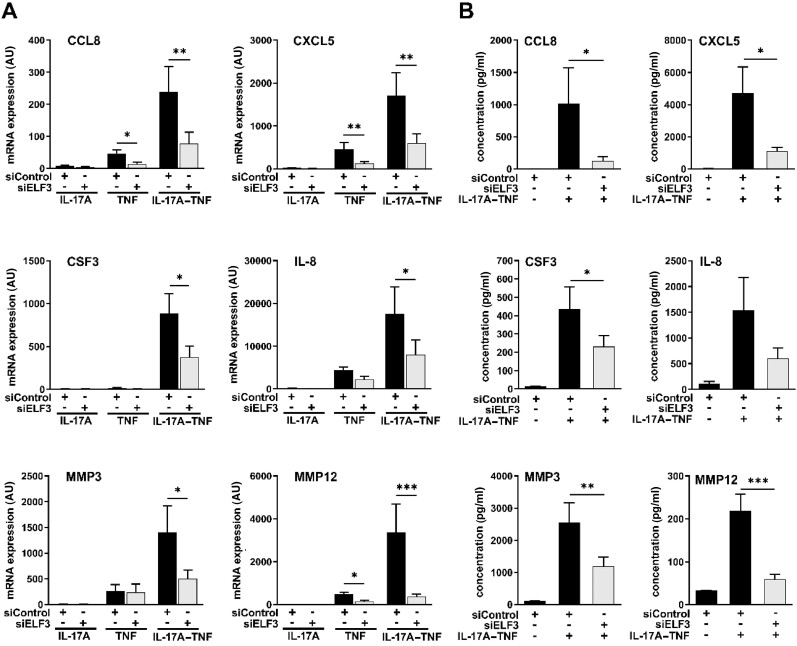
To examine the role of ELF3 in the regulation of proinflammatory mediators produced by synovial fibroblasts, siRNA was used to knock down ELF3 before stimulating cells with IL-17A and/or TNF. ELF3 siRNA significantly reduced mRNA expression of CCL8, CXCL5, CSF3, IL-1A, IL-8, IL-23A, MMP3, MMP10 and MMP12 induced by IL-17A–TNF in OA synovial fibroblasts (Fig. 5A and Supplementary Fig. S7, available at Rheumatology online). Despite showing strong synergistic induction of expression by IL-17A–TNF, IL-6 mRNA was unaffected by ELF3 silencing (Supplementary Fig. S7, available at Rheumatology online). The effects of ELF3 depletion were verified at the protein level by measuring proteins readily secreted by synovial fibroblasts, including CCL8, CXCL5, CSF3, IL-8, MMP3 and MMP12. Except for IL-8, depletion of ELF3 significantly reduced the release of all studied factors into the culture medium in response to IL-17A–TNF (Fig. 5B). Similar effects of ELF3 silencing were observed in synovial fibroblasts of RA patients in the regulation of CCL8, IL-23A and MMP12, representing chemokines, cytokines and MMPs (Supplementary Fig. S8, available at Rheumatology online).
Fig. 5.
ELF3 silencing reduces the induction of inflammatory factors by the combination of IL-17A and TNF
Human primary synovial fibroblasts from OA patients were transfected with ELF3 siRNA or with negative control siRNA. After 48 h the cells were stimulated for 24 h with IL-17A (50 ng/ml) and/or TNF (10 ng/ml). (A) mRNA expression of the indicated cytokines and MMPs analysed by qRT-PCR. (B) Protein concentration of the indicated cytokines and MMPs in the cell supernatant analysed by Bio-Plex multiplex immunoassay. Data represent means ± SEM of six individual experiments with cells from different donors. *P < 0.05, **P < 0.01, ***P < 0.001. CCL8: chemokine (C-C motif) ligand 8; CSF3: colony-stimulating factor-3; CXCL5: chemokine (C-X-C motif) ligand-5; ELF3: E74-like factor-3.
ELF3 induction downstream of IκBζ regulates CCL8 expression
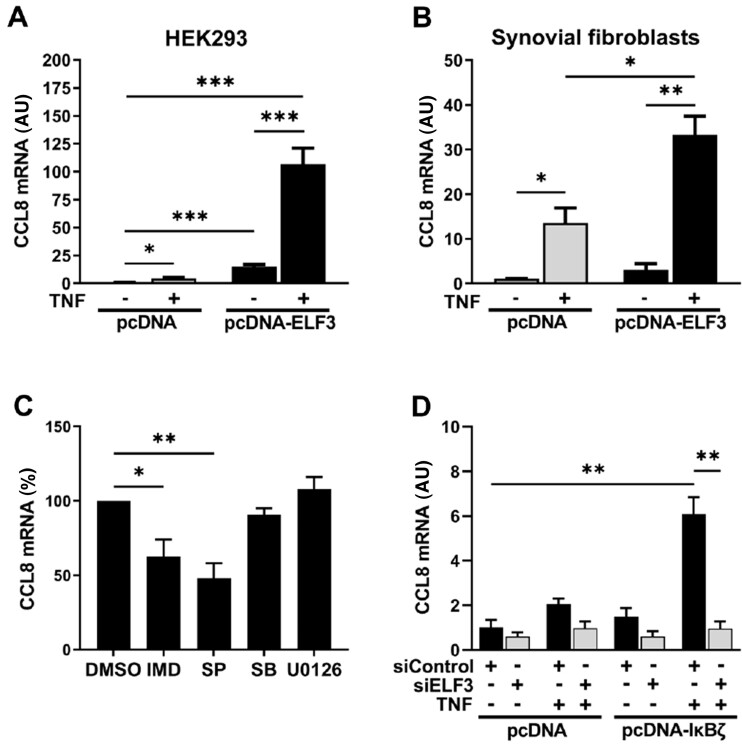
ELF3 acts in conjunction with other transcription factors including NF-κB and AP-1, and moreover extracellular-signal-regulated kinase (ERK) pathway modulates ELF3 activity [20, 21, 36]. Therefore, we wanted to study the effect of forced expression of ELF3 on gene expression in the presence or absence of external stimuli. HEK293 cells, transiently overexpressing ELF3, were stimulated with TNF or left unstimulated. Due to limited potential of active transcription in HEK293 cells, regulation of a relatively small subset of genes can be studied. CCL8 was selected as a suitable target based on pilot experiments. CCL8 mRNA expression was only modestly induced by TNF alone, whereas ELF3 overexpression alone showed a more significant effect (Fig. 6A). Most importantly, the combination of ELF3 overexpression and TNF stimulation resulted in a clear synergistic effect and high expression of CCL8 mRNA. The result was validated in human synovial fibroblasts. In these cells, TNF alone significantly induced CCL8 mRNA (Fig. 6B). However, as in HEK293 cells, overexpression of ELF3 together with TNF stimulation resulted in marked synergistic increase of CCL8 mRNA expression.
Fig. 6.
ELF3 induction downstream of IκBζ regulates CCL8 mRNA expression
(A and B) HEK293 cells and OA synovial fibroblasts were transfected with plasmid coding for ELF3 or with empty vector. After 48 h cells were stimulated for 24 h with TNF (10 ng/ml) or left untreated. (A) CCL8 mRNA expression in HEK293 cells and (B) CCL8 mRNA expression in human OA primary synovial fibroblasts. (C) HEK293 cells were transfected with plasmid coding for ELF3. After 48 h dimethyl sulfoxide (DMSO), IMD-0354 (IMD; 2 μM), SP600125 (SP; 10 μM), SB202190 (SB; 10 μM) or U0126 (10 μM) was added for 1 h in the cell culture medium, followed by stimulation with TNF (10 ng/ml) for 8 h. CCL8 mRNA expression was analysed by qRT-PCR. (D) HEK293 cells were transfected with plasmid coding for IκBζ or with empty vector for 24 h, after which siRNA targeting ELF3 or negative control siRNA was introduced to the cells. After 24 h the cells were stimulated with TNF (10 ng/ml) or left untreated. CCL8 mRNA at 24 h after stimulation was analysed by qRT-PCR. Data represent means ± SEM of four (A, C) or three (B, D) individual experiments. *P < 0.05, **P < 0.01, ***P < 0.001. CCL8: chemokine (C-C motif) ligand 8; ELF3: E74-like factor-3; IκBζ: NF-κB inhibitor-ζ.
Although ELF3 overexpression alone was sufficient for CCL8 mRNA induction, its effect was clearly potentiated by signalling pathways activated by TNF. To reveal which signalling pathways are involved, HEK293 cells overexpressing ELF3 were stimulated with TNF in the presence of inhibitors of NF-κB and mitogen-activated protein kinases JNK, p38 and ERK. The expression of CCL8 mRNA in cells overexpressing ELF3 was significantly inhibited by JNK or NF-κB inhibitors but not by inhibitors of p38 or ERK (Fig. 6C).
IκBζ is involved in the induction of subset of inflammatory genes [28]. However, based on our results these effects could be either direct or mediated by the induction of ELF3. Like ELF3 silencing, also IκBζ siRNA reduced the mRNA expression of CCL8, CSF3 and MMP12 in synovial fibroblasts. However, in contrast to ELF3 silencing, IκBζ depletion also reduced the expression of IL-6 mRNA (Supplementary Fig. S9, available at Rheumatology online). To provide direct evidence for the contribution of ELF3 in IκBζ controlled gene expression, we measured CCL8 mRNA in HEK293 cells transfected with IκBζ expression vector and ELF3 siRNA. The combination of IκBζ overexpression and TNF resulted in increased expression of CCL8 mRNA, and this induction was dependent on IκBζ mediated upregulation of ELF3 expression as revealed by almost complete abrogation of CCL8 mRNA expression in cells treated with ELF3 siRNA (Fig. 6D).
Discussion
The synergy between IL-17A and TNF contributes to inflammation and tissue destructive processes in chronic arthritis. In the present work we addressed the role of ELF3 in the synergy between IL-17A and TNF through characterizing the effects of IL-17A and TNF on the regulation of ELF3 expression and studying how the expression of proinflammatory mediators is influenced by ELF3.
In contrast to high induction of ELF3 in synovial fibroblasts by IL-1β, the effects of TNF and IL-17A were minor. However, combining IL-17A with TNF resulted in high and sustained expression. The signalling leading to ELF3 induction appears not to be disease-specific, as ELF3 was equally regulated in synovial fibroblasts of RA and OA patients. The similarities in ELF3 regulation by IL-1β and IL-17A–TNF are intriguing, as these treatments can induce a highly similar transcriptional program [32]. ELF3-mediated transcriptional regulation might at least partly explain these observed similarities.
In accordance with previous studies [20, 36], we found that the NF-κB pathway was central for ELF3 induction. However, the upregulation of ELF3 mRNA by IL-17A–TNF in synovial fibroblasts was sensitive to cycloheximide, thus indicating its dependency on de novo protein synthesis. IκBζ is an important coactivator of secondary response genes, and is involved in the synergistic regulation of several genes by IL-17A and TNF [26, 27, 37]. We observed that not only was the expression of IκBζ mRNA induced more strongly by IL-17A–TNF as compared with either cytokine alone, but also IκBζ mRNA half-life was increased in cells treated with IL-17A–TNF compared with TNF alone. Degradation and translational silencing of IκBζ mRNA has been shown to be mediated by Regnase-1 (also known as MCPIP1) [38–40]. The mRNA destabilization ability of Regnase-1 is subject to complex and stimulus specific regulation. In response to IL-1β, IL-36α or Toll-like receptor stimulation, Regnase-1 is rapidly phosphorylated by IL-1 receptor-associated kinase-1 (IRAK1) leading to its degradation [41–43]. In contrast, IL-17A stimulation only weakly causes Regnase-1 degradation, and the main mechanism for Regnase-1 suppression instead involves phosphorylation of Regnase-1 by TANK-binding kinase 1 (TBK1) and inducible IκB kinase (IKKi), leading to its translocation from the endoplasmic reticulum to the cytoplasm [43]. Because Regnase-1-mediated mRNA decay requires active translation, it is inhibited by cycloheximide [39]. Consistent with this, we observed increased IκBζ mRNA expression in response to cycloheximide treatment. Although our results in synovial fibroblasts are thus compatible with IL-17A-induced suppression of Regnase-1 activity, leading to IκBζ mRNA stabilization, work providing direct mechanistic evidence was beyond the scope of this study.
Besides being required for IκBζ induction, NF-κB is also involved in transcriptional regulation mediated by IκBζ [44]. While overexpression of IκBζ resulted in increase of ELF3 promoter activation and gene expression in HEK293 cells, the presence of TNF enhanced these effects. The critical role of IκBζ in regulation of ELF3 expression was observed also in synovial fibroblasts, as depletion of IκBζ completely abrogated ELF3 mRNA induction by IL-17A–TNF. Our result with IκBζ overexpression is similar to the effect reported for IκBζ in IL-6 regulation and in line with regulation of hBD2, Lipocalin-2, G-CSF and C/EBPδ, although the regulation of these factors was critically dependent on TNF stimulation or overexpression of NF-κB p50 subunit [33, 44, 45]. Clearly the role of NF-κB for IκBζ-mediated transcriptional control is important, despite the small differences owing probably to variability of the promoter structures or the cell types used.
In addition to NF-κB, binding of C/EBPβ on the gene promoter is prerequisite for IκBζ-mediated transcriptional activation [33]. Accordingly, we observed that C/EBPβ plays an important role in regulation of ELF3 mRNA expression. However, TNF stimulation causes similar induction of C/EBPβ mRNA as does IL-17A–TNF, yet TNF alone does not significantly affect ELF3 expression. In addition, C/EBPβ-depletion affects ELF3 mRNA expression regardless of the treatment. Thus, although C/EBPβ contributes to regulation of ELF3, the transcriptional regulation of C/EBPβ does not seem to have a significant role in the synergistic induction of ELF3 by IL-17A and TNF. Our results agree with Yamazaki et al. [45] showing that in cells in which IκBζ is expressed independently of inflammatory stimuli, the induction of other primary response genes by TNF is not required for IκBζ-mediated gene regulation. Taken together, the data suggest that the coordinated actions of NF-κB, C/EBPβ and IκBζ are required for ELF3 mRNA induction, with IL-17A providing the crucial signal leading to IκBζ mRNA stabilization, which is needed for high and sustained expression of ELF3.
ELF3 mediates inflammatory responses by IL-1α and IL-1β [20, 21, 36]. Our results indicate that it also plays a significant role in the synergy between IL-17A and TNF. Silencing of ELF3 in primary synovial fibroblasts markedly reduced the production of several inflammatory cytokines and proteinases induced by IL-17A–TNF. Overexpression studies showed a modest effect for ELF3 alone, but a potent synergistic effect with TNF in regulation of CCL8 mRNA expression in HEK293 cells and in synovial fibroblasts. Accordingly, CCL8 mRNA upregulation in cells overexpressing ELF3 was dependent on AP-1 activation by JNK, as wells as on NF-κB signalling. These results are consistent with previous studies wherein ELF3 was described to act in synergy with NF-κB in regulation of lipocalin-2 and with AP-1 to regulate MMP13 expression [21, 46], and highlights the importance of combinational control, a characteristic property of the ETS family [47], for ELF3-mediated transcriptional activation.
We established that at least some of the genes regulated by IκBζ are completely dependent on ELF3, as evidenced by the lack of induction of CCL8 mRNA in response to IκBζ overexpression and TNF in cells depleted of ELF3. The induction of ELF3 expression as a part of IκBζ-mediated signalling could in theory serve as the means for expanding the set of regulated genes, due to the ability of ELF3 to collaborate with AP-1 in addition to NF-κB. The findings expand the knowledge of how IκBζ mediates the effects of IL-17A and TNF [48] by providing additional signalling events downstream of IκBζ induction. The sustained nature of ELF3 induction implies that it might also contribute to prolonged production of inflammatory mediators. Although a role in cartilage degradation was shown for both ELF3 and IκBζ in meniscectomy-induced OA experimental model [49, 50], there are no studies in models of inflammatory arthritis, which would better reveal their contribution to inflammatory signalling. Findings establishing IκBζ as a key regulator of IL-17A-driven effects in psoriasis [27] are intriguing, considering that psoriasis, AS and PsA share pathogenic mechanisms including the role of IL-17A. Consequently, in addition to their role in the inflammatory signalling in RA and OA synovium, ELF3 and IκBζ might also play an important role in AS and PsA.
In conclusion, this study reveals the important role of transcription factor ELF3 in mediating the synergy between IL-17A and TNF in human synovial fibroblasts. Moreover, we demonstrate that the co-factor of NF-κB, IκBζ, is involved in the regulation of ELF3 expression. The results could help in understanding better the diseases in which IL-17A plays a significant role, as the ability of IL-17A to enhance the responses to TNF is probably one of the main proinflammatory mechanisms of this important cytokine.
Supplementary Material
Acknowledgements
We thank MSc Marcelina Bilicka for her skilful technical support in cell culture and qRT-PCR. V-P.K. is a recipient of grants from the Finska Läkaresällskapet, Finnish Cultural Foundation, Orion Research Foundation, Maire Lisko Foundation, Reumatautien tutkimussäätiö and Otto A. Malm Foundation. K.K.E. is a recipient of grants from Stockmann Foundation, Instrumentarium Science Foundation and Finska Läkaresällskapet, and Arthritis Society of Canada and the Canadian Institutes of Health Research (THC 135230). K.N. is a recipient of Yrjö Jahnsson Foundation (20217434, 20207306), Päivikki and Sakari Sohlberg Foundation, and Maire Lisko Foundation grants.
Funding: This work was supported by Helsinki University Hospital research funds and Academy of Finland.
Disclosure statement: The authors have declared no conflicts of interest.
Contributor Information
Vesa-Petteri Kouri, Department of Medicine, University of Helsinki and Helsinki University Hospital; Translational Immunology Research Program, Research Programs Unit, University of Helsinki; Department of Clinical Chemistry, University of Helsinki and Helsinki University Hospital.
Juri Olkkonen, Department of Medicine, University of Helsinki and Helsinki University Hospital; Translational Immunology Research Program, Research Programs Unit, University of Helsinki.
Katariina Nurmi, Department of Medicine, University of Helsinki and Helsinki University Hospital; Translational Immunology Research Program, Research Programs Unit, University of Helsinki.
Nitai Peled, Department of Medicine, University of Helsinki and Helsinki University Hospital; Translational Immunology Research Program, Research Programs Unit, University of Helsinki.
Mari Ainola, Department of Medicine, University of Helsinki and Helsinki University Hospital; Translational Immunology Research Program, Research Programs Unit, University of Helsinki.
Jami Mandelin, Department of Medicine, University of Helsinki and Helsinki University Hospital; Translational Immunology Research Program, Research Programs Unit, University of Helsinki.
Dan C Nordström, Department of Medicine, University of Helsinki and Helsinki University Hospital; Translational Immunology Research Program, Research Programs Unit, University of Helsinki; Department of Internal Medicine and Rehabilitation.
Kari K Eklund, Department of Medicine, University of Helsinki and Helsinki University Hospital; Translational Immunology Research Program, Research Programs Unit, University of Helsinki; Inflammation Center, Division of Rheumatology, Helsinki University Hospital; ORTON Orthopaedic Hospital of the Orton Foundation, Helsinki, Finland.
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Bottini N, Firestein GS.. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol 2013;9:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robinson WH, Lepus CM, Wang Q. et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol 2016;12:580–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kalliolias GD, Ivashkiv LB.. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol 2016;12:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Armaka M, Apostolaki M, Jacques P. et al. Mesenchymal cell targeting by TNF as a common pathogenic principle in chronic inflammatory joint and intestinal diseases. J Exp Med 2008;205:331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lubberts E, Koenders MI, van den Berg WB.. The role of T-cell interleukin-17 in conducting destructive arthritis: lessons from animal models. Arthritis Res Ther 2005;7:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Faust HJ, Zhang H, Han J. et al. IL-17 and immunologically induced senescence regulate response to injury in osteoarthritis. J Clin Invest 2020;130:5493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fossiez F, Djossou O, Chomarat P. et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med 1996;183:2593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kehlen A, Thiele K, Riemann D, Langner J.. Expression, modulation and signalling of IL-17 receptor in fibroblast-like synoviocytes of patients with rheumatoid arthritis. Clin Exp Immunol 2002;127:539–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hwang SY, Kim JY, Kim KW. et al. IL-17 induces production of IL-6 and IL-8 in rheumatoid arthritis synovial fibroblasts via NF-κB- and PI3-kinase/Akt-dependent pathways. Arthritis Res Ther 2004;6:R120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Honorati MC, Bovara M, Cattini L, Piacentini A, Facchini A.. Contribution of interleukin 17 to human cartilage degradation and synovial inflammation in osteoarthritis. Osteoarthr Cartil 2002;10:799–807. [DOI] [PubMed] [Google Scholar]
- 11. Chabaud M, Durand JM, Buchs N. et al. Human interleukin-17: a T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum 1999;42:963–70. [DOI] [PubMed] [Google Scholar]
- 12. Zhang X, Yuan Y, Pan Z. et al. Elevated circulating IL-17 level is associated with inflammatory arthritis and disease activity: a meta-analysis. Clin Chim Acta 2019;496:76–83. [DOI] [PubMed] [Google Scholar]
- 13. Deligne C, Casulli S, Pigenet A. et al. Differential expression of interleukin-17 and interleukin-22 in inflamed and non-inflamed synovium from osteoarthritis patients. Osteoarthr Cartil 2015;23:1843–52. [DOI] [PubMed] [Google Scholar]
- 14. Snelling SJB, Bas S, Puskas GJ. et al. Presence of IL-17 in synovial fluid identifies a potential inflammatory osteoarthritic phenotype. PLoS One 2017;12:e0175109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Katz Y, Nadiv O, Beer Y.. Interleukin-17 enhances tumor necrosis factor α-induced synthesis of interleukins 1,6, and 8 in skin and synovial fibroblasts: a possible role as a "fine-tuning cytokine" in inflammation processes. Arthritis Rheum 2001;44:2176–84. [DOI] [PubMed] [Google Scholar]
- 16. Robert M, Miossec P.. IL-17 in rheumatoid arthritis and precision medicine: from synovitis expression to circulating bioactive levels. Front Med (Lausanne) 2018;5:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koshy PJ, Henderson N, Logan C. et al. Interleukin 17 induces cartilage collagen breakdown: novel synergistic effects in combination with proinflammatory cytokines. Ann Rheum Dis 2002;61:704–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oettgen P, Alani RM, Barcinski MA. et al. Isolation and characterization of a novel epithelium-specific transcription factor, ESE-1, a member of the ets family. Mol Cell Biol 1997;17:4419–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rudders S, Gaspar J, Madore R. et al. ESE-1 is a novel transcriptional mediator of inflammation that interacts with NF-κB to regulate the inducible nitric-oxide synthase gene. J Biol Chem 2001;276:3302–9. [DOI] [PubMed] [Google Scholar]
- 20. Grall F, Gu X, Tan L. et al. Responses to the proinflammatory cytokines interleukin-1 and tumor necrosis factor α in cells derived from rheumatoid synovium and other joint tissues involve nuclear factor κB-mediated induction of the Ets transcription factor ESE-1. Arthritis Rheum 2003;48:1249–60. [DOI] [PubMed] [Google Scholar]
- 21. Otero M, Plumb DA, Tsuchimochi K. et al. E74-like factor 3 (ELF3) impacts on matrix metalloproteinase 13 (MMP13) transcriptional control in articular chondrocytes under proinflammatory stress. J Biol Chem 2012;287:3559–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aletaha D, Neogi T, Silman AJ. et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580–8. [DOI] [PubMed] [Google Scholar]
- 23. Ainola MM, Mandelin JA, Liljeström MP, Li TF. et al. Pannus invasion and cartilage degradation in rheumatoid arthritis: involvement of MMP-3 and interleukin-1β. Clin Exp Rheumatol 2005;23:644–50. [PubMed] [Google Scholar]
- 24. Olkkonen J, Kouri V, Hynninen J, Konttinen YT, Mandelin J.. Differentially expressed in chondrocytes 2 (DEC2) increases the expression of IL-1β and is abundantly present in synovial membrane in rheumatoid arthritis. PLoS One 2015;10:e0145279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nurmi K, Niemi K, Kareinen I. et al. Native and oxidised lipoproteins negatively regulate the serum amyloid A-induced NLRP3 inflammasome activation in human macrophages. Clin Transl Immunology 2021;10:e1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karlsen JR, Borregaard N, Cowland JB.. Induction of neutrophil gelatinase-associated lipocalin expression by co-stimulation with interleukin-17 and tumor necrosis factor-α is controlled by IκB-ζ but neither by C/EBP-β nor C/EBP-δ. J Biol Chem 2010;285:14088–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johansen C, Mose M, Ommen P. et al. IκBζ is a key driver in the development of psoriasis. Proc Natl Acad Sci USA 2015;112:E5825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Willems M, Dubois N, Musumeci L, Bours V, Robe PA.. IκBζ: an emerging player in cancer. Oncotarget 2016;7:66310–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yamazaki S, Muta T, Matsuo S, Takeshige K.. Stimulus-specific induction of a novel nuclear factor-κB regulator, IκB-ζ, via Toll/interleukin-1 receptor is mediated by mRNA stabilization. J Biol Chem 2005;280:1678–87. [DOI] [PubMed] [Google Scholar]
- 30. Eto A, Muta T, Yamazaki S, Takeshige K.. Essential roles for NF-κB and a Toll/IL-1 receptor domain-specific signal(s) in the induction of IκB-ζ. Biochem Biophys Res Commun 2003;301:495–501. [DOI] [PubMed] [Google Scholar]
- 31. Yamazaki S, Muta T, Takeshige K.. A novel IκB protein, IκB-ζ, induced by proinflammatory stimuli, negatively regulates nuclear factor-κB in the nuclei. J Biol Chem 2001;276:27657–62. [DOI] [PubMed] [Google Scholar]
- 32. Sparna T, Rétey J, Schmich K. et al. Genome-wide comparison between IL-17 and combined TNF-alpha/IL-17 induced genes in primary murine hepatocytes. BMC Genomics 2010;11:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matsuo S, Yamazaki S, Takeshige K, Muta T.. Crucial roles of binding sites for NF-κB and C/EBPs in IκB-ζ-mediated transcriptional activation. Biochem J 2007;405:605–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee C, Huang C.. LASAGNA-Search 2.0: integrated transcription factor binding site search and visualization in a browser. Bioinformatics 2014;30:1923–5. [DOI] [PubMed] [Google Scholar]
- 35. Moore JE, Purcaro MJ, Pratt HE. et al. ; ENCODE Project Consortium. Expanded encyclopaedias of DNA elements in the human and mouse genomes. Nature 2020;583:699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu J, Duan R, Cao H. et al. Regulation of epithelium-specific Ets-like factors ESE-1 and ESE-3 in airway epithelial cells: potential roles in airway inflammation. Cell Res 2008;18:649–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Amatya N, Garg AV, Gaffen SL.. IL-17 signaling: the Yin and the Yang. Trends Immunol 2017;38:310–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Behrens G, Winzen R, Rehage N. et al. A translational silencing function of MCPIP1/Regnase-1 specified by the target site context. Nucleic Acids Res 2018;46:4256–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mino T, Murakawa Y, Fukao A. et al. Regnase-1 and roquin regulate a common element in inflammatory mRNAs by spatiotemporally distinct mechanisms. Cell 2015;161:1058–73. [DOI] [PubMed] [Google Scholar]
- 40. Muromoto R, Tawa K, Ohgakiuchi Y. et al. IκB-ζ expression requires both TYK2/STAT3 activity and IL-17-regulated mRNA stabilization. Immunohorizons 2019;3:172–85. [DOI] [PubMed] [Google Scholar]
- 41. Takaishi M, Satoh T, Akira S, Sano S.. Regnase-1, an immunomodulator, limits the IL-36/IL-36R autostimulatory loop in keratinocytes to suppress skin inflammation. J Invest Dermatol 2018;138:1439–42. [DOI] [PubMed] [Google Scholar]
- 42. Iwasaki H, Takeuchi O, Teraguchi S. et al. The IκB kinase complex regulates the stability of cytokine-encoding mRNA induced by TLR-IL-1R by controlling degradation of regnase-1. Nat Immunol 2011;12:1167–75. [DOI] [PubMed] [Google Scholar]
- 43. Tanaka H, Arima Y, Kamimura D. et al. Phosphorylation-dependent Regnase-1 release from endoplasmic reticulum is critical in IL-17 response. J Exp Med 2019;216:1431–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yamamoto M, Yamazaki S, Uematsu S. et al. Regulation of Toll/IL-1-receptor-mediated gene expression by the inducible nuclear protein IκBζ. Nature 2004;430:218–22. [DOI] [PubMed] [Google Scholar]
- 45. Yamazaki S, Matsuo S, Muta T. et al. Gene-specific requirement of a nuclear protein, IκB-ζ, for promoter association of inflammatory transcription regulators. J Biol Chem 2008;283:32404–11. [DOI] [PubMed] [Google Scholar]
- 46. Conde J, Otero M, Scotece M. et al. E74-like factor 3 and nuclear factor-κB regulate lipocalin-2 expression in chondrocytes. J Physiol (Lond) 2016;594:6133–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Verger A, Duterque-Coquillaud M.. When Ets transcription factors meet their partners. Bioessays 2002;24:362–70. [DOI] [PubMed] [Google Scholar]
- 48. Slowikowski K, Nguyen HN, Noss EH. et al. CUX1 and IκBζ (NFKBIZ) mediate the synergistic inflammatory response to TNF and IL-17A in stromal fibroblasts. Proc Natl Acad Sci USA 2020;117:5532–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Choi M, MaruYama T, Chun C, Park Y.. Alleviation of murine osteoarthritis by cartilage-specific deletion of IκBζ. Arthritis Rheumatol (Hoboken, NJ) 2018;70:1440–9. [DOI] [PubMed] [Google Scholar]
- 50. Wondimu EB, Culley KL, Quinn J. et al. Elf3 contributes to cartilage degradation in vivo in a surgical model of post-traumatic osteoarthritis. Sci Rep 2018;8:6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.