Abstract
Objective
To quantify preferences for preventive therapies for rheumatoid arthritis (RA) across three countries.
Methods
A web-based survey including a discrete choice experiment was administered to adults recruited via survey panels in the UK, Germany and Romania. Participants were asked to assume they were experiencing arthralgia and had a 60% chance of developing RA in the next 2 years and completed 15 choices between no treatment and two hypothetical preventive treatments. Treatments were defined by six attributes (effectiveness, risks and frequency/route of administration) with varying levels. Participants also completed a choice task with fixed profiles reflecting subjective estimates of candidate preventive treatments. Latent class models (LCMs) were conducted and the relative importance of attributes, benefit–risk trade-offs and predicted treatment uptake was subsequently calculated.
Results
Completed surveys from 2959 participants were included in the analysis. Most participants preferred treatment over no treatment and valued treatment effectiveness to reduce risk more than other attributes. A five-class LCM best fitted the data. Country, perceived risk of RA, health literacy and numeracy predicted class membership probability. Overall, the maximum acceptable risk for a 40% reduction in the chance of getting RA (60% to 20%) was 21.7%, 19.1% and 2.2% for mild side effects, serious infection and serious side effects, respectively. Predicted uptake of profiles reflecting candidate prevention therapies differed across classes.
Conclusion
Effective preventive pharmacological treatments for RA were acceptable to most participants. The relative importance of treatment attributes and likely uptake of fixed treatment profiles were predicted by participant characteristics.
Keywords: RA, preventive treatment, patient preferences, discrete choice experiment
Rheumatology key messages.
Acceptable preventive treatments for RA could reduce pain, disability and societal costs on a huge scale.
This study quantifies preferences for treatments to reduce the risk of RA development across three countries.
This guides trial design and development of decision-making support for individuals at risk of RA.
Introduction
Increased understanding of the biological mechanisms involved in the pre-clinical stages of rheumatoid arthritis (RA) [1] and the ability to identify individuals at risk of developing RA [2] have highlighted opportunities for preventive intervention [3–5]. There is emerging research interest in assessing whether a short course of therapy will prevent the onset of RA in at-risk individuals. B cell depletion, with a single infusion of 1000 mg of rituximab, has been shown to delay the onset of RA in individuals with arthralgia and autoantibody positivity [6]. The preventive efficacy of abatacept in this group [7] and HCQ in asymptomatic autoantibody-positive individuals [8] is under investigation and novel cellular therapies to prevent RA are being explored [9].
Difficulties in recruitment to RA prevention trials have been reported [10]. A trial assessing the efficacy of 40 mg atorvastatin daily for 3 years was prematurely discontinued due to a low inclusion rate resulting from unwillingness to participate [11]. Recent EULAR recommendations highlight the importance of understanding the perspectives of at-risk groups to facilitate tailored strategies to enhance engagement with prevention studies and increase understanding about what types of preventive treatments are acceptable [12]. There is growing awareness of the value of insights into patient preferences for drug development, approval and reimbursement [13–15]. Increased understanding of the relative importance of treatment attributes and acceptable benefit–risk trade-offs for preventive treatments could prevent investment in studies of unacceptable treatments, guide selection of outcomes and endpoints and inform the development of resources to support decision making around participation in prevention trials.
While preferences for treatments for established RA have been well studied [16, 17], few studies have quantified preferences for preventive treatment of at-risk groups [18]. Among those at risk for RA, there is uncertainty regarding not only treatment effectiveness and safety, but also the individual’s risk of developing RA, the timeline for that risk and the severity of disease if RA does develop [19]. Qualitative studies have explored stakeholder perceptions of preventive treatment for RA [20]. A survey of individuals with arthralgia and autoantibody positivity found that 40% reported a willingness to accept preventive treatments that were 100% effective with minor side effects of immune suppression if their risk of developing RA was 70% [21]. A pilot study in Switzerland [22] and two Canadian studies [23, 24] of preventive treatment choices found that treatments with mild side effects were acceptable, although the relative importance of treatment attributes varied across studies. The aim of this study was to quantify preferences and preference heterogeneity for preventive treatments for RA in large samples of the general population across three European countries.
Methods
This study is part of a case study for the Innovative Medicines Initiative (IMI) project ‘Patient Preferences in Benefit-Risk Assessments during the Medical Product Lifecycle’ (PREFER), which aims to develop evidence-based recommendations on how and when preference studies can inform decision making during drug development [13]. The study was approved by the London-Hampstead Research Ethics Committee (19/LO/0407) and the Ethics Committee of the Friedrich-Alexander-Universität Erlangen-Nürnberg (92_17 B). The protocol has been published [25].
Participant identification and recruitment
Participants were recruited in the UK, Germany and Romania via online survey panels (surveyengine.com) using age- and gender-matched samples informed by a prior study of individuals at risk of RA [26]. These countries were chosen to reflect a geographically and culturally diverse range across Europe to facilitate assessment of preference consistency across populations with distinct healthcare systems. Members of online survey panels in each country received an e-mail invitation (Supplementary Data S1, available at Rheumatology online) to take part in and link to the online survey. The initial questions of the survey confirmed respondent’s eligibility to participate (age ≥18 years and without a diagnosis of RA). Eligible respondents were provided with participant information and asked to provide anonymous electronic informed consent. Participants provided written informed consent. After completing the survey, panel members were credited with panel points (equivalent to ∼€2–3). Translation of the survey from English to German and Romanian was undertaken in accordance with ISO17100 standards [27]. Recruitment continued until 1000 surveys were completed in each country. For sample size assessment details see Supplementary Data S1, available at Rheumatology online.
Survey development
A discrete choice experiment (DCE) within a web-based survey was developed following best practices [28, 29]. This method has been widely applied to elicit preferences for healthcare interventions [30, 31]. The DCE consists of a series of choice tasks. For each task, participants were asked to imagine they were experiencing joint pain that was impacting on their daily activities and had received test results indicating their risk of developing RA in the next 2 years was 60%; their doctor has suggested they consider taking a treatment to reduce their risk; and treatment would be for 1 year. Participants were asked to choose between two treatment options or to opt out (no treatment), where treatments could lower the chance of getting RA in exchange for a risk of side effects and different modes and frequencies of administration. Each treatment option was described by their level of six attributes. See Supplementary Data S1, available at Rheumatology online for an example choice task.
DCE attribute and level selection and experimental design
The selection of treatment attributes included in the DCE was informed by a previous literature review [18], qualitative study and attribute ranking survey [25]. Final attributes (Table 1) were agreed upon by an international team of clinical researchers, consultant rheumatologists, preference elicitation experts and patient partners. Attribute levels were estimated with input from clinical experts, including researchers leading the development and/or clinical trials of preventive treatments for RA. The text used to describe attributes and levels to participants is available in Supplementary Data S1, available at Rheumatology online. The combinations of attribute levels that define each treatment option and the set of treatment options in each choice question of a DCE constitute the experimental design. This must have statistical properties that allow estimation of the preference weights of interest obtained from responses to a number of choice tasks that is feasible for participants to complete. Ngene (ChoiceMetrics, Sydney, NSW, Australia) was used to construct a Bayesian D-efficient fractional factorial design [32]. Prior information on attribute importance was initially based on previous literature and expert best guesses, then the outcomes of conditional logit analysis of a pilot survey (N = 100) were used as priors for the final experimental design. Sixty unique choice tasks were generated, divided over four blocks. Each participant answered one block (15 choice tasks) only. Participants were randomized to blocks upon entering the survey using the minimization method to ensure equal proportions of participants across blocks. The design was restricted to exclude the following combinations of attribute levels: taking a pill monthly or every 6 months, having an injection daily or having a drip daily or weekly. Interactions between effectiveness and chance of a serious infection and between effectiveness and the chance of serious side effects were included.
Table 1.
Discrete choice task background scenario, attributes and levels
| Choice task background: When answering, we ask you to imagine that you are experiencing some joint symptoms (joint pain and stiffness) that are starting to impact on your daily activities and that the results of a blood test show you have a 60% chance of developing rheumatoid arthritis in the next 2 years. Your doctor suggests that you consider taking one of the following treatments for 1 year. In this case, would you prefer treatment A, treatment B or no treatment? | ||
|---|---|---|
| Treatment attribute | Levels | |
| Chance of developing RA reduced from 60% to | 10% | 10 in 100 |
| 20% | 20 in 100 | |
| 30% | 30 in 100 | |
| 40% | 40 in 100 | |
| How the treatment is taken | A shallow injection under the skin | |
| A drip into the vein | ||
| One or two tablets | ||
| How often the medication has to be taken | Daily | |
| Weekly | ||
| Monthly | ||
| Every 6 months | ||
| Chance of reversible mild side effects (e.g. nausea, skin rashes or muscle pain) | 2% | 2 in 100 people |
| 5% | 5 in 100 people | |
| 10% | 10 in 100 people | |
| Chance of a serious infection (e.g. pneumonia) | 0% | 0 in 100 people |
| 1% | 1 in 100 people | |
| 5% | 5 in 100 people | |
| Chance of a serious, potentially irreversible side effect (e.g. brain inflammation, lymphoma or retinopathy) | 0.001% | 1 in 100 000 people |
| 0.02% | 20 in 100 000 people | |
| 0.1% | 100 in 100 000 people | |
Survey content
The survey was co-developed with patient partners and pretested in a convenience sample (N = 15) using ‘think-aloud’ interviews. It included background information about RA (see Supplementary Data S1, available at Rheumatology online), questions to assess comprehension of the background material, introduction to the choice tasks and treatment attributes and levels, a guided ‘walk-through’ choice task example, warm-up choice tasks and 15 DCE choice tasks. During completion of each task, participants could choose to view the explanation of each attribute and its levels. Participants then completed an additional choice task including four treatment options with fixed profiles, which reflected drug characteristics and known risks of agents under investigation as preventive treatments for RA (HCQ, atorvastatin, abatacept and tolerogenic cell-based therapy). Treatment effectiveness was estimated with input from researchers leading the development and/or clinical trials of the relevant agents.
The survey included measures of sociodemographic variables, the Single-Item Health Literacy Screener [33] and the three-item version of the Subjective Numeracy Scale [34] and participants assessed their lifetime risk of developing RA using a five-item Likert-type scale (‘very unlikely’ to ‘very likely’). Upon completion of the survey, all respondents were provided with information about RA for those who are at risk developed by an international team of patient partners, rheumatologists and researchers as part of a previous project [35].
Data analysis
The outcomes of interest were treatment preferences and preference heterogeneity, relative preference weights for levels of treatment attributes, estimated risk equivalents [maximum acceptable risk (MAR) for changes in treatment effectiveness] and choice frequency of fixed treatment profiles. Results were considered statistically significant if P ≤ 0.05. Analyses were carried out using SPSS version 27.0 (IBM, Armonk, NY, USA) and NLogit (Econometric Software, Plainview, NY, USA). Participants who exhibited non-attendance to the survey were removed from the analysis, including those who completed the survey in 5 min and those who took 5–9 min to answer the survey, answered two or more of three background comprehension questions incorrectly and showed ‘flat-lining’ behaviour (always choosing ‘treatment A’ or always choosing ‘treatment B’) in the DCE choice tasks.
Panel latent class models (LCMs) were used on a pooled dataset combining data across countries. All attributes were considered non-linear and recoded using effects codes [36, 37]. Based on model fit tests [Akaike information criterion (AIC), log likelihood], the most suitable model was selected (models ranging from one to six classes were tested). See Supplementary Data S1, available at Rheumatology online, for the final utility equation.
A class assignment model was fitted to assess the contribution of participant characteristics (i.e. country, perceived risk of developing RA, smoking, age, gender, health literacy, numeracy and educational level). A significant variable in the class assignment model indicates that this variable contributes to the class assignment (e.g. if the coefficient of the health literacy variable is positive and significant for class 1, participants with a higher health literacy score are more likely to belong to class 1).
Relative importance scores for the attributes relative to the most important attribute were calculated based on the results of the LCMs, separately for all classes. The class-adjusted relative importance was calculated by computing the relative importance score of all attributes in each class separately, after which they were weighted according to class assignment probability.
The MAR for a 40% reduction in the chance of getting RA (60% to 20%) was calculated for each of the three risk attributes according to the equation:
For these calculations an LCA model was used, assuming linearity for all attributes included. The MAR was calculated separately for all classes after which they were weighted according to class assignment probability. Predicted choice [1/(1+exp−v)] between the four hypothetical treatment profiles with fixed profiles and no treatment was tabulated using LCM estimates for each class.
Patient and public involvement
A panel of eight patient research partners [based in the UK (n = 4), Germany (n = 2), The Netherlands (n = 1) and Sweden (n = 1)] contributed to the development of research objectives, selection of attributes and levels, development of choice tasks and survey content and pretesting.
Results
Responses of 982 individuals (332 males, 650 females) in the UK, 984 (325 males, 656 females, 2 gender not reported) in Germany and 993 (340 males, 652 females, 1 other) in Romania were included. Participant characteristics are summarized in Table 2. Health literacy scores were significantly lower in the sample in Romania compared with samples in Germany and the UK. Subjective numeracy scores were significantly higher in the sample in Romania compared with those in both Germany and the UK. Participants in Germany perceived their chances of developing RA to be significantly lower than those in either the UK or Romania.
Table 2.
Participant characteristics per country
| Characteristics | UK (n = 982), n (%) | Germany (DE; n = 984), n (%) | Romania (RO; n = 993), n (%) | Multiple comparisons | |
|---|---|---|---|---|---|
| Age (years) [χ2 (10, N = 2959) = 2.58, P = 0.990] | 18–29 | 220 (22.4) | 218 (22.2) | 228 (23.0) | |
| 30–39 | 212 (21.6) | 212 (21.5) | 217 (21.9) | ||
| 40–49 | 229(23.3) | 228 (23.2) | 231 (23.3) | ||
| 50–59 | 203 (20.7) | 208 (21.1) | 206 (20.7) | ||
| 60–69 | 84 (8.6) | 84 (8.5) | 87 (8.8) | ||
| ≥70 | 34 (3.5) | 34 (3.5) | 24 (2.4) | ||
| Highest level of education [χ2 (12, N = 2959) = 551.74, P < 0.001] | None | 12 (1.2) | 7 (0.7) | 4 (0.4) | UK > RO |
| Primary school | 2 (0.2) | 15 (1.5) | 6 (0.6) | DE > UK and RO | |
| Secondary school | 207 (21.1) | 377 (38.3) | 36 (3.6) | UK > RO | |
| DE > UK and RO | |||||
| Sixth form | 271 (27.6) | 295 (30.0) | 256 (25.8) | DE > RO | |
| Degree or vocational | 373 (38.0) | 118 (12.0) | 501 (50.5) | UK > DE | |
| RO > UK and DE | |||||
| Postgraduate | 109 (11.1) | 169 (17.2) | 184 (18.5) | DE > UK | |
| RO > UK | |||||
| Other | 8 (0.8) | 3 (0.3) | 6 (0.6) | ||
| Employed/self-employed [χ2 (2, N = 2959) = 9.81, P = 0.007] | Yes | 641 (65.3) | 700 (71.1) | 702 (70.7) | DE > UK |
| RO > UK | |||||
| No | 341 (34.7) | 284 (28.9) | 291 (29.3) | UK > DE and RO | |
| Family history of RA [χ2 (8, N = 2959) = 106.18, P < 0.001] | Definitely not | 210 (21.4) | 308 (31.3) | 176 (16.8) | DE > UK and RO |
| UK > RO | |||||
| Probably not | 205 (20.9) | 231 (23.5) | 159 (16.0) | UK > RO | |
| DE > RO | |||||
| Don’t know | 245 (24.9) | 212 (21.5) | 304 (30.6) | RO > UK and DE | |
| Probably | 163 (16.6) | 118 (12.0) | 197 (19.8) | UK > DE | |
| RO > DE | |||||
| Definitely | 159 (16.2) | 115 (11.7) | 166 (16.7) | UK > DE | |
| RO > DE | |||||
| Smoking [χ2 (4, N = 2959) = 127.68, P < 0.001] | Yes | 218 (22.2) | 360 (36.6) | 393 (39.6) | DE > UK |
| RO > UK | |||||
| Never | 536 (54.6) | 408 (41.5) | 310 (31.2) | UK > DE and RO | |
| No, but have in the past | 228 (23.2) | 216 (22.0) | 290 (29.2) | RO > UK and DE | |
| Median (mean rank) | Median (mean rank) | Median (mean rank) | |||
|---|---|---|---|---|---|
| Single-Item Health Literacy Scale [H (2, N = 2959) = 38.26, P < 0.001] | 5.00 (1603.06) | 5.00 (1439.54) | 4 (1398.40) | UK > RO | |
| DE > RO | |||||
| Three-item subjective numeracy scale [H (2, N = 2959) = 57.22, P < 0.001] | 14.0 (1411.82) | 13.0 (1381.08) | 15.0 (1645.45) | RO > UK | |
| RO > DE | |||||
| Perceived likelihood of developing RA in the future [H (2, N = 2959) = 35.81, P < 0.001] | 3.00 (1560.30) | 3.00 (1359.07) | 3.00 (1520.42) | UK > DE | |
| RO > DE | |||||
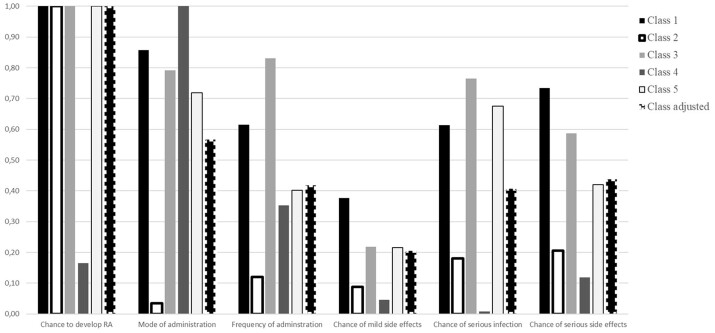
Five classes were fitted in the LCM (see Table 3 for model coefficients). The average probabilities of belonging to classes 1–5 were 51%, 10%, 26%, 4% and 10%, respectively. In all classes, participants preferred treatments that were more effective and had less risk of serious infection and serious side effects. For classes 1, 2 and 3, preventive treatment was preferred over no treatment, whereas in classes 4 and 5, no treatment was preferred. The relative importance of treatment attributes in each class is summarized in Fig. 1. Treatment effectiveness was the most important attribute in all classes except for class 4, where the method of administration was more important. The least important attribute was mild side effects in all classes except for class 2, where the method of administration was least important. In classes 1, 3 and 4, oral tablets were preferred over injection or infusion, while in class 2, this attribute was not significant. In class 5, infusion was preferred over oral tablets and tablets over injections. In the same class, the frequency of administration did not significantly impact on preferences, while in the other classes, the least frequent attribute level of every 6 months was preferred. A preference for the lowest level of mild side effects was found in classes 1–3, with no significant impact on preferences in classes 4 and 5.
Table 3.
Parameter estimates from the LCM of preferences for preventive treatment of RA
| Attribute | Level | Class 1 |
Class 2 |
Class 3 |
Class 4 |
Class 5 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | s.e. | Estimate | s.e. | Estimate | s.e. | Estimate | s.e. | Estimate | s.e. | ||
| No treatment (opt out) | −4.14*** | 0.08 | −3.81*** | 0.12 | −0.12*** | 0.02 | 1.08*** | 0.07 | 3.58*** | 0.06 | |
| Chance of developing RA | 40% (ref) | −0.42*** | 0.04 | −3.31*** | 0.12 | −0.29*** | 0.02 | −0.47*** | 0.09 | −0.52*** | 0.15 |
| 30% | −0.10*** | 0.02 | −0.79*** | 0.07 | −0.05** | 0.02 | 0.03 | 0.12 | −0.20 | 0.16 | |
| 20% | 0.13*** | 0.02 | 0.87*** | 0.07 | 0.11*** | 0.02 | 0.09 | 0.10 | 0.34** | 0.13 | |
| 10% | 0.40*** | 0.02 | 3.24*** | 0.13 | 0.23*** | 0.02 | 0.36*** | 0.09 | 0.39*** | 0.12 | |
| Mode of administration | Tablet (ref) | 0.38*** | 0.04 | −0.11 | 0.10 | 0.20*** | 0.03 | 3.17*** | 0.18 | 0.04 | 0.18 |
| Injection | −0.06*** | 0.01 | 0.00 | 0.04 | 0.02 | 0.02 | −1.30*** | 0.10 | −0.35*** | 0.11 | |
| Infusion | −0.32*** | 0.02 | 0.12 | 0.07 | −0.22*** | 0.02 | −1.87*** | 0.14 | 0.31** | 0.15 | |
| Frequency of administration | Daily (ref) | −0.21*** | 0.06 | −0.32*** | 0.10 | −0.22*** | 0.04 | −0.37** | 0.18 | 0.20 | 0.22 |
| Weekly | −0.17*** | 0.02 | −0.08 | 0.06 | −0.13*** | 0.02 | −0.61** | 0.15 | −0.11 | 0.15 | |
| Monthly | 0.09*** | 0.02 | −0.08 | 0.07 | 0.14*** | 0.03 | −0.19 | 0.19 | −0.17 | 0.18 | |
| Every 6 months | 0.29*** | 0.02 | 0.47*** | 0.06 | 0.21*** | 0.03 | 1.17*** | 0.15 | 0.07 | 0.16 | |
| Chance of mild side effects | 2% (ref) | 0.14*** | 0.03 | 0.27*** | 0.04 | 0.05*** | 0.02 | −0.03 | 0.08 | 0.03 | 0.10 |
| 5% | 0.03*** | 0.01 | 0.03 | 0.04 | 0.02 | 0.02 | 0.13 | 0.09 | −0.11 | 0.13 | |
| 10% | −0.17*** | 0.01 | −0.30*** | 0.04 | −0.07*** | 0.02 | −0.10 | 0.08 | 0.08 | 0.10 | |
| Chance of serious infection | 0% (ref) | 0.22*** | 0.02 | 0.55*** | 0.05 | 0.21*** | 0.02 | 0.00 | 0.10 | 0.36*** | 0.09 |
| 1% | 0.06*** | 0.02 | 0.07 | 0.05 | −0.02 | 0.02 | 0.02 | 0.09 | −0.11 | 0.12 | |
| 5% | −0.28*** | 0.01 | −0.62*** | 0.05 | −0.19*** | 0.02 | −0.02 | 0.08 | −0.25** | 0.11 | |
| Chance of serious side effects | 1 in 100 000 (ref) | 0.32*** | 0.03 | 0.67*** | 0.05 | 0.20*** | 0.01 | −0.12 | 0.09 | 0.22** | 0.09 |
| 20 in 100 000 | −0.04*** | 0.01 | 0.02 | 0.03 | −0.09*** | 0.02 | 0.36*** | 0.09 | −0.16 | 0.11 | |
| 100 in 100 000 | −0.28*** | 0.01 | −0.69*** | 0.05 | −0.11*** | 0.02 | −0.24*** | 0.09 | −0.06 | 0.11 | |
| Average class probability | 0.51 | 0.10 | 0.26 | 0.04 | 0.10 | ||||||
| Class membership modela | |||||||||||
| Constant | 0.98*** | 0.34 | −11.06*** | 1.02 | 1.39*** | 0.35 | −2.72*** | 0.65 | |||
| Country | Germany | −0.59*** | 0.15 | 0.17 | 0.19 | −0.11 | 0.16 | −0.04 | 0.25 | – | – |
| Romania | −0.69*** | 0.14 | −0.34* | 0.19 | −1.00*** | 0.16 | −0.82*** | 0.27 | – | – | |
| High health literacy | −0.15 | 0.15 | −0.11 | 0.23 | −0.52*** | 0.16 | 0.35 | 0.30 | – | – | |
| High numeracy | 0.73*** | 0.13 | 6.00*** | 0.56 | 0.47*** | 0.14 | 0.89*** | 0.22 | – | – | |
| High perceived risk of developing RA | 0.08 | 0.14 | −0.99*** | 0.20 | −0.16 | 0.15 | −0.16 | 0.24 | – | – | |
| Model fit measures | Loglikelihood | −48050.65 | |||||||||
| AIC | 96299.3 | ||||||||||
Class 5 is the reference category.
P < 0.10,
P < 0.05,
P < 0.001.
Fig. 1.
Relative importance of treatment attributes stratified by class in the LCM of preferences for preventive treatments for RA
The model fit significantly improved when including country, health literacy, numeracy and perceived risk of developing RA in the class assignment model. Participants from Germany were less likely to belong to class 1, whereas participants from Romania were most likely to belong to class 5. Participants with low health literacy were more likely to belong to class 3, whereas respondents with high numeracy were more likely to belong to classes 1–4. Respondents with a high perceived risk of developing RA were less likely to belong to class 2. The MAR for a 40% reduction in the chance of getting RA (60% to 20%) was 21.7% (s.e. 5.2), 19.1% (s.e. 37.7) and 2.2% (s.e. 2.6) for mild side effects, serious infection and serious side effects, respectively.
The attribute levels used to describe the fixed treatment profiles estimating candidate preventive treatments for RA, the frequency with which each profile was chosen, and uptake of each treatment predicted by our model are summarized in Table 4. The treatment profile designed to approximate treatment with abatacept was most often chosen, and the least likely choice was to opt out of treatment. While participants who belong to the largest class are expected to (always) choose treatment, 90% of participants in class 5 would be expected to choose no treatment. Predicted treatment choice varied across classes.
Table 4.
Choice frequencies and predicted choices per class (based on LCM output) for additional choice task with fixed attribute levelsa
| Characteristics | No treatment | Treatment A | Treatment B | Treatment C | Treatment D |
|---|---|---|---|---|---|
| Chance of developing RA reduced from 60% to … | 60 in 100 | 20 in 100 | 40 in 100 | 30 in 100 | 30 in 100 |
| How the treatment is taken | Not taken | Shallow injection under the skin | One or two tablets | One or two tablets | Shallow injection under the skin |
| How often the treatment has to be taken | Never | Weekly | Daily | Daily | Monthly |
| Chance of a mild side effect | 0 | 5 in 100 people | 10 in 100 people | 10 in 100 people | 10 in 100 people |
| Chance of a serious infection due to treatment | 0 | 1 in 100 people | 0 in 100 people | 0 in 100 people | 1 in 100 people |
| Chance of a serious side effect that is potentially irreversible | 0 | 20 in 100 000 people | 1 in 100 000 people | 100 in 100 000 people | 1 in 100 000 people |
| Choice frequency, n (%) | 425 (14) | 818 (28) | 606 (20) | 468 (16) | 642 (22) |
| Predicted uptake, % | |||||
| Class 1 | 0 | 23 | 27 | 21 | 28 |
| Class 2 | 1 | 73 | 2 | 6 | 19 |
| Class 3 | 18 | 18 | 20 | 19 | 25 |
| Class 4 | 12 | 1 | 35 | 51 | 1 |
| Class 5 | 90 | 2 | 4 | 4 | 1 |
| Class adjusted | 14 | 24 | 18 | 18 | 14 |
Treatment choices were unlabelled (i.e. participants chose between treatment A, B, C and D or no treatment). Attribute levels in each treatment profile reflected fixed profiles that reflected drug characteristics and known risks of agents under investigation as preventive treatments for RA (abatacept, atorvastatin, HCQ and tolerogenic cell-based therapy, respectively). Treatment effectiveness was estimated with input from researchers leading the development and/or clinical trials of the relevant agents.
Discussion
Treatments to reduce the risk of RA were acceptable to most participants and treatment effectiveness was the most important determinant of participants’ choices overall. The importance of effectiveness aligns with two of three previous choice-based studies of preferences for preventive treatment [22, 24]. A third study found that serious side effects were more important than effectiveness, although most participants in that study were patients being treated for established RA [23]. Previous qualitative studies have suggested that positive viewpoints of preventive therapies are associated with high expectations of their effectiveness [38–41], highlighting the need to increase understanding of the true likely effectiveness of interventions to reduce RA risk.
A previous stated-choice study of preferences for preventive treatment of RA found that the probability of mild side effects and the method of treatment administration did not significantly determine participants’ choice [22], which does not align with the current findings. This discrepancy across studies could be due to differences in the definition of attributes and levels, preference method chosen and/or the study samples.
Preference heterogeneity was predicted by participant characteristics. Respondents with lower health literacy were more likely to prefer no treatment and more likely to focus on mode and frequency of administration. Others who preferred no treatment were also sensitive to the chance of serious side effects or infection and more likely to report low numeracy. Participants in Romania were more likely to belong to a class where no treatment was preferred than those in Germany and the UK. Another group whose choices were focused on treatment effectiveness to reduce RA risk were more likely to have high perceived risk of developing RA. These findings suggest that developing effective communication tools tailored to individuals’ needs and illness beliefs is essential to facilitate shared decision making by at-risk individuals about taking part in prevention studies or accepting future treatments in clinical settings. The finding that country explained the probability of class membership suggests that treatment preferences are not generalizable across countries and further investigation of treatment preferences across a wide range of countries and the sociocultural and other (e.g. healthcare system related) factors that drive preference variability across countries is needed.
Predicted uptake of treatment profiles intended to estimate treatments under consideration for RA prevention was similar across treatments but differed across subgroups identified by the LCM. Participants with high perceived risk of RA were more likely to belong to the group predicted to prefer treatment profiles estimating biologic and cell-based therapy. Predicted uptake was higher than in two previous studies [23, 24] that included healthcare professional endorsement as a treatment attribute. In this study, this attribute was held constant in the choice task scenario, as it was felt that few people would accept a therapy their doctor did not endorse. Uptake estimates are strongly influenced by subjective attribute levels assigned to treatment profiles. In this study, these were chosen with input from investigators leading prevention trials. A treatment profile chosen to reflect abatacept was most often chosen, reflecting the assignment of the highest level of treatment effectiveness in the fixed choice task. Two previous studies concluded that biologic treatments are unlikely to be acceptable for RA prevention [23, 24]. However, the treatment profiles used in these studies to describe biologic therapies estimated them to be less effective at reducing the risk of RA than other treatments. They also included rituximab, which is administered via an infusion, i.e. the least preferred route for most participants. Rituximab, as a single infusion of 1000 mg, has been shown to delay, but not prevent RA development [6] and thus was not included in the current study. Further investigation is warranted to establish preferences for preventive treatment benefits other than effectiveness to reduce risk, including delayed disease development and impact on early symptoms that may precede the diagnosis of RA.
The current study focused on preferences for preventive pharmacological interventions, as there are/have been several clinical trials of drugs to assess their ability to reduce the risk of RA development. Preventive lifestyle interventions were not addressed here. However, there is a rationale for certain preventive behavioural/lifestyle approaches, including smoking cessation [42], diet, exercise and weight management. Combined behavioural and pharmacological strategies may be effective in future clinical applications [43]. There is also converging evidence across qualitative studies that lifestyle interventions would be preferred over preventive drug therapies [20]. The efficacy of lifestyle approaches to prevent RA development [44] and preferences for such interventions are therefore important areas for future studies.
This study has several strengths, including large samples across three countries, robust preference elicitation methodology and input from clinical researchers, methodological experts and patient partners. The treatment attributes and levels included were based on extensive literature review, qualitative investigation and expert consultation, in line with best practice [28, 29, 45]. As with all preference elicitation studies, the results are generalizable to treatments well represented by these attributes and levels and not to others. For example, alternative methods of treatment administration were not addressed in the present study.
A limitation is that participants were members of the general population asked to assume a hypothetical risk of RA. Previous research has highlighted common public misperceptions regarding RA that are likely to impact beliefs about the need for preventive treatment and associated decision making [46, 47]. We addressed this possibility by close collaboration with patient partners during the development of background information for survey participants and the inclusion of comprehension questions to check participant understanding of this material. Further research is needed to directly assess preferences of individuals with an elevated risk of RA, including those with RA-related autoantibodies and inflammatory-type joint symptoms. A further limitation is that participants were members of online panels who received a small payment to complete the survey and may not be fully representative of the general population. This was addressed to some extent by detailed analysis of data quality and exclusion of responses that did not meet our quality criteria. However, further investigation is needed to establish differences in the treatment preferences of survey panel members compared with samples recruited via alternative methods. This is being explored in a separate analysis of the current case study [25]. The use of an online survey further limits recruitment to those with access to web-based technology and a degree of literacy that may limit the ability to generalize our findings to other populations. Although this study benefits from input from patient partners across a range of European countries, no patient partner from Romania contributed to the survey design, although they were included in a previous related project that informed the clinical objectives of this study [48].
In conclusion, this study establishes that effective treatments to reduce the risk of RA are acceptable, informs the selection of acceptable candidate therapies and guides the design of prevention trials and the development of tailored risk communication tools for those considering preventive treatment.
Supplementary Material
Acknowledgements
This article and its contents reflect the PREFER project’s view and not the view of the IMI, European Union (EU) or European Federation of Pharmaceutical Industries and Associations (EFPIA). The authors are grateful to members of the extended PREFER RA case study team and the PREFER RA case study patient research partners for their important contributions to the development and design of this study. The protocol has also been reviewed and approved by the IMI PREFER expert review board and steering committee. The study was conducted in compliance with guidelines for Good Clinical Practice, the Research Governance Framework for Health and Social Care and the Data Protection Act 1998. Personal data protection in this study was compliant with the EU General Data Protection Regulation 2016/679 and the information security policies of the Universities of Birmingham and Erlangen.
Funding: This work was supported by IMI 2 Joint Undertaking (grant 115966), which receives support from the EU’s Horizon 2020 Research and Innovation Program and European Federation of Pharmaceutical Industries and Associations (EFPIA). K.R. is supported by the National Institute for Health and Care Research Birmingham Biomedical Research Centre.
Disclosure statement: R.L.D. is a shareholder of Johnson & Johnson and an employee of Janssen R&D (of Johnson & Johnson). M.E. is a member of speakers bureaus for AbbVie, Chugai, Eli Lilly, Novartis, Roche, Sanofi and Mundipharma; a paid instructor for AbbVie, Chugai and Roche and a consultant for AbbVie, Novartis, Roche and Sanofi and has received grant/research support from Roche and Chugai. C.R. is a shareholder and an employee of Eli Lilly. K.R. has received personal fees from AbbVie and Sanofi and grant/research support from Bristol Myers Squibb. B.H. is an employee of Pfizer. All other authors have declared no conflicts of interest.
Contributor Information
Gwenda Simons, Rheumatology Research Group, Institute of Inflammation and Ageing, College of Medical and Dental Sciences, University of Birmingham, Birmingham, UK.
Jorien Veldwijk, Erasmus School of Health Policy and Management and Erasmus Choice Modelling Centre, Erasmus University Rotterdam, Rotterdam; Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, The Netherlands.
Rachael L DiSantostefano, Janssen R&D, Titusville, NJ, USA.
Matthias Englbrecht, Freelance Data Scientist, Eckental, Germany.
Christine Radawski, Eli Lilly and Company, Indianapolis, IN, USA.
Karin Schölin Bywall, Centre for Research Ethics and Bioethics, Uppsala University, Uppsala, Sweden.
Larissa Valor Méndez, Department of Internal Medicine 3-Rheumatology and Immunology, Friedrich Alexander University (FAU) Erlangen-Nurnberg and Universitatsklinikum Erlangen, Erlangen, Germany.
Brett Hauber, Pfizer, Inc., New York, NY; Comparative Health Outcomes, Policy, and Economics Institute, University of Washington School of Pharmacy, Seattle, WA, USA.
Karim Raza, Rheumatology Research Group, Institute of Inflammation and Ageing, College of Medical and Dental Sciences, University of Birmingham, Birmingham, UK; Research into Inflammatory Arthritis Centre Versus Arthritis and MRC-Versus Arthritis Centre for Musculoskeletal Ageing Research, University of Birmingham; Sandwell and West Birmingham NHS Trust, Birmingham, UK.
Marie Falahee, Rheumatology Research Group, Institute of Inflammation and Ageing, College of Medical and Dental Sciences, University of Birmingham, Birmingham, UK.
Data availability statement
Data are available upon reasonable request.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Tracy A, Buckley CD, Raza K.. Pre-symptomatic autoimmunity in rheumatoid arthritis: when does the disease start? Semin Immunopathol 2017;39:423–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Boheemen L, van Schaardenburg D.. Predicting rheumatoid arthritis in at-risk individuals. Clin Ther 2019;41:1286–98. [DOI] [PubMed] [Google Scholar]
- 3. Raza K, Klareskog L, Holers VM.. Predicting and preventing the development of rheumatoid arthritis. Rheumatology 2016;55:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Steenbergen HW, da Silva JAP, Huizinga TWJ, van der Helm-van Mil AHM.. Preventing progression from arthralgia to arthritis: targeting the right patients. Nat Rev Rheumatol 2018;14:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stanway JA, Isaacs JD.. Tolerance-inducing medicines in autoimmunity: rheumatology and beyond. Lancet Rheumatol 2020;2:E565–75. [DOI] [PubMed] [Google Scholar]
- 6. Gerlag DM, Safy M, Maijer KI. et al. Effects of B-cell directed therapy on the preclinical stage of rheumatoid arthritis: the PRAIRI study. Ann Rheum Dis 2019;78:179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Al-Laith M, Jasenecova M, Abraham S. et al. Arthritis prevention in the pre-clinical phase of RA with abatacept (the APIPPRA study): a multi-centre, randomised, double-blind, parallel-group, placebo-controlled clinical trial protocol. Trials 2019;20:429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Institute of Allergy and Infectious Diseases. Strategy to Prevent the Onset of Clinically-Apparent Rheumatoid Arthritis (StopRA). https://clinicaltrials.gov/ct2/show/NCT02603146 (21 July 2022, date last accessed).
- 9. Isaacs JD, Iqbal K.. Potential pharmacologic targets for the prevention of rheumatoid arthritis. Clin Ther 2019;41:1312–22. [DOI] [PubMed] [Google Scholar]
- 10. van Boheemen L, Ter Wee MM, Seppen B, van Schaardenburg D.. How to enhance recruitment of individuals at risk of rheumatoid arthritis into trials aimed at prevention: understanding the barriers and facilitators. RMD Open 2021;7:e001592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Boheemen L, Turk S, Beers-Tas M. et al. Atorvastatin is unlikely to prevent rheumatoid arthritis in high risk individuals: results from the prematurely stopped STAtins to Prevent Rheumatoid Arthritis (STAPRA) trial. RMD Open 2021;7:e001591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mankia K, Siddle HJ, Kerschbaumer A. et al. EULAR points to consider for conducting clinical trials and observational studies in individuals at risk of rheumatoid arthritis. Ann Rheum Dis 2021;80:1286–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Bekker-Grob E, Berlin C, Levitan B. et al. Giving patients’ preferences a voice in medical treatment life cycle: the PREFER public–private project. Patient 2017;10:263–6. [DOI] [PubMed] [Google Scholar]
- 14. Ho MP, Gonzalez JM, Lerner HP. et al. Incorporating patient-preference evidence into regulatory decision making. Surg Endosc 2015;29:2984–93. [DOI] [PubMed] [Google Scholar]
- 15. Marsh K, van Til JA, Molsen-David E. et al. Health preference research in Europe: a review of its use in marketing authorization, reimbursement, and pricing decisions-report of the ISPOR stated preference research special interest group. Value Health 2020;23:831–41. [DOI] [PubMed] [Google Scholar]
- 16. Durand C, Eldoma M, Marshall DA, Bansback N, Hazlewood GS.. Patient preferences for disease-modifying antirheumatic drug treatment in rheumatoid arthritis: a systematic review. J Rheumatol 2020;47:176–87. [DOI] [PubMed] [Google Scholar]
- 17. Bywall KS, Kihlbom U, Hansson M. et al. Patient preferences on rheumatoid arthritis second-line treatment: a discrete choice experiment of Swedish patients. Arthritis Res Ther 2020;22:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simons G, Caplan J, DiSantostefano R. et al. Systematic review of quantitative preference studies of treatments for rheumatoid arthritis among patients and at risk populations. Arthritis Res Ther 2022;24:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Falahee M, Raza K.. Rheumatoid arthritis prevention: any takers? RMD Open 2021;7:e001633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Siddle HJ, Chapman LS, Mankia K. et al. Perceptions and experiences of individuals at-risk of rheumatoid arthritis (RA) knowing about their risk of developing RA and being offered preventive treatment: systematic review and thematic synthesis of qualitative studies. Ann Rheum Dis 2022;81:159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Boheemen L, Bolt JW, Ter Wee MM. et al. Patients’ and rheumatologists’ perceptions on preventive intervention in rheumatoid arthritis and axial spondyloarthritis. Arthritis Res Ther 2020;22:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Finckh A, Escher M, Liang MH, Bansback N.. Preventive treatments for rheumatoid arthritis: issues regarding patient preferences. Curr Rheumatol Rep 2016;18:51. [DOI] [PubMed] [Google Scholar]
- 23. Harrison M, Bansback N, Aguiar M. et al. Preferences for treatments to prevent rheumatoid arthritis in Canada and the influence of shared decision-making. Clin Rheumatol 2020;39:2931–41. [DOI] [PubMed] [Google Scholar]
- 24. Harrison M, Spooner L, Bansback N. et al. Preventing rheumatoid arthritis: preferences for and predicted uptake of preventive treatments among high risk individuals. PLoS One 2019;14:e0216075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Falahee M, Simons G, DiSantostefano RL. et al. Treatment preferences for preventive interventions for rheumatoid arthritis: protocol of a mixed methods case study for the Innovative Medicines Initiative PREFER project. BMJ Open 2021;11:e045851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wells I, Zemedikun DT, Simons G. et al. Predictors of interest in predictive testing for rheumatoid arthritis amongst first degree relatives of rheumatoid arthritis patients. Rheumatology 2022;61:3223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. International Organization for Standardization. ISO 17100:2015 Translation Services – Requirements for Translation Services. Technical Committee ISO/TC37. https://www.iso.org/standard/59149.html (21 July 2022, date last accessed).
- 28. Bridges JFP, Hauber AB, Marshall D. et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health 2011;14:403–13. [DOI] [PubMed] [Google Scholar]
- 29. Reed Johnson F, Lancsar E, Marshall D. et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis experimental design good research practices task force. Value Health 2013;16:3–13. [DOI] [PubMed] [Google Scholar]
- 30. de Bekker-Grob E, Ryan M, Gerard K.. Discrete choice experiments in health economics: a review of the literature. Health Econ 2012;21:145–72. [DOI] [PubMed] [Google Scholar]
- 31. Soekhai V, de Bekker-Grob E, Ellis A, Vass C.. Discrete choice experiments in health economics: past, present and future. Pharmacoeconomics 2019;37:201–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ChoiceMetrics. Ngene 1.2. User Manual & Reference Guide. Sydney, NSW, Australia: ChoiceMetrics, 2012. http://www.choice-metrics.com/NgeneManual120.pdf.
- 33. Morris NS, MacLean CD, Chew LD, Littenberg B.. The Single Item Literacy Screener: evaluation of a brief instrument to identify limited reading ability. BMC Fam Pract 2006;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McNaughton CD, Cavanaugh KL, Kripalani S, Rothman RL, Wallston KA.. Validation of a short, 3-item version of the subjective numeracy scale. Med Decis Making 2015;35:932–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Euro-Team. Towards early diagnosis and biomarker validation in arthritis management. Final report. FP7-HEALTH project ID 305549. https://cordis.europa.eu/project/id/305549/reporting (27 July 2022, date last accessed).
- 36. Bech M, Gyrd-Hansen D.. Effects coding in discrete choice experiments. Health Econ 2005;14:1079–83. [DOI] [PubMed] [Google Scholar]
- 37. Hensher DA, Rose JM, Greene WH.. Applied choice analysis: a primer. New York: Cambridge University Press, 2005. [Google Scholar]
- 38. Falahee M, Simons G, Buckley CD. et al. Patients’ perceptions of their relatives’ risk of developing rheumatoid arthritis and of the potential for risk communication, prediction, and modulation. Arthritis Care Res (Hoboken) 2017;69:1558–65. [DOI] [PubMed] [Google Scholar]
- 39. Simons G, Stack RJ, Stoffer-Marx M. et al. Perceptions of first-degree relatives of patients with rheumatoid arthritis about lifestyle modifications and pharmacological interventions to reduce the risk of rheumatoid arthritis development: a qualitative interview study. BMC Rheumatol 2018;2:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mosor E, Stoffer-Marx M, Steiner G. et al. I would never take preventive medication! Perspectives and information needs of people who underwent predictive tests for rheumatoid arthritis. Arthritis Care Res (Hoboken) 2020;72:360–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Munro S, Spooner L, Milbers K. et al. Perspectives of patients, first-degree relatives and rheumatologists on preventive treatments for rheumatoid arthritis: a qualitative analysis. BMC Rheumatol 2018;2:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kallberg H, Ding B, Padyukov L. et al. Smoking is a major preventable risk factor for rheumatoid arthritis: estimations of risks after various exposures to cigarette smoke. Ann Rheum Dis 2011;70:508–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zaccardelli A, Friedlander HM, Ford JA, Sparks JA.. Potential of lifestyle changes for reducing the risk of developing rheumatoid arthritis: is an ounce of prevention worth a pound of cure? Clin Ther 2019;41:1323–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Walrabenstein W, van der Leeden M, Weijs P. et al. The effect of a multidisciplinary lifestyle program for patients with rheumatoid arthritis, an increased risk for rheumatoid arthritis or with metabolic syndrome-associated osteoarthritis: the “Plants for Joints” randomized controlled trial protocol. Trials 2021;22:715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Coast J, Horrocks S.. Developing attributes and levels for discrete choice experiments using qualitative methods. J Health Serv Res Policy 2007;12:25–30. [DOI] [PubMed] [Google Scholar]
- 46. Simons G, Mason A, Falahee M. et al. Qualitative exploration of illness perceptions of rheumatoid arthritis in the general public. Musculoskelet Care 2017;15:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Simons G, Belcher J, Morton C. et al. Symptom recognition and perceived urgency of help-seeking for rheumatoid arthritis and other diseases in the general public: a mixed method approach. Arthritis Care Res 2017;69:633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Birch R, Simons G, Wahamaa H. et al. Development and formative evaluation of patient research partner involvement in a multi-disciplinary European translational research project. Res Involv Engagem 2020;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.