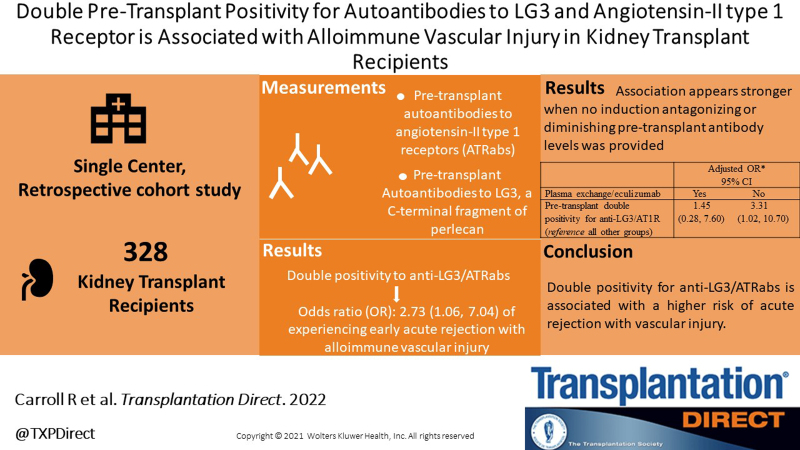
Background.
Both angiotensin II receptor autoantibodies (ATRabs) and autoantibodies to LG3 have been linked to kidney graft rejection with alloimmune vascular injury (AVI). We aimed to examine whether positivity for both anti-LG3 and ATRabs is associated with rejection with AVI in kidney transplant recipients.
Methods.
We performed a retrospective cohort study including consecutive kidney transplant recipients between 2013 and 2017 at a single center. The primary outcome was acute rejection with AVI (Banff grade 2 or 3 T-cell-mediated rejection and/or antibody-mediated rejection) in the first 3 mo posttransplant. The secondary outcome was death-censored allograft loss. The independent variables, anti-LG3 and ATRab, were measured pretransplant.
Results.
Among the 328 study participants, 68 experienced acute rejection with AVI and 23 experienced graft loss over a median follow-up of 4.5 y. In a multivariable model, double pretransplant positivity for anti-LG3/ATRab was associated with acute rejection with AVI (odds ratio: 2.73, 95% confidence interval: 1.06-7.05). We did not observe an association between double positivity for anti-LG3/ATRab and death-censored graft loss.
Conclusions.
Double positivity for anti-LG3/ATRabs pretransplant is associated with a higher risk of acute rejection with AVI. Whether therapies that remove antibodies could decrease that risk remains to be studied.
Supplemental Visual Abtract: http://links.lww.com/TXD/A494.
Kidney allograft rejection with vascular involvement, especially when antibody-mediated, is associated with poor response to current antirejection treatment and shorter graft survival.1 Although donor-specific antibodies (DSAs) are associated with microvascular damage in kidney transplant recipients, a role for non-HLA antibodies and autoantibodies in mediating allograft vascular injury is gaining increasing attention.2,3 Antiangiotensin 2 type 1 receptor autoantibodies (ATRabs) have been linked to severe endarteritis in kidney transplant recipients and produced a similar phenotype when passively transferred in an animal model of kidney transplantation.2 Anti-LG3 autoantibodies have been linked to rejection with endarteritis in kidney transplant recipients and enhanced vascular injury and inflammation when passively transferred in a murine model of major histocompatibility complex-mismatched aortic transplantation.3
Both ATRab and anti-LG3 have been linked to poorer graft survival in liver repeat transplants, wherein a synergistic adverse impact on graft survival was also noted in patients who were both anti-LG3 and ATRab positive pretransplant.4,5 A synergistic interaction between DSA and ATRab has also been reported, where the presence of both DSA and ATRab was associated with an increased risk rejection and poorer kidney graft survival.6 Furthermore, our group has previously shown that in patients with rejection affecting the allograft vasculature, the presence of both DSA and anti-LG3 was associated with poorer graft survival.3 Here, we ask whether the presence of ATRab and anti-LG3 is associated with an increased risk of rejection with alloimmune vascular injury (AVI) and whether this translates into poorer graft survival.
MATERIALS AND METHODS
Patients
We performed a retrospective cohort study including all consecutive adult patients who received a kidney transplantation at the Royal Adelaide transplant center, Australia, between January 1, 2013, and December 31, 2017. Recipients of combined organ transplants were excluded, as well as those with no biological material to measure anti-LG3 levels. Recipients were followed until death, graft loss or May 29, 2020, whichever occurred first. Patients who died or who had early nonimmunological loss within the first 3 mo posttransplant were excluded. The standard maintenance immunosuppression protocol includes tacrolimus, mycophenolate mofetil and corticosteroids in decreasing doses, continuing with prednisone 5–7.5 mg/d over the long-term. Basiliximab is used as standard induction for low immunological risk patients, whereas those at high immunological risk because of clinically significant pretransplant DSA (usually approaching 4000 MFI or multiple low-level DSA) or ABO-incompatibility (>1:16 titer) have received plasma exchange and thymoglobulin, or as part of a trial, thymoglobulin and eculizumab. Patients with weak pretransplant DSA (1000 to <4000 MFI) or low anti-ABO titer (<1:16) received standard induction. Starting in November 2014, all patients with ATRab >25 U/mL were treated with thymoglobulin 3 mg/kg and 1 plasma volume plasma exchange preoperative and 2 postoperative, with a mix of fresh–frozen plasma and albumin. Recipients of living donors with ATRab > 25 U/mL were given 3 plasma exchange sessions before transplant. Patients with ATRab 17–25 U/mL were treated with thymoglobulin but not plasma exchange. All patients with >17 U/mL were also treated with 4–16 mg of candesartan peri- and postoperatively as tolerated.7 The project was approved by the Royal Adelaide ethics review board committee (project number HREC/13/RAH/494).
Measurements
The primary outcome was the occurrence of acute rejection with AVI, defined as Banff grade 2 or 3 cell-mediated rejection and/or antibody-mediated rejection, occurring in the first 3 mo posttransplant. We also included cases of microvascular inflammation (ie, histology suggestive of antibody-mediated rejection but not meeting the 3 criteria) as suspected antibody-mediated rejections. Rejections were reclassified according to the Banff 2019 classifications8 as Banff lesions were available to reclassify rejections that had been scored according to previous Banff versions. The secondary outcome was death-censored graft survival, defined as time between transplantation and return to dialysis or retransplantation, whichever occurred first. Patients who died with graft function were censored at the time of death without having had an event.
The independent variables were anti-LG3 and ATRabs, measured once on pretransplant sera. Anti-LG3 were measured with a locally developed ELISA as described previously.3 Anti-LG3 levels were classified as elevated when above the median.3 ATRabs were measured pretransplant using an ELISA Immunoassay kit (One Lambda, Canoga Park, CA) following published protocol9 and performed by the Australian Red Cross Blood Service (ARCBS) in Adelaide. We used a cutoff of 17 for ATRab positivity, as recommended by the manufacturer. We first classified patients in 4 groups according to the presence of both autoantibodies (group 1: positivity for both anti-LG3 and ATRab, group 2: positivity for anti-LG3 but not ATRab, group 3: positivity for ATRab but not anti-LG3, group 4: both anti-LG3 and ATRab negative). Clinical data on donor (age, type [living, neurologically deceased, donation after cardiocirculatory arrest], HLA type), recipient (age, sex, race, first transplant, chronic kidney disease), HLA type, immunological risk (ABO- and/or DSA-incompatible, pretransplant and peak panel reactive antibodies), induction and maintenance immunosuppression, delayed graft function, and procedure (date/era, cold ischemic time) characteristics were collected through hospital chart review.
Statistical Analyses
Continuous variables are reported as means and standard deviations or medians and interquartile ranges, depending on their distribution. Categorical variables are summarized as proportions. We analyzed between-group crude differences in nonnormally distributed continuous variables with Wilcoxon rank-sum (or Kruskal–Wallis) tests or with ANOVAs/T tests when normally distributed. We performed chi-square (or Fisher’s exact) tests to analyze the between-group differences in categorical variables. We evaluated correlations between continuous variables using Spearman correlation coefficients. For the first primary outcome, we fit a logistic regression model wherein the dependent variable was the occurrence of acute rejection with AVI, and the main exposure was positivity to pretransplant anti-LG3 and to ATRab (versus positivity to anti-LG3 alone, to ATRab alone or to neither). We then explored whether the association between pretransplant positivity for anti-LG3/ATRab was different in patients who received or did not receive induction therapies that had an impact on the level (plasma exchange) or the action (eculizumab) of autoantibodies by presenting subgroup analyses. We also performed sensitivity analyses excluding (1) 100 ABO-incompatible, DSA-incompatible transplants with any high- or low-level DSA pretransplant and (2) 6 suspected cases of antibody-mediated rejection. We assessed the association between death-censored graft loss and pretransplant positivity for anti-LG3/ATRab by fitting a Cox regression model. To adjust for confounding, in all models, we examined the association between all covariates listed above and (1) the exposure (pretransplant high anti-LG3 antibodies and high ATRab) and (2) the outcome for each aim (acute rejection with AVI, death-censored graft survival). To account for confounding in the multivariable model for the primary outcome, we included all the covariates that were associated with either the exposure or the outcome with a P value ≤0.15, except for delayed graft function, as it could be a manifestation of early vascular rejection or be in the pathophysiological pathway between autoantibodies and rejection. Two patients had missing value for cold ischemic time and were imputed the mean value. We did not need to simplify the full multivariable model for the main outcome as there were few covariates that met the selection criteria. For the secondary outcome, due to the limited number of events (n = 24), we only included anti-LG3/ATRab, the main independent variable of interest, and use of plasma exchange/eculizumab, since it was the only other variable that was associated with death-censored graft loss with a P value <0.05 in univariable analyses. We did not include early rejection with vascular injury in the multivariable model for graft loss as it would be in the pathophysiological pathway between pretransplant autoantibodies and graft loss. The analyses were performed with SAS version 9.4 (SAS institute, Cary, NC).
RESULTS
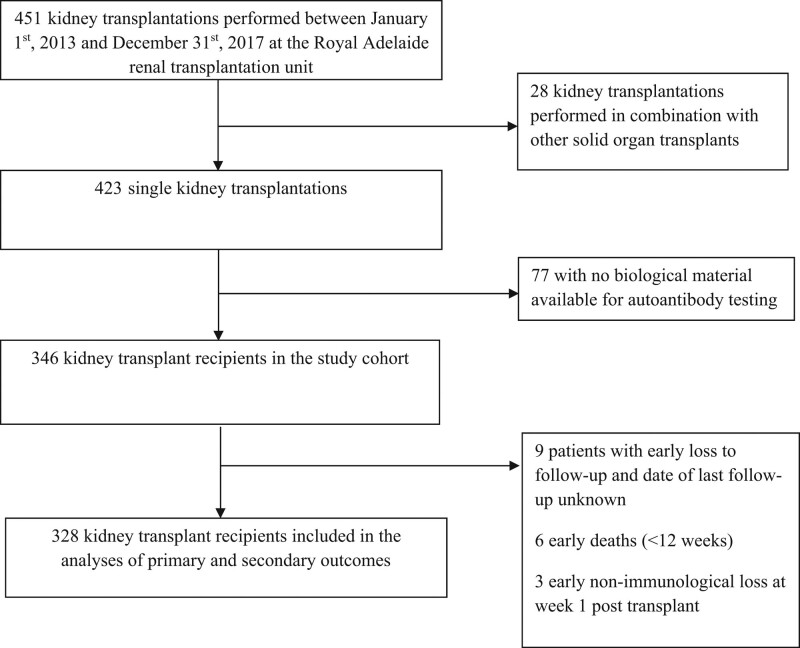
Among the 451 patients who received a kidney transplantation at the Royal Adelaide transplant center, 346 patients had available biological material, 328 of whom were included in the analyses (Figure 1). Recipient, donor, and procedure-related characteristics for study participants are provided in Table 1 categorized by pretransplant autoantibody status. Patients with double positivity for anti-LG3/ATRab were less likely to be transplanted in the 2015–2017 study period and had shorther cold ischemic time. Those with double positivity for anti-LG3/ATRab and single positivity for ATRab were more likely to receive induction that could lower the level of autoantibodies (plasma exchange with or without IvIg) or impede their action (eculizumab) than other groups. The vast majority of patients (n = 323, 99%) were on tacrolimus and mycophenolate mofetil, whereas 2 patients were on sirolimus and mycophenolate mofetil, 2 patients were on tacrolimus and sirolimus, 1 received tacrolimus without an adjuvant, and 1 was on cyclosporine and mycophenolate mofetil. Anti-LG3 was positively associated with pretransplant ATRab (ρ = 0.14, P = 0.01).
FIGURE 1.
Flow chart of patients.
TABLE 1.
Recipient, donor, and procedure characteristics, n = 328 stratified according to pretransplant anti-LG3 and AT1R positivity
| Recipient/donor/procedure characteristics | Anti-LG3 + AT1R +, n = 23 | Anti-LG3 + AT1R –, n = 140 | Anti-LG3 – AT1R +, n = 25 | Anti-LG3 – AT1R –, n = 140 |
|---|---|---|---|---|
| Mean age at transplant, y (SD) | 52 (12) | 50 (13) | 47 (14) | 52 (13) |
| Male sex, n (%) | 17 (74) | 99 (71) | 17 (68) | 100 (71) |
| Cause of CKD, n (%) | ||||
| Glomerular diseases | 6 (26) | 41 (29) | 5 (20) | 39 (28) |
| Diabetes | 6 (26) | 21 (15) | 3 (12) | 19 (13) |
| Polycystic kidney disease | 5 (22) | 20 (14) | 6 (24) | 23 (16) |
| Hypertension/vascular | 0 (0) | 12 (9) | 1 (4) | 13 (9) |
| Autoimmune | 3 (13) | 5 (4) | 6 (24) | 7 (5) |
| Other or unknown | 3 (13) | 41 (29) | 4 (16) | 39 (28) |
| Median pretransplant panel reactive antibodies (IQR) | 0 (0–3) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Transplant date 2015–2017,a n (%) | 8 (35) | 72 (51) | 19 (76) | 87 (62) |
| Induction by immunological risk, n (%) | ||||
| ABO-compatible and no pretransplant donor-specific antibody | 16 (70) | 102 (74) | 15 (60) | 108 (77) |
| Plasma exchange (with or without IvIg) and thymoglobulin | 2 (13) | 0 (0) | 4 (27) | 1 (1) |
| Thymoglobulin alone | 5 (31) | 7 (7) | 3 (12) | 6 (6) |
| Plasma exchange (with or without IvIg) and basiliximab | 1 (6) | 2 (2) | 1 (4) | 0 (0) |
| Basiliximab alone | 8 (50) | 93 (91) | 7 (28) | 100 (93) |
| ABO-incompatible or positive pretransplant DSA | 7 (30) | 38 (27) | 10 (40) | 32 (23) |
| Plasma exchange (with or without IvIg) and thymoglobulin | 4 (57) | 4 (11) | 7 (70) | 2 (6) |
| Plasma exchange (with or without IvIg) and rituximab | 0 (0) | 3 (8) | 0 (0) | 1 (3) |
| Thymoglobulin and eculizumab | 1 (14) | 5 (13) | 0 (0) | 3 (9) |
| Thymoglobulin alone | 0 (0) | 6 (16) | 2 (20) | 11 (34) |
| Basiliximab alone | 2 (29) | 20 (53) | 1 (10) | 15 (47) |
| Induction with either plasma exchange and/or eculizumaba | 8 (35) | 14 (10) | 12 (48) | 7 (5) |
| No. HLA mismatches, n (%) | ||||
| 0–2 | 4 (17) | 20 (14) | 5 (26) | 15 (11) |
| 3–4 | 8 (35) | 46 (33) | 9 (36) | 49 (35) |
| 5–6 | 11 (48) | 74 (53) | 11 (44) | 76 (55) |
| Mean cold ischemic time, ha (SD) | 8 (6) | 13 (6) | 11 (4) | 12 (7) |
| Mean donor age, y (SD) | 49 (16) | 45 (17) | 47 (15) | 46 (16) |
| First transplantation, n (%) | 21 (91) | 119 (85) | 22 (88) | 122 (88) |
| Donor type, n (%) | ||||
| Living donor | 5 (22) | 16 (11) | 3 (12) | 13 (9) |
| Neurologically deceased | 18 (78) | 104 (74) | 18 (72) | 105 (75) |
| Donor after cardiocirculatory arrest | 0 (0) | 20 (15) | 4 (16) | 22 (16) |
| Delayed or slow graft function, n (%) | 17 (74) | 97 (69) | 20 (80) | 102 (73) |
aP value <0.05 for a difference between the groups.
CKD, chronic kidney disease; DSA, donor-specific antibody; IQR, interquartile range.
We observed 68 episodes of acute graft rejection involving the allograft vasculature (this could therefore include V1,2,3 lesions associated with either T-cell-mediated rejection (i and t scores >1), definite and suspected cases of antibody-mediated rejection, or both. The Banff classification of all acute rejection episodes (with and without vascular injury) can be found in Table 2, including the description of the 6 suspected cases. Univariable analyses for the associations between early acute rejection with vascular injury and all independent variables are reported in Table S1, SDC, http://links.lww.com/TXD/A493. As we observed that single positivity for either anti-LG3 or ATRab was not associated with early acute rejection with vascular injury (Table S1, SDC, http://links.lww.com/TXD/A493), we performed subsequent analyses categorizing patients into 2 groups: those who were positive for both anti-LG3/ATRab pretransplant versus all other patients. We found that when compared with all other patients, those who showed double pretransplant positivity for anti-LG3/ATRab were more likely to experience acute rejection with vascular injury (odds ratio [OR]: 2.73, 95% confidence interval [CI] 1.06-7.05) in a multivariable analysis that adjusted for donor age, donor type, cold ischemic time, transplant period, and induction with plasma exchange/eculizumab (Table 3).
TABLE 2.
Characteristics of early (within 90 d) rejection episodes
| Characteristics of rejection episodes | |
|---|---|
| Rejection episodes | |
| With vascular involvement, n (%) | 68 (21) |
| T-cell-mediated Banff grade 2A, n (%) | 28 (41) |
| Pretransplant or de novo donor-specific antibody, n (%) | 5 (17) |
| Positive C4d staining in peritubular capillaries, n (%) | 0 (0) |
| T-cell-mediated Banff grade 2B, n (%) | 14 (21) |
| Pretransplant or de novo donor-specific antibody, n (%) | 1 (7) |
| Positive C4d staining in peritubular capillaries, n (%) | 0 (0) |
| T-cell-mediated Banff grade 3, n (%) | 3 (4) |
| Pretransplant or de novo donor-specific antibody, n (%) | 0 (0) |
| Positive C4d staining in peritubular capillaries, n (%) | 0 (0) |
| Antibody-mediated rejection, n (%) | 15 (22) |
| Pretransplant or de novo donor-specific antibody, n (%)a | 12 (80) |
| Positive C4d staining in peritubular capillaries, n (%) | 6 (40) |
| Suspected antibody-mediated rejection, n (%)a | 6 (9) |
| Without vascular involvement, n (%) | 44 (13) |
| T-cell-mediated borderline changes, n (%)a | 33 (75) |
| T-cell-mediated Banff grade 1A, n (%) | 5 (11) |
| T-cell-mediated Banff grade 1B, n (%) | 6 (14) |
aThree antibody-mediated rejection cases were ABO-incompatible and donor-specific antibody negative; the 6 suspected antibody-mediated rejection episodes showed significant microvascular inflammation without meeting the 3 criteria for antibody-mediated rejection (g+ptc ≥ 2 with g ≥ 1 in the absence of positive C4d staining and donor-specific antibodies, n = 4; ptc3 in the presence of donor-specific antibody and C4d negative, n = 1; ptc 3 in the absence of C4d staining and donor-specific antibody, n = 1), 1 borderline rejection had positive C4d staining.
TABLE 3.
Association between double positivity for AT1R and anti-LG3 and early acute rejection with vascular injurya
| Recipient/donor/procedure characteristics | Odds ratio (95% confidence interval) | P |
|---|---|---|
| Pretransplant double positivity for AT1R and anti-LG3 (reference all other groups) |
2.73 (1.06, 7.05) | 0.04 |
| Transplant date 2015–2017 (vs 2013–2014) | 0.72 (0.40, 1.27) | 0.26 |
| Cold ischemic time (per 1-h higher) | 1.05 (1.00, 1.04) | 0.06 |
| Use of plasma exchange with or without intravenous immunoglobulins and/or eculizumab (vs none) | 1.52 (0.70, 3.30) | 0.28 |
| Donor type (reference living donor) | ||
| Neurologically deceased | 0.55 (0.22, 1.37) | 0.20 |
| Donor after cardiac arrest | 0.83 (0.27, 2.57) | 0.74 |
| Donor age (per 1-y higher) | 1.02 (1.00-1.04) | 0.04 |
aThe multivariable model comprises all and only the variables listed in this table based on their association with the exposure (Table 1) and the outcome (Table S1, SDC, http://links.lww.com/TXD/A493).
We then performed exploratory subgroup analyses to verify whether the association between double positivity for anti-LG3/ATRab and early rejection with vascular injury was modified by induction that could either lower the level (plasma exchange) of autoantibodies or impede their action (eculizumab). In patients who had received these therapies, we found no association between double positivity for anti-LG3/ ATRab and early acute rejection with vascular injury (OR: 1.45, 95% CI 0.28-7.60), whereas this association was strong in patients who were not treated with plasma exchange/eculizumab (OR: 3.38, 95% CI 1.04=10.94) (Table 4). In the subgroup of recipients who had pretransplant DSA or ABO antibodies but who did not receive antibody-lowering therapies because the pretransplant antibodies were not deemed significant (n = 57), we observed a positive, albeit not significant, association between double positivity for anti-LG3/ATRab and early rejection with vascular injury (OR: 5.11, 95% CI 0.29-89.46). We did not perform multivariable analyses given the small number of patients with rejection with vascular injury (n = 10) in this subgroup. Last, our sensitivity analyses (Table S2, SDC, http://links.lww.com/TXD/A493) show that the association between double positivity for anti-LG3/ATRab and acute rejection involving the graft vasculature was maintained when ABO- or DSA-incompatible transplants were excluded, as well as when suspected but unconfirmed cases of antibody-mediated rejection were excluded.
TABLE 4.
Stratified analyses by plasma exchange/eculizumab for the association between double positivity for anti-LG3/AT1R and early acute rejection with vascular injury
| Unadjusted OR (95% CI) | Adjusted ORa (95% CI) | |||
|---|---|---|---|---|
| Plasma exchange/eculizumab | Yes | No | Yes | No |
| Pretransplant double positivity for anti-LG3/AT1R(reference all other groups) | 1.60 (0.32, 8.11) | 2.96 (1.01,8.70) | 1.45 (0.28, 7.60) | 3.31 (1.02, 10.70) |
aAdjusted model also included transplant period, cold ischemic time, donor age, donor type (neurologically deceased, deceased after cardiocirculatory arrest vs living). As there were only 12 acute rejection episodes with vascular injury in patients who received plasma exchange/eculizumab, the multivariate model may be overfit.
CI, confidence interval; OR, odds ratio.
Last, we examined whether double positivity for anti-LG3/ATRab pretransplant was associated with death-censored graft loss. Over a median follow-up of 4.5 y, 24 patients experienced death-censored graft loss. We found no association between double positivity for anti-LG3/ATRab and death-censored graft survival (hazard ratio: 0.71, 95% CI 0.16-3.09) (Table 5). The only factor that showed an association with death-censored graft survival was use of pretransplant plasma exchange (with or without IvIg) and/or eculizumab, a marker of high immunological risk transplants. The results of univariable analyses are found in Table S3, SDC, http://links.lww.com/TXD/A493. Although it was associated with graft loss in univariable analyses (hazard ratio: 2.95, 95% CI 1.22-7.13), we did not include early rejection with vascular injury in the multivariable model for graft loss as it would be in the pathophysiological pathway between pretransplant autoantibodies and graft loss.
TABLE 5.
Association between pretransplant double positivity for AT1R and anti-LG3 and death-censored graft loss in the multivariable model
| Recipient/donor/procedure characteristics | Univariable model HR (95% CI) | P | Multivariable model HR (95% CI) | P |
|---|---|---|---|---|
| Pretransplant double positivity for AT1R and anti-LG3 (reference all other groups) | 0.95 (0.22, 4.07) | 0.95 | 0.71 (0.16, 3.09) | 0.64 |
| Use of plasma exchange (with or without intravenous immunoglobulins) or eculizumab (vs none) | 2.95 (1.22, 7.13) | 0.02 | 3.09 (1.26, 7.57) | 0.01 |
CI, confidence interval; HR, hazard ratio.
DISCUSSION
Although anti-HLA DSA are associated with both macro- and microvascular injury in antibody-mediated rejection of solid organ transplantation,8,10 a large body of literature has also shown associations between autoantibodies and acute rejection with vascular injury in the past decade.2-5,11-14 Here, we have found that pretransplant positivity for both anti-LG3/ATRabs was associated with early kidney graft rejection with vascular injury. Furthermore, this association was weaker and not significant in the subgroup of patients who had received therapies that could either lower the levels of antibodies or impede their action in the perioperative period. Collectively, these data suggest that both autoantibodies could act synergistically to injure the allograft vasculature in the context of kidney transplantation, and that their removal or antagonism may reduce the risk of acute rejection with vascular injury.
The main outcome assessed in this study is AVI, whether macro- or microvascular. This is the motivation for our choice of pooling T-cell-mediated rejections with v lesions and rejections with g or ptc lesions, in the presence or absence of DSA, as an outcome definition. The reasons underlying this choice are that both macrovascular v lesions and microvascular (g/ptc) lesions tend to cluster together8,15 and can be associated with both allo- and autoantibodies.1-3,8 Furthermore, vascular injury, whether micro- or macrovascular, is associated with worse graft survival, especially when antibody-mediated.1,16,17
In the present work, we did not observe a higher risk of acute rejection with vascular injury in patients who were only anti-LG3-positive pretransplant. This contrasts with our earlier data in a different patient cohort, wherein such an association was found.3 There are several possible explanations for these discrepant findings. First, we had not measured pretransplant ATRabs in our earlier work, and hence, it is not possible for us to determine whether a proportion of anti-LG3 positive patients also had ATRabs and would have been double positive for anti-LG3/ATRabs. In line with this possibility, another group recently reported that 4 of 11 hypersensitized patients with no pretransplant DSA and who developed antibody-mediated rejection had anti-LG3 antibodies, with 2 also having ATRabs.14 Second, our previous study included patients transplanted in earlier eras (1985–2008), when maintenance immunosuppressive agents had weaker potency than the regimens that are currently used. The deleterious impact of pretransplant anti-LG3 may be weaker in the presence of stronger immunosuppression such as that given in the current study. In the current study, positivity to ATRabs alone was not associated with early rejection with vascular injury, which contrasts with observations from other reports.2,13 This is most likely due to the unique pretransplant protocol implemented at the Royal Adelaide Hospital since 2015, where pretransplant ATRabs are measured and where plasma exchange is performed pretransplant and recipients treated with candesartan when ATRabs are found positive. Indeed, this protocol has similar medium-term graft survival stratified above or below ATRab 17 U/mL.18 In this context, one can speculate that the association between ATRab positivity and acute rejection with vascular injury would be weakened, and that the presence of both antibodies could be needed to mediate graft rejection with AVI.
Although we are the first to report on a synergy between anti-LG3 and ATRabs in augmenting the risk of rejection with AVI, the presence of such synergy has been reported between DSAs and ATRabs in kidney transplant recipients,6,19,20 and another group has also reported a synergistic adverse impact of double positivity to anti-LG3 and ATRabs on liver graft survival.4 Furthermore, as the possibility of ATRabs being a risk factor for the development of DSAs been raised,20 we also observed a correlation between ATRabs and anti-LG3. This could be due the propensity of transplant candidates to develop autoantibodies and/or to the presence of one of these autoantibodies with vascular tropism favoring the development of the other. We could not demonstrate that double positivity for anti-LG3/ATRabs was associated with lower death-censored graft survival, but the wide CIs due to the small number of graft losses (n = 24) preclude any definite conclusion. Early rejection with vascular injury, however, was associated with graft loss in univariable analysis. Hence, larger studies would be needed to examine the association between anti-LG3/ATRabs and graft loss.
In our earlier work on the association between anti-LG3 and acute rejection with vascular injury, none of the recipients received high-risk transplants.3 In contrast, the current study includes 27% high-immunological-risk ABO- or DSA-incompatible transplants. These patients were induced with plasma exchange or a combination of thymoglobulin and eculizumab when the pretransplant DSA was deemed significant, but could also be induced with basiliximab or thymoglobulin alone when low median fluorescence intensity antibodies were found. Our exploratory subgroup analyses suggest that the strength of association between double positivity for anti-LG3/ATRabs and acute rejection with vascular injury is diminished when therapies that can lower or modulate the level of pretransplant antibodies are used. The strength of the association between double positivity for anti-LG3/ATRabs and acute rejection with vascular injury was within the same order of magnitude in patients with weak pretransplant DSAs or ABO antibodies who did not receive plasma exchange/eculizumab than in patients who had no pretransplant DSAs or ABO antibodies. This suggests that induction therapies received by patients with the highest immunological risk diminishes the strength of association between anti-LG3/ATRabs and acute rejection with AVI. Our subgroup analysis in low-immunological-risk patients by DSA/ABO criteria shows a strong association between double positivity to anti-LG3/ATRabs and acute rejection with AVI. Measuring these autoantibodies may help uncover an immunological risk that would not be detected otherwise.
Our study is limited by the relatively small number of events, which precludes a definite conclusion on the impact of the autoantibodies of interest on graft survival and limits formal interaction tests to address effect modification by plasma exchange/eculizumab. Our study only addresses the association between pretransplant antibodies and early clinical outcomes, whereas de novo autoantibodies could also have an impact on allograft outcomes. Last, this is a single-center study in which a unique protocol is used when ATRabs are found positive pretransplant, which may limit the generalizability of our findings.
In conclusion, our results suggest that double positivity for anti-LG3/ATRabs before transplantation is associated with a higher risk of acute rejection with AVI, and that this risk may be lower when therapies that lower/modulate antibodies are used in the perioperative period. Further studies will be needed to examine the clinical relevance of screening for anti-LG3 and ATRabs before transplantation, in combination with strategies such as plasma exchange when levels are found elevated.
Supplementary Material
Footnotes
This work was funded by the Kidney Foundation of Canada (H.C.) and by the Canadian Institutes of Health Research (PJT-14884 and PJT-155985) to M.-J.H. and H.C. H.C. is a Fonds de recherche du Québec (Santé) scholar. M.-J.H. and H.C. hold jointly the Shire Chair in Nephrology, Transplantation and Renal Regeneration. M.-J.H. thanks Fondation J.-Louis Lévesque for renewed support.
The authors declare no conflicts of interest.
All authors agree with the content of the manuscript and have made substantial contributions to the work; R.C., M.-J.H., and H.C. participated in research design, and S.D., T.E., F.B., S.J., and A.K.R. participated in the performance of the research. B.G. and A.T.-B. participated in the clinical data collection. R.C., J.T., M.-J.H., and H.C. participated in data analysis and in the writing of the paper. All authors provided critical feedback and helped shape the research, analysis, and manuscript.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Lefaucheur C, Loupy A, Vernerey D, et al. Antibody-mediated vascular rejection of kidney allografts: a population-based study. Lancet. 2013;381:313–319. [DOI] [PubMed] [Google Scholar]
- 2.Dragun D, Muller DN, Brasen JH, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352:558–569. [DOI] [PubMed] [Google Scholar]
- 3.Cardinal H, Dieude M, Brassard N, et al. Antiperlecan antibodies are novel accelerators of immune-mediated vascular injury. Am J Transplant. 2013;13:861–874. [DOI] [PubMed] [Google Scholar]
- 4.Xu Q, McAlister VC, House AA, et al. Autoantibodies to LG3 are associated with poor long-term survival after liver retransplantation. Clin Transplant. 2021;35:e14318. [DOI] [PubMed] [Google Scholar]
- 5.Xu Q, McAlister VC, Leckie S, et al. Angiotensin II type I receptor agonistic autoantibodies are associated with poor allograft survival in liver retransplantation. Am J Transplant. 2020;20:282–288. [DOI] [PubMed] [Google Scholar]
- 6.Malheiro J, Tafulo S, Dias L, et al. Deleterious effect of anti-angiotensin II type 1 receptor antibodies detected pretransplant on kidney graft outcomes is both proper and synergistic with donor-specific anti-HLA antibodies. Nephrology (Carlton). 2019;24:347–356. [DOI] [PubMed] [Google Scholar]
- 7.Carroll RP, Riceman M, Hope CM, et al. Angiotensin II type-1 receptor antibody (AT1Rab) associated humoral rejection and the effect of peri operative plasma exchange and candesartan. Hum Immunol. 2016;77:1154–1158. [DOI] [PubMed] [Google Scholar]
- 8.Loupy A, Haas M, Roufosse C, et al. The Banff 2019 Kidney Meeting Report (I): updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am J Transplant. 2020;20:2318–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reinsmoen NL, Lai CH, Heidecke H, et al. Anti-angiotensin type 1 receptor antibodies associated with antibody mediated rejection in donor HLA antibody negative patients. Transplantation. 2010;90:1473-1477. [DOI] [PubMed] [Google Scholar]
- 10.Lefaucheur C, Loupy A. Antibody-mediated rejection of solid-organ allografts. N Engl J Med. 2018;379:2580–2582. [DOI] [PubMed] [Google Scholar]
- 11.Mahesh B, Leong HS, McCormack A, et al. Autoantibodies to vimentin cause accelerated rejection of cardiac allografts. Am J Pathol. 2007;170:1415–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porcheray F, Fraser JW, Gao B, et al. Polyreactive antibodies developing amidst humoral rejection of human kidney grafts bind apoptotic cells and activate complement. Am J Transplant. 2013;13:2590–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lefaucheur C, Viglietti D, Bouatou Y, et al. Non-HLA agonistic anti-angiotensin II type 1 receptor antibodies induce a distinctive phenotype of antibody-mediated rejection in kidney transplant recipients. Kidney Int. 2019;96:189–201. [DOI] [PubMed] [Google Scholar]
- 14.Riesco L, Irure J, Rodrigo E, et al. Anti-perlecan antibodies and acute humoral rejection in hypersensitized patients without forbidden HLA specificities after kidney transplantation. Transpl Immunol. 2019;52:53–56. [DOI] [PubMed] [Google Scholar]
- 15.Sis B, Einecke G, Chang J, et al. Cluster analysis of lesions in nonselected kidney transplant biopsies: microcirculation changes, tubulointerstitial inflammation and scarring. Am J Transplant. 2010;10:421–430. [DOI] [PubMed] [Google Scholar]
- 16.Kozakowski N, Herkner H, Bohmig GA, et al. The diffuse extent of peritubular capillaritis in renal allograft rejection is an independent risk factor for graft loss. Kidney Int. 2015;88:332–340. [DOI] [PubMed] [Google Scholar]
- 17.Viglietti D, Loupy A, Aubert O, et al. Dynamic prognostic score to predict kidney allograft survival in patients with antibody-mediated rejection. J Am Soc Nephrol. 2018;29:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carroll RP, Deayton S, Emery T, et al. Proactive treatment of angiotensin receptor antibodies in kidney transplantation with plasma exchange and/or candesartan is safe and associated with excellent graft survival at 4 years: a single centre Australian experience. Hum Immunol. 2019;80:573–578. [DOI] [PubMed] [Google Scholar]
- 19.Taniguchi M, Rebellato LM, Cai J, et al. Higher risk of kidney graft failure in the presence of anti-angiotensin II type-1 receptor antibodies. Am J Transplant. 2013;13:2577–2589. [DOI] [PubMed] [Google Scholar]
- 20.Crespo M, Llinas-Mallol L, Redondo-Pachon D, et al. Non-HLA antibodies and epitope mismatches in kidney transplant recipients with histological antibody-mediated rejection. Front Immunol. 2021;12:703457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.