EXECUTIVE SUMMARY
In adult cardiac surgery with cardiopulmonary bypass (CPB), avoiding hyperthermic perfusion (>37°C) is recommended to reduce the risk of cardiac surgery-associated acute kidney injury (CSA-AKI). (Class of Recommendation: I, Level of Evidence: B-R)
In adult cardiac surgery with CPB, a goal-directed oxygen delivery strategy is recommended to reduce the risk of CSA-AKI. (Class of Recommendation: I, Level of Evidence: B-R)
In adult cardiac surgery with CPB, it is reasonable to adopt the Kidney Disease Improving Global Outcomes (KDIGO) practice guidelines for patients at high risk of AKI to reduce the risk of CSA-AKI. (Class of Recommendation IIA; Level of Evidence B-R)
In adult cardiac surgery with CPB, fenoldopam may be reasonable to reduce the risk of CSA-AKI, as long as hypotension is avoided. (Class of Recommendation: IIB, Level of Evidence: B-R)
In adult cardiac surgery with CPB, it might be reasonable to use minimally invasive extracorporeal circulation (MiECC) techniques to reduce the risk of CSA-AKI. (Class of Recommendation: IIB, Level of Evidence: B-R)
In adult cardiac surgery with CPB, dopamine infusion alone, during CPB and the perioperative period, is not recommended to reduce the risk of CSA-AKI. (Class of Recommendation III: No Benefit, Level of Evidence: A)
In adult cardiac surgery with CPB, mannitol is not recommended to reduce the risk of CSA-AKI. (Class of Recommendation III: No Benefit, Level of Evidence: B-R)
Cardiac surgery-associated acute kidney injury (CSA-AKI) occurs in 15–50% of adults undergoing cardiac surgery and is characterized by a .3 mg/dL or 50% increase in serum creatinine from baseline or oliguria (1–4). There is wide regional, national, and international variation in rates of CSA-AKI (5,6). This is likely due to the lack of use of standardized definitions of AKI, such as the Kidney Disease Improving Global Outcomes (KDIGO) (4), the risk, injury, failure, loss, end-stage (RIFLE), or Acute Kidney Injury Network criteria (Table 1) (7–9), and to the fact that there have been limited attempts to synthesize the current evidence for strategies to prevent and mitigate AKI after adult cardiac surgery (10,11).
Table 1.
Adult cardiac surgery-associated acute kidney injury definitions.
| Variable | Serum Creatinine | Urine Output* | ||
|---|---|---|---|---|
| Diagnostic | ||||
| RIFLE | AKIN | KDIGO | ||
| No explicit criteria | ≥.3 mg/dL or ≥1.5 × baseline within 48 hours | ≥.3 mg/dL within 48 hours or ≥1.5 × baseline within 7 days | <.5 mL/kg/h for ≥6 hours† | |
| Staging | ||||
| Stage 1 or risk | ≥1.5 × baseline or eGFR decreased >25% | ≥.3 mg/dL or ≥1.5 × to 2.0 × baseline | ≥.3 mg/dL or ≥1.5 × to 1.9 × baseline | <.5 mL/kg/h for 6–12 hours |
| Stage 2 or injury | ≥2.0 × baseline or eGFR decreased >50% | ≥2.0 × to 3.0 × baseline | ≥2.0–2.9 × baseline | <.5 mL/kg/h for ≥12 hours |
| Stage 3 or failure | ≥3.0 × baseline or increase by >.5 mg/dL to >4.0 mg/dL or eGFR decreased >50% | >3.0 × baseline or increase by >.5 mg/dL to ≥4.0 mg/dL or initiation of RRT | ≥3.0 × baseline or increase by ≥.3 mg/dL to ≥4.0 mg/dL or initiation of RRT or if <18 years, eGFR <35 mL/min/1.73 m2 | <.3 mL/kg/h for >24 hours or anuria for ≥12 hours |
| Clinical outcomes | ||||
| Loss | RRT >4 weeks | |||
| ESRD | RRT >3 months | |||
AKIN, Acute Kidney Injury Network; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; KDIGO, Kidney Disease Improving Global Outcomes; RIFLE, risk, injury, failure, loss, end-stage renal disease; RRT, renal replacement therapy.
All staging criteria for urine output match for RIFLE, AKIN, and KDIGO.
This criterion applies only to AKIN and KDIGO.
Using a comprehensive and updated review of the literature on the prevention of CSA-AKI and other renal complications in adult cardiac surgery patients, the committee developed this joint guideline on renal protection strategies. These guidelines were created through a multispecialty partnership among the Society of Thoracic Surgeons, the Society of Cardiovascular Anesthesiologists, and the American Society of Extracorporeal Technology.
The multidisciplinary task force synthesized the evidence for renal-protective strategies using the highest level of literature review and scoring consistent with other Society of Thoracic Surgeons guidelines (12,13). Specifically, the task force synthesized and scored the evidence on pharmacologic strategies, fluid management, transfusion, cardiopulmonary bypass (CPB) management, and other more targeted strategies (e.g., remote ischemic preconditioning and prophylactic dialysis).
METHODOLOGY
An initial comprehensive literature search of the MEDLINE database (National Library of Medicine) was performed in 2015, with the intent of selecting only randomized trials of adult cardiac surgery patients who underwent cardiac surgery with CPB with assessment of clinical measures of renal function as primary outcomes. The results were limited to randomized controlled trials (RCTs) and meta-analyses published in English between January 1, 2000 and March 31, 2014. This search was further expanded and updated in 2017 using a new data management tool. As a final survey of the current literature, manuscripts through March 30, 2021, were included if they met the inclusion/exclusion criteria. The strategies for conducting the 2015 and the 2017 literature searches are listed in Appendix A.
The initial literature search resulted in 365 publications that were reviewed by a team of multidisciplinary authors (R.B., J.B., K.S., and C.M.). A total of 173 studies met the inclusion criteria for full-paper review, and data for 78 were extracted into the tables (Appendix B).
The second literature search identified an additional 592 publications, from which a team of authors (R.B., J.B., K.W.L., S.L., L.M., L.S.L., A.F., F.D., and S.F.) selected 50 studies and extracted data from them into the evidence tables (Appendix B). Reference lists of identified research papers were also scanned manually to identify any additional relevant studies that might have been missed in the MEDLINE query. All relevant studies were appraised for risk of bias using a customized checklist for RCTs and meta-analyses (Appendix C).
Meta-analyses were conducted where RCT data were deemed adequate and sufficiently similar to be pooled using random-effect models requiring a minimum of two studies reporting comparable renal outcomes (e.g., CSA-AKI, change in serum creatinine, new onset of dialysis). Only published meta-analyses are included in the main guideline document. Meta-analyses conducted by the task force are located in the supplementary materials.
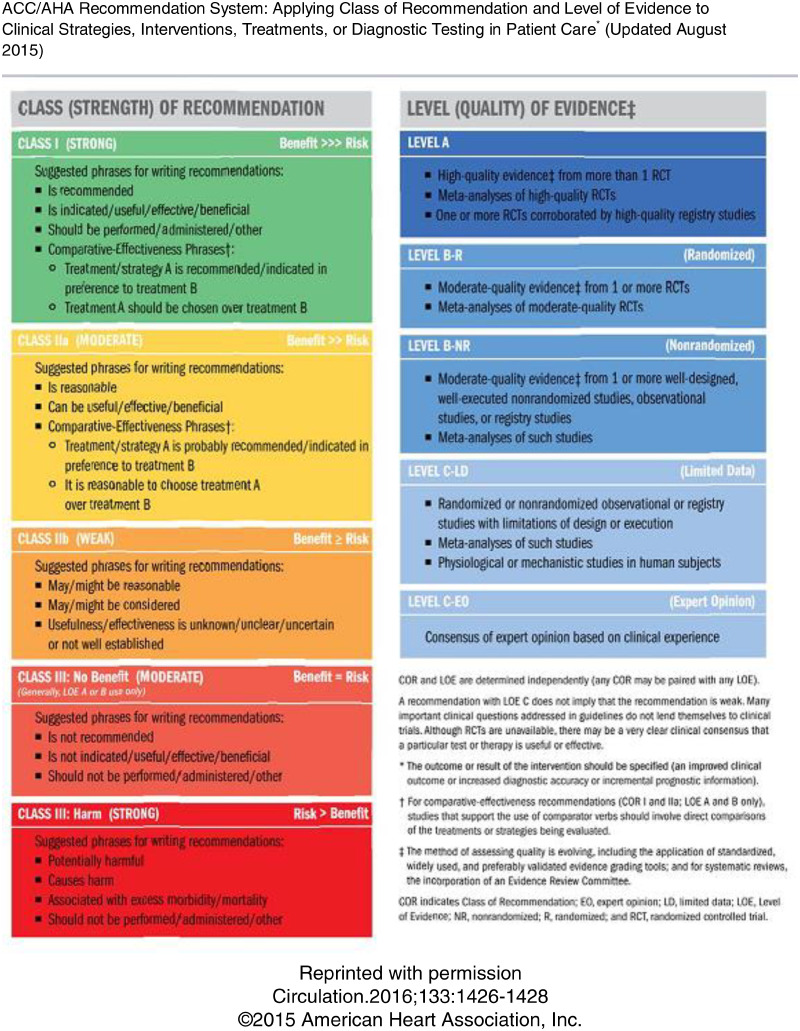
Data were reviewed by all authors, and recommendations were first drafted by each subtopic author group and then refined by using a modified Delphi consensus process. The recommendations are graded according to the American College of Cardiology/American Heart Association Recommendation System (14), included as Appendix D. A simplified list of the recommendations is included as Table 2 to aid in implementation. In addition, a number of proposed recommendations, which were omitted due to lack of consensus or clear clinical value, are included in Appendix E.
Table 2.
Brief overview of recommendations to prevent acute kidney injury and initiation of dialysis by phase of care intraoperative recommendations.
| For patients undergoing cardiac surgery with CPB | Avoid hyperthermic perfusion (arterial catheter >37°C) (Class I, Level B-R) Avoid nadir DO2 <270 mL/min/m2 (Class I, Level B-R) Consider minimally invasive extracorporeal circulation techniques (Class IIB, Level B-R) Consider fenoldopam infusion during CPB and perioperatively (Class IIB, Level B-R) … DO NOT USE dopamine infusion for renal protection during CPB and perioperatively (Class III: No Benefit, Level A) DO NOT USE mannitol to prime CPB for renal protection (Class III: No Benefit, Level B-R) |
| Postoperative recommendations | |
| High AKI-risk patients: elevations in [TIMP-2]*[IGFBP7] ≥.3 | KDIGO practice guidelines can be effective:
|
ACEi, angiotensin-converting enzyme inhibitor; AKI, acute kidney injury; ARB, angiotensin-receptor blocker; CPB, cardiopulmonary bypass; DO2, oxygen delivery; IGFBP7, insulin-like growth factor-binding protein 7; KDIGO, Kidney Disease Improving Global Outcomes; TIMP2, tissue inhibitor of metalloproteinases 2.
CPB STRATEGIES
The literature summarized subsequently recommends intraoperative CPB strategies decrease the risk of developing acute renal injury after cardiac surgery. These interventions include avoiding hyperthermic perfusion (>37°C), avoiding low delivery of oxygen (DO2), and the adoption of minimally invasive extracorporeal circulation (MiECC) techniques. There is currently not enough evidence to support use of heparin-coated circuits, leukocyte filtration, pulsatile flow during CPB, intraoperative hemofiltration and ultrafiltration, relative hypertension during CPB, or multipass hemoconcentration (vs. centrifugation of residual CPB blood) to decrease acute renal injury after cardiac surgery. These strategies may be beneficial for other outcomes.
Class I
In adult cardiac surgery with CPB, avoiding hyperthermic perfusion (>37°C) is recommended to reduce the risk of CSA-AKI. (Level of Evidence: B-R)
In adult cardiac surgery with CPB, a goal-directed DO2 strategy is recommended to reduce the risk of CSA-AKI. (Level of Evidence: B-R)
Class IIB
In adult cardiac surgery with CPB, the use of MiECC techniques to minimize the risk of CSA-AKI might be reasonable. (Level of Evidence: B-R)
Temperature Management
A randomized trial by Boodhwani and colleagues (15) of 223 low-risk coronary artery bypass graft (CABG) surgery patients found that rewarming on CPB from 32°C to a nasopharyngeal target of 37°C vs. 34°C resulted in significantly higher postoperative serum creatinine values. The authors identified rewarming as an independent risk factor for renal dysfunction, suggesting differential rewarming and potential hyperthermia as proposed mechanisms for this difference (15).
Newland and colleagues (16) performed a single-center retrospective cohort study of 1,393 consecutive adult patients undergoing valve, CABG, or combined CABG and valve surgery and found that increased number of minutes on CPB with arterial outlet temperature >37°C and higher postoperative intensive care unit (ICU) arrival temperatures were associated with a significantly increased incidence of CSA-AKI (CPB hyperthermia odds ratio [OR], 1.03 per minutes increase, 95% CI, 1.01–1.05; ICU admission temperature OR, 1.44 per degree increase, 95% CI 1.13–1.85). This finding was reproduced in a multicenter study of 8,407 patients that found in both cohort- and propensity-matched studies that duration of rewarming temperature >37°C (hyperthermic perfusion) was independently associated with RIFLE risk classification or greater (OR, 1.42; 95% CI, 1.09–1.77; p = .012) and injury classification or greater AKI (OR, 1.52; 95% CI, 1.09–1.97; p = .016) in the entire cohort, and injury classification or greater AKI (OR, 1.51; 95% CI, 1.15–1.90; p = .006) in propensity-matched patients (17).
DO2 Strategy
Ranucci and colleagues (18) demonstrated in a single-center prospective observational study of 1,048 individuals that the minimum DO2 index (DO2i) during CPB was independently associated with CSA-AKI requiring renal replacement therapy, with an optimal diagnostic threshold for DO2i identified as 272 mL/min/m2. Newland and Baker (19) supported this finding in an observational study of 210 patients in which they suggested that the integral of the amount and time for DO2 below a critical threshold was an independent predictor of AKI. They reported a DO2i threshold of 270 mL/min/m2 (19). Mukaida and associates (20) reported that increased time <300 mL/min/m2 was significantly associated with increased AKI. A small propensity-matched study demonstrated that when targeted DO2 (>300 mL/min/m2) was included as part of a multifaceted goal-directed strategy, CSA-AKI was reduced (20).
In 2018, Ranucci and investigators (21) published a multicenter RCT in which goal-directed perfusion aimed to avoid a CPB nadir DO2i of <280 mL/min/m2 resulted in significantly lower AKI stage 1. The goal-directed perfusion intervention aimed to maintain DO2i at ≥280 mL/min/m2 by adjusting arterial flow according to hematocrit value to maintain DO2 above the prespecified threshold (280 mL/min/m2). When low hematocrit resulted in an inability to achieve the desired threshold, red blood cells were transfused to increase DO2 (21). In 2019, the Australian and New Zealand Collaborative Perfusion Registry group published findings from >19,000 adult cardiac surgery patients and reported that nadir DO2i <270 mL/min/m2 was associated with significantly increased odds of developing AKI by 52% (OR, 1.52; 95% CI, 1.29–1.77; p < .001) (22).
The goal-directed perfusion investigations include a range of temperatures and target DO2i, thereby limiting precise specification of goals. Despite these limitations, goal-directed perfusion correlates with a reduction in CSA-AKI and is recommended.
Minimally Invasive Extracorporeal Circulation
MiECC is a strategy that requires coordinated efforts among surgeons, anesthesiologists, and perfusionists and has been proposed as an alternative approach to undertaking cardiac surgery using standard CPB (23). The Minimal Invasive Extra-Corporeal Technologies International Society defined that in addition to core components for CPB (membrane oxygenator, heat exchanger, cardioplegia system), an MiECC system should include a closed system, biologically inert (coated) circuit coating, have reduced priming volume, use a centrifugal pump, contain a venous bubble trap/venous air removal device, and incorporate a shed blood management system. Additional features and components, such as vents and reservoirs, may be included.
The results of randomized trials using MiECC circuits have been conflicting. The literature is limited by the heterogeneity in the techniques reported for both the intervention (MiECC techniques) and the control arm (CPB) of the trials reported. Two meta-analyses have reported on the impact of MiECC on the renal outcome of cardiac surgery with CPB, with conflicting findings. Sun and associates (24) reported no difference in acute renal failure in six studies, with a relative risk of .922 (95% CI, .388–1.01; p = .854; I2 = 11.9%); however, a more recent meta-analysis reported MiECC reduced the odds of CSA-AKI by >50% compared with conventional CPB (OR, .47; 95% credibility interval, .24–.89) (25). More recent RCTs have not reported CSA-AKI outcomes.
PHARMACOLOGIC STRATEGIES
Recommendations
Class IIB
In adult cardiac surgery with CPB, fenoldopam may be reasonable to reduce the risk of CSA-AKI, as long as hypotension is avoided. (Level of Evidence: B-R)
Class III: No Benefit
In adult cardiac surgery with CPB, dopamine infusion alone, during CPB and the perioperative period, is not recommended to reduce the risk of CSA-AKI. (Class of Recommendation III: No Benefit, Level of Evidence: A)
In adult cardiac surgery with CPB, mannitol is not recommended to provide protection against CSA-AKI. (Level of Evidence: B-R)
Diuretics.
Dopamine is a widely studied diuretic (26). At low doses (.3–5 μg/kg/min), dopamine has been purported to increase renal blood flow and promote natriuresis and diuresis. Consequently, there has been substantial interest in using a “renal-dose” dopamine infusion to improve kidney perfusion and prevent acute renal injury in patients undergoing CPB. Most of the single-center randomized clinical trials (27–34) have not demonstrated benefit with dopamine infusion to prevent AKI.
Two other diuretic agents, mannitol and furosemide, have been studied in randomized trials and consistently demonstrated no benefit (28,31,35–37). Furthermore, one trial raises concerns that furosemide infusion may have deleterious effects on postoperative renal function (28).
In a randomized clinical trial involving patients with normal preoperative renal function, patients who received a dopamine infusion (2 μg/kg/min) during the cardiac operation and in the early postoperative period had similar postoperative renal function and clinical outcomes as those who received placebo treatment (30). Most of the other trials investigating low-dose dopamine infusion during CPB have relied on urinary biomarkers to determine whether dopamine reduced subclinical levels of renal injury. The findings of biomarker studies have been inconsistent; overall, they have not yielded evidence supporting a renal-protective effect of dopamine.
In a randomized clinical trial involving patients with normal preoperative renal function, those who received a dopamine infusion (2.5 μg/kg/min) during cardiac surgery and in the early postoperative period had significantly lower levels of urinary retinol-binding protein on postoperative day 1, but not on day 2 or 5, compared with patients who received placebo; however, there were no significant differences in clinical outcomes or in other biomarker levels (31).
A study focused on patients at high risk for postoperative renal dysfunction found that patients who received a dopamine infusion (3 μg/kg/min) during their cardiac operations and during the early postoperative period had a significantly more negative fluid balance than those who received placebo, but there were no significant differences in clinical outcomes or biomarkers, including urinary retinol-binding protein, during the 6 days after surgery (32).
In a trial comparing treatment strategies in patients with normal preoperative renal and cardiac function who were undergoing elective CABG with CPB, patients were randomly assigned to receive or not receive a dopamine infusion (2 μg/kg/min) during the perioperative period (33). The authors reported that the dopamine infusion was associated with significantly higher urinary β2-microglobulin excretion—an indicator of renal tubular injury—on postoperative day 3 compared with controls; there were no significant differences in clinical outcomes or other biomarkers.
Similarly, in the randomized clinical trial by Carcoana and colleagues (28), the authors compared four treatment strategies—dopamine infusion (2 μg/kg/min) throughout the CPB period, mannitol (1 g/kg) added to the pump prime, both dopamine infusion and mannitol, or placebo—in patients with normal preoperative renal function who were undergoing CABG with CPB. Although there were no differences in renal function indicators or clinical outcomes, the infusion of dopamine (alone or in combination with mannitol) was associated with significantly higher β2-microglobulin excretion at 1 hour after CPB compared with placebo; this difference did not persist at 6 or 24 hours after CPB. Logistic regression analysis revealed that the dopamine infusion was associated with up to a 7.7-fold increase in the odds of an increased β2-microglobulin excretion rate at 1 hour after CPB (28).
No studies have demonstrated that dopamine reduces the incidence of clinical AKI or renal replacement therapy. In fact, few studies include major clinical end points. In the meta-analysis performed by Patel and colleagues (27), only 4 of the 11 randomized clinical trials focusing on dopamine reported clinical outcomes. Although the analysis revealed that dopamine infusion was associated with a small decline in renal function as measured by creatinine clearance, the effect size was small and was not evident when low-quality studies were excluded.
One small clinical trial among 60 patients with normal preoperative renal function who were undergoing CABG with CPB evaluated dopamine with diltiazem. Patients were assigned to receive 1 of 4 treatments during the perioperative period: dopamine infusion (2 μg/kg/min), diltiazem infusion (2 μg/kg/min), both dopamine and diltiazem infusion, or neither infusion (controls). Importantly, patients who received dopamine alone or diltiazem alone exhibited significantly higher urinary β2-microglobulin levels at 24 hours than controls; in contrast, patients who received combined dopamine and diltiazem infusion had urinary β2-microglobulin levels that were similar to controls. Furthermore, compared with the other groups, the patients who received the combined dopamine and diltiazem infusion exhibited significantly higher creatinine clearance and osmotic clearance 24 hours after surgery and significantly higher free water clearance at 24 and 72 hours after surgery (34).
Two randomized clinical trials focusing on the impact of mannitol in providing renal protection in two different patient populations were reported. In the first study, 40 patients with normal preoperative renal function were randomized to have mannitol (500 mg/kg) or Hartmann solution added to the CPB prime during elective cardiac operations (37). Compared with the control group, the use of mannitol was not associated with differences in postoperative renal function or in urinary retinol-binding protein or microalbumin levels. In the second study, 47 patients with preoperative renal dysfunction (serum creatinine 130–250 μmol/L) undergoing elective cardiac operations were randomized to the same two groups, and the use of mannitol was not associated with differences in postoperative renal function compared with controls (36).
Another single-center randomized clinical trial compared treatment strategies in patients with normal preoperative renal function who were undergoing CABG with CPB (28). Patients were assigned to receive mannitol (1 g/kg) added to the pump prime, dopamine infusion (2 μg/kg/min) throughout the CPB period, both dopamine infusion and mannitol, or placebo. Compared with placebo, the use of mannitol without dopamine was not associated with any differences in β2-microglobulin excretion, renal function variables, or clinical end points, including major postoperative events and ICU and hospital lengths of stay. The group that received both dopamine and mannitol exhibited significantly higher β2-microglobulin excretion 1 hour after CPB compared with placebo. Logistic regression analysis revealed that the combination of mannitol and dopamine infusion was associated with a 5.3-fold increase in the odds of an increased β2-microglobulin excretion rate (p = .008) (28).
Lassnigg and colleagues (30) observed no significant differences between the placebo and diuretic treatments of dopamine and furosemide, specifically in length of stay in the ICU or hospital mortality. Significance for other clinical outcomes was not measured, but for groups undergoing furosemide treatments, two died of myocardial infarction and two underwent renal replacement therapy.
When testing dopexamine, Dehne and colleagues (29) observed no significant clinical complications in their cohort regarding death or renal replacement therapy; other key clinical outcomes were not measured. Sumeray and colleagues (31) made no conclusions about clinical outcomes after dopamine infusion in their study. Woo and coworkers (32) observed no significant differences between placebo and dopamine treatments for postoperative renal replacement therapy or ICU length of stay. Yavuz and colleagues (34) recorded no patients with clinical complications, including in-hospital mortality, cardiac complications, respiratory issues, or renal dysfunction, during their dopamine trial, which was consistent with a follow-up study by Yavuz and colleagues (34) published in the same year. Carcoana and coworkers (28) observed nonsignificant differences in clinical outcomes, including ICU length of stay, among the treatment groups for dopamine or for furosemide.
Vasodilators.
Several different types of vasodilators have been studied to determine whether they provide renal protection during CPB. There is some clinical evidence suggesting that the use of several agents—including fenoldopam, nitroprusside, diltiazem combined with dopamine, and prostaglandin I2 analogues—may improve renal outcomes (27,34,38–46). Clinical evidence in support of using angiotensin-receptor blockers (ARBs), angiotensin-converting enzyme inhibitors (ACEi), or nifedipine is lacking (47–49). Importantly, although some studies have indicated that the use of vasodilators may improve various indicators of postoperative renal function, very few have demonstrated that vasodilators reduce the incidence of acute renal failure after CPB.
Of the various vasodilators, the most frequently studied has been fenoldopam, a selective dopamine receptor D1 agonist. Three principal trials yielded differing results. One single-center randomized clinical trial comparing perioperative fenoldopam (.05 μg/kg/min) vs. dopamine (2.5 μg/kg/min) infusion in high-risk patients undergoing cardiac surgery found no differences in renal outcomes between the groups (39). This study was interrupted by the safety monitoring board because of “the documented lack of efficacy of fenoldopam in improving renal outcome, the significant increased incidence of hypotension during CPB, and the suggestive intraoperative trend toward the use of vasoconstrictors” (39).
In a double-blind randomized clinical trial that compared complex cardiac surgery patients who received perioperative fenoldopam infusion (.1 μg/kg/min) vs. placebo, the difference in the incidence of AKI did not reach statistical significance (0 of 38 in the fenoldopam group vs. 4 of 40 in controls; p = .1) (44). Interestingly, in the subgroup analysis of patients who received inotropic support to treat low cardiac output syndrome immediately after surgery, the patients who received fenoldopam had a significantly lower incidence of AKI than those who received placebo (0 of 11 vs. 4 of 6; p = .006).
Cogliati and colleagues (40) published the largest randomized clinical trial regarding the use of fenoldopam during cardiac operations. This double-blind randomized clinical trial compared perioperative fenoldopam infusion (.1 μg/kg/min for 24 hours) vs. placebo in patients who underwent elective CABG and/or valve operations with CPB. Cogliati and colleagues (40) found that fenoldopam administration significantly reduced the incidence of AKI (12 of 95 vs. 27 of 98; p = .02) and renal replacement therapy (0 of 95 vs. 8 of 98; p = .007). The patients who received fenoldopam also had a shorter mean ICU stay than controls (2.3 ± 1.1 days vs. 4.2 ± 3.1 days; p < .0005). No drug-related episodes of hypotension were noted during the study.
In 2019, a meta-analysis of seven trials using trial sequential analysis to reduce random error also demonstrated a benefit of fenoldopam with respect to renal insufficiency but documented an increased occurrence of hypotension (50). Sun and colleagues (50) did not confirm an association between fenoldopam use and reduced incidence of renal replacement therapy after cardiac operations, suggesting that most studies were small and variable and that there is not enough evidence to support it.
However, a benefit has been reported in cohort studies and in three other meta-analyses (27,43,45,46,51). Roasio and colleagues (46) found a similar decrease in need for dialysis in patients treated with fenoldopam (17% vs. 39%; p = .037) but did not find ICU stay to be significantly different. In the systematic review by Patel and colleagues (27), fenoldopam was not found to significantly reduce mortality (OR, 1.36; 95% CI, .29–6.36; p-value not published) but did reduce the need for postoperative dialysis (OR, .35; 95% CI, .13–.96; p-value not published) (27).
Although the previously described results support the potential benefit of using fenoldopam during CPB, the three principal studies had substantial differences in patient populations, fenoldopam dosage, infusion timing, study design, and control group treatment. The impact of fenoldopam appears positive in the cardiac surgery literature, but the marked heterogeneity of the studies weakens the strength of the collective evidence and precludes a recommendation stronger than IIB. The KDIGO recommendation to not use fenoldopam to prevent or treat AKI (2C) may seem inconsistent with our recommendation. The preponderance of data in cardiac surgery does show benefit in reducing AKI and renal replacement therapy, presuming hypotension can be avoided. Analysis of the totality of the literature, including studies in contrast-induced nephropathy and critically ill patients, have not shown universal benefit and most likely contribute to the KDIGO 2C recommendation.
TARGETED THERAPIES
Recommendations
Class IIA
In adult cardiac surgery with CPB, it is reasonable to adopt the KDIGO practice guidelines for patients at high risk of AKI to reduce the incidence of AKI. (Level of Evidence B-R)
KDIGO Guideline Implementation.
In patients at high risk of AKI, the use of the KDIGO guideline recommendations in practice can be useful to reduce the incidence of AKI (9,11). The PrevAKI (Prevention of Cardiac Surgery-Associated Acute Kidney Injury by Implementing the KDIGO Guidelines in High-Risk Patients Identified by Biomarkers) trial is a single-center trial that randomized patients at 4 hours after surgery found to be at high risk of AKI to receive either the KDIGO surgical bundle or to control. The KDIGO bundle includes close monitoring to optimize volume status and hemodynamics, avoidance of nephrotoxic drugs, stopping ACEi and ARBs for 48 hours, measurement of serum creatinine and urine output, prevention of hyperglycemia for 72 hours, and use of alternatives to constant agents (11). Controls received standard of care in addition to specifications to maintain mean arterial pressure >65 mmHg and central venous pressure between 8 and 10 mmHg, with ACEi and ARBs continued according to American College of Cardiology recommendations (Table 3) (9,11).
Table 3.
Standard of care and kidney disease improving global outcomes bundle intervention.
| Control | Intervention KDIGO Bundle |
|---|---|
| Standard care | Discontinuation of all nephrotoxic agents if possible |
| ACEi and ARBs continued according to ACC recommendations | Discontinuation of ACE inhibitors and ARBs for the first 2 days after surgery |
| MAP will be kept >65 mmHg | Close monitoring of serum creatinine and urinary output |
| CVP between 8 and 10 mmHg | Avoidance of hyperglycemia by close monitoring |
| _ | Consideration of alternatives to radio contrast agents |
| _ | Hemodynamic monitoring and optimization according to a hemodynamic algorithm |
ACC, American College of Cardiology; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; CVP, central venous pressure; KDIGO, Kidney Disease: Improving Global Outcomes; MAP, mean arterial pressure.
The PrevAKI trial demonstrated a statistically significant reduction in AKI overall (55.1% intervention vs. 71.7% control; OR, .48; 95% CI, .29–.80; p = .004) significant reductions in moderate to severe AKI as well (29.7% vs. 44.9%; p = .009). However, there were no differences in the need for renal replacement therapy, 90-day mortality, ICU length of stay, or major adverse kidney events end points (11). A retrospective, bundled approach by Engelman and colleagues (52) demonstrated that hemodynamic monitoring, a liberal transfusion threshold, and avoidance of nephrotoxic drugs used in high-risk cardiac surgery patients (identified with AKI biomarkers) mitigated the risk of AKI. These efforts were coordinated through a multidisciplinary acute kidney response team and demonstrated a relative risk reduction of 89% for stage 2/3 CSA-AKI. Magruder and colleagues (53) reported a small retrospective propensity-matched study in which a goal-directed perfusion initiative to reduce CSA-AKI demonstrated a significant reduction in AKI (8 of 88 vs. 21 of 88) and reduced creatinine increase after cardiac surgery.
Abbreviations and Acronyms
- ACEi
= angiotensin-converting enzyme inhibitors
- ARBs
= angiotensin-receptor blockers
- CABG
= coronary artery bypass graft
- CPB
= cardiopulmonary bypass
- CSA-AKI
= cardiac surgery-associated acute kidney injury
- DO2
= delivered oxygen
- DO2i
= delivered oxygen index
- KDIGO
= Kidney Disease Improving Global Outcomes
- ICU
= intensive care unit
- MiECC
= minimally invasive extracorporeal circulation
- OR
= odds ratio
- RCT
= randomized controlled trial
- RIFLE
= risk, injury, failure, loss, end-stage
Appendix A—Search Strategy
(((((prevention OR protection OR (outcome assessment) OR (risk factors))) AND (((((renal OR kidney) AND (hematocrit OR hemodilution OR oxygen)) OR ((kidney diseases) OR (kidney failure) OR (Kidney Function Tests)) AND ((Humans[Mesh]) AND (English[lang]))) OR (hemoglobins AND ((Humans[Mesh]) AND (English[lang]))) OR (anemia AND ((Humans[Mesh]) AND (English[lang])))))) AND (((((cardiac surgery OR (((““cardiopulmonary bypass””[TIAB] NOT Medline[SB]) OR ““cardiopulmonary bypass””[MeSH Terms] OR (““coronary artery bypass””[TIAB] NOT Medline[SB]) OR ““coronary artery bypass””[MeSH Terms]) OR (valve OR valvular) AND surgery) OR ““Heart-lung machine””[MeSH Terms] OR ((hemofiltration OR ultrafiltration) AND (cardiac OR heart)) AND ((Humans[Mesh]) AND (English[lang]))) NOT (cardiac surgery OR (((““cardiopulmonary bypass””[TIAB] NOT Medline[SB]) OR ““cardiopulmonary bypass””[MeSH Terms] OR (““coronary artery bypass””[TIAB] NOT Medline[SB]) OR ““coronary artery bypass””[MeSH Terms]) OR (valve OR valvular) AND surgery) OR ““Heart-lung machine””[MeSH Terms] OR ((hemofiltration OR ultrafiltration) AND (cardiac OR heart)) AND ((Humans[Mesh]) AND (English[lang]) AND ((infant[MeSH] OR child[MeSH] OR adolescent [MeSH]))))) OR ((cardiac surgery OR (((““cardiopulmonary bypass””[TIAB] NOT Medline[SB]) OR ““cardiopulmonary bypass””[MeSH Terms] OR (““coronary artery bypass””[TIAB] NOT Medline[SB]) OR ““coronary artery bypass””[MeSH Terms]) OR (valve OR valvular) AND surgery) OR ““Heart-lung machine””[MeSH Terms] OR ((hemofiltration OR ultrafiltration) AND (cardiac OR heart)) AND ((Humans[Mesh]) AND (English[lang]) AND ((infant[MeSH] OR child[MeSH] OR adolescent[MeSH])))) AND (cardiac surgery OR (((““cardiopulmonary bypass””[TIAB] NOT Medline[SB]) OR ““cardiopulmonary bypass””[MeSH Terms] OR (““coronary artery bypass””[TIAB] NOT Medline[SB]) OR ““coronary artery bypass””[MeSH Terms]) OR (valve OR valvular) AND surgery) OR ““Heart-lung machine””[MeSH Terms] OR ((hemofiltration OR ultrafiltration) AND (cardiac OR heart)) AND ((Humans[Mesh]) AND (English[lang]) AND (adult[MeSH]))))) NOT (((cardiac surgery OR (((““cardiopulmonary bypass””[TIAB] NOT Medline[SB]) OR ““cardiopulmonary bypass””[MeSH Terms] OR (““coronary artery bypass””[TIAB] NOT Medline[SB]) OR ““coronary artery bypass””[MeSH Terms]) OR (valve OR valvular) AND surgery) OR ““Heart-lung machine””[MeSH Terms] OR ((hemofiltration OR ultrafiltration) AND (cardiac OR heart)) AND ((Humans[Mesh]) AND (English[lang]))) NOT (cardiac surgery OR (((““cardiopulmonary bypass””[TIAB] NOT Medline[SB]) OR ““cardiopulmonary bypass””[MeSH Terms] OR (““coronary artery bypass””[TIAB] NOT Medline[SB]) OR ““coronary artery bypass””[MeSH Terms]) OR (valve OR valvular) AND surgery) OR ““Heart-lung machine””[MeSH Terms] OR ((hemofiltration OR ultrafiltration) AND (cardiac OR heart)) AND ((Humans[Mesh]) AND (English[lang]) AND ((infant[MeSH] OR child[MeSH] OR adolescent[MeSH]))))) OR ((cardiac surgery OR (((““cardiopulmonary bypass””[TIAB] NOT Medline[SB]) OR ““cardiopulmonary bypass””[MeSH Terms] OR (““coronary artery bypass””[TIAB] NOT Medline[SB]) OR ““coronary artery bypass””[MeSH Terms]) OR (valve OR valvular) AND surgery) OR ““Heart-lung machine””[MeSH Terms] OR ((hemofiltration OR ultrafiltration) AND (cardiac OR heart)) AND ((Humans[Mesh]) AND (English[lang]) AND ((infant[MeSH] OR child[MeSH] OR adolescent[MeSH])))) AND (cardiac surgery OR (((““cardiopulmonary bypass””[TIAB] NOT Medline[SB]) OR ““cardiopulmonary bypass””[MeSH Terms] OR (““coronary artery bypass””[TIAB] NOT Medline[SB]) OR ““coronary artery bypass””[MeSH Terms]) OR (valve OR valvular) AND surgery) OR ““Heart-lung machine””[MeSH Terms] OR ((hemofiltration OR ultrafiltration) AND (cardiac OR heart)) AND ((Humans[Mesh]) AND (English[lang]) AND (adult[MeSH])))) AND ((Editorial[ptyp] OR Letter[ptyp] OR Comment[ptyp] OR Festschrift[ptyp] OR Historical Article[ptyp] OR Lectures[ptyp] OR Legal Cases[ptyp] OR Legislation[ptyp] OR News[ptyp] OR Newspaper Article [ptyp] OR Patient Education Handout[ptyp]))))))) AND (Humans[Mesh] AND (Meta-Analysis[ptyp] OR Randomized Controlled Trial[ptyp]) AND English[lang] AND adult[MeSH]) AND (Humans[Mesh] AND (Meta-Analysis[ptyp] OR Randomized Controlled Trial[ptyp]) AND English[lang] AND adult[MeSH]).
Appendix B. Data table.
Appendix table will be available at the journal’s website.
Appendix C. RCT and meta-analysis appraisal.
| Author | Title | Year | Did the Trial Address a Clearly Focused Issue? | Method of Randomization Is Described | Adequate Method of Allocation Concealment Is Described | Adequate Follow-Up at the Conclusion of the Study | Were Patients, Health Workers and Study Personnel “Blind” to Treatment? | Were the Groups Similar at the Start of the Trial? | Aside from the Experimental Intervention, Were the Groups Treated Equally? | Can the Results be Applied in Your Context or to a Local Population? | Were All Clinically Important Outcomes Considered? | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vasodilators | ||||||||||||
| Lassnigg, A et al. | Lack of renoprotective effects of dopamine and furosemide during cardiac surgery | 2000 | ⊕ | ⊕ | ⊕ | ⊕ | ? | ⊕ | ⊕ | ? | ⊕ |
|
| Dehne, MG et al. | Impairment of renal function after cardiopulmonary bypass is not influenced by dopexamine | 2001 | ⊕ | ⊖ | ⊖ | ⊕ | ⊖ | ⊕ | ⊕ | ⊖ | ⊖ |
|
| Sumeray, M et al. | Low dose dopamine infusion reduces renal tubular injury following cardiopulmonary bypass surgery | 2001 | ⊕ | ⊕ | ⊖ | ⊕ | ⊕ | ⊕ | ⊕ | ⊖ | ⊖ |
|
| Yavuz, S et al. | Renal dose dopamine in open heart surgery. Does it protect renal tubular function? | 2002 | ⊕ | ⊖ | ⊖ | ⊕ | ⊖ | ⊕ | ⊕ | ⊖ | ⊕ | Small, unblinded study (two groups n = 11 each) with no power calculation. |
| Yavuz, S et al. | Effect of combined dopamine and diltiazem on renal function after cardiac surgery | 2002 | ⊕ | ⊖ | ⊖ | ⊕ | ⊖ | ⊕ | ⊕ | ⊖ | ⊕ | Small, unblinded study (four groups n = 15 each) with no power calculation. |
| Bergman, AS et al. | Diltiazem infusion for renal protection in cardiac surgical patients with preexisting renal dysfunction | 2002 | ⊕ | ⊖ | ⊖ | ⊕ | ? | ⊕ | ⊕ | ⊖ | ⊖ |
|
| Woo, EB et al. | Dopamine therapy for patients at risk of renal dysfunction following cardiac surgery: Science or fiction? | 2002 | ⊕ | ⊖ | ⊖ | ⊕ | ⊖ | ⊕ | ⊕ | ⊖ | ⊖ | Eight patients excluded after randomization |
| Caimmi, PP et al. | Fenoldopam for renal protection in patients undergoing cardiopulmonary bypass | 2003 | ⊕ | ⊖ | ⊖ | ⊕ | ⊖ | ⊕ | ⊕ | ⊖ | ⊖ | |
| Carcoana, OV et al. | Mannitol and dopamine in patients undergoing cardiopulmonary bypass: A randomized clinical trial | 2003 | ⊕ | ⊕ | ⊖ | ⊕ | ⊕ | ⊕ | ⊕ | ? | ⊖ | 35 withdrawals prior to allocation |
| Turker, H et al. | Effects of enalaprilat infusion on hemodynamics and renal function in patients undergoing cardiac surgery | 2004 | ⊕ | ⊖ | ⊖ | ⊕ | ⊖ | ⊕ | ⊕ | ⊖ | ⊖ |
|
| Bove, T et al. | Renoprotective action of fenoldopam in high-risk patients undergoing cardiac surgery: A prospective, double-blind, randomized clinical trial | 2005 | ⊕ | ⊕ | ⊕ | ⊕ | ? | ⊕ | ⊕ | ? | ⊕ |
|
| Kaya, K et al. | The effect of sodium nitroprusside infusion on renal function during reperfusion period in patients undergoing coronary artery bypass grafting: A prospective randomized clinical trial | 2007 | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | Data from two separate centers |
| Cogliati, AA et al. | Fenoldopam infusion for renal protection in high-risk cardiac surgery patients: A randomized clinical study | 2007 | ⊕ | ⊕ | ? | ⊕ | ⊕ | ⊕ | ⊕ | ? | ⊖ |
|
| Witczak, BJ et al. | Renal function after cardiopulmonary bypass surgery in patients with impaired renal function. A randomized study of the effect of nifedipine | 2008 | ⊕ | ⊕ | ? | ⊕ | ⊖ | ⊕ | ⊕ | ⊖ | ⊖ |
|
| Ranucci, M et al. | Effects of fenoldopam infusion in complex cardiac surgical operations: A prospective, randomized, double-blind, placebo-controlled study | 2010 | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊖ | ? | ⊕ |
|
| Anti-Inflammatory | ||||||||||||
| Tang, AT et al. | Leukodepletion reduces renal injury in coronary revascularization: A prospective randomized study | 2002 | ⊕ | ⊖ | ⊖ | ⊕ | ⊖ | ⊕ | ⊕ | ⊖ | ⊖ | Statistically significant difference in renal injury, although this did not translate to any statistical difference in renal function, and no renal impairment was reported in any patient. Statistical power to detect a difference was not calculated. |
| McBride, WT et al. | Methylprednisolone favourably alters plasma and urinary cytokine homeostasis and subclinical renal injury at cardiac surgery | 2004 | ⊕ | ⊖ | ⊖ | ⊕ | ⊖ | ⊕ | ⊕ | ⊖ | ⊖ | Patients and laboratory staff were blinded, but the periop physicians knew the allocation. |
| Loef, BG et al. | Effect of dexamethasone on perioperative renal function impairment during cardiac surgery with cardiopulmonary bypass | 2004 | ⊕ | ⊖ | ⊖ | ⊕ | ? | ⊖ | ⊕ | ⊖ | ⊖ |
|
| Burns, KE et al. | Perioperative N-acetylcysteine to prevent renal dysfunction in high-risk patients undergoing CABG surgery: A randomized controlled trial | 2005 | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ |
|
| Ristikankare, A et al. | Lack of renoprotective effect of i.v. N-acetylcysteine in patients with chronic renal failure undergoing cardiac surgery | 2006 | ⊕ | ? | ⊖ | ⊕ | ? | ⊕ | ⊕ | ? | ⊖ | Paper refers to study as double-blinded, but few details of blinding reported. Only states “a similar volume of saline” was given to control groups. |
| Bolcal, C et al. | Leukodepletion improves renal function in patients with renal dysfunction undergoing on-pump coronary bypass surgery: A prospective randomized study | 2007 | ⊕ | ⊖ | ⊖ | ? | ⊖ | ⊕ | ⊕ | ⊖ | ⊖ | Unclear how many patients had follow-up data. |
| Haase, M et al. | Phase II, randomized, controlled trial of high-dose N-acetylcysteine in high-risk cardiac surgery patients | 2007 | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | Data from two separate centers |
| Sisillo, E et al. | N-acetylcysteine for prevention of acute renal failure in patients with chronic renal insufficiency undergoing cardiac surgery: A prospective, randomized, clinical trial | 2008 | ⊕ | ⊕ | ⊖ | ⊕ | ⊕ | ⊕ | ⊕ | ? | ⊕ |
|
| Barr LF and Kolodner, K | N-acetylcysteine and fenoldopam protect the renal function of patients with chronic renal insufficiency undergoing cardiac surgery | 2008 | ⊕ | ? | ⊖ | ⊕ | ? | ⊕ | ⊕ | ⊖ | ⊕ |
|
| Adabag, AS et al. | Utility of N-acetylcysteine to prevent acute kidney injury after cardiac surgery: A randomized controlled trial | 2008 | ⊕ | ⊕ | ⊖ | ⊕ | ⊕ | ⊕ | ⊕ | ? | ⊕ |
|
| Amr, YM et al. | Effects of dexamethasone on pulmonary and renal functions in patients undergoing CABG with cardiopulmonary bypass | 2009 | ⊕ | ⊖ | ⊖ | ? | ? | ⊕ | ⊕ | ⊖ | ⊖ | Small single-center study with a high risk of bias due to lack of details on methodology. |
| Natriuretics/Diuretics | ||||||||||||
| Mahesh, B et al | Does furosemide prevent renal dysfunction in high-risk cardiac surgical patients? Results of a double-blinded prospective randomised trial | 2008 | ⊕ | ⊖ | ⊖ | ⊕ | ? | ⊕ | ⊕ | ⊖ | ⊕ |
|
| Yallop, KG et al. | The effect of mannitol on renal function following cardio-pulmonary bypass in patients with normal pre-operative creatinine | 2008 | ⊕ | ⊕ | ⊖ | ⊕ | ⊕ | ⊕ | ⊕ | ⊖ | ⊖ |
|
| Smith, MN et al. | The effect of mannitol on renal function after cardiopulmonary bypass in patients with established renal dysfunction | 2008 | ⊕ | ⊕ | ⊖ | ⊕ | ⊖ | ⊕ | ⊕ | ⊖ | ⊖ | Perfusionist was not blinded, but the rest of the study personnel were. |
| Ejaz, AA et al. | Prophylactic nesiritide does not prevent dialysis or all-cause mortality in patients undergoing high-risk cardiac surgery | 2009 | ⊕ | ⊕ | ? | ⊕ | ? | ⊕ | ⊕ | ? | ⊕ |
|
| Sezai, A et al. | Influence of continuous infusion of low-dose human atrial natriuretic peptide on renal function during cardiac surgery: A randomized controlled study | 2009 | ⊕ | ? | ? | ? | ? | ⊕ | ⊕ | ? | ⊖ |
|
| Operative Techniques | ||||||||||||
| Kocakulak, M et al. | Pulsatile flow improves renal function in high-risk cardiac operations | 2005 | ⊕ | ⊖ | ⊖ | ⊕ | ⊖ | ? | ⊕ | ⊖ | ⊖ |
|
| Sajja, LR et al. | Coronary artery bypass grafting with or without cardiopulmonary bypass in patients with preoperative non-dialysis dependent renal insufficiency: A randomized study | 2007 | ⊕ | ⊕ | ⊖ | ⊕ | ⊖ | ⊕ | ⊕ | ? | ⊖ | |
| Boodhwani, M et al. | Effects of mild hypothermia and rewarming on renal function after coronary artery bypass grafting | 2009 | ⊕ | ⊕ | ⊕ | ⊕ | ⊖ | ⊕ | ⊕ | ? | ⊕ | Single-center pilot study designed to study neurologic outcomes of temperature management on CPB. Post hoc analysis for renal outcomes. |
| Presta, P et al. | Can pulsatile cardiopulmonary bypass prevent perioperative renal dysfunction during myocardial revascularization in elderly patients? | 2009 | ⊕ | ⊕ | ⊖ | ⊕ | ⊖ | ⊕ | ⊕ | ? | ⊖ | |
| Remote Ischemic Preconditioning | ||||||||||||
| Venugopal, V et al. | Effect of remote ischemic preconditioning on acute kidney injury in nondiabetic patients undergoing coronary artery bypass graft surgery: A secondary analysis of 2 small randomized trials | 2010 | ⊕ | ⊕ | ⊖ | ⊕ | ⊕ | ⊕ | ⊕ | ? | ⊖ | Results could have been confounded by significantly longer clamp times in the control group, although a further analysis of AKI on those who only underwent CABG remained significant. |
| Choi, YS et al. | Effect of remote ischemic preconditioning on renal dysfunction after complex valvular heart surgery: A randomized controlled trial | 2011 | ⊕ | ⊕ | ⊖ | ⊕ | ⊕ | ⊕ | ⊕ | ? | ⊖ | Small single-center study. |
| Zimmerman, RF et al. | Ischemic preconditioning at a remote site prevents acute kidney injury in patients following cardiac surgery | 2011 | ⊕ | ⊕ | ⊕ | ⊕ | ⊖ | ⊕ | ⊕ | ? | ⊖ | |
| Sodium Bicarbonate | ||||||||||||
| Haase, M et al. | Sodium bicarbonate to prevent increases in serum creatinine after cardiac surgery: A pilot double-blind, randomized controlled trial | 2009 | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ? | ⊕ | Single-center pilot study |
| Kristeller, JL et al. | Lack of effectiveness of sodium bicarbonate in preventing kidney injury in patients undergoing cardiac surgery: A randomized controlled trial | 2013 | ⊕ | ⊕ | ? | ? | ⊕ | ⊕ | ⊕ | ? | ⊖ |
|
| Haase, M et al. | Prophylactic perioperative sodium bicarbonate to prevent acute kidney injury following open heart surgery: A multicenter double-blinded randomized controlled trial | 2013 | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊖ | ⊕ | ⊕ | ⊕ |
|
| McGuinness, SP et al. | Sodium bicarbonate infusion to reduce cardiac surgery-associated acute kidney injury: A phase II multicenter double-blind randomized controlled trial | 2013 | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | Some aspects of CPB were not standardized across institutions. |
| Other | ||||||||||||
| Durmaz, I et al. | Prophylactic dialysis in patients with renal dysfunction undergoing on-pump coronary artery bypass surgery | 2003 | ⊕ | ⊕ | ⊖ | ⊕ | ⊖ | ⊕ | ⊕ | ⊖ | ⊖ | Method of randomization (based on the last digit of the medical record number) is questionable. |
| Marathias, KP et al. | Preoperative intravenous hydration confers renoprotection in patients with chronic kidney disease undergoing cardiac surgery | 2006 | ⊕ | ⊖ | ⊖ | ⊕ | ⊖ | ⊕ | ⊕ | ⊖ | ⊖ | |
| Song, YR et al. | Prevention of acute kidney injury by erythropoietin in patients undergoing coronary artery bypass grafting: A pilot study | 2009 | ⊕ | ⊕ | ? | ⊕ | ⊕ | ⊕ | ⊕ | ? | ⊖ |
|
| Nouri-Majalan, N et al. | Effects of allopurinol and vitamin E on renal function in patients with cardiac coronary artery bypass grafts | 2009 | ⊕ | ⊖ | ⊖ | ? | ? | ⊕ | ⊕ | ⊖ | ⊖ | |
| Prowle, JR et al. | Pilot double-blind, randomized controlled trial of short-term atorvastatin for prevention of acute kidney injury after cardiac surgery | 2011 | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ? | ⊖ |
|
| Oh, SW et al. | Erythropoietin improves long-term outcomes in patients with acute kidney injury after coronary artery bypass grafting | 2012 | ⊕ | ⊕ | ⊕ | ? | ⊕ | ⊕ | ⊕ | ? | ⊖ | |
| Ejaz, AA et al. | Effect of uric acid lowering therapy on the prevention of acute kidney injury in cardiovascular surgery | 2013 | ⊕ | ⊕ | ? | ⊕ | ? | ⊕ | ⊕ | ? | ⊖ |
|
| Darcin, OT et al. | Effect of iloprost on renal function in patients undergoing coronary artery bypass grafting: A clinical study | 2013 | ⊕ | ⊖ | ⊖ | ? | ⊖ | ⊕ | ⊕ | ? | ⊖ | Single-center study lacking in methodology details. |
| Meta-Analysis Appraisal | ||||||||||||
| Landoni, G et al. | Fenoldopam reduces the need for renal replacement therapy and in-hospital death in cardiovascular surgery: A meta-analysis | 2008 | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | Results had low heterogeneity in the primary outcome of interest (RRT). | |
| Adabag, AS et al. | Efficacy of N-acetylcysteine in preventing renal injury after heart surgery: A systematic review of randomized trials | 2009 | ⊕ | ? | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ |
|
|
| Seabra, VF et al. | Off-pump coronary artery bypass surgery and acute kidney injury: A meta-analysis of randomized controlled trials | 2010 | ⊕ | ? | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | MEDLINE was only database searched for relevant papers. | |
| Patel, NL et al. | Pharmacological therapies for the prevention of acute kidney injury following cardiac surgery: A systematic review | 2011 | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊖ | ⊕ | ⊕ | Significant heterogeneity for several outcomes. | |
| Li, L et al. | The role of remote ischemic preconditioning on postoperative kidney injury in patients undergoing cardiac and vascular interventions: A meta-analysis | 2013 | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊖ | ⊕ | COI section reports funding from a foundation grant, but unclear if individual authors had any RWIs worth noting. | |
Appendix D. ACC Recommendation System.
APPENDIX E—STS/SCA/AmSECT Clinical Practice Guidelines for the Prevention of Adult Cardiac Surgery-Associated Acute Kidney Injury
INTRODUCTION
As outlined in the practice guideline, a systematic review of the literature, and meta-analysis, where appropriate, was performed for all recommendations listed. Several strategies to prevent AKI after cardiac surgery have been studied repeatedly or continue to be practiced widely despite weak or mixed evidence supporting their use. These interventions, with either conflicting evidence or insufficient evidence to support a recommendation are listed herein.
The strategies fall into one of three categories: 1) cardiopulmonary bypass techniques; 2) fluid management and transfusion; 3) pharmacological interventions. Each intervention and the available evidence are reviewed in detail below. A brief executive summary of the evidence is provided in the following Tables.
SUMMARY OF EVIDENCE
| Strategy | Rationale | Evidence |
|---|---|---|
| Cardiopulmonary Bypass Strategies | ||
| Leukocyte filtration | Reduce inflammatory response & associated AKI | Several small, non-randomized studies (n < 100) with some reduction in biomarkers & RRT |
| Arterial cannula with filter | Reduce micro-emboli to kidneys | 1 RCT (n = 1,289) showing reduced incidence of AKI in high-risk patients |
| Arterial perfusion pressure | Maintaining higher renal perfusion pressure | Several studies (randomized & non-randomized) showing no effect |
| Surface heparin coating | Varying levels of anticoagulation & associated inflammatory response | Several studies (non-randomized) showing no effect |
| Hemo-/ultrafiltration | Reduce inflammatory mediators & increase hematocrit | 1 RCR (n = 199) suggesting lower biomarkers immediately postoperatively (not later) in patients with reduced eGFR |
| Fluid Management & Transfusion | ||
| Autologous CPB priming | Reduce hemodilution | 1 RCT (n = 72) showing no benefit |
| Volume expanders (HES) | Maintaining intravascular volume/oncotic pressure | 4 small RCTs not showing any difference for AKI |
| Blood salvage/autotransfusion | Reduce hemodilution | 2 small RCTs (n = 34, n = 80) not showing a difference for AKI |
| Maintaining a hematocrit target | Reduce hemodilution | 1 RCT (n = 54) showing no benefit to prevent AKI |
| Preoperative hydration | Maintaining intravascular volume | 1 RCT (n = 45) randomizing patients with moderate—severe CKG suggesting lower incidence of AKI/dialysis |
| Transfusion for low hematocrit | Reduce hemodilution | 4 RCT (including TITre2 & TRACS) showing no difference for AKI |
| Remote ischemic preconditioning | Unclear | 10 RCT, 2 showing benefit, 8 showing no benefit; meta-analysis with pooled effect suggesting no benefit (RR .95; .86–1.06) for AKI |
| Prophylactic dialysis | Unclear | 1 RCT (n = 44) with questionable study design suggesting benefit (reduced dialysis) |
| Pharmacological Strategies. | ||
| Diuretics | Increased diuresis | 3 small RCT (n = 24, n = 126, n = 50) not showing any benefit in terms of AKI |
| Vasodilators | Increased renal perfusion | Several small RCTs all showing increased urine output, decreased biomarkers (creatinine) but no reduction in RRT |
CARDIOPULMONARY BYPASS STRATEGIES
Leukocyte Filtration (Depletion)
Leukocyte depletion is a strategy designed to minimize the inflammatory response to CPB. Several small, single-center randomized trials have reported an improvement in renal function when leukodepletion was used, although with varying methods for leukodepletion. Bolcal et al. studied 50 CABG surgery patients with preoperative renal dysfunction (creatinine 1.5–2.0 mg/dL) undergoing CPB with or without leukodepletion in the arterial line and reported reduced tubular postoperative serum creatinine, blood urea nitrogen, and α-glutathione S-transferase in the depleted group (1). Rubino and coworkers studied 82 CABG patients undergoing CPB with or without leukodepletion in the arterial and cardioplegia lines and reported higher eGFR and lower creatinine and BUN and less patients requiring renal replacement therapy in the treatment group (2). Similarly, Tang and colleagues studied 40 patients undergoing CPB with or without leukodepletion in the arterial line throughout CPB. The group that underwent leukocyte filtration had significantly less increase in urinary markers of renal injury (retinol-binding protein and microalbumin) but no difference in serum creatinine or BUN (3).
Cannula Selection
Banbury and colleagues randomized 1,289 patients undergoing CABG, aortic valve replacement, or mitral valve repair/replacement to either with an integrated deployable filter or standard arterial cannula (4). Patients with moderate or greater preoperative risk (Higgins score ≥5) and treated with the deployable filter arterial cannula had a lower incidence of renal complications. The proposed mechanism was a reduction in particulate emboli captured by the 120-μm filter used with the deployable filter cannula (4). Patients treated with the filter cannula had a lower incidence of renal insufficiency (13.7% vs. 23.9%, p = .04) (4). Renal insufficiency was defined as an elevation of the serum creatinine above 2.0 mg/dL in patients with normal creatinine levels preoperatively, a >50% increase in creatinine above an abnormal baseline level, or the need for dialysis (4).
Perfusion Pressure
Sirvinskas et al. studied 179 patients with normal preoperative renal function undergoing CABG, valve, or valve/CABG surgery with CPB (5). Patients were randomized to one of three groups based on a target perfusion pressure during CPB: 1) <60 mmHg, 2) 60–69.9 mmHg, and 3) ≥70 mmHg. No differences were reported in the incidence of acute kidney injury, serum creatinine levels or creatinine clearance during surgery or the first 48 hours after surgery (5). More recently Vedel 2018 and Kandler 2019 both reported randomized trials which evaluated renal outcomes. Vedel in a study designed to evaluate cerebral outcomes achieved a mean pressure of 66.8 ± 4.9 mmHg in the high pressure group compared with 44.77 ± 4.7 mmHg in the control, and reported no differences in detailed renal outcomes including serum creatinine and dialysis (6). Kandler 2019 achieved a mean pressure of 61 ± 4 mmHg in the high pressure group (compare with 47 ± 5 mmHg in control) and reported no differences in AKI, serum creatinine, glomerular filtration rates or dialysis.
Surface Coating
Svenmarker and colleagues reported a reduction in serum creatinine at discharge in CABG patients that underwent CPB with heparin-coated circuits and lower heparinization (ACT >250 seconds) vs. patients managed with uncoated circuits and standard heparinization (ACT >480 seconds) (7). Allen and coworkers found no difference in renal outcomes or biomarker release in a small randomized study using coated circuits (8). Mullen and colleagues compared renal outcomes in three groups of patients undergoing CABG: 1) uncoated CPB circuits and standard heparinization (ACT >400 seconds), 2) heparin-coated and standard heparinization, or 3) heparin-coated and lower heparinization (ACT 180–400) and found no difference in postoperative creatinine levels (9).
Hemofiltration/Ultrafiltration
Zero-balance ultrafiltration (Z-BUF) is a technique that filters blood on CPB, removes volume, and then replaces that volume with an equal volume of crystalloid. The purpose is to reduce inflammatory mediators circulating during and after CPB that may mediate end-organ injury. Matata et al. performed a single-center randomized controlled trial of 199 cardiac surgical patients who had a preoperative estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 but >15 mL/min/1.73 m2. Subjects were randomized to undergo Z-BUF while on CPB vs. standard CPB protocol (10). Urinary NGAL/Cr, eGFR, and serum Cr and urea were compared between groups on ICU arrival and at 24, 48, and 72 hours after ICU arrival. These AKI markers were all significantly lower in the Z-BUF group at ICU arrival but at no other postoperative time points.
FLUID MANAGEMENT AND TRANSFUSION
Autologous Priming
Kiessling and colleagues randomized 72 patients >75 years old and/or LVEF <40% and undergoing CABG and/or aortic valve replacement (11). The treatment group had whole blood removed via central line (mean 280 mL) which was used to prime the CPB circuit just prior to initiating CPB, and the control group had a standard crystalloid prime with no autologous blood. There were no differences in the increase in creatinine or the incidence of postoperative dialysis between groups (11).
Hydroxyethyl Starch (HES)
Ooi and colleagues randomized 90 patients undergoing elective CABG with CPB (12). One group received 6% HES 130/.4 in the CPB prime and for volume replacement when needed, and the other group received 4% succinylated gelatin. There was no difference in postoperative eGFR between groups (12).
Kuitunen et al. randomized 45 patients undergoing elective CABG with CPB (13). Group 1 received 20 mL/kg of low molecular weight HES (120) in the CPB prime, group 2 received 20 mL/kg of high molecular weight HES (400) in the CPB prime, and group 3 received 4% albumin in the CPB prime. There was no difference in urine output between groups. However, the groups receiving either of the HES solutions experienced less stable clot as measured by thromboelastography (13).
Sethi et al. randomized 80 consecutive patients undergoing elective CABG on CPB to 1 of four groups (n = 20 per group). The total prime was 1,500 mL in all groups and were as follows: Group 1 = 1,000 mL HESp (potato-derived)+ 500 mL Ringer’s lactate; group 2 = 1,500 mL HESp only; Group 3 = 1,000 mL HESM (maize-derived)+ 500 mL Ringer’s lactate; group 4 = 1,500 mL HESM only. In this aim to distinguish balanced HES between these two different source material starches (potato starch vs. waxy maize starch) and the effect on renal function, the authors found no significant changes from baseline in the postoperative serum creatinine values in all groups. In fact, despite the differences in physiochemical properties, the two solutions exerted the same effect (14).
Lomivorotov et al. evaluated the influence of HES compared with normal saline on kidney integrity. This was a prospective, randomized, single-blind pilot study. Patients were randomized to receive once either 7.2% NaCl/6% HES 200/.5 (HSH group, n = 20) or placebo (.9% NaCl; n = 20) at a dose of 4 mL/kg for 30 minutes after anesthesia induction. The incidence of AKI within 48 hours was similar between the groups at 20% in the HSH group and 30% in the placebo group (NS). There was a significantly lower peak value for serum cystatin C in the HSH group (.83 [.73–.89] mg/L) compared with the control group (1.02 [.88–1.15] mg/L; p = .001). HSH does not impact AKI incidence when used for the volume therapy in on-pump coronary artery bypass surgery and had no effect on tubular injury or glomerular filtration (15).
Blood Salvage/Autotransfusion
Scrascia et al. randomized 34 patients undergoing elective CABG with CPB (16). In the control group, residual blood in the CPB circuit was discarded and no autotransfusion was used before or after CPB. In the treatment group residual CPB blood and blood collected before and after CPB was processed in an autotransfusion device and reinfused to the patient. The incidence of renal replacement therapy was not different between groups (16).
Niranjan and colleagues randomized 80 patients undergoing elective CABG to one of four groups: 1) Off-Pump CABG with autotransfusion, 2) Off-Pump CABG without autotransfusion, 3) On CPB CABG with autotransfusion, or 4) On CPB CABG without autotransfusion. The incidence of renal complications was not different between groups (17).
Myocardial infarction and multi-organ failure were not mentioned in either paper, other clinical outcomes either mentioned no occurrence or were not significantly different between experimental groups.
Hematocrit Target
von Heymann and coworkers randomized 54 low-risk patients >70 kg with preoperative HCT >36% and undergoing elective CABG with CPB (18). One group was maintained during CPB with a minimum HCT of 20% and the other group maintained with a minimum HCT of 25%. The median CPB flow index was 3.2 L/min/m2 in both groups. There was no difference in the incidence of renal failure or postoperative creatinine levels between groups. The authors concluded that DO2 did not fall below critical levels in either group (18). They recorded no significant difference for all clinical outcomes.
Preoperative Hydration
Marathias et al. randomized 45 patients with moderate to severe chronic kidney disease (eGFR <45 mL/min) and undergoing CABG (13% OPCAB) and/or valve replacement surgery (19). The treatment group received preoperative hydration with half-isotonic saline at a rate of 1 mL/kg/h for 12 hours before the operation and the control group was maintained on fluid restriction. The incidence of acute renal failure defined as a 25% increase in peak creatinine was 53% in the fluid restriction group compared with 30% in the hydration group. No patients in the hydration group requiring dialysis (p < .01) (19). There was either no occurrence, no mention, or no significant difference for clinical outcomes, there were no significant differences between groups for postoperative myocardial infarction, and time spent in ICU.
Perioperative Transfusion Strategy
Karkouti and colleagues randomized 60 anemic (HGB 10–12 g/dL) patients undergoing CABG and/or valve replacement with CPB (20). The treatment group prophylactically received 2 units packed red blood cells 1–2 days prior to surgery and the control group was transfused during or after surgery as needed. Perioperative anemia and transfusion rates were decreased in the treatment group. The incidence of AKI was similar between groups (20).
Murphy and the TITre2 Investigators found no significant difference in ischemic events-stroke, MI, gut, or AKI-with restrictive transfusion threshold (Hemoglobin <7.5 mg/dL) compared to liberal transfusion threshold (Hemoglobin <9.0 mg/dL) (21).
Nakamura et al. in a TRACS sub-study, found no difference in acute renal injury with a restrictive transfusion threshold (Hemoglobin <8 mg/dL) compared to liberal transfusion threshold (Hemoglobin <10 mg/dL) (22). Song et al. studied their transfusion protocol in 409 patients and report a low incidence of renal dysfunction, defined as a doubling of creatinine or renal replacement therapy. The restrictive transfusion threshold was Hemoglobin <6 mg/dL, and transfusion for Hemoglobin 8–10 mg/dL required evidence of end organ ischemia (23).
To summarize, the reviewed papers demonstrate the safety of autologous priming, hydroxyethyl starch, blood salvage and autotransfusion, and preoperative hydration. Transfusion for preoperative anemia was ineffective in reducing the incidence of AKI in cardiac surgery. Overall, most fluid management techniques for AKI and renal complications have little effect on clinical outcomes and the evidence to date is not strong enough to warrant clinical guideline recommendations (21,22).
Remote Ischemic Preconditioning (RIPC)
Remote ischemic preconditioning (RIPC) is conducted after induction of anesthesia prior to cardiac surgery. Therapy includes 3–4 cycles of limb ischemic for 5 minutes by using a blood pressure cuff for inflation around one upper arm or thigh followed by 5 minutes of reperfusion in each cycle. The preoperative use of remote ischemic preconditioning is believed to prevent ischemia, reperfusion, and inflammatory injury to organs during surgery through rapid cycles of brief ischemia and reperfusion (24–31).
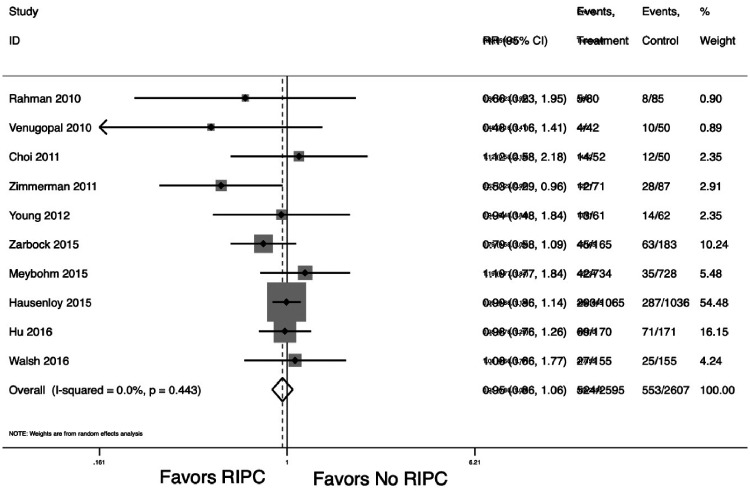
Multiple randomized trials have evaluated the efficacy of remote ischemic preconditioning in various cardiac surgery populations. A meta-analysis of these 10 trials suggests RIPC does not reduce the risk of AKI (RR .95; 95% CI: .86, 1.06; p = .376, Figure 1) (27,32–37). All trials used 3–4 cycles of 5 minutes of ischemia followed by 5 minutes of reperfusion in either an upper or lower limb. Two randomized controlled trials reported statistically significant reduction in acute kidney injury favoring remote ischemic preconditioning (36,37). Zarbock and colleagues evaluated remote ischemic preconditioning (3 cycles of 5 minutes each) in the upper arm (n = 120) compared to placebo sham controls (n = 120) in a multicenter randomized controlled trial among high-risk cardiac surgery patients. Overall remote ischemic preconditioning significantly reduced acute kidney injury reduced by 15% (95% CI, 2.56–27.44%; p = .02), renal replacement therapy was reduced by 10% (95% CI, 2.25–17.75%; p = .01), and intensive care unit stay was reduced by 1 day (p = .04) (36). Zimmerman and colleagues conducted a single-center randomized controlled trial among 120 elective patients undergoing cardiac surgery using 3 cycles of ischemia–reperfusion for 5 minutes in a lower limb. Remote ischemic preconditioning significantly reduced acute kidney injury by 27% (95% CI .10–.42; p = .004) (37). Choi and colleagues conducted a small randomized control trial of 76 adult patients undergoing complex valve surgery using 3 cycles of 5-minute ischemia–reperfusion in the lower limb but found no benefit in reducing acute kidney injury (37%) compared with controls (32%, p = .7) (32). Young and colleagues conducted a pilot randomized controlled trial among 96 adults undergoing high-risk cardiac surgery using 3 cycles of 5 minutes of ischemia–reperfusion in an upper limb yet found no difference in acute kidney injury (32,35). Two randomized trials, Rahman and Venugopal, reported on randomized placebo-controlled trials in elective or urgent nondiabetic CABG patients using 3 cycles of 5 minutes each in upper arm remote ischemic preconditioning (33,34). Both reported nonsignificant trends toward remote ischemic preconditioning benefit in preventing acute kidney injury (33,34). Hu and colleagues in the remote ischemic preconditioning (RIPerc) study randomized valve surgery patients with rheumatic heart disease to three cycles of 5 minutes each in the right thigh during surgery or control (29). The RIPerc study found no difference in AKI (68% in RIPerc group compared to 71% in controls, p = .67) (29). In the Remote IMPACT trial, Walsh randomized patients to 3 cycles of 5 minutes each on one thigh or to a sham procedure (31). They found no difference in AKI with RR 1.10 (95% CI: .68, 1.78), however found a higher incidence of acute dialysis in the RIPC group at 4.9% compared to 1.6% in the sham group (31). The ERICCA trial by Hausenloy and colleagues randomized patients to 4 cycles of 5 minutes each of RIPC to the upper arm or to control (28). The ERICCA trial found no difference in AKI (38.0% in RIPC and 38.3% in control, p = .98) (28). The RIPHeart study by Meybohm and colleagues randomized elective patients to 4 cycles of 5 minutes each of RIPC to the upper limb or to a sham procedure (30). The RIPHeart study found no difference in AKI (RIPC 6.1% compared to sham 5.1%, p = .45).
Figure 1.
Meta-analysis RIPC and AKI.
The strength of the evidence is insufficient to recommend remote ischemic preconditioning as a targeted prophylactic therapy to prevent acute kidney injury among elective or urgent cardiac surgery patients. Additional trials powered to detect differences in the requirement for new dialysis and clarify efficacy among nondiabetic and high-risk valve populations are needed.
Prophylactic Dialysis
Prophylactic perioperative hemodialysis has been considered a targeted therapy to prevent AKI and postoperative dialysis among patients in acute renal failure at the time of surgery but without a history of dialysis. For these patients at highest risk of developing acute kidney injury, it is believed that initiating dialysis in the perioperative setting will prevent acute and long-term renal injury.
One trial by Durmaz and colleagues randomized 44 patients with a baseline serum creatinine >2.5 (mg/dL) not requiring dialysis to either prophylactic hemodialysis or control. In the prophylactic hemodialysis group, hemodialysis was conducted twice within 72 hours of the surgery and postoperatively only if the serum creatinine was >10% elevated over baseline. In the control group, hemodialysis was initiated postoperatively only if the serum creatinine was >50% elevated over baseline. However postoperative initiation of hemodialysis was only started in 1 (4.8%) patient in the prophylactic arm and started in 8 (34.8%) patients in the control arm (p = .023). In addition, in-hospital mortality was significantly lower among the prophylactic hemodialysis patients with only one death (4.8%) compared to seven deaths (30.4%) among the control patients (p = .048). ICU length of stay was also shorter in the prophylactic dialysis group at 39.47 ± 21.87 days compared with 85.34 ± 68.89 days in the control group (p = .005) (38). Prophylactic dialysis among patients with preexisting renal dysfunction with a serum creatinine >2.5 (mg/dL) demonstrates potential in preventing postoperative dialysis and in-hospital mortality. However, additional trials are needed (38).
PHARMACOLOGIC STRATEGIES
Diuretics
Dopexamine, a synthetic analogue of dopamine, has been studied. In a small randomized clinical trial, 24 patients with preoperative renal dysfunction (serum creatinine ≥1.5 mg/dL) and 24 patients without renal dysfunction were randomly assigned to either receive or not receive dopexamine infusion (1 μg/kg/min) during cardiac operations. Regardless of preoperative renal function status, patients who received dopexamine infusion exhibited increased cardiac index and urine output, but did not exhibit biomarker evidence of reduced renal injury compared with those who did not receive dopexamine (39).
In a single-center, double-blind randomized clinical trial, 126 patients with normal preoperative renal function were assigned to receive furosemide infusion (.5 μg/kg/min), dopamine infusion (2 μg/kg/min), or placebo during their cardiac operations and the early postoperative period. Patients who received furosemide exhibited the highest maximum increase in serum creatinine (p = .001) and had the highest incidence of acute renal injury (6/41, p = .01), defined as a maximum increase in serum creatinine >.5 mg/dL (40). More recently, Mahesh and colleagues conducted a single-center, double-blind randomized clinical trial to evaluate the use of furosemide in patients at high risk for postoperative renal dysfunction (41). Fifty patients undergoing cardiac surgery were assigned to receive furosemide infusion (4 mg/h) or placebo during the operation and the early postoperative period. Patients who received furosemide had a higher urine output and higher fluid requirement, but no differences in the incidence of renal dysfunction compared to controls. Although the furosemide group had lower postoperative urinary retinol-binding protein levels, urinary retinol-binding protein/creatinine ratios were similar (41).
Additional studies have tested mannitol and furosemide for adverse effects on clinical outcomes. Mahesh and colleagues recorded no significant differences between mannitol groups for death and postoperative dialysis, nor was there a need for postoperative dialysis for the study done by Mahesh et al. (41).
Vasodilators
One single-center, blinded, placebo-controlled randomized clinical trial involving 240 patients who underwent CABG showed beneficial effects of administering sodium nitroprusside during the rewarming period of CPB (titrated after starting dose of .1 μg/kg/h) (42). Compared to controls, patients who received sodium nitroprusside exhibited significantly higher intraoperative urine output, lower incidence of increased postoperative serum creatinine, lower incidence of reduced postoperative creatinine clearance, and higher postoperative glomerular filtration rates. Doubling of postoperative creatinine levels occurred less frequently in patients who received sodium nitroprusside (2/124) than control patients (8/116; p = .053). There were no significant differences in clinical complications in this trial (42).
The results of two small randomized clinical trials suggest that the combination of diltiazem and dopamine may provide renal protection. In the first trial, in low-risk patients with normal preoperative renal function undergoing CABG with CPB, perioperative combined administration of diltiazem and dopamine (both at 2 μg/kg/min) resulted in higher creatinine clearance, osmotic clearance, and free water clearance values on postoperative day 1 compared to those who received diltiazem only, dopamine only, or neither drug; these differences did not persist at day 7 (43). In the second randomized clinical trial, perioperative administration of diltiazem (.25 μg/kg bolus followed by 1.7 μg/kg/min infusion) in patients with mild-to-moderate preoperative renal dysfunction was associated with improved GFR (as measured by iohexol clearance) at 3 weeks after CAB and/or valve procedures; there were no differences in serum creatinine levels or urinary N-acetyl-β-glucosaminidase concentrations between the treatment and control groups (44). All patients in this study received perioperative dopamine infusion (2 μg/kg/min) (44).
Bergman et al. observed that the patients receiving Diltiazem treatments did not have any significant changes from the placebo group for the time spent in the ICU, they also recorded no occurrence of respiratory complications (44). Yavuz and coworkers observed no differences in treatment groups for in-hospital mortality, cardiac complications, respiratory issues, or renal dysfunction during their dopamine trial (43).
In one single-center, non-blinded randomized clinical trial (45), intravenous administration of a synthetic analog of prostacyclin PGI2 (1.25–2.5 ng/kg/min), during the rewarming phase of CPB, was associated with higher intraoperative urine output, lower incidence of increased postoperative serum creatinine, and a lower incidence of reduced postoperative creatinine clearance compared to controls. The trial listed nonsignificant differences among mortality, stroke, and postoperative dialysis (45).
Disclosures
Rakesh C. Arora reports relationships with Mallinckrodt LLC and Edwards Lifesciences Corporation that include consulting or advisory and a relationship with Abbott Nutrition that includes travel reimbursement. Chirag R. Parikh reports a relationship with Renalytix that includes equity or stocks, and relationships with Genfit SA and Novartis Pharmaceuticals Corporation that include consulting or advisory. Richard Solomon reports a relationship with MediBeacon that includes consulting or advisory and a relationship with Sonogenix that includes funding grants. Kevin W. Lobdell reports a relationship with Medtronic that includes consulting or advisory. The other authors have no conflicts of interest to disclose.
The Appendices can be viewed in the online version of this article [https://dx.doi.org/10.1016/j.athoracsur.2022.06.054] on https://www.annalsthoracicsurgery.org.
FUNDING
Dr. Brown has received support from the NIH, including: NHLBI R01HL199664, R01HL130828, R01HL157130, NIDDK R01DK113201, R01DK122073, and NIAID R01AI155752. Dr Fox has received support from the NIH, R01HL148448. There was no funding for these guidelines.
REFERENCES
- 1.Brown JR, Cochran RP, Dacey LJ, et al. Perioperative increases in serum creatinine are predictive of increased 90-day mortality after coronary artery bypass graft surgery. Circulation. 2006;114(suppl):I409–13. [DOI] [PubMed] [Google Scholar]
- 2.Brown JR, Cochran RP, Leavitt BJ, et al. Multivariable prediction of renal insufficiency developing after cardiac surgery. Circulation. 2007;116(suppl):I139–43. [DOI] [PubMed] [Google Scholar]
- 3.Vives M, Wijeysundera D, Marczin N, et al. Cardiac surgery-associated acute kidney injury. Interact Cardiovasc Thorac Surg. 2014;18:637–45. [DOI] [PubMed] [Google Scholar]
- 4.Kellum JA, Lameire N. Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (part 1). Crit Care. 2013;17:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown JR, Kramer RS, Coca SG, et al. Duration of acute kidney injury impacts long-term survival after cardiac surgery. Ann Thorac Surg. 2010;90:1142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown JR, Kramer RS, MacKenzie TA, et al. Determinants of acute kidney injury duration after cardiac surgery: An externally validated tool. Ann Thorac Surg. 2012;93:570–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagshaw SM, George C, Bellomo R. A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23:1569–74. [DOI] [PubMed] [Google Scholar]
- 8.Joannidis M, Metnitz B, Bauer P, et al. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med. 2009;35:1692–702. [DOI] [PubMed] [Google Scholar]
- 9.Acute Kidney Injury Work Group . Kidney disease: Improving global outcomes (KDIGO). KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2(suppl 1):1–138. [Google Scholar]
- 10.Nadim MK, Forni LG, Bihorac A, et al. Cardiac and vascular surgery-associated acute kidney injury: The 20th International Consensus Conference of the ADQI (Acute Disease Quality Initiative) group. J Am Heart Assoc. 2018;7:e008834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meersch M, Schmidt C, Hoffmeier A, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: The PrevAKI randomized controlled trial. Intensive Care Med. 2017;43:1551–61. Published correction appears in Intensive Care Med. 2017;43:1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aldea GS, Bakaeen FG, Pal J, et al. The Society of Thoracic Surgeons clinical practice guidelines on arterial conduits for coronary artery bypass grafting. Ann Thorac Surg. 2016;101:801–9. [DOI] [PubMed] [Google Scholar]
- 13.Badhwar V, Rankin JS, Damiano RJ Jr, et al. The Society of Thoracic Surgeons 2017 clinical practice guidelines for the surgical treatment of atrial fibrillation. Ann Thorac Surg. 2017;103:329–41. [DOI] [PubMed] [Google Scholar]
- 14.Halperin JL, Levine GN, Al-Khatib SM, et al. Further evolution of the ACC/AHA clinical practice guideline recommendation classification system: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2016;133:1426–8. [DOI] [PubMed] [Google Scholar]
- 15.Boodhwani M, Rubens FD, Wozny D, et al. Effects of mild hypothermia and rewarming on renal function after coronary artery bypass grafting. Ann Thorac Surg. 2009;87:489–95. [DOI] [PubMed] [Google Scholar]
- 16.Newland RF, Tully PJ, Baker RA. Hyperthermic perfusion during cardiopulmonary bypass and postoperative temperature are independent predictors of acute kidney injury following cardiac surgery. Perfusion. 2013;28:223–31. [DOI] [PubMed] [Google Scholar]
- 17.Newland RF, Baker RA, Mazzone AL, et al. Rewarming temperature during cardiopulmonary bypass and acute kidney injury: A multicenter analysis. Ann Thorac Surg. 2016;101:1655–62. [DOI] [PubMed] [Google Scholar]
- 18.Ranucci M, Romitti F, Isgro G, et al. Oxygen delivery during cardiopulmonary bypass and acute renal failure after coronary operations. Ann Thorac Surg. 2005;80:2213–20. [DOI] [PubMed] [Google Scholar]
- 19.Newland RF, Baker RA. Low oxygen delivery as a predictor of acute kidney injury during cardiopulmonary bypass. J Extra Corpor Technol. 2017;49:224–30. [PMC free article] [PubMed] [Google Scholar]
- 20.Mukaida H, Matsushita S, Kuwaki K, et al. Time-dose response of oxygen delivery during cardiopulmonary bypass predicts acute kidney injury. J Thorac Cardiovasc Surg. 2019;158:492–9. [DOI] [PubMed] [Google Scholar]
- 21. Ranucci M, Johnson I, Willcox T, et al. Goal-directed perfusion to reduce acute kidney injury: A randomized trial. J Thorac Cardiovasc Surg. 2018;156:1918–27.e1912. [DOI] [PubMed] [Google Scholar]
- 22.Newland RF, Baker RA, Woodman RJ, et al. Predictive capacity of oxygen delivery during cardiopulmonary bypass on acute kidney injury. Ann Thorac Surg. 2019;108:1807–14. [DOI] [PubMed] [Google Scholar]
- 23.Anastasiadis K, Murkin J, Antonitsis P, et al. Use of minimal invasive extracorporeal circulation in cardiac surgery: Principles, definitions and potential benefits. A position paper from the Minimal Invasive Extra-Corporeal Technologies International Society (MiECTiS). Interact Cardiovasc Thorac Surg. 2016;22:647–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Y, Gong B, Yuan X, et al. What we have learned about minimized extracorporeal circulation versus conventional extracorporeal circulation: An updated meta-analysis. Int J Artif Organs. 2015;38:444–53. [DOI] [PubMed] [Google Scholar]
- 25.Kowalewski M, Pawliszak W, Raffa GM, et al. Safety and efficacy of miniaturized extracorporeal circulation when compared with off-pump and conventional coronary artery bypass grafting: Evidence synthesis from a comprehensive Bayesian-framework network meta-analysis of 134 randomized controlled trials involving 22,778 patients. Eur J Cardiothorac Surg. 2016;49:1428–40. [DOI] [PubMed] [Google Scholar]
- 26.Choi MR, Kouyoumdzian NM, Rukavina Mikusic NL, et al. Renal dopaminergic system: Pathophysiological implications and clinical perspectives. World J Nephrol. 2015;4:196–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel NN, Rogers CA, Angelini GD, et al. Pharmacological therapies for the prevention of acute kidney injury following cardiac surgery: A systematic review. Heart Fail Rev. 2011;16:553–67. [DOI] [PubMed] [Google Scholar]
- 28.Carcoana OV, Mathew JP, Davis E, et al. Mannitol and dopamine in patients undergoing cardiopulmonary bypass: A randomized clinical trial. Anesth Analg. 2003;97:1222–9. [DOI] [PubMed] [Google Scholar]
- 29.Dehne MG, Klein TF, Muhling J, et al. Impairment of renal function after cardiopulmonary bypass is not influenced by dopexamine. Ren Fail. 2001;23:217–30. [DOI] [PubMed] [Google Scholar]
- 30.Lassnigg A, Donner E, Grubhofer G, et al. Lack of renoprotective effects of dopamine and furosemide during cardiac surgery. J Am Soc Nephrol. 2000;11:97–104. [DOI] [PubMed] [Google Scholar]
- 31.Sumeray M, Robertson C, Lapsley M, et al. Low dose dopamine infusion reduces renal tubular injury following cardiopulmonary bypass surgery. J Nephrol. 2001;14:397–402. [PubMed] [Google Scholar]
- 32.Woo EB, Tang AT, el-Gamel A, et al. Dopamine therapy for patients at risk of renal dysfunction following cardiac surgery: Science or fiction? Eur J Cardiothorac Surg. 2002;22:106–11. [DOI] [PubMed] [Google Scholar]
- 33.Yavuz S, Ayabakan N, Dilek K, et al. Renal dose dopamine in open heart surgery. Does it protect renal tubular function? J Cardiovasc Surg (Torino). 2002;43:25–30. [PubMed] [Google Scholar]
- 34.Yavuz S, Ayabakan N, Goncu MT, et al. Effect of combined dopamine and diltiazem on renal function after cardiac surgery. Med Sci Monit. 2002;8:PI45–50. [PubMed] [Google Scholar]
- 35.Mahesh B, Yim B, Robson D, et al. Does furosemide prevent renal dysfunction in high-risk cardiac surgical patients? Results of a double-blinded prospective randomized trial. Eur J Cardiothorac Surg. 2008;33:370–6. [DOI] [PubMed] [Google Scholar]
- 36.Smith MN, Best D, Sheppard SV, et al. The effect of mannitol on renal function after cardiopulmonary bypass in patients with established renal dysfunction. Anaesthesia. 2008;63:701–4. [DOI] [PubMed] [Google Scholar]
- 37.Yallop KG, Sheppard SV, Smith DC. The effect of mannitol on renal function following cardio-pulmonary bypass in patients with normal pre-operative creatinine. Anaesthesia. 2008;63:576–82. [DOI] [PubMed] [Google Scholar]
- 38.Bergman AS, Odar-Cederlof I, Westman L, et al. Diltiazem infusion for renal protection in cardiac surgical patients with preexisting renal dysfunction. J Cardiothorac Vasc Anesth. 2002;16:294–9. [DOI] [PubMed] [Google Scholar]
- 39.Bove T, Landoni G, Calabrò MG, et al. Renoprotective action of fenoldopam in high-risk patients undergoing cardiac surgery: A prospective, double-blind, randomized clinical trial. Circulation. 2005;111:3230–5. [DOI] [PubMed] [Google Scholar]
- 40.Cogliati AA, Vellutini R, Nardini A, et al. Fenoldopam infusion for renal protection in high-risk cardiac surgery patients: A randomized clinical study. J Cardiothorac Vasc Anesth. 2007;21:847–50. [DOI] [PubMed] [Google Scholar]
- 41.Darcin OT, Zor MH, Sahin V, et al. Effect of iloprost on renal function in patients undergoing coronary artery bypass grafting: A clinical study. Ann Thorac Cardiovasc Surg. 2013;19:12–7. [DOI] [PubMed] [Google Scholar]
- 42.Kaya K, Oguz M, Akar AR, et al. The effect of sodium nitroprusside infusion on renal function during reperfusion period in patients undergoing coronary artery bypass grafting: A prospective randomized clinical trial. Eur J Cardiothorac Surg. 2007;31:290–7. [DOI] [PubMed] [Google Scholar]
- 43.Landoni G, Biondi-Zoccai GG, Marino G, et al. Fenoldopam reduces the need for renal replacement therapy and in-hospital death in cardiovascular surgery: A meta-analysis. J Cardiothorac Vasc Anesth. 2008;22:27–33. [DOI] [PubMed] [Google Scholar]
- 44.Ranucci M, De Benedetti D, Bianchini C, et al. Effects of fenoldopam infusion in complex cardiac surgical operations: A prospective, randomized, double-blind, placebo-controlled study. Minerva Anestesiol. 2010;76:249–59. [PubMed] [Google Scholar]
- 45.Ranucci M, Soro G, Barzaghi N, et al. Fenoldopam prophylaxis of postoperative acute renal failure in high-risk cardiac surgery patients. Ann Thorac Surg. 2004;78:1332–7, discussion 1337–8. [DOI] [PubMed] [Google Scholar]
- 46.Roasio A, Lobreglio R, Santin A, et al. Fenoldopam reduces the incidence of renal replacement therapy after cardiac surgery. J Cardiothorac Vasc Anesth. 2008;22:23–6. [DOI] [PubMed] [Google Scholar]
- 47.Flesch M, Knipp S, Kessler G, et al. ARTA: At1-receptor blocker therapy in patients undergoing coronary artery bypass grafting. Clin Res Cardiol. 2009;98:33–43. [DOI] [PubMed] [Google Scholar]
- 48.Türker H, Dönmez A, Zeyneloğlu P, et al. Effects of enalaprilat infusion on hemodynamics and renal function in patients undergoing cardiac surgery. Anadolu Kardiyol Derg. 2004;4:296–300. [PubMed] [Google Scholar]
- 49.Witczak BJ, Hartmann A, Geiran OR, et al. Renal function after cardiopulmonary bypass surgery in patients with impaired renal function. A randomized study of the effect of nifedipine. Eur J Anaesthesiol. 2008;25:319–25. [DOI] [PubMed] [Google Scholar]
- 50.Sun H, Xie Q, Peng Z. Does fenoldopam protect kidney in cardiac surgery? A systemic review and meta-analysis with trial sequential analysis. Shock. 2019;52:326–33. [DOI] [PubMed] [Google Scholar]
- 51.Zangrillo A, Biondi-Zoccai GG, Frati E, et al. Fenoldopam and acute renal failure in cardiac surgery: A meta-analysis of randomized placebo-controlled trials. J Cardiothorac Vasc Anesth. 2012;26:407–13. [DOI] [PubMed] [Google Scholar]
- 52.Engelman DT, Crisafi C, Germain M, et al. Using urinary biomarkers to reduce acute kidney injury following cardiac surgery. J Thorac Cardiovasc Surg. 2020;160:1235–46.e2. [DOI] [PubMed] [Google Scholar]
- 53.Magruder JT, Crawford TC, Harness HL, et al. A pilot goal-directed perfusion initiative is associated with less acute kidney injury after cardiac surgery. J Thorac Cardiovasc Surg. 2017;153:118–25. e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
REFERENCES
- 1.Bolcal C, Akay HT, Bingol H, et al. Leukodepletion improves renal function in patients with renal dysfunction undergoing on-pump coronary bypass surgery: A prospective randomized study. Thorac Cardiovasc Surg. 2007;55:89–93. [DOI] [PubMed] [Google Scholar]
- 2.Rubino AS, Serraino GF, Mariscalco G, et al. Leukocyte depletion during extracorporeal circulation allows better organ protection but does not change hospital outcomes. Ann Thorac Surg. 2011;91:534–40. [DOI] [PubMed] [Google Scholar]
- 3.Tang AT, Alexiou C, Hsu J, et al. Leukodepletion reduces renal injury in coronary revascularization: A prospective randomized study. Ann Thorac Surg. 2002;74:372–7, discussion 377. [DOI] [PubMed] [Google Scholar]
- 4.Banbury MK, Kouchoukos NT, Allen KB, et al. Emboli capture using the Embol-X intraaortic filter in cardiac surgery: A multicentered randomized trial of 1,289 patients. Ann Thorac Surg. 2003;76:508–15, discussion 515. [DOI] [PubMed] [Google Scholar]
- 5.Sirvinskas E, Andrejaitiene J, Raliene L, et al. Cardiopulmonary bypass management and acute renal failure: Risk factors and prognosis. Perfusion. 2008;23:323–7. [DOI] [PubMed] [Google Scholar]
- 6.Vedel AG, Holmgaard F, Rasmussen LS, et al. High-target versus low-target blood pressure management during cardiopulmonary bypass to prevent cerebral injury in cardiac surgery patients: A randomized controlled trial. Circulation. 2018;137:1770–80. [DOI] [PubMed] [Google Scholar]
- 7.Svenmarker S, Haggmark S, Jansson E, et al. Use of heparin-bonded circuits in cardiopulmonary bypass improves clinical outcome. Scand Cardiovasc J. 2002;36:241–6. [DOI] [PubMed] [Google Scholar]
- 8.Allen S, McBride WT, Young IS, et al. A clinical, renal and immunological assessment of surface modifying additive treated (smart) cardiopulmonary bypass circuits. Perfusion. 2005;20:255–62. [DOI] [PubMed] [Google Scholar]
- 9.Mullen JC, Bentley MJ, Gelfand ET, et al. Coronary artery bypass surgery with heparin-coated perfusion circuits and low-dose heparinization. Can J Surg. 2002;45:166–72. [PMC free article] [PubMed] [Google Scholar]
- 10.Matata BM, Scawn N, Morgan M, et al. A single-center randomized trial of intraoperative zero-balanced ultrafiltration during cardiopulmonary bypass for patients with impaired kidney function undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2015;29:1236–47. [DOI] [PubMed] [Google Scholar]
- 11.Kiessling AH, Wedde S, Keller H, et al. Pre-filling of the extracorporeal circuit with autologous blood is safe, but not effective in optimizing biocompatibility in high-risk patients. Perfusion. 2012;27:371–7. [DOI] [PubMed] [Google Scholar]
- 12.Ooi JS, Ramzisham AR, Zamrin MD. Is 6% hydroxyethyl starch 130/0.4 safe in coronary artery bypass graft surgery? Asian Cardiovasc Thorac Ann. 2009;17:368–72. [DOI] [PubMed] [Google Scholar]
- 13.Kuitunen AH, Hynynen MJ, Vahtera E, et al. Hydroxyethyl starch as a priming solution for cardiopulmonary bypass impairs hemostasis after cardiac surgery. Anesth Analg. 2004;98:291–7, table of contents. [DOI] [PubMed] [Google Scholar]
- 14.Sethi BS, Chauhan S, Bisoi AK, et al. Comparison of a waxy maize and a potato starch-based balanced hydroxyethyl starch for priming in patients undergoing coronary artery bypass grafting. J Cardiothorac Vasc Anesth. 2014;28:690–7. [DOI] [PubMed] [Google Scholar]
- 15.Lomivorotov VV, Fominskiy EV, Efremov SM, et al. Infusion of 7.2% NaCl/6% hydroxyethyl starch 200/0.5 in on-pump coronary artery bypass surgery patients: A randomized, single-blind pilot study. Shock. 2014;41:193–9. [DOI] [PubMed] [Google Scholar]
- 16.Scrascia G, Rotunno C, Nanna D, et al. Pump blood processing, salvage and re-transfusion improves hemoglobin levels after coronary artery bypass grafting, but affects coagulative and fibrinolytic systems. Perfusion. 2012;27:270–7. [DOI] [PubMed] [Google Scholar]
- 17.Niranjan G, Asimakopoulos G, Karagounis A, et al. Effects of cell saver autologous blood transfusion on blood loss and homologous blood transfusion requirements in patients undergoing cardiac surgery on-versus off-cardiopulmonary bypass: A randomised trial. Eur J Cardiothorac Surg. 2006;30:271–7. [DOI] [PubMed] [Google Scholar]
- 18.von Heymann C, Sander M, Foer A, et al. The impact of an hematocrit of 20% during normothermic cardiopulmonary bypass for elective low risk coronary artery bypass graft surgery on oxygen delivery and clinical outcome—A randomized controlled study [ISRCTN35655335]. Crit Care. 2006;10:R58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marathias KP, Vassili M, Robola A, et al. Preoperative intravenous hydration confers renoprotection in patients with chronic kidney disease undergoing cardiac surgery. Artif Organs. 2006;30:615–21. [DOI] [PubMed] [Google Scholar]
- 20.Karkouti K, Wijeysundera DN, Yau TM, et al. Advance targeted transfusion in anemic cardiac surgical patients for kidney protection: An unblinded randomized pilot clinical trial. Anesthesiology. 2012;116:613–21. [DOI] [PubMed] [Google Scholar]
- 21.Murphy GJ, Pike K, Rogers CA, et al. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med. 2015;373:193. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura RE, Vincent JL, Fukushima JT, et al. A liberal strategy of red blood cell transfusion reduces cardiogenic shock in elderly patients undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2015;150:1314–20. [DOI] [PubMed] [Google Scholar]
- 23.Song HK, von Heymann C, Jespersen CM, et al. Safe application of a restrictive transfusion protocol in moderate-risk patients undergoing cardiac operations. Ann Thorac Surg. 2014;97:1630–5. [DOI] [PubMed] [Google Scholar]
- 24.Birnbaum Y, Hale SL, Kloner RA. Ischemic preconditioning at a distance: Reduction of myocardial infarct size by partial reduction of blood supply combined with rapid stimulation of the gastrocnemius muscle in the rabbit. Circulation. 1997;96:1641–6. [DOI] [PubMed] [Google Scholar]
- 25.Gho BC, Schoemaker RG, van den Doel MA, et al. Myocardial protection by brief ischemia in noncardiac tissue. Circulation. 1996;94:2193–200. [DOI] [PubMed] [Google Scholar]
- 26.Kharbanda RK, Nielsen TT, Redington AN. Translation of remote ischaemic preconditioning into clinical practice. Lancet. 2009;374:1557–65. [DOI] [PubMed] [Google Scholar]
- 27.Li L, Li G, Yu C, et al. The role of remote ischemic preconditioning on postoperative kidney injury in patients undergoing cardiac and vascular interventions: A meta-analysis. J Cardiothorac Surg. 2013;8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hausenloy DJ, Candilio L, Evans R, et al. Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med. 2015;373:1408–17. [DOI] [PubMed] [Google Scholar]
- 29.Hu Q, Luo W, Huang L, et al. Multiorgan protection of remote ischemic perconditioning in valve replacement surgery. J Surg Res. 2016;200:13–20. [DOI] [PubMed] [Google Scholar]
- 30.Meybohm P, Bein B, Brosteanu O, et al. A multicenter trial of remote ischemic preconditioning for heart surgery. N Engl J Med. 2015;373:1397–407. [DOI] [PubMed] [Google Scholar]
- 31.Walsh M, Whitlock R, Garg AX, et al. Effects of remote ischemic preconditioning in high-risk patients undergoing cardiac surgery (remote impact): A randomized controlled trial. Can Med Assoc J. 2016;188:329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi YS, Shim JK, Kim JC, et al. Effect of remote ischemic preconditioning on renal dysfunction after complex valvular heart surgery: A randomized controlled trial. J Thorac Cardiovasc Surg. 2011;142:148–54. [DOI] [PubMed] [Google Scholar]
- 33.Rahman IA, Mascaro JG, Steeds RP, et al. Remote ischemic preconditioning in human coronary artery bypass surgery: From promise to disappointment? Circulation. 2010;122(suppl):S53–9. [DOI] [PubMed] [Google Scholar]
- 34.Venugopal V, Laing CM, Ludman A, et al. Effect of remote ischemic preconditioning on acute kidney injury in nondiabetic patients undergoing coronary artery bypass graft surgery: A secondary analysis of 2 small randomized trials. Am J Kidney Dis. 2010;56:1043–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young PJ, Dalley P, Garden A, et al. A pilot study investigating the effects of remote ischemic preconditioning in high-risk cardiac surgery using a randomised controlled double-blind protocol. Basic Res Cardiol. 2012;107:256. [DOI] [PubMed] [Google Scholar]
- 36.Zarbock A, Schmidt C, Van Aken H, et al. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: A randomized clinical trial. J Am Med Assoc. 2015;313:2133–41. [DOI] [PubMed] [Google Scholar]
- 37.Zimmerman RF, Ezeanuna PU, Kane JC, et al. Ischemic preconditioning at a remote site prevents acute kidney injury in patients following cardiac surgery. Kidney Int. 2011;80:861–7. [DOI] [PubMed] [Google Scholar]
- 38.Durmaz I, Yagdi T, Calkavur T, et al. Prophylactic dialysis in patients with renal dysfunction undergoing on-pump coronary artery bypass surgery. Ann Thorac Surg. 2003;75:859–64. [DOI] [PubMed] [Google Scholar]
- 39.Dehne MG, Klein TF, Muhling J, et al. Impairment of renal function after cardiopulmonary bypass is not influenced by dopexamine. Ren Fail. 2001;23:217–30. [DOI] [PubMed] [Google Scholar]
- 40.Lassnigg A, Donner E, Grubhofer G, et al. Lack of renoprotective effects of dopamine and furosemide during cardiac surgery. J Am Soc Nephrol. 2000;11:97–104. [DOI] [PubMed] [Google Scholar]
- 41.Mahesh B, Yim B, Robson D, et al. Does furosemide prevent renal dysfunction in high-risk cardiac surgical patients? Results of a double-blinded prospective randomised trial. Eur J Cardiothorac Surg. 2008;33:370–6. [DOI] [PubMed] [Google Scholar]
- 42.Kaya K, Oguz M, Akar AR, et al. The effect of sodium nitroprusside infusion on renal function during reperfusion period in patients undergoing coronary artery bypass grafting: A prospective randomized clinical trial. Eur J Cardiothorac Surg. 2007;31:290–7. [DOI] [PubMed] [Google Scholar]
- 43.Yavuz S, Ayabakan N, Goncu MT, et al. Effect of combined dopamine and diltiazem on renal function after cardiac surgery. Med Sci Monit. 2002;8:PI45–50. [PubMed] [Google Scholar]
- 44.Bergman AS, Odar-Cederlof I, Westman L, et al. Diltiazem infusion for renal protection in cardiac surgical patients with preexisting renal dysfunction. J Cardiothorac Vasc Anesth. 2002;16:294–9. [DOI] [PubMed] [Google Scholar]
- 45.Darcin OT, Zor MH, Sahin V, et al. Effect of iloprost on renal function in patients undergoing coronary artery bypass grafting: A clinical study. Ann Thorac Cardiovasc Surg. 2013;19:12–7. [DOI] [PubMed] [Google Scholar]