ABSTRACT
We have evaluated the immunogenicity of two dose of Covaxin given at a one-month interval to two adult populations, i.e. COVID-19 naïve-vaccinated individuals (n = 118) and COVID-19 recovered individuals (n = 128) with the vaccination. The immune response in the study population were assessed at three follow-ups, namely at one month post first dose, one and six months after the second dose. The persistence of S1RBD IgG and neutralizing antibodies for six months post vaccination was observed at different time intervals. The enhanced immune response was observed in both the participant groups. The study emphasizes the need for a booster dose post six months of vaccination.
KEYWORDS: Sars-CoV-2, Covaxin, adult population, immunogenicity
To the editor
COVID-19 affected millions of lives and had a devastating impact on the public health system across the globe. India had also witnessed the dreadful effects of the COVID-19 pandemic.1,2 Many SARS-CoV-2 vaccines with different platforms were developed within a short span of time. These vaccines were found effective in curbing the mortality and severity of the disease.3 One such whole-virion inactivated SARS-CoV-2 vaccine, named Covaxin (BBV152) was indigenously developed in India.4 The immunogenicity of Covaxin has been demonstrated, and it is now the second most widely used vaccine in India.4,5 Even with the rigorous national COVID-19 vaccination campaign in India, many breakthrough and reinfection cases were reported during the second and third waves of the pandemic.6 Several studies have found that the immune response to the currently available COVID-19 vaccines is waning, resulting in breakthroughs and reinfections.6,7 Although these vaccines helped to lessen the severity of the disease, they provided less protection against newly emerged SARS-CoV-2 variants. Recent studies have also shown a significant increase in the humoral and neutralizing antibody responses following the administration of the booster dose against the Omicron variant. There appears to be little data on the long-term persistence of the immune response to Covaxin in an Indian context.
Considering this, we have evaluated an immunogenicity of Covaxin and compared its humoral immune responses in two categories of adults through a prospective cohort study. Prior to the immunization with Covaxin, we defined the study population (18–80-year old, median 32 years) in two groups who were COVID-19 naïve individuals (presumably those who never had SARS-CoV-2 infection proven by PCR or serology, n = 118) and COVID-19 recovered individuals (presumably those who had SARS-CoV-2 infection proven by PCR or antigen test and minimum 14 days interval since recovery, n = 128). The two cohorts and the study participants were chosen considering the baseline antibody titers against SARS-CoV-2, the COVID-19 positivity rate, sample power, and loss to follow-up.2 All the individuals received two doses of Covaxin at one month intervals. The study population was assessed during three follow-ups one month post first dose, one month and six month post second dose. At each follow-up, oro/nasopharyngeal swabs (OPS/NPS) and blood samples were collected from the available subjects to determine the immune responses of the Covaxin at vaccination center, Pune from June 2021 to March 2022 (Figure 1). The cohort retention was found to be >85%, >70% and >40% in 1st, 2nd and 3rd follow-up respectively (Figure 1). The third wave of the pandemic with omicron variant coincided with the losses from the 3rd follow-up at six month. The immune response in terms of IgG and neutralizing antibodies (NAbs) was determined using S1-RBD IgG ELISA for all individuals and the plaque reduction neutralization test (PRNT).
Figure 1.
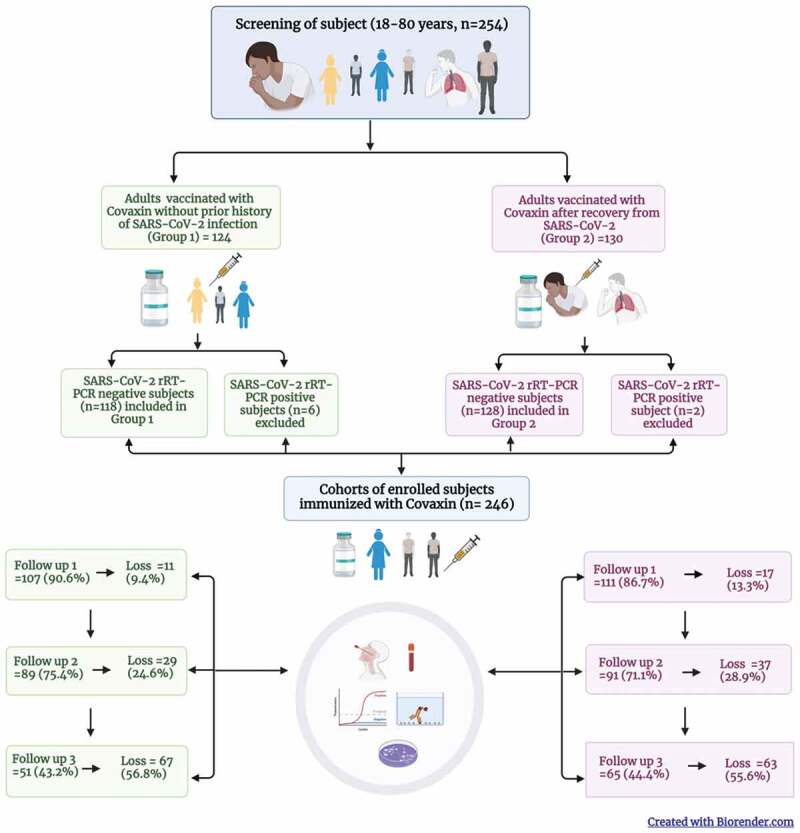
Cohort of adults immunized with Covaxin (BBV152) in Pune, India and their follow up during June 2021 – March 2022.
Briefly, 96-well ELISA plates (Nunc, Germany) were coated with SARS-CoV-2 specific antigens (S1-RBD at a concentration of 1.5 µg/well in PBS pH 7.4). The plates were blocked with a Liquid Plate Sealer (CANDOR Bioscience GmbH, Germany) and Stabilcoat (Surmodics, USA) for 2 h at 37°C. The plates were washed two times with 10 mM PBS, pH 7.4 with 0.1% Tween-20 (PBST) (Sigma-Aldrich, USA). The sera of study subjects (1:50 dilution) were serially four-fold diluted and added to the antigen-coated plates and incubated at 37°C for 1 h.8 These wells were washed five times using 1× PBST and followed by addition 50 μl/well of anti-human IgG horseradish peroxidase (HRP) (Sigma-Aldrich, USA) diluted in Stabilzyme Noble (Surmodics, USA). The plates were incubated for half an hour at 37°C and then washed as described above. Further, 100 μl of TMB substrate was added and incubated for 10 min. The reaction was stopped by 1 N H2SO4, and the absorbance values were measured at 450 nm using an ELISA reader. The cutoff for the assays was set at twice of average optical density value of negative control. The endpoint titer of a sample is defined as the reciprocal of the highest dilution that has a reading above the cutoff value.8
The neutralizing antibody titer was determined for 10% of the study population in both groups at all the follow-up time points. Briefly, the sera of study subjects were serially four-fold diluted and further mixed with an equal amount of live SARS-CoV-2 B.1, Delta, Omicron BA.1 and BA.2 variants (Source: ICMR-NIV, Pune) separately. Post the incubation at 37°C for 1 hr, each virus-diluted serum sample (0.1 ml) was inoculated onto duplicate wells of a 24-well tissue culture plate of Vero CCL-81 cells. After incubating the plate at 60 min, an overlay medium consisting of 2% Carboxymethyl cellulose (CMC) with 2% fetal calf serum (FCS) in 2 × MEM was added to the cell monolayer after incubation of the plate at 37°C for 1 hr. The plate was further incubated at 37°C with 5% CO2 for 5 days. At assay termination, plates were stained with 1% amido black for an hour. Antibody titers were defined as the highest serum dilution that resulted in >50 (PRNT50) reduction in the number of plaques.9
In both groups, age, gender and co-morbidity were not significantly associated with SARS-CoV-2 positivity (Supplementary Table S1). A rise in the geometrical mean titer (GMT) of S1-RBD IgG was observed amongst the participants of both groups at one month post immunization compared to the GMTs one month post first dose (Group 1: S1-RBD: 154.4 to 446.3, Group 2 S1-RBD: 918 to 1127). However, the GMTs at six months post vaccination found to be slightly raised in Group 1 (S1-RBD: 446.3 to 685.9), whereas it slightly declined in Group 2 (S1-RBD: 1127 to 839.8) compared to the one month follow-up (Figure 2a,b). Besides this, more than 90% of participants in both groups had S1-RBD IgG antibodies six months after two doses of vaccination.
Figure 2.
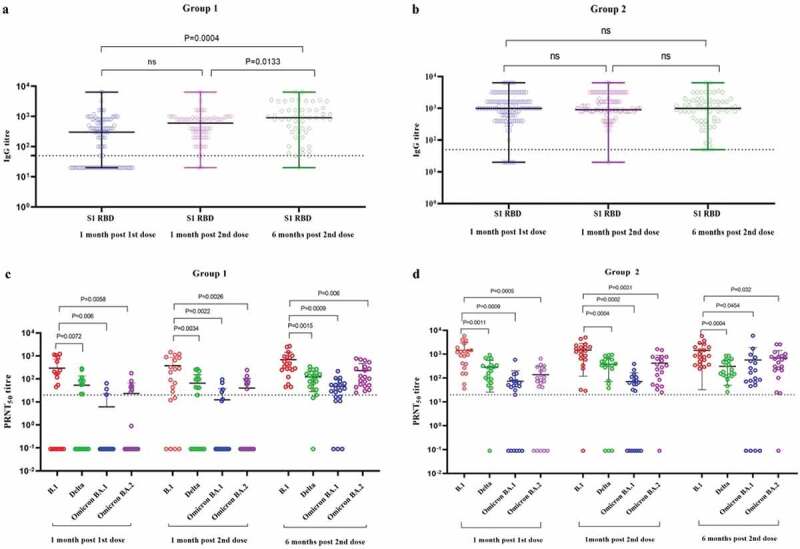
The S1-RBD IgG antibody titer of the sera of study subjects at one month post first dose, 1 month post second dose, and 6 months post second dose from a) Group 1 (COVID-19 naïve individuals) and b) Group 2 (COVID-19 recovered individuals) vaccinated with two doses of Covaxin. The neutralizing antibody titer of sera of study subjects at second dose, 1 month post second dose, and 6 months post second dose from c) Group 1 and d) Group 2 against SARS-CoV-2 B.1, Delta, Omicron BA.1 and BA.2 variants. Differences across time points, categories, ELISA and SARS-CoV-2 variants were analyzed using paired t-tests; p-value less than 0.05 were considered to be statistically significant. The dotted line on the figures indicates the limit of detection of the assays. The data is presented as mean value ± standard deviation. All the analyses were performed using GraphPad PRISM version 9.0.
Considering the hybrid immunity in group 2 participants, the GMTs of NAbs were higher than those of group 1 participants at each follow-up against B.1, Delta, Omicron BA.1, and BA.2. When compared to B.1, both groups had significantly lower levels of NAbs against Delta, Omicron BA.1 and BA.2. The NAb titer increased after six months of follow-up compared to one month of follow-up for all variants. The lowest GMTs of NAbs were observed against the BA.1 variant (Figure 2c,d). This is associated with the high immune escape potential of Omicron variants as compared to Delta.10 Except for the BA.1 variant, the NAbs persisted for six months, with 90% of participants in both categories. A recent study also reported persistence of IgGs and NAb till 1 year after two doses of Covaxin and protective efficacy against variant lineages of SARS-CoV-2.11
In conclusion, a two-dose regimen of the Covaxin enhanced the humoral immune response in adults with/without past COVID-19 infection. Additionally, IgG and NAb responses persisted for six months post-vaccination. Over the period of COVID-19 pandemic, SARS-CoV-2 has constantly evolved, leading to the emergence of new variants. Hence, it is necessary to carry out such prospective study for a longer duration to check the immunogenicity of the vaccine against the emerging SARS-CoV-2 variants. This data is also consistent with the current COVID-19 vaccination policy, which suggests that a booster dose is required six months after the initial vaccination. The primary immunization followed by the booster doses would definitely help in reducing disease severity of SARS-CoV-2 and diminishing the socio-economic impact and burden on the public health system.
Supplementary Material
Acknowledgments
The authors would like to gratefully acknowledge the staff of ICMR-NIV, Pune including Mrs Asha Salunkhe, Mr Chetan Patil, Mrs Savita Patil, Mrs Triparna Majumdar, Mrs Ashwini Waghmare, Mrs Priyanka Waghmare, Mrs Shilpa Ray for extending the excellent technical support. We thank all study participants and staff of district hospital, Aundh, Pune for their support during the study.
Funding Statement
Financial support was provided by the Indian Council of Medical Research (ICMR), New Delhi at ICMR-National Institute of Virology, Pune under intramural funding “COVID-19.”
Disclosure statement
No potential conflict of interest was reported by the authors.
Authors contribution
PDY and RRS contributed to study design, data analysis, interpretation and writing. RD, AS, DYP, AMS, GNS, AN, MK, YKG, JSG, GRD, RJ, RH, KK, JY, PG and contributed to data collection, data analysis, interpretation and writing. PDY, RRS, DYP, RD, AMS and PA contributed to the critical review and finalization of the paper.
Data availability statement
All relevant data are within the manuscript.
Ethical approval
The study was approved by the Institutional Human Ethics Committee of ICMR-NIV, Pune, India under the project “Comparative assessment of BBV152 vaccine (COVAXIN™) antibody and antigen-specific responses in immunized population without past COVID-19 infection, individuals vaccinated after recovery from COVID-19 and non-vaccinated individuals with past COVID-19 infection [NIV/IEC/May/2021/D-11].”
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2156753
References
- 1.Fisayo T, Tsukagoshi S.. Three waves of the COVID-19 pandemic. Postgrad Med J. 2021. May 1;97(1147):332. doi: 10.1136/postgradmedj-2020-138564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogam P, Joshi A, Nagarkar S, Jain D, Gupte N, Shashidhara LS, Monteiro JM, Mave V. Burden of COVID-19 and case fatality rate in Pune, India: an analysis of the first and second wave of the pandemic. IJID Regions. 2022;2:74–4. doi: 10.1016/j.ijregi.2021.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagy A, Alhatlani B. An overview of current COVID-19 vaccine platforms. Comput Struct Biotechnol J. 2021;19:2508–17. doi: 10.1016/j.csbj.2021.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ella R, Vadrevu KM, Jogdand H, Prasad S, Reddy S, Sarangi V, Ganneru B, Sapkal G, Yadav P, Abraham P, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect Dis. 2021;21(5):637–46. doi: 10.1016/S1473-3099(20)30942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization . Annexes to the interim recommendations for use of the bharat biotech BBV152 COVAXIN® vaccine against COVID-19. [accessed 2022 Sep15]. https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE-recommendation-bbv152-covaxin-annexes.
- 6.Arora G, Taneja J, Bhardwaj P, Goyal S, Naidu K, Yadav SK, Saluja D, Jetly S. Adverse events and breakthrough infections associated with COVID-19 vaccination in the Indian population. J Med Virol. 2022;94(7):3147–54. doi: 10.1002/jmv.27708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menni C, May A, Polidori L, Louca P, Wolf J, Capdevila J, Hu C, Ourselin S, Steves CJ, Valdes AM, et al. COVID-19 vaccine waning and effectiveness and side-effects of boosters: a prospective community study from the ZOE COVID study. Lancet Infect Dis. 2022. Apr 8;22(7):1002–10. doi: 10.1016/S1473-3099(22)00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deshpande G, Kaduskar O, Deshpande K, Bhatt V, Yadav PD, Gurav Y, Potdar V, Khutwad K, Vidhate S, Salunke A, et al. Longitudinal clinic-serological analysis of anti-nucleocapsid and anti-receptor binding domain of spike protein antibodies against SARS-CoV-2. Int J Infect Dis. 2021;112:103–10. doi: 10.1016/j.ijid.2021.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deshpande GR, Sapkal GN, Tilekar BN, Yadav PD, Gurav Y, Gaikwad S, Kaushal H, Deshpande KS, Kaduskar O, Sarkale P, et al. Neutralizing antibody responses to SARS-CoV-2 in COVID-19 patients. Indian J Med Res. 2020. Jul & Aug;152(1 & 2):82–87. doi: 10.4103/ijmr.IJMR_2382_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahay RR, Patil DY, Sapkal GN, Shete AM, Yadav PD. Cases of SARS-CoV-2 reinfection with Omicron BA. 2 post breakthrough infection with Delta and kappa variants. Infect Dis. 2022 Aug 22:1–4. doi: 10.1080/23744235.2022.2114538. [DOI] [PubMed] [Google Scholar]
- 11.Kumar NP, Banurekha VV, Kumar CG, Nancy A, Padmapriyadarsini C, Shankar S, Hanna LE, Murhekar M, Devi KU, Babu S. Inactivated COVID-19 vaccines: durability of Covaxin/BBV152 induced immunity against variants of concern. J Travel Med. 2022;29(6):taac088. doi: 10.1093/jtm/taac088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the manuscript.


