Abstract
Objective.
Tapered low-volume, low-pressure (LVLP) cuffs have been introduced to improve sealing and reduce injury from tracheostomy and endotracheal intubation compared to traditional cylindrical high-volume, low-pressure (HVLP) cuffs. The objective of this study is to develop a swine model of tracheostomy injury and to compare live tissue response following LVLP and HVLP tracheostomy placement.
Study Design.
In vivo animal study.
Setting.
Academic institution.
Methods.
Swine underwent tracheostomy followed by placement of LVLP and HVLP tracheostomy cuffs at 30 cm H2O. After 24 and 48 hours, tracheal specimens underwent histopathological analysis including cilia, lamina propria and epithelial thickness, and mucosal injury score.
Results.
In all cuff contact areas, mean epithelial thickness for both tracheostomy cohorts was decreased compared to control epithelium at 24 and 48 hours (P < .01). HVLP proximal epithelium thickness was decreased at 24 and 48 hours relative to LVLP sections (P < .05). Lamina propria thickness in proximal LVLP sections was less than HVLP sections at 24 hours and 48 hours (P < .05). Mucosal injury score at areas of cuff contact was increased in tracheostomy cohorts relative to controls (P < .001), with HVLP injury score greater than LVLP at the proximal cuff (P < .05).
Conclusion.
In a swine model, tracheostomy resulted in increased mucosal injury compared to normal tracheal mucosa. LVLP cuffs resulted in less injury than HVLP cuffs, with reduced mucosal inflammation and improved health of epithelium and lamina propria. The wider proximal LVLP cuff demonstrated improved mucosal health compared to the HVLP cylindrical cuff.
Keywords: tracheostomy cuff, cuff design, endotracheal tube, tracheal injury, swine model, endotracheal. intubation
Endotracheal intubation and tracheostomy are necessary interventions to gain airway control and appropriate patient ventilation.1 Tracheostomy is indicated after long-term intubation of 7 to 14 days to limit laryngeal injury and increase comfort by removing the endotracheal tube (ETT) from the oral cavity, oropharynx, and larynx. Tracheostomy rates are approximately 10% in long-term ventilated patients and 1.3% of all intubated patients.2 While necessary to ventilate and oxygenate critically ill patients, prolonged ETT intubation and tracheostomy can cause significant injury to the larynx and trachea. In particular, cuff injury from both ETT and tracheostomy tubes occurs due to pressure exerted on the tracheal mucosa, which can cause tissue necrosis and lead to laryngotracheal stenosis.3
The ideal cuff inflation pressure to minimize both tracheal injury and aspiration as a consequence of improper sealing correlates with tracheal capillary perfusion pressure.4,5 Laryngotracheal stenosis at the cuff site was a major complication in the 1960s when low-volume, high-pressure cuffs were employed in ETT and tracheostomy tubes. This occurred because high-pressure cuffs used surpassed the tracheal capillary perfusion pressure of 20 to 30 cmH2O. During the 1970s, the advent of high-volume, low-pressure (HVLP) cuffs markedly reduced the incidence of tracheal stenosis by an order of magnitude.6–8 Furthermore, design aspects of ETT and tracheostomy cuffs, such as material, pressure, and contact surface, are the main determinants of sealing efficacy, which limits the risk of ventilatory-associated pneumonia. Yet, a high-pressure seal may also lead to injury of the tracheal mucosa. In addition, high cuff pressure may develop from inadequate positioning of the tube or incorrect sizing, leading to cuff overinflation.9
In recent years, the large diameter of HVLP cuffs has been identified as a key problem, as it is 1.5 to 2 times larger than the trachea, leading to longitudinal cuff folds that develop upon inflation, resulting in incomplete sealing and leakage of gastric contents past the cuff. In addition, overinflation causes increased tracheal pressure around these folds, leading to regional mucosal injury.10 To address these concerns and improve cuff design, low-volume, low-pressure (LVLP) cuffs were introduced. One LVLP tracheostomy tube design is the Shiley Flexible Adult Tracheostomy Tubes with TaperGuard cuff (Medtronic). LVLP cuffs have a tapered shape to fit into the trachea and minimize cuff folds and improve the seal compared to HVLP cuffs.10,11 LVLP cuffs also reduce the area of cuff-trachea contact, which has been associated with increased discomfort and hoarseness.12 These benefits have been documented using ex vivo bench testing, but there are little data on the impact of LVLP cuff design on live tissue.
While some studies have quantified the impact of ETT design on acute tracheal injury,13–15 there are limited data on acute tissue damage caused by tracheostomy following placement of LVLP cuffs. Acute cuff injury is commonly studied using an animal model in intubated anesthetized swine,16,17 which have similar airway anatomy to humans and enable histopathological analysis of live tissue response. The study of tracheostomy cuffs as opposed to ETT would allow for assessment at longer periods of cuff pressure than in intubated swine.13,14,18,19 In addition, these animal models have primarily examined histopathological changes using qualitative assessments of mucosal injury and lack objective measures of injury. The objective of this study is to use a swine model to measure acute tracheal injury from tracheostomy tubes to evaluate the impact of LVLP cuff design compared to HVLP analogs. In addition, the quantitative histopathological metrics used to objectively assess injury can be applied to future models of cuff injury from endotracheal and tracheostomy tubes. We hypothesize that LVLP cuffs will lead to reduced mucosal injury and inflammation compared to HVLP cuffs, as measured by epithelial damage, lamina propria edema, and acute inflammation.
Methods
Animal Cohort and Tracheostomy Intervention
Institutional Animal Care and Use Committee approval (SW20M176) was obtained, and the care and handling of the animals were in accord with National Institutes of Health guidelines for ethical animal treatment. Experimental sus scrofa pigs were anesthetized and sedated with ketamine and xylazine, with 1% to 5% isoflurane maintenance anesthesia. Pigs underwent tracheotomy with vertical skin incision followed by subcutaneous tissue and strap muscle separation, thyroid traction, and tracheostomy tube placement between the first and second tracheal ring. Intraoperative bronchoscopy was performed to ensure adequate placement. Immediately postoperatively, animals were continuously monitored with tracheostomy tubes maintained at 30 cmH2O. Tracheostomy tubes included an LVLP design (Shiley Flexible Adult Tracheostomy Tubes with TaperGuard cuff; Medtronic) and an HVLP design (Shiley DCT Tracheostomy Tube; Medtronic). The inner canula was changed every 4 hours, along with flexible bronchoscopy to monitor airway secretions and ensure a patent airway. In addition, 1 to 2 cc of saline was used to flush the tube to loosen secretions and prevent mucus plugging. Suctioning was performed when evidence of obstructing material was observed during bronchoscopy or respiratory distress. Following 24 or 48 hours, pigs were euthanized using a barbiturate-based commercial euthanasia solution given intravenously while anesthetized.
Tissue Harvest and Processing
The larynx and trachea were harvested as a single unit. The location of the inflated tracheostomy cuff was recorded in situ, using dye to demarcate the proximal and distal boundaries. Photos of the gross tracheal injury were captured prior to fixation. Tissue samples at the proximal cuff (B1), cuff midpoint (B2), and distal cuff (B3), as well as control tissue and at the distal trachea (internal control), were collected (Figure 1). To account for possible differences in mucosal anatomy between the distal (internal control) trachea, 1 rapid processed autopsy (RPA) was used as an external control from an uninjured swine, with 3 sections taken in the proximal trachea. All tissue samples were preserved in 10% formalin for 48 hours before trimming, embedding in paraffin, and sectioning at a 5-μm thickness.
Figure 1.
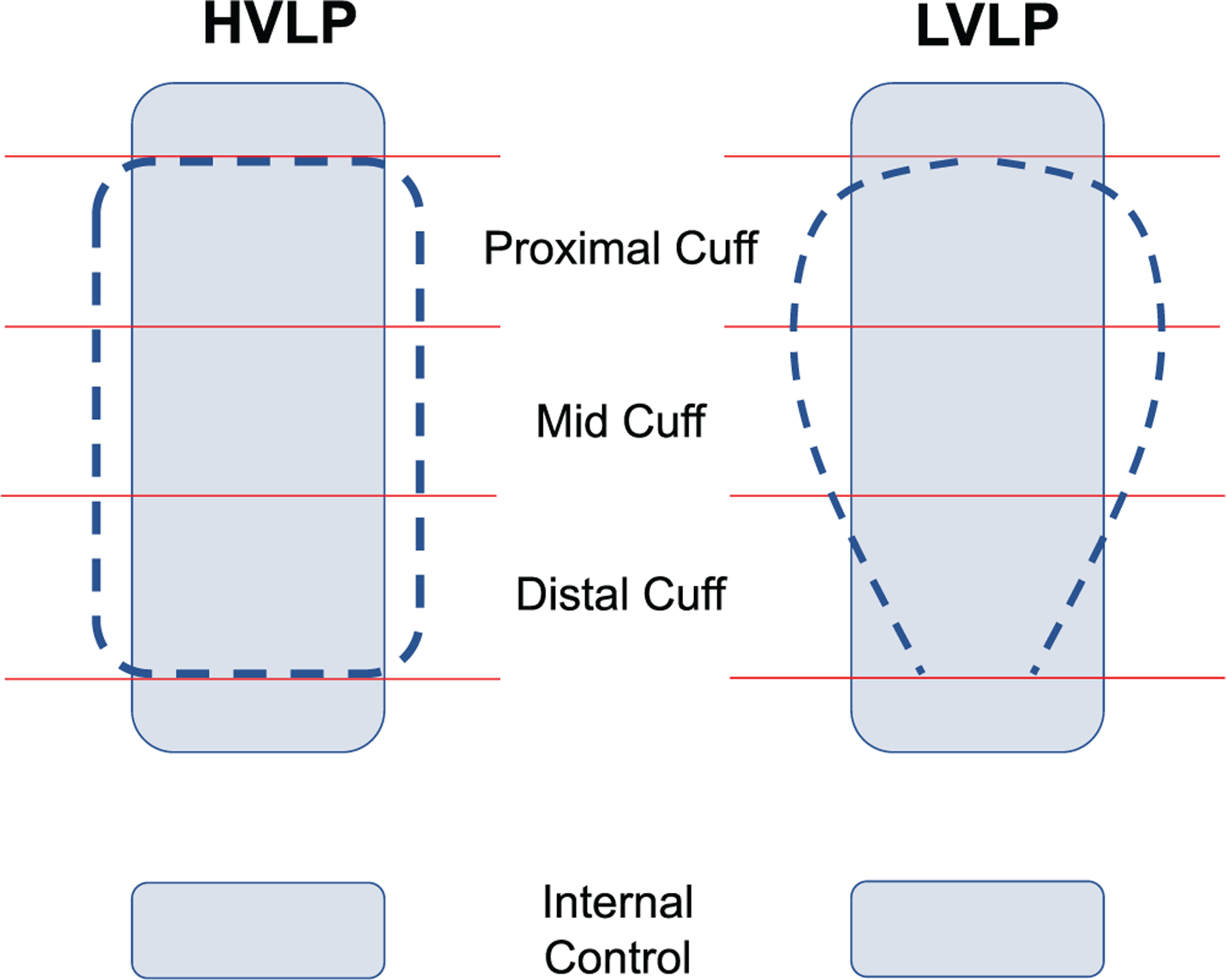
High-volume, low-pressure (HVLP) and low-volume, low-pressure (LVLP) cuffs (dotted) and tracheal wall (rectangle). Contact points/internal control demarcated by solid lines.
Histopathology and Immunohistochemistry
Tissue sections were processed for histopathology using hemoxylin and eosin (H&E) staining. For epithelial marker staining, formalin-fixed, paraffin-embedded (FFPE) tissue sections (5 μm) were deparaffinized and rehydrated using a xylene/ethanol-based protocol. To recover antigenicity, a heat-induced epitope retrieval procedure using a sodium citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0) and a steamer was performed. To block nonspecific binding sites, slides were washed with 1 mL phosphate-buffered saline, incubated for 30 minutes in Dulbecco’s modified Eagle medium with 10% fetal bovine serum, and then incubated with pan-cytokeratin (AE1/AE3) and TUBA-PE (sc23950) to identify ciliated epithelium. A confocal microscope was used to visualize and image positive staining.
Quantitative Histopathological Image Analysis
To measure epithelial coverage, the entire tracheal ring was imaged at 10× power, and the percentage of the basement membrane covered with epithelium was computed using ImageJ (National Institutes of Health) image measurement software. Five areas of present epithelium were randomly imaged at locations evenly spaced along the tracheal ring at 40× power. The average number of epithelial cell layer thickness was quantified. Five random locations evenly spaced along the tracheal ring were imaged at 10× power, and the largest length from epithelial cell layer to cartilaginous ring was measured for an average lamina propria thickness. The ratio of lamina propria thickness at cuff contact to the control (lamina propriacuff/lamina propriacontrol) was computed. Each tissue section was evaluated for degree of mucosal injury using a score developed by Gordin et al.15 graded from 0 to 4 (0, normal; 1, epithelial compression; 2, epithelial loss; 3, sub-epithelial/glandular necrosis; 4, perichondrium involvement).
Statistical Analysis
GraphPad Prism (GraphPad Software) was used for statistical analyses. All values are depicted as mean ± standard deviation. All normally distributed data were statistically analyzed using unpaired 2-tailed t test with α set at .05 significance.
Results
Pilot Animal Cohort
The postoperative course of preliminary pilot animals (n = 4) is detailed in Table 1, with 1 animal surviving until a planned euthanasia at 48 hours. Times of death for the other 3 animals occurred at 20 to 42 hours following tracheostomy. Causes of death included overnight mucus plug (n = 2) and cardiopulmonary arrest with unsuccessful suctioning of necrotic tissue obstructing the trachea (n = 1). These preliminary data led to changes in postoperative care, including continuous monitoring, suctioning, and bronchoscopy every 4 hours and airway humidification to ensure survival to 24 to 48 hours. Results from these 4 animals informed the postoperative monitoring protocols and were not included in data analysis.
Table 1.
Subset of 4 Pilot Pig Experiments That Established the Postoperative Monitoring Protocol.a
| Swine No. | Tracheostomy type | Monitoring details | Postoperative course | Necropsy result | Time of death | Cause of death |
|---|---|---|---|---|---|---|
| 1 | Size 4 HVLP | Checks every 8 hours with scheduled suctioning | Acute apneic event at 5 hours postoperatively, resolved with suctioning of blood from the trachea, CXR confirmed proper tracheostomy tube placement. | Mucus plug occluded tracheostomy tube and extended into distal trachea. Tracheitis and necrosis of tracheal mucosa. | POD 1 (20 hours) | Airway obstruction from mucus plug |
| 2 | Size 6 LVLP | Checks every 8 hours with suctioning as needed | Recovery from surgery was uneventful. No adverse events. | Mucus plug occluded distal tracheostomy tube and extended into distal trachea. Necrotic tissue noted in distal trachea. | POD 2 (42 hours) | Airway obstruction from mucus plug |
| 3 | Size 4 LVLP | Continuous monitoring; inner canula changed every 4 hours and scheduled suctioning every 8 hours | Recovery from surgery was uneventful. No adverse events. | No blood or mucus in tracheostomy. Minimal mucosal changes to the trachea. | POD 2 (48 hours) | Planned euthanasia |
| 4 | Size 4 HVLP | Continuous monitoring; inner canula changed every 4 hours, with suctioning as needed | Respiratory distress, stridor, O2 desaturation at 16 hours postoperatively. On tracheal suctioning, mucus plug expectorated with recovery of and O2 levels and resolution of stridor. | Necrotic tissue occluding the distal trachea to the carina. No mucus plug in tracheostomy tube. | POD 1 (32 hours) | Airway obstruction from necrotic tissue |
Abbreviations: CXR: chest X-ray; HVLP, high volume, low pressure; LVLP, low volume, low pressure; POD, postoperative day.
Data from these pilot pigs were not included in the study.
Study Animal Cohort
Experimental swine (n = 20) with a mean weight of 22.0 ± 2.6 kg underwent tracheostomy with either HVLP or LVLP and were included in the analysis of tracheal injury following 24 hours (n = 10) and 48 hours (n = 10) of placement.
Immunohistochemistry and H&E and Gross Specimen Analysis
Tracheal specimens demonstrated loss of ciliated epithelial cells in the majority of tracheostomy sections compared to the control tracheal specimen. Gross specimens exhibited larger areas of necrotic tissue in contact points of the tracheostomy tube, with friable and edematous regions worse in the HVLP specimens compared to LVLP specimens (Figure 2). Pan-cytokeratin and TUBA staining demonstrated loss of ciliated epithelium in both LVLP and HVLP cuff conditions compared to controls. There was less epithelial damage seen in the LVLP experimental condition in the proximal cuff, B1 (Figure 3A). There were multifocal areas of epithelial flattening and ulceration within the trachea and a complete absence of cilia for both LVLP and HVLP cohorts.
Figure 2.
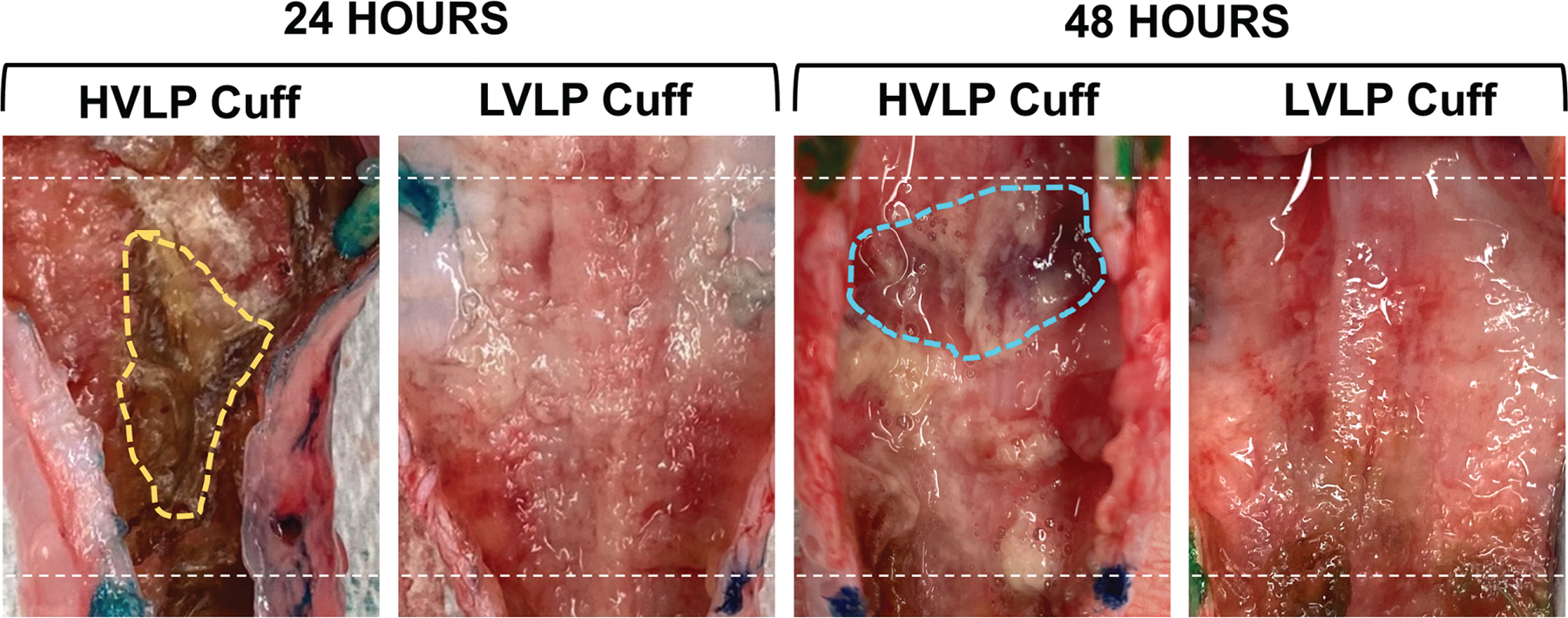
Gross tracheal specimen mucosa following tracheostomy cuff placement. Following 24 and 48 hours, there was marked necrotic debris (yellow) and edematous tissue (blue) noted in high-volume, low-pressure trachea mucosa. Dotted line indicates proximal/distal cuff margins. HVLP, high volume, low pressure; LVLP, low volume, low pressure.
Figure 3.
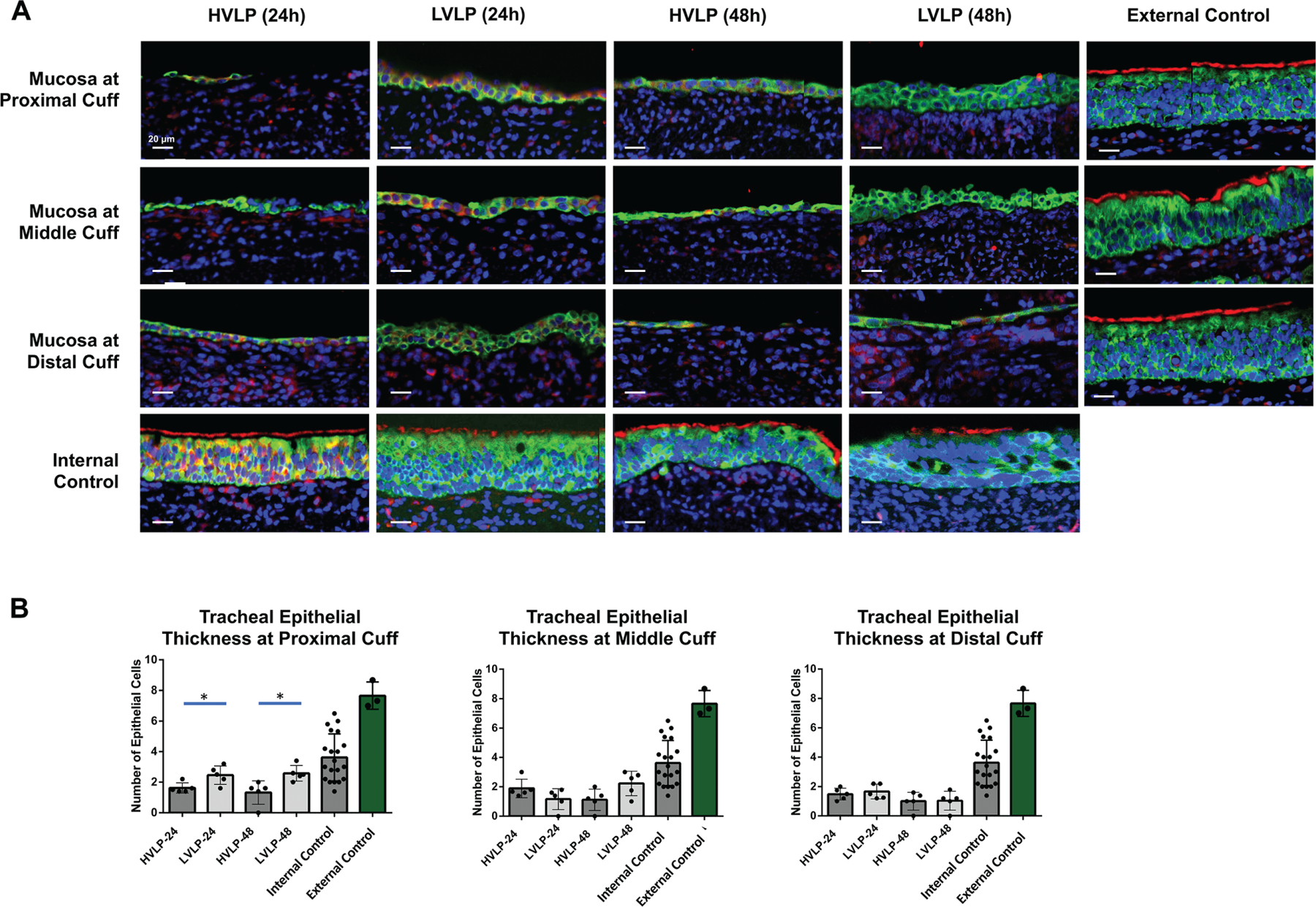
Tracheal mucosa at cuff contact compared to control. (A) Representative TUBA/panCK staining in normal pig trachea with uninjured ciliated epithelium vs cuff. (B) Epithelial cell thickness following 24-hour/48-hour cuff contact compared to controls. *P <. 05. HVLP, high volume, low pressure; LVLP, low volume, low pressure.
Quantitative Histopathological Analysis
In all cuff contact areas, mean epithelial layer thickness for both tracheostomy cohorts was decreased compared to noninjured external control epithelium thickness (3.6 ± 1.5 cells, P < .01) and RPA noninjured controls (7.7 ± 0.9 cells, P < .01) at both 24 and 48 hours (Figure 3B). In comparing the 2 tracheostomy groups, LVLP proximal epithelium sections were increased at 24 hours relative to HVLP sections (2.5 ± 0.6 and 1.6 ± 0.3, respectively, P = .03; Figure 3B). Similarly, at 48 hours, the mean epithelial layer thickness at the LVLP cuff’s proximal section was also increased compared to HVLP proximal sections (2.6 ± 0.5 and 1.3 ± 0.8, respectively, P = .02; Figure 3B). In the middle and distal cuff, there were no significant differences between epithelial thickness in HVLP and LVLP at 24 hours (P = .12 [middle], P = .55 [distal]) or 48 hours (P = .06 [middle], P = .92 [distal]; Figure 3B). Figure 4A shows representative images of proximal lamina propria thicknesses, which were increased in the HVLP trachea sections. At 24 hours, the HVLP lamina propria were increased compared to uninjured RPA in the proximal, middle, and distal cuff contact points (P = .01, P = .01, and P = .02, respectively) while only the distal LVLP sections had increased lamina propria compared to uninjured RPA controls (P = .03; Figure 4B). Both tracheostomy cohorts had increased lamina propria thickness compared to uninjured RPA controls at 48 hours in proximal, middle, and distal cuffs (P < .05, Figure 4B). However, the mean lamina propria thickness in proximal LVLP sections was less than HVLP sections at both 24 hours and 48 hours (P = .02 and P = .02; Figure 4B). The Gordin mucosal injury score was increased in tracheostomy cohorts relative to uninjured controls for all cuff contact points (P < .001) but was decreased in LVLP relative to HVLP cohorts at both 24 and 48 hours (P = .01 and P = .04) in the proximal cuff (Figure 5).
Figure 4.
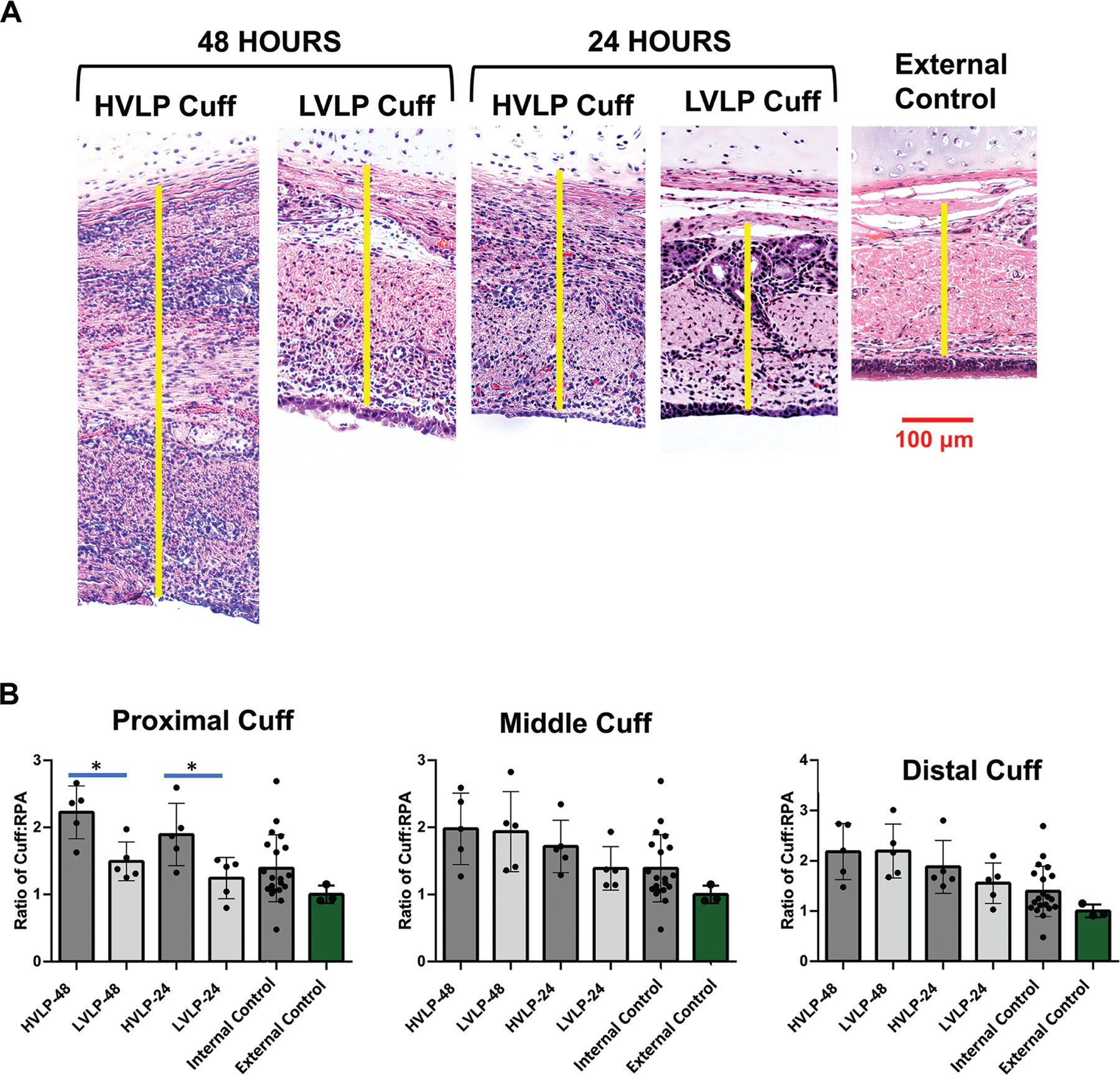
Lamina propria thickness of tracheostomy cuff mucosa. (A) Representative images of lamina propria thickness in cuff cohorts compared to controls (yellow), measured from the epithelium to cartilage. (B) Mean ± SD of lamina propria thickness. *P < .05. HVLP, high volume, low pressure; LVLP, low volume, low pressure.
Figure 5.
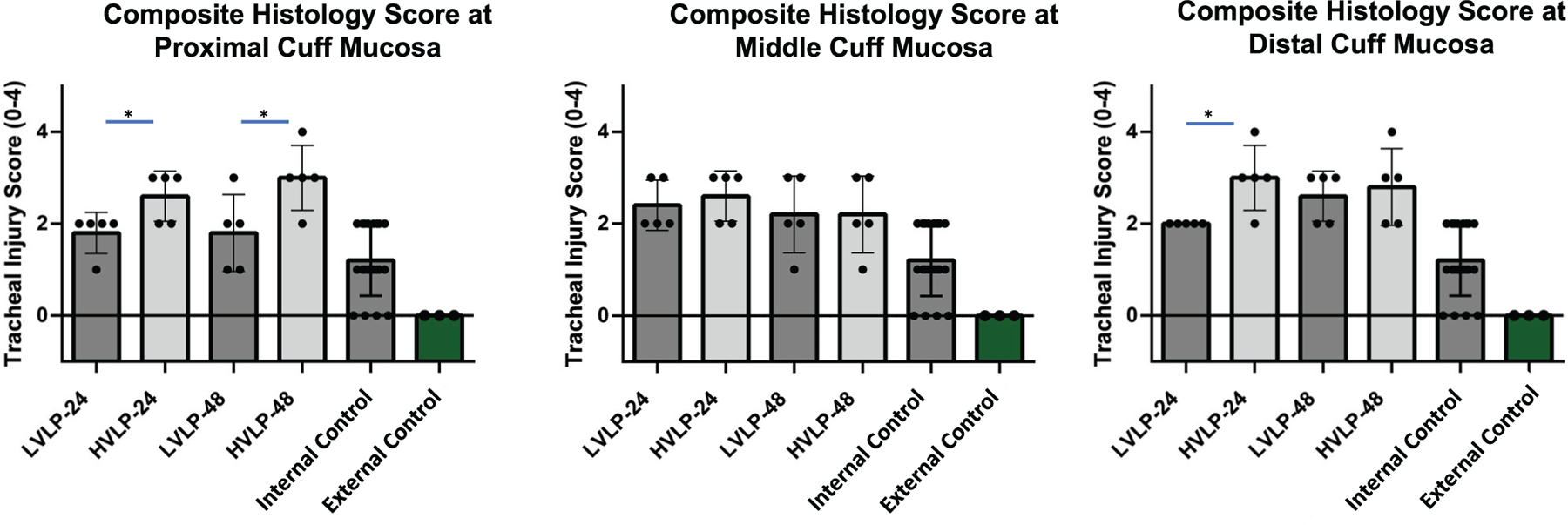
Composite histology score following tracheostomy. Increased injury observed in all cuffs at 24/48 hours, defined by Gordin score. In proximal (24/48 hours) and distal (48 hours) cuff, there was decreased injury in LVLP compared to HVLP. *P < .05. HVLP, high volume, low pressure; LVLP, low volume, low pressure.
Discussion
This study demonstrated significant acute mucosal damage following short-term tracheostomy placement. Overall, tracheostomy resulted in increased epithelial injury, lamina propria thickness, and mucosal injury score compared to uninjured controls, both at 24 and 48 hours following placement. In a comparison of cuff types, LVLP cuffs resulted in less acute injury than HVLP cuffs as measured by epithelial and lamina propria thickness and mucosal injury score. Interestingly, the proximal aspect of the LVLP cuff, which is designed to be the only part in contact with the tracheal mucosa, actually demonstrated less injury compared to the proximal HVLP contact location.
Acute laryngotracheal injury in the context of intubation has been well documented, but less is known about the progression of mucosal injury following tracheostomy. We found that the trachea specimens had several key changes following tracheostomy placement: (1) epithelial layer disruption, (2) regional tissue edema, and (3) inflammatory changes. Our findings are similar to parallel studies conducted after prolonged intubation of large animals to study the impact of ETT on tracheal injury. Following 3 hours of intubation, Sinha et al14 observed loss of cilia in epithelial cells, with significant epithelial flattening, ulceration, and inflammation indicative of acute, suppurative, and ulcerative tracheitis. In studies of 24-hour intubation, significant laryngeal injury was present at tube-mucosa contact points, with an associated increase in histological inflammation, ulceration, and necrosis.13 Gordin et al.16 observed significant tracheal injury only 4 hours after intubation, which was worsened by hypoxic conditions. Disruption of the epithelial barrier is important clinically because it allows for invasion of antigens, leading to tracheal neutrophil activation in response to microbial threats. Epithelial injury also causes a disruption of mucus transport, leading to mucostasis and increased antigen concentration in those areas of epithelial barrier loss.20
Our findings highlight that LVLP cuffs cause less tracheal mucosal injury compared to HVLP cuffs. Reduced injury from LVLP cuffs may be a direct consequence of a lower pressure exerted on the tracheal wall with the taper-shaped vs the cylindrical cuff. In addition, ex vivo bench tests demonstrated the LVLP has a more equal distribution of pressure compared to HVLP cuffs, which have been shown to exert areas of higher pressure on adjacent tissue.21 In particular, LVLP cuffs are composed of a thinner, more compliant, and lower friction material, which limits the cuff folds that increase lateral force in localized regions of contact. The insertion force for the taper-shaped cuff is 40% less than that of the cylindrical-shaped cuff, which decreases trauma as the tracheostomy tube is inserted, further reducing the risk of epithelial injury.21 While studies showed tapered-cuff ETTs require a lower pressure to achieve a seal compared with cylindrical-shaped ETTs, those studies were not performed in patients requiring high ventilator pressures.22–24 Further studies are necessary to determine if taper-shaped cuffs significantly decrease the incidence of ventilator-associated pneumonia found in intensive care unit patients.25 Anecdotal observations of tapered-cuff tracheostomies have reported some difficulties maintaining a seal in patients requiring high ventilator pressures. This appears to be due to the short cranial-caudal length of the tapered cuff that is in contact with the tracheal wall, which lacks the redundancy of traditional HVLP tracheostomy tubes. This may be addressed by careful positioning or upsizing to a larger tube in patients with increased ventilator pressures.
One interesting finding was that the LVLP tracheostomy cuff resulted in less injury in the proximal cuff area compared to the HVLP cuff, despite the larger diameter of the tapered design. In vitro studies have demonstrated that the cuff is protective against fluid leakage, largely owing to the proximal large diameter and distal small diameter, allowing the geometry to fit into the trachea to form a seal with minimal folds.26–28 The locations in which the cuff contacts the trachea are presumed to be the most vulnerable due to transmural pressure injury. While the proximal part of the LVLP cuff is in complete contact with the tracheal wall, it has shown less injury than the middle and distal portion. One possible explanation for this paradoxical finding is that the proximal portion has less movement during head motion or tube manipulation, such as during suctioning. This is in contrast to the middle and distal portions being in partial contact with the trachea, which may cause greater shear force and frictional injury as well as pressure changes during swine movement.29
While the present study investigated indwelling tracheostomy injury after 24 to 48 hours, the findings are relevant to prolonged endotracheal intubation. Laryngeal injury following intubation is a common complication ranging from self-limiting edema to subglottic stenosis (SGS), with increasing duration often correlating with worse injury. One prospective cohort study reported that 57% of patients had acute laryngeal injury (ulceration/granulation tissue),30 but most injuries did not lead to long-term outcomes.30 Tracheal stenosis can occur as a late complication weeks to months after initial intubation, with an incidence ranging from 0.3% to 11%.31,32 While our findings demonstrate that LVLP cuffs lead to less injury after 24 to 48 hours of tracheostomy placement, many other risk factors affect long-term outcomes in patients with ETT and tracheostomy, including comorbidities (diabetes, obesity), duration, tube diameter, and cuff pressures. It is important to address these risk factors in conjunction with cuff design to minimize injury and use an animal model to study development of SGS following ETT or tracheostomy.
While this study quantified acute tracheal injury following tracheostomy, there are limitations. This study was conducted in swine, which reproduced human airway dynamics during tracheostomy and enabled endoscopic and histopathological assessment of live tissue. In particular, swine have similar-sized airways to humans, which is crucial for testing tracheostomy tubes in clinical practice.33,34 However, tracheostomy care in swine presented specific challenges. Pilot animals demonstrated that tracheostomized pigs are at risk for rapid-onset airway obstruction due to edema and necrosis, and they require continuous postoperative monitoring due to mucus secretions and, at times, extensive mucosal injury. To provide visual confirmation of a patent airway and to guide suctioning frequency, we performed flexible tracheobronchoscopy at least every 4 hours along with suctioning and gentle irrigation to prevent obstruction from secretions. These adjustments allowed swine to survive 48 hours after surgery and assessment of mucosal injury at the peak inflammatory phase of wound healing. Due to this shorter timeline of assessment, further work is needed to assess the impact of longer tracheostomy placement, particularly if acute inciting injury leads to SGS. Given the potential for mucosal injury in swine and high risk of airway obstruction, another large animal model would be better to assess long-term tracheostomy injury.
This study established an acute mucosal injury model of tracheostomy, allowing for endoscopic assessment and histopathological evaluation after prolonged tracheal cuff pressure, which is relevant to both tracheostomy and intubation without necessitating prolonged anesthesia. Greater injury to the epithelium and inflammation was observed in areas of the tracheostomy cuff after 24 to 48 hours compared to noninjured controls. In addition, LVLP tracheostomy cuffs were associated with improved histology scores, increased epithelial thickness, and decreased edema compared to HVLP cuffs. Evaluation of tracheostomy-associated injury can help elucidate aspects of cuff design that affect inflammation and tissue damage, such as insertion force, dimensions, and pressure exerted on the trachea. Objective metrics (epithelial cell thickness, lamina propria thickness, mucosal injury histology score) and swine tracheostomy model can be used in future validation of tracheostomy tube designs and also be applied to ETTs to evaluate for pressure injury.
Funding source:
Research reported in this publication was supported by the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under award number 1R01DC018567. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Competing interests:
Alexander T. Hillel has a sponsored research agreement with Medtronic to investigate tracheostomy tube injury to the trachea. Medtronic manufactured both the tracheostomy tubes investigated in this study, including low-volume, low-pressure design (Shiley Flexible Adult Tracheostomy Tubes with TaperGuard cuff) and high-volume, low-pressure design (Shiley DCT Tracheostomy Tube). The remaining authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1.Prokakis C, Koletsis EN, Panagiotis D, et al. Airway trauma: a review on epidemiology, mechanisms of injury, diagnosis and treatment. J Cardiothorac Surg. 2014;9(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fischler L, Erhart S, Kleger GR, et al. Prevalence of tracheostomy in ICU patients: a nation-wide survey in Switzerland. Intensive Care Med. 2000;26(10):1428–1433. [DOI] [PubMed] [Google Scholar]
- 3.Cipriano A, Mao ML, Hon HH, et al. An overview of complications associated with open and percutaneous tracheostomy procedures. Int J Crit Illn Inj Sci. 2015;5(3):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knowlson GT, Bassett HF. The pressures exerted on the trachea by endotracheal inflatable cuffs. Br J Anaesth. 1970;42(10):834–837. [DOI] [PubMed] [Google Scholar]
- 5.Kim DM, Shin MJ, Kim SD, et al. What is the adequate cuff volume for tracheostomy tube? A pilot cadaver study. Ann Rehabil Med. 2020;44(5):402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber AL, Grillo HC. Tracheal stenosis: an analysis of 151 cases. Radiol Clin North Am. 1978;16(2):291–308. [PubMed] [Google Scholar]
- 7.Lewis FR, Schiobohm RM, Thomas AN. Prevention of complications from prolonged tracheal intubation. Am J Surg. 1978; 135(3):452–457. [DOI] [PubMed] [Google Scholar]
- 8.Heffner JE, Miller KS, Sahn SA. Tracheostomy in the intensive care unit. Part 2: Complications. Chest. 1986;90(3):430–436. [DOI] [PubMed] [Google Scholar]
- 9.Hess DR. Tracheostomy tubes and related appliances. Respir Care. 2005;50(4):497–510. [PubMed] [Google Scholar]
- 10.Bassi LG, Ranzani OT, Marti LB, et al. An in vitro study to assess determinant features associated with fluid sealing in the design of endotracheal tube cuffs and exerted tracheal pressures. Crit Care Med. 2013;41(2):518–526. [DOI] [PubMed] [Google Scholar]
- 11.Zanella A, Scaravilli V, Isgrò S, et al. Fluid leakage across tracheal tube cuff, effect of different cuff material, shape, and positive expiratory pressure: a bench-top study. Intensive Care Med. 2011;37(2):343–347. [DOI] [PubMed] [Google Scholar]
- 12.Chang JE, Kim H, Han SH, et al. Effect of endotracheal tube cuff shape on postoperative sore throat after endotracheal intubation. Anesth Analg. 2017;125(4):1240–1245. [DOI] [PubMed] [Google Scholar]
- 13.Shah MD, Nguyen LH, Campisi P, et al. Piloting a novel porcine model for endolaryngeal injury following prolonged intubation. Int J Pediatr Otorhinolaryngol. 2007;71(9):1399–1406. [DOI] [PubMed] [Google Scholar]
- 14.Sinha R, Correia R, Gardner D, et al. Mucosal injury following short-term tracheal intubation: a novel animal model and composite tracheal injury score. Laryngoscope Invest Otolaryngol. 2018;3(4):257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordin A, Chadha NK, Campisi P, et al. Effect of a novel anatomically shaped endotracheal tube on intubation-related injury. Arch Otolaryngol Head Neck Surg. 2010;136(1):54–59. [DOI] [PubMed] [Google Scholar]
- 16.Gordin A, Chadha NK, Campisi P, et al. An animal model for endotracheal tube–related laryngeal injury using hypoxic ventilation. Otolaryngol Head Neck Surg. 2011;144(2):247–251. [DOI] [PubMed] [Google Scholar]
- 17.Chadha NK, Gordin A, Luginbuehl I, et al. Automated cuff pressure modulation: a novel device to reduce endotracheal tube injury. Arch Otolaryngol Head Neck Surg. 2011;137(1): 30–34. [DOI] [PubMed] [Google Scholar]
- 18.Weymuller EA. Laryngeal injury from prolonged endotracheal intubation. Laryngoscope. 1988;98(8, pt 2)(suppl 45):1–15. [DOI] [PubMed] [Google Scholar]
- 19.Kim JH, Ahn JJ, Jegal Y, et al. Rapid establishment of tracheal stenosis in pigs using endotracheal tube cuff overpressure and electrocautery. Curr Med Sci. 2021;41(2):329–335. [DOI] [PubMed] [Google Scholar]
- 20.Alexopoulos C, Jansson B, Lindholm CE. Mucus transport and surface damage after endotracheal intubation and tracheostomy: an experimental study in pigs. Acta Anaesthesiol Scand. 1984; 28(1):68–76. [DOI] [PubMed] [Google Scholar]
- 21.Kim S Comparison of the cuff pressures of a TaperGuard endotracheal tube during ipsilateral and contralateral rotation of the head: a randomized prospective study. Medicine. 2018;97(42): e12702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuboi S, Miyashita T, Yamaguchi Y, et al. The TaperGuard™ endotracheal tube intracuff pressure increase is less than that of the Hi-Lo™ tube during nitrous oxide exposure: a model trachea study. Anesth Analg. 2013;116(3):609–612. [DOI] [PubMed] [Google Scholar]
- 23.Dave MH, Frotzler A, Spielmann N, et al. Effect of tracheal tube cuff shape on fluid leakage across the cuff: an in vitro study. Br J Anaesth. 2010;105(4):538–543. [DOI] [PubMed] [Google Scholar]
- 24.D’Haese J, Keukeleire TD, Remory I, et al. Assessment of intraoperative microaspiration: does a modified cuff shape improve sealing? Acta Anaesthesiol Scand. 2013;57(7):873–880. [DOI] [PubMed] [Google Scholar]
- 25.Huang WM, Huang XA, Du YP, et al. Tapered cuff versus conventional cuff for ventilator-associated pneumonia in ventilated patients: a meta-analysis of randomized controlled trials. Can Respir J. 2019;2019:7876417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madjdpour C, Mauch J, Dave MH, et al. Comparison of air-sealing characteristics of tapered- vs. cylindrical-shaped high-volume, low-pressure tube cuffs. Acta Anaesthesiol Scand. 2012; 56(2):230–235. [DOI] [PubMed] [Google Scholar]
- 27.In J, Shim S, Chung S. The effect of positive end-expiratory pressure on air leakage: comparison of cuff designs. Korean J Crit Care Med. 2014;29(1):3–6. [Google Scholar]
- 28.Dave MH, Frotzler A, Spielmann N, et al. Effect of tracheal tube cuff shape on fluid leakage across the cuff: an in vitro study. Br J Anaesth. 2010;105(4):538–543. [DOI] [PubMed] [Google Scholar]
- 29.Komasawa N, Mihara R, Imagawa K, et al. Comparison of pressure changes by head and neck position between high-volume low-pressure and taper-shaped cuffs: a randomized controlled trial. BioMed Res Int. 2015;2015:386080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shinn JR, Kimura KS, Campbell BC, et al. Incidence and outcomes of acute laryngeal injury after prolonged mechanical ventilation. Crit Care Med. 2019;47(12):1699–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.House JC, Noordzij JP, Murgia RD, et al. Laryngeal injury from prolonged intubation: a prospective analysis of contributing factors (vol 121, 596, 2011). Laryngoscope. 2020;130(9):2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schweiger C, Marostica PJ, Smith MM, et al. Incidence of post-intubation subglottic stenosis in children: prospective study. J Laryngol Otol. 2013;127(4):399–403. [DOI] [PubMed] [Google Scholar]
- 33.Jiang JJ, Raviv JR, Hanson DG. Comparison of the phonation-related structures among pig, dog, white-tailed deer, and human larynges. Ann Otol Rhinol Laryngol. 2016;110(12):1120–1125. [DOI] [PubMed] [Google Scholar]
- 34.Murison PJ, Jones A, Mitchard L, et al. Development of perioperative care for pigs undergoing laryngeal transplantation: a case series. Lab Anim. 2009;43(4):338–343. [DOI] [PubMed] [Google Scholar]


