Abstract
The role of donor and recipient Coronavirus disease 2019 (COVID-19) immunologic status pre-transplantation has not been fully investigated in allogeneic hematopoietic stem cell transplantation (HSCT) recipients. Given the poor immunogenicity to vaccines in this population and the serious outcomes of COVID-19, adoptive transfer of immunity may offer important insight into improving protection for this vulnerable population. In this study, we evaluated the role of adoptive transfer of immunity at 1 month post-transplantation and 6 months post-transplantation after vaccination of recipients, based on pre-transplantation severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination and infection exposures of both recipient and donor. Using banked specimens from related donor allogeneic HSCT recipients and clinical data from both donors and recipients, anti-Spike (S) IgG titers were analyzed at 1, 3, and 6 months post-transplantation according to prior SARS-CoV-2 immunologic exposures. Recipients were excluded if they had received SARS-CoV-2 monoclonal antibodies or had infection in the first 6 months post-transplantation. Of the 53 recipient-donor pairs, 29 donors and 24 recipients had prior SARS-CoV-2 immunologic exposure. Recipient-donor pairs with no prior SARS-CoV-2 exposure (D0R0) had significantly lower anti-S IgG titers at 1 month compared to those with prior exposures (D1R1) (D0R0: median, 2.43 [interquartile range (IQR), .41 to 3.77]; D1R1: median, 8.42; IQR, 5.58 to 12.20]; P = .008). At 6 months, anti-S IgG titers were higher in recipients who were vaccinated at 3 months post-transplantation in the D1R1 cohort (median IgG, 148.34; IQR, 92.36 to 204.33) compared with the D0R0 cohort (median IgG, 38.74; IQR, 8.93 to 119.71). Current strategies should be optimized to enhance SARS-CoV-2 protection for HSCT recipients, including augmentation of the immune response for both donors and recipients prior to transplantation.
Key Words: Adoptive transfer, SARS-CoV-2, humoral response, vaccine-induced immunity, HSCT
INTRODUCTION
Recipients of allogeneic hematopoietic stem cell transplantation (HSCT) have an impaired immune response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination, owing to both the underlying hematologic malignancy and prior chemotherapy and conditioning regimens [1,2]. As a result, morbidity and mortality due to Coronavirus disease 2019 (COVID-19) are higher in this population [3,4]. These patients also exhibit increased and prolonged viral shedding, which likely has public health implications for the development of new variants of concern [5,6]. In addition, although cell populations repopulate after transplantation, early reduction of T cells and natural killer cells and prolonged humoral defects occur [7], further compromising the ability to create a robust and protective immune response to vaccination. New strategies are urgently needed to improve protection for this vulnerable population, especially in the context of continued evolution of variants.
Vaccination of donors and recipients prior to HSCT may be one method for improving protection against COVID-19. This approach has been examined for other pathogens, with varying results 8, 9, 10, 11. Both the donor and recipient's prior immunization history are known to be important, as demonstrated by potentiated antibody responses against hepatitis B when both the donor and recipient were immunized with hepatitis B vaccine prior to transplantation but no difference in antibody responses when only the donor was previously immunized [12]. Post-transplantation antibody-level enhancement is also dependent on the type of vaccine. A greater potentiation effect has been observed for highly immunogenic vaccine antigens, such as tetanus toxoid, with a reduced effect seen for less immunogenic vaccines, such as pneumococcal polysaccharide vaccines [11].
We sought to examine the effects of vaccination in the allogeneic HSCT recipient population with the mRNA platform, which is highly immunogenic, to evaluate the adoptive transfer of immunity post-transplantation for SARS-CoV-2 specifically. A prior study has suggested that pre-HSCT donor vaccination may have an impact on the post-HSCT humoral response to SARS-CoV-2 vaccination after transplantation, although the sample size was relatively small, and the data require further validation [13]. Current recommendations suggest restarting the COVID-19 mRNA vaccine primary series (which consists of 3 doses for immunocompromised patients) at 3 months post-transplantation [14]; however, the data to support this recommendation are minimal and do not take into account prior exposures pre-transplantation. Furthermore, as new mRNA COVID-19 vaccines are developed and implemented that target specific variants, such as the bivalent ancestral/Omicron vaccine [15,16], guidelines on vaccination for HSCT recipients become even more opaque. In the present study, using banked specimens from allogeneic HSCT recipients, we evaluated how donor and recipient COVID-19 immunologic status pre-transplantation impacts recipient SARS-CoV-2 antibody response post-transplantation.
METHODS
The study was approved by the Institutional Review Board at the Dana-Farber Cancer Institute (DFCI). Adult patients who underwent allogeneic HSCT with related donors between January 1 and December 31, 2021 were identified and selected using available cryopreserved plasma samples that had been collected for a biospecimen repository research protocol at 1 month, 3 months, and 6 months post-transplantation. Since the COVID-19 vaccines were approved under Emergency Use Authorization (EUA) from December 2020 to February 2021 and initially prioritized only for high-risk individuals, and the first full licensure occurred in August 2021 [17], selecting the year 2021 allowed us to capture both individuals who had been vaccinated and those who had not been vaccinated prior to harvest. In accordance with current recommendations, recipients were vaccinated or revaccinated at the oncology clinic at 100 days post-transplantation with either the mRNA-1273 (Moderna; 100 g) or BNT162b2 (Pfizer; 30 μg) vaccine series. Using the single-molecule array (Simoa) technology [1,18], quantitative detection of anti-Spike (S; full spike protein) and anti-Nucleocapsid (N) IgG was assessed for the recipients at 1 month, 3 months, and 6 months post-transplantation measured in normalized average enzymes per bead (nAEB). Donor and recipient COVID-19 histories and vaccinations were extracted using the electronic medical record and clinical database associated with the tissue bank. Donors and recipients were categorized based on their SARS-CoV-2 immunologic exposure prior to transplant: SARS-CoV-2 vaccination and/or SARS-CoV-2 infection (defined by documented SARS-CoV-2 PCR in the electronic medical record, reported history of SARS-CoV-2, or positive anti-N IgG). HSCT recipients who received SARS-CoV-2 monoclonal antibodies during the study period were excluded, as well as recipients who tested positive for SARS-CoV-2 from the time of transplant through six months.
Descriptive and graphical summaries were performed. Fisher's exact tests (categorical characteristics) and Mann Whitney U tests (continuous characteristics) were used, with two-sided P-values <0.05 considered statistically significant after accounting for multiple comparisons using false discovery rate (FDR) control. All analyses were conducted in R version 4.1.2 (https://www.R-project.org/).
RESULTS
Demographic, Disease, and Treatment Characteristics
A total of 93 allogeneic related donor HSCTs were performed at Dana-Farber Cancer Institute during 2021. Of those, 55 individuals had banked recipient plasma samples identified from the biospecimen repository. Among these, 53 were included for analysis; 1 was excluded due to SARS-CoV-2 exposure and receipt of monoclonal antibodies post-transplantation, and 1 was excluded because the individual underwent a second transplantation (from an unrelated donor) during the study period. The recipients had a median age of 58.10 years (IQR, 52.87 to 66.32 years), and 19 (35.85%) were female (Table 1 ). Several disease types were represented, with acute myeloid leukemia and myelodysplastic syndrome the main indications for transplantation (33.96% and 20.75%, respectively). Additional characteristics including transplant type, conditioning regimen, graft-versus-host-disease (GVHD) prophylaxis, and occurrence of GVHD in the first 6 months post-transplantation are described in Table 1.
Table 1.
Demographics and Treatment Characteristics (N = 53)
| Characteristic | Value |
|---|---|
| Female sex, n (%) | 19 (35.85) |
| Age at time of transplant, yr, median (IQR) | 58.10 (52.87-66.32) |
| Disease (indication for HSCT), n (%) | |
| Acute lymphoblastic leukemia | 5 (9.43) |
| Acute myeloid leukemia | 18 (33.96) |
| Chronic myeloid leukemia | 2 (3.77) |
| Hemoglobinopathy | 1 (1.89) |
| Hodgkin lymphoma | 4 (7.55) |
| Myelodysplastic syndrome | 11 (20.75) |
| Myeloproliferative neoplasm | 4 (7.55) |
| Non-Hodgkin lymphoma | 3 (5.66) |
| Other acute leukemia | 2 (3.77) |
| Other leukemia | 3 (5.66) |
| Transplant type, n (%) | |
| Matched related | 29 (54.72) |
| Mismatched related | 1 (1.89) |
| Haploidentical related | 23 (43.40) |
| Conditioning regimen type | |
| Reduced intensity | 35 (66.04) |
| Myeloablative | 18 (33.96) |
| GVHD prophylaxis regimen, n (%) | |
| Cyclophosphamide, mycophenolate mofetil, tacrolimus | 26 (49.06) |
| Methotrexate, sirolimus, tacrolimus | 1 (1.89) |
| Methotrexate, tacrolimus | 24 (45.28) |
| None* | 2 (3.77) |
| History of acute GVHD, n (%) | 23 (43.40) |
| History of chronic GVHD in first 6 mo, n (%) | 3 (5.66) |
Participants were in a clinical trial to study immunosuppression-free regulatory T cell graft-engineered haploidentical HSCT.
COVID-19 Immunologic Exposures
Twenty-eight of the 53 donors (52.83%) were vaccinated prior to stem cell harvest, with a median of 122 days (IQR, 58.5 to 159.0 days) from the last vaccine dose to transplantation, and 1 donor had a history of SARS-CoV-2 infection prior to transplantation (Table 2 ). Twenty-two recipients (41.51%) were vaccinated prior to transplantation, with a median of 140 days (IQR, 97 to 181 days) from the last vaccine dose to transplantation. Four recipients (7.55%) had an infection prior to transplantation, with 2 of these recipients also receiving vaccination prior to transplantation. The donor (D) and recipient (R) pairs based on immunologic status (0, no prior SARS-CoV-2 vaccine or infection; 1, prior SARS-CoV-2 vaccine or infection) included D0R0 (n = 17), D1R1 (n = 18), D0R1 (n = 7), D1R0 (n = 7), and D1Runknown (n = 4). Vaccination types and number of doses are described in Supplementary Table S1.
Table 2.
Donor and Recipient COVID-19 Immunologic Exposures (Vaccination or Infection) Pre-transplantation
| Parameter | Donor SARS-CoV-2 Exposures (N = 53) | Recipient SARS-CoV-2 Exposures (N = 53) | Recipient Vaccination or Infection Between 3 and 6 mo Post-Transplantation) (N = 53) |
|---|---|---|---|
| Vaccination, n (%) | 28 (52.83) | 22 (41.51) | 28 (51.85) |
| No vaccine, n (%) | 24 (45.28) | 25 (47.17) | 25 (48.15) |
| Infection, n (%) | 1 (1.87) | 4 (7.55)* | 0 (0) |
| Vaccine status unknown, n (%) | 0 (0) | 4 (7.55) | 0 (0) |
| Time from last vaccine dose to transplant, d, median (IQR) | 122 (58.5, 159) | 140 (97, 181) | N/A |
N/A indicates not applicable.
Two recipients had both vaccination and infection prior to transplantation.
Anti–S IgG Antibody Titers in Recipients Post-Transplant
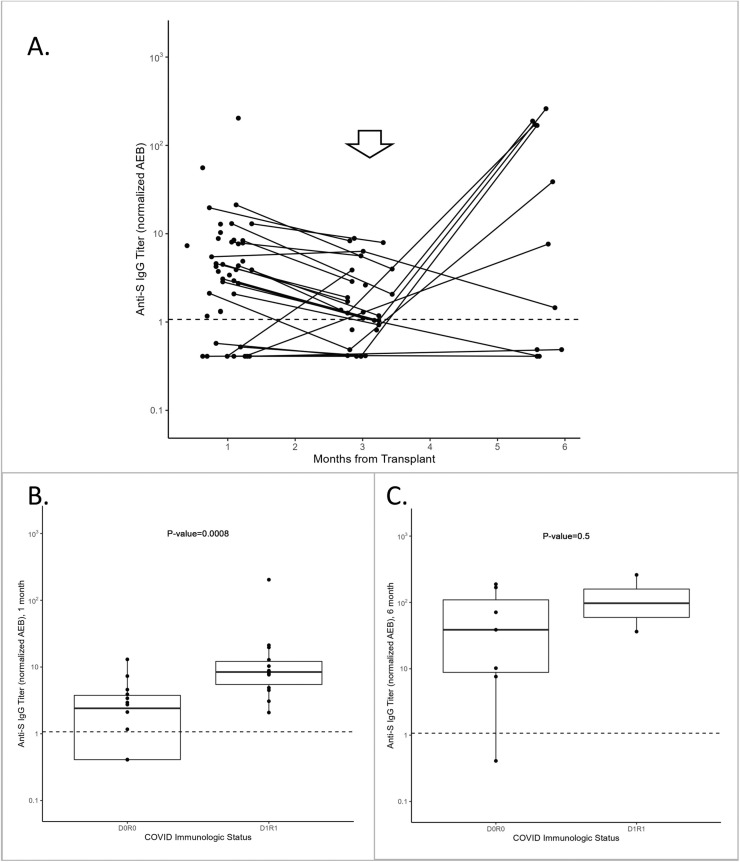
Anti-S IgG titers were measured in recipients at approximately 1-, 3-, and 6-months post-transplantation. There was evidence of passive antibody transfer or retention at month one in some recipients, followed by antibody decay for the subsequent 2 months and an increased titer at 6 months after COVID-19 vaccination at 3 months post-transplantation (Figure 1 A). No recipients had a documented COVID-19 history or positive anti-N values from the time of transplantation to 6 months post-transplantation, although 4 recipients had a history of COVID-19 prior to transplantation (accounted for in the immunologic status).
Figure 1.
(A) Anti-S IgG titers, in nAEB, in all recipients (n = 53) at 1, 3, and 6 months post-transplantation. The arrow indicates the approximate time of vaccination post-transplantation (∼3 months). (B) Anti-S IgG titers were analyzed at 1 month post-transplantation by immunologic status (D, donor; R, recipient; 0, no prior SARS-CoV-2 vaccination or infection before transplantation; 1, prior SARS-CoV-2 vaccination and/or infection before transplantation) for D0R0 (n = 14) and D1R1 (n = 14). The median titer was significantly higher for the D1R1 group compared with the D0R0 group (P = .008). (C) The anti-S IgG titers for recipients who were vaccinated between 3 and 6 months post-transplantation are represented by COVID-19 immunologic status, D0R0 (n = 7) or D1R1 (n = 2). Anti-S titers are shown at 6 months post-transplantation. No significant difference is observed between the D0R0 and D1R1 groups (P = .5). Values are median and IQR, and the dotted line at 1.07 nAEB represents the assay cutoff.
Passive Antibody Transfer or Retention at 1 Month
To further evaluate the effects of donor and recipient SARS-CoV-2 immunologic exposure on passive antibody transfer or retention, anti-S IgG titers in recipients were evaluated at 1 month post-transplantation and stratified by exposure history (Figure 1B). Recipients in the D0R0 group had significantly lower anti-S IgG titers compared to those in the D1R1 group (D0R0: median, 2.43 [IQR, .41 to 3.77]; D1R1: median, 8.42; IQR, 5.58 to 12.20]; P = .008). There was no significant difference in 1-month anti-S IgG titers between the D0R0 group and the D0R1 group (median, 4.25; P = .058) or D1R0 group (median, 1.71; P = .788) (Supplementary Figure S1).
Vaccine Response at 6 Months Post-Transplantation
Anti-S IgG titers were measured at 6 months post-transplantation in recipients who were vaccinated at 3 months post-transplantation (n = 28). To determine whether donor or recipient memory B cells were present with a recall response, anti-S IgG titers were compared by immunologic exposure (Figure 1C). The D1R1 group (n = 2) had the highest median anti-S response at 148.34 (IQR 92.36-204.33), although this was nonsignificant compared with the D0R0 group (n = 7; median anti-S IgG, 38.74; IQR, 8.93 to 119.71), possibly related to the small sample size. These findings should be further explored with a larger sample size. Of note, the median titer of anti-S IgG in the D1R1 group exceeded that in allogeneic HSCT recipients and in healthy adults at 1 month after the initial 2-dose series (20.27 nAEB versus 65.70 nAEB, respectively), as we described previously [1], which suggests a possible augmented effect. There was no significant difference in the median antibody titer between D0R0 and D0R1 (median anti-S, 1.45; IQR, 1.45 to 1.45) or D1R0 (median anti-S, 1.21; IQR, .74 to 44.27) (Supplementary Figure S2).
DISCUSSION
Overall, our study demonstrates both passive anti-S IgG transfer from donors to recipients and retention of antibodies in recipients with prior SARS-CoV-2 exposure at 1 month post-transplantation. Our data suggest that optimizing vaccination for both the donor and recipient prior to transplantation may aid the development of antibodies that can be protective in vulnerable individuals 3 months after transplantation. Furthermore, for the recipients vaccinated at 3 months post-transplantation, recipients were able to produce an immune response, as evidenced by the 6-month post-transplantation titers, regardless of prior immunologic exposure for either the donor or the recipient. These data support the current guidelines recommending revaccination at around 100 days post-transplantation. Although there was no significant difference in anti-S titer values between the D0R0 and D1R1 groups at 6 months in recipients who were vaccinated post-transplantation, there was a trend toward a higher magnitude of anti-S IgG in the D1R1 group. However, this is an underpowered observation that should be explored further with a larger sample population.
Similar to other studies that have investigated transfer of immunity to HSCT recipients, our study reports on immunogenicity, which is not necessarily reflective of protection against infection 19. Future studies should examine clinical efficacy in the context of donor and/or recipient transfer of immunity. Interestingly, low-level antibody positivity was seen in some recipients at 1 month post-transplantation in the D0R0 group. This may be due to cross-reactivity with other coronaviruses or to asymptomatic SARS-CoV-2 infections in donors or recipients that were not reported. In addition, the transfer of B cell-mediated immunity likely is dependent on many factors, including the timing and type of vaccination, GVHD therapies, and prior chemotherapy and immunomodulatory treatments received, which our study was not powered to describe. Although we suspect that the healthy donors likely had robust responses to SARS-CoV-2 mRNA vaccination, the titer of anti-S IgG or memory B cells at the time of transplantation was not known in this study. Our pilot study highlights additional areas that should be explored in a larger population of HSCT donor-recipient pairs.
Our results provide valuable insight into the complex dynamics of donor and recipient immunity with regard to the COVID-19 mRNA vaccines. The unique immunologic properties of the donor-recipient pair in HSCT may offer another method to augment SARS-CoV-2 immunity. Determining the optimal timing of vaccination pre-transplantation for both donors and recipients and identifying other factors that can improve immune response and durability will be essential next steps.
ACKNOWLEDGMENTS
Financial disclosure: Sample processing and cryopreservation was supported by the Pasquarello Tissue Bank in Hematologic Malignancies at the Dana-Farber Cancer Institute. The project described was supported by Grant 1UL1TR002541-01 to the Harvard Clinical and Translational Science Center from the National Center for Advancing Translational Science, as well as funding from Barbara and Amos Hostetter and the Chleck Foundation.
Conflict of interest statement: A.C.S. reports support for the present work from the Chleck Foundation and Barbara and Amos Hostetter and is involved in human immunodeficiency virus (HIV), coronavirus (COVID), and other vaccine clinical trials conducted in collaboration with the National Institutes of Health (NIH), HIV Vaccine Trials Network, COVID Vaccine Prevention Network, International AIDS Vaccine Initiative, Crucell/Janssen, and Moderna. G.Z. reports support from the University of British Columbia/British Columbia Children's Hospital for statistical consulting on multiple projects involving continuous physiologic monitoring to improve pediatric care in resource-limited settings, funded by the Bill & Melinda Gates Foundation. D.R.W. has a financial interest in Quanterix, a company developing an ultrasensitive digital immunoassay platform; is an inventor of the SIMOA technology, a founder of the company, and a member of its board of directors; and received financial support for the present work from Barbara and Amos Hostetter and the Chleck Foundation. L.R.B. reportes research support from the NIH (including the National Institute of Allergy and Infectious Diseases and the National Center for Advancing Translational Sciences), the Wellcome Trust, and the Bill & Melinda Gates Foundation, outside the submitted work; has served on data and safety monitoring boards, safety monitoring committees, and advisory committees for the NIH and US Food and Drug Administration; and is involved in HIV, COVID, and other vaccine clinical trials conducted in collaboration with the NIH, HIV Vaccine Trials Network, COVID Vaccine Prevention Network, International AIDS Vaccine Initiative, Crucell/Janssen, Moderna, Military HIV Research Program, the Bill & Melinda Gates Foundation, and the Ragon Institute. R.J.S. reports consulting for Cugene, Takeda, Jasper, Jazz Pharmaceuticals, Precision Biosciences, Alexion, and Rheos Therapeutics and serving on a data safety monitoring board for Juno Therapeutics, on the board of directors for Kiadis, on a career development award committee for Gilead, and on the board of directors for the National Marrow Donor Program—Be the Match. N.C.I. reports funding from AiCuris, Merck, Fujifilm, Astellis, and GSK.
Authorship statement: A.C.S. designed and performed the research, analyzed the data, and wrote the manuscript. C.C. and Z.S. performed the laboratory analyses and edited the manuscript. D.R.W. supported the laboratory components and created the antibody assay, assisted with the analysis design, and edited the manuscript. G.Z. assisted with analysis consultation and statistical methods. X.L. contributed to the laboratory processing and analysis. N.C.I. assisted with the study design and editing the manuscript. L.R.B. and R.J.S. assisted with the study design and analysis and were involved with drafting the manuscript. L.R.B. and R.S. contributed equally as co-senior authors.
Footnotes
Abbreviations: SARS-CoV-2, severe acute respiratory syndromecoronavirus2; HSCT, Hematopoietic stem cell transplantation
Financial disclosure: See Acknowledgments on page 337.e5.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jtct.2023.01.025.
Appendix. Supplementary materials
REFERENCES
- 1.Sherman AC, Desjardins M, Cheng CA, et al. Severe acute respiratory syndrome coronavirus 2 messenger RNA vaccines in allogeneic hematopoietic stem cell transplant recipients: immunogenicity and reactogenicity. Clin Infect Dis. 2022;75:e920–e923. doi: 10.1093/cid/ciab930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mamez AC, Pradier A, Giannotti F, et al. Antibody responses to SARS-CoV2 vaccination in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2021;56:3094–3096. doi: 10.1038/s41409-021-01466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma A, Bhatt NS, St Martin A, et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol. 2021;8:e185–e193. doi: 10.1016/S2352-3026(20)30429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vijenthira A, Gong IY, Fox TA, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136:2881–2892. doi: 10.1182/blood.2020008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark SA, Clark LE, Pan J, et al. SARS-CoV-2 evolution in an immunocompromised host reveals shared neutralization escape mechanisms. Cell. 2021;184 doi: 10.1016/j.cell.2021.03.027. 2605-2617.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nabel KG, Clark SA, Shankar S, et al. Structural basis for continued antibody evasion by the SARS-CoV-2 receptor binding domain. Science. 2022;375:eabl6251. [DOI] [PMC free article] [PubMed]
- 7.Ogonek J, Kralj Juric M, Ghimire S, et al. Immune reconstitution after allogeneic hematopoietic stem cell transplantation. Front Immunol. 2016;7:507. doi: 10.3389/fimmu.2016.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris AE, Styczynski J, Bodge M, Mohty M, Savani BN, Ljungman P. Pretransplant vaccinations in allogeneic stem cell transplantation donors and recipients: an often-missed opportunity for immunoprotection? Bone Marrow Transplant. 2015;50:899–903. doi: 10.1038/bmt.2015.49. [DOI] [PubMed] [Google Scholar]
- 9.Molrine DC, Antin JH, Guinan EC, et al. Donor immunization with pneumococcal conjugate vaccine and early protective antibody responses following allogeneic hematopoietic cell transplantation. Blood. 2003;101:831–836. doi: 10.1182/blood-2002-03-0832. [DOI] [PubMed] [Google Scholar]
- 10.Molrine DC, Guinan EC, Antin JH, et al. Donor immunization with Haemophilus influenzae type b (HIB)-conjugate vaccine in allogeneic bone marrow transplantation. Blood. 1996;87:3012–3018. [PubMed] [Google Scholar]
- 11.Storek J, Dawson MA, Lim LC, et al. Efficacy of donor vaccination before hematopoietic cell transplantation and recipient vaccination both before and early after transplantation. Bone Marrow Transplant. 2004;33:337–346. doi: 10.1038/sj.bmt.1704336. [DOI] [PubMed] [Google Scholar]
- 12.Wimperis JZ, Brenner MK, Prentice HG, et al. Transfer of a functioning humoral immune system in transplantation of T-lymphocyte-depleted bone marrow. Lancet. 1986;1:339–343. doi: 10.1016/s0140-6736(86)92315-9. [DOI] [PubMed] [Google Scholar]
- 13.Leclerc M, Redjoul R, Le Bouter A, et al. Impact of donor vaccination on recipient response to early SARS-CoV-2 mRNA vaccination after allogeneic HSCT. Lancet Haematol. 2022;9:e318–e321. doi: 10.1016/S2352-3026(22)00097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Society of Hematology. ASH-ASTCT COVID-19 vaccination for HCT and CAR T cell recipients. Available at: https://www.hematology.org:443/covid-19/ash-astct-covid-19-vaccination-for-hct-and-car-t-cell-recipients. Accessed June 15, 2021.
- 15.Chalkias S, Harper C, Vrbicky K, et al. A bivalent omicron-containing booster vaccine against Covid-19. N Engl J Med. 2022;387 doi: 10.1056/NEJMoa2208343. 1279-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Food and Drug Administration. COVID-19 bivalent vaccine boosters. 2022. Available at: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-bivalent-vaccine-boosters. Accessed September 17, 2022.
- 17.US Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccines and comirnaty information. 2022. Available at: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/comirnaty-and-pfizer-biontech-covid-19-vaccine. Accessed April 1, 2022.
- 18.Norman M, Gilboa T, Ogata AF, et al. Ultrasensitive high-resolution profiling of early seroconversion in patients with COVID-19. Nat Biomed Eng. 2020;4:1180–1187. doi: 10.1038/s41551-020-00611-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherman AC, Desjardins M, Baden LR. Vaccine-induced severe acute respiratory syndrome coronavirus 2 antibody response and the path to accelerating development (determining a correlate of protection) Clin Lab Med. 2022;42:111–128. doi: 10.1016/j.cll.2021.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.