Abstract
We present an unusual case of monkeypox (MPOX) virus transmission to a dermatology resident during examination of affected patients. Viral DNA sequencing led to the identification of the most likely contact. This case, along with a review of all published cases so far, emphasizes the possible hazard of MPOX transmission to health care personnel, even when wearing personal protective equipment. It also emphasizes the need for maintaining high index of suspicion when examining patients with new dermatological lesions and strict compliance with the revised Centers for Disease Control and Prevention recommendations for specimen collection from such patients.
Key Words: Monkeypox virus, Occupational exposure, Personal protective equipment, Felon finger, Viral DNA sequencing
Introduction
Since May 2022 the world is facing the largest MPOX (formerly monkeypox) virus outbreak described so far in nonendemic countries, considered by the World Health Organization as a public health emergency of international concern.1
MPOX virus transmission may occur through direct contact with infected skin lesions, respiratory secretions, or even objects and surfaces.2 Thus far, in the current outbreak, sexual activity or intimate contact, particularly among men who have sex with men, seem to be the main routes of disease transmission.3, 4 - 5
The possibility of MPOX nosocomial transmission to health care providers (HCP) in nonendemic areas was recently evaluated by Zachary et al.,6 who performed a systemic review of literature and concluded that the risk of transmission following exposure in well-resourced health care settings was low, with only 1 published case of such transmission in 2018 in the United Kingdom.7 Furthermore, Marshall et al.,8 analyzed the risk of infection during the current outbreak among exposed HCPs and similarly found a very low risk of transmission, even with incomplete adherence to recommended personal protective equipment (PPE).
In spite of these reassuring previous findings, reports of HCP occupational infections are emerging monthly since July 2022.9, 10, 11, 12, 13 - 14 Here, we characterized a case of nosocomial transmission of MPOX virus using molecular typing, and review the current literature.
Case report
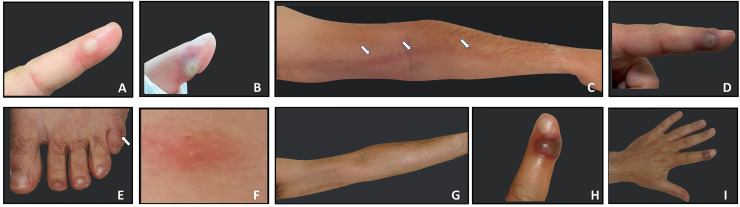
A 37-year-old healthy heterosexual male dermatology resident at a large tertiary hospital, noticed a small painful vesicle on the tip of his left index finger. As it was first identified during a family vacation that included rafting, he attributed the occurrence of the lesion to mechanical irritation following intensive rowing. During the following 9 days the lesion gradually grew in size with aggravated pain, local edema and the development of axillar lymphadenitis on the same side (Fig 1 A). In the absence of other lesions or symptoms at first, the resident treated himself topically with mupirocin ointment and adhesive water-resistant dressings.
Fig 1.
Progression of clinical manifestations. Index finger lesion at presentation. A single tender vesiculopustular lesion on the left index finger was observed on day 9. The lesion was 6 millimeters in diameter, with no fluctuation or local heat on palpation (A); Needling of the left index finger lesion was performed on day 10, leading to hematoma development at the puncture site (B); On day 12, significant lymphangitis developed, extending up to the left axilla (C) associated with slight enlargement of the primary lesion (D), and appearance of 2 additional lesions on the fifth left toe (E) and middle back (F); During days 13-16, the aforementioned systemic and lymphangitic (G) manifestations subsided, while the primary lesion continued to enlarge up to 10 millimeters in diameter (H,I).
On the ninth day, mild systemic symptoms including myalgia and malaise appeared. Given that this lesion developed during the early weeks of the MPOX outbreak, during which the resident had actively participated in the diagnosis of numerous MPOX-positive patients, a vesicle fluid sample was obtained for bacterial culture and MPOX polymerase chain reaction (PCR) analysis. Bacterial cultures were sterile but the PCR test turned to be positive for MPOX virus.
On the 12th day, painful lymphangitis developed over the forearm extending up to the ipsilateral axilla. In addition, 2 small pruritic popular lesions were noted on the back and on left fifth toe (Fig 1 B-H). Pharyngeal samples were negative by PCR for MPOX while samples taken from the papule on the back yielded a positive MPOX virus PCR. Laboratory tests were significant for elevated levels of liver enzymes (ALT 191 U/L, GGT 272 U/L), and increased C-reactive protein (up to16.98 mg/L, normal range 0-5). Complete blood count and chest x-ray were normal.
The patient's asymptomatic wife and 2 daughters were tested for MPOX by throat swab PCR on day 11 and were found to be disease-free. According to the instructions of the Israeli Ministry of Health, they received a single dose of the JYNNEOS smallpox/MPOX vaccine on day 13. On 1 month follow-up no signs or symptoms of MPOX were noted among the resident's family members.
A thorough epidemiological investigation was conducted. The resident did not receive smallpox vaccination during childhood, neither was he vaccinated for MPOX prior to the event. No history of skin abrasion or minor trauma prior to appearance of the lesion was reported by the affected resident. No history of close contacts with MPOX affected individuals, apart from contact with patients seen in the emergency room, was elicited. During the reported period of June to July, 160 PCR samples were collected from 37 suspected patients at our medical center. The specimens were taken from the skin, oropharynx, and in a few cases, also from the rectum or semen. Of these, 21 cases were confirmed to be positive. The resident performed medical examinations and specimen collection from 13 patients suspected of being infected with MPOX during this time. Out of these, 11 were found to be MPOX PCR positive, of whom 9 were examined prior to the appearance of the resident's first lesion.
As the MPOX outbreak was announced worldwide, a protocol for patient management, isolation and PPE use was gathered and distributed throughout the hospital. The order and mode of protective gear wearing and removal was similar to the order which was used during COVID outbreaks, thus the resident was well trained in donning and doffing PPE. Specimen collection was also performed according to the hospital's protocol: after collection, the swabs were inserted into a closed-cap container and placed into 2 plastic bags, with the outermost layer containing a biohazard sign. Then, inserted into a screw-on plastic container and placed in a designated location for collection by a hospital porter in a sterile manner to be transported to the laboratory. The cleaning protocol for rooms occupied by suspected or positive MPOX cases in the emergency room was to clean and disinfect with a hypochlorite solution (1,000 ppm). Shared equipment used in these rooms were cleaned using chlorine wipes, while equipment not suitable for such sterilization was cleaned in 2 rounds using Quaternary ammonium wipes.
Although the resident did not recall any repeated contact with the collected specimens after removing PPE, we believe that the most reasonable mode of infection in our case was inadvertent contact with infected fomites in the vicinity of an infected individual, or with the contaminated surface of incorrectly removed PPE. Other less likely modes of transmission were: (1) minor trauma penetrating both glove and skin during the examination or crust sampling (performed by a scalpel that may have created micro tears in the glove of the supporting hand. Although such a breach of PPE was not noticed by the resident). (2) Minor inadequacies in the use of PPE. The resident followed institutional guidelines for protective measures when examining patients suspected of contracting MPOX, including the use of a disposable gown, gloves, N-95 mask, and glasses. However, a face shield was not used while the HCP was wearing only glasses, and in some cases, the gowns were permeable. (3) Breaches in the process of transferring samples to the laboratory were also found which may have led to environmental contamination. (4) Finally, exposure to an undiagnosed MPOX-affected patient is also possible.
Since the resident had been wearing a waterproof bandage to cover the lesion, the risk of transmission of MPOX to other patients was considered as low. However, 6 patients who underwent surgical procedures performed by the resident, and 2 HCPs who worked closely with him were defined as potentially exposed and were requested to monitor body temperature and appearance of new skin lesions. All other HCPs who worked with the resident were also asked to report new skin lesions. None of the exposed patients or HCPs developed any MPOX signs or symptoms.
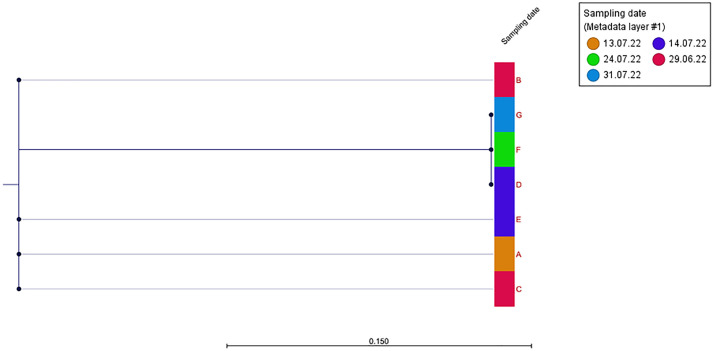
Examination of PCR data from samples obtained from all MPOX-positive patients examined by the affected physician yielded 7 samples which were considered adequate for whole genome sequencing (WGS) (Cycle Threshold (CT) Values <35). WGS of the aforementioned 7 samples along with the sample obtained from the affected physician was performed (approved by the institutional review board in accordance with the principles of the Declaration of Helsinki. For detailed methods of the WGS please refer to the attached supplementary file). All samples were identified as hMPOX-1, lineage B.1. A list of shared single nucleotide polymorphism (SNP) is presented in supplementary Table 1. Phylogenetic analysis revealed maximal proximity between the physician's samples (Fig 2 , Sample G) and samples obtained from patients D and F. Since only patient D was examined by the physician prior to symptom onset, this patient was identified as the most likely source of disease transmission. This was a 41-year-old man who had sex with men (MSM), generally healthy. He was referred to the emergency room due to the emergence of 2 lesions on his genital area during the preceding several days, suspected to be infected with MPOX. Other than that he was asymptomatic. Recent relevant exposures of patient D were unprotected sexual and oral intercourse. He was examined by the resident–who performed a full body examination, followed by lesion unroofing and sampling.
Fig 2.
Single nucleotide polymorphism based phylogeny of human mpox virus samples. The maximum likelihood tree of patients (n = 6) was rooted in a reference physician's isolate (G).
Sampling dates are marked for each sample.
Of note, is that whole genome sequencing has certain limitations in determining the source of transmission. Of the 13 MPOX-positive patients evaluated by the resident, only 7 were suitable for the analysis and only 5 were chronologically relevant (ie, evaluated before symptom onset). As depicted in Figure 2, the proximity was observed not only to patient D, but also to Patient F, indicating the likelihood of infection within a close cluster. This suggests that it is possible that additional patients, who were evaluated by the resident but not included in the analysis, may have been the source of transmission.
Discussion
MPOX virus is transmitted through direct contact with infected lesions, respiratory secretions, or objects.2 Although its main mode of transmission during the current outbreak has been through sexual contacts, several recent reports have described occupational transmission to HCP as well.
In a review of recent publications dating from the current MPOX outbreak, 6 cases of occupational HCP infection have been reported in 5 different countries (2 in Brazil and United States of America, 1 in France and Portugal) (Table 1 ). In all cases the first lesion appeared on a finger, where exposure to the virus had presumably occurred. Although transmission mode was mainly associated with using sharp instruments for unroofing and sampling the patients' lesions followed by HCP's skin injury with the affected instrument, transmission through contact with contaminated fomites was also suspected in some of the cases. Disease course was very similar among most occupational HCP cases, with the appearance of the first lesion at the inoculation site 4-5 days after contact, followed by systemic manifestations during the viremic phase and then additional distant lesions. Our case, along with 4 of 6 previously reported cases, illustrates a possible advantage of administering postexposure prophylaxis vaccination in a timely manner. When the vaccination was not given, a systemic spread of the disease occurred. However, in instances where the vaccination was administered, HCPs experienced only a single lesion (resembling a Jennerian pustule15 at the site of inoculation), with no systemic symptoms. These results suggest that prompt administration of postexposure prophylaxis vaccination may provide a sufficient immunologic response to prevent the virus from spreading throughout the body.
Table 1.
Demographic and clinical characteristics, course of disease and treatment provided in published cases of occupational MPOX virus infections during the current outbreak.
| Location and timing | Mode of exposure | Clinical symptoms | PCR Swabs | MPOX related treatment and vaccination | Use of PPE | |
|---|---|---|---|---|---|---|
| Carvalho et al., Emerg Infect Dis, 2022 | Brazil July 2022 | Needlestick injury (finger). while gathering materials to discard in a sharps container when a needle perforated the glove | - Inoculation site after 5 d (nodule turned to vesicle) - Spread of lesions (hands, thigh, face). Total 7 lesions. Preceded generalized symptoms of fever and lymphadenopathy |
Positive from the lesion Positive from OPX. | None | Wearing personal protective equipment, including gown, gloves, goggles, and mask. |
| Caldas JP et al., Emerg Infect Dis, 2022 | Portugal July 2022 | Needlestick injury (finger). There was no wound or bleeding. | - Inoculation site after 4 d(vesicle) | Positive from the lesion. Negative from OPX. | Since no signs appeared after the injury, at first the incident was not reported as an occupational exposure and was not considered for postexposure prophylaxis treatment. | Wearing the recommended personal protective equipment; the gloves appeared intact. |
| Mendoza et al., Emerg Infect Dis, 2022 | Florida July 2022 | Needlestick injury (finger), while recapping the used needle after using it to create an opening in the vesicular lesion to facilitate direct contact of the swab with fluid in the lesion. | - Inoculation site after 10 d. - No additional lesions or other clinical signs or symptoms were reported |
Positive from the lesion. | 15 h after exposure first dose of a 2-dose JYNNEOS vaccination series was given for postexposure prophylaxis. | Not mentioned |
| Salvato et al., Emerg Infect Dis, 2022 | Brazil July 2022 | Suspected to be transmitted through fomite exposure with surfaces in the patient's home, own PPE, or outer surfaces of the specimen transport box. | HCP 1: - after 5 d - single lesion on finger. - systemic symptoms (lymphangitis in her left upper arm and worsened hyperemia). - Another local lesion. HCP 2: - after 5 d – single lesion on the forearm. - Systemic symptoms (fever and lymphadenopathy) - Spread of lesions (face). |
Positive from the lesion. Selected samples from the patient and HCP-1 for whole-genome sequencing analysis which showed that the sequenced genomes were 100% identical. | none | HCPs wore PPE, including safety glasses, disposable isolation gowns, and N95 respiratory masks – during the sample collections. However, during the interview with the patient – did not wear gloves. |
| Le Pluart et al, Open Forum Infect Dis, 2022 | France July 2022 | Needlestick injury (Right thumb) during swab collection by medical resident. | - Inoculation site after 4 d – 1 single lesion (vesicle). - No systemic symptoms. |
Positive from the lesion. Negative from OPX. | Within 3 h after exposure - received a dose of third-generation smallpox vaccine (Imvanex) for postexposure prophylaxis. The HCP's flat mates were also vaccinated. | Wearing appropriate PPE consisting of disposable gown, disposable gloves, FFP2 mask, and goggles. |
| Alarcón et al., Emerging infectious diseases, 2022 | USA August 2022 | Inadvertent contamination during specimen collection, contact with contaminated environmental surfaces or unrecognized skin contamination during glove doffing. | - Short prodrome of myalgia, fatigue, and mild headache. - Small, raised skin lesion on her left middle finger progressed to a blister with umbilication. - Systemic symptoms (fever, cough, sore throat) - Spread of lesions throughout her body (10 lesions). |
Positive from the lesion. | 2 wk course of oral tecovirimat. (It should be noted HCP's medical history significant for rheumatoid arthritis - treated with etanercept (Anti-TNF). | Wearing full PPE (N95 respirator, gown, and eye protection) when examining suspected patients and swabbing lesions. However, in 2 cases the HCP did not wear full PPE at first, and only when patient's symptoms raised her suspicion, changed to full PPE before swabbing the lesions. |
| Our case | Israel July 2022 | Contact with infected fomites in the patient's vicinity, or minor and unnoticed trauma penetrating both glove and skin during specimen collection. | - After 4 d – 1 vesicle (index finger left hand). - Systemic symptoms (weakness and ascending lymphangitis in left arm) - Spread of lesions (back and toe). |
Positive from the lesion. Negative from OPX. Whole genome sequencing were analyzed, comparing the mpox positive patients along with the sample from the affected physician identifying the most likely source of disease transmission. | On day 13, family members were vaccinated with third-generation smallpox vaccine (JYNNEOS) | Wearing appropriate PPE consisting of disposable gown, gloves, N-95 mask, and glasses. |
PCR, polymerase chain reaction; PPE, personal protective equipment; USA, United States of America; OPX, oropharynx; HCP, health care personnel; TNF, tumor necrosis factor; d, days; h, hours; wk, weeks.
None of the HCPs presented with oral lesions, however oropharyngeal MPOX PCR sampling was conducted in 4 of 7 cases, including our own. Of these, only 1 test yielded a positive result.
Based on those cases, using PPE when caring for a patient with MPOX infection prevents transmission in most cases, unless there is a breach in the protective gear. The Centers for Disease Control and Prevention (CDC) has recently revised its recommendations for collecting MPOX specimens.16 The revisions advise that unroofing the lesions prior to swabbing is unnecessary and should be avoided to reduce the risk of HCP infection. To minimize the risk of environmental contamination and transmission, the guidelines also recommend that HCPs remove gloves, perform hand hygiene, and put on a new pair of gloves after completing the specimen collection.
It is important to note that even if the recent recommendations of the CDC are strictly followed, and the use of sharp instruments is avoided, transmission might occur during the removal of contaminated PPE or through direct contact with contaminated surfaces. In addition, infection might occur while caring for an undiagnosed MPOX infected patient without the use of full PPE as MPOX infection might be missed due to its highly variable clinical presentation, especially during the current outbreak.3 , 5 , 17
To conclude, by December 2022, more than 70,000 cases of MPOX infection have been reported worldwide, while only 6 cases of proven MPOX transmission to HCPs have been described thus far. It is unclear whether nosocomial infection with MPOX is rare as suggested by these numbers or whether infection of HCPs with MPOX is underdiagnosed. A high index of suspicion for early MPOX infection signs and symptoms as well as strict adherence to current recommendation is mandatory in order to minimize HCP infection risk.
Footnotes
Conflicts of interest: None to report.
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.ajic.2023.01.006.
Appendix. Supplementary materials
References
- 1.WHO Director-General's statement at the press conference following IHR Emergency Committee regarding the multi-country outbreak of monkeypox - 23 July 2022 [Internet]. [cited 2022 Sep 20]. Accessed February 19, 2023. https://www.who.int/director-general/speeches/detail/who-director-general-s-statement-on-the-press-conference-following-IHR-emergency-committee-regarding-the-multi–country-outbreak-of-monkeypox–23-july-2022
- 2.Titanji BK, Tegomoh B, Nematollahi S, Konomos M, Kulkarni PA. Monkeypox: A contemporary review for healthcare professionals. Open Forum Infect Dis. 2022;9:ofac310. doi: 10.1093/ofid/ofac310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries - april-june 2022. N Engl J Med. 2022;387:679–691. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 4.Del Rio C, Malani PN. Update on the monkeypox outbreak. JAMA. 2022;328:921–922. doi: 10.1001/jama.2022.14857. [DOI] [PubMed] [Google Scholar]
- 5.Gessain A, Nakoune E, Yazdanpanah Y. Monkeypox. N Engl J Med. 2022;387:1783–1793. doi: 10.1056/NEJMra2208860. [DOI] [PubMed] [Google Scholar]
- 6.Zachary KC, Shenoy ES. Monkeypox transmission following exposure in healthcare facilities in nonendemic settings: Low risk but limited literature. Infect Control Hosp Epidemiol. 2022;43:920–924. doi: 10.1017/ice.2022.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaughan A, Aarons E, Astbury J, et al. Human-to-human transmission of monkeypox virus, United Kingdom, October 2018. Emerging Infect Dis. 2020;26:782–785. doi: 10.3201/eid2604.191164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marshall KE, Barton M, Nichols J, et al. Health care personnel exposures to subsequently laboratory-confirmed monkeypox patients - Colorado, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1216–1219. doi: 10.15585/mmwr.mm7138e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Pluart D, Ruyer-Thompson M, Ferré VM, et al. A healthcare-associated infection with monkeypox virus of a healthcare worker during the 2022 outbreak. Open Forum Infect Dis. 2022;9 doi: 10.1093/ofid/ofac520. ofac520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salvato RS, Rodrigues Ikeda ML, Barcellos RB, et al. Possible occupational infection of healthcare workers with monkeypox virus. Brazil. Emerging Infect Dis. 2022;28:2520–2523. doi: 10.3201/eid2812.221343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendoza R, Petras JK, Jenkins P, et al. Monkeypox virus infection resulting from an occupational needlestick - Florida, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1348–1349. doi: 10.15585/mmwr.mm7142e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alarcón J, Kim M, Balanji N, et al. Occupational monkeypox virus transmission to healthcare worker, california, USA, 2022. Emerging Infect Dis. 2022;29:435–437. doi: 10.3201/eid2902.221750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caldas JP, Valdoleiros SR, Rebelo S, Tavares M. Monkeypox after occupational needlestick injury from pustule. Emerging Infect Dis. 2022;28:2516–2519. doi: 10.3201/eid2812.221374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.arvalho LB, Casadio LVB, Polly M, et al. Monkeypox virus transmission to healthcare worker through needlestick injury, Brazil. Emerging Infect Dis. 2022;28:2334–2336. doi: 10.3201/eid2811.221323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breman JG. Smallpox. J Infect Dis. 2021;224(12):S379–S386. doi: 10.1093/infdis/jiaa588. Suppl 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guidelines for Collecting and Handling Specimens for Mpox Testing | Mpox | Poxvirus | CDC [Internet]. [cited 2022 Dec 9]. Accessed February 19, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/prep-collection-specimens.html
- 17.Guarner J, Del Rio C, Malani PN. Monkeypox in 2022-What clinicians need to know. JAMA. 2022;328:139–140. doi: 10.1001/jama.2022.10802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.