Abstract
Objective
To systematically evaluate the efficacy and safety of the Shuangdan Mingmu capsule in the treatment of diabetic retinopathy (DR).
Methods
Common Chinese and English databases, including PubMed, Medline, Embase, VIP, Wanfang, and the Chinese National Knowledge Infrastructure (CNKI), were searched from their inception to May 31, 2022. According to the Cochrane Handbook, two reviewers independently evaluated and collected data on the included studies. Meta-analysis was performed by RevMan software 5.4.
Results
Seven trials with a total of 835 patients were included. The clinical effectiveness rate was defined as the primary outcome, and the TCM symptom score, Chinese-Version Low Vision Quality of Life Questionnaire (CLVQOL) scores, macular thickness, hemorrhagic spot area, vascular endothelial growth factor levels, platelet-derived growth factor levels, and the incidence of adverse effects were the secondary outcome. The results of the meta-analysis showed that, compared with conventional medical treatment alone, the Shuangdan Mingmu capsule combined with conventional treatment could significantly improve the clinical effectiveness rate of treating DR (OR = 4.07, 95% CI (2.10, 7.89), p < 0.0001), and reduce the incidence of adverse reactions in DR patients (OR = 0.47, 95% CI (0.26, 0.86), p=0.01). In addition, other results showed that TCM symptom score(OR = −3.47, 95% CI (−3.84, −3.10), p < 0.00001); CLVQOL scores (OR = 23.93, 95% CI (21.37, 26.49), p < 0.00001); macular thickness (OR = −47.34, 95% CI (−50.67, 44.00), p < 0.00001); hemorrhagic spot area (OR = −0.91, 95% CI (−1.01, −0.81), p < 0.00001); vascular endothelial growth factor levels (OR = −45.76, 95% CI (−49.74, 41.79), p < 0.00001); platelet-derived growth factor levels (OR = −1.73, 95% CI (−2.15, −1.31), p < 0.00001).
Conclusion
Compared with conventional treatment alone, the Shuangdan Mingmu capsule combined with conventional treatment is more effective and safer in the treatment of diabetic retinopathy. However, due to the limitations of the included studies, more high-quality studies are still needed to further assess the efficacy and safety of the Shuangdan Mingmu capsule in the treatment of diabetic retinopathy.
1. Introduction
Diabetes mellitus is a disease characterized by chronic hyperglycemia caused by metabolism disorders of sugar, fat, and protein due to insufficient insulin secretion or defective function. Diabetic retinopathy (DR) is a microangiopathy that mainly involves small blood vessels, causing microvascular occlusion, bleeding, and hemorrhage and eventually leading to retinal detachment, which seriously affects the quality of life of patients [1]. In 2019, diabetes and its complications became one of the major causes of death worldwide, and its prevalence in the world reached 10.2% [2, 3]. In 2020, the number of patients with DR in China reached 6 million, and 1.34 million were at risk of visual impairments.
At present, the conventional treatment for DR is mainly Western medicine. Calcium dobesilate, which inhibits vasoactive substances and improves microvascular circulation, is a common drug for treating DR. However, the efficacy of calcium dobesilate alone in the treatment of DR is not ideal. In China, a combination of traditional Chinese medicine (TCM) and conventional treatment has often been prescribed for DR patients with the aim of improving efficacy [4].
Shuangdan Mingmu capsule (SDMMC) is a Chinese patent medicine prepared from traditional Chinese medicines such as Nvzhenzi (Frustus Ligustri Lucidi) and Mohanlian (Yerbadetajo Herb), which have the activities of invigorating the blood and brightening the eyes, benefiting the kidney, and nourishing the liver. An increasing number of clinical trials have assessed the efficacy and safety of SDMMC combined with conventional treatment for the treatment of DR; most studies suggest that SDMMC can improve the clinical effectiveness rate (CER), TCM symptom score, and quality of life (QOL), and can also reduce adverse events [5]. However, the effects of SDMMC combined with conventional treatment for patients with DR have never been systematically evaluated.
In SDMMC, Ligustrum lucidum and Moxanthus are rich in flavonoids, which are rich in phenolic hydroxyl groups and have strong antioxidant capacity. Several studies have found that Ligustrum lucidum and Moxanthus have hepatoprotective effects on acute liver injury, and both of them can reduce the serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in a dose-dependent manner in a liver injury model. It can reduce the content of malondialdehyde (MDA) in liver tissue homogenate and enhance the activity of superoxide dismutase (SOD). The combination of the two drugs can effectively improve the pathological changes in liver tissue [6].
In this study, the efficacy and safety of SDMMC combined with conventional treatment for DR patients were systematically evaluated, aiming to provide evidence for clinical practice.
2. Materials and Methods
2.1. Protocol and Registration
The present systematic review and meta-analysis of RCTs were performed following the PRISMA guidelines [7]. The protocol of this study was registered in PROSPERO with the registration number CRD42022361851 (https://crd.york.ac.uk/PROSPERO/display_record.php?RecordID=361851).
2.2. Criteria for Selection of Studies
2.2.1. Inclusion Criteria
(1) Type of study: randomized controlled trial (RCT); (2) study population: patients with DR: refer to the clinical guidelines for DR issued by the American Academy of Ophthalmology in 2018 (Diabetic Retinopathy Preferred Practice Pattern, DR PPP); (3) experimental intervention: SDMMC combined with conventional treatment; (4) control intervention: conventional treatment. (5) outcomes: the clinical efficacy rate, TCM symptom score, CLVQOL scores, macular thickness, hemorrhagic spot area, vascular endothelial growth factor levels, platelet-derived growth factor levels, blood glucose, and adverse reactions.
2.2.2. Exclusion Criteria
(1) The studies were non-RCTs; (2) patients had not been diagnosed as DR or did not have a clear diagnosis; (3) duplicate publications; (4) studies without valid outcome measures; and (5) reviews, reports of basic experiments, and reports of expert experience.
2.3. Search Strategy
A computerized search of English and Chinese databases, such as PubMed, Medline, Embase, VIP Database, Wanfang Database, and Chinese National Knowledge Infrastructure (CNKI), was performed. The literature search was not restricted to languages, and the search time was from the inception of these databases to May 31, 2022. Search terms included diabetic retinopathy, SDMMC, randomized controlled trial, treatment, etc. The search results were double-checked, and the references included in the literature were screened to prevent omissions.
2.4. Literature Screening and Data Extraction
The literature was independently screened and evaluated using Microsoft Excel by two reviewers (J.A.D and Y.Q.M). Data were extracted from the studies that met the inclusion criteria and exclusion criteria, and in case of disagreement, a third reviewer (L.L.Y, or Q.B.W) was consulted.
2.5. Risk of Bias Evaluation
The methodological quality of the included literature was evaluated. The risk of bias assessment tool recommended by the Cochrane Handbook was used to assess the risk of bias, which was classified into 3 levels: low risk, unclear risk, and high risk. The assessment was based on the following seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases [8–10].
2.6. Statistical Analysis Methods
RevMan software 5.4 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020) was applied for statistical processing. The odds ratio (OR) with 95% confidence intervals (CI) was used for count data (the clinical efficacy rate, adverse effects), and the mean difference (MD) with 95% CI was used for continuous outcomes. If I2 ≤ 50%, homogeneity was suggested, and a fixed-effects model was used; conversely, if I2 > 50%, substantial heterogeneity was suggested, and a random-effects model was used for synthetic analysis. Differences were considered statistically significant if p < 0.05 [11–13].
2.7. Publication Bias
Funnel plots were performed to examine the potential bias in the RCTs included in the meta-analysis when the number of the included RCTs was more than 10 [8, 12].
3. Results
3.1. Literature Search and Screening Results
A total of 96 papers were searched; 7 studies [14–20] met the criteria and were included after eliminating duplicates and intensive reading of the abstract and full text; all the included RCTs were performed in China (Figure 1).
Figure 1.
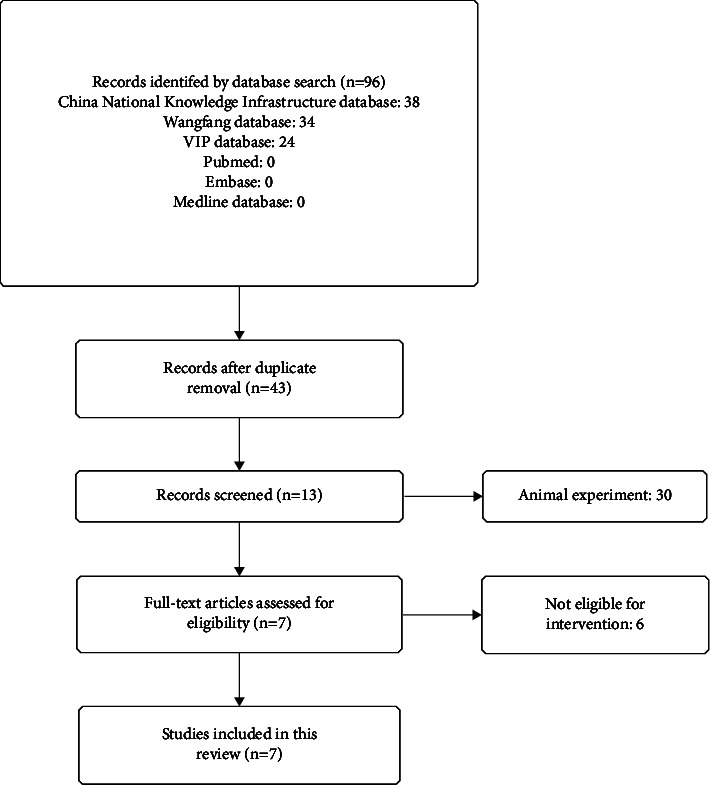
A systematic review and meta-analysis diagram of the search.
3.2. Basic Characteristics of the Included Literature
According to the inclusion and exclusion criteria, 7 RCTs with a total of 835 patients were included, including 419 cases in the test group and 416 cases in the control group [14–20]. The test group was treated with SDMMC combined with conventional treatment, and the control group was treated with conventional treatment alone (Table 1).
Table 1.
The characteristics of the included studies.
| Reference | Design | Sample size (T/C) | Age (T/C) | Interventions (T/C) | Outcomes |
|---|---|---|---|---|---|
| Tu et al. 2009 [19] | RCT | 102/100 | 1870 | SDMMC + doxium/doxium | ①② |
| Pang 2015 [18] | RCT | 40/40 | 49.4 ± 5.7/49.6 ± 5.3 | SDMMC + doxium/doxium | ① |
| Jin and Zhang 2019 [17] | RCT | 72/71 | 63.07 ± 8.08/62.39 ± 8.34 | SDMMC + calcium dobesilate/calcium dobesilate | ①②④⑤ |
| Fu 2019 [15] | RCT | 40/40 | 56.37 ± 11.21 | SDMMC + calcium dobesilate/calcium dobesilate | ③ |
| Liu et al. 2019 [16] | RCT | 60/60 | 57.54 ± 8.11/57.10 ± 9.26 | SDMMC + calcium dobesilate/calcium dobesilate | ①④⑤⑥⑦ |
| Zhang et al. 2021 [14] | RCT | 53/53 | 69.70 ± 2.12/69.70 ± 2.13 | SDMMC + compound anisodine hydrobromide injection/compound anisodine hydrobromide injection | ② |
| Ji and Liu 2022 [20] | RCT | 52/52 | 56.63 ± 4.02/56.53 ± 4.09 | SDMMC + calcium dobesilate/calcium dobesilate | ①⑥⑦ |
Notes: ① clinical effective rate; ② eyesight; ③ blood glucose; ④ TCMsymptomscore; ⑤ CLVQOL scores; ⑥ macular thickness and hemorrhagic spot area; ⑦ vascular endothelial growth factor levels and platelet-derived growth factor levels RCT, randomized controlled trial; T/C, treatment group/control group.
3.3. Assessment of the Quality of the Included Studies
The baseline indicators of the seven included studies were largely consistent, but the random assignment method was unclear, and some did not mention allocation concealment and blinding. The primary outcomes of all studies were fully consistent with the expected reporting. There was no selective report bias and no other risks of bias in the included literature (Table 2, Figures 2 and 3).
Table 2.
The methodologic quality of the included trials assessed using the cochrane risk of bias tool.
| References | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| Tu et al. 2009 [19] | ? | ? | + | + | + | + | + |
| Pang 2015 [18] | ? | ? | ? | ? | + | + | + |
| Jin and Zhang 2019 [17] | ? | ? | ? | ? | + | + | + |
| Fu 2019 [15] | ? | ? | ? | ? | + | + | + |
| Liu et al. 2019 [16] | + | ? | ? | ? | + | + | + |
| Zhang et al. 2021 [14] | + | ? | ? | ? | + | + | + |
| Ji and Liu 2022 [20] | + | ? | ? | ? | + | + | + |
Notes: + = low risk of bias; ? = unclear risk of bias; − = high risk of bias.
Figure 2.
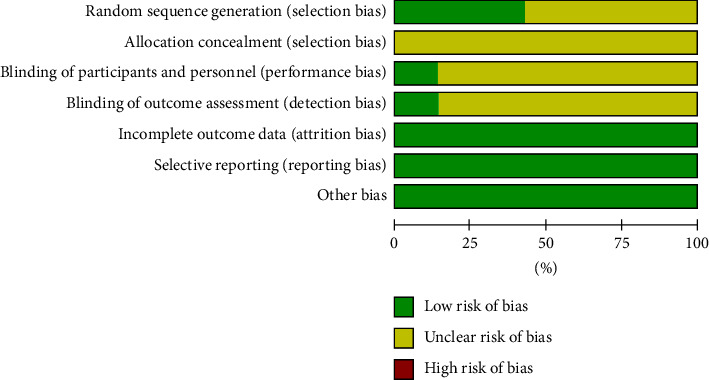
Risk of bias graph.
Figure 3.
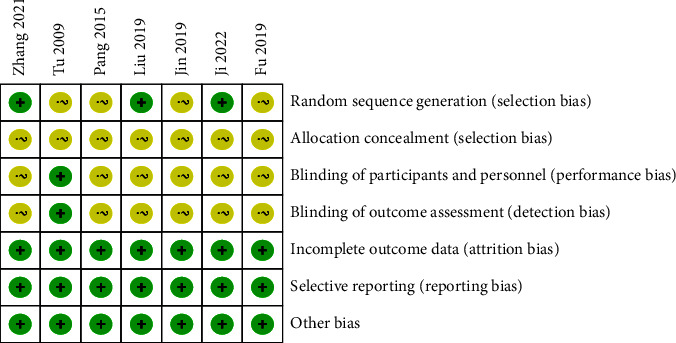
Risk of bias summary.
3.4. The Clinical Effective Rate
The clinical effectiveness rate was described in 5 studies included in this study [16–20], including 649 patients. The results of the meta-analysis showed a statistically significant difference in the clinical effectiveness rate of the test group compared with the control group (OR = 4.07, 95% CI (2.10, 7.89), p < 0.0001). This result indicated that SDMMC could improve the clinical effectiveness rate of treating DR (Figure 4). There was homogeneity for this outcome (I2 = 0%), and a fixed-effects model was used.
Figure 4.
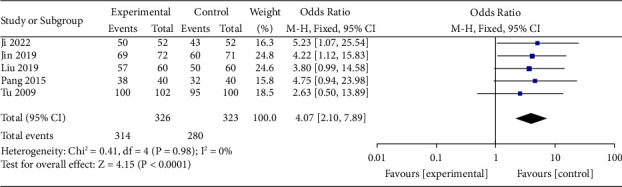
Clinical effective rate.
3.5. TCM Symptom Score
Two studies assessed the effect of different interventions on TCM syndromes [16, 17]. Meta-analysis showed a statistically significant difference in the TCM syndrome score between the test group and the control group (OR = −3.47, 95% CI (−3.84, −3.10), p < 0.00001). This result indicated that the SDMMC could improve the TCM symptom score of DR patients (Figure 5). There was homogeneity for this outcome (I2 = 39%), and a fixed-effects model was used.
Figure 5.
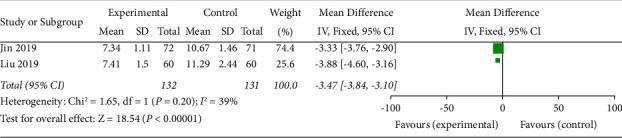
TCM symptom score.
3.6. Chinese-Version Low Vision Quality of Life Questionnaire (CLVQOL) Scores
CLVQOL scores were described in 2 studies [16, 17]. The results of the meta-analysis showed a statistically significant difference in CLVQOL scores between the test group and the control group (OR = 23.93, 95% CI (21.37, 26.49), p < 0.00001). This indicated that the SDMMC improved the CLVQOL scores of DR patients (Figure 6). There was homogeneity for this outcome (I2 = 0%), and a fixed-effects model was used.
Figure 6.
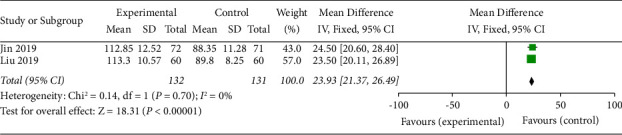
CLVQOL scores.
3.7. Macular Thickness
Macular thickness was described in 2 studies [16, 20]. The results of the meta-analysis showed a statistically significant difference in macular thickness between the test group and the control group (OR = −47.34, 95% CI (−50.67, 44.00), p < 0.00001). This result indicated that the SDMMC could reduce the macular thickness in DR patients (Figure 7). There was homogeneity for this outcome (I2 = 18%), and a fixed-effects model was used.
Figure 7.
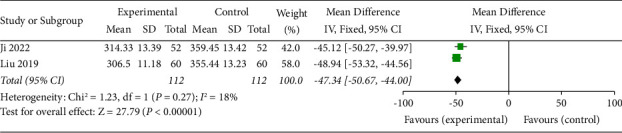
Macular thickness.
3.8. Hemorrhagic Spot Area
The bleeding spot area was described in 2 included studies [16, 20]. The results of the meta-analysis showed a statistically significant difference in bleeding spot area in the test group compared with the control group (OR = −0.91, 95% CI (−1.01, −0.81), p < 0.00001). This result indicated that SDMMC could reduce the area of bleeding spots in DR patients (Figure 8). There was homogeneity for this outcome (I2 = 0%), and a fixed-effects model was used.
Figure 8.
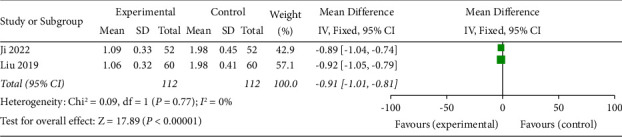
Hemorrhagic spot area.
3.9. Vascular Endothelial Growth Factor Levels (VEGF)
VEGF levels were described in 2 studies [16, 20]. The results of the meta-analysis showed that SDMMC could significantly downregulate VEGF levels in DR patients (OR = −45.76, 95% CI (−49.74, 41.79), p < 0.00001) (Figure 9). There was homogeneity for this outcome (I2 = 0%), and a fixed-effects model was used.
Figure 9.
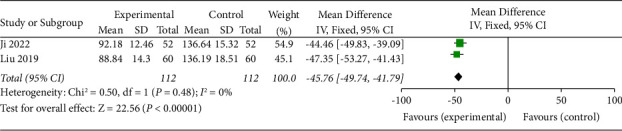
Vascular endothelial growth factor levels.
3.10. Platelet-Derived Growth Factor Levels (PDGF)
PDGF levels were described in 2 studies [16, 20]. The results of the meta-analysis showed a statistically significant difference in PDGF levels in the test group compared with the control group (OR = −1.73, 95% CI (−2.15, −1.31), p < 0.00001). This result indicated that SDMMC significantly downregulated PDGF levels in DR patients (Figure 10). There was substantial heterogeneity for this outcome (I2 = 51%), and a random-effects model was applied.
Figure 10.
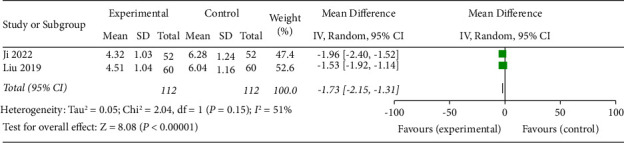
Platelet-derived growth factor levels.
3.11. Blood Glucose
Among these studies, only one study described blood glucose [15]. Comparing fasting blood glucose (FBG) and 2 h postprandial blood glucose (2 h PBG) before and after treatment between the two groups, the differences between the groups were statistically significant (p < 0.05). Meta-analysis was not possible for this outcome.
3.12. Adverse Reactions
Six studies described adverse reactions [14–17, 19, 20]. Meta-analysis using a fixed-effects model showed a statistically significant difference in adverse reactions between the groups (OR = 0.47, 95% CI (0.26, 0.86), p=0.01). This result indicated that SDMMC could reduce the incidence of adverse reactions in DR patients (Figure 11).
Figure 11.
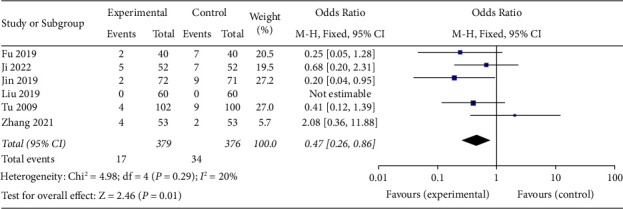
Adverse reactions.
4. Discussion
Diabetic retinopathy is a common complication of diabetes mellitus, which mainly manifests as blurred vision, fundus exudation, and retinal edema. According to traditional Chinese medicine, DR is associated with “abrupt blindness (Bao Mang),” “dimness of vision (Shi Zhan Hun Miao),” “internal obstruction (Nei Zhang),” and “blood filling the pupil (Xue Guan Tong Shen),” etc. [21]. In 2011, the Chinese Diabetes Society summarized the etiology and pathogenesis of DR, considering yin deficiency of the liver and kidney as the underlying pathogenesis and blood stasis blocking the veins as the surface pathogenesis [22]. According to TCM theory, the functions of the SDMMC include nourishing the qi and yin of the liver and kidney, promoting blood circulation, and brightening the eyes, which work against the pathogenesis of DR.
SDMMC is derived from the combination of the Erzhi pill in the medical book “Yibian” of the Ming Dynasty and the Liuwei Dihuang pill in the “Straight Guide to Pediatric Medicine (Xiaoer Yaozheng Zhijue)” written by Qian Yi of the Song Dynasty. The main herbs of the formula are Nvzhenzi (Frustus Ligustri Lucidi) and Mohanlian (Yerbadetajo Herb), which can nourish the yin of the liver and kidney. In addition, Mohanlian (Yerbadetajo Herb) is also good at cooling the blood to stop bleeding. Shanzhuyu (Fructus Corni) and Shanyao (Rhizoma Dioscoreae) can nourish the kidney and liver and invigorate the spleen. Danshen (Radix Salviae Miltiorrhizae) and Sanqi (Sanchi) can invigorate and promote blood circulation to remove blood stasis. Mudanpi (Cortex Moutan), Zexie (Rhizoma Alismatis), and Fuling (Poria) have the activities of clearing the liver and draining fire, as well as drying dampness. Niuxi (Radix Achyranthis Bidentatae) can invigorate blood circulation, remove blood stasis, and strengthen tendons and bones; it is also good at preventing the rising of qi and blood [23, 24]. The combination of these Chinese medicinal herbs is beneficial to the kidney and liver, blood circulation, and eyesight, and is especially suitable for the treatment of diabetic retinopathy caused by yin deficiency of the liver and kidney and blood stasis [25] (Table 3).
Table 3.
The Chinese medicinal herbs contained in SDMMC.
| Chinese name | English name | Latin name | Family | Plant part | Processing |
|---|---|---|---|---|---|
| Nvzhenzi | Frustus Ligustri Lucidi | Ligustrum lucidum Ait. | Oleaceae | Fruit seed | Dried |
| Mohanlian | Yerbadetajo Herb | Eclipta prostrate L. | Compositae | Whole plant | Dried |
| Shanzhuyu | Fructus Corni | Cornus officinalis Sieb. et Zucc. | Cornaceae | Sarcocarp | Dried |
| Shanyao | Rhizoma Dioscoreae | Dioscorea opposita Thunb. | Dioscoreaceae | Root stock | Dried |
| Danshen | Radix Salviae Miltiorrhizae | Salvia miltiorrhiza Bunge. | Labiatae | Root stock and root | Dried |
| Sanqi | Sanchi | Panax notoginseng (Burk.) F. H. Chen | Araliaceae | Root | Dried |
| Mudanpi | Cortex Moutan | Paeonia suffruticosa Andr. | Ranunculaceae | Root bark | Dried |
| Zexie | Rhizoma Alismatis | Alisma orientalis (Sam.) Juzep | Alismataceae | Tuber | Dried |
| Fuling | Poria | Poria cocos (Schw.) Wolf | Polyporaceae | Sclerotium | Dried |
| Niuxi | Radix Achyranthis Bidentatae | Achyranthes bidentata Blume. | Amaranthaceae | Root | Dried |
The results of this study showed that the clinical effectiveness rate of patients in the test group was significantly higher than that of the control group; the TCM symptom score was significantly lower than that of the control group; the CLVQOL score was significantly higher than that of the control group; the macular thickness and hemorrhagic spot area were significantly smaller than those of the control group; the abovementioned differences were statistically significant (p < 0.05). These findings indicate that SDMMC combined with conventional treatment is effective for DR, which can improve the overall clinical efficacy rate, symptoms of patients, quality of life, and some objective signs of patients with DR.
This study also showed that the serum VEGF and PDGF levels of patients in the test group were significantly lower than those in the control group, and the differences were statistically significant (p < 0.05). VEGF enhances retinal capillary permeability and affects patients' visual acuity [26]. PDGF is a peptide that can be produced by a variety of cellular stimuli and can induce cell proliferation and promote extracellular matrix accumulation and monocyte-macrophage infiltration. Numerous studies have shown that PDGF is involved in the process of glucose metabolism in the diabetic state and can promote its own expression, thus forming a vicious cycle. The combination of SDMMC with conventional medicine downregulated the VEGF and PDGF levels, which might bring long-term benefits to DR patients [27, 28].
In addition, in this study, six of the seven included studies observed adverse reactions, and the meta-analysis showed that the incidence of adverse reactions in the test group was significantly lower than that in the control group, indicating that the combination of SDMMC with conventional treatment was safe and could reduce the incidence of adverse reactions in DR patients [29, 30].
There were some of the following limitations in this study: (a) the number of studies that met the inclusion criteria of this study was relatively small, all of which were written in Chinese; (b) only three of the included studies specified the randomization method, and the rest did not specify the randomization grouping method, which might have led to selective bias; (c) most of the included studies did not mention blinding, allocation concealment, follow-up, or prognosis; (d) only one study described blood glucose, meta-analysis was not possible for this outcome; (e) for all outcomes, the number of included studies was less than 10; therefore, funnel plots were not performed.
5. Conclusions
Compared with conventional treatment alone, the Shuangdan Mingmu capsule combined with conventional treatment is more effective and safer in the treatment of diabetic retinopathy. However, due to the limitations of the included studies, more high-quality studies are still needed to further assess the efficacy and safety of the Shuangdan Mingmu capsule in the treatment of diabetic retinopathy.
Acknowledgments
This work was supported by the National Administration of Traditional Chinese Medicine-Traditional Chinese Medicine Science and Technology Research Special Project (GZY-KJS-2021-005), National Natural Science Foundation of China ( 81603454), and the Science and Technology Development Fund, Macau SAR ( 0098/2021/A2).
Contributor Information
Qibiao Wu, Email: qbwu@must.edu.mo.
Lili Yu, Email: llyu@must.edu.mo.
Data Availability
The raw data utilized in this study are available publicly from VIP Database (https://qikan.cqvip.com/) , Wanfang Database (https://www.wanfangdata.com.cn/), Chinese National Knowledge Infrastructure (CNKI, https://www.cnki.net/), PubMed (https://pubmed.ncbi.nlm.nih.gov/), Medline (https://www.nlm.nih.gov/medline/index. html) and Embase (https://www.embase.com/landing?status=grey).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Qibiao Wu, Lili Yu, and Jiaan Du conceptualized the study. Jiaan Du performed methodology, investigation, formal analysis, and wrote the original draft. Yingqi Mao carried out the investigation, validation, and formal analysis and also wrote and edited the article. Validation, investigation, and formal analysis were done by Youhua Xu. Validation and investigation were done by Kai Qu and AiweiHan. Methodology, validation, investigation, review, and correction of the draft and supervision were done by Qibiao Wu and Lili Yu.
References
- 1.Yi S., Zhou Q. Interpretation of clinical guidelines for diabetic retinopathy of the American Academy ofOphthalmology 2018. Rec Adv Ophthalmol . 2019;39(6):501–506. [Google Scholar]
- 2.Laddha Ap, Kulkarni Ya. Tannins and vascular complications of Diabetes: an update. Phytomedicine . 2019;56:229–245. doi: 10.1016/j.phymed.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Klein B. E. K. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiology . 2007;14(4):179–183. doi: 10.1080/09286580701396720. [DOI] [PubMed] [Google Scholar]
- 4.Chinese Diabetes Society Retinopathy Group. Expert consensus on prevention and treatment of diabetic retinopathy. Chin J Diabetes Mellitus . 2018;10(4):241–247. [Google Scholar]
- 5.Qin Y., Fang Li, Tu L., et al. Multicentric clinical study of Shuangdan Mingmu capsule on diabetic retinopathy. Journal of TCM Univ. of Hunan . 2010;30(1):46–51. [Google Scholar]
- 6.Hu D., Chen X., Lu Y., Fang M., Wang J., Wen A. Research progress on hepatoprotective effects of Erzhiwan and its components. Journal of Pharmaceutical Practice . 2016;34(04):289–291. [Google Scholar]
- 7.Matthew J, McK E, Patrick M., Boutron I. The PRISMA 2020 Statement: An Updated Guidance for Reporting Systematic Reviews. BMJ . 2021 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higgins J. P. T., Thomas J., Chandler J., et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 . Cochrane, 2022, https://www.training.cochrane.org/handbook. [Google Scholar]
- 9.Chen H., Yao X., Li T., et al. Compound Kushen injection combined with platinum-based chemotherapy for stage III/IV non-small cell lung cancer: a meta-analysis of 37 RCTs following the PRISMA guidelines. Journal of Cancer . Jan 20 2020;11(7):1883–1898. doi: 10.7150/jca.40267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y. W., Zhang J., Hu J. Q., et al. Neuraxial adjuvants for prevention of perioperative shivering during cesarean section: a network meta-analysis following the PRISMA guidelines. World Journal of Clinical Cases . Aug 26 2019;7(16):2287–2301. doi: 10.12998/wjcc.v7.i16.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin S., An X., Guo Y., et al. Meta-analysis of astragalus-containing traditional Chinese medicine combined with chemotherapy for colorectal cancer: efficacy and safety to tumor response. Frontiers Oncology . Aug 13 2019;9:p. 749. doi: 10.3389/fonc.2019.00749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H., Yao X., Liu Z., et al. Efficacy and safety of Shenqi Fuzheng injection combined with platinum-based chemotherapy for stage III/IV non-small cell lung cancer: a protocol for systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) . Sep 2019;98(39) doi: 10.1097/md.0000000000017350.e17350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu M., Yuan G., Luo G., et al. Network pharmacology analysis and experimental verification strategies reveal the action mechanism of danshen decoction in treating ischemic cardiomyopathy. Evidence-based Complementary and Alternative Medicine . 2022;2022:1–15. doi: 10.1155/2022/7578055.7578055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y., Lu C., Guan S., Hui C. Effect of Shuangdan Mingmu capsule combined with compound camptothecin injection in the treatment of diabetic retinopathy. Heilongjiang Medicine Journal . 2021;34(5):1108–1110. [Google Scholar]
- 15.Fu Z. Safety of shuangdan Mingmu capsules combined with calcium dobesilate in the treatment of diabetic retinopathy [J] World Latest Medicine Information . 2019;19(102):205–206. [Google Scholar]
- 16.Liu J., Kong Y., Wang L. Clinical efficacy of shuangdan Mingmu capsules combined with calcium dobesilate in the treatment of diabetic retinopathy and its effects on serum levels of vascular EndothelialGrowth factor, platelet-derived growth factor and interleukin-1[J] Evaluation and analysis of drug-use in hospitals of China . 2019;19(11):1332–1338. [Google Scholar]
- 17.Jin L., Zhang Li-J. Clinical study on Shuangdan Mingmu Capsules combined with calcium dobesilate in treatment of diabetic retinopathy. Drugs & Clinic . 2019;34(1):159–163. [Google Scholar]
- 18.Pang Y. Clinical observation on Shuangdan Mingmu capsule in the treatment of diabetic retinopathy. Diabetes New World . 2015;3:p. 39. [Google Scholar]
- 19.Tu L., Wang W., Wang Y., Zang M., Huang W. Efficacy and safety of Shuangdan Mingmu capsule in treating diabetic retinopathy. Chinese Journal of New Drugs . 2009;18(24):2331–2336. [Google Scholar]
- 20.Ji X., Liu W. Safety and efficacy of Shuangdan Mingmu capsule combined with calcium dobesilate dispersible tablets in the treatment of diabetic retinopathy. Clinical Research and Practice . 2022;7(11):85–87. [Google Scholar]
- 21.Li C. Clinical study on Qingre Huayu Recipe for nonproliferative diabetic retinopathy. Int Eye Sci . 2019;19(1):157–159. [Google Scholar]
- 22.Chinese Diabetes Society Retinopathy Group. Diabetic retinopathy Chinese medicine treatment standards. World Journal of Integrated Traditional and Western Medicine . 2011;6(7):p. 633. [Google Scholar]
- 23.National Compilation of Chinese Herbal Medicines Writing Group. National Compilation of Chinese Herbal Medicine . Beijing, China: People’s Medical Publishing House; 1975. [Google Scholar]
- 24.Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia . Beijing, China: The medicine science and technology press of China; 2015. [Google Scholar]
- 25.Peng J., Xinrn W., Pan K., Liu Z., Qin Y., Peng Q. Effects of Shuangdan Mingmu Capsule on the expressions of VEGF-a, VEGF-b, VEGF-c and its receptor Flk-1 in the retina in a diabetic rat model. China Journal of Traditional Chinese Medicine and Pharmacy . 2019;34(8):3447–3450. [Google Scholar]
- 26.Pei Y., Zhang X., Zhang Li, Jin Li. Study on the relationship between HMW-ADP, TNF-α, VEGF and diabetic retinopathy. CHINA MEDICAL HERALD . 2019;16(9):106–109. [Google Scholar]
- 27.Xie C., Cui Q., Yan F. Vitreous levels of vascular endothelial growth factor and platelet-derived growth factor and their correlation in patients with proliferative diabetic retinopathy. Journal of Clinical Ophthalmology . 2018;26(4):293–295. [Google Scholar]
- 28.Zhang R., Pan T., Xiang Y., et al. β-Elemene reverses the resistance of p53-deficient colorectal cancer cells to 5-fluorouracil by inducing pro-death autophagy and cyclin D3-dependent cycle arrest. Frontiers in Bioengineering and Biotechnology . 2020;8:p. 378. doi: 10.3389/fbioe.2020.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang J., Jiang Z., Wang Y., et al. Modulation of gut microbiota to overcome resistance to immune checkpoint blockade in cancer immunotherapy. Current Opinion in Pharmacology . 2020;54:1–10. doi: 10.1016/j.coph.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Zhang R., Pan T., Xiang Y., et al. Curcumenol triggered ferroptosis in lung cancer cells via lncRNA H19/miR-19b-3p/FTH1 axis. Bioactive Materials . 2022;13:23–36. doi: 10.1016/j.bioactmat.2021.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data utilized in this study are available publicly from VIP Database (https://qikan.cqvip.com/) , Wanfang Database (https://www.wanfangdata.com.cn/), Chinese National Knowledge Infrastructure (CNKI, https://www.cnki.net/), PubMed (https://pubmed.ncbi.nlm.nih.gov/), Medline (https://www.nlm.nih.gov/medline/index. html) and Embase (https://www.embase.com/landing?status=grey).


