Abstract
Lung cancer with complex epidermal growth factor receptor (EGFR) and CTNNB1 comutations is rare, and the efficacy of tyrosine kinase inhibitors (TKIs) is generally poor. Here, we encountered a lung cancer patient with complex EGFR (L858R and E709X) and CTNNB1 comutations who successfully responded to afatinib. A 78‐year‐old woman visited our hospital with a cough and bloody sputum that had worsened over the past year. She had multiple mass shadows in both lungs and nodular shadows in the bronchi. The patient was diagnosed with lung adenocarcinoma cT4N3M1c stage IVB. A genetic analysis of the primary tumor using the Oncomine Dx target test multi‐CDx system revealed positivity for EGFR (L858R and E709X) and CTNNB1 mutations. The expression of programmed death ligand 1 (22C3 clones) in tumor cells was negative by immunostaining. The patient was treated with afatinib as first‐line therapy and achieved clinical improvement and a partial response and is continuing treatment 1 year later. Case reports of lung cancer patients with EGFR/CTNNB1 comutations are rare, and TKIs are not considered to be effective. We herein present the first case report of lung cancer with the co‐occurrence of uncommon and complex EGFR (L858R and E709X) and CTNNB1 mutations that was successfully treated with afatinib.
Keywords: afatinib, CTNNB1 mutation, E709X, L858R, non–small cell lung cancer
INTRODUCTION
The use of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) to treat EGFR mutation‐positive non‐small cell lung cancer (NSCLC) has markedly improved its prognosis. 1 , 2 , 3 , 4 However, previous studies have demonstrated that disease progression was earlier and overall survival was shorter in patients with uncommon EGFR mutations than in those with common EGFR mutations. 5 , 6 Afatinib has previously been shown to be effective against NSCLC tumors with some uncommon EGFR mutations. 7 , 8 CTNNB1 mutations have previously been detected in 5.3% of lung adenocarcinomas and 2% of NSCLC. 9 , 10 They have been identified as a critical resistance mechanism to EGFR‐TKIs. 11 , 12 However, there have been no case reports of lung cancer with uncommon and complex EGFR (L858R and E709X) and CTNNB1 comutations. We herein report the first case report of lung cancer with the co‐occurrence of uncommon and complex EGFR (L858R and E709X) and CTNNB1 mutations that was successfully treated with afatinib.
CASE REPORT
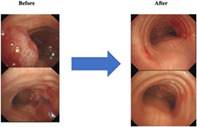
A 78‐year‐old woman was referred to our hospital with a cough and bloody sputum. She was a nonsmoker with a family history of lung cancer. She also had a history of hypertension. Her Eastern Cooperative Oncology Group performance status was one. The results of blood tests were as follows: white blood cell count 11 600/μl, red blood cell count 403 × 104/μl, platelet count 24.0 × 104/μl, carcinoembryonic antigen 17.4 ng/ml, and cytokeratin 19 fragments 10.0 ng/ml. Chest X‐ray (Figure FIGURE 1a) and contrast‐enhanced computed tomography (CT) (Figure FIGURE 1b‐d) showed mass shadows in the right upper, middle, and lower lobes, multiple nodular shadows in both lungs, and intramural nodules in the trachea and right bronchus. Bronchoscopy with standard observations revealed multiple tumors in the trachea, and a tumor which obstructed the right main bronchus (Figure FIGURE 2). Transbronchial tumor biopsies showed adenocarcinoma. Contrast‐enhanced computed tomography showed metastases in the lung, pleura, and liver. The patient was diagnosed with right upper lobe adenocarcinoma cT4N3M1c stage IVB. A genetic analysis of the primary tumor using the Oncomine Dx target test multi‐CDx aystem revealed positivity for EGFR (L858R and E709X) and CTNNB1 mutations. The allele frequencies of L858R, E709X, and CTNNB1 were 14.8, 14.7, and 13.7%, respectively. The expression of programmed death ligand 1 (22C3 clones) in tumor cells was negative by immunostaining. Based on these findings, the patient was treated with afatinib (40 mg/day) as first‐line therapy. She achieved clinical improvement and a partial response. The only side effects that developed were grade 1 diarrhea and skin rash, and, thus the patient continued treatment. Seven months later, bronchoscopy showed that the tumor in the trachea had disappeared (Figure 3). One year later, other tumors had shrunk and tumor markers had normalized (Figure FIGURE 4).
FIGURE 1.
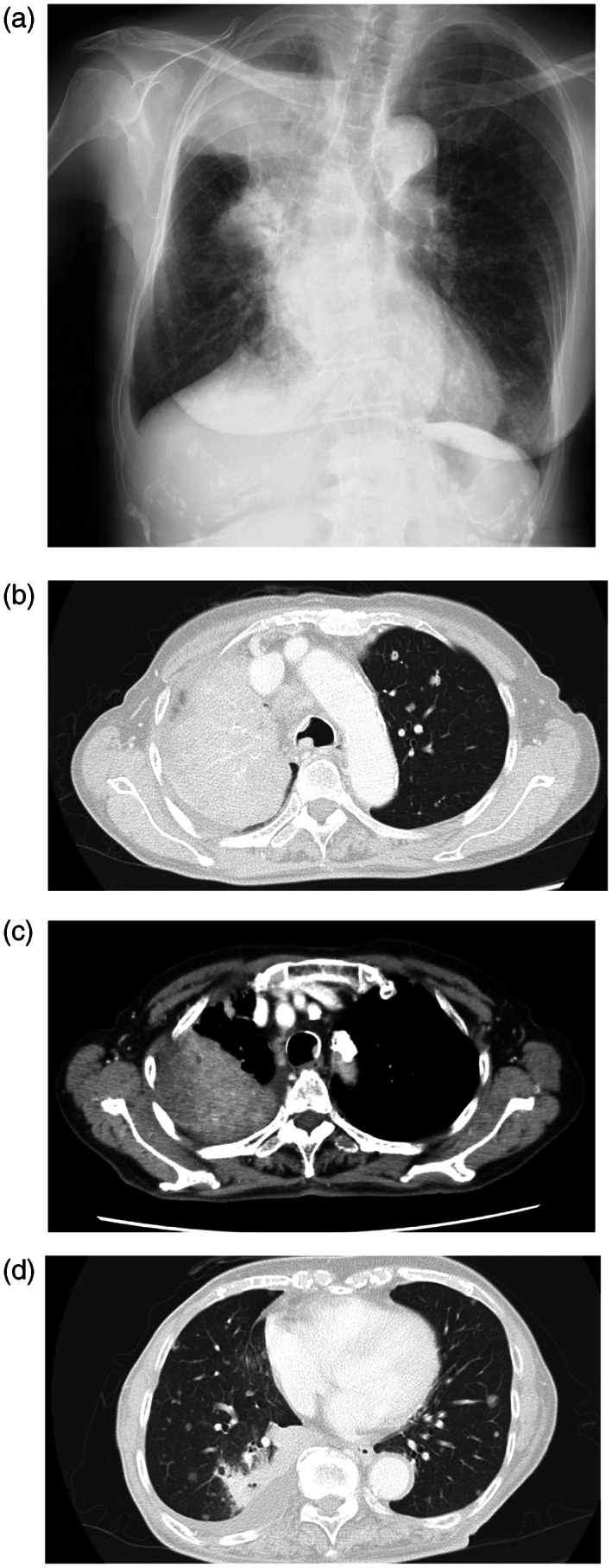
(a) Chest X‐ray and (b, c, d) contrast‐enhanced computed tomography (CT) at first admission. Mass shadows in the right upper, middle, and lower lobes, multiple nodular shadows in both lungs, and intramural nodules in the trachea and right bronchus. Right pleural effusion was noted.
FIGURE 2.
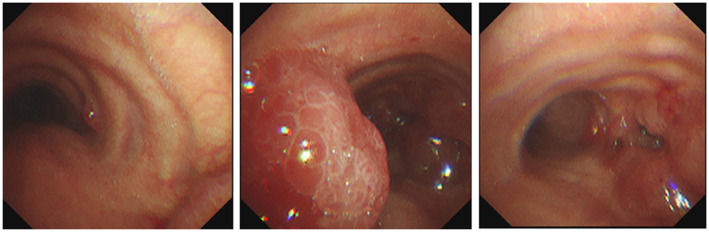
Bronchoscopy with standard observation revealed multiple tumors in the trachea and a tumor which obstructed the right main bronchus.
FIGURE 3.
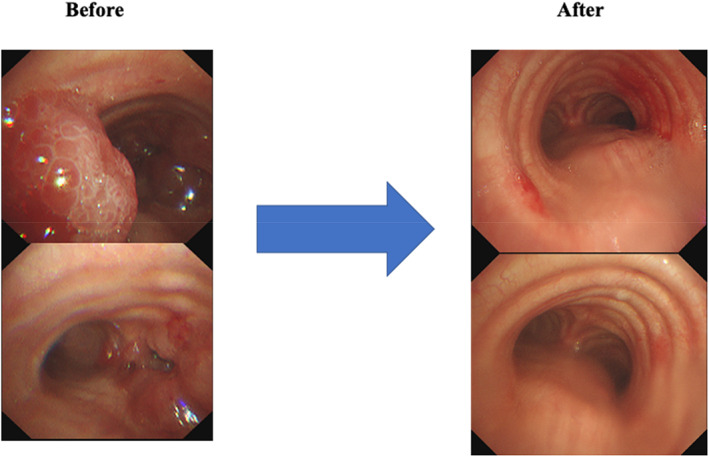
Bronchoscopy findings before and 7 months after treatment.
FIGURE 4.
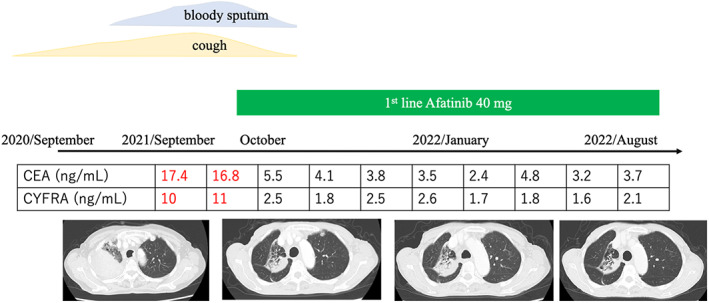
The clinical course of the patient.
DISCUSSION
We encountered a lung cancer patient with uncommon and complex EGFR (L858R and E709X) and CTNNB1 comutations. To the best of our knowledge, this is the first case report of lung cancer with the co‐occurrence of uncommon and complex EGFR (L858R and E709X) and CTNNB1 mutations. This patient successfully responded to afatinib.
EGFR‐TKIs for EGFR mutation‐positive NSCLC have markedly improved its prognosis. 1 , 2 , 3 , 4 Progression‐free survival after EGFR‐TKI therapy has previously been reported to be significantly longer in patients with a mutant allele frequency of L858R (MAFLR) of >9% than in those with a MAFLR of ≤9%. 13 However, previous studies demonstrated that disease progression was earlier and overall survival was shorter in patients with uncommon EGFR mutations than in those with common EGFR mutations. 5 , 6 Afatinib was found to be effective against NSCLC tumors with some uncommon EGFR mutations. 7 , 8 It has also been recommended for complex mutations involving E709X, S768I, or G719X. 14 The present case with uncommon and complex EGFR (L858R and E709X) and CTNNB1 comutations responded to afatinib and has continued treatment for 1 year.
Beta‐catenin, encoded by the CTNNB1 gene, is essential for establishing and maintaining the epithelial layer and is a key downstream component of the canonical Wnt signaling pathway. 15 CTNNB1 mutations are oncogenic in several cancers. 15 CTNNB1 mutations in the Wnt/β‐catenin pathway and RAS, RAF, and upstream EGFR mutations in the RAS–ERK pathway have been shown to play essential roles in tumorigenesis. 16 Among 564 patients with lung adenocarcinoma, 30 (5.3%) harbored CTNNB1 mutations. 9 Another study detected CTNNB1 mutations in 11 (2%) out of 546 NSCLC patients, commonly in conjunction with EGFR mutations. 10 A previous study reported that females and nonsmokers with lung cancer were more likely to harbor CTNNB1 mutations. 9 CTNNB1 mutations are an important factor in EGFR‐TKI resistance. 11 , 12 Furthermore, they have been shown to contribute to the development of a noninflamed tumor microenvironment. 17 , 18 Recurrence‐free survival was found to be significantly shorter in patients with CTMNNB1/EGFR comutations than in those with a single EGFR mutation. 19 In lung adenocarcinomas, CTNNB1 expression is associated with shorter survival. 20 Therefore, our patient needs to be carefully observed.
In conclusion, we present a lung cancer patient with uncommon and complex EGFR (L858R and E709X) and CTNNB1 comutations. The patient successfully responded to afatinib and has continued treatment for 1 year. CTNNB1 mutations have been identified as a crucial resistance mechanism to EGFR‐TKIs, and, thus, careful observations are warranted.
AUTHOR CONTRIBUTION
MK drafted the original manuscript. ET reviewed the manuscript draft and revised it critically on intellectual content. SI, NK, YO, HM, NH, KN and TS reviewed the manuscript draft. All authors approved the final version of the manuscript to be published.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Kunishige M, Ichihara S, Kadota N, Okano Y, Machida H, Hatakeyama N, et al. Non‐small cell lung cancer with EGFR (L858R and E709X) and CNNB1 mutations responded to afatinib. Thorac Cancer. 2023;14(4):423–426. 10.1111/1759-7714.14775
REFERENCES
- 1. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. [DOI] [PubMed] [Google Scholar]
- 2. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802): a multicentre, open‐label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42. [DOI] [PubMed] [Google Scholar]
- 3. Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–34. [DOI] [PubMed] [Google Scholar]
- 4. Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, et al. Afatinib versus cisplatin plus gemcitabine for first‐line treatment of Asian patients with advanced non‐small‐cell lung cancer harbouring EGFR mutations (LUX‐lung 6): an open‐label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–22. [DOI] [PubMed] [Google Scholar]
- 5. Wu JY, Yu CJ, Chang YC, Yang CH, Shih JY, Yang PC. Effectiveness of tyrosine kinase inhibitors on "uncommon" epidermal growth factor receptor mutations of unknown clinical significance in non‐small cell lung cancer. Clin Cancer Res. 2011;17:3812–21. [DOI] [PubMed] [Google Scholar]
- 6. Mehta A, Vasudevan S. Rare epidermal growth factor receptor gene alterations in non‐small cell lung cancer patients, tyrosine kinase inhibitor response and outcome analysis. Cancer Treat Res Commun. 2021;28:100398. [DOI] [PubMed] [Google Scholar]
- 7. Yang JCH, Sequist LV, Geater SL, Tsai CM, Mok TSK, Schuler M, et al. Clinical activity of afatinib in patients with advanced non‐small‐cell lung cancer harbouring uncommon EGFR mutations: a combined post‐hoc analysis of LUX‐lung 2, LUX‐lung 3, and LUX‐lung 6. Lancet Oncol. 2015;16:830–8. [DOI] [PubMed] [Google Scholar]
- 8. Popat S, Hsia TC, Hung JY, Jung HA, Shih JY, Park CK, et al. Tyrosine kinase inhibitor activity in patients with NSCLC harboring uncommon EGFR mutations: a retrospective international cohort study (UpSwinG). Oncologist. 2022;27:255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou C, Li W, Shao J, Zhao J, Chen C. Analysis of the clinicopathologic characteristics of lung adenocarcinoma with CTNNB1 mutation. Front Genet. 2019;10:1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sequist LV, Heist RS, Shaw AT, Fidias P, Rosovsky R, Temel JS, et al. Implementing multiplexed genotyping of non‐small‐cell lung cancers into routine clinical practice. Ann Oncol. 2011;22:2616–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao J, Lin G, Zhuo M, Fan Z, Miao L, Chen L, et al. Next‐generation sequencing based mutation profiling reveals heterogeneity of clinical response and resistance to osimertinib. Lung Cancer. 2020;141:114–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thomas de Montpréville V, Lacroix L, Rouleau E, Mamodaly M, Leclerc J, Tutuianu L, et al. Non‐small cell lung carcinomas with CTNNB1 (beta‐catenin) mutations: a clinicopathological study of 26 cases. Ann Diagn Pathol. 2020;46:151522. [DOI] [PubMed] [Google Scholar]
- 13. Ono A, Kenmotsu H, Watanabe M, Serizawa M, Mori K, Imai H, et al. Mutant allele frequency predicts the efficacy of EGFR‐TKIs in lung adenocarcinoma harboring the L858R mutation. Ann Oncol. 2014;25:1948–53. [DOI] [PubMed] [Google Scholar]
- 14. Kohsaka S, Nagano M, Ueno T, Suehara Y, Hayashi T, Shimada N, et al. A method of high‐throughput functional evaluation of EGFR gene variants of unknown significance in cancer. Sci Transl Med. 2017;9:eaan6566. [DOI] [PubMed] [Google Scholar]
- 15. Gao C, Wang Y, Broaddus R, Sun L, Xue F, Zhang W. Exon 3 mutations of CTNNB1 drive tumorigenesis: a review. Oncotarget. 2018;9:5492–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jeong WJ, Ro EJ, Choi KY. Interaction between Wnt/beta‐catenin and RAS‐ERK pathways and an anti‐cancer strategy via degradations of beta‐catenin and RAS by targeting the Wnt/beta‐catenin pathway. NPJ Precis Oncol. 2018;2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Luke JJ, Bao R, Sweis RF, Spranger S, Gajewski TF. WNT/beta‐catenin pathway activation correlates with immune exclusion across human cancers. Clin Cancer Res. 2019;25:3074–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Takeuchi Y, Tanegashima T, Sato E, Irie T, Sai A, Itahashi K, et al. Highly immunogenic cancer cells require activation of the WNT pathway for immunological escape. Sci Immunol. 2021;6:eabc6424. [DOI] [PubMed] [Google Scholar]
- 19. Kim IA, Hur JY, Kim HJ, et al. Targeted next‐generation sequencing analysis predicts the recurrence in resected lung adenocarcinoma harboring EGFR mutations. Cancers (Basel). 2021;13 :3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Woenckhaus M, Merk J, Stoehr R, Schaeper F, Gaumann A, Wiebe K, et al. Prognostic value of FHIT, CTNNB1, and MUC1 expression in non‐small cell lung cancer. Hum Pathol. 2008;39:126–36. [DOI] [PubMed] [Google Scholar]


