Abstract
Objective:
Randomized trials and retrospective data suggest that covered balloon-expandable (CBE) stents have better short-term patency compared with balloon-expandable bare-metal stents (BMSs) in the treatment of iliac artery disease. This study evaluated midterm outcomes of BMSs vs CBE stents placed in the common iliac artery (CIA) for aortoiliac occlusive disease.
Methods:
All endovascular interventions for symptomatic peripheral arterial occlusive disease performed at a single institution from 2006 to 2012 were reviewed. Patients undergoing stent placement in the CIA segment were included in the analysis. Demographic data, TransAtlantic Inter-Society Consensus (TASC) classification, stent type, patency, and limb reinterventions were compared.
Results:
For treatment of de novo distal aorta or CIA stenosis, 254 procedures were performed in 162 patients. BMSs were used in 190 arteries; CBE stents were used in 64 arteries. There was no difference in age, gender, or TASC classification between the two groups. Mean follow-up was 22 ± 16 months. Primary patency, assisted patency, and secondary patency were significantly better in the BMS group. CIAs treated with covered stents were more likely at 1 year or longer to require repeated intervention (hazard ratio, 2.5; 95% confidence interval, 1.2-5.3; P = .009). TASC classification did not predict need for reintervention in either group. Multivariate analysis revealed dual antiplatelet therapy to be the only other factor to affect patency during long-term follow-up.
Conclusions:
In this study, BMSs had significantly better patency compared with CBE stents for treatment of aortoiliac occlusive disease. A randomized trial comparing patency as well as restenosis rates with long-term follow-up is needed to determine if there is any benefit from use of covered stents in the aortoiliac segment.
Endovascular therapy has evolved as a first-line treatment of debilitating aortoiliac occlusive disease and has been shown to substantially improve health-related quality of life.1 Studies comparing open surgical bypass with endovascular therapy in patients with comparable disease patterns have equivalent outcomes at 3 years between the two groups.2 Open surgical treatment is maximally invasive and carries a mortality rate of up to 4.4%.3 Additional morbidity from aortobifemoral bypass ranges from 1% to 15%, with an infection rate of 0.5% to 5%.4,5 Endovascular treatment avoids the physiologic stress of surgery and has reduced infection rates.
Early research on aortoiliac interventions focused on angioplasty vs angioplasty with bare-metal stent (BMS) placement. These studies indicated that BMS placement was associated with improved long-term patency over angioplasty alone6; however, BMS restenosis remains a significant problem. Improving long-term patency has the advantage of reducing the need for reintervention and improving patient satisfaction. Use of stents covered with expanded polytetrafluoroethylene (ePTFE) not only supports the treated vessel but also excludes the injured atheromatous plaque from intraluminal macrophages that can migrate through the stent interstices and lead to inflammation.7 This proposed mechanism in theory provides the potential of improved patency because of decreased restenosis after stent placement. This has not been substantiated by the current literature, however.8 Studies of ePTFE-covered stents in the infrainguinal segment have demonstrated rates of reintervention as high as 43% at 1 year, but patency may be improved by long-term use of dual antiplatelet agents.9
Thus far, support for the use of covered stents within the aortoiliac segment is based on one small retrospective review and one industry-sponsored randomized controlled trial. The initial retrospective review consisted of only 54 patients but demonstrated a statistically improved patency rate with the use of covered balloon-expandable (CBE) stents (92%) compared with balloon-expandable BMSs (72%) at 2 years for aortoiliac bifurcation disease.10 The Covered vs Balloon Expandable Stent Trial (COBEST) included treatment of the entire aortoiliac segment, and the primary outcomes for this study were binary restenosis and target vessel revascularization at 18 months. In addition, both balloon-expandable and self-expanding BMSs were compared with CBE stents.11 The clinical significance of the outcomes from COBEST are uncertain and may not accurately reflect how patients are treated in real-world practice. This study therefore sought to compare the effectiveness of balloon-expandable BMSs with CBE stents implanted in the distal aorta and common iliac arteries (CIAs) for treatment of aortoiliac occlusive disease.
METHODS
The University of California Davis maintains a retrospective database of all patients treated for lower extremity arterial disease from June 2006 to December 2012. The database collects demographic, procedural, and outcomes data for patients who undergo endovascular or surgical treatment. Maintenance and analysis of the database are approved by the University of California Davis Institutional Review Board.
Patients.
All patients in the database who underwent endovascular treatment of the CIA segment from June 2006 to December 2012 were identified. Thirteen patients treated for recurrent disease, iliac aneurysms, or graft stenosis were excluded. An additional 13 patients treated with self-expanding stents in the CIA were also excluded. Two patients with attempted endovascular therapy had subsequent open surgical treatment and were also excluded. The remaining cohort consisted of 162 patients and 254 CIAs treated with either balloon-expandable BMSs (n = 190) or CBE stents (n = 64).
Variables recorded in the database include gender, comorbidities, preprocedure symptoms, indications for the intervention, Rutherford classification of disease, medications, assessment of prior treatment, and results of noninvasive vascular laboratory testing. Hyperlipidemia includes both patients with the diagnosis and those being treated with medication. Patients on dialysis are characterized as having end-stage renal disease. At the time of entry into the database, a board-certified vascular surgeon or interventional cardiologist with peripheral arterial experience reviews all angiographic imaging to assign the appropriate TransAtlantic Inter-Society Consensus (TASC) II classification of disease and to assess runoff. For patients treated with peripheral arterial disease, the resting ankle-brachial index (ABI) is typically obtained preoperatively and at clinical follow-up examinations. The typical follow-up strategy for these patients is a duplex ultrasound (DUS) examination or ABI within 1 to 3 months after the procedure and at 6 and 12 months. Patients are typically seen every 6 to 12 months in the second year and then yearly thereafter. At each clinical visit, noninvasive testing with DUS or ABI is obtained. DUS of the treated area is performed if the patient’s body habitus permits. Patients who develop clinical symptoms are reevaluated sooner than those without symptoms.
Interventions.
A board-certified vascular surgeon or an interventional cardiologist specializing in peripheral artery disease at the University of California Davis Vascular Center performed all interventions. The choice of CBE stents vs BMSs was decided on a case-by-case basis at the discretion of the treating physician. A subset of patients underwent treatment of multiple arterial segments, including the femoropopliteal segment or tibial vessels, during the index procedure. For the purpose of analysis, patients were characterized as having undergone single-vessel (CIA only) or multivessel (additional arterial segments treated at the index procedure) interventions. Multivessel interventions included both suprainguinal and infrainguinal interventions. All treatment was performed with fixed imaging under local anesthesia with conscious sedation, except in cases in which a hybrid surgical procedure necessitated general anesthesia. Heparin is routinely administered to elevate the activated clotting time above 250 seconds before intervention, and the dose is repeated as needed throughout the course of the procedure.
In the case of bilateral CIA interventions or distal aortic interventions, a “kissing balloon” technique is used to deploy the stents simultaneously. Both CBE stents and BMSs were minimally oversized relative to the treated artery. In cases in which subintimal angioplasty was performed after reentry into the aorta, the aortic bifurcation was elevated to cover the entire area of disease. If the combined stent diameter was larger than the size of the distal aorta, smaller stents were selected and postdilated with care. In patients with unilateral disease, the stent covered the entire length of the stenosis or occlusion. BMSs included the Express LD (Boston Scientific, Natick, Mass), Omnilink (Abbott Vascular, Abbott Park, Ill), and Genesis Palmaz (Cordis, Bridgewater, NJ). The CBE stent used in all cases was the iCAST (Atrium Medical, Hudson, NH) stent with an external cover of ePTFE.
All patients were prescribed aspirin after the procedure at a dose ranging from 81 to 325 mg. Clopidogrel, at 75 mg daily, was routinely prescribed after the procedure, with dual antiplatelet therapy continued for at least 6 weeks. At the discretion of the treating physician, a loading dose of 300 mg of clopidogrel was administered at the time of the procedure. Patients receiving anticoagulation therapy were prescribed clopidogrel for 6 weeks in addition to the anticoagulation. After this time, patients were transitioned to long-term anticoagulation and aspirin therapy. Patients who were receiving clopidogrel before the procedure were not given a loading dose, and clopidogrel was continued indefinitely.
Outcomes.
Technical success was defined as less than 30% residual stenosis within the CIA segment. Primary, primary-assisted, and secondary patency were defined as recommended by the Society for Vascular Surgery guidelines.12 Loss of primary patency was defined as any stent that underwent reintervention to prevent thrombosis or any stent that thrombosed primarily. The need for reintervention was determined by a change in a previously palpable pulse, recurrent symptoms, drop in the ABI >0.15, Doppler ultrasound findings indicating a >50% stenosis defined as >100% increase in the peak systolic velocity relative to the adjacent segments,13 or any combination of these findings. Decision for reintervention was made on a case-by-case basis. Only major amputations, defined as at the level of the ankle or more proximal, were considered for outcomes. Minor amputations, defined as an amputation that preserved a functional foot, were recorded but not analyzed. Overall survival was recorded for all patients. For patients with limited follow-up, the Social Security Death Index was used to determine mortality and date of death.
Data analysis.
Categorical data were described by frequency and percentage and compared by χ2 tests. Continuous variables were described with the mean and standard deviation. Survival, freedom from reintervention, and patency estimates were determined by Kaplan-Meier analysis. Log-rank testing was used to compare patency estimates. Multivariable analysis was used to identify risk factors for loss of primary patency. A P value of .05 or less was considered to be statistically significant. All statistical analysis for this study was performed with R software (v 3.0.1).
RESULTS
Overall demographics between the two groups, including age, hypertension, diabetes mellitus, and coronary artery disease, were similar with the exception that more patients in the BMS group had congestive heart failure (Table I). Only 10% of patients in each group were life-long nonsmokers. One quarter of patients in both groups were not receiving any antiplatelet treatment before the procedure. Women represented 49% of the patients in the CBE stent group and 54% of the patients in the BMS group. The most common indication for treatment was lifestyle-limiting claudication; however, a significantly greater number of patients in the BMS group (35% vs 15%; P = .02) were treated for critical limb ischemia. Only 10% in the BMS vs 3% in the CBE stent group were treated for acute limb ischemia (P = .15). Mean follow-up for the cohort was 22 months (standard deviation ± 16).
Table I.
Patient demographic characteristics by stent type
| Variable | BMS (n = 125), No. (%) | CBE stent (n = 37), No. (%) | P value |
|---|---|---|---|
| Mean age ± SD, years | 65 ± 11 | 64 ± 11 | .69 |
| Male gender | 58 (46) | 19 (51) | .73 |
| Hypertension | 97 (77) | 32 (86) | .34 |
| Dyslipidemia | 88 (70) | 27 (73) | .92 |
| Coronary artery disease | 66 (53) | 17 (46) | .56 |
| Congestive heart failure | 28 (22) | 2 (5) | .04 |
| End-stage renal disease | 14 (11) | 0 | .07 |
| Diabetes mellitus | 50 (40) | 9 (24) | .12 |
| Stoke | 25 (20) | 7 (19) | 1.00 |
| Statin | 86 (69) | 22 (60) | .39 |
| Smoking status | |||
| Current | 55 (44) | 13 (35) | .44 |
| Prior | 57 (46) | 20 (54) | .47 |
| Never | 13 (10) | 4 (10) | 1.00 |
| Anticoagulation | 11 (9) | 1 (5) | .34 |
| Antiplatelet therapy | |||
| Aspirin | 49 (39) | 19 (51) | .26 |
| Clopidogrel | 6 (5) | 2 (5) | 1.00 |
| Aspirin + clopidogrel | 37 (30) | 7 (19) | .28 |
| None | 33 (26) | 9 (24) | .96 |
| Clinical indication | |||
| Claudication | 72 (58) | 27 (73) | .13 |
| Critical limb ischemia | 44 (35) | 5 (14) | .02 |
| Acute limb ischemia | 4 (3) | 4 (10) | .15 |
BMS, Bare-metal stent; CBE, covered balloon-expandable; SD, standard deviation.
Of the 254 CIAs treated, no significant difference between lesion length or TASC II classification was observed in comparing the CBE stent and BMS groups (Table II). As expected, 30 arteries (61%) treated with covered stents and 81 arteries (63%) treated with BMSs were for TASC A and B disease. The CIA was the sole treated segment in 44 arteries (69%) of the CBE stent group vs 118 arteries (62%) of the BMS group. Multivessel interventions were performed in 20 arteries (31%) initially treated with CBE stents. Only one of these was for concomitant infrainguinal disease; the remaining 19 were for external iliac artery (EIA) disease. Within the BMS group, 72 (38%) of the CIAs treated had additional arterial segments treated during the index procedure; 22 (31%) of these multivessel interventions were for infrainguinal occlusive disease.
Table II.
Comparison of CIA lesion and intervention characteristics
| N = 254 | CBE stents (n = 64), No. (%) | BMS (n = 190), No. (%) | P value |
|---|---|---|---|
| Mean lesion length ° SD, mm | 42 ± 18 | 39 ± 18 | .22 |
| TASC II classification | |||
| A | 18 (28) | 55 (29) | 1.00 |
| B | 21 (33) | 64 (34) | 1.00 |
| C | 11 (17) | 46 (24) | .32 |
| D | 14 (22) | 25 (13) | .14 |
| Single-vessel intervention | 44 (69) | 118 (62) | .42 |
| Multivessel intervention | 20 (31) | 72 (38) | .42 |
| Suprainguinal | 19 (30) | 50 (26) | .72 |
| Infrainguinal | 1 (1) | 16 (8) | .11 |
| Both | 0 (0) | 6 (3) | .34 |
BMS, Bare-metal stent; CBE, covered balloon-expandable; CIA, common iliac artery; SD, standard deviation; TASC, TransAtlantic Inter-Society Consensus.
Access site complications occurred in one patient (1.6%) treated with a CBE stent and three patients (1.6%) treated with BMSs. Closure devices were used in the one patient in the CBE stent group and in two of the three patients in the BMS group. The patient in the CBE stent group developed a hematoma that did not require any further intervention. Two patients in the BMS group developed pseudoaneurysms. One was treated with ultrasound-guided compression, and the second was treated with thrombin injection. Both resolved with these measures. One patient in the BMS group did not have a closure device used and developed a hematoma with ongoing bleeding that required surgical repair.
Although 15% of the original cohort was lost to long-term follow-up, overall primary patency at 3 years was 81% (Table III). Limbs treated with BMSs had significantly better primary patency than those treated with CBE stents (89 ± 3% vs 72 ± 6%; P = .008; Fig 1). Secondary patency was also significantly better in the BMS group (98 ± 1% vs 92 ± 3%; P = .03) compared with CBE stents (Fig 2). Five arteries in the CBE stent group and four arteries in the BMS group thrombosed. Although reintervention was attempted in all but two patients with BMSs, none provided long-term patency. Two patients in each group represented with acute limb ischemia when the CIA stents thrombosed. All seven patients who had a failed reintervention went on to require open surgical bypass, and one patient needed a major amputation.
Table III.
Patency and limb salvage by limb; survival by patient
|
Year, % (95% confidence interval)
|
|||||
|---|---|---|---|---|---|
| Measure | Group | 1 year | 2 years | 3 years | P value |
| Primary patency | Overall | 90 (86-95) | 84 (79-90) | 81 (75-88) | .008 |
| BMS | 92 (88-97) | 89 (84-95) | 89 (84-95) | ||
| CBE | 85 (76-95) | 72 (61-86) | 72 (61-86) | ||
| Primary-assisted patency | Overall | 97 (95-100) | 96 (93- 99) | 96 (93-99) | .04 |
| BMS | 98 (96-100) | 98 (96-100) | 98 (96-100) | ||
| CBE | 94 (88-100) | 90 (82- 99) | 90 (82-99) | ||
| Secondary patency | Overall | 98 (94-99) | 97 (93-99) | 97 (87-98) | .03 |
| BMS | 99 (97-100) | 98 (95-100) | 98 (91-100) | ||
| CBE | 96 (91-100) | 92 (85-99) | 92 (85-99) | ||
| Limb salvage | Overall | 98 (96-100) | 95 (92-99) | 95 (92-99) | .93 |
| BMS | 98 (95-100) | 95 (92-99) | 95 (91-99) | ||
| CBE | 98 (95-100) | 95 (88-100) | 95 (88-100) | ||
| Survival | Overall | 92 (87-97) | 80 (72-89) | 73 (64-84) | .10 |
| BMS | 90 (85-96) | 75 (66-86) | 71 (61-83) | ||
| CBE | 97 (91-100) | 97 (91-100) | 81 (62-100) | ||
BMS, Bare-metal stent; CBE, covered balloon-expandable.
Fig 1.
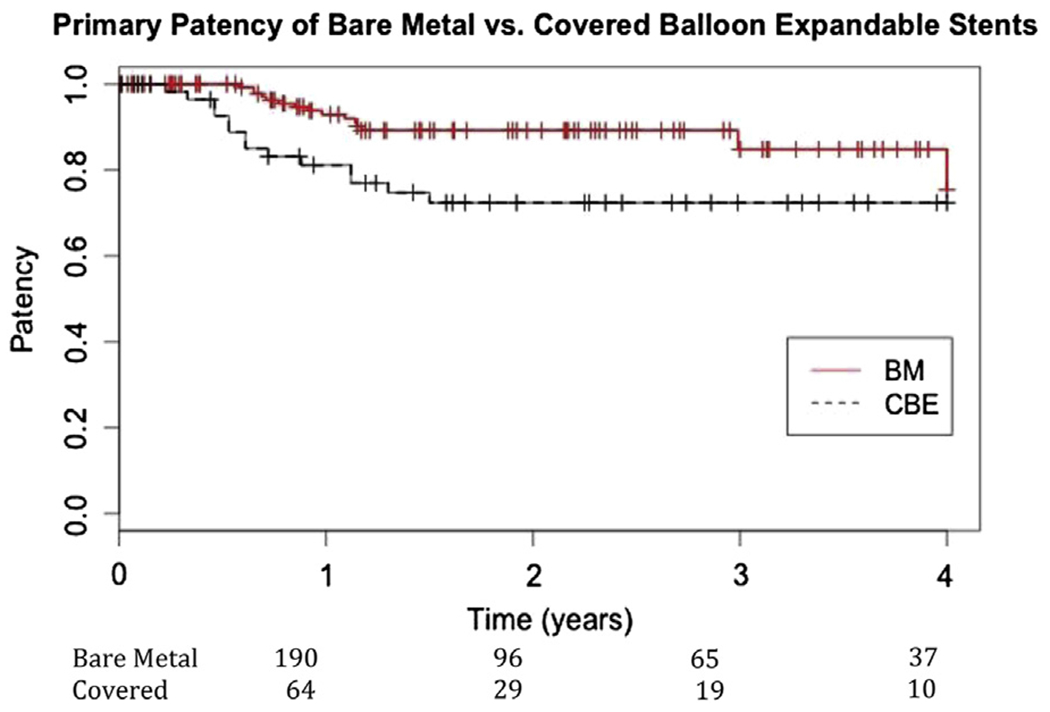
Primary patency of bare-metal (BM) stents (89% ± 3%) compared with covered balloon-expandable (CBE) stents (72% ± 6%) in the common iliac artery (CIA) (P = .008).
Fig 2.
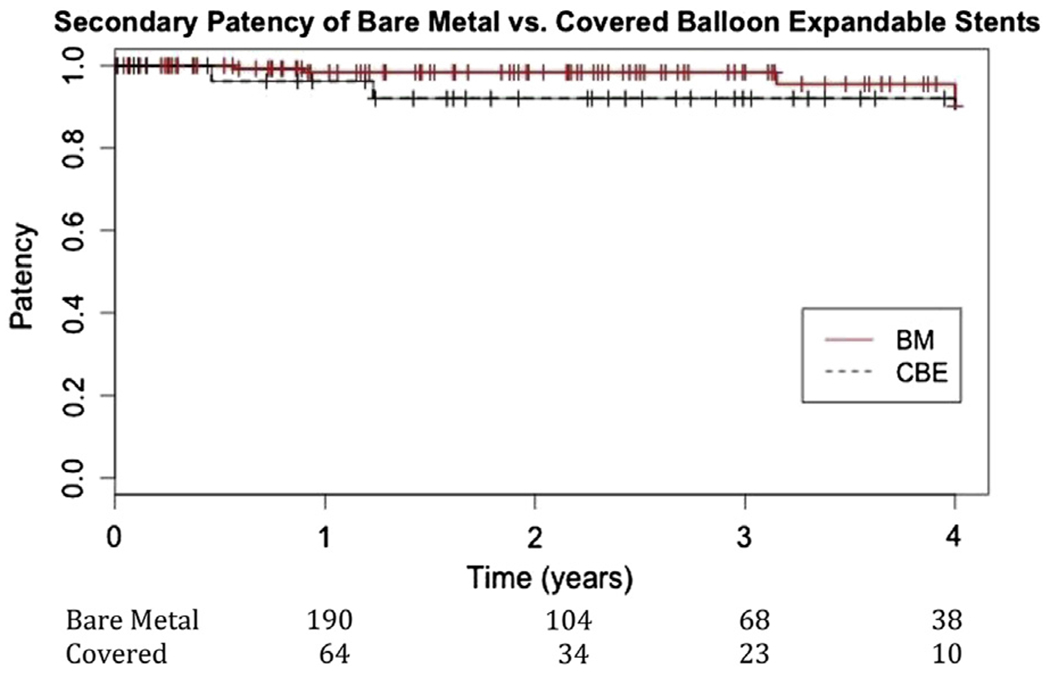
Secondary patency of bare-metal (BM) stents (98% ± 1%) compared with covered balloon-expandable (CBE) stents (92% ± 3%) in the common iliac artery (CIA) by limb (P = .03).
Limb salvage in both groups was 95% (P = .93). Six patients (5%) in the BMS group and two patients (5%) in the CBE stent group required amputation. Among the patients in the BMS group who required amputation, five were for progression of critical limb ischemia. In the CBE stent group, amputation was required in one patient who originally presented with acute limb ischemia. Symptomatic claudication was the original presentation for two patients, one in the BMS group and one in the CBE stent group, which ultimately underwent amputation. The overall survival for the entire cohort at 3 years was 73%. Survival was lower in the BMS group compared with the CBE stent group (71% vs 81%); however, this was not statistically significant (P = .10).
Gender, continued use of statin medications, smoking status, bilateral CIA treatment, multivessel treatment, lesion length, and TASC classification were not predictive of primary patency on multivariate analysis. In univariate analysis, use of anticoagulation predicted long-term patency, but this did not hold up on multivariate analysis. The only identified predictors of primary patency were continued use of dual antiplatelet therapy at 12 months and original indication for the procedure. Patients not taking clopidogrel and aspirin at 12 months were more likely to undergo reintervention or to experience thrombosis at 3 years (hazard ratio, 4.25; P = .003). In addition to dual antiplatelet therapy, original indication for the procedure predicted loss of primary patency. Patients treated for claudication were more likely to require reintervention at 3 years than those treated for critical limb ischemia (hazard ratio, 2.6; 95% confidence interval, 1.14-6.09; P = .02).
DISCUSSION
This study represents the largest reported comparison of balloon-expandable BMSs to CBE stents in the CIA for the treatment of aortoiliac occlusive disease. The main finding was that BMSs were associated with improved primary and secondary patency compared with CBE stents.
COBEST, currently the only randomized controlled trial of the two stent types, randomized 168 limbs, but the primary outcome was binary restenosis and not primary patency. In addition, the study did not separate CIAs and EIAs in the analysis.11 Other studies of patients with CIA disease have shown primary patency rates that range from 63% to 86% at 4 years.14,15 Our study showed similar results, with an overall 3-year primary patency of 81%. In a study of 54 patients treated with bilateral kissing stents for aortoiliac bifurcation disease, Sabri showed the primary patency of covered stents to be 92% compared with 62% in the BMS group.10 The low primary patency of BMSs in this study raises questions about including deployment of stents sized below 8 mm in diameter and extending BMSs more than 1 cm above the aortic bifurcation. In COBEST as well as in other trials, BMSs that extended more than 1 cm above the bifurcation had poorer outcomes. This suggests that for the kissing technique or in treatment of bilateral disease, CBE stents may be more beneficial. We did not find that patients with bilateral BMSs had worse outcomes than those with CBE stents, although an analysis of the distance that stents extended into the aorta for bilateral disease was not performed.
The secondary patency of CBE stents in our study was 97% with follow-up to 3 years. The lower primary patency and high primary-assisted and secondary patency likely reflect the aggressive use of routine DUS screening and willingness to intervene early in identified stenosis. Although routine postprocedure evaluation has been questioned because of the added cost, the difference in secondary patency rates may very well support its use. All patients in our study who had an intervention before stent thrombosis remained patent after treatment. The significant difference in improved secondary patency of BMSs compared with CBE stents may be the result of higher numbers of patients treated with BMSs. Gandini et al found that long-term secondary patency for stents in the aortoiliac segment was 90% at 5 years but decreased to 78% at 10 years.16 They used a follow-up schedule of 1, 3, 6, and 12 months for the first year and then yearly after that, similar to our study. Although DUS was the primary mode to detect restenosis, they also routinely obtained computed tomography angiography at 6 and 12 months or if DUS showed any evidence of restenosis. In most cases, the CIA segment can be imaged adequately. In patients in whom body habitus precluded DUS, a drop in ABI of more than 0.15 has been shown to be predictive of restenosis and potential thrombosis. The increased radiation associated with computed tomography angiography does not support its use for surveillance imaging.
Surgery has traditionally been recommended for patients with TASC C and D lesions because of the improved durability of the intervention.17 However, several studies have shown that patients with TASC C and D iliac lesions do better with primary stenting. Primary patency rates in these patients range from 72% to 89% at 3 years, with secondary patency rates between 83% and 93%.6,18,19 COBEST demonstrated that patients with TASC C and D disease treated with covered stents had lower binary restenosis than those treated with BMSs, although the study was not adequately powered for subgroup analysis. In that study, 51% of the patients in the covered stent group and 43% in the BMS group had advanced disease. In our study, TASC C and D lesions represented only 39% of the limbs treated with covered stents and 37% of the BMS group. These lower numbers may not be adequate to detect a difference in patency on univariate analysis.
EIA disease tends to be long-segment disease, is best treated completely, and may require multiple overlapping stents. Gandini et al retrospectively reviewed 138 patients with aortoiliac disease and found that stents in the EIA had worse patency than those in the CIA.16 In addition, when both the CIA and EIA were treated simultaneously, patency was significantly lower than when the CIA was the isolated treatment segment. We analyzed our population by isolated treatment of the CIA segment compared with multivessel treatment of other arterial segments during the initial procedure, whether suprainguinal or infrainguinal. No difference was found in the primary patency of the CBE stent or BMS when the EIA was also treated at the same time. This may reflect our use of newer, long, single, self-expanding stents in the EIA segment compared with prior studies, in which multiple CBE stents or BMSs were required and self-expanding stents were not as conformable in the tortuous EIA.
There was no difference in limb salvage between the two groups in this study. Other studies have demonstrated an amputation rate after iliac artery stenting as high as 12%.20 The overall amputation rate in this study was 5%. However, in terms of absolute numbers, more patients in the BMS group underwent a major amputation. The 3% amputation rate in the BMS group is likely to be due to initial treatment indication rather than to stent thrombosis. More patients in the BMS group were treated for critical limb ischemia. Of the eight limbs amputated during the follow-up period, seven had primary patency of the CIA stents. The one patient whose BMS thrombosed was originally treated for symptomatic claudication with bilateral CIA stents. The patient re-presented with stent thrombosis and underwent surgical bypass. The amputation occurred 1 year after surgical treatment.
Women represented 49% of the patients in the CBE stent group and 54% of those in the BMS group. Gender has been associated with worse outcomes after endovascular treatment in both the femoropopliteal region and the iliac arteries. This has been most pronounced in women with critical limb ischemia. Using administrative data, Goode et al evaluated more than 23,000 iliac interventions performed on the iliac segment in England during a 5-year period. On multivariate analysis, they found that women had worse outcomes than men (odds ratio, 4.98 [2.09-13.25]).21 Our study found no difference in primary or secondary patency of CBE stents or BMSs in the CIAs on the basis of gender. The group was further stratified to compare only the 133 patients with critical limb ischemia. Again, gender was not predictive of loss of primary or secondary patency.
The use of dual antiplatelet therapy was one of the only factors found to improve the overall primary patency. In a study of 87 limbs treated with stent grafts in the femoropopliteal segment, Johnston et al found that continued use of dual antiplatelet therapy was protective against major adverse limb events.9 This study focused on the femoropopliteal segment, which is subjected to many different forces with movement. The CIA segment does not move in the same manor, but our results would also support continued use of dual antiplatelet therapy.
Our study has several limitations. First, this study is retrospective, and although attempts were made to limit the patient population to a homogeneous group by excluding patients not treated specifically for aortoiliac occlusive disease, subtle factors that influenced stent selection may not have been recorded. Moreover, there are markedly more patients in the BMS group, and this difference persists throughout the follow-up period. The statistical difference in outcomes could be the result of the unequal cohorts and smaller size at later dates of follow-up. Our facility has on hand advanced reentry equipment that may make our technical success rate potentially higher than that of other facilities. Only two patients in the original cohort were not successfully treated and went on to require open surgical treatment. Finally, 15% of patients in our study were lost to follow-up, and these patients could represent significant stent failures or patients who needed surgical revision.
CONCLUSIONS
In this single-center retrospective experience, BMSs were found to be superior to CBE stents for the treatment of CIA disease at 3 years. Currently, the DISCOVER trial (Dutch Iliac Stent Trial: COVERed balloon-expandable vs uncovered balloon-expandable stents in the common iliac artery) is randomizing patients with iliac disease to definitively answer the question of whether covered stents in the CIAs are better than BMSs. Our results are in contrast to reports that show CBE stents are superior to BMSs, and at this time there is not sufficient evidence to support the use of more costly CBE stents for treatment of atherosclerotic disease of the CIA.
DISCUSSION.
Dr Scott E. Musicant (La Mesa, Calif). I would really like to thank the program committee for the opportunity to discuss this paper. And, thank you to Dr Humphries for providing the manuscript to me prior to the meeting.
Dr Humphries and her colleagues at UC Davis have performed a retrospective analysis of their experience using balloon-expandable stents for the treatment of common iliac artery disease. They have compared their experience with covered balloon-expandable and bare-metal balloon expandable stents in 162 patients and 254 arteries treated over a 6.5-year period.
The vast majority (75%) of these were patients treated with balloon-expandable bare-metal stents. Only 25% of patients were treated with covered stents. They had a mean follow-up of 22 months. And, as she showed, a significantly higher proportion of patients in the bare metal stent group presented with CLI.
Their results showed that primary, assisted-primary, and secondary patency were significantly better for bare-metal stents compared with covered stents. This was irrespective of TASC classification, lesion length, or adjunctive procedures performed above or below the inguinal crease. Mortality and limb salvage were not statistically different between the two groups. However, I was saddened to see that two patients who initially presented with claudication ended up requiring amputations.
Their results are in direct contrast to multiple other studies which have shown improved outcomes in the common iliac segment using covered BE stents. The COBEST trial was a prospective, multicenter, randomized controlled trial published in JVS in 2011 from Australia that showed significantly less restenosis and fewer reinterventions in the group treated with covered stents. Their subgroup analysis showed that patients with TASC C and D lesions actually did the best with covered stents. Similar results were found in other series as well. So, I have just fourteen questions for you. No, actually I only have four.
First, you found an equally small number of stents in each group that experienced thrombosis. If that is true, then what was the primary method of failure in the covered stent group? Were these lesions at the proximal or distal ends of the stent grafts or de novo lesions? And, since you were following these patients closely with DUS, were their differences in flow velocities on follow-up duplex that could have identified those stents at risk of failure?
Second, more patients in the bare-metal group underwent concomitant SFA interventions. Despite the fact that this did not quite reach statistical significance, is it possible that these patients had improved outflow that accounted for the improved patency? Similarly, do you have any information on the degree of disease in the hypogastric or external iliac artery that could be of relevance?
Third, how do you account for the completely opposite results from the findings of other studies that show superior patency rates of covered stents in this population? Typically, we see patients with CLI have worse outcomes than patients with claudication; however, you had much higher percentage of CLI patients that received bare-metal stents. The COBEST trial had less that 6% of self-expanding bare-metal stents in their cohort, so that is not a great explanation for the discrepant findings.
Finally, what is your recommendation for me the next time I succeed in recannalizing a TASC D CIA lesion? Should I follow your data or those of many others?
I enjoyed reading your paper and again appreciate the opportunity to discuss this paper.
Dr Misty D. Humphries. Thank you for the questions. I will address them in the order presented.
We did not assess the specific failure mode for each either group, although those patients with repeat interventions have angiograms that can be reviewed. The presumed mechanism for failure for covered stents is thought to be at the ends of the stents vs bare-metal stents, which develop diffuse stenosis throughout the stent. Although most patients are followed with duplex ultrasound, documentation of the specific mode of failure was not recorded. As a follow-up to this work, we would like to investigate the mechanism of failure for both stent types as well as the difference between stent restenosis and patency.
As far as differences based on outflow disease, we attempted to characterize procedures as multiple intervention procedures involving the supra- and/or infrainguinal region vs single-intervention procedures involving only the common iliac segment. No difference was found in the outcomes for patients with multiple interventions compared to those with only single interventions, but we did not look at all angiograms to specifically assess the femoral outflow. Unfortunately, we do not have angiographic evaluation of the SFA in all patients. We could go back to evaluate the internal iliac arteries, but in all these patients, the external iliac arteries were either treated or patent. Therefore, utility of reporting the internal iliac arteries specifically is unclear. As far as the degree of disease in the iliac arteries, the TASC class reported here is based on the iliac angiography and we found no difference in the outcomes with worsening TASC classification.
Our work is in opposition to the only RCT on covered vs bare-metal stents, but we evaluated the group by patency. Other studies have looked at binary restenosis and used duplex ultrasound criteria that may or may not be considered a stenosis in a stented region by those of us in this room. Covered stents develop less binary restenosis according to the COBEST trial, but not all patients get a reintervention immediately when they develop a duplex identified stenosis that is >250cm/sec. A stenosis at this level may be watched for progression or the development of recurrent symptoms before a patient gets a reintervention. Our work presents pragmatic results of bar- metal and covered stents. We are showing that in a typical practice, bare-metal stents are used more frequently and the patency is not worse than covered stents.
Finally, the next time you recanalize a stenosis for a TASC D CIA lesion, consider my work as a guide for you but not a definitive answer. I do not think I can tell you to use a covered stent or a bare-metal stent. Further work needs to look at the cost effectiveness of covered and uncovered stents, which we are working on. Patency captures the initial reintervention the patient has after stent placement, but if two or three interventions are needed to maintain the patency for a bare-metal stent, the lower cost may not be justified. How much do these repeat procedures cost? We don’t know the answers to these questions and we need to in order to make a definitive recommendation about what stents should be used in the common iliac arteries and all peripheral arteries. Our work simply shows that with regard to patency, bare-metal stents are not necessarily worse than covered. It also pushes the question of how do we translate data from randomized control trials into practice, and whether we should use data from trials that consider outcomes we may not agree with to influence our practice. The DISCOVER trial that is ongoing in The Netherlands may be able to answer the question of which stents develop stenosis, but again, these types of explanatory trials can’t always translate into the pragmatic setting that we practice in. Until a more comprehensive approach to evaluating restenosis, patency, reinterventions, and cost effectiveness is available, my practice will not change.
Author conflict of interest:
J.L. receives consulting fees from Abbot Vascular, Boston Scientific, Bard, Covidien, and Medtronic.
Footnotes
Presented at the plenary session of the Twenty-eighth Annual Meeting of the Western Vascular Society, Jasper, Alberta, Canada, September 21-24, 2013.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
REFERENCES
- 1.Bosch JL, van der Graaf Y, Hunink MG. Health-related quality of life after angioplasty and stent placement in patients with iliac artery occlusive disease: results of a randomized controlled clinical trial. The Dutch Iliac Stent Trial Study Group. Circulation 1999;99:3155–60. [DOI] [PubMed] [Google Scholar]
- 2.Kashyap VS, Pavkov ML, Bena JF, Sarac TP, O’Hara PJ, Lyden SP, et al. The management of severe aortoiliac occlusive disease: endovascular therapy rivals open reconstruction. J Vasc Surg 2008;48:1451-7.1457.e1-3. [DOI] [PubMed] [Google Scholar]
- 3.de Vries SO, Hunink MG. Results of aortic bifurcation grafts for aortoiliac occlusive disease: a meta-analysis. J Vasc Surg 1997;26:558–69. [DOI] [PubMed] [Google Scholar]
- 4.van den Akker PJ, van Schilfgaarde R, Brand R, Hajo van Bockel J, Terpstra JL. Long-term results of prosthetic and non-prosthetic reconstruction for obstructive aorto-iliac disease. Eur J Vasc Surg 1992;6:53–61. [DOI] [PubMed] [Google Scholar]
- 5.Mingoli A, Sapienza P, Feldhaus RJ, Di Marzo L, Burchi C, Cavallaro A. Comparison of femorofemoral and aortofemoral bypass for aortoiliac occlusive disease. J Cardiovasc Surg (Torino) 2001;42:381–7. [PubMed] [Google Scholar]
- 6.AbuRahma AF, Hayes JD, Flaherty SK, Peery W. Primary iliac stenting versus transluminal angioplasty with selective stenting. J Vasc Surg 2007;46:965–70. [DOI] [PubMed] [Google Scholar]
- 7.Jamshidi P, Mahmoody K, Erne P. Covered stents: a review. Int J Cardiol 2008;130:310–8. [DOI] [PubMed] [Google Scholar]
- 8.Elsner M, Auch-Schwelk W, Britten M, Walter DH, Schachinger V, Zeiher AM. Coronary stent grafts covered by a polytetrafluoroethylene membrane. Am J Cardiol 1999;84:335–8. A8. [DOI] [PubMed] [Google Scholar]
- 9.Johnston PC, Vartanian SM, Runge SJ, Hiramoto JS, Eichler CM, Owens CD, et al. Risk factors for clinical failure after stent graft treatment for femoropopliteal occlusive disease. J Vasc Surg 2012;56:998–1007.e1. [DOI] [PubMed] [Google Scholar]
- 10.Sabri SS, Choudhri A, Orgera G, Arslan B, Turba UC, Harthun NL, et al. Outcomes of covered kissing stent placement compared with bare metal stent placement in the treatment of atherosclerotic occlusive disease at the aortic bifurcation. J Vasc Interv Radiol 2010;21:995–1003. [DOI] [PubMed] [Google Scholar]
- 11.Mwipatayi BP, Thomas S, Wong J, Temple SE, Vijayan V, Jackson M, et al. A comparison of covered vs bare expandable stents for the treatment of aortoiliac occlusive disease. J Vasc Surg 2011;54:1561–70. [DOI] [PubMed] [Google Scholar]
- 12.Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg 1997;26:517–38. [DOI] [PubMed] [Google Scholar]
- 13.Moneta GL, Zaccardi MJ, Olmsted KA. Strandness’s duplex scanning in vascular disorders. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 14.Cikrit DF, Dalsing MC, Harris VJ, Lalka SG, Sawchuk AP, Trerotola SO, et al. Long-term follow-up of the Palmaz stent for iliac occlusive disease. Surgery 1995;118:608–13. [DOI] [PubMed] [Google Scholar]
- 15.Sacks D, Marinelli DL, Martin LG, Spies JB. Reporting standards for clinical evaluation of new peripheral arterial revascularization devices. J Vasc Interv Radiol 2003;14(Pt 2):S395–404. [DOI] [PubMed] [Google Scholar]
- 16.Gandini R, Fabiano S, Chiocchi M, Chiappa R, Simonetti G. Percutaneous treatment in iliac artery occlusion: long-term results. Cardiovasc Intervent Radiol 2008;31:1069–76. [DOI] [PubMed] [Google Scholar]
- 17.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg 2007;33(Suppl 1):S1–75. [DOI] [PubMed] [Google Scholar]
- 18.Leville CD, Kashyap VS, Clair DG, Bena JF, Lyden SP, Greenberg RK, et al. Endovascular management of iliac artery occlusions: extending treatment to TransAtlantic Inter-Society Consensus class C and D patients. J Vasc Surg 2006;43:32–9. [DOI] [PubMed] [Google Scholar]
- 19.De Roeck C, Hendriks JMH, Delrue F, Lauwers P, Van Schil P, De Maeseneer M, et al. Long-term results of primary stenting for long and complex iliac artery occlusions. Acta Chir Belg 2006;106:187–92. [DOI] [PubMed] [Google Scholar]
- 20.Cambria RA, Farooq MM, Mewissen MW, Freischlag JA, Seabrook GR, Crain MR, et al. Endovascular therapy of iliac arteries: routine application of intraluminal stents does not improve clinical patency. Ann Vasc Surg 1999;13:599–605. [DOI] [PubMed] [Google Scholar]
- 21.Goode S, Keltie K, Burn J, Patrick H, Cleveland TJ, Campbell B, et al. Effect of procedure volume on outcomes after iliac artery angioplasty and stenting. Br J Surg 2013;100:1189–96. [DOI] [PubMed] [Google Scholar]


