Abstract
Objective
Previous studies have sought to determine the effects of total knee arthroplasty (TKA) using kinematic alignment (KA) versus mechanical alignment (MA) to reproduce the native knee alignment and soft tissue envelope for improved patient satisfaction. There are limited studies that compare acute perioperative outcomes between KA and MA patients as it pertains to pain‐related opioid consumption and hospital length of stay (LOS). This study aims to compare early KA and MA in restoring function and rehabilitation after surgery to reduce hospitalization and opioid consumption.
Methods
A retrospective review of 42 KA and 58 MA primary TKA patients performed by a single surgeon between 2020–2021 was conducted. Demographics were controlled between groups and radiographic measurements and functional outcomes were compared. Pain was evaluated with inpatient/outpatient morphine milligram equivalents (MME) and visual analogue scale (VAS) scores. Mobility was assessed using multiple measures by a physical therapist. Mean preoperative and 3‐month postoperative flexion range of motion (ROM) were analyzed, and overall complications, LOS, and non‐home discharge between groups compared. Continuous variables were compared using the Wilcoxon rank‐sum test, and categorical variables were compared using the chi‐square or Fisher exact test. Statistical significance was set at P < 0.05.
Results
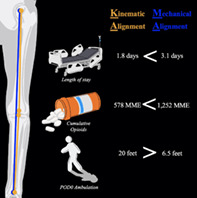
KA patients had shorter LOS (1.8 vs 3.1 days) and less cumulative opioid requirements compared to MA patients (578 vs 1253 MME). On postoperative day 0, KA patients ambulated on average twice the distance of MA patients (20 vs 6.5 feet). KA patients had residual tibia component in varus (1.4° vs −0.3°), femoral component in valgus (−1.9° vs 0.2°), and valgus joint line obliquity compared with MA (−1.5° vs 0.2°). There were no significant differences between 3‐month postoperative flexion arc motion, discharge destination, KOOS or SF‐12 outcomes, and surgical complication rates between groups.
Conclusions
By restoring the native joint line obliquity and minimizing the frequency of ligament releases, KA for TKA may improve pain relief, early mobility, and decreased length of stay compared with traditional methods of establishing neutral limb axis by MA.
Keywords: Kinematic alignment, Mechanical alignment, Opioid, Patient reported outcome, Total knee arthroplasty
By restoring the native joint line obliquity and minimizing the frequency of ligament releases, kinematic alignment for total knee arthroplasty may decrease length of stay, improve pain relief, and hasten early mobility compared with traditional methods of establishing neutral limb axis by mechanical alignment.
Introduction
Although total knee arthroplasty (TKA) is a well‐established option for improving patients' quality of life through pain alleviation, restoration of function and range of motion, and correction of deformity, patient satisfaction has been less predictable with reports of up to a 20% dissatisfaction rate. 1 , 2 , 3 , 4 , 5 , 6 This rate of dissatisfaction has remained static despite significant improvements in surgical technique, biomaterials, implant designs, and technology. 2 , 7 While the use of navigation and computer assisted technology has resulted in more reliable, accurate bone cuts, the persistence of dissatisfaction after TKA has led surgeons to question the reliability and effect of placing components in all patients according to a neutral mechanical alignment. In fact, Bellemans et al. found that around 32% of men, and 17% of women have a natural constitutional varus alignment of 3 degrees of varus or more, which suggests that for any given patient there may be patient‐specific target values that will best distribute TKA load that is not universal. 8 , 9 Patients may have a combination of muscular, skeletal, and neurologic imbalances that impact gait and dynamic loading of the joint in a patient specific manner which may be different from the overall mechanical axis. 10 Previous studies have sought to determine the effects of TKA using kinematic alignment (KA) versus mechanical alignment (MA) to reproduce and preserve the native knee alignment and soft tissue envelope for improved patient satisfaction.
While KA theoretically improves pain, function, and recovery by restoring constitutional alignment of the limb and joint line without large soft tissue releases, there are concerns over varus implantation of tibial components leading to loosening, increased contact stresses, and polyethylene wear. 11 Outcome studies comparing KA and MA TKA have mixed results and focus on patient‐reported outcome measures (PROM) and implant survivorship. 11 , 12 , 13 , 14 There are limited studies that compare acute perioperative outcomes between KA and MA patients as it pertains to reported pain‐related opioid consumption, hospital length of stay (LOS), and functional mobility scores. Previous studies have found prolonged postoperative use of opioids after TKA to be associated with increased rates of stiffness, poor functional outcomes, and limitations on quality of life. 15 With the new emphasis on enhanced recovery pathways and value‐based health care models, it is important for providers to optimize pain, improve return to functional recovery, and decrease hospital LOS.
In this retrospective review, we assess for: (i) inpatient and outpatient opioid consumption; (ii) rehabilitation mobility as assessed by a physical therapist; (iii) discharge disposition; and (iv) subjective functional outcome scores to understand the dynamics of KA and MA on short and long‐term recovery. By reproducibly restoring the patient specific joint‐line orientation and native soft tissue balance, we predict that patients undergoing TKA by KA will have improved functional mobility scores, ambulation distance, less opioid requirements, and shorter LOS compared to patients with a neutral mechanical axis.
Methods
Study Design
This study is a retrospective cohort analysis comparing patients undergoing kinematically aligned TKA with mechanically aligned TKA from 2020–2021 by a single surgeon (David H. So). The primary outcomes of the study were LOS, functional mobility scores, patient reported outcomes, and opioid analgesics use reported in milligram morphine equivalents (MME). The secondary outcomes were any kind of complication after surgery. Preoperative and postoperative radiographic measurements of component alignment and joint line obliquity were compared between groups, and the correlation between radiographic deformity and functional outcomes were assessed. The study was performed according to the strengthening the reporting of observational studies in epidemiology (STROBE) guidelines. 16
Patient Population and Ethical Considerations
A retrospective chart review of 101 patients, with diagnosed knee osteoarthritis, who underwent primary TKA by a fellowship‐trained orthopedic surgeon (David H. So) between 2020 and 2021 was performed. Patients with: (i) knee pain other than a primary diagnosis of osteoarthritis; (ii) below the age of 18; (iii) pre‐existing pain regimen managed by a third‐party pain specialist; (iv) missing patient data; and (v) pregnant patients were excluded from our study. Forty‐four patients underwent KA TKA, and 57 age‐ and sex‐matched MA patients were chosen for analysis. The decision for KA was determined by the performing surgeon and criteria included all patients who did not have: (i) any prior hardware around the knee; (ii) prior osteochondral procedures; and (iii) a contralateral mechanically aligned TKA. The study protocol was reviewed by the University of California, Irvine Institutional Review Board and approved under number 20216513. Given the retrospective nature of the study, the Institutional Review Board granted the study authors a waiver of informed consent.
Data Abstraction
Demographic variables included age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) status, and history of chronic pain and opioid use before surgery. Perioperative variables obtained included estimated blood loss, cumulative MME requirements, LOS, discharge destination, distance walked inpatient, flexion range of motion, and Boston AM‐PAC scores. Cumulative MME was calculated using the total dose of opioid analgesics administered normalized to MME. Patients who had subjective visual analogue scale (VAS) pain scores of 0–3 were treated with acetaminophen, while VAS scores of 4–7 were considered to have moderate pain and were given 5mg of oxycodone. Patients with VAS scores of 7–10 were considered to have severe pain and were given 10mg of oxycodone, and breakthrough pain not relieved with oxycodone were treated with hydromorphone. Total distance walked was a summation of the reported patient distance traveled with physical therapy teams. The postoperative outcomes variables collected were inpatient and outpatient prescribed MME, clinical complications, and range of motion as reported by the treating providers. PROMs included 3‐month postoperative subjective pain and functional outcome scores as outlined in Knee Injury and Osteoarthritis Scores (KOOS JR) and Short Form Survey (SF 12), which have been validated by previous studies. 17 , 18 , 19
KA Technique
All TKAs were performed by a fellowship trained arthroplasty surgeon (David H. So) between 2020–2021 at a single tertiary referral center. In the KA group, surgery was carried out according to the standard MicroPort Orthopedics Evolution MP system protocol, and a standard medial parapatellar approach was utilized and cruciate ligaments excised. The depth of the distal femoral condyle was measured, and appropriate corresponding distal depth resection and valgus angle cuts were made. A caliper was used to verify the thickness of the femoral resections matched the KA targeted resections. After trial femur impaction, gap spacers were placed to tension the medial and lateral compartments. The tibial varus/valgus cut angle was measured according to native deformity, and cut angle maintained within 1–2 degrees of native residual deformity that would allow for spacer block fit in both extension and flexion. Tibial component rotation was set to the junction of the medial and middle third of the tubercle, and the patella was then resurfaced.
MA Technique
The MA technique was performed as previously described in the literature utilizing computer navigation to guide measured reaction of bone with the goal of achieving overall neutral coronal limb alignment with femur and tibia cuts perpendicular to the mechanical axis of each bone. 20
Radiographic Measurements
Preoperative and postoperative full length coronal standing films were utilized to measure the hip knee ankle (HKA) angle, femoral component relative to the mechanical axis (FCRTMA), tibia component relative to the mechanical axis (TCRTMA), and joint line orientation angle (JLOA) of both TKA groups. The HKA angle was defined as the angle subtended by a line drawn from the center of the femoral head to the center of the knee and a line drawn from the center of the knee to the center of the talus. The FCRTMA and TCRTMA was defined as the angle between the MA of the femur and tibia compared with the transcondylar line of the femur and tibia components respectively. The JLOA was defined as the angle formed between the joint line and a line parallel to the floor. 21 Valgus deformity was indicated with a “‐” and varus deformity with “+.”
Statistical Analysis
Continuous variables were described with mean ± standard deviation or median (minimum, maximum), whereas categorical variables were reported with absolute and relative frequency. 22 The Wilcoxon rank‐sum test was conducted to compare continuous variables, while binary outcomes were compared using the chi‐square or Fisher exact test as appropriate. For all tests, P < 0.05 was considered statistically significant. Stata 17 (StataCorp LLC, College Station, TX, USA) was used as statistical software for all analyses.
Results
Baseline Demographics and Radiographic Measurements
The study population had a median age of 68 years, and there were no significant differences in gender, BMI, ASA status, history of chronic pain or opioid use, and estimated blood loss between the KA and MA groups (Table 1). The JLOA of the KA group was significantly more valgus at −1.5 degrees compared to the MA group at 0.2 degrees of varus (P < 0.001) (Table 2). Compared to the MA group, the KA group had a significantly more valgus FCRTMA at −1.9 degrees (P < 0.001) (Table 2). The KA group also had significantly more varus TCRTMA at 1.4 degrees compared to the MA group at −0.3 degrees of valgus (P<0.001) (Table 2).
TABLE 1.
Demographic and medical history characteristics of KA vs MA cohorts
| Kinematic axis (n = 44) | Mechanical axis (n = 57) | Mean difference | 95% CI of the difference | Chi‐square | P | |
| Age, median ± SD | 68.5 ± 7.5 | 68.2 ± 7.7 | 0.268 | (−2.770, 3.305) | 0.861 | |
| Gender, n (%) | 0.018 | 1.000 | ||||
| Male | 16 (36.4) | 20 (35.1) | ||||
| Female | 20 (63.6) | 37 (64.9) | ||||
| BMI, mean ± SD | 31.0 ± 6.6 | 32.6 ± 5.6 | −1.592 | (−4.002, 0.818) | 0.193 | |
| ASA, n (%) | 2.186 | 0.535 | ||||
| 0 | 0 (0) | 1 (1.8) | ||||
| 1 | 0 (0) | 0 (0) | ||||
| 2 | 16 (36.4) | 26 (47.3) | ||||
| 3 | 27 (61.4) | 27 (49.1) | ||||
| 4 | 1 (2.2) | 1 (1.8) | ||||
| History of chronic pain | 10 (22.7) | 21 (39.6) | 3.156 | 0.085 | ||
| History of Opioid Use | 5 (11.4) | 10 (18.9) | 1.036 | 0.402 | ||
| EBL, mean ± SD | 105.1 ± 65.9 | 83.6 ± 63.0 | 21.556 | (−4.610, 47.722) | 0.105 |
TABLE 2.
Preoperative and postoperative radiographic measurements in KA vs MA groups
| Kinematic axis (n = 44) | Mechanical axis (n = 57) | Mean difference | 95% CI of the difference | Chi‐ square | P | |
| Preoperative HKA angle | ||||||
| Degrees, Mean ± SD | 0.7 ± 8.7 | 2.6 ± 8.3 | −1.961 | (−5.788, 1.867) | 0.311 | |
| Degrees, |Mean| ± SD | 6.9 ± 5.2 | 7.3 ± 4.6 | −0.349 | (−2.558, 1.860) | 0.754 | |
| Deformity, n (%) | 0.462 | |||||
| Valgus | 21 (58.3) | 27 (65.9) | 0.638 | |||
| Varus | 15 (41.7) | 14 (34.1) | ||||
| Postoperative HKA angle | ||||||
| Degrees, Mean ± SD | −0.7 ± 3.1 | 0.3 ± 4.1 | −0.965 | (−2.584, 0.654) | 0.239 | |
| Degrees, |Mean| ± SD | 2.7 ± 1.8 | 3.0 ± 2.7 | −0.343 | (−1.398, 0.712) | 0.519 | |
| Deformity, n (%) | 0.017 | 1.000 | ||||
| Valgus | 13 (37.1) | 18 (33.3) | ||||
| Varus | 22 (62.9) | 33 (66.7) | ||||
| ∆HKA Angle (postoperative‐preoperative) | ||||||
| Degrees, Mean ± SD | −1.3 ± 8.0 | −2.3 ± 7.0 | 1.042 | (−2.332, 4.416) | 0.540 | |
| Degrees, |Mean| ± SD | 6.3 ± 4.9 | 6.1 ± 4.0 | 0.229 | (−1.791, 2.250) | 0.822 | |
| Deformity, n (%) | 0.332 | 0.619 | ||||
| Maintained | 22 (64.7) | 27 (71.1) | ||||
| Opposite | 12 (35.3) | 11 (28.9) | ||||
| Postoperative joint line angle | ||||||
| Degrees, Mean ± SD | −1.5 ± 2.0 | 0.2 ± 1.3 | −1.630 | (−2.390, −0.870) | <0.001 | |
| Degrees, |Mean| ± SD | 1.9 ± 1.6 | 0.8 ± 1.0 | 1.045 | (0.438, 1.652) | 0.001 | |
| Deformity, n (%) | 8.766 | 0.007 | ||||
| Valgus | 6 (18.2) | 12 (57.1) | ||||
| Varus | 27 (81.8) | 9 (42.9) | ||||
| Postoperative Fem component to Fem axis | ||||||
| Degrees, Mean ± SD | −1.9 ± 2.6 | 0.2 ± 2.2 | −2.066 | (−3.124, −1.007) | <0.001 | |
| Degrees, |Mean| ± SD | 2.5 ± 1.9 | 1.3 ± 1.7 | 1.176 | (0.373, 1.979) | 0.005 | |
| Deformity, n (%) | 5.625 | 0.022 | ||||
| Valgus | 9 (23.7) | 15 (51.7) | ||||
| Varus | 29 (76.3) | 14 (48.3) | ||||
| Postoperative Tib component to Tib axis | ||||||
| Degrees, Mean ± SD | 1.4 ± 1.8 | −0.3 ± 1.2 | 1.644 | (0.963, 2.325) | <0.001 | |
| Degrees, |Mean| ± SD | 1.8 ± 1.3 | 0.7 ± 1.1 | 1.108 | (0.571, 1.646) | <0.001 | |
| Deformity, n (%) | 13.092 | 0.001 | ||||
| Valgus | 7 (18.9) | 15 (65.2) | ||||
| Varus | 30 (81.1) | 8 (34.8) |
Hospital Length of Stay, Ambulation, and Opioid Consumption
Compared to the MA group, KA patients had a statistically significant shorter LOS (1.8 vs 3.1 days) and triple the postoperative day 0 median ambulation distance (20 vs 6.5 feet) (p < 0.005) (Table 3). Although KA patients had significantly more MME inpatient requirement, the cumulative overall 3‐month MME consumption was significantly less compared to the MA patients (Table 3).
TABLE 3.
Postoperative outcomes KA vs MA cohorts
| Kinematic axis (n = 44) | Mechanical axis (n = 57) | Mean difference | 95% CI of the difference | Chi‐square | Mann–Whitney U | P | |
|---|---|---|---|---|---|---|---|
| Objective outcomes | |||||||
| Discharge POD, mean ± SD | 1.8 ± 1.1 | 3.1 ± 2.2 | −1.259 | (−1.988, 0.530) | 0.001 | ||
| Morphine equivalents inpatient, mean ± SD | 128.4 ± 141.1 | 32.5 ± 24.0 | 95.936 | (52.564, 139.309) | <0.001 | ||
| Morphine equivalents 1st postop Visit, mean ± SD | 405.1 ± 317.3 | 661.6 ± 383.1 | −256.527 | (−402.922, −110.132) | 0.001 | ||
| Morphine equivalents 2nd postop Visit, mean ± SD | 44.9 ± 118.6 | 432.5 ± 559.2 | −387.589 | (−550.329, −224.850) | <0.001 | ||
| Morphine equivalents 3rd postop visit, mean ± SD | 21.7 ± 85.8 | 216.0 ± 433.4 | −194.325 | (−325.534, −63.117) | 0.005 | ||
| Cumulative morphine equivalents, mean ± SD | 578.1 ± 397.3 | 1252.8 ± 998.0 | −675.692 | (−970.573, −378.811) | <0.001 | ||
| POD0 distance, median (min, max) | 20.0 (0, 300) | 6.5 (0, 160) | 801.5 | 0.039 | |||
| % Gait assistance, median (min, max) | |||||||
| POD0 | 25.0 (0, 100.0) | 25.0 (0, 75.0) | 805.5 | 0.748 | |||
| POD1 | 25.0 (0, 37.5) | 25.0 (0, 37.5) | 933.0 | 0.774 | |||
| POD2 | 25.0 (0, 75.0) | 25.0 (0, 37.5) | 388.5 | 0.510 | |||
| Discharge destination, n (%) | 0.109 | 0.743 | |||||
| Home | 40 (90.9) | 51 (92.7) | |||||
| Not Home | 4 (9.1) | 4 (7.3) | |||||
| Complications | |||||||
| Surgical site infection, median (min, max) | 0 (0, 0) | 0 (0, 1) | 1166.0 | 0.367 | |||
| Postop pulmonary complication, median (min, max) | 0 (0, 0) | 0 (0, 1) | 1122.0 | 0.358 | |||
| Postop cardiac complication, median (min, max) | 0 (0, 0) | 0 (0, 1) | 1100.0 | 0.191 | |||
| Subjective outcomes | |||||||
| Boston PAC score, mean ± SD | |||||||
| POD0 | 15.9 ± 3.0 | 16.3 ± 2.8 | −0.391 | (−1.597, 0.815) | 0.521 | ||
| POD1 | 17.2 ± 3.0 | 17.2 ± 2.5 | 0.044 | (−2.792, 2.880) | 0.975 | ||
| POD2 | 16.9 ± 2.8 | 18.5 ± 0.7 | −1.630 | (−5.862, 2.601) | 0.434 | ||
| VAS pain score, median (min, max) | |||||||
| POD0 | 5.0 (0, 9) | 4.0 (0, 10) | 1169.5 | 0.662 | |||
| POD1 | 5.5 (0, 10) | 5.0 (0, 9) | 1151.5 | 0.859 | |||
| POD2 | 5.0 (0, 9) | 6.0 (0, 10) | 562.0 | 0.985 | |||
| KOOS interval, mean ± SD | 76.5 ± 16.2 | 83.0 ± 12.1 | −0.425 | (−13.557, 12.708) | 0.261 | ||
| PCS‐12, mean ± SD | 33.9 ± 9.5 | 40.8 ± 10.7 | −6.872 | (−15.110, 1.366) | 0.098 | ||
| MCS‐12, mean ± SD | 52.2 ± 7.5 | 48.5 ± 10.4 | 3.710 | (−3.735, 11.156) | 0.315 |
Mobility Scores and Patient Reported Outcomes
There were no significant differences among the groups in discharge destination, % gait assistance during therapy gait sessions, Boston AM‐PAC scores, and VAS pain scores. There were no significant differences between 3‐month postoperative KOOS or SF‐12 outcomes, and surgical complication rate, including surgical site infections and cardiac or pulmonary complications, was similar between groups (Table 3). Postoperative flexion ROM measurements and interval change compared to the preoperative measurements were also similar between alignment groups (Table 4).
TABLE 4.
Preoperative and postoperative range of motion KA vs MA cohorts
| Kinematic axis (n = 44) | Mechanical axis (n = 57) | Mean difference | 95% CI of the difference | P | |
| Preoperative ROM, mean ± SD | 103.9 ± 22.0 | 105.9 ± 16.6 | −2.049 | (−9.772, 5.675) | 0.600 |
| 1st Postop Visit ROM, mean ± SD | 92.7 ± 10.4 | 90.3 ± 14.2 | 2.392 | (−2.816, 7.599) | 0.364 |
| 2nd Postop Visit ROM, mean ± SD | 107.2 ± 14.3 | 108.0 ± 11.8 | −0.814 | (−6.293, 4.665) | 0.769 |
| 3rd Postop Visit ROM, mean ± SD | 116.2 ± 10.8 | 114.3 ± 11.4 | 1.911 | (−3.088, 6.910) | 0.449 |
| ∆ROM (1st postop‐preop), mean ± SD | −11.3 ± 22.8 | −14.1 ± 17.0 | 2.753 | (−5.592, 11.098) | 0.514 |
| ∆ROM (2nd postop‐preop), mean ± SD | 3.1 ± 20.2 | 2.4 ± 18.9 | 0.635 | (−7.650, 8.919) | 0.879 |
| ∆ROM (3rd postop‐preop), mean ± SD | 8.6 ± 13.6 | 8.4 ± 16.3 | 0.225 | (−6.676, 7.125) | 0.948 |
Discussion
As previous studies have demonstrated mixed results in terms of outpatient long‐term follow‐up PROMs, this study focuses on the acute perioperative improvements seen in opioid consumption, ambulation distance, and LOS between KA and MA patients. 23 , 24 Management of pain is an important part of patient satisfaction and care, and identifying techniques to reduce postoperative opioid dependence improves the physician‐patient communication on expected outcomes and discharge planning. In this retrospective review, we assess inpatient and outpatient opioid consumption, subjective functional outcome scores, and rehabilitation mobility as assessed by a physical therapist in order to understand the dynamics of KA versus MA on short‐ and long‐term recovery. While the consensus on sustained 10 to 15 year subjective knee functional scores and survivorship of KA TKA is still unknown, this study aims to highlight the benefits of KA in terms of reducing hospital costs and faster rehabilitation in a time where it is important for surgeons to identify ways to shorten LOS.
Inpatient and Outpatient Opioid Consumption
Although KA patients had increased inpatient opioid requirements, the cumulative 3‐month postoperative opioid consumption among KA patients was less than MA patients. The decreased MME requirement is clinically significant since fewer opioids overall decreases the risk of respiratory complications, falls, nausea, vomiting, urinary retention, and cognitive impairment, which are side effects that can significantly prolong the rehabilitation process and increase medical costs. 25 No prior studies have evaluated pain after KA TKA as a function of opioid consumption, and our results may reflect the ability of KA to reestablish more normal native knee kinematics with preserving the soft tissue envelope and minimizing the frequency of ligament release. 26 , 27 By restoring the knee to the more normal pre‐arthritic alignment that the patient has been accustomed to throughout the years, our patients may have had fewer overall instances of moderate and severe pain requiring opioid relief within the 3‐month follow‐up. 28 Perhaps the reason why our cohorts had no significant difference in average overall VAS pain scores may be explained by the generally low pain at rest for both groups, as the daily VAS score for each group was recorded as an average mostly while resting in bed rather than during therapy.
Rehabilitation Mobility
In fact, the KA patients ambulated nearly twice as much compared to the MA patients on the same day of surgery. Perhaps by restoring the natural laxity of the knee with minimal ligament releases, KA patients were able to recover faster and had improved gait endurance. 29 By reproducing the patient‐specific femoral‐tibial joint line orientation and native soft tissue balance and physiologic strain, it is possible our KA patients had improved postoperative day 0 ambulation by reducing the occurrence of knee‐balance related complications and poor kinematics that traditionally affect MA TKA. 30 Similarly, Dossett et al. found KA patients walked on average 50 feet further than MA patients prior to hospital discharge, suggesting the role of maintaining native alignment for better pain relief and function. 13 It is possible the increased exertion and faster clearance of ambulating 150 feet in our KA group may be related to the increased inpatient opioid consumption in our KA group compared to MA. The MA patients were slower to clear physical therapy and thus may have exerted themselves less than the KA group and had less moderate–severe pain requiring opioid relief. In addition, although we predicted the KA group to have significantly improved % gait assistance when ambulating with physical therapy due to proposed restoration of native kinematics, there was no significant difference compared to MA patients. However, the reason why our cohorts had no significant difference in % gait assistance may be explained by the generally minimal gait assistance <25% in both groups as evaluated by a therapist. Furthermore, although the Boston AM‐PAC scores, which evaluate amount of dependence needed to complete activities of daily living, were not significantly improved in the KA group, the Boston AM‐PAC scores evaluate tasks such as bed mobility and upper body grooming, which may not necessarily reflect functional recovery after TKA.
Discharge Disposition
Overall reduced opioid intake combined with faster return to baseline ambulation may contribute to greater overall participation during therapy sessions that leads to earlier and safer discharge to home. 31 In our study, KA patients were discharged on average 1.3 days sooner than MA patients, which is clinically significant as the extended stay is important to consider from a billing, hospital bed space, and hospital quality metrics perspective. 32 In our patients, a combination of safe ambulation as deemed by a therapist and well‐controlled pain on oral medications are major requirements for discharge to home after TKA. Compared to MA, our KA patients were able to reach criteria for discharge faster which is in alignment with recent emphasis on enhanced recovery after surgery protocols to improve the efficiency of postoperative care, reduce the risk of nosocomial infections, and limit hospital complications.
Functional Outcomes
Although previous studies found KA patients to have better knee ROM by minimizing the need for complex ligament releases, our study did not show any significant inpatient or 3‐month follow‐up differences in knee arc ROM. 13 , 14 , 33 Our findings are in alignment with Waterson et al. who found no statistically significant differences in ROM, timed up and go, and two‐minute distance tolerances between alignment groups. 12 While previous studies suggested early functional recovery in the KA group with significantly improved peak torque in quadriceps and hamstrings, the sustained improvements were not significant at 1‐year follow‐up, which may reflect our findings of only early significant benefit in functional recovery from KA. 12 , 20 In fact, even with PROMs, there are conflicting results in terms of 1, 2, and 5 year follow‐up of KA patients in terms of KOOS, EQ‐5D, WOMAC, and OKS scores between KA and MA. 11 , 13 , 14 , 20
Similar to prior studies, KA patients on postoperative radiographic measurements had statistically significant residual TCRTMA in varus, FCRTMA in valgus, and JLOA in valgus compared with MA patients. 13 , 21 While this study suggests KA may help shorten inpatient LOS, reduce opioid consumption, and improve postoperative day 0 ambulation distance, this study does not evaluate the long‐term outcome and survivability of implants. Although our KA patients had tibial components inserted in varus, surgeons should be cautious in potential long‐term concerns over loosening as biomechanical evidence suggests varus tibial alignment increases contact stresses and bone‐implant loads. 34 Despite concerns over residual varus implantation resulting in increased polyethylene wear and stresses, the orientation of the joint line remains relatively parallel to the floor on a long‐leg weight bearing radiograph despite the range of obliquity to the mechanical axis. 35
Strengths and Limitations
There are several limitations to consider in this study, including its retrospective design, nonrandomized nature, and lack of blinding of patients which may limit the generalizability of our results. Due to the relatively new implementation of the technique performed in a single surgeon cohort, there were limited number of patients in the analysis and many PROMs were not fully completed. The patients were not blinded to the planned alignment technique performed and determined by the operating surgeon which may influence patient reported satisfaction outcomes. It is possible that many outcomes deemed not statistically significant may not have been powered to elucidate associations due to the small sample size. In addition, although outpatient opioids prescribed may have been used for other sources of pain and may not truly reflect the actual use of the narcotic, prescribed amounts of MME at clinic visits were used in this study as reflective of opioid requirements. Strengths of the study include surgeries performed by a single surgeon within a short time frame and the uniformity of postoperative ROM measurements by the same provider, which help decrease variations of surgeon specific techniques and postoperative protocols.
Conclusion
With the emphasis on enhanced recovery protocols and return to early functional recovery, a KA TKA may help facilitate out of bed mobilization, reduce overall opioid consumption, and shorten LOS. By restoring the native joint line obliquity and minimizing the frequency of ligament releases, KA for TKA may improve pain relief and rehabilitation compared with traditional methods of establishing neutral limb axis by MA. KA may contribute to greater early participation during therapy sessions that lead to shorter inpatient hospitalization and reduced costs.
Author Contributions
All contributing authors participated in the study formulation and design. Megan R. Donnelly, Kylie Callan, Maddison McLellan, Arya Amirhekmat participated in initial study preparation, data collection, and statistical analysis. The initial draft of the manuscript was written by Brandon E. Lung. The draft was edited by William C. McMaster, Steven Yang, and David H. So. All authors commented on previous versions of the manuscript, and all authors read and approved the final manuscript.
References
- 1. Scott CEH, Howie CR, MacDonald D, Biant LC. Predicting dissatisfaction following total knee replacement: a prospective study of 1217 patients. J Bone Jt Surg. 2010;92:1253–8. 10.1302/0301-620X.92B9.24394 [DOI] [PubMed] [Google Scholar]
- 2. Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KDJ. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468:57–63. 10.1007/s11999-009-1119-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robertsson O, Dunbar M, Pehrsson T, Knutson K, Lidgren L. Patient satisfaction after knee arthroplasty: a report on 27,372 knees operated on between 1981 and 1995 in Sweden. Acta Orthop Scand. 2000;71:262–7. 10.1080/000164700317411852 [DOI] [PubMed] [Google Scholar]
- 4. Noble PC, Conditt MA, Cook KF, Mathis KB. The John Insall award: patient expectations affect satisfaction with total knee arthroplasty. Clin Orthop Relat Res. 2006;452:35–43. [DOI] [PubMed] [Google Scholar]
- 5. Gandhi R, Davey JR, Mahomed NN. Predicting patient dissatisfaction following joint replacement surgery. J Rheumatol. 2008;35:2415–8. 10.3899/jrheum.080295 [DOI] [PubMed] [Google Scholar]
- 6. Kim TK, Chang CB, Kang YG, Kim SJ, Seong SC. Causes and predictors of patient's dissatisfaction after uncomplicated total knee arthroplasty. J Arthroplasty. 2009;24:263–71. 10.1016/j.arth.2007.11.005 [DOI] [PubMed] [Google Scholar]
- 7. Nam D, Nunley RM, Barrack RL. Patient dissatisfaction following total knee replacement: a growing concern? Bone Jt J. 2014;96:96–100. 10.1302/0301-620X.96B11.34152 [DOI] [PubMed] [Google Scholar]
- 8. Bellemans J, Colyn W, Vandenneucker H, Victor J. The Chitranjan Ranawat award: is neutral mechanical alignment normal for all patients? The concept of constitutional varus. Clin Orthop Relat Res. 2012;470(1):45–53. 10.1007/s11999-011-1936-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parratte S, Pagnano MW, Trousdale RT, Berry DJ. Effect of postoperative mechanical axis alignment on the fifteen‐year survival of modern, cemented total knee replacements. J Bone Jt Surg. 2010;92(12):2143–9. 10.2106/JBJS.I.01398 [DOI] [PubMed] [Google Scholar]
- 10. Otsuki T, Nawata K, Okuno M. Quantitative evaluation of gait pattern in patients with osteoarthrosis of the knee before and after total knee arthroplasty. Gait analysis using a pressure measuring system. J Orthop Sci. 1999;4:99–105. [DOI] [PubMed] [Google Scholar]
- 11. Young SW, Sullivan NPT, Walker ML, Holland S, Bayan A, Farrington B. No difference in 5‐year clinical or radiographic outcomes between kinematic and mechanical alignment in TKA: a randomized controlled trial. Clin Orthop Relat Res. 2020;478(6):1271–9. 10.1097/CORR.0000000000001150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Waterson HB, Clement ND, Eyres KS, Mandalia VI, Toms AD. The early outcome of kinematic versus mechanical alignment in total knee arthroplasty: a prospective randomised control trial. Bone Jt J. 2016;98(10):1360–8. 10.1302/0301-620X.98B10.36862 [DOI] [PubMed] [Google Scholar]
- 13. Dossett HG, Estrada NA, Swartz GJ, LeFevre GW, Kwasman BG. A randomised controlled trial of kinematically and mechanically aligned total knee replacements: two‐year clinical results. Bone Jt J. 2014;96:907–13. 10.1302/0301-620X.96B7.32812 [DOI] [PubMed] [Google Scholar]
- 14. Gao ZX, Long NJ, Zhang SY, Yu W, Dai YX, Xiao C. Comparison of kinematic alignment and mechanical alignment in total knee arthroplasty: a meta‐analysis of randomized controlled clinical trials. Orthop Surg. 2020;12(6):1567–78. 10.1111/os.12826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Urban JA, Dolesh K, Martin E. A multimodal pain management protocol including preoperative cryoneurolysis for total knee arthroplasty to reduce pain, opioid consumption, and length of stay. Arthroplast Today. 2021;12(10):87–92. 10.1016/j.artd.2021.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–9. 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 17. Mihalko WM, Kerkhof AL, Ford MC, Crockarell JR, Harkess JW, Guyton JL. Cryoneurolysis before total knee arthroplasty in patients with severe osteoarthritis for reduction of postoperative pain and opioid use in a single‐center randomized controlled trial. J Arthroplasty. 2021;36:1590–8. 10.1016/j.arth.2020.11.013 [DOI] [PubMed] [Google Scholar]
- 18. Dasa V, Lensing G, Parsons M, Harris J, Volaufova J, Bliss R. Percutaneous freezing of sensory nerves prior to total knee arthroplasty. Knee. 2016;23:523–8. 10.1016/j.knee.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 19. Lyman S, Lee YY, Franklin PD, Li W, Cross MB, Padgett DE. Validation of the KOOS, JR: a short‐form knee arthroplasty outcomes survey. Clin Orthop Relat Res. 2016;474(6):1461–71. 10.1007/s11999-016-4719-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Young SW, Walker ML, Bayan A, Briant‐Evans T, Pavlou P, Farrington B. The Chitranjan S. Ranawat Award: no difference in 2‐year functional outcomes using kinematic versus mechanical alignment in TKA: a randomized controlled clinical trial. Clin Orthop Relat Res. 2017;475(1):9–20. 10.1007/s11999-016-4844-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yoon JR, Han SB, Jee MK, Shin YS. Comparison of kinematic and mechanical alignment techniques in primary total knee arthroplasty: a meta‐analysis. Medicine. 2017. Sep;96(39):e8157. 10.1097/MD.0000000000008157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sappey‐Marinier E, Pauvert A, et al. Kinematic versus mechanical alignment for primary total knee arthroplasty with minimum 2 years follow‐up: a systematic review. SICOT J. 2020;6:18. 10.1051/sicotj/2020014 Epub 2020 Jun 17. PMID: 32553101; PMCID: PMC7301633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu B, Feng C, Tu C. Kinematic alignment versus mechanical alignment in primary total knee arthroplasty: an updated meta‐analysis of randomized controlled trials. J Orthop Surg Res. 2022;17(1):201. 10.1186/s13018-022-03097-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oderda GM, Said Q, Evans RS, et al. Opioid‐related adverse drug events in surgical hospitalizations: impact on costs and length of stay. Ann Pharmacother. 2007;41:400–7. [DOI] [PubMed] [Google Scholar]
- 26. Howell SM, Papadopoulos S, Kuznik K, et al. Does varus alignment adversely affect implant survival and function six years after kinematically aligned total knee arthroplasty? Int Orthop. 2015;39:2117–24. [DOI] [PubMed] [Google Scholar]
- 27. Hutt J, LeBlanc MA, Massé V, et al. Kinematic TKA using navigation: surgical technique and initial results. Orthop Traumatol Surg Res. 2016;102:99–104. [DOI] [PubMed] [Google Scholar]
- 28. Vanlommel L, Vanlommel J, Claes S, Bellemans J. Slight undercorrection following total knee arthroplasty results in superior clinical outcomes in varus knees. Knee Surg Sports Traumatol Arthrosc. 2013;21(10):2325–30. 10.1007/s00167-013-2481-4 [DOI] [PubMed] [Google Scholar]
- 29. Gu Y, Roth JD, Howell SM, Hull ML. How frequently do four methods for mechanically aligning a total knee arthroplasty cause collateral ligament imbalance and change alignment from normal in white patients? J Bone Jt Surg. 2014;96(12):e101. [DOI] [PubMed] [Google Scholar]
- 30. Blakeney W, Clément J, Desmeules F, Hagemeister N, Rivière C, Vendittoli PA. Kinematic alignment in total knee arthroplasty better reproduces normal gait than mechanical alignment. Knee Surg Sports Traumatol Arthrosc. 2019;27(5):1410–7. 10.1007/s00167-018-5174-1 [DOI] [PubMed] [Google Scholar]
- 31. Tess BH, Glenister HM, Rodrigues LC, Wagner MB. Incidence of hospital‐acquired infection and length of hospital stay. Eur J Clin Microbiol Infect Dis. 1993;12(2):81–6. 10.1007/BF01967579 [DOI] [PubMed] [Google Scholar]
- 32. Mukand JA, Cai C, Zielinski A, Danish M, Berman J. The effects of dehydration on rehabilitation outcomes of elderly orthopedic patients. Arch Phys Med Rehabil. 2003;84(1):58–61. 10.1053/apmr.2003.50064 [DOI] [PubMed] [Google Scholar]
- 33. Howell SM, Hull ML. Kinematic alignment in total knee arthroplasty. In: Scott WN, editor. Insall and Scott Surgery of the Knee. Philadelphia, PA: Elsevier; 2012. p. 1255–68. [Google Scholar]
- 34. Ishikawa M, Kuriyama S, Ito H, Furu M, Nakamura S, Matsuda S. Kinematic alignment produces near‐normal knee motion but increases contact stress after total knee arthroplasty: a case study on a single implant design. Knee. 2015;22:206–12. [DOI] [PubMed] [Google Scholar]
- 35. Victor JM, Bassens D, Bellemans J, et al. Constitutional varus does not affect joint line orientation in the coronal plane. Clin Orthop Relat Res. 2014;472:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]


