Abstract
The loss of skeletal muscle mass and function is defined as sarcopenia, which might develop in elderly patients with cancers. It has been indicated as a potential negative factor in the survival of patients with malignant tumours. The aim of this systematic review and meta‐analysis was to evaluate the associations between sarcopenia and survival outcomes or postoperative complications in patients with oesophageal cancer (EC). Web of Science, Embase, Medline, and Cochrane Library databases were searched until 10 May 2022, using keywords: sarcopenia, oesophageal cancer, and prognosis. Studies investigating the prognostic value of sarcopenia on EC survival were included. Forest plots and summary effect models were used to show the result of this meta‐analysis. The quality of included studies was evaluated with the Newcastle‐Ottawa Scale (NOS). A total of 1436 studies were identified from the initial search of four databases, and 41 studies were included for the final quantitative analysis. This meta‐analysis revealed a significant association between sarcopenia and overall survival (OS) [hazard ratios (HR):1.68, 95% confidence interval (CI):1.54–1.83, P = 0.004, I 2 = 41.7%] or disease‐free survival (DFS) 1.97 (HR: 1.97, 95% CI: 1.44–2.69, P = 0.007, I 2 = 61.9%) of EC patients. Subgroup analysis showed that sarcopenia remained a consistent negative predictor of survival when stratified by different treatment methods, populations, or sarcopenia measurements. Sarcopenia was also a risk factor for postoperative complications with a pooled odds ratio of 1.47 (95% CI: 1.21–1.77, P = 0.094, I 2 = 32.7%). The NOS scores of all included studies were ≥6, and the quality of the evidence was relatively high. The results from the study suggested that sarcopenia was significantly associated with both survival outcomes and postoperative complications in EC patients. Sarcopenia should be appropriately diagnosed and treated for improving short‐term and long‐term outcomes of patients with EC.
Keywords: Sarcopenia, Oesophageal cancer, Skeletal muscle index, Prognosis, Meta‐analysis
Introduction
Oesophageal cancer (EC), one of the most malignant tumours, is the 6th most deadly cancer around the world. 1 The two main pathological subtypes of EC are oesophageal squamous cell carcinoma (ESCC) and oesophageal adenocarcinoma (EAC), which are most prevalent in Eastern Asia and Western countries, respectively. 2 Despite the significant improvement in treatment strategies during the last decade, the prognosis of patients with EC remained relatively poor, with below 30% 5 year survival rate. 3 , 4 Therefore, identifying a specific predictor of survival outcome and postoperative complication risk is vital for improving the outcome of EC patients. The introduction of novel predictors of prognosis is helpful for the decision‐making of therapy and overall risk stratification.
Loss of muscle mass, commonly referred to as sarcopenia, is an essential feature of patients with malnutrition. Sarcopenia is usually found in elderly people with cancer, which is caused by a negative balance between insufficient food intake and high tumour metabolism. 5 Various methods have been utilized to evaluate sarcopenia. The skeletal muscle index (SMI) is a commonly used method to measure sarcopenia, calculated as the total muscle area observed on computed tomography (CT) scan at the level of the third lumbar vertebra divided by the height squared.
Patients with EC are likely to present with reduced food intake because of tumour blocking or anatomical changes after esophagogastrostomy. Thus, patients with EC are prone to develop sarcopenia because of insufficient nutritional support. 6 It was reported that sarcopenia had a high incidence rate of 75% for EC patients, 7 and early studies had indicated that sarcopenia was a risk factor for postoperative complications and EC prognosis. 8 , 9 , 10 However, considering the limited sample size in single clinical research, the impact of sarcopenia on postoperative complications and survival of EC patients needed to be further investigated through the meta‐analysis of all available data. Deng et al. conducted a meta‐analysis to detect sarcopenia effects on long‐term survival of patients with EC, 11 however, they only included 11 studies with 1520 patients, and the selected studies were dated up to 2018. In addition, Uzair et al. 12 had conducted another meta‐analysis, including 21 studies published before 2020 about sarcopenia assessed solely by CT‐based body imaging and included patients who underwent surgery with curative intent. Therefore, with more up to date research, this study aimed to make a significant contribution to quantitatively summarize all the existing evidence and achieve a better understanding of the clinical significance of sarcopenia with regard to prognosis and postoperative complication prediction in patients with EC.
Materials and methods
Search design
This systemic review and meta‐analysis were conducted according to the Preferred Reporting Items for Systematic reviews and Meta‐Analyses (PRISMA statement). 13 Web of Science, Embase, Medline, and Cochrane Library were independently searched. The search was completed on 18 April 2022. Case reports, reviews, expert opinions, and conference abstracts were excluded.
Study selection
The included studies met the following criteria: (i) studies of patients who were pathologically diagnosed with EC; (ii) studies must report the measurement of sarcopenia; (iii) studies divided patients into different groups according to the standard of sarcopenia; (iv) studies reported hazard ratios (HRs) or odds ratios (ORs) of postoperative complications, survival outcomes of patients with EC and 95% confidence intervals (95% CIs); (v) The Newcastle‐Ottawa Scale (NOS) scores of studies were ≥6. During the selection process, studies were excluded if (i) the study duplicated research; (ii) patients had non‐oesophageal cancer or had no pathological diagnosis; (iii) animal experiments; (iv) studies reporting incomplete data. Two researchers (P. F. and J. Z.) searched the databases independently, and each was blinded to the other's results. If disagreements occurred, the results were delivered to the third researcher (X. X.) to evaluate and come to an agreement.
Data extraction
Data extraction was conducted independently by P. F. and J. Z. The following data were retrieved: first author name, publication year, sample size, cancer types, tumour stage, the measurement of sarcopenia, correlated HRs and ORs of postoperative complications with 95% CIs, and other factors. The details of included studies are shown in Table 1. HRs were primarily extracted from multivariate analyses, otherwise from univariate analyses or software to extract from the survival analysis charts.
Table 1.
Basic information of included studies
| Name | Year | N (Male/Female) | Age | Population | Cancer type | Treatment modalities | Stage | Measurement | Result | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|
| Benadon 36 | 2020 | 104 (72/32) | Mean 63 | French | EC | nCRT and surgery | I‐IV | SMI | OS | 7 |
| Boshier 37 | 2021 | 97 (74/23) | 63.2 ± 10.8 | American and British | EC | Surgery and CRT | I‐IV | SMI | OS | 6 |
| Elliott 7 | 2017 | 192 (156/36) | 61.6 ± 9.3 | Irish | EC | nCRT and surgery | I‐IV | SMI | OS, DFS | 6 |
| Fehrenbach 38 | 2021 | 85 (75/10) | 64.3 ± 9.8 | German | EAC | nCRT and surgery | I‐IV | SMI | OS, DFS | 7 |
| Gabiatti 39 | 2019 | 123 (108/15) | 59.3 ± 11.7 | Brazilian | EC | CRT | NA | SMI | OS, PFS | 6 |
| Grotenhuis 40 | 2016 | 120 (88/32) | 19–78; median 62 | Dutch | EC | nCRT and surgery | 0‐IV | SMI | OS | 7 |
| Harada 41 | 2016 | 325 (298/27) | <66: 153; ≥66: 172 | Japanese | ESCC | dCRT and surgery | I‐IV | SMI | OS | 8 |
| Huang 42 | 2020 | 107 (101/6) | 54.1 ± 7.5 | Chinese | ESCC | nCRT and surgery | I‐III | SMI | OS, DFS | 7 |
| Ishida 43 | 2021 | 333 (294/39) | 35–83 | Japanese | EC | NAC and surgery | I‐IV | PMI | OS | 8 |
| Jarvinen 44 | 2018 | 118 (NA/NA) | 65.8 ± 9.9 | Finn | EC | Surgery and chemoradiotherapy | NA | SMI | OS | 7 |
| Jarvinen 45 | 2018 | 115 (86/29) | 63 ± 9 | Finn | EC | nCRT and surgery | I‐IV | SMI | OS, RFS | 7 |
| Kamitani 46 | 2019 | 90 (77/13) | NA | Japanese | EC | nCRT and surgery | I‐III | SMI | OS | 6 |
| Liu 47 | 2016 | 84 (72/12) | <65: 51; ≥65: 33 | Japanese | ESCC | nCRT and surgery | I‐III | PMI | OS | 6 |
| Ma 48 | 2018 | 198 (190/8) | 36–91; median 67 | Korean | EC | cCRT and surgery | I‐IV | SMI | OS | 8 |
| Maeda 49 | 2020 | 72 (65/7) | 43–83; median 66 | Japanese | ESCC | Surgery and CRT | I‐IV | PMI | OS, PFS | 6 |
| Makiura 50 | 2017 | 98 (83/15) | 43–83; median 67 | Japanese | EC | nCRT and surgery | I‐IV | PMI, grip strength and gait speed | OS | 7 |
| Mallet 51 | 2020 | 97 (81/16) | 63.61 ± 11.12 | Japanese | EC | CRT | I‐IV | SMI | OS | 7 |
| Matsunaga 14 | 2019 | 163 (128/35) | 64.7 ± 8 | Japanese | EC | nCRT and surgery | 0‐IV | SMM | OS, RFS | 7 |
| Mayanagi 10 | 2017 | 66 (57/9) | 63.3 ± 8 | Japanese | EC | Neoadjuvant chemotherapy and surgery | I‐IV | SMI | OS, RFS | 6 |
| Nakashima 9 | 2018 | 175 (147/28) | <65 | Japanese | EC | nCRT and surgery | 0‐IV | SMI | OS | 8 |
| Nakashima 9 | 2018 | 166 (142/24) | ≥65 | Japanese | EC | nCRT and surgery | 0‐IV | SMI | OS | 8 |
| Nishigori 52 | 2016 | 199 (164/35) | NA | Japanese | ESCC | Neoadjuvant chemotherapy and surgery | II‐IV | SMI | OS | 7 |
| Ozawa 53 | 2019 | 194 (171/23) | 43–86; mean 64.1 | Japanese | ESCC | Surgery | I‐IV | SMI | OS, DFS | 8 |
| Ozawa 54 | 2019 | 82 (71/11) | 63.5 ± 7.5 | Japanese | ESCC | nCRT and surgery | I‐IV | PMI | DFS | 6 |
| Paireder 55 | 2016 | 130 (106/24) | 30.8–81; median 61.4 | Austrian | EC | nCRT and surgery | I‐IV | SMI | OS, DFS | 7 |
| Panje 56 | 2019 | 60 (56/4) | 38–75; median 61 | Swiss | EC | Surgery and CRT | I‐IV | SMI | OS | 6 |
| Park 57 | 2017 | 124 (NA/NA) | 63.38 ± 8.47 | Korean | EC | Surgery | I‐III | PMA | OS | 7 |
| Saeki 58 | 2018 | 157 (122/35) | Mean: 64.9 for sarcopenic patients and 63.3 for nonsarcopenic patients | Japanese | EC | nCRT and surgery | II‐III | SMI | OS | 7 |
| Sakai 59 | 2021 | 89 (77/12) | 42–81; mean 65 | Japanese | EC | Surgery | I‐IV | SMI | OS | 6 |
| Sato 60 | 2018 | 48 (32/16) | Mean: 65.5 for sarcopenic patients and 70 for nonsarcopenic patients | Japanese | EC | CRT | III | SMI | OS | 6 |
| Siegal 61 | 2018 | 173 (144/29) | Mean: 65.7 for sarcopenic patients and 65.4 for nonsarcopenic patients | American | EC | nCRT and surgery | 0‐III | SMI | OS, DFS | 8 |
| Soma 62 | 2018 | 102 (89/13) | Mean: 69.2 for sarcopenic patients and 65.8 for nonsarcopenic patients | Japanese | ESCC | nCRT and surgery | I‐IV | SMI | NA | 6 |
| Srpcic 63 | 2020 | 139 (117/22) | 63.9 ± 9.5 | Slovenian | EC | nCRT and surgery | I‐IV | SMI | OS | 7 |
| Sugawara 64 | 2020 | 411 (350/61) | 41–92 | Japanese | EC | Surgery and CRT | 0‐III | SMI | OS | 8 |
| Takahashi 65 | 2019 | 316 (265/51) | 71 ± 4.4 | Japanese | EC | nCRT and surgery | 0‐IV | SMI | OS, RFS | 8 |
| Takahashi 66 | 2021 | 229 (199/30) | NA | Japanese | EC | nCRT and surgery | I‐IV | SMI | OS, RFS | 7 |
| Tamandl 8 | 2016 | 200 (151/49) | 56.6–70; median 63.9 | Austrian | EC | Neoadjuvant chemotherapy and surgery | I‐IV | SMI | OS | 8 |
| Wakefield 67 | 2021 | 52 (45/7) | Mean: 66 for sarcopenic patients and 62 for nonsarcopenic patients | American | EC | nCRT and surgery | 0‐IV | SMI | OS, DFS | 7 |
| Watanabe 68 | 2022 | 131 (113/18) | 43–82; median 67 | Japanese | EC | Surgery and CRT | I‐IV | SMI | OS, DFS | 6 |
| Yip 69 | 2014 | 35 (30/5) | 34–78; mean 63 | British | EC | nCRT and surgery | II‐IV | SMI | OS | 7 |
| Yoon 70 | 2020 | 248 (NA/NA) | 63.46 ± 7.63 | Korean | ESCC | nCRT and surgery | I‐IV | SMI | OS | 8 |
Note: All stages in the table were divided according to the latest version of TNM staging in the corresponding study year.
Abbreviations: nCRT, neoadjuvant chemoradiotherapy; cCRT, concurrent chemoradiotherapy; dCRT, definitive chemoradiotherapy; CRT, chemoradiotherapy; NAC, neoadjuvant therapy; EC, oesophageal cancer; ESCC, oesophageal squamous cell cancer; EAC, oesophageal adenocarcinoma; OS, overall survival; DFS, disease‐free survival; PFS, progression‐free survival; RFS, recurrence‐free survival; CT, computed tomography; SMI, skeletal muscle index; SMM, skeletal muscle mass; BMI, body mass index; PMI, psoas muscle index; IMAC, intracellular muscle adipose tissue content; SD, standard deviation; NA, information not afforded.
Quality assessment
Quality assessment of the included studies was evaluated according to the standard of the NOS. Studies with scores of ≥6 were considered of high quality. Two researchers extracted data independently to reduce risk and other biases. After completion of data extraction, all the search findings and the review processes were evaluated by the third researcher, who also determined the inclusion criteria of the studies.
Statistical analysis
Forest plots were conducted to show the effect size of selected studies in our meta‐analysis and to visualize the results. In the forest plot, the effects size of each included study was utilized to pool an effects summary using a proper effects model. I 2 statistics were used to test the heterogeneity among studies. When the heterogeneity analysis was completed, an appropriate effects summary model was chosen to analyse according to the I 2 value. If the I 2 value was ≥50%, we considered that the study had significant heterogeneity, and the random‐effects model was used to pool the effect size (I 2 ≥ 50%). If the I 2 value was <50%, we considered that the research heterogeneity was acceptable (I 2 < 50%), and the fixed‐effects model was applied. The log HR and OR with their standard errors (SE) were recognized as effects size to pool in summary, with the pooled results value showing the influence of sarcopenia on EC prognosis. Generally, the pooled HR and OR values of >1 indicated worse survival and risk factors. To test the reliability of the pooled effects size in the meta‐analysis, a sensitivity analysis of the included studies was conducted by removing one single study in turn. To estimate the publication bias among the studies, Begg's test was applied by assessing the P value and asymmetry of an inverted funnel plot, and it is considered that there was no publication bias among the included studies when the P value was >0.05. For studies that had not directly reported the exact value of HRs, the Engauge Digitizer was utilized to retrieve the data from the survival curves. Analysis of the data in this meta‐analysis was conducted by utilizing the STATA 12.0 software package.
Results
Search results
The flow chart shown in Figure 1 demonstrates the selection of studies included in this meta‐analysis. A total of 1436 studies were found through the initial search of the electronic database. After removing duplicates, 713 studies were excluded. When reviewing the titles and abstracts, 637 studies were further excluded because of irrelevant contents, and the last 86 relevant studies underwent full‐text screening. After the full‐text screening, 45 studies were excluded for ineligibility because of failure to meet the inclusion criteria or because the data in the studies could not be retrieved. Ultimately, a total of 41 studies were included in this meta‐analysis (Figure 1).
Figure 1.
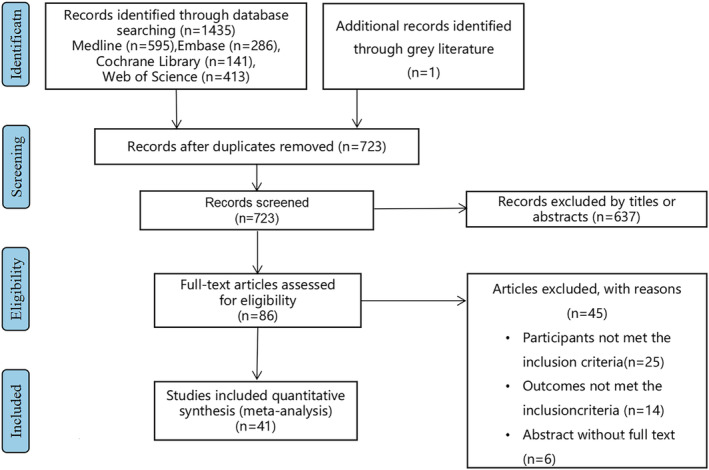
The flow diagram indicated the process of study selection.
Study characteristics
Table 1 shows the main characteristics and details extracted from all included studies. The total number of included patients was 5965, with the median age ranging from 54.1 to 69.2 years and sample sizes ranging from 35 to 411. Over half of the patients included in this meta‐analysis were from Japan, and a large proportion of studies did not strictly divide EC subtypes according to the pathologic diagnosis. Most patients with EC had surgical resection combined with neoadjuvant therapy. Four studies investigated patients who underwent chemoradiotherapy only, patients in three studies had undergone surgery combined with adjuvant therapy, and three studies reported patients treated with surgery combined with neoadjuvant and adjuvant treatment. In only two studies, patients with EC underwent surgery only. The follow‐up time varied in different studies ranging from 21 to 120 months. In addition, various measurements of sarcopenia were applied in different studies. A large proportion of studies applied the SMI as a standard to evaluate sarcopenia through CT images to measure the total skeletal muscle mass at the third lumbar level normalized by the square of height value. In addition, some studies used the psoas muscle index (PMI) to measure sarcopenia, whereas Matsunaga et al. 14 utilized electrodes to calculate the skeletal muscle mass (SMM) automatically and patients with SMM value lower than 90% standard SMM were grouped into the sarcopenia group. Thirty‐nine studies used overall survival (OS) as the survival outcomes of patients with EC and reported their HRs, while nine studies reported HR for disease‐free survival (DFS). The progression‐free survival (PFS) and recurrence‐free survival (RFS) were reported in two and five studies, respectively. Seventeen studies reported the incidence of postoperative complications in patients with or without sarcopenia, from which the OR could be calculated. More details about each study are available in Online Resource S1.
Quality assessment
All included research in our meta‐analysis were cohort studies, and the quality assessment and risk of bias were conducted in accordance with the NOS. The NOS score of each included study is listed in Table 1; all studies evaluated were of high quality (NOS score ≥6).
Prognostic value of sarcopenia in patients with EC
Association of sarcopenia with overall survival
The results of this meta‐analysis show that sarcopenia could significantly predict survival outcomes in patients with EC. Patients with sarcopenia were significantly associated with poorer OS with a pooled HR of 1.68 (95% CI: 1.54–1.83) (Figure 2). According to the value of I 2 (I 2 = 41.7%), we considered that there was an acceptable heterogeneity among studies that reported the HRs for OS, hence a fixed‐effects model was used to evaluate the data.
Figure 2.
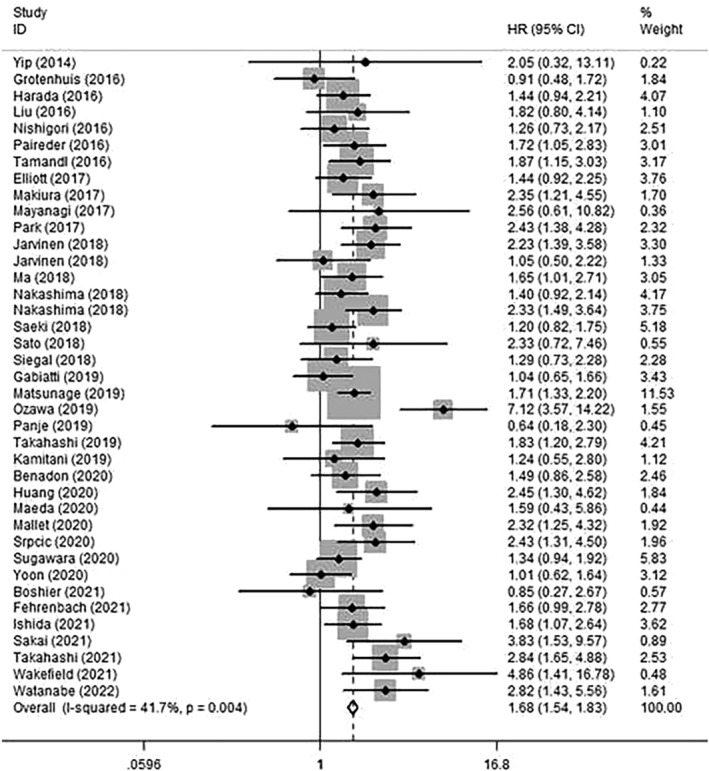
Forest plot of studies evaluating hazard ratios of sarcopenia and the overall survival of oesophageal cancer.
Association of sarcopenia with disease‐free survival and recurrence‐free survival
Nine studies investigated the association between sarcopenia and DFS, while five studies investigated the RFS; among the EC patients, sarcopenia was also shown to be an unfavourable predictor, with the pooled HR for DFS being 1.97 (95% CI: 1.44–2.69) compared with those patients without sarcopenia (Figure 3). A random‐effects model was used to evaluate for moderate heterogeneity, and it was found that the I 2 value was 61.9%. However, such statistical significance was not detected in RFS for the 95% CI incorporated with the null line (HR: 1.50, 95% CI: 0.93–2.42) (Online Resource S2).
Figure 3.
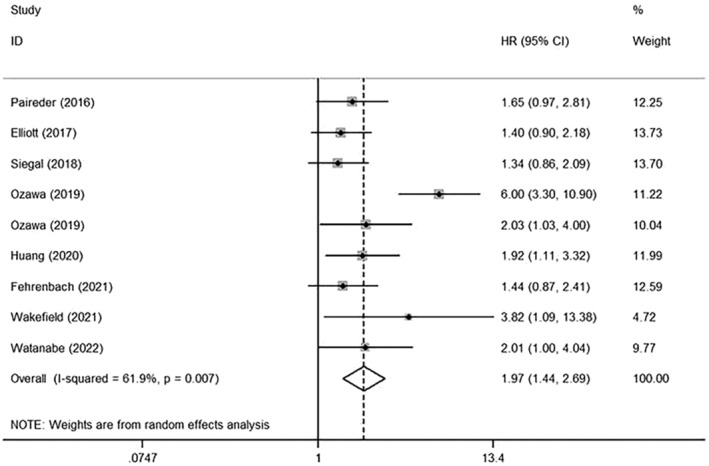
Forest plot of studies evaluating hazard ratios of sarcopenia and the disease‐free survival of oesophageal cancer.
Subgroup analysis of sarcopenia in EC prognosis
To investigate the prognostic value of sarcopenia in depth, we also conducted subgroup analyses based on different clinical features. Subgroup analysis by treatment modalities showed sarcopenia was significantly associated with poor OS in those patients who underwent neoadjuvant therapy followed by surgery (HR: 1.67, 95% CI: 1.48–1.88) and those who received neoadjuvant therapy and surgery combined with adjuvant therapy (HR: 1.47, 95% CI: 1.10–1.95). For EC patients undergoing non‐surgical treatments, the pooled HR for OS was 1.59 (95% CI: 1.07–2.35). However, no statistical significance was found in patients who underwent surgery only (HR: 2.15, 95% CI: 0.84–5.53) or patients treated with surgery combined with postoperative adjuvant therapy (HR: 2.49, 95% CI: 0.81–7.64) (Figure 4A). After analysing subgroups based on different populations, we verified that sarcopenia was associated with a worse prognosis for patients from either Asian or non‐Asian countries, the pooled HR was 1.75 (95% CI: 1.58–1.94) or 1.52 (95% CI: 1.30–1.77) respectively (Figure 4B). When stratified by the measurements of sarcopenia, most studies utilized the SMI to assess sarcopenia at the level of the third lumbar vertebra through CT scanning (HR: 1.64, 95% CI: 1.49–1.81), and six studies applied non‐SMI parameters to identify sarcopenia (HR: 1.82, 95% CI: 1.51–2.20) (Figure 4C). In addition, nine studies had only investigated the impact of sarcopenia on ESCC patients, and the pooled HR for OS was 1.77 (95% CI: 1.21–2.58) (Figure 5), suggesting that sarcopenia was still a negative indicator of ESCC patients.
Figure 4.
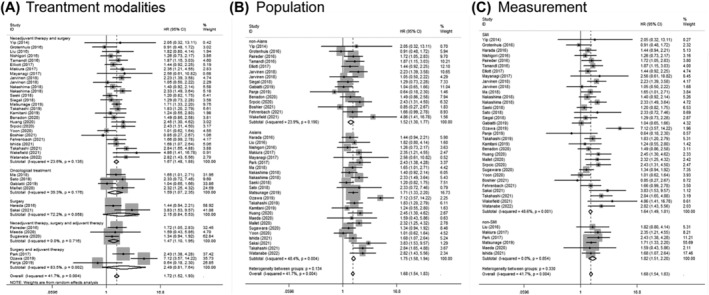
Forest plot of studies evaluating hazard ratios of sarcopenia and the overall survival of oesophageal cancer, stratified by (A) treatment modalities; (B) population; (C) sarcopenia measurement.
Figure 5.
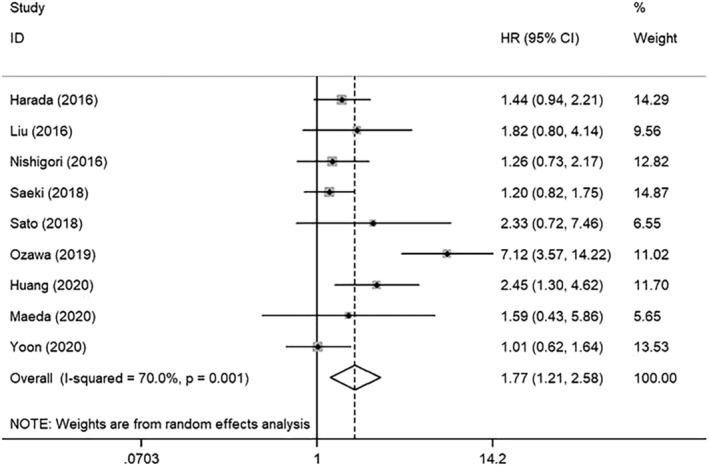
Forest plot of studies evaluating hazard ratios of sarcopenia and the overall survival of oesophageal squamous cell cancer.
Sarcopenia and postoperative complications
In this meta‐analysis, 17 studies reported the OR of postoperative complications in patients with EC. Based on the value of I 2 (I 2 = 32.7%), we considered the heterogeneity was low, and the fixed‐effects model was used. The results demonstrated that the pooled OR was 1.47 (95% CI: 1.21–1.77), indicating a significantly higher risk of postoperative complications for patients with sarcopenia compared with those patients without sarcopenia (Figure 6A).
Figure 6.
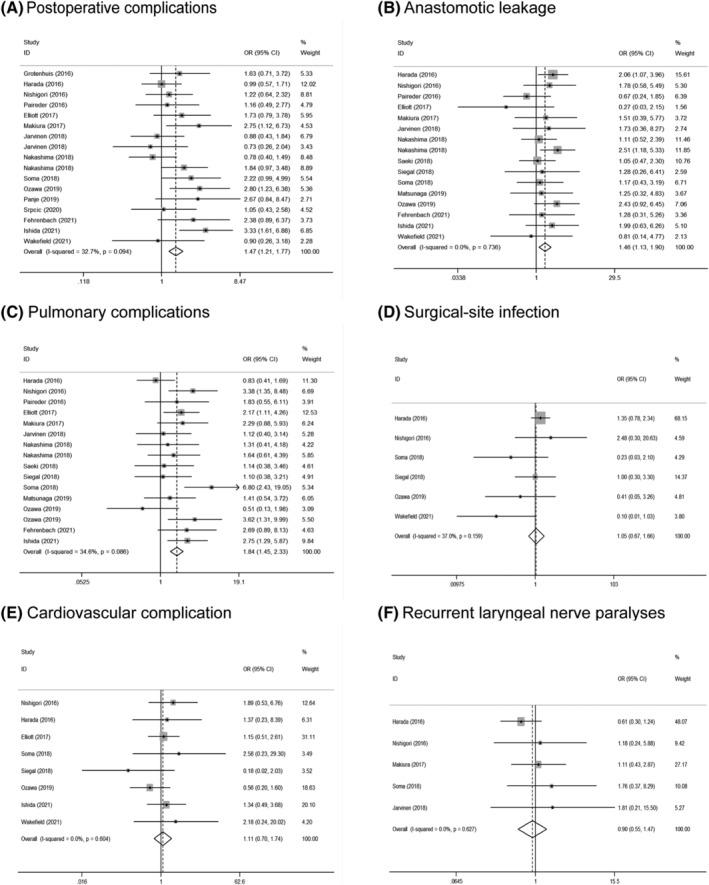
(A) Forest plot of odds ratios for postoperative complications in sarcopenic patients compared with non‐sarcopenic patients, and subgroup stratified by (B) anastomotic leakage; (C) pulmonary complications; (D) surgical‐site infection; (E) cardiovascular complication; (F) recurrent laryngeal nerve paralyses.
Among those studies which had reported the OR of sarcopenia on detailed postoperative complications, including anastomotic leak, pulmonary complications, surgical‐site infection, cardiovascular complication, and recurrent laryngeal nerve paralyses, the results showed sarcopenia was a risk factor for the anastomotic leak (OR = 1.46; 95% CI: 1.13–1.90) (Figure 6B) and pulmonary complications (OR = 1.84; 95% CI: 1.45–2.33) (Figure 6C), but no statistical significance was found for surgical‐site infection (OR = 1.05; 95% CI: 0.67–1.66) (Figure 6D), cardiovascular complication (OR = 1.11; 95% CI: 0.70–1.74) (Figure 6E) or recurrent laryngeal nerve paralyses (OR = 0.90; 95% CI: 0.55–1.47) (Figure 6F).
Sensitivity analysis and publication bias
To assess the stability and reliability of the original analysis, a sensitivity analysis was applied through sequential removal of each study. The result showed that the survival outcome of the prime analysis was not impacted by removing any single study, even when removing studies of relatively low quality. Moreover, hidden publication bias was tested using Begg's test. A symmetrical appearance was checked in the funnel plot. The P value of Begg's test was 0.091 for OS and 0.140 for DFS (Figure 7). Therefore, no notable publication bias was found in the meta‐analysis.
Figure 7.
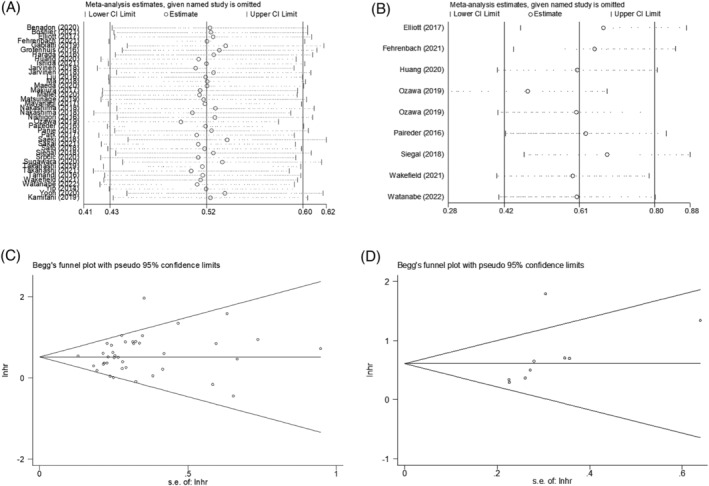
(A) Sensitivity analysis for meta‐analysis of sarcopenia for OS. (B) Sensitivity analysis for meta‐analysis of sarcopenia for DFS. (C) Funnel plots of publication bias for meta‐analysis of sarcopenia for OS. (D) Funnel plots of publication bias for meta‐analysis of sarcopenia for DFS.
Discussion
Most EC patients with advanced stage are found with the syndrome of dysphagia, and the risk of malnutrition in these patients is excessively increased. 15 , 16 It is imperative to identify a reliable index to evaluate the nutritional status of EC patients and supplement with proper nutritional support. Traditional nutritional body mass index (BMI) had some limitations, as it failed to reflect the precise nutrition status and body composition. 17 Some other indexes, such as the malnutrition screening tool (MST), patient‐generated subjective global assessment (PG‐SGA), and nutritional risk screening (NRS), have been utilized in clinics to evaluate the nutritional level of patients with cancer. 18 However, these indexes had limited predictive value and have not been widely utilized in clinical practice, and it is of great necessity for confirming an indicator to precisely predict the malnutritional status of EC patients. Recently, sarcopenia, first proposed by Rosenberg in 1989 to describe the phenomenon of muscle mass reduction with advancing age, has attracted our interest as a new nutritional indicator because of its superior predictive properties. 19 The main characteristic of sarcopenia is the infiltration of connective tissue and fat into muscle tissue with a decreased number of type 1 and 2 fibres and reduced motor units. 20 In recent years, sarcopenia has been recognized as a core standard for evaluating nutritional levels, especially in patients with cancer. 21 It has been shown to be closely correlated with adverse clinical outcomes in patients who underwent chemotherapy. 22 In terms of EC, previous studies have shown that the incidence rate of sarcopenia could be up to 75%. 23 Because of the high prevalence of sarcopenia in EC, it is important to determine if sarcopenia has prognostic value in patients with EC. Thus, this meta‐analysis was conducted to explore the influence of sarcopenia on patients with EC.
In our study, we included 41 studies with a total of 5965 patients with EC. According to the results of the meta‐analysis, forest plots clearly showed that sarcopenia could significantly predict worse OS and DFS. Sarcopenia was also shown to be a risk factor for postoperative complications. Furthermore, based on the results of the subgroup analysis, sarcopenia was significantly associated with poor OS with different therapies, populations, and even different sarcopenia measurement subgroups. It was also identified as a risk factor for major postoperative complications including anastomotic leak and pulmonary complications. Still, sarcopenia was not significantly associated with surgical‐site infections, cardiovascular complications, and recurrent laryngeal nerve paralysis. Such discrepancy might partly be attributed to the low incidence of these complications after esophagectomy as well as the limited number of relevant clinical research, which may make the results biased to a certain extent. In terms of subgroup analysis, in patients who only underwent surgery and those who were treated with surgery combined with postoperative adjuvant therapy, no statistical association was found between sarcopenia and their prognosis. Such results might partly be explained by the imbalanced clinical baseline of patients within different subgroups and the limited number of studies. Meanwhile, according to the I 2 value of these two subgroups (I 2 = 72.2% and I 2 = 83.5%), high heterogeneity existed among the two subgroups, which might result in the pooled results deviating from the actual outcomes, and more studies are needed to confirm this conclusion. Taken together, we consider sarcopenia to be a negative predictor for patients with EC and a risk factor for postoperative complications. We suggest that sarcopenia is a robust predictive factor and should be incorporated in the routine evaluation of patients with EC, which might be useful for clinicians to adjust treatment strategies and deliver appropriate nutrition support in time.
Besides sarcopenia, myosteatosis is also an important indicator of muscle depletion. Myosteatosis, characterized by decreasing muscle radiodensity and abnormally fat infiltration in skeletal muscle, could be analysed through an advanced CT scan. Some early studies had indicated that myosteatosis was correlated with worse clinical outcomes in patients with pancreatic cancer. 24 Interestingly, conflicting results were reported in studies conducted by Gabiatti et al. and Srpcic et al. They had investigated the myosteatosis for EC patients by mean muscle attenuation (MA). Srpcic et al. demonstrated in their study that myosteatosis in patients who underwent esophagectomy was associated with poorer OS. However, in the study of Gabiatti et al., EC patients with myosteatosis presented a favourable prognosis. Unfortunately, the number of studies investigating myosteatosis in EC patients was limited, and these results could not be systematically analysed in our meta‐analysis. Therefore, more high‐quality clinical research is necessary to investigate the impact of myosteatosis on EC patients and to get a convincing conclusion.
There are two relevant studies published before. Deng et al. conducted a meta‐analysis to detect sarcopenia effects on the long‐term survival of patients with EC. 11 However, they only included 11 studies with 1520 patients, and the selected studies were dated till 2018. In addition, Uzair et al. 12 had conducted a meta‐analysis focusing on sarcopenia determined by SMI. They reported that sarcopenia was associated with poor survival outcomes in EC patients who had undergone surgical therapy. However, it only included 21 studies involving 3966 patients published before 2020, and significant heterogeneities among studies could be seen in their meta‐analysis. However, there are several new findings that greatly surpass these previously reported meta‐analyses: (1) Our analysis included the largest number of studies and sample size. The amount of included studies was up to 41 with 5965 EC patients; (2) We also included the latest literature published till 2022; (3) We provided more comprehensive information than previous studies. In Uzair's study, they included only EC patients treated with surgery but not the patients who underwent non‐surgery methods such as chemoradiotherapy. In addition, all included studies in Uzair's research utilized SMI as the only parameter to measure sarcopenia. To our knowledge, diagnosing sarcopenia using CT imaging has not yet been established, and the optimal measurement of sarcopenia has not reached a consensus. Thus, we also incorporated studies applying other parameters in measuring sarcopenia, such as the sex‐specific 25th percentile for the PMI and the standard of SMM. We have made subgroup analyses based on different treatment modalities and investigated the association between survival and sarcopenia in depth. In addition, they did not report the data on the uncommon postoperative complications such as surgical‐site infection, cardiovascular complications, and recurrent laryngeal nerve paralysis, which we had also evaluated. So, our manuscript represents the most up‐to‐date and comprehensive evidence available for elucidating the impact of sarcopenia on the prognosis of EC patients.
In addition to patients with EC, sarcopenia has also been shown to act as a negative factor in other malignancies such as colorectal cancer, breast cancer, and lung cancer. 25 , 26 , 27 However, the actual mechanism of how sarcopenia is involved in EC prognosis remains elusive. During the last two decades, the concept of skeletal muscle acting only as a motor unit has been challenged. An increasing number of studies have verified that muscle could serve as a modulator of immune regulation by releasing peptides and cytokines. 28 , 29 Traditionally, it is believed that sarcopenia is complicated with decreasing nutritional levels and suppressed immune function. 30 Growing evidence have identified that muscle can produce two cytokines IL‐7 and IL‐15, which are essential in maintaining immune function and developing immature lymphocytes. 31 Interestingly, both cytokines have been shown to be inversely associated with skeletal mass, suggesting the link between sarcopenia and decreasing immune function. 31 , 32 Moreover, sarcopenia may also be related to the sensitivity of tumour cells to immunotherapy. Nishioka et al. found that in patients with advanced non‐small‐cell lung cancer treated with immune checkpoint inhibitors therapy, patients with sarcopenia were less likely to achieve better immunotherapy outcomes, 33 which indicated that sarcopenia might impair the immune system in anti‐tumour response. Apart from immunity resistance, some scholars hold the view that patients with sarcopenia are prone to develop insulin resistance, and previous studies have shown that insulin resistance involves various pathways of tumour progression. 34 Consequently, it is imperative to conduct more research to investigate the exact mechanism through which sarcopenia impacts the prognosis of EC.
Although this meta‐analysis compiles the evidence and illustrates that sarcopenia acts as a critical factor with prognostic value in EC, it still has certain limitations. First of all, ESCC and EAC are the two main histological types of EC. In this meta‐analysis, we had conducted a subgroup analysis among nine studies that had only included ESCC patients. However, most of the selected studies did not analyse two subtypes of EC separately, so we could only analyse them together. And there was one study that included EAC patients solely. Without enough studies, the subgroup analysis on EAC patients could not be achieved. Because the oncological features of ESCC and EAC are relatively different, this may weaken the reliability of the conclusion to some extent. In addition, few studies applied the same cut‐off values of SMI to evaluate sarcopenia, and the subgroup analysis based on cut‐off values of SMI was unable to be achieved. Therefore, more multicentre clinical studies with strong evidence are required to confirm the prognostic value of sarcopenia in EC, and well‐recognized measurements or standards to evaluate sarcopenia should be further established.
Conclusions
This systematic review and meta‐analysis confirmed that sarcopenia was significantly associated with both survival outcomes and postoperative complications in EC patients. We suggest that sarcopenia should be incorporated in routine evaluation and appropriately diagnosed for patients with EC, which might be helpful for clinicians to adjust treatment and deliver timely nutrition support for improving patients' short‐term and long‐term outcomes.
Conflict of interest
None declared.
Funding
This work was supported by the National Nature Science Foundation of China (81970481 and 82000514), the Sichuan Science and Technology Program (2022YFS0048), 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (2020HXFH047, ZYJC18010, 20HXJS005, and 2018HXFH020), and China Postdoctoral Science Foundation (2020M673241).
Supporting information
Data S1. Supporting Information
Data S2. Supporting Information
Acknowledgements
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle. 35
Fang P., Zhou J., Xiao X., Yang Y., Luan S., Liang Z., Li X., Zhang H., Shang Q., Zeng X., and Yuan Y. (2023) The prognostic value of sarcopenia in oesophageal cancer: A systematic review and meta‐analysis, Journal of Cachexia, Sarcopenia and Muscle, 14, 3–16, 10.1002/jcsm.13126
Pinhao Fang, Jianfeng Zhou, and Xin Xiao have contributed equally to this work.
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 2. Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet 2017;390:2383–2396. [DOI] [PubMed] [Google Scholar]
- 3. Taziki MH, Rajaee S, Behnampour N, Tadrisee M, Mansourian AR. Esophageal cancer: 5‐year survival rate at south‐east of Caspian sea of northern Iran. J Cancer Res Ther 2011;7:135–137. [DOI] [PubMed] [Google Scholar]
- 4. Li X, Chen L, Luan S, Zhou J, Xiao X, Yang Y, et al. The development and progress of nanomedicine for esophageal cancer diagnosis and treatment. Semin Cancer Biol 2022;86:873–885. [DOI] [PubMed] [Google Scholar]
- 5. Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre‐cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia‐anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr (Edinburgh, Scotland) 2010;29:154–159. [DOI] [PubMed] [Google Scholar]
- 6. Mariette C, De Botton ML, Piessen G. Surgery in esophageal and gastric cancer patients: what is the role for nutrition support in your daily practice? Ann Surg Oncol 2012;19:2128–2134. [DOI] [PubMed] [Google Scholar]
- 7. Elliott JA, Doyle SL, Murphy CF, King S, Guinan EM, Beddy P, et al. Sarcopenia: Prevalence, and Impact on Operative and Oncologic Outcomes in the Multimodal Management of Locally Advanced Esophageal Cancer. Ann Surg 2017;266:822–830. [DOI] [PubMed] [Google Scholar]
- 8. Tamandl D, Paireder M, Asari R, Baltzer PA, Schoppmann SF, Ba‐Ssalamah A. Markers of sarcopenia quantified by computed tomography predict adverse long‐term outcome in patients with resected oesophageal or gastro‐oesophageal junction cancer. Eur Radiol 2016;26:1359–1367. [DOI] [PubMed] [Google Scholar]
- 9. Nakashima Y, Saeki H, Nakanishi R, Sugiyama M, Kurashige J, Oki E, et al. Assessment of Sarcopenia as a Predictor of Poor Outcomes After Esophagectomy in Elderly Patients With Esophageal Cancer. Ann Surg 2018;267:1100–1104. [DOI] [PubMed] [Google Scholar]
- 10. Mayanagi S, Tsubosa Y, Omae K, Niihara M, Uchida T, Tsushima T, et al. Negative Impact of Skeletal Muscle Wasting After Neoadjuvant Chemotherapy Followed by Surgery on Survival for Patients with Thoracic Esophageal Cancer. Ann Surg Oncol 2017;24:3741–3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deng HY, Zha P, Peng L, Hou L, Huang KL, Li XY. Preoperative sarcopenia is a predictor of poor prognosis of esophageal cancer after esophagectomy: a comprehensive systematic review and meta‐analysis. Dis Esophagus 2019;32. [DOI] [PubMed] [Google Scholar]
- 12. Jogiat UM, Sasewich H, Turner SR, Baracos V, Eurich DT, Filafilo H, et al. Sarcopenia Determined by Skeletal Muscle Index Predicts Overall Survival, Disease‐free Survival, and Postoperative Complications in Resectable Esophageal Cancer: A Systematic Review and Meta‐analysis. Ann Surg 2022;276:e311–e318. [DOI] [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matsunaga T, Miyata H, Sugimura K, Motoori M, Asukai K, Yanagimoto Y, et al. Prognostic Significance of Sarcopenia and Systemic Inflammatory Response in Patients With Esophageal Cancer. Anticancer Res 2019;39:449–458. [DOI] [PubMed] [Google Scholar]
- 15. Cao J, Xu H, Li W, Guo Z, Lin Y, Shi Y, et al. Nutritional assessment and risk factors associated to malnutrition in patients with esophageal cancer. Curr Probl Cancer 2021;45:100638. [DOI] [PubMed] [Google Scholar]
- 16. Sheetz KH, Zhao L, Holcombe SA, Wang SC, Reddy RM, Lin J, et al. Decreased core muscle size is associated with worse patient survival following esophagectomy for cancer. Dis Esophagus 2013;26:716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clarys JP, Martin AD, Marfell‐Jones MJ, Janssens V, Caboor D, Drinkwater DT. Human body composition: A review of adult dissection data. Am J Hum Biol 1999;11:167–174. [DOI] [PubMed] [Google Scholar]
- 18. Castillo‐Martínez L, Castro‐Eguiluz D, Copca‐Mendoza ET, Pérez‐Camargo DA, Reyes‐Torres CA, Ávila EA, et al. Nutritional Assessment Tools for the Identification of Malnutrition and Nutritional Risk Associated with Cancer Treatment. Rev Invest Clin 2018;70:121–125. [DOI] [PubMed] [Google Scholar]
- 19. Rosenberg IH. 1989 Herman Award lecture. Folate absorption: clinical questions and metabolic answers. Am J Clin Nutr 1990;51:531–534. [DOI] [PubMed] [Google Scholar]
- 20. Kamel HK. Sarcopenia and aging. Nutr Rev 2003;61:157–167. [DOI] [PubMed] [Google Scholar]
- 21. Meza‐Valderrama D, Marco E, Dávalos‐Yerovi V, Muns MD, Tejero‐Sánchez M, Duarte E, et al. Sarcopenia, Malnutrition, and Cachexia: Adapting Definitions and Terminology of Nutritional Disorders in Older People with Cancer. Nutrients 2021;13:761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Davis MP, Panikkar R. Sarcopenia associated with chemotherapy and targeted agents for cancer therapy. J Palliat Med 2019;8:86–101. [DOI] [PubMed] [Google Scholar]
- 23. Boshier PR, Heneghan R, Markar SR, Baracos VE, Low DE. Assessment of body composition and sarcopenia in patients with esophageal cancer: a systematic review and meta‐analysis. Dis Esophagus 2018;31. [DOI] [PubMed] [Google Scholar]
- 24. van Dijk DP, Bakens MJ, Coolsen MM, Rensen SS, van Dam RM, Bours MJ, et al. Low skeletal muscle radiation attenuation and visceral adiposity are associated with overall survival and surgical site infections in patients with pancreatic cancer. J Cachexia Sarcopenia Muscle 2017;8:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Trejo‐Avila M, Bozada‐Gutiérrez K, Valenzuela‐Salazar C, Herrera‐Esquivel J, Moreno‐Portillo M. Sarcopenia predicts worse postoperative outcomes and decreased survival rates in patients with colorectal cancer: a systematic review and meta‐analysis. Int J Colorectal Dis 2021;36:1077–1096. [DOI] [PubMed] [Google Scholar]
- 26. Zhang XM, Dou QL, Zeng Y, Yang Y, Cheng ASK, Zhang WW. Sarcopenia as a predictor of mortality in women with breast cancer: a meta‐analysis and systematic review. BMC Cancer 2020;20:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang M, Shen Y, Tan L, Li W. Prognostic Value of Sarcopenia in Lung Cancer: A Systematic Review and Meta‐analysis. Chest 2019;156:101–111. [DOI] [PubMed] [Google Scholar]
- 28. Afzali AM, Müntefering T, Wiendl H, Meuth SG, Ruck T. Skeletal muscle cells actively shape (auto)immune responses. Autoimmun Rev 2018;17:518–529. [DOI] [PubMed] [Google Scholar]
- 29. Giudice J, Taylor JM. Muscle as a paracrine and endocrine organ. Curr Opin Pharmacol 2017;34:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsukioka T, Izumi N, Kyukwang C, Komatsu H, Toda M, Hara K, et al. Loss of Muscle Mass is a Novel Predictor of Postoperative Early Recurrence in N2‐Positive Non‐Small‐Cell Lung Cancer. Ann Thorac Cardiovasc Surg 2018;24:121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duggal NA, Pollock RD, Lazarus NR, Harridge S, Lord JM. Major features of immunesenescence, including reduced thymic output, are ameliorated by high levels of physical activity in adulthood. Aging Cell 2018;17:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marzetti E, Carter CS, Wohlgemuth SE, Lees HA, Giovannini S, Anderson B, et al. Changes in IL‐15 expression and death‐receptor apoptotic signaling in rat gastrocnemius muscle with aging and life‐long calorie restriction. Mech Ageing Dev 2009;130:272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nishioka N, Uchino J, Hirai S, Katayama Y, Yoshimura A, Okura N, et al. Association of Sarcopenia with and Efficacy of Anti‐PD‐1/PD‐L1 Therapy in Non‐Small‐Cell Lung Cancer. J Clin Med 2019;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Buoite Stella A, Gortan Cappellari G, Barazzoni R, Zanetti M. Update on the Impact of Omega 3 Fatty Acids on Inflammation, Insulin Resistance and Sarcopenia: A Review. Int J Mol Sci 2018;19:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the journal of cachexia, sarcopenia and muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Benadon B, Servagi‐Vernat S, Quero L, Cattan P, Guillerm S, Hennequin V, et al. Sarcopenia: An important prognostic factor for males treated for a locally advanced esophageal carcinoma. Dig Lover Dis 2020;52:1047–1052. [DOI] [PubMed] [Google Scholar]
- 37. Boshier PR, Klevebro F, Jenq W, Puccetti F, Muthuswamy K, Hanna GB, et al. Long‐term variation in skeletal muscle and adiposity in patients undergoing esophagectomy. Dis Esophagus 2021;34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fehrenbach U, Wuensch T, Gabriel P, Segger L, Yamaguchi T, Auer TA, et al. CT Body Composition of Sarcopenia and Sarcopenic Obesity: Predictors of Postoperative Complications and Survival in Patients with Locally Advanced Esophageal Adenocarcinoma. Cancer 2021;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gabiatti CTB, Martins MCL, Miyazaki DL, Silva LP, Lascala F, Macedo LT, et al. Myosteatosis in a systemic inflammation‐dependent manner predicts favorable survival outcomes in locally advanced esophageal cancer. Cancer Med 2019;8:6967–6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grotenhuis BA, Shapiro J, van Adrichem S, de Vries M, Koek M, Wijnhoven BP, et al. Sarcopenia/Muscle Mass is not a Prognostic Factor for Short‐ and Long‐Term Outcome After Esophagectomy for Cancer. World J Surg 2016;40:2698–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Harada K, Ida S, Baba Y, Ishimoto T, Kosumi K, Tokunaga R, et al. Prognostic and clinical impact of sarcopenia in esophageal squamous cell carcinoma. Dis Esophagus 2016;29:627–633. [DOI] [PubMed] [Google Scholar]
- 42. Huang CH, Lue KH, Hsieh TC, Liu SH, Wang TF, Peng TC. Association Between Sarcopenia and Clinical Outcomes in Patients With Esophageal Cancer Under Neoadjuvant Therapy. Anticancer Res 2020;40:1175–1181. [DOI] [PubMed] [Google Scholar]
- 43. Ishida T, Makino T, Yamasaki M, Yamashita K, Tanaka K, Saito T, et al. Quantity and Quality of Skeletal Muscle as an Important Predictor of Clinical Outcomes in Patients with Esophageal Cancer Undergoing Esophagectomy after Neoadjuvant Chemotherapy. Ann Surg Oncol 2021;28:7185–7195. [DOI] [PubMed] [Google Scholar]
- 44. Järvinen T, Ilonen I, Kauppi J, Volmonen K, Salo J, Räsänen J. Low skeletal muscle mass in stented esophageal cancer predicts poor survival: A retrospective observational study. Thorac Cancer 2018;9:1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Järvinen T, Ilonen I, Kauppi J, Salo J, Räsänen J. Loss of skeletal muscle mass during neoadjuvant treatments correlates with worse prognosis in esophageal cancer: a retrospective cohort study. World J Surg Oncol 2018;16:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kamitani N, Migita K, Matsumoto S, Wakatsuki K, Kunishige T, Nakade H, et al. Association of skeletal muscle loss with the long‐term outcomes of esophageal cancer patients treated with neoadjuvant chemotherapy. Surg Today 2019;49:1022–1028. [DOI] [PubMed] [Google Scholar]
- 47. Liu J, Motoyama S, Sato Y, Wakita A, Kawakita Y, Saito H, et al. Decreased Skeletal Muscle Mass After Neoadjuvant Therapy Correlates with Poor Prognosis in Patients with Esophageal Cancer. Anticancer Res 2016;36:6677–6685. [DOI] [PubMed] [Google Scholar]
- 48. Ma DW, Cho Y, Jeon MJ, Kim JH, Lee IJ, Youn YH, et al. Relationship Between Sarcopenia and Prognosis in Patient With Concurrent Chemo‐Radiation Therapy for Esophageal Cancer. Front Oncol 2019;9:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Maeda N, Shirakawa Y, Tanabe S, Sakurama K, Noma K, Fujiwara T. Skeletal muscle loss in the postoperative acute phase after esophageal cancer surgery as a new prognostic factor. World J Surg Oncol 2020;18:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Makiura D, Ono R, Inoue J, Fukuta A, Kashiwa M, Miura Y, et al. Impact of Sarcopenia on Unplanned Readmission and Survival After Esophagectomy in Patients with Esophageal Cancer. Ann Surg Oncol 2018;25:456–464. [DOI] [PubMed] [Google Scholar]
- 51. Mallet R, Modzelewski R, Lequesne J, Mihailescu S, Decazes P, Auvray H, et al. Prognostic value of sarcopenia in patients treated by Radiochemotherapy for locally advanced oesophageal cancer. Radiat Oncol (London, England) 2020;15:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nishigori T, Okabe H, Tanaka E, Tsunoda S, Hisamori S, Sakai Y. Sarcopenia as a predictor of pulmonary complications after esophagectomy for thoracic esophageal cancer. J Surg Oncol 2016;113:678–684. [DOI] [PubMed] [Google Scholar]
- 53. Oguma J, Ozawa S, Kazuno A, Yamamoto M, Ninomiya Y, Yatabe K. Prognostic significance of sarcopenia in patients undergoing esophagectomy for superficial esophageal squamous cell carcinoma. Dis Esophagus 2019;32. [DOI] [PubMed] [Google Scholar]
- 54. Ozawa Y, Nakano T, Taniyama Y, Sakurai T, Onodera Y, Kamiya K, et al. Evaluation of the impact of psoas muscle index, a parameter of sarcopenia, in patients with esophageal squamous cell carcinoma receiving neoadjuvant therapy. Esophagus 2019;16:345–351. [DOI] [PubMed] [Google Scholar]
- 55. Paireder M, Asari R, Kristo I, Rieder E, Tamandl D, Ba‐Ssalamah A, et al. Impact of sarcopenia on outcome in patients with esophageal resection following neoadjuvant chemotherapy for esophageal cancer. Eur J Surg Oncol 2017;43:478–484. [DOI] [PubMed] [Google Scholar]
- 56. Panje CM, Höng L, Hayoz S, Baracos VE, Herrmann E, Garcia Schüler H, et al. Skeletal muscle mass correlates with increased toxicity during neoadjuvant radiochemotherapy in locally advanced esophageal cancer: A SAKK 75/08 substudy. Radiat Oncol (London, England) 2019;14:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Park SY, Yoon JK, Lee SJ, Haam S, Jung J. Prognostic value of preoperative total psoas muscle area on long‐term outcome in surgically treated oesophageal cancer patients. Interact Cardiovasc Thorac Surg 2017;24:13–19. [DOI] [PubMed] [Google Scholar]
- 58. Saeki H, Nakashima Y, Kudou K, Sasaki S, Jogo T, Hirose K, et al. Neoadjuvant Chemoradiotherapy for Patients with cT3/Nearly T4 Esophageal Cancer: Is Sarcopenia Correlated with Postoperative Complications and Prognosis? World J Surg 2018;42:2894–2901. [DOI] [PubMed] [Google Scholar]
- 59. Sakai M, Sohda M, Saito H, Ubukata Y, Nakazawa N, Kuriyama K, et al. Impact of combined assessment of systemic inflammation and presarcopenia on survival for surgically resected esophageal cancer. Am J Surg 2021;221:149–154. [DOI] [PubMed] [Google Scholar]
- 60. Sato S, Kunisaki C, Suematsu H, Tanaka Y, Miyamoto H, Kosaka T, et al. Impact of Sarcopenia in Patients with Unresectable Locally Advanced Esophageal Cancer Receiving Chemoradiotherapy. In Vivo 2018;32:603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Siegal SR, Dolan JP, Dewey EN, Guimaraes AR, Tieu BH, Schipper PH, et al. Sarcopenia is not associated with morbidity, mortality, or recurrence after esophagectomy for cancer. Am J Surg 2018;215:813–817. [DOI] [PubMed] [Google Scholar]
- 62. Soma D, Kawamura YI, Yamashita S, Wake H, Nohara K, Yamada K, et al. Sarcopenia, the depletion of muscle mass, an independent predictor of respiratory complications after oncological esophagectomy. Dis Esophagus 2019;32. [DOI] [PubMed] [Google Scholar]
- 63. Srpcic M, Jordan T, Popuri K, Sok M. Sarcopenia and myosteatosis at presentation adversely affect survival after esophagectomy for esophageal cancer. Radiol Oncol 2020;54:237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sugawara K, Yamashita H, Urabe M, Okumura Y, Yagi K, Aikou S, et al. Geriatric Nutrition Index Influences Survival Outcomes in Gastric Carcinoma Patients Undergoing Radical Surgery. JPEN J Parenter Enteral Nutr 2021;45:1042–1051. [DOI] [PubMed] [Google Scholar]
- 65. Takahashi K, Watanabe M, Kozuki R, Toihata T, Okamura A, Imamura Y, et al. Prognostic Significance of Skeletal Muscle Loss During Early Postoperative Period in Elderly Patients with Esophageal Cancer. Ann Surg Oncol 2019;26:3727–3735. [DOI] [PubMed] [Google Scholar]
- 66. Takahashi K, Nishikawa K, Furukawa K, Tanishima Y, Ishikawa Y, Kurogochi T, et al. Prognostic Significance of Preoperative Osteopenia in Patients Undergoing Esophagectomy for Esophageal Cancer. World J Surg 2021;45:3119–3128. [DOI] [PubMed] [Google Scholar]
- 67. Wakefield CJ, Hamati F, Karush JM, Arndt AT, Geissen N, Liptay MJ, et al. Sarcopenia after induction therapy is associated with reduced survival in patients undergoing esophagectomy for locally‐advanced esophageal cancer. J Thorac Dis 2021;13:861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Watanabe A, Oshikiri T, Sawada R, Harada H, Urakawa N, Goto H, et al. Actual Sarcopenia Reflects Poor Prognosis in Patients with Esophageal Cancer. Ann Surg Oncol 2022;29:3670–3681. [DOI] [PubMed] [Google Scholar]
- 69. Yip C, Goh V, Davies A, Gossage J, Mitchell‐Hay R, Hynes O, et al. Assessment of sarcopenia and changes in body composition after neoadjuvant chemotherapy and associations with clinical outcomes in oesophageal cancer. Eur Radiol 2014;24:998–1005. [DOI] [PubMed] [Google Scholar]
- 70. Yoon HG, Oh D, Ahn YC, Noh JM, Pyo H, Cho WK, et al. Prognostic Impact of Sarcopenia and Skeletal Muscle Loss During Neoadjuvant Chemoradiotherapy in Esophageal Cancer. Cancer 2020;12:925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information
Data S2. Supporting Information


