Abstract
Objectives
The transtibial pull‐out repair (TP) is a relatively new method for treating meniscal root tear; however, the clinical evaluation of its healing effect remains controversial. Due to ethical constraints and limitations of imaging techniques in humans, here we dynamically observe the healing effects of TP and TP with platelet‐rich plasma gel (PRG) at the histological level using an animal model.
Methods
Platelet‐rich plasma (PRP) and PRG of rabbits were prepared. Platelet‐derived growth factor (PDGF) and transforming growth factor‐β1 (TGF‐β1) levels in PRP and PRG were determined using an enzyme‐linked immunosorbent assay. A rabbit model of anterior horn tear of the medial meniscus and TP surgery were created. PRG was injected between the anterior horn of the medial meniscus and the tibial tunnel. Rabbits were divided into three groups: the anterior horn tear group (Tear group), the anterior horn tear + TP group (TP group), and the anterior horn tear + TP + PRG group (TP + PRG group). The healing effect was observed dynamically using histopathological studies and biomechanical experiments.
Results
The platelet content in PRP significantly increased to approximately 4.57 times that of whole blood. PDGF and TGF‐β1 concentrations in PRG increased to 2.46 and 4.15 times those in PRP, respectively. Hematoxylin and eosin (H&E) and Masson staining showed that the number of inflammatory cells in healing tissue decreased and the collagen fibers significantly increased in TP and TP + PRG groups at 4, 8, and 12 weeks postoperatively compared to those in Tear group. Neatly arranged, interlaced, and dense collagen fibers were found between the anterior horn and bone at 12 weeks. H&E and toluidine blue staining showed that the injury to the femoral condyle cartilage was alleviated. The healing performance in TP + PRG group was better and faster than that in TP group. The maximum tensile fracture strength of the meniscus progressively increased at 8 and 12 weeks postoperatively.
Conclusions
Anterior horn injury of the medial meniscus in rabbits can be repaired using the TP technique, and the addition of autologous PRG to the bone tunnel promotes early healing of the meniscus and bone postoperatively. Meanwhile, both treatments can reduce the secondary damage to the cartilage due to osteoarthritis.
Keywords: Medial Meniscus, Platelet‐Rich Plasma Gel, Rabbit, Root Tear, Transtibial Pull‐Out Repair
Anterior horn injury of the medial meniscus in rabbits can be repaired using transtibial pull‐out repair (TP), and the addition of autologous platelet‐rich plasma gel (PRG) to the bone tunnel promotes early healing of the meniscus and bone at 4, 8, and 12 weeks postoperatively.
Introduction
The meniscus is a crescent‐shaped fibrocartilage tissue located between the femoral condyle and tibial plateau that is composed of chondrocytes and collagen fibers. 1 The meniscus is firmly fixed to the tibial plateau through the meniscal root, and the integrity of the root is critical for the function of the meniscus. Meniscal root tear is a common injury of the knee joint. 2 , 3 , 4 With improvement in the success rate of meniscal root tear evaluation using imaging modalities and a deeper understanding of meniscal function, increasing attention has been paid to treating meniscal root tears.
Currently, methods for treating meniscal root tears include conservative treatment, partial meniscectomy, and meniscal repair. Although partial meniscectomy relieves clinical symptoms in most patients, it fails to restore the function of the meniscus or prevent the progression of osteoarthritis. 5 Capillary plexus and small blood vessels are connected between the meniscal root and tibia, and the collagen fibers in the meniscal root are radial; thus, the meniscal root has good blood supply 6 and sufficient tensile strength. 7 These characteristics provide the anatomical basis for meniscal repair. Increasingly, in magnetic resonance imaging (MRI) examinations or secondary arthroscopic reexaminations, transtibial pull‐out repair (TP) has been shown to result in anatomical reduction of the injured meniscus and alleviate secondary damage to the articular cartilage. 8 , 9 , 10 , 11 , 12 However, some studies have suggested this may not occur postoperatively. 13 , 14 , 15 Due to ethical constraints and the limitations of imaging techniques, the healing effects of this repair technique cannot be accurately observed in humans at the histopathological level. To learn more about the healing process of the medial meniscus and the secondary changes in knee cartilage after TP, we aimed to dynamically observe the surgical effects of TP at the histological level using an animal model.
It often takes a long time for the meniscal root to heal in the bone tunnel. Recently, biological methods for treating meniscal injuries have become a popular area of research. Platelet‐rich plasma (PRP) is a platelet concentrate obtained by centrifuging autologous whole blood. Platelet‐rich plasma gel (PRG) is formed by adding an activator to PRP. As a blood derivative containing a supraphysiological concentration of platelets, PRG is rich in platelet‐derived growth factor (PDGF), transforming growth factor‐β1 (TGF‐β1), vascular endothelial growth factor, fibroblast growth factor, and other growth factors. PRG can promote various types of cell migration and proliferation, and it provides a good microenvironment for tissue repair and regeneration. Moreover, PRG is simple to prepare and easy to apply in clinical practice; thus, the role of PRG in the healing of bones, cartilage, and tendons is attracting increasing attention. 16 , 17 However, most studies have focused on the autogenous repair of menisci using PRG. Whether the addition of PRG into the bone tunnel can promote the healing between the meniscal root and bone has not been reported.
This study aimed to: (i) observe the healing of the torn anterior horn of medial meniscus to the bone tunnel after TP surgery in a rabbit model; (ii) explore whether the addition of autologous PRG to the bone tunnel can promote early healing of the meniscus and bone postoperatively.
Materials and Methods
Experimental Animals
The animal experimental protocol was established according to the appropriate ethical guidelines and approved by the Ethics Committee of Hebei Medical University (IACUC‐Hebmu‐PD‐2016012). Seventy healthy male New Zealand rabbits weighing approximately 2.5–3.0 kg (supplied by Changyang Xishan Farm in Beijing, China, SCXK [Jing] 2016‐0007) were selected. Seven rabbits were used to compare the maximum tensile rupture strength of the anterior root of the medial meniscus (MM) between the left and right legs. The remaining 63 animals were randomly divided into three groups: the anterior horn tear of the MM group (Tear group), the anterior horn tear of the MM + TP surgery group (TP group), and the anterior horn tear of the MM + TP surgery + PRG group (TP + PRG group). Three rabbits in each group were selected for general observation and histopathological experiments at 4, 8, and 12 weeks after surgery. To examine the maximum tensile fracture strength of the meniscal anterior horn, biomechanical experiments were performed on six rabbits in each group at 8 and 12 weeks postoperatively.
Preparation of PRP and PRG
PRP was prepared using the procedure described by Landesberg et al. 18 In brief, 6 mL of blood was extracted from the central artery of the rabbit ear, of which 1 mL was tested for the platelet count and 5 mL was used to prepare PRP. The blood was spun at 200 g for 10 min, and the upper plasma (more than 3 mm below the interface) was extracted into another sterile tube. After centrifugation for another 10 min (200 g), the upper three quarters of the liquid was removed, and the remaining one quarter of the liquid was PRP. Then, 0.3 mL of PRP was taken for counting, and the remainder was reserved for the next experiments.
A PRP activator was prepared by adding 1 mL of 10% calcium chloride into 1000 units of bovine thrombin. PRG is the mixture of PRP and the PRP activator.
Platelet Content Detection
The platelet content in 0.3 mL of whole blood or PRP was detected using fully automatic hematology analyzers (Sysmex Corporation, Japan).
Detection of Rabbit PDGF and TGF‐β1
The levels of PDGF and TGF‐β1 in PRP and PRG were determined using an enzyme‐linked immunosorbent assay (ELISA) kits (Shanghai Enzyme‐linked Biotechnology Co., Ltd., China). PRP and PRG were prepared from the same animal blood samples on the same day and stored at −80°C until they were used. All specimens were thawed at room temperature on the experimental day. Then, 100 μL of the supernatants were withdrawn, and the concentrations of rabbit PDGF and TGF‐β1 were detected using ELISA kits, according to the manufacturer's instructions. The optical density (OD) value of each sample was determined using a microplate reader (FLUOstar® Omega, BMG LABTECH Ltd., Germany). The standard solutions in the ELISA kit were used to draw the standard curve and obtain the linear regression equation. The OD value of each sample was entered into an equation to calculate the concentration of PDGF or TGF‐β1.
Surgical Technique
Rabbits were anesthetized using pentobarbital sodium (30 mg/kg) intravenously. Considering postoperative fixation, the operation was performed on only one knee of each rabbit, while the other knee was not treated. Penicillin (400,000 units) was intramuscularly injected once a day for 3 consecutive days.
Establishment of a model of anterior horn tear of the MM: The anterior horn of the MM was selected for the tear as the anterior and posterior stops of the MM are on the tibial plateau, while the posterior root of the lateral meniscus is attached to the femur in rabbits (different from humans). The anterior horn of the MM can be fully exposed after opening the articular cavity, which allowed fixation to be performed in the closest anatomical position. A medial patellar rim incision was made, the joint cavity was opened, the patella was dislocated laterally, and the knee was maintained in flexion. The anterior root of the MM was exposed completely, and the meniscus was cut at the junction of the anterior root of the meniscus and the inserted ligament (Figure 1A). Before the wound was closed, the joint cavity was rinsed with saline. An aseptic dressing was applied to cover and bind the wound, and the leg was fixed with plaster in the knee flexion position.
Fig. 1.
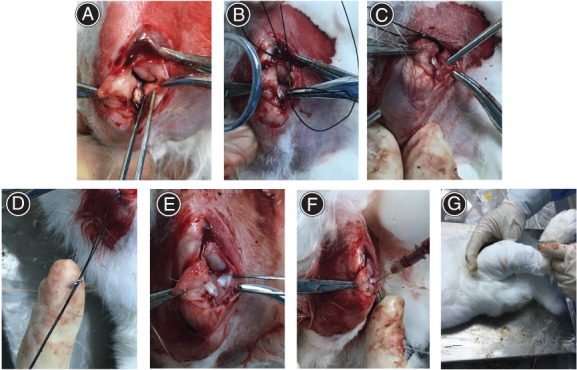
The anterior horn tear of the medial meniscus was repaired using the transtibial pull‐out repair technique. (A) The anterior horn of the medial meniscus in a rabbit was cut from the tibial plateau. (B) The modified Mason–Allen suturing technique was performed at the anterior horn end of the torn meniscus. (C) A tibial tunnel was drilled. (D) The sutures were firmly fixed in the bone tunnel using a self‐made steel wire. (E) The horn of the medial meniscus was pulled into the tibial tunnel. (F) The mixture (platelet‐rich plasma gel) was injected into the tibial tunnel. (G) The operative leg was fixed in the knee flexion position using plaster
TP technique: Cross‐horizontal and vertical mattress sutures were performed using two 4–0 operation lines at the anterior angular end of the torn meniscus (Figure 1B), known as the modified Mason–Allen (MMA) stitch. A drill bit was used to make a 2‐mm diameter bone tunnel toward the lateral tibial tubercle, starting from the position of the tibial plateau below the torn anterior horn of the meniscus (Figure 1C). A steel wire was used to pull the sutures through the tunnel. With the knee in flexion, the sutures were firmly fixed in the bone tunnel using a self‐made S‐shaped steel wire (Figure 1D). This caused the anterior horn end of the meniscus to be pulled into the bone marrow by approximately 2 mm, resulting in a tight fit with the tunnel (Figure 1E).
PRG injection: Preoperatively, PRP was prepared from blood drawn from the rabbit ear artery using the Landesberg method. 12 Then, 0.4 mL of PRP and 0.1 mL of activator were drawn up using syringes. After TP, the PRP and activator were injected into the tibial tunnel simultaneously (Figure 1F), and the joint cavity was closed layer by layer. The aseptic dressing of the wound and plaster fixation of the knee were the same as described in the previous section (Figure 1G).
General Observation and Histological Staining of the Meniscus and Articular Cartilage
Three rabbits in each group were euthanized using excessive anesthetics at 4, 8, and 12 weeks. The gross morphologies of the medial condyle of the femur, meniscus, and bone tunnel of the tibia were observed in both the left and right knees. For each animal, a segment of the femur was truncated 2 cm above the distal femur, and a segment of the tibia was truncated 2 cm under the tibial platform. The distal femur with articular cartilage and the tibia with tibial plateau were fixed with paraformaldehyde and decalcified. The anterior horn of the meniscus and the 2‐mm bone wall around the bone tunnel were embedded in paraffin sections. Hematoxylin and eosin (H&E) and Masson staining were performed to observe cell growth and fiber formation and distribution, between the anterior horn of the meniscus and the bone wall. Without harming the articular cartilage, the medial part of the medial condyle of the femur was cut along the sagittal plane of the specimen, and H&E and toluidine blue stain staining were performed to observe changes in the femoral cartilage.
Biomechanical Experiment
At 8 and 12 weeks after surgery, six rabbits in each group were euthanized, and the left and right knee joints were stored in the refrigerator at −20°C for biomechanical experimentation.
The specimens were thawed at room temperature on testing day. The muscle, adipose tissue, and ligaments were removed, and only the MM attachment and tibia platform were left intact. The meniscal body was fixed to the fixture of a biomechanical tester (BOSE, ElectroForce 3200, USA), and the tibia was fixed on a self‐made tube grip. The tensile test was conducted with an acceleration of 0.2 mm/s and a maximum load not exceeding 200 N. The maximum tensile fracture strength was recorded when the meniscus was pulled out or broken from the tibial tunnel.
Statistical Analysis
Data and images were processed using the Statistical Package for the Social Sciences Version 21.0, Origin 7.5, and Adobe Illustrator CS6 software. The results were expressed as mean ± standard deviation. The paired t‐test or Wilcoxon test was used for comparing two groups. One‐way analysis of variance was used to compare three groups, with post hoc Tukey tests to compare two groups when the difference was significant. A statistically significant difference was defined as p < 0.05.
Results
Platelet Content Test
The platelet content in PRP significantly increased to approximately 4.57 times that of whole blood, and relative platelet concentrations were 4.57 ± 1.11 and 1.00 ± 0.44, respectively (p < 0.01). Moreover, the effective concentration of PRP was approximately 3–5 times that of whole blood 19 ; thus, the PRP prepared in this experiment conformed to the standard.
Detection of Rabbit PDGF and TGF‐β1 Levels
The concentrations of PDGF and TGF‐β1 in PRG increased to 2.46 times and 4.15 times those in PRP (p < 0.01), respectively (Figure 2).
Fig. 2.
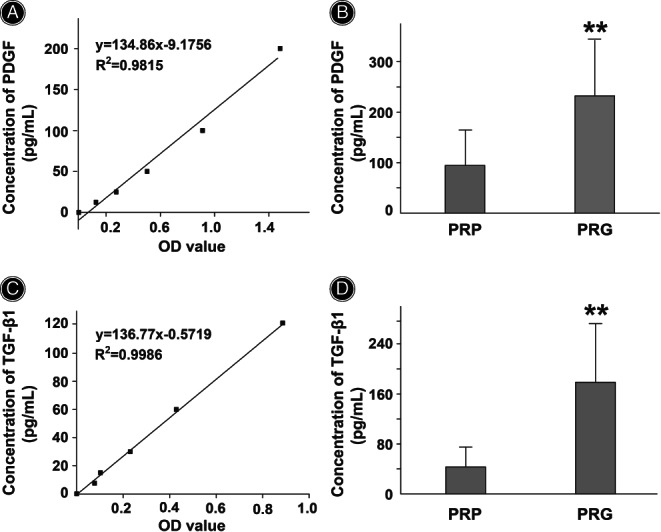
Comparison of platelet‐derived growth factor (PDGF) or transforming growth factor‐β1 (TGF‐β1) levels between platelet‐rich plasma (PRP) and platelet‐rich plasma gel (PRG). (A) Standard curve of PDGF. (B) PDGF concentrations in both PRP and PRG were detected. (C) Standard curve of TGF‐β1. (D) TGF‐β1 concentrations in both PRP and PRG were detected. Data are presented as mean ± standard deviation. N = 14 in each group. The paired t‐test or Wilcoxon test was used to compare the two groups. ** p < 0.01 vs. PRP
General Observation of the Rabbit Knee Joint
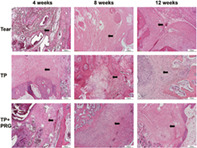
In Tear group, the position of the meniscus was slightly lateral postoperatively. The healing tissue at the anterior attachment appeared dull in color as time progressed. In TP and TP + PRG Groups at 4, 8, and 12 weeks postoperatively, the anterior horn of the meniscus inserted into the bone marrow was not found protruding from the passage, and the color and shape of the meniscus were normal (Figure 3). At the gross inspection, the articular cartilage of the distal femur had a similar appearance in all three groups.
Fig. 3.
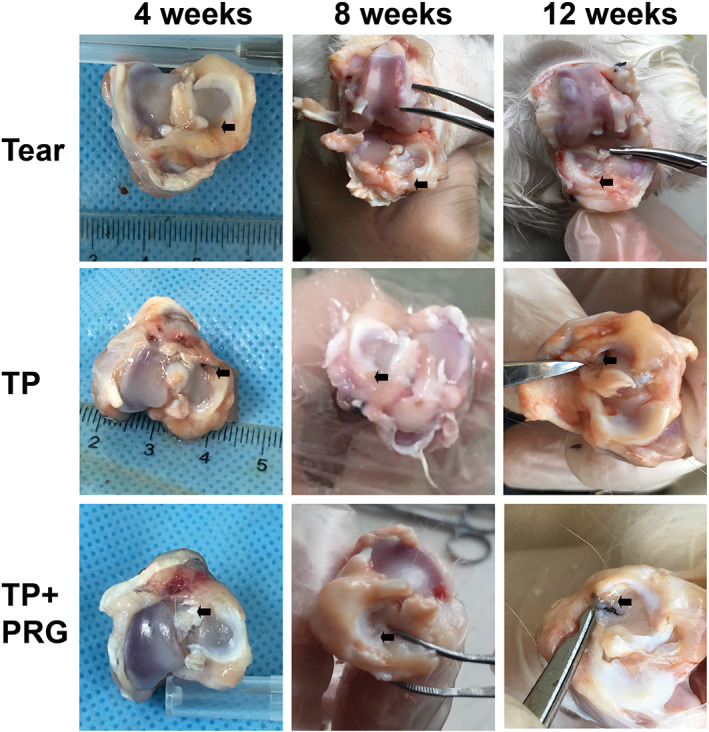
Morphological changes in the rabbit meniscus. The general appearances of the menisci, including their position, shape, and color, were shown in Tear group, TP group and TP + PRG group at 4, 8, and 12 weeks after surgery. The black arrow points to the tear in the anterior horn of meniscus. Tear: the anterior horn tear of the medial meniscus; TP: the anterior horn tear + TP surgery; TP + PRG: the anterior horn tear + TP surgery + PRG
H&E and Masson Staining of the Meniscus
In conventional H&E staining, the basic tissues, such as nuclei, were stained blue, and the acidic tissues, such as the cytoplasm, were stained red. Collagen fibers were red, and the inter‐fibrillary bundles were weakly alkaline with H&E staining. With Masson staining, collagen fibers were green or blue.
At 4 weeks after surgery, the healed tissues in Tear group showed acute inflammation, with a disordered tissue structure in the injured area, dilated small veins and capillaries, inflammatory cells that infiltrated the tissue space, and few disordered collagen fibers in the anterior horn. In TP group, the space between the anterior horn and bone was filled with a few inflammatory cells, and the collagen fibers between them were arranged irregularly. In TP + PRG group, inflammation was significantly reduced, and the distribution of collagen fibers was loose in the gap (Figures 4 and 5).
Fig. 4.
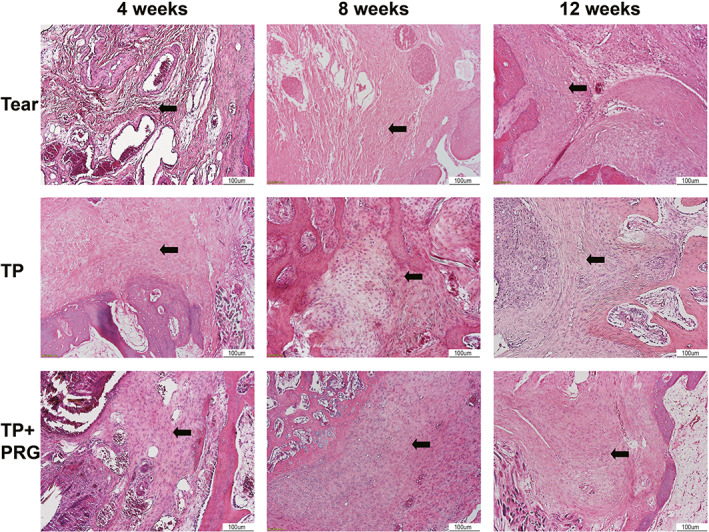
Histopathological changes in healing tissue in the anterior horn of the meniscus evaluated using hematoxylin and eosin staining. The cell growth and fiber formation and distribution between the anterior horn of the meniscus and the bone wall can be seen in Tear group, TP group, and TP + PRG group at 4, 8, and 12 weeks after surgery. The black arrows indicate collagen fibers. Tear: the anterior horn tear of the medial meniscus; TP: the anterior horn tear + TP surgery; TP + PRG: the anterior horn tear + TP surgery + PRG
Fig. 5.
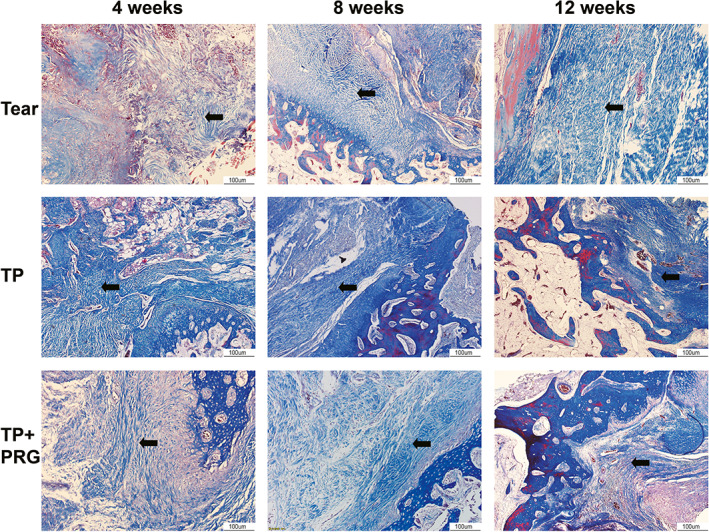
Histopathological changes in healing tissue in the anterior horn of the meniscus evaluated using Masson staining. Collagen fibers between the anterior horn of the meniscus and the bone wall can be seen in Tear group, TP group, and TP + PRG group at 4, 8, and 12 weeks after surgery. The black arrows indicate collagen fibers. Tear: the anterior horn tear of the medial meniscus; TP: the anterior horn tear + TP surgery; TP + PRG: the anterior horn tear + TP surgery + PRG
At 8 weeks postoperatively, the inflammatory changes in healing tissue in Tear group were alleviated. The number of inflammatory cells and telangiectasia decreased, and collagen fibers began to increase. In the same period, hyperplasia of fibrous tissue in the interstitial space in TP group was more obvious than that in the interstitial space in Tear group, and the arrangement of collagen fibers was slightly disordered. Meanwhile, the fibrous tissue in TP + PRG group was dense, and the fibroblasts were arranged regularly compared with those in Tear group. The number of collagen fibers between the anterior horn and bone increased, and some collagen fibers were connected to the bone (Figures 4 and 5).
At 12 weeks after surgery, Tear group was in the recovery stage of inflammation with obvious fibrous tissue hyperplasia. In TP group, many collagen fibers were observed to be arranged in an orderly distribution in the crevices, and a few collagen fibers were attached to the anterior horn and bone. In TP + PRG group, the number of fibroblasts decreased, and more neatly arranged, interlaced, and dense collagen fibers filled the gap between the anterior horn and bone tunnel (Figures 4 and 5).
H&E and Toluidine Blue Staining of the Condylar Cartilage of the Femur
Figure 6 shows the H&E staining of the femoral condyle cartilage. In the healthy control group, the surface of the cartilage was smooth, and the layers were clear. The tidal lines were intact, the chondrocytes were arranged neatly, and the matrix was evenly stained. Compared with the healthy control group, 4 weeks after surgery, the surface of the cartilage in Tear group was rough and the layers were unclear. The tide line was blurred. The chondrocytes were disordered, and some gathered in clusters. The cartilage surfaces in Groups B and C were slightly uneven, and the cells were arranged neatly without chondrocyte clustering. At 8 weeks postoperatively, the cartilage layer in Tear group became thinner. The tidal line was blurred and discontinuous. The number of chondrocytes decreased, and a necrosis cluster appeared in the surface cells. The surfaces of the cartilage layers in TP and TP + PRG Groups were uneven. Compared with Tear group, the chondrocytes were arranged in order and distributed in multiple layers, and the tidal line was clear. At 12 weeks after surgery, the surface of the cartilage layer in Tear group was severely deficient, thinned, and uneven and it had an exposed subchondral bone in some areas. The tide line was blurred and discontinuous. The number of chondrocytes decreased, they were disordered, and some were necrotic. The lacunae of the cartilage were empty. Although there was some damage and thinning of the cartilage layer in Groups B and C, they were significantly improved compared with Tear group. TP + PRG group showed better results than TP group in the same period.
Fig. 6.
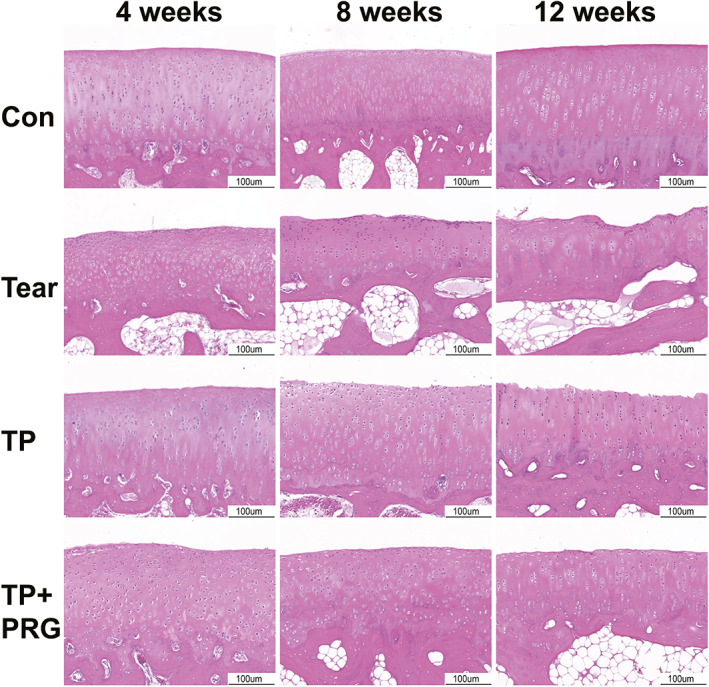
Histopathological changes in the femoral cartilage in rabbits evaluated using hematoxylin and eosin staining. The changes in the cartilage layer at the end of the femur were shown in the Con group, Tear group, TP group, and TP + PRG group at 4, 8, and 12 weeks after surgery. Con: the healthy control; Tear: the anterior horn tear of the medial meniscus; TP: the anterior horn tear + TP surgery; TP + PRG: the anterior horn tear + TP surgery + PRG
The proteoglycan content can be indicated by the color and thickness of the cartilage in toluidine blue staining. The healthy control group tissues had a thicker cartilage layer, larger, blue‐stained area, and darker staining. With the extension of the postoperative time (4, 8, and 12 weeks), the surface defects in the cartilage layer in Tear group gradually worsened, and the blue‐stained area became thinner and lighter. At the same period after surgery, the cartilage surface wear in TP group and TP + PRG group was reduced, and the blue‐stained cartilage area were larger and darker. Furthermore, cartilage staining in TP + PRG group was better than that in TP group at the same period, although neither group reached the level of staining in the healthy control group (Figure 7).
Fig. 7.
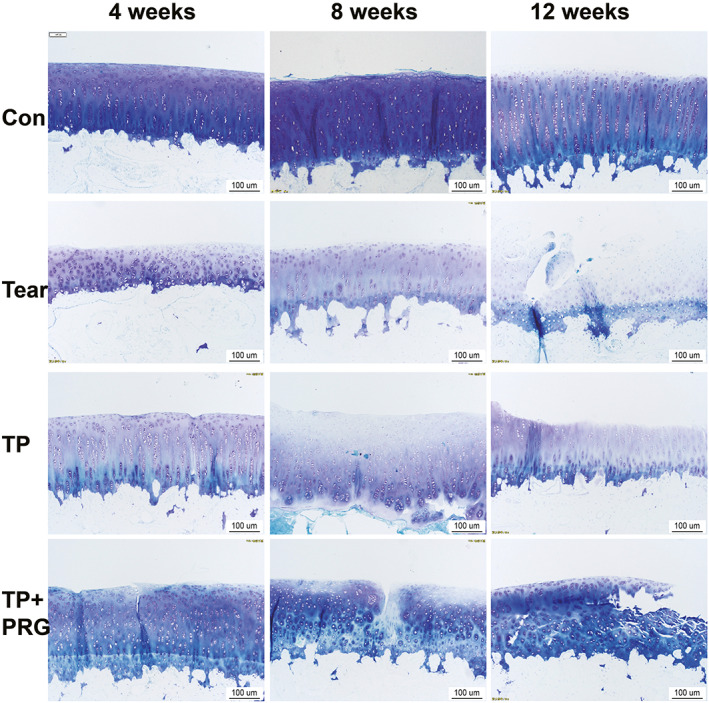
Histopathological changes in the femoral cartilage in rabbits evaluated using toluidine blue staining. The changes in the femoral condyle cartilage, especially those in the proteoglycan content, were shown in the Con group, Tear group, TP group, and TP + PRG group at 4, 8, and 12 weeks after surgery. Con: the healthy control; Tear: the anterior horn tear of the medial meniscus; TP: the anterior horn tear + TP surgery; TP + PRG: the anterior horn tear + TP surgery + PRG
Biomechanical Experiment
Figure 8A shows no statistically significant difference in the maximum tensile fracture strength of the MM between the right and left knees in healthy rabbits, which were 102.0 ± 17.9 N and 92.8 ± 24.7 N, respectively (p > 0.05).
Fig. 8.
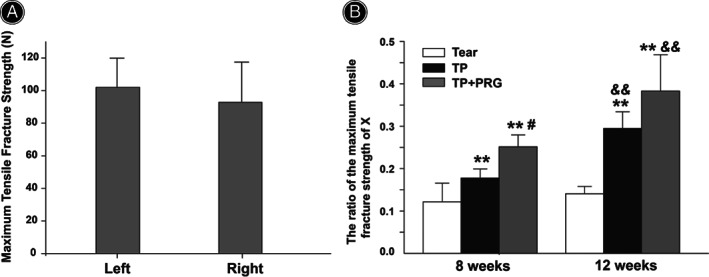
Biomechanical experiment of the anterior horn of the medial meniscus. (A) No statistically significant difference in the maximum tensile fracture strength of the medial meniscus was observed between the right and left knees of healthy rabbits (seven rabbits). (B) Comparisons of the maximum tensile fracture strength of the anterior horn of the medial meniscus among the three groups (i.e., Tear, TP, and TP + PRG groups) 8 and 12 weeks after surgery. X: operative leg to nonoperative leg in the same animal. Tear: the anterior horn tear of the medial meniscus; TP: the anterior horn tear + TP surgery; TP + PRG: the anterior horn tear + TP surgery + PRG. N = 6 in each group. **p < 0.01 vs. Tear group for the same period; # p < 0.05 vs. TP group for the same period; and & p < 0.01 vs. 8 weeks postoperatively in the same group
At 8 and 12 weeks postoperatively, the maximum tensile fracture strengths in TP and TP + PRG groups were significantly increased compared to those in Tear group (Figure 8B). At 8 weeks after surgery, the strengths in TP and TP + PRG groups were 1.46 and 2.07 times that in Tear group, respectively (p < 0.01). Meanwhile, the intensity in TP and TP + PRG groups had increased to 2.10 and 2.72 times that in Tear group, respectively, 12 weeks after surgery (p < 0.01).
At 8 weeks postoperatively, the maximum tensile fracture strength of the MM in TP + PRG group was significantly increased, and it was 41.58% higher than that in TP group (p < 0.05). The strength in TP + PRG group at 12 weeks after surgery showed an increasing trend compared with that in TP group; however, no significant difference was observed. The meniscal strength in TP + PRG group at 8 and 12 weeks after surgery reached 25.14% and 38.31% of the normal meniscal strength, respectively (Figure 8A,B).
Furthermore, Figure 8B shows no significant differences in the maximum tensile fracture strengths of the meniscus observed 8 or 12 weeks after surgery in Tear group. However, with extension of the postoperative time, the mechanical strength of the meniscus 12 weeks after surgery was stronger than that after 8 weeks postoperatively, increasing by 65.97% in TP group and 52.4% in TP + PRG group (p < 0.01).
Discussion
The aim of this study was to dynamically observe the healing effects of simple TP and TP with platelet‐rich gel (PRG) using histopathological studies and biomechanical experiments. Our study found that anterior horn injury of the MM could be repaired in rabbits using the TP technique, and the addition of autologous PRG to the bone tunnel promoted early healing of the meniscus and bone postoperatively. In addition, both could reduce secondary damage to the cartilage due to osteoarthritis.
TP Surgery in Humans
With an increased understanding of the structure and function of the meniscus and the development of arthroscopic technology, the damaged root of the meniscus should be repaired as much as possible to preserve the function of the meniscus. Currently, there are three main methods to repair meniscal root tears using arthroscopy, including TP, 8 anchor suture repair, and side‐to‐side suture repair, among which some clinical experience with TP has been obtained. Although some clinical studies have focused on the repair of meniscal root tears using TP, their conclusions remain controversial. It has been reported that the clinical and imaging impacts of MM traction repair are significantly better than those of partial meniscectomy. Moreover, the annular tension of the meniscus recovers well and the healing is good based on MRI and secondary arthroscopy findings. 9 Lee et al. found that after 24 months, the Hospital for Special Surgery and Lysholm scores increased from 61.1 to 93.8 and 57.0 to 93.1, respectively, in 21 cases of knee meniscal repair, and none of the patients developed arthritis. 10 Nine patients with meniscal root tear accompanied by ligament injury were followed up for an average with of 41.1 months after surgery, and root healing was confirmed by arthroscopy in five patients and MRI in four patients. 11 Biomechanical tests showed that when the posterior root of the lateral meniscus was torn, the average peak contact pressure increased from 2.8 MPa to 4.2 MPa, and the contact area decreased from 451 mm2 to 304 mm2 compared with a healthy meniscus. After repair, the peak contact pressure decreased to 2.9 MPa, and the contact area also increased to 386 mm2; thus, meniscal repair can achieve a good outcome. 12 However, some studies have found that although the clinical prognosis scores increased after meniscal root repair, most menisci were only partially healed, with increased extrusion and progression of the articular cartilage defect grade on follow‐up MRI. 13 During a postoperative follow‐up study of 11 patients for an average of 13.4 months, it was found that none of them healed completely, including loose healing of five knees, scar repair of four knees, and nonunion of two knees. 14 After an average of 33 months of follow‐up for 51 patients, Moon et al. found that extrusion increased from 3.6 mm to 5.0 mm on MRI. 15
TP Surgery in Animal Model
Confined by ethics and imaging techniques, clinical studies cannot accurately observe the effects of the repair operation at the histopathological level in humans. Animal studies on repair of meniscal root injuries using TP are rare. Gao et al. reported that there was healing tissue between the fixed attachment and bone after surgery; however, the refixed meniscal attachment did not improve the biomechanical properties of the meniscus or alleviate injuries related to secondary osteoarthritis. 20 Another group of authors used a similar surgical technique to repair the meniscal root of rabbits in which the cartilage of the tibial plateau was removed and fixed on the tibial plateau, and they found a gap (no attachment) between the fixed anterior horn of the meniscus and the tibial plateau. 21 When evaluating the use of TP surgery for meniscal repair using animal experiments, the following problems should be fully considered in animal experiments: (1) anatomical characteristics of the animal meniscus, (2) method for establishing the bone tunnel, (3) suturing method, (4) single or double knee surgery, (5) whether postoperative plaster fixation should be applied, (6) postoperative observation time, and (7) how to select an appropriate control group (e.g., due to the large individual differences among animals, it is better to choose the nonoperative leg of the same animal as the control in biomechanical experiments). Overall, the most fundamental and important question is how to ensure that the repaired anterior horn of the meniscus is firmly attached to the bone tunnel.
In this study, we made various choices to ensure the success of the operation. First, it was necessary to consider whether one or both knees should be operated on and whether the affected limb should be fixed in plaster after surgery. It has been reported that when a rabbit limb is immobilized for a long time, the meniscus can have varying degrees of degenerative changes (especially after 8 weeks of immobilization) that do not improve with activity. 22 In the preliminary experiment, we found that animals undergoing double knee surgery were not in good condition, and the survival rate was low. Moreover, if the operated leg was not fixed, the anterior horn of the meniscus would be pulled out from the bone channel as the animal moved, and subsequent experiments could not be performed. Therefore, we selected a single knee joint for surgery and fixed it using a long leg cast in the flexion position as rabbits use the squatting position of lower limb flexion in cages. The cast was removed after 4 weeks, with negligible impact on mobility. Second, similar to the results described by Goertzen et al., we found that the maximum tensile fracture strength of the healthy MM anterior attachment was not significantly different between the knees of the rabbits, so we could select either leg for surgery. 23 Third, the anterolateral area below the tibial plateau was chosen as the exit of the bone tunnel and the position under the torn anterior horn was chosen as the entrance to the bone tunnel because there is less bone at the entrance to the bone tunnel under the anterior meniscal horn. The bone tunnel could be destroyed if the anterior medial side of the tibial plateau was chosen as the exit. Moreover, the muscle tissue is attached below the tibial plateau, which can cover the fixed steel wire, allowing it to avoid contact with the skin and reducing the risk of infection. Fourth, the MMA suturing technique had superior biomechanical properties and could better pull the anterior horn of the meniscus into the bone tunnel. 24 , 25 , 26 These choices ensured that the anterior horn could firmly attach to the anatomical position.
The Effects of TP Surgery
Our findings showed that the maximum tensile fracture strength of the repaired meniscus began to increase by 8 weeks after surgery and had doubled by 12 weeks. This may be because the collagen fibers between the refixed anterior horn of the meniscus and the bone gradually increased, from a loose arrangement at 4 weeks to a neat, interlaced, and dense arrangement by 12 weeks postoperatively. There are abundant blood vessels, nerves, and cytokines in the bone marrow. Bone marrow mesenchymal stem cells may differentiate into fibroblasts and fibrochondrocytes, which can synthesize and secrete collagen fibrils and matrices. 27 , 28 , 29 This is due to the existence of shear force in the bone marrow (the broken end of the meniscus can move slightly during intramedullary bone fixation). Therefore, the healing tissue could change from granulation to fibrocartilage tissue. 20 Due to the enhancement of meniscal function and the decrease in tibiofemoral peak pressure, the cartilage injury of the medial part of the femoral condyle significantly reduced in the operation group.
The Effect of PRG
At the same time periods, the histological staining of the meniscus in the PRG group showed more collagen fibers between the anterior horn of the meniscus and the bone than the operation group. Meanwhile, the maximum tensile fracture strength of the meniscus increased, the injury to the femoral condyle cartilage decreased, and healing speed was evidently accelerated. The main structure of PRG consists of a fibrin network and platelets. This typical reticular scaffold structure is conducive to the cell obtaining nutrients and the flow of metabolites. A high concentration of platelets can produce more than 30 growth factors, including PDGF and TGF‐β1, which play important roles in promoting cell proliferation and differentiation. 30 , 31 , 32 However, because PRG can only be injected once during surgery, the long‐term effects are not clear.
Limitations and Strengths
There are several limitations to the present study. First, the most common root tear in clinical practice is posterior root tear of the medial meniscus. Although there is essentially no difference between the anterior and posterior roots of the meniscus, there are some differences in the types and proportions of collagen fibers as well as the mechanical strength of the cartilage. In future, larger animal models should be used to better simulate clinical scenarios. Second, it has been shown that the change in the distribution pattern of collagen fibers might be as important as the change in the number of collagen fibers; therefore, we plan to explore the mechanism in which the torn horn of the medial meniscus heals to the bone wall in our next study. The study will provide an experimental basis and theoretical support for the use of this surgical technique in clinical practice and the application of PRG in this technique.
Conclusion
In conclusion, anterior horn injuries of the MM can be repaired using TP. The addition of autologous PRG to the bone tunnel promotes early healing of the meniscus and bone after surgery. Simultaneously, both can reduce the secondary damage to the cartilage related to osteoarthritis. Meniscal healing is a long‐term process. Although the maximum tensile fracture strength of the meniscus after repair did not reach the value of healthy controls, we believe that the function of the meniscus would gradually recover as time passes.
Disclosure
The authors declare that they have no conflict of interest.
Authors' Contributions
PC and BHS established a model of meniscus anterior horn tear and carried out follow‐up experiments. PC, BHS, and LSZ analyzed the results. PC, BHS, CXS, and XFW wrote the manuscript. YFD, TYC, JLS, KS, LSZ participated in the experiment. XFW and CXS designed and guidance the study. All authors read and approved the final manuscript.
Peng Cui and Bai‐hai Sun these authors contributed equally to this work.
Grant Sources: This study was supported by the Science and Technology Program of Hebei Province (No. 16277785D).
Contributor Information
Chen‐xia Shi, Email: chenxiashi@hotmail.com.
Xiao‐feng Wang, Email: wangxf2012313@163.com.
References
- 1. Markes AR, Hodax JD, Ma CB. Meniscus form and function. Clin Sports Med. 2020;39:1–12. [DOI] [PubMed] [Google Scholar]
- 2. Jones JC, Burks R, Owens BD, Sturdivant RX, Svoboda SJ, Cameron KL. Incidence and risk factors associated with meniscal injuries among active‐duty US military service members. J Athl Train. 2012;47:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bin SI, Kim JM, Shin SJ. Radial tears of the posterior horn of the medial meniscus. Arthroscopy. 2004;20:373–8. [DOI] [PubMed] [Google Scholar]
- 4. Allaire R, Muriuki M, Gilbertson L, Harner CD. Biomechanical consequences of a tear of the posterior root of the medial meniscus. Similar to total meniscectomy. J Bone Jt Surg. 2008;90:1922–31. [DOI] [PubMed] [Google Scholar]
- 5. Krych AJ, Johnson NR, Mohan R, Dahm DL, Levy BA, Stuart MJ. Partial meniscectomy provides no benefit for symptomatic degenerative medial meniscus posterior root tears. Knee Surg Sports Traumatol Arthrosc. 2018;26:1117–22. [DOI] [PubMed] [Google Scholar]
- 6. Arnoczky SP, Warren RF. Microvasculature of the human meniscus. Am J Sports Med. 1982;10:90–5. [DOI] [PubMed] [Google Scholar]
- 7. Kim YM, Joo YB. Pullout failure strength of the posterior horn of the medial meniscus with root ligament tear. Knee Surg Sports Traumatol Arthrosc. 2013;21:1546–52. [DOI] [PubMed] [Google Scholar]
- 8. Kim YM, Rhee KJ, Lee JK, Hwang DS, Yang JY, Kim SJ. Arthroscopic pullout repair of a complete radial tear of the tibial attachment site of the medial meniscus posterior horn. Arthroscopy. 2006;22(795):e1–4. [DOI] [PubMed] [Google Scholar]
- 9. Kim SB, Ha JK, Lee SW, Kim DW, Shim JC, Kim JG, et al. Medial meniscus root tear refixation: comparison of clinical, radiologic, and arthroscopic findings with medial meniscectomy. Arthroscopy. 2011;27:346–54. [DOI] [PubMed] [Google Scholar]
- 10. Lee JH, Lim YJ, Kim KB, Kim KH, Song JH. Arthroscopic pullout suture repair of posterior root tear of the medial meniscus: radiographic and clinical results with a 2‐year follow‐up. Arthroscopy. 2009;25:951–8. [DOI] [PubMed] [Google Scholar]
- 11. Kim YJ, Kim JG, Chang SH, Shim JC, Kim SB, Lee MY. Posterior root tear of the medial meniscus in multiple knee ligament injuries. Knee. 2010;17:324–8. [DOI] [PubMed] [Google Scholar]
- 12. Schillhammer CK, Werner FW, Scuderi MG, Cannizzaro JP. Repair of lateral meniscus posterior horn detachment lesions: a biomechanical evaluation. Am J Sports Med. 2012;40:2604–9. [DOI] [PubMed] [Google Scholar]
- 13. Kaplan DJ, Alaia EF, Dold AP, Meislin RJ, Strauss EJ, Jazrawi LM, et al. Increased extrusion and ICRS grades at 2‐year follow‐up following transtibial medial meniscal root repair evaluated by MRI. Knee Surg Sports Traumatol Arthrosc. 2018;26:2826–34. [DOI] [PubMed] [Google Scholar]
- 14. Seo HS, Lee SC, Jung KA. Second‐look arthroscopic findings after repairs of posterior root tears of the medial meniscus. Am J Sports Med. 2011;39:99–107. [DOI] [PubMed] [Google Scholar]
- 15. Moon HK, Koh YG, Kim YC, Park YS, Jo SB, Kwon SK. Prognostic factors of arthroscopic pull‐out repair for a posterior root tear of the medial meniscus. Am J Sports Med. 2012;40:1138–43. [DOI] [PubMed] [Google Scholar]
- 16. Lopez‐Vidriero E, Goulding KA, Simon DA, Sanchez M, Johnson DH. The use of platelet‐rich plasma in arthroscopy and sports medicine: optimizing the healing environment. Arthroscopy. 2010;26:269–78. [DOI] [PubMed] [Google Scholar]
- 17. Shibata M, Takagi G, Kudo M, Kurita J, Kawamoto Y, Miyagi Y, et al. Enhanced sternal healing through platelet‐rich plasma and biodegradable gelatin hydrogel. Tissue Eng, Part A. 2018;24:1406–12. [DOI] [PubMed] [Google Scholar]
- 18. Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet‐rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58:297–300. [DOI] [PubMed] [Google Scholar]
- 19. Taylor DW, Petrera M, Hendry M, Theodoropoulos JS. A systematic review of the use of platelet‐rich plasma in sports medicine as a new treatment for tendon and ligament injuries. Clin J Sport Med. 2011;21:344–52. [DOI] [PubMed] [Google Scholar]
- 20. Gao J, Wei X, Messner K. Healing of the anterior attachment of the rabbit meniscus to bone. Clin Orthop Relat Res. 1998;348:246–58. [PubMed] [Google Scholar]
- 21. Hong JH, Park JI, Kim KH, Kim YM, Joo YB, Jeon YS. Repair of the complete radial tear of the anterior horn of the medial meniscus in rabbits: a comparison between simple pullout repair and pullout repair with human bone marrow stem cell implantation. Knee Surg Relat Res. 2011;23:164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ochi M, Kanda T, Sumen Y, Ikuta Y. Changes in the permeability and histologic findings of rabbit menisci after immobilization. Clin Orthop Relat Res. 1997;334:305–15. [PubMed] [Google Scholar]
- 23. Goertzen D, Gillquist J, Messner K. Tensile strength of the tibial meniscal attachments in the rabbit. J Biomed Mater Res. 1996;30:125–8. [DOI] [PubMed] [Google Scholar]
- 24. Lee DW, Jang SH, Ha JK, Kim JG, Ahn JH. Meniscus root refixation technique using a modified Mason‐Allen stitch. Knee Surg Sports Traumatol Arthrosc. 2013;21:654–7. [DOI] [PubMed] [Google Scholar]
- 25. Chung KS, Choi CH, Bae TS, Ha JK, Jun DJ, Wang JH, et al. Comparison of tibiofemoral contact mechanics after various transtibial and all‐inside fixation techniques for medial meniscus posterior root radial tears in a porcine model. Arthroscopy. 2018;34:1060–8. [DOI] [PubMed] [Google Scholar]
- 26. LaPrade RF, LaPrade CM, Ellman MB, Turnbull TL, Cerminara AJ, Wijdicks CA. Cyclic displacement after meniscal root repair fixation: a human biomechanical evaluation. Am J Sports Med. 2015;43:892–8. [DOI] [PubMed] [Google Scholar]
- 27. Zellner J, Pattappa G, Koch M, Lang S, Weber J, Pfeifer CG, et al. Autologous mesenchymal stem cells or meniscal cells: what is the best cell source for regenerative meniscus treatment in an early osteoarthritis situation? Stem Cell Res Ther. 2017;8:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ding Z, Huang H. Mesenchymal stem cells in rabbit meniscus and bone marrow exhibit a similar feature but a heterogeneous multi‐differentiation potential: superiority of meniscus as a cell source for meniscus repair. BMC Musculoskelet Disord. 2015;16:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vangsness CT Jr, Farr J 2nd, Boyd J, Dellaero DT, Mills CR, LeRoux‐Williams M. Adult human mesenchymal stem cells delivered via intra‐articular injection to the knee following partial medial meniscectomy: a randomized, double‐blind, controlled study. J Bone Jt Surg. 2014;96:90–8. [DOI] [PubMed] [Google Scholar]
- 30. Okuda K, Kawase T, Momose M, Murata M, Saito Y, Suzuki H, et al. Platelet‐rich plasma contains high levels of platelet‐derived growth factor and transforming growth factor‐beta and modulates the proliferation of periodontally related cells in vitro. J Periodontol. 2003;74:849–57. [DOI] [PubMed] [Google Scholar]
- 31. Qi Y, Tang R, Shi Z, Feng G, Zhang W. Wnt5a/Platelet‐rich plasma synergistically inhibits IL‐1beta‐induced inflammatory activity through NF‐kappaB signaling pathway and prevents cartilage damage and promotes meniscus regeneration. J Tissue Eng Regener Med. 2021;15:612–24. [DOI] [PubMed] [Google Scholar]
- 32. Jalowiec JM, D'Este M, Bara JJ, Denom J, Menzel U, Alini M, et al. An in vitro investigation of platelet‐rich plasma‐gel as a cell and growth factor delivery vehicle for tissue engineering. Tissue Eng, Part C Methods. 2016;22:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]


