Abstract
Background
There is a large body of evidence linking muscular weakness, as determined by low grip strength, to a host of negative ageing‐related health outcomes. Given these links, grip strength has been labelled a ‘biomarker of aging’; and yet, the pathways connecting grip strength to negative health consequences are unclear. The objective of this study was to determine whether grip strength was associated with measures of DNA methylation (DNAm) age acceleration.
Methods
Middle age and older adults from the 2006 to 2008 waves of the Health and Retirement Study with 8–10 years of follow‐up were included. Cross‐sectional and longitudinal regression modelling was performed to examine the association between normalized grip strength (NGS) and three measures of DNAm age acceleration, adjusting for cell composition, sociodemographic variables and smoking. Longitudinal modelling was also completed to examine the association between change in absolute grip strength and DNAm age acceleration. The three DNAm clocks used for estimating age acceleration include the established DunedinPoAm, PhenoAge and GrimAge clocks.
Results
There was a robust and independent cross‐sectional association between NGS and DNAm age acceleration for men using the DunedinPoAm (β: −0.36; P < 0.001), PhenoAge (β: −8.27; P = 0.01) and GrimAge (β: −4.56; P = 0.01) clocks and for women using the DunedinPoAm (β: −0.36; P < 0.001) and GrimAge (β: −4.46; P = 0.01) clocks. There was also an independent longitudinal association between baseline NGS and DNAm age acceleration for men (β: −0.26; P < 0.001) and women (β: −0.36; P < 0.001) using the DunedinPoAm clock and for women only using the PhenoAge (β: −8.20; P < 0.001) and GrimAge (β: −5.91; P < 0.001) clocks. Longitudinal modelling revealed a robust association between change in grip strength from wave 1 to wave 3 was independently associated with PhenoAgeAA (β: −0.13; 95% CI: −0.23, −0.03) and GrimAgeAA (β: −0.07; 95% CI: −0.14, −0.01) in men only (both P < 0.05).
Conclusions
Our findings provide some initial evidence of age acceleration among men and women with lower NGS and loss of strength over time. Future research is needed to understand the extent to which DNAm age mediates the association between grip strength and chronic disease, disability and mortality.
Keywords: Grip strength, DNA methylation, Strength training, Ageing
Introduction
There is a large body of evidence linking muscular weakness, as determined by low grip strength, to a host of negative ageing‐related health outcomes including diabetes, 1 , 2 physical disability, 3 , 4 , 5 cognitive decline (including Alzheimer's disease 6 ) 7 , 8 and early all‐cause mortality. 9 , 10 , 11 , 12 Given these findings, grip strength has been labelled a ‘biomarker of aging’ 13 ; and yet, this crude metric provides no direct biological plausibility or mechanism linking weakness with chronic disease, physical and cognitive impairments or specific causes of mortality.
Ageing is indeed a complex, multidimensional phenomenon that manifests differently between individuals throughout the lifespan and is highly conditional on interactions between genetic, environmental, behavioural and demographic characteristics. 14 The prevailing context for ageing research has been backward‐looking, taking into consideration only the chronological age in years since birth. 15 However, there is a well‐established literature demonstrating that certain subgroups of the population age at different rates, 16 , 17 and thus better understanding these subgroups, and the underlying mechanisms contributing to accelerated ageing phenotypes could lead to improved early detection systems, development of targeted interventions and ultimately a decrease in population disability‐adjusted life years. A growth in research evidence documents that epigenetic phenomena, such as DNA methylation (DNAm), are highly implicated in the development of disease 18 and rate of biological ageing. Given that methylation profiles are thought to be modifiable by lifestyle and other environmental factors, it has been proposed that DNAm age is a robust biological ageing clock providing a superior estimate of true biological age over chronological age. 19 , 20 A number of epigenetic clock measures have been generated for measuring an individual's epigenetic age, and in particular, to indicate accelerated or decelerated biological ageing (i.e. as compared with chronological age). 21 , 22 The objective of this study was to determine the association between grip strength and DNAm age acceleration derived from three epigenetic clocks trained on phenotypic ageing (i.e. age‐related health conditions/profiles) and mortality.
Methods
Cohort
We used a longitudinal panel study [Health and Retirement Study (HRS)] to determine the cross‐sectional and longitudinal association between muscle strength DNAm age acceleration in middle‐aged and older adults. The HRS is a prospective epidemiological resource gathering extensive questionnaires, physical and cognitive measures and biological samples in a cohort of more than 43 000 participants. HRS is a multistage area probability survey of non‐institutionalized, community‐dwelling Americans aged 51 years and older. Study details have been previously described, and all questionnaires are available on the HRS website (http://hrsonline.isr.umich.edu). Briefly, the HRS is the longest running longitudinal study of older adults in the USA, with consistently high response rates. The HRS follows respondents longitudinally until death, and new cohorts have been enrolled since the 1992 baseline interviews, in order to maintain the population representativeness of the study sample. Starting in 2006, a random one‐half sample of HRS participants was selected for an enhanced face‐to‐face interview that included physical and biological measurements (e.g. hand grip strength, blood and saliva collection), and the other half completed only the core interview. The other random one‐half completed the enhanced face‐to‐face interview that included physical and biological measurements in 2008. These half‐sample alternate waves are done so that longitudinal information from the enhanced face‐to‐face interview is available every 4 years at the individual level, and the expanded content is available every wave on a nationally representative half‐sample of the longitudinal panel. This rotating design has continued in all biennial HRS waves since 2006, resulting in follow‐up data on the physical, biomarker and cognitive health measures every 4 years for the subsamples. Our initial study sample of n = 14 781 included all HRS participants aged 51 or older (70.0 years) interviewed in 2006/2008, when physical measure and biomarker collection began in the HRS, and with an 8‐year follow‐up assessment in 2014/2016. The final cohort of n = 1275 reflect only participants with full data on all exposures, covariates and the three dependent DNAm age acceleration clocks, only available on a subsample of the 2016 respondents. The age, sex and racial breakdown of our sample is consistent with that of Americans aged 50 years and older in the USA, based on U.S. census data. 23
Exposures
Grip strength
In HRS, hand grip strength was assessed using a Smedley spring‐type hand dynamometer (Scandidact, Odder, Denmark). Participants were instructed to squeeze the device with maximal effort and then let go. Grip strength assessments were administered while participants were standing with their arm at their side and with the elbow flexed at a 90° angle. After one practice trial, two measurements were taken with each hand, alternating hands. The maximum measurement from the four trials was considered maximal grip strength capacity. Normalized grip strength (NGS) as grip strength per body mass was examined as a continuous predictor.
The HRS methylation sample
DNA methylation assays were done on a non‐random subsample (N = 4018) of people who participated in the 2016 Health and Retirement Venous Blood Study (VBS), 24 as described thoroughly in HRS documentation (https://hrsonline.isr.umich.edu/modules/meta/vbs/2016/desc/HRS2016VBSDD.pdf). 21
Methods for DNAm
DNA methylation data are based on assays using the Infinium MethylationEPIC BeadChip completed at the Advanced Research and Diagnostics Laboratory at the University of Minnesota. Samples were randomized across plates by key demographic variables (i.e. age, cohort, sex, education and race/ethnicity) with 39 pairs of blinded duplicates, as previously described thoroughly in HRS documentation (https://hrsdata.isr.umich.edu/sites/default/files/documentation/data‐descriptions/EPICLOCKS_DD.pdf). 25
Subsample weights
Respondents with at least one valid venous blood result were assigned a VBS weight. The weights were adjusted for the differential probabilities of participation by dividing the HRS 2016 sample weight by the predicted probability of having a valid venous blood result among community‐dwelling 2016 HRS respondents born prior to 1960. A separate respondent level weight was created for the subsample of respondents in the DNAm sample to adjust for selection and was used for analyses of data from that sample (VBSI16WGTRA). 25 The weighted sample represents community‐dwelling adults 56 years and over in the USA.
DNAm measures
Thirteen epigenetic clocks have been estimated from the HRS data. Nine of these clocks are ‘first generation’ clocks trained on chronological age, and four are ‘second generation’ clocks trained on health‐related outcomes (Zhang, PhenoAge, GrimAge, DunedinPoAm). Of these four, we chose three (PhenoAge, GrimAge, DunedinPoAm). PhenoAge and GrimAge were regressed on age to obtain DNAm ‘accelerated age’ estimates, whereas DunedinPoAm was originally created as a pace‐of‐ageing clock.
PhenoAge was developed by Morgan Levine using composite clinical biomarkers combined into a multi‐system measure of biological age, called phenotypic age, which was developed to estimate an individual's mortality risk using nine markers of tissue and immune function [albumin, creatinine, serum glucose, CRP, lymphocyte percent, mean (red) cell volume, red cell distribution width, alkaline phosphatase, white blood cell count] and age. 25 Phenotypic age was predicted by PhenoAge based on 513 CpGs in whole blood from the same sample. Although this clock was developed using whole blood data, values from all tested tissues and cells are correlated with age and predict mortality better than chronological age‐based clocks. 26 PhenoAge has been shown to predict multiple ageing outcomes such as mortality, cancer, lifespan, physical function and Alzheimer's disease. Moreover, PhenoAge acceleration (PhenoAgeAA) is related to biomarkers such as high CRP, glucose, triglycerides, waist‐to‐hip ratio and low HDL cholesterol. 26
GrimAge was constructed by HRS staff member Jonah Fisher with assistance of Steve Horvath and was developed based on the 7 DNAm surrogates of plasma proteins and smoking pack‐years in a two‐stage procedure. 25 , 27 First, surrogate DNAm biomarkers of physiological risk and stress factors were defined with plasma proteins [adrenomedullin, CRP, plasminogen activation inhibitor 1 (PAI‐1) and growth differentiation factor 15 (GDF15)] and DNAm‐based estimator of smoking pack‐years. Then, time‐to‐death was regressed on these biomarkers and an estimator of smoking years to estimate a composite biomarker of lifespan—GrimAge. High values of this measure (GrimAgeAA) are associated with morbidity and mortality risk. 27
DunedinPoAm was developed to represent individual variation in the pace of biological ageing. Based on data from the Illumina 450K Array run on samples from the Dunedin cohort, estimates were derived by using elastic net regression models to calculate a methylation pace‐of‐ageing (mPoA) score. 28 The pace of ageing was calculated with composited slopes across the 18 biomarkers that measure the rate of ageing in the cardiovascular, metabolic, renal, hepatic, pulmonary, periodontal and immune systems. Then, the pace‐of‐ageing composite was scaled to represent the mean trend in the cohort among Dunedin study members with methylation data at age 38. The mPoA algorithm was trained on three waves of biomarker data from participants, including data collected at ages 26, 32 and 38. DunedinPoAm is estimated in years per chronological year (years/chronological year). 25
Covariates
The following sociodemographic measures were included in the primary analytic approach as independent covariates: age, self‐reported race/ethnicity (White, Black, Hispanic, other), marital status, education (<high school graduate, high school graduate, some college, college graduate and ≥college graduate), household wealth, history of smoking (never vs. ever smoked) and cell composition as described. 29
Analyses
Descriptive statistics were used to explore the distribution, central tendency and variation of each baseline NGS measurement, with an emphasis on graphical methods such as histograms, scatterplots and boxplots. Weighted multivariate linear regression analyses were conducted, using the SAS surveyreg procedure to determine the unadjusted and adjusted association between NGS and DNAm age acceleration for each clock. First, a cross‐sectional analysis was performed to examine the association between NGS and DNAm age acceleration across all clocks in the same discreet time period (2016). Thereafter, we explored a retrospective longitudinal association between baseline (‘wave 1’) NGS (2006/2008) and DNAm age acceleration across all clocks at 8‐year follow‐up (‘wave 3’). Finally, to examine if change in strength was associated with DNAm age acceleration, longitudinal modelling was completed to examine the association between changes in absolute grip strength from wave 1 to wave 3 and DNAm age acceleration for each clock at wave 3. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC), and for all analyses, P < 0.05 was considered statistically significant.
Results
Descriptive and health data are presented as weighted means, standard errors and percentages for men and women in Tables 1 and 2 for the wave 3 full study population and final sample subset, respectively. The average age of study participants was 70 years. Approximately 45.1% of men and 44.3% of women were classified as obese (body mass index >30 kg/m2). On average, men had a stronger grip strength relative to body mass than women (0.44 kg/kg vs. 0.33 kg/kg, respectively). The majority of participants were non‐Hispanic White and reported being married and having some college or greater educational attainment. The distribution of household wealth was similar in men and women.
Table 1.
Demographic and health characteristics of the weighted, cross‐sectional full study population by sex at wave 3 (2014/2016)
| Men | Women | |
|---|---|---|
| n = 5888 | n = 8893 | |
| Age, years | 70.3 (0.23) | 69.6 (0.22) |
| Body mass index (BMI), kg/m2 | 28.9 (0.16) | 28.6 (0.16) |
| Obesity (BMI >30), % | 45.1 | 42.3 |
| Level of education | ||
| Less than H.S., % | 12.5 | 14.6 |
| High school graduate, % | 30.8 | 32.8 |
| Some college, % | 21.7 | 26.8 |
| College graduate, % | 35.0 | 25.7 |
| Married, % | 70.3 | 55.7 |
| Race | ||
| White, % | 89.3 | 85.8 |
| Black, % | 6.9 | 7.9 |
| Unknown/other, % | 3.8 | 6.4 |
| Ethnicity | ||
| Hispanic, % | 6.7 | 8.7 |
| Net worth | ||
| Quartile 1, % | 16.4 | 20.1 |
| Quartile 2, % | 21.0 | 21.0 |
| Quartile 3, % | 25.7 | 22.6 |
| Quartile 4, % | 37.0 | 36.4 |
| NGS (relative to body mass) | 0.44 (0.003) | 0.33 (0.002) |
Table 2.
Demographic and health characteristics of the weighted, cross‐sectional final subset population by sex at wave 3
| Men | Women | |
|---|---|---|
| n = 528 | n = 746 | |
| Age, years | 69.0 (0.32) | 69.6 (0.33) |
| Body mass index (BMI), kg/m2 | 29.0 (0.24) | 28.6 (0.24) |
| Obesity (BMI >30), % | 45.9 | 43.7 |
| Level of education | ||
| Less than H.S., % | 11.0 | 13.8 |
| High school graduate, % | 27.9 | 33.9 |
| Some college, % | 23.0 | 27.1 |
| College graduate, % | 38.2 | 25.2 |
| Married, % | ||
| Race | ||
| White, % | 88.9 | 84.1 |
| Black, % | 8.7 | 9.1 |
| Unknown/other, % | 2.4 | 6.8 |
| Ethnicity | ||
| Hispanic, % | 7.4 | 8.6 |
| Net worth | ||
| Quartile 1, % | 19.8 | 23.8 |
| Quartile 2, % | 24.5 | 24.0 |
| Quartile 3, % | 26.7 | 24.0 |
| Quartile 4, % | 29.1 | 28.2 |
| NGS (relative to body mass) | 0.44 (0.005) | 0.34 (0.003) |
Cross‐sectional analyses
For the unadjusted cross‐sectional analyses, greater grip strength was inversely associated with DNAm age acceleration. Specifically, for men, there was a statistically significant association between NGS and DunedinPoAm, PhenoAgeAA and GrimAgeAA (all P < 0.01). For women, there was a statistically significant association between NGS and DunedinPoAm only. Moreover, in models adjusted for age, race, education, marital status, income, cell composition and smoking, there was an independent cross‐sectional association between NGS and DNAm age acceleration for men and women using the DunedinPoAm clock, for men only using the PhenoAgeAA and for men and women using the GrimAgeAA clock (Table 3).
Table 3.
Cross‐sectional regression models were completed stepwise for each DNAm age acceleration clock to examine the effects of incremental adjustment on the exposure variable (NGS)
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| Men | ||||
| PhenoAgeAA | −7.95 (−13.92, −1.98)* | −9.71 (−15.84, −3.57)* | −9.16 (−15.26, −3.05)* | −8.27 (−14.25, −2.29)* |
| GrimAgeAA | −5.52 (−9.56, −1.48)* | −2.89 (−6.93, 1.15) | −2.69 (−6.79, 1.41) | −4.56 (−8.45, −0.68)* |
| DunedinPoAm | −0.41 (−0.55, −0.28)* | −0.34 (−0.49, −0.19)* | −0.34 (−0.49, −0.19)* | −0.36 (−0.51, −0.21)* |
| Women | ||||
| PhenoAgeAA | −6.50 (−13.39, 0.39) | −4.55 (−11.86, 2.75) | −4.26 (−11.56, 3.05) | −4.96 (−12.45, 2.53) |
| GrimAgeAA | −5.98 (−9.99, 1.57) | −2.54 (−6.43, 1.35) | −2.62 (−6.43, 1.18) | −4.46 (−7.81, −1.11)* |
| DunedinPoAm | −0.45 (−0.60, −0.29)* | −0.34 (−0.48, −0.19)* | −0.33 (−0.48, −0.19)* | −0.36 (−0.50, −0.22)* |
Model 1: Unadjusted. Model 2: Model 1 + demographic variables (age, race, education, marital status, income). Model 3: Model 1 + Model 2 + cell composition. Model 4: Model 1 + Model 2 + Model 3 + smoking.
P < 0.05.
Longitudinal analyses
Greater grip strength was inversely associated with DNAm age acceleration in longitudinal models. Specifically, for men, there was a statistically significant longitudinal association between wave 1 NGS and wave 3 DunedinPoAm and GrimAgeAA (all P < 0.01). For women, there was a statistically significant association between wave 1 NGS and wave 3 DunedinPoAm, PhenoAgeAA and GrimAgeAA. Moreover, in models adjusted for age, race, education, marital status, income, cell composition and smoking, there was an independent longitudinal association between wave 1 NGS and wave 3 DNAm age acceleration for men and women using the DunedinPoAm clock and for women only using the PhenoAgeAA and GrimAgeAA clocks (Table 4).
Table 4.
Longitudinal regression models were completed stepwise to examine the effects of incremental adjustment on the exposure variable (NGS) at baseline to 8‐year DNAm age acceleration
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| Men | ||||
| PhenoAgeAA | −3.59 (−8.14, 0.96) | −2.94 (−7.99, 2.10) | −3.15 (−8.22, 1.92) | −3.17 (−8.24, 1.90) |
| GrimAgeAA | −4.48 (−7.64, −1.32)* | −1.81 (−4.92, 1.30) | −1.68 (−4.84, 1.47) | −2.28 (−5.20, 0.63) |
| DunedinPoAm | −0.32 (−0.42, −0.22)* | −0.26 (−0.37, −0.15)* | −0.26 (−0.36, −0.15)* | −0.26 (−0.37, −0.16)* |
| Women | ||||
| PhenoAgeAA | −9.00 (−13.76, −4.24)* | −7.92 (−12.63, −3.21)* | −7.73 (−12.39, −3.08)* | −8.20 (−12.73, −3.31)* |
| GrimAgeAA | −7.29 (−10.13, −4.46)* | −5.10 (−8.07, −2.14)* | −4.91 (−7.84, −1.98)* | −5.91 (−8.55, −3.26)* |
| DunedinPoAm | −0.43 (−0.55, −0.31)* | −0.36 (−0.48, −0.24)* | −0.35 (−0.47, −0.23)* | −0.36 (−0.48, −0.24)* |
Model 1: Unadjusted. Model 2: Model 1 + demographic variables (age, race, education, marital status, income). Model 3: Model 1 + Model 2 + cell composition. Model 4: Model 1 + Model 2 + Model 3 + smoking.
P < 0.05
Finally, change in grip strength from wave 1 to wave 3 was independently associated with PhenoAgeAA (β: −0.13; 95% CI: −0.23, −0.03) and GrimAgeAA (β: −0.07; 95% CI: −0.14, −0.01) in men only (both P < 0.05) (Model 5: Table S1).
Discussion
The principal finding of the current study is that among middle‐aged and older men and women, lower NGS was associated with DNAm age acceleration across several clocks. These results suggest the DNAm clocks examined in this study represent clinically relevant biomarkers of age acceleration. Perhaps more importantly, our findings represent the first evidence and largest study to date demonstrating that greater NGS may be protective against DNAm age acceleration (see conceptual model; Figure 1). The limited literature on DNAm age acceleration and functional assessments of ageing is mixed. 30 , 31 One study in a German cohort found that accumulating deficits (comprehensive frailty) were associated with increasing age acceleration derived from the Horvath DNAm clock, 31 which used DNA from multiple cell and tissue types and trained on chronological age. 32 However, another study in an older German cohort did not find an association between measures of intrinsic and extrinsic 33 epigenetic age acceleration and frailty or functional assessments. 30 Most recently, phenotypic and mortality trained epigenetic clocks were found to be associated with cross‐sectional and 3‐year change in frailty outcomes in the Canadian Longitudinal Study on Aging (CLSA). 31 The heterogeneity of findings observed in these studies is likely due to employing DNAm age acceleration measures derived from different clocks. Our results, which are consistent with the CLSA, suggest that thoughtful selection of DNAm clocks to be included in future analyses of age‐related functional decline or frailty is warranted. DNAm clocks designed to represent phenotypic ageing and mortality are better suited for understanding the association between grip strength and health‐related outcomes.
Figure 1.
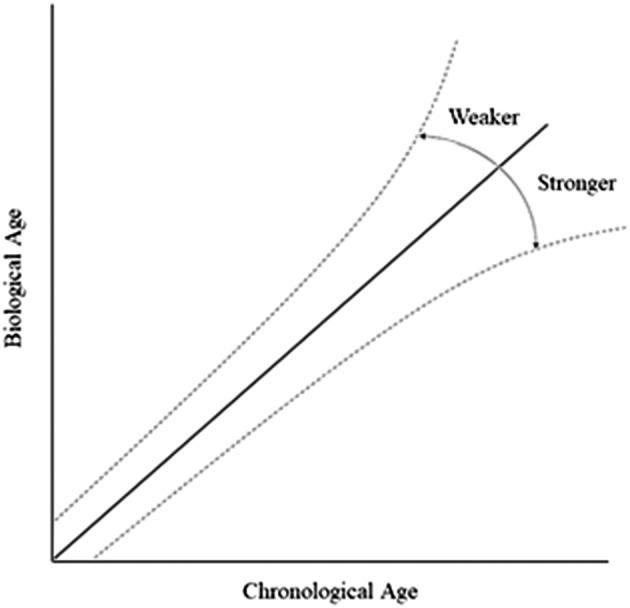
Conceptual model demonstrating the deviations in DNAm age (‘biological age’) from chronological age associated with weaker or stronger NGS.
Our findings further revealed that associations between NGS and DNAm age acceleration clocks were not consistent across sex categories in the fully adjusted models. In the cross‐sectional analysis, stronger NGS was inversely associated with GrimAgeAA and DunedinPoAmAA in both men and women, but only PhenoAA in men. In our longitudinal models, only DunedinPoAmAA was statistically significantly associated with NGS in both men and women, whereas GrimAgeAA and PhenoAA were both statistically significant in women. This suggests that future studies should include sex‐stratified models to inform potential differences in ageing indicators by sex.
As a biological marker of ageing, the specific mechanisms underlying observed correlations between lower grip strength and negative health outcomes remain unclear. DNAm patterns may be one pathophysiological pathway by which grip strength and morbidity are related. There is also a growing consensus that the low‐grade chronic inflammation in ageing (‘inflammaging’) is a highly significant risk factor for both morbidity and mortality in elderly people 34 and may represent a strong mechanism linking age‐related increases in adiposity and metabolic dysregulation with sarcopenia and muscle weakness. 35 Indeed, we have previously shown that chronic inflammation was associated with lower NGS in both men and women and also emerged as a significant causal mediator between lower NGS and both incident disability and chronic disease multimorbidity. 5 We have also demonstrated that muscle weakness and testosterone deficiency were highly correlated and independently associated with multi‐morbidity in young and older men. 36 Moreover, the largest study to date to examine the genome‐wide association studies of grip strength revealed that a genetic variant in a chromosomal region that regulates myotube differentiation and muscle repair may contribute to variability in grip strength in the elderly. 37 The extent to which immune, endocrine and genetic mechanisms combine to contribute to age‐related weakness, DNAm age acceleration, chronic disease and cause‐specific mortality is an important future research directive.
However, muscle weakness and DNAm age acceleration may also just simply represent proxies of ageing, resulting in their correlation. Conventional ageing research ignores changes in life‐expectancy at the population level, actual life expectancy at the individual level and characteristics of people throughout time and has led to an incomplete understanding of the factors that influence altered ageing trajectories. For example, why is someone aged 50 years considered to be ‘middle‐aged’ in today's society, whereas 150 years ago a person of the same age would be considered elderly? It has been conjectured that certain lifestyles (e.g. regular physical activity and healthy dietary habits) may produce these effects by altering typical ageing trajectories of DNAm that underwrite health and functional status into older adulthood. Recently, a conceptual model was proposed for the sequence of events and outcomes that surround the role of muscle weakness in the disease and disabling processes. 36 However, future studies are certainly needed that provide specific evidence for how muscle strength and clinically relevant health outcomes are mediated or moderated by other factors, as well as specific hypothesized connections between grip strength and DNAm age acceleration to elucidate potential biological mechanisms. Future research is also needed to examine a potential causal mediation pathway between grip strength (exposure), physical activity/nutritional status (mediators) and accelerated/decelerated DNAm age.
Regardless, grip strength is a clinically relevant biomarker of ageing, and global DNAm is relatively stable over time. 38 Thus, the potential exists for combining measures of grip strength and DNAm age acceleration to screen individuals for future risk of chronic disease and functional decline before it occurs. Doing so would allow for the design of interventions to delay or prevent their onset and progression. Although this is an exciting possibility, extensive research of longitudinal DNAm age acceleration measurements must be undertaken, as well as additional replication of our findings in other U.S. and international cohorts.
We acknowledge that our study has several limitations. Firstly, we had DNAm at only a single time point (wave 3). Future studies are needed to examine earlier onset deviations in DNAm acceleration/deceleration and factors that contribute to these alterations. Secondly, we cannot rule out time‐varying confounding because baseline measurements of all covariates were included in our final models. Thus, whether declines in NGS ‘cause’ a risk for DNAm age acceleration, or if incident disease processes (e.g. diabetes) themselves, are a cause of diminished muscle function and altered biological age is an interesting topic. We were also unable to determine if other competing risks or unmeasured confounding [i.e. other risk factors (e.g. lack of physical activity) or existing diseases (e.g. cancer)] may have influenced the observed findings. We further recognize the potential bias introduced by losing a substantial number of the participants when modelling for the independent association between NGS and prevalent/longitudinal DNAm age acceleration. Future efforts are needed to validate and extend these findings in other/larger cohorts to improve our understanding of the underlying mechanisms linking weakness and accelerated biological ageing. Lastly, a limitation in this study was not using any biomarkers of pathophysiology. For example, there are multiple markers of chronic inflammation and oxidative stress, including TNF‐ , IL‐6, IL‐12, IL‐1ß and reactive oxygen species. Future studies could examine multiple markers simultaneously, as chronic inflammation and oxidative stress are both highly dependent on complex signalling and lead to age‐related diseases.
Despite these limitations, this study has various strengths. Most notably, we used grip strength as our primary exposure, which is a cost‐effective, reliable proxy for total body muscle strength that can be easily administered in the clinical or community settings and across populations of children, adolescents and adults. 11 , 39 , 40 , 41 Moreover, the results of this study are generalizable to community‐dwelling older Americans because the sample used in this study is from the longest running, longitudinal, nationally representative cohort of older adults in the USA. Our analysis is superior to the results from the recently published findings from the CLSA, because we stratified by sex and examined findings at a longer time horizon (i.e. 8 years instead of 3‐year follow‐up). Lastly and most importantly, our findings demonstrating that lower NGS was associated with DNAm age acceleration across several clocks were independent of smoking status—a known risk factor for negative health outcomes and strong predictor of DNAm patterns.
Conclusion
Our findings provide some initial evidence of age acceleration among men and women with lower NGS and loss of strength over time. Preservation of muscle strength may positively influence healthy ageing by protecting against DNAm age acceleration. Future research is needed to understand the extent to which DNAm age mediates the association between grip strength and chronic disease, disability and early mortality, as well as the extent to which lifestyle/behavioural factors (e.g. physical activity and dietary status) can mediate the association between grip strength and accelerated/decelerated DNAm age.
Conflicts of interest
The authors declare that no conflict of interest relevant to this article exists.
Supporting information
Table S1. Model 5: Association between changes in absolute grip strength from wave 1 to wave 3, and DNAm age acceleration for each clock at wave 3.
Acknowledgements
This work was supported in part by the National Institutes of Health (NIH) grant (R01 AG060110 to J.F.). The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle.
Peterson M. D., Collins S., Meier H. C. S., Brahmsteadt A., and Faul J. D. (2023) Grip strength is inversely associated with DNA methylation age acceleration, Journal of Cachexia, Sarcopenia and Muscle, 14, 108–115, 10.1002/jcsm.13110
References
- 1. Peterson MD, Zhang P, Choksi P, Markides KS, al Snih S. Muscle weakness thresholds for prediction of diabetes in adults. Sports Med 2016;46:619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Karvonen‐Gutierrez CA, Peng Q, Peterson M, Duchowny K, Nan B, Harlow S. Low grip strength predicts incident diabetes among mid‐life women: The Michigan study of women's health scross the nation. Age Ageing 2018;47:685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Duchowny KA, Clarke PJ, Peterson MD. Muscle weakness and physical disability in older Americans: Longitudinal findings from the U.S. health and retirement study. J Nutr Health Aging 2018;22:501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McGrath RP, Ottenbacher KJ, Vincent BM, Kraemer WJ, Peterson MD. Muscle weakness and functional limitations in an ethnically diverse sample of older adults. Ethn Health 2017;26:1–12. [DOI] [PubMed] [Google Scholar]
- 5. Peterson MD, Casten K, Collins S, Hassan H, Garcia‐Hermoso A, Faul J. Muscle weakness is a prognostic indicator of disability and chronic disease multimorbidity. Exp Gerontol 2021;152:111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community‐dwelling older persons. Arch Neurol 2009;66:1339–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alfaro‐Acha A, al Snih S, Raji MA, Kuo YF, Markides KS, Ottenbacher KJ. Handgrip strength and cognitive decline in older Mexican Americans. J Gerontol A Biol Sci Med Sci 2006;61:859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duchowny K, Ackley S, Brenowitz W, Wang J, Zimmerman SC, Caunca MR, et al. Associations between handgrip strength and dementia risk, cognition, and neuroimaging outcomes in the UK biobank cohort study. JAMA Open Netw 2022;5:e2218314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peterson M, Zhang P, Duchowny K, Markides K, Ottenbacher K, Snih SA. Declines in strength and mortality risk among older Mexican Americans: Joint modeling of survival and longitudinal data. J Gerontol A Biol Sci Med Sci 2016;In Press;71:1646–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu Y, Wang W, Liu T, Zhang D. Association of grip strength with risk of all‐cause mortality, cardiovascular diseases, and cancer in community‐dwelling populations: A meta‐analysis of prospective cohort studies. J Am Med Dir Assoc 2017;18:551.e17–551.e35. [DOI] [PubMed] [Google Scholar]
- 11. Whitney DG, Peterson MD. The association between differing grip strength measures and mortality and cerebrovascular event in older adults: National health and giang trends study. Front Physiol 2018;9:1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. García‐Hermoso A, Cavero‐Redondo I, Ramírez‐Vélez R, Ruiz JR, Ortega FB, Lee DC, et al. Muscular strength as a predictor of all‐cause mortality in an apparently healthy population: A systematic review and meta‐analysis of data from approximately 2 million men and women. Arch Phys Med Rehabil 2018;99:2100–2113.e5. [DOI] [PubMed] [Google Scholar]
- 13. Sayer AA, Kirkwood TB. Grip strength and mortality: A biomarker of ageing? Lancet 2015;386:226–227. [DOI] [PubMed] [Google Scholar]
- 14. Ben‐Shlomo Y, Cooper R, Kuh D. The last two decades of life course epidemiology, and its relevance for research on ageing. Int J Epidemiol 2016;45:973–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scherbov S, Sanderson WC. New approaches to the conceptualization and measurement of age and aging. J Aging Health 2016;28:1159–1177. [DOI] [PubMed] [Google Scholar]
- 16. Lopez‐Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013;153:1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hamczyk MR, Nevado RM, Barettino A, Fuster V, Andres V. Biological versus chronological aging: JACC focus seminar. J Am Coll Cardiol 2020;75:919–930. [DOI] [PubMed] [Google Scholar]
- 18. Ligthart S, Marzi C, Aslibekyan S, Mendelson MM, Conneely KN, Tanaka T, et al. DNA methylation signatures of chronic low‐grade inflammation are associated with complex diseases. Genome Biol 2016;17:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda SV, et al. Genome‐wide methylation profiles reveal quantitative views of human aging rates. Mol Cell 2013;49:359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Christiansen L, Lenart A, Tan QH, Vaupel JW, Aviv A, McGue M, et al. DNA methylation age is associated with mortality in a longitudinal Danish twin study. Aging Cell 2016;15:149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Crimmins EM, Thyagarajan B, Levine ME, Weir DR, Faul J. Associations of age, sex, race/ethnicity, and education with 13 epigenetic clocks in a nationally representative U.S. sample: The health and retirement study. J Gerontol A Biol Sci Med Sci 2021;76:1117–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jain P, Binder A, Chen B, Parada H Jr, Gallo LC, Alcaraz J, et al. Analysis of epigenetic age acceleration and healthy longevity among older US women. JAMA Netw Open 2022;5:e2223285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bureau UC . An aging nation: the older population in the United States. 2014:25‐1140. Accessed April 4, 2020. https://www.census.gov/prod/2014pubs/p25‐1140.pdf
- 24. Crimmins EM, Faul J, Thyagarajan B, Weir D. Venous blood collection and assay protocol in the 2016 health and retirement study 2016 venous blood study (VBS). 2017. http://hrsonline.isr.umich.edu/modules/meta/vbs/2016/desc/HRS2016VBSDD.pdf
- 25. Crimmins E, Kim J, Fisher J, Faul J. HRS epigenetic clocks. 2020. https://hrsdata.isr.umich.edu/sites/default/files/documentation/data‐descriptions/EPICLOCKS_DD.pdf
- 26. Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY) 2018;10:573–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lu AT, Quach A, Wilson JG, Reiner AP, Aviv A, Raj K, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY) 2019;11:303–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Belsky DW, Caspi A, Arseneault L, Baccarelli A, Corcoran DL, Gao X, et al. Quantification of the pace of biological aging in humans through a blood test, the DunedinPoAm DNA methylation algorithm. Elife 2020;9:e54870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Campbell KA, Colacino JA, Park SK, Bakulski KM. Cell types in environmental epigenetic studies: Biological and epidemiological frameworks. Curr Environ Health Rep 2020;7:185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vetter VM, Spira D, Banszerus VL, Demuth I. Epigenetic clock and leukocyte telomere length are associated with vitamin D status but not with functional Assessments and frailty in the Berlin aging study II. J Gerontol A Biol Sci Med Sci 2020;75:2056–2063. [DOI] [PubMed] [Google Scholar]
- 31. Verschoor CP, Lin DTS, Kobor MS, Mian O, Ma J, Pare G, et al. Epigenetic age is associated with baseline and 3‐year change in frailty in the Canadian longitudinal study on aging. Clin Epigenetics 2021;13:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Horvath S. DNA methylation age of human tissues and cell types. Genome Biol 2013;14:R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Quach A, Levine ME, Tanaka T, Lu AT, Chen BH, Ferrucci L, et al. Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging (Albany NY) 2017;9:419–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age‐associated diseases. J Gerontol A Biol Sci Med Sci 2014;69:S4–S9. [DOI] [PubMed] [Google Scholar]
- 35. Kalinkovich A, Livshits G. Sarcopenic obesity or obese sarcopenia: A cross talk between age‐associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev 2016;35:200–221. [DOI] [PubMed] [Google Scholar]
- 36. McGrath RP, Kraemer WJ, Al Snih S, Peterson MD. Handgrip strength and health in aging adults. Sports Med 2018;48:1993–2000. [DOI] [PubMed] [Google Scholar]
- 37. Matteini AM, Tanaka T, Karasik D, Atzmon G, Chou WC, Eicher JD, et al. GWAS analysis of handgrip and lower body strength in older adults in the CHARGE consortium. Aging Cell 2016;15:792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bjornsson HT, Sigurdsson MI, Fallin MD, Irizarry RA, Aspelund T, Cui H, et al. Intra‐individual change over time in DNA methylation with familial clustering. JAMA 2008;299:2877–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ramirez‐Velez R, Tordecilla‐Sanders A, Correa‐Bautista JE, Peterson MD, Garcia‐Hermoso A. Handgrip strength and ideal cardiovascular health among Colombian children and adolescents. J Pediatr 2016;179:82–89.e1. [DOI] [PubMed] [Google Scholar]
- 40. Duchowny KA, Peterson MD, Clarke PJ. Cut points for clinical muscle weakness among older Americans. Am J Prev Med 2017;53:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Peterson MD, Duchowny K, Meng Q, Wang Y, Chen X, Zhao Y. Low normalized grip strength is a biomarker for cardiometabolic disease and physical disabilities among U.S. and Chinese adults. J Gerontol A Biol Sci Med Sci 2017;72:1525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Model 5: Association between changes in absolute grip strength from wave 1 to wave 3, and DNAm age acceleration for each clock at wave 3.


