Abstract
Background
Frailty development is partly dependent on multiple factors like low levels of nutrients and high levels of oxidative stress (OS) and inflammation potentially leading to a muscle‐catabolic state. Measures of specific biomarker patterns including nutrients, OS and inflammatory biomarkers as well as muscle related biomarkers like 3‐methylhistidine (3MH) may improve evaluation of mechanisms and the complex networks leading to frailty.
Methods
In 220 multi‐morbid patients (≥ 60 years), classified as non‐frail (n = 104) and frail (n = 116) according to Fried's frailty criteria, we measured serum concentrations of fat‐soluble micronutrients, amino acids (AA), OS, interleukins (IL) 6 and 10, 3MH (biomarker for muscle protein turnover) and serum spectra of fatty acids (FA). We evaluated biomarker patterns by principal component analysis (PCA) and their cross‐sectional associations with frailty by multivariate logistic regression analysis.
Results
Two biomarker patterns [principal components (PC)] were identified by PCA. PC1 was characterized by high positive factor loadings (FL) of carotenoids, anti‐inflammatory FA and vitamin D3 together with high negative FL of pro‐inflammatory FA, IL6 and IL6/IL10, reflecting an inflammation‐related pattern. PC2 was characterized by high positive FL of AA together with high negative FL of 3MH‐based biomarkers, reflecting a muscle‐related pattern. Frail patients had significantly lower factor scores than non‐frail patients for both PC1 [median: −0.27 (interquartile range: 1.15) vs. 0.27 (1.23); P = 0.001] and PC2 [median: −0.15 (interquartile range: 1.13) vs. 0.21 (1.38); P = 0.002]. Patients with higher PC1 or PC2 factor scores were less likely to be frail [odds ratio (OR): 0.62, 95% CI: 0.46–0.83, P = 0.001 for PC1; OR: 0.64, 95% CI: 0.48–0.86, P = 0.003 for PC2] compared with patients with lower PC1 or PC2 factor scores. This indicates that increasing levels of anti‐inflammatory biomarkers and increasing levels of muscle‐anabolic biomarkers are associated with a reduced likelihood (38% and 36%, respectively) for frailty. Significant associations remained after adjusting the regression models for potential confounders.
Conclusions
We conclude that two specific patterns reflecting either inflammation‐related or muscle‐related biomarkers are both significantly associated with frailty among multi‐morbid patients and that these specific biomarker patterns are more informative than single biomarker analyses considering frailty identification.
Keywords: Biomarker, Frail, Inflammation, Methylhistidine, Micronutrients, Muscle
Introduction
Frailty, a multifactorial geriatric syndrome, is associated with an increased risk for adverse outcomes and mortality, 1 , 2 even in younger age groups (45–55 years), 3 and is more prevalent in older ages. 4 Nutrient inadequacies as well as age‐related oxidative stress (OS) and inflammation 1 , 5 are among the key factors in frailty development. These might contribute to impairments in muscle structure and functionality, further leading to a decrease in muscle mass, strength and physical performance 6 , 7 and hence, to sarcopenia, which is both a contributor and a component of frailty. 8 , 9
Essential and branched‐chain amino acids (eAA and BCAA), which are crucial for keeping muscle protein synthesis (MPS; anabolic state) and breakdown (MPB; catabolic state) balanced, 10 were found to be lower in frail compared with non‐frail adults. 11 Moreover, lower eAA and BCAA concentrations were related to lower skeletal muscle index, muscle strength and sarcopenia. 12 Low micronutrient and high OS concentrations were linked to higher prevalence and incidence of frailty and to lower muscle mass, strength and physical performance. 12 , 13 , 14 Low plasma vitamin D3 (VitD3) and carotenoids together with high plasma protein carbonyl (PrCarb, a biomarker of OS) concentrations were associated with pre‐frailty and frailty in community‐dwelling old. 15 Furthermore, a circulating biomarker pattern including fat‐soluble vitamins and carotenoids was related to a higher risk for frailty. 16 Inflammaging, the age‐related state of chronic inflammation, is suggested to play a pivotal role in sarcopenia and frailty development, 9 and especially, elevated pro‐inflammatory interleukin‐6 (IL6) was associated with frailty. 17 Fatty acids (FA) can affect inflammation and might be involved in muscle‐related mechanisms leading to skeletal muscle loss, sarcopenia and frailty. Lower circulating polyunsaturated fatty acids (PUFA) such as EPA (eicosapentaenoic acid; C20:5 n‐3) or DHA (docosahexaenoic acid; C22:6 n‐3) were related to lower muscle parameters and with sarcopenia as well as with frailty in older individuals. 12 , 18 3‐Methylhistidine (3MH) concentrations and 3MH/Crea ratios (3MH‐to‐creatinine ratio) were found to be useful as biomarkers for muscle protein turnover. 19 , 20 Moreover, plasma 3MH, 3MH/Crea and 3MH/eGFR (3MH‐to‐estimated glomerular filtration rate ratio) were positively associated with frailty, 21 and thus, circulating 3MH‐based biomarkers are considered as suitable biomarkers for frailty identification and to elucidate a potential muscle‐catabolic state.
Considering the complexity of the multifactorial aetiology of frailty, simultaneous measures of a variety of circulating biomarkers and biomarker patterns might improve the evaluation of underlying mechanisms and the complex networks leading to frailty. However, data on such measures and multi‐biomarker patterns including both frailty‐associated dietary biomarkers and OS, inflammation and muscle‐related biomarkers are scarce, particularly in the hospital patients. Therefore, we aimed to measure a variety of circulating biomarkers, to identify biomarker patterns and to evaluate cross‐sectional associations between biomarker patterns and frailty status in a cohort of non‐frail and frail in‐hospital patients.
Methods
Study population
In this study, we assessed a total of 220 older in‐hospital patients from a clinical cohort from the Protestant Geriatric Center Berlin (EGZB) in cooperation with the Department of Geriatrics and Medical Gerontology at the Charité—Universitätsmedizin Berlin and was approved by the ethics committee according to the principles of the Declaration of Helsinki. All participants signed a written consent.
Inclusion criteria were age ≥60 years, signed informed consent, Mini Mental State Examination (MMSE) ≥ 24 points, life expectancy > 3 months according to assessment by the attending physician and proficiency in German language.
Patients' characteristics were assessed at discharge. Patients are discharged from hospital when in a stable condition. Blood samples were only drawn in the morning after an overnight fast at discharge from hospital, and serum samples were then stored at −80°C until biomarker measurements.
Patients characteristics
Patients' information and characteristics included frailty status according to Fried's frailty criteria, 1 sex (as female and male), age (years), body mass index (BMI) (kg/m2), estimated glomerular filtration rate (eGFR) (mL/min/1.73 m2), nutrient intake data (kcal, energy% or g per kg body weight), number of co‐morbidities (n) and medication intake (drugs/day).
BMI was calculated as followed: BMI = body weight/height2. Body weight (kg) was measured by a calibrated chair scale (KERN MCC 250K100M, KERN & SOHN GmbH, Balingen‐Frommern, Germany). Study participants were weighed with an empty bladder and light clothing on. Body height (cm) was measured by a mobile stadiometer (seca217, seca GmbH & Co. KG, Hamburg, Germany). Nutrient intake was determined by a 24‐h recall upon discharge from hospital and then evaluated using EbisPro software (based on the German Federal Food Code; Version 3.0). The eGFR, as an estimation of kidney function, was calculated according to Levey et al. 22 considering creatinine concentrations, sex and age of the patients. In regard to the basic documentation, age, sex, type (ICD classification) and number of diseases and the number of medications were documented.
Frailty classification
Patients were classified into robust, pre‐frail and frail using criteria by Fried et al. 1 Briefly, patients exhibiting ≥3 of the 5 following criteria were considered as frail: weight loss, exhaustion, low physical activity, slowness and weakness; but those exhibiting one to two of these criteria were considered as pre‐frail, and those exhibiting none of these criteria were considered as robust. For statistical analyses, robust patients and pre‐frail patients were grouped together, as non‐frail patients, because there were only n = 14 robust patients compared with n = 90 pre‐frail patients and n = 116 frail patients.
Biomarker analyses
Serum concentrations of retinol, α‐ and γ‐tocopherol, lycopene, α‐carotene and β‐carotene, lutein/zeaxanthin, and β‐cryptoxanthin were measured by high‐performance liquid chromatography (HPLC) according to Stuetz et al. 23 and Weber et al. 24 Serum concentrations of VitD3 (25‐OH‐vitamin D3) were measured by ultra‐performance liquid chromatography–tandem mass spectrometry (UPLC‐MS/MS) according to van den Ouweland et al. 25 with modifications. Briefly, 50 μL serum was mixed with 10 μL of an internal standard solution (1.36 μM d6‐25OH‐D3) and vortexed. To support release of 25‐OH‐vitamin D3 from its protein bond, 25 μL of 2 M sodium hydroxide was added, and samples were incubated for 20 min at room temperature. Protein precipitation was achieved by adding 300 μL of acetonitrile/methanol 9:1 (v/v). Samples were centrifuged for 10 min at 17.000 g at 4°C, and subsequently, solid‐phase extraction (SPE) was performed using Oasis PRiME HLB 1 cc, 30 mg cartridges. Samples were diluted, loaded onto the SPE cartridges, washed with 1 mL Milli‐Q and eluted with 1 mL acetonitrile. Samples were then evaporated to dryness for 1.5 h using a vacuum centrifuge and reconstituted in 50 μL of methanol/Milli‐Q 1:1 (v/v) before 5 μL was injected into the LC system.
Fatty acid spectra in serum phospholipids were measured by a strongly modified method using extraction with tert‐butyl methyl ether/methanol, solid‐phase separation, hydrolysis and methylation with trimethylsulfonium hydroxide and subsequent analysis by gas chromatography. 26
Serum concentrations of PrCarb and protein bound 3‐nitrotyrosine (3‐NT) were measured by non‐commercial in‐house ELISA methods according to Weber et al. 24 , 27 Serum concentrations of MDA were measured by HPLC. 24 Serum concentrations of IL6 and IL10 were measured by commercial ELISA methods (IL6: intra‐assay CV: 4.2–5.1%, inter‐assay CV: 4.7–5.0%; IL10: intra‐assay CV: 1.9–2.0%, inter‐assay CV: 3.7–4.8%; both BioVendor, Brno, Czech Republic) according to the manufacturer's instructions, and IL6/IL10 ratio was calculated.
Serum concentrations of amino acids, 3MH and 1MH (as biomarker for meat intake) were measured by UPLC‐MS/MS according to Prinsen et al. 28 with modifications. Briefly, 10 μL of serum was mixed with 40 μL of internal standard mix (14 labelled amino acids, each between 15 and 62.5 μM in 90% acetonitrile) and vortexed for 30 s. To support protein precipitation, samples were placed at −20°C for 10 min before being centrifuged for 10 min at 17.000 g and 4°C. Forty microlitres of sample supernatant was then transferred into an autosampler vial with a 100‐μL glass insert, and 2 μL was injected into the LC system. Serum creatinine was measured by ABX Pentra Creatinine 120 CP kit (HORIBA ABX SAS, Montpellier, France), which is based on kinetic Jaffé method, according to the manufacturer's instructions. Five ratios were then calculated: 3MH/Crea, 1MH/Crea, 3MH/eGFR, 1MH/eGFR and 3MH/1MH.
All measured biomarker concentrations and calculated ratios are shown in Table S1 .
Statistical analyses
Patients' characteristics, serum biomarker concentrations and fatty acid spectra of patients are reported according to frailty status. Comparisons between characteristics, serum biomarker concentrations and fatty acid spectra were performed by Χ2 test (for categorical variables) and by Mann–Whitney U test or Student's t‐test (for continuous variables). Data of continuous variables are shown as median [interquartile range (IQR)] (Table 1 ).
Table 1.
Characteristics and nutrient intake data by frailty status among 220 patients
| Descriptive data | Total | Non‐frail | Frail | P‐value |
|---|---|---|---|---|
| Frailty status | 220 (100) | 104 (47.3) | 116 (52.7) | ‐ |
| Sex [n (%)] | ||||
| Male | 99 (45.4) | 49 (47.1) | 50 (43.9) | 0.550 a |
| Female | 124 (54.6) | 55 (52.9) | 66 (56.9) | |
| Age [years] b | 78.3 (10.8) | 76.5 (9.5) | 80.2 (10.8) | 0.001 |
| BMI [kg/m2] c | 26.1 (7.5) | 25.5 (6.4) | 27.8 (8.0) | 0.005 |
| eGFR [mL/min/1.73 m2] b | 62.3 (33.0) | 68.3 (28.5) | 56.8 (32.6) | 0.001 |
| Co‐morbidities [n] b | 6.0 (6.0) | 6.0 (6.0) | 7.0 (6.0) | 0.001 |
| Medication [drugs/day] b | 10.0 (5.0) | 9.0 (6.0) | 10.0 (5.0) | 0.002 |
| Nutrient intake data | ||||
| Energy [kcal] b | 1354.3 (643.8) | 1450.9 (666.0) | 1275.2 (632.4) | 0.023 |
| Protein [g/kg body weight] c | 0.77 (0.46) | 0.85 (0.47) | 0.66 (0.45) | 0.001 |
| Protein [energy%] b | 17.0 (5.8) | 17.0 (5.0) | 16.0 (5.0) | 0.492 |
| Fat [energy%] b | 40.0 (9.0) | 40.0 (10.8) | 40.0 (9.0) | 0.562 |
| Carbohydrate [energy%] b | 43.0 (12.0) | 42.0 (13.0) | 43.5 (11.0) | 0.363 |
BMI, body mass index; eGFR, estimated glomerular filtration rate.
Data are shown as median (interquartile range, IQR).
Differences between frailty groups for categorical variables determined by χ 2 test.
Differences between frailty groups for continuous variables determined by Student's t‐test for normally distributed data.
Differences between frailty groups for continuous variables determined by Mann–Whitney U test for non‐normally distributed data; statistically significant different at P < 0.05.
Principal component analysis (PCA) including 40 analytes was performed to derive biomarker patterns [principal components (PC)] based on circulating serum biomarker concentrations and fatty acid spectra among all patients with complete data. Biomarker concentrations and fatty acid spectra were z‐standardized (z‐score) before PCA was performed, because concentrations and spectra were quantified in different units. PC need to fulfil the following requirements to be considered for further statistical analyses: eigenvalue ≥1, explaining at least 10% of variance and interpretable pattern structure with at least three biomarkers with factor loading (FL) ≥ 0.3 per PC. Based on the standardized biomarker values weighted by the FL in each PC, individual PC factor scores for each patient were calculated. In principle, higher scores are related to higher biomarker concentrations. Subsequently, PC factor scores were compared between frailty groups by Student's t‐test.
Multiple logistic regression models (crude and adjusted for age, sex, medication intake, number of co‐morbidities and energy intake), using frailty status as dependent dichotomous variable and PC factor scores as independent variable, were performed to evaluate cross‐sectional associations, described as odds ratios (OR) with 95% confidence interval (95% CI), of each biomarker pattern with frailty. The OR describes the likelihood to be frail compared with be non‐frail per increase in unit (increase per standard deviation) of the respective PC factor score. An OR < 1 indicates a lower likelihood to be frail than to be non‐frail with increasing factor scores, whereas an OR > 1 indicates a higher likelihood to be frail than to be non‐frail with increasing factor scores.
Differences and associations were considered statistically significant at P < 0.05. All statistical analyses were carried out using SPSS software (SPSS Inc., Chicago, IL, USA; Version 25.0.0.2). For figure preparation, Microsoft PowerPoint and Excel (Microsoft Office 2019, Microsoft Corporation, Redmond, WA, USA) were additionally used.
Results
In our study, 47.3% and 52.7% of a total of 220 in‐hospital patients were non‐frail and frail, respectively, and further patients' characteristics are shown in Table 1 . Frail patients were statistically significantly older [median: 80.2 years (IQR: 10.8) vs. 76.5 years (9.5)] and had significantly higher BMI, medication intake, more co‐morbidities and lower eGFR, total energy intake and protein intake per kg of body weight compared with non‐frail patients, whereas sex did not differ between frailty status. Furthermore, the majority of these patients were admitted due to orthopaedic disorders (45.0%), followed by cardiovascular disease (19.5%), cancer disease (10.5%), lung disease (8.2%), gastrointestinal disease (6.8%) and other causes (10.0%), and the distribution of main causes of hospital admissions did not differ between frail and non‐frail patients.
Circulating biomarker concentrations differed significantly between frailty groups, as shown in Figures 1 and 2 and Table S1 . Frail patients had significantly higher serum IL6 and IL6/IL10 as well as significantly lower serum levels of β‐cryptoxanthin and β‐carotene than non‐frail patients (Figure 1 ) implying higher inflammation as well as less anti‐oxidative capacity in frail patients. Serum concentrations of PrCarb, MDA, 3‐NT and IL10 as well as vitamins A, E and D3, lutein/zeaxanthin, lycopene and α‐carotene were similar between frailty groups. Serum concentrations of 3MH, 3MH/Crea and 3MH/eGFR were significantly higher in frail compared with non‐frail patients indicating a higher muscle protein turnover in frail patients (Figure 2 ). In contrast, the BCAAs valine and leucine (Figure 2 ) together with histidine, lysine and tryptophan serum concentrations were significantly lower in frail than in non‐frail patients. Furthermore, linoleic acid (C18:2 n6), α‐linolenic acid (C18:3 n3) and eicosadienoic acid (C20:2 n6) were higher in non‐frail than in frail patients, whereas pro‐inflammatory arachidonic acid (C20:4 n‐6) was higher in frail than in non‐frail patients (Figure 1 ). Serum EPA and DHA were both similar between frailty groups.
Figure 1.
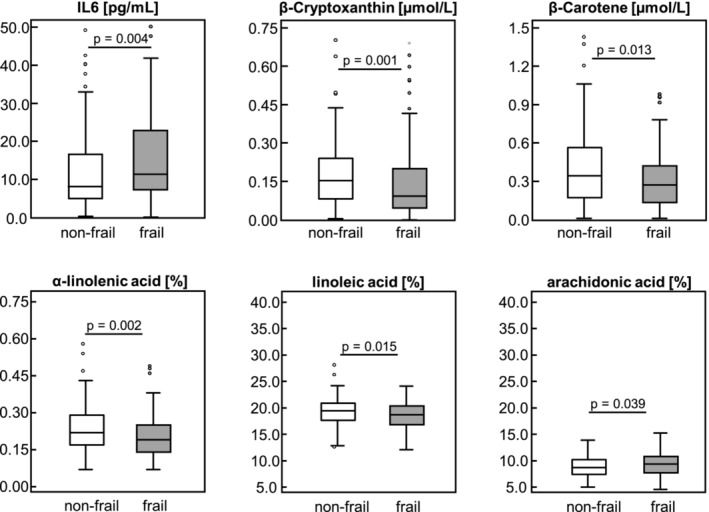
Circulating interleukin 6 and carotenoid concentrations, and fatty acid percentages by frailty status among 220 patients. Data are shown as boxplot; differences between frailty groups were determined by Student's t‐test for normally distributed data or by Mann–Whitney U test for non‐normally distributed data; statistically significant different at P < 0.05.
Figure 2.
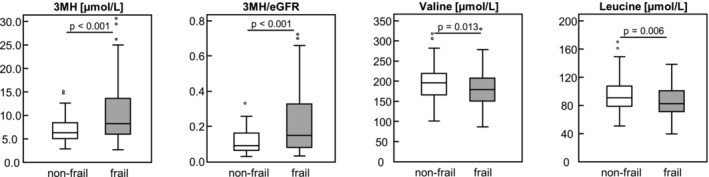
Circulating 3MH‐related biomarker and BCAA concentrations by frailty status among 220 patients. Data are shown as boxplot; differences between frailty groups were determined by Student's t‐test for normally distributed data or by Mann–Whitney U test for non‐normally distributed data; statistically significant different at P < 0.05. 3MH, 3‐methylhistidine; BCAA, branched‐chain amino acids; eGFR, estimated glomerular filtration rate.
With PCA, two biomarker patterns (PC1 and PC2; Table 2 ) were derived exploratorily. PC1 was characterized by high positive FL of anti‐oxidative and anti‐inflammatory carotenoids and FA and VitD3 together with high negative FL of pro‐inflammatory FA, IL6 and IL6/IL10 (inflammation‐related biomarker pattern). Higher PC1 factor scores are therefore associated with higher concentrations of anti‐inflammatory biomarkers. PC2 was characterized by high positive FL of AA, especially BCAA, together with high negative FL of 3MH/eGFR, 3MH and creatinine (muscle‐related biomarker pattern). Hence, higher PC2 factor scores are associated with higher concentrations of biomarkers related to muscle anabolism. Frail patients had significantly lower factor scores than non‐frail individuals for both PC1 [median: −0.27 (IQR: 1.15) vs. 0.27 (1.23)] and PC2 [median: −0.15 (IQR: 1.13) vs. 0.21 (1.38)] (Figure 3 ).
Table 2.
Principal components 1 and 2 with respective factor loadings of the components among 220 patients
| PC1 | Factor loading | PC2 | Factor loading |
|---|---|---|---|
| β‐Carotene | 0.760 | Valine | 0.824 |
| α‐Carotene | 0.711 | Leucine | 0.782 |
| Lutein/zeaxanthin | 0.655 | Methionine | 0.756 |
| β‐Cryptoxanthin | 0.619 | Lysine | 0.735 |
| Lycopene | 0.577 | Tryptophan | 0.668 |
| Eicosadienoic acid | 0.565 | Isoleucine | 0.623 |
| α‐Linolenic acid | 0.538 | Histidine | 0.573 |
| Linoleic acid | 0.533 | Phenylalanine | 0.501 |
| α‐Tocopherol | 0.406 | Threonine | 0.468 |
| Histidine | 0.397 | Proline | 0.336 |
| Vitamin D3 | 0.394 | 3MH/eGFR | −0.415 |
| Retinol | 0.311 | 3MH | −0.367 |
| Tryptophan | 0.300 | Creatinine | −0.350 |
| Arachidonic acid | −0.527 | ||
| Palmitic acid | −0.436 | ||
| IL6 | −0.392 | ||
| Isoleucine | −0.362 | ||
| IL6/IL10 | −0.334 |
3MH, 3‐methylhistidine; eGFR, estimated glomerular filtration rate; IL, interleukin; PC, principal component.
Components with factor loadings between 0.300 and −0.300 are not shown in this table; components and factor loadings in italics represent negative loading; PC1: eigenvalue = 5.579, variance = 13.95%; PC2: eigenvalue = 4.654, variance = 11.63%.
Figure 3.
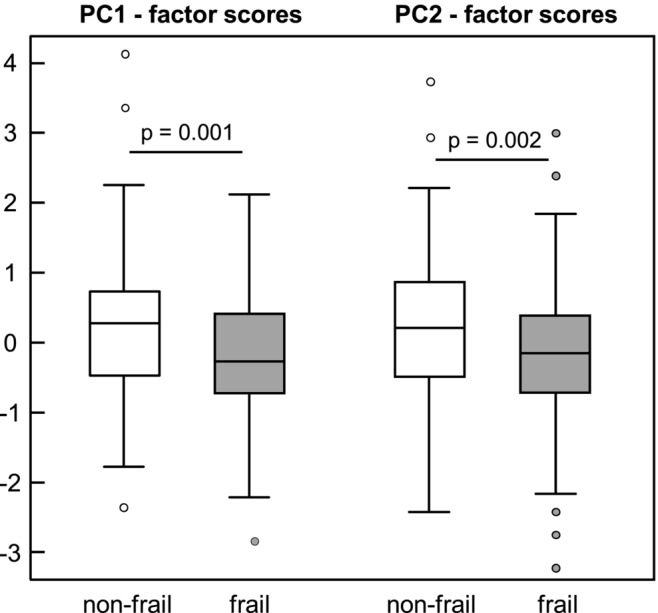
Factor scores of principal components 1 and 2 by frailty status among 220. Data are shown as boxplot; differences between frailty groups were determined by Student's t‐test; statistically significant different at P < 0.05. PC, principal component.
Significant cross‐sectional associations of both PC1 and PC2 factor scores with frailty were observed (Tables 3 and 4 ). Patients with higher PC1 factor scores had significantly lower odds for frailty (OR: 0.62; 95% CI: 0.46–0.83; P = 0.001; Table 1 , crude model) compared with patients with lower PC1 factor scores. This OR indicates that increasing serum levels of anti‐inflammatory biomarkers such as carotenoids and selective FA are associated with a reduced likelihood (approximately 38%) for frailty. Statistically significantly lower odds for frailty were still present for patients with higher PC1 factor scores after adjusting the regression model for potential confounders such as age, sex, energy intake and medication intake and co‐morbidities (Table 3 , Models 1–4). Furthermore, patients with higher PC2 factor scores had significantly lower odds for frailty (OR: 0.64; 95% CI: 0.48–0.86; P = 0.003; Table 2 , crude model) compared with patients with lower PC2 factor scores. This indicates that increasing serum concentrations of muscle‐anabolic eAA and BCAA are associated with a reduced likelihood (approximately 36%) for frailty. This association remained significant after adjusting the regression model for potential confounders (Table 4 , Models 1–4).
Table 3.
Cross‐sectional associations between biomarker pattern PC1 (considered as per increase in factor score units) and frailty status assessed by multivariate logistic regression models
| OR | 95% CI | P‐value | |
|---|---|---|---|
| Crude model | 0.62 | 0.46; 0.83 | 0.001 |
| Model 1 | 0.54 | 0.38; 0.75 | 0.000 |
| Model 2 | 0.58 | 0.41; 0.82 | 0.002 |
| Model 3 | 0.54 | 0.38; 0.78 | 0.001 |
| Model 4 | 0.60 | 0.41; 0.86 | 0.005 |
Results are displayed as odds ratios (OR with 95% confidence interval). Crude model: frailty status (non‐frail and frail) as dependent variable and PC1 factor score (per unit) as independent variable; Model 1: crude model + age + sex; Model 2: crude model + age + sex + medication intake + co‐morbidities; Model 3: crude model + age + sex + energy intake; Model 4: crude model + age + sex + energy intake + medication intake + co‐morbidities.
Table 4.
Cross‐sectional associations between biomarker pattern PC2 (considered as per increase in factor score units) and frailty status assessed by multivariate logistic regression models
| OR | 95% CI | P‐value | |
|---|---|---|---|
| Crude model | 0.64 | 0.48; 0.86 | 0.003 |
| Model 1 | 0.65 | 0.48; 0.87 | 0.004 |
| Model 2 | 0.69 | 0.51; 0.94 | 0.016 |
| Model 3 | 0.66 | 0.49; 0.88 | 0.006 |
| Model 4 | 0.70 | 0.52; 0.95 | 0.021 |
Results are displayed as odds ratios (OR with 95% confidence interval). Crude model: frailty status (non‐frail and frail) as dependent variable and PC2 factor score (per unit) as independent variable; Model 1: crude model + age + sex; Model 2: crude model + age + sex + medication intake + co‐morbidities; Model 3: crude model + age + sex + energy intake; Model 4: crude model + age + sex + energy intake + medication intake + co‐morbidities.
Discussion
In our study, we evaluated cross‐sectional associations of biomarker patterns including nutrition‐related fat‐soluble micronutrients, FA and AA, as well as biomarkers of OS, inflammation and muscle‐related biomarkers among older, multi‐morbid and hospitalized patients. It is, to the best of our knowledge, the first study to simultaneously measure and evaluate this unique panel of circulating biomarkers considering the complexity of the multifactorial mechanisms leading to frailty. Main outcome revealed two biomarker patterns that were both significantly associated with frailty. One reflecting a mainly inflammation‐related biomarker pattern being characterized by high FL of carotenoids, fatty acids and interleukins. The second reflecting a more muscle‐related biomarker pattern characterized mainly by high FL of BCAA (and other eAA), 3MH and 3MH/eGFR. Hence, our results contribute to improve the understanding of the complex intertwined mechanisms involved in frailty development and elucidate nutritional influences on frailty.
The finding that biomarker patterns are significantly associated with frailty and the likelihood of frailty, although some single nutrients and metabolites are not significantly different between frail and non‐frail patients, indicates that such multi‐biomarker approaches are favourable compared with single nutrients or metabolites as biomarkers of frailty. This is in line with previous suggestions for frailty biomarkers. 29
An inflammatory signature, 30 an AA profile 31 and biomarker patterns considering analytes involved in inflammation, muscle and neuromuscular metabolism and AAs 32 were found for PF&S (physical frailty and sarcopenia) and non‐PF&S patients. Furthermore, a pattern low in α‐tocopherol and γ‐tocopherol and retinol and high in carotenes was associated with higher odds for frailty. 16 However, contrarily to our study, no combination of nutrients, oxidative stress, inflammation or muscle‐related biomarkers involved in frailty development was analysed, 16 , 30 , 31 and thus, possible intertwined effects were not considered in previous studies. Studies investigating metabolic profiles with regard to the PF&S phenotype were performed in generally healthier, less morbid and less medicated older adults, 30 , 31 , 32 in contrast to our study. Our investigations in hospitalized patients of a clinical cohort, which represents a vulnerable, multi‐morbid old population that is further characterized by a high medication intake, are of importance, because frailty is positively associated with both co‐morbidities 3 and medication intake. 33 Among our in‐hospital cohort, 52.7% patients were frail, which is similar with up to 60% within hospitalized, multi‐morbid Spanish individuals, 34 but in contrast to 3.0–7.7% 4 , 35 in community‐dwelling older adults. Previous studies on biomarkers and metabolic profiles were performed within community‐dwelling adults, whereas studies in hospitalized, multi‐morbid patients are scarce. We revealed significant cross‐sectional associations of nutrition‐related biomarker patterns with frailty despite the heterogeneity of patients, who are multi‐morbid and have a high medication intake. Hence, our study is adding new information to this gap in frailty research and underlines the importance of an adequate nutrient supply in higher age.
Maintenance of muscle mass relies on the balance between muscle protein synthesis and breakdown. Essential AA and BCAA, in particular leucine, stimulate MPS and insulin inhibits MPB; however, the response to muscle‐anabolic stimuli might be reduced (anabolic resistance) in aged muscles, potentially leading to both a muscle‐catabolic state (MPB > MPS) and muscle atrophy 10 and subsequently contributing to frailty development. Patients with a biomarker profile characterized by low levels of BCAA together with high levels of 3MH‐based metabolites were more likely to be frail in our study. Lower circulating eAA and BCAA concentrations, like valine and leucine, were associated with lower muscle mass, strength, slowness and sarcopenia among community‐dwelling older adults 12 and were significantly lower in frail than in non‐frail community‐dwelling older men. 11 In our study, non‐frail and frail patients could be differentiated by 3MH, 3MH/Crea and 3MH/eGFR, indicating 3MH‐based biomarkers as suitable for frailty identification, as previously suggested. 21 Our results further indicate that frail patients are in a muscle‐catabolic state, implying that a higher intake of proteins, eAA or BCAA is beneficial regarding muscle protein synthesis, which is in line with recommendations for higher protein intakes in older adults. 36
Inflammation, that is, higher IL6, was inversely associated with skeletal muscle quality, strength, function and physical performance in mobility‐limited older individuals, 37 and higher IL6 and CRP concentrations have been associated with pre‐frailty and frailty. 17 In our study, frail patients also had significantly higher serum IL6 and IL6/IL10 concentrations than non‐frail patients. Furthermore, we observed a significantly positive correlation between IL6 and 3MH (Spearman correlation ρ = 0.213; P = 0.001) as well as 3MH/eGFR (ρ = 0.213; P = 0.001), hence supporting the link between inflammation and higher MPB, muscle catabolism and frailty. Besides inflammation, OS was previously shown to be associated with frailty. 15 Both pre‐frail and frail individuals had significantly higher plasma PrCarb compared with robust individuals (aged >65 years), 14 , 15 whereas 3‐NT levels were not different between frailty groups. 15 Higher plasma PrCarb were also positively associated with pre‐frailty and frailty. 14 , 15 MDA concentrations were significantly higher in frail than in non‐frail adults and were significantly associated with frailty. 14 However, in our study, serum concentrations of PrCarb, 3‐NT and MDA were similar between frailty groups. Because our non‐frail group consists primarily of pre‐frail patients and only few non‐frail patients, it is not surprising that OS is similar between the two frailty groups. There were similar PrCarb, MDA and 3‐NT concentrations between pre‐frail and frail individuals in previous studies. 14 , 15
Fat‐soluble vitamins and carotenoids as well as selective FA exert anti‐oxidative or anti‐inflammatory properties, thus potentially protecting older adults against OS and inflammation and hence against frailty. Lower α‐carotene and β‐carotene, lycopene, lutein/zeaxanthin and β‐cryptoxanthin concentrations were associated with a higher likelihood for frailty in individuals of four European cohorts. 15 Significantly lower β‐cryptoxanthin and β‐carotene concentrations in frail than in non‐frail patients in our study strengthen previous findings. 15 Higher dietary intake and higher circulating carotenoid concentrations both individually and cumulatively attenuated the likelihood for physical frailty. 38 Beneficial effects of carotenoids are based on their anti‐oxidative capacity and function to counteract oxidative stress and thus might also be involved in subsequent inflammatory processes. Significant correlations between serum IL6 and carotenoid concentrations (β‐cryptoxanthin: ρ = −0.304, β‐carotene: ρ = −0.308, lycopene: ρ = −0.279, α‐carotene: ρ = −0.250 and lutein/zeaxanthin: ρ = −0.178; all P < 0.01) in our study indicate that carotenoids might have anti‐inflammatory function and that carotenoid‐rich fruits and vegetables exert protective actions against age‐related physical decline.
Among FA, arachidonic acid has pro‐inflammatory effects, whereas EPA and DHA have anti‐inflammatory effects. 39 An inverse association between levels of EPA and DHA with higher odds for frailty was found in community‐dwelling older Korean adults. 18 Our data show that a biomarker profile, characterized by high levels of carotenoids, α‐linolenic acid and linoleic acid together with low levels of pro‐inflammatory metabolites such as arachidonic acid, palmitic acid (C16:0) and IL6, was associated with lower odds for frailty and supports these previous findings. However, in contrast to previous data, EPA and DHA were neither different between frailty groups nor highly loading on PC1 in our study. Finally, our results indicate that frail patients exhibit a pro‐inflammatory phenotype and that an anti‐inflammatory and anti‐oxidative diet might decelerate frailty development.
Because frailty itself and the biomarkers we measured in our study can be affected by age, sex as well as co‐morbidities or medication intake, 3 , 4 , 33 , 40 , 41 , 42 and because frail patients were significantly older and had significantly more co‐morbidities as well as higher medication intake than non‐frail patients, we adjusted the statistical analyses for the confounding variables age, sex, number of co‐morbidities and medication intake. Interestingly, we observed significant correlations of both the number of co‐morbidities and medication intake with IL6 (ρ = 0.305 and ρ = 0.279, respectively), IL6/IL10 (ρ = 0.272 and ρ = 0.275, respectively), 3MH (ρ = 0.484 and ρ = 0.291, respectively) and 3MH/eGFR (ρ = 0.468 and ρ = 0.280, respectively; all P < 0.01), linking multi‐morbidity and polypharmacy to both higher inflammation and a muscle catabolic state. Furthermore, the number of co‐morbidities was significantly correlated with levels of β‐cryptoxanthin (ρ = −0.304), β‐carotene (ρ = −0.199), lycopene (ρ = −0.166) and α‐carotene (ρ = −0.187) and vitamin E (data not shown). Medication intake was also significantly correlated with β‐cryptoxanthin, β‐carotene (ρ = −0.189), lycopene (ρ = −0.137) and lutein/zeaxanthin (ρ = −0.184) and vitamin E concentrations (data not shown). This indicates a strong effect of multi‐morbidity and polypharmacy on micronutrient levels and points out that older adults/patients might benefit from an adequate dietary nutrient supply as well as by a reduction in medication intake (‘deprescribing’). However, examinations on possible interactions between specific diseases, single medications, inflammation and nutrition were out of scope of this study but remain important for future studies in the context of frailty. Furthermore, the prevalence of main diseases had no significant impact on frailty classification, and, to take the influence of the number of diseases and medications into account, we adjusted our statistical models for these potential confounders. Because circulating AA, FA and fat‐soluble micronutrient concentrations are influenced by diet, we compared nutrient intake data obtained by 24‐h recall between frailty groups and further adjusted the statistical analyses for energy intake. Finally, statistical analyses were adjusted for age, sex, energy intake, medication intake and number of co‐morbidities. Because circulating 3MH concentrations might be influenced by dietary meat intake 19 or kidney function, 20 we also measured 1MH as biomarker for meat intake 19 and eGFR as estimate of kidney function. 22 We observed that 3MH/1MH was similar between frailty groups (Table S1), suggesting a similar impact of possible meat intake on serum 3MH in both groups. To overcome possible kidney function limitations that might occur with ageing, we normalized 3MH to eGFR.
There are some limitations within our study. Analysis strategy limits the comparability of our findings with previous and other studies on biomarker patterns, because the availability of analytical methods might vary between studies. Cross‐sectional associations in our study are not able to reveal causal effects and are not able to explore details in mechanisms that are involved in the development of frailty, and therefore, longitudinal studies are needed in the future. However, we did consider a broad variety of possible confounding variables in our statistical analyses, thus strengthening our results. We were only able to compare two frailty groups (non‐frail vs. frail) due to a small number of robust patients that were grouped together with pre‐frail patients as non‐frail patients. However, differences found in our study can be interpreted as differences primarily between pre‐frail and frail patients. Also, both patterns are independent and do not necessarily have to be present simultaneously in the same frail patient, and thus, we cannot conclude that frailty or frail patients are always characterized by both a pro‐inflammatory and a muscle‐catabolic pattern at the same time. In our study, mainly Caucasian older patients were investigated, and hence, identified biomarker patterns and associations need to be further validated in other ethnic groups. Furthermore, we did not measure and evaluate the full spectrum of biomarkers that are involved in frailty development, and thus, there might be alternative or stronger biomarker patterns than ours. Eventually, omics‐based analyses might reveal deeper insights into frailty development. However, our specific biomarker patterns reflect important nutritional aspects (like anti‐oxidative and anti‐inflammatory micronutrients and fatty acids as well as muscle‐related amino acids) together with intertwined underlying mechanisms for frailty development (like inflammation and oxidative stress as well as muscle protein turnover) in multi‐morbid patients. Whether these findings are transferrable to other populations, such as community‐dwelling old adults, or whether improvements in nutritional status, muscle strength and reduced inflammation are reflected by changes in the biomarker patterns identified in this study, remains to be seen. We used data at hospital discharge to preclude effects of acute disease that are difficult to disentangle from the effects of frailty, and, therefore, possible changes of metabolite concentrations during hospital stay cannot be evaluated. Also, the impact of food intake in hospital that might differ from the habitual diet in the study participants cannot be quantified.
Conclusion
This is the first study, to our knowledge, that simultaneously measures this broad panel of fat‐soluble micronutrients, fatty acids and amino acids, as well as oxidative stress, inflammation and 3MH‐based biomarkers, and evaluates cross‐sectional associations of respective biomarker patterns with frailty in old hospital patients. We identified two biomarker patterns, characterized either by inflammation‐related or by muscle‐related circulating biomarkers, that are both associated with frailty among multi‐morbid patients. Interestingly, associations of both biomarker patters with frailty are similarly pronounced. We conclude that frail patients are in a pro‐inflammatory or a muscle‐catabolic state and that analyses of such specific biomarker patterns are more informative than single biomarker analyses in the identification of frailty. However, prospective analyses are necessary to further detect potential causal relationships.
Conflict of interest
All authors declare that they have no conflict of interest.
Supporting information
Table S1. Circulating biomarker concentrations, ratios and percentages by frailty status among 220 patients
Acknowledgements
This work was supported by the NutriAct‐Competence Cluster Nutrition Research Berlin‐Potsdam funded by the Federal Ministry of Education and Research (FKZ: 01EA1806A‐H). The authors certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 43
Open Access funding enabled and organized by Projekt DEAL. WOA Institution: DEUTSCHES INSTITUT FUR ERNAHRUNGSFORSCHUNG Consortia Name : Projekt DEAL
Open Access funding enabled and organized by Projekt DEAL. WOA Institution: DEUTSCHES INSTITUT FUR ERNAHRUNGSFORSCHUNG Consortia Name : Projekt DEAL
Kochlik B., Franz K., Henning T., Weber D., Wernitz A., Herpich C., Jannasch F., Aykaç V., Müller‐Werdan U., Schulze M. B., Grune T., and Norman K. (2023) Frailty is characterized by biomarker patterns reflecting inflammation or muscle catabolism in multi‐morbid patients, Journal of Cachexia, Sarcopenia and Muscle, 14, 157–166, 10.1002/jcsm.13118
References
- 1. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 2. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013;381:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hanlon P, Nicholl BI, Jani BD, Lee D, McQueenie R, Mair FS. Frailty and pre‐frailty in middle‐aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK Biobank participants. Lancet Public Health 2018;3:e323–e332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Manfredi G, Midao L, Paul C, Cena C, Duarte M, Costa E. Prevalence of frailty status among the European elderly population: Findings from the survey of health, aging and retirement in Europe. Geriatr Gerontol Int 2019;19:723–729. [DOI] [PubMed] [Google Scholar]
- 5. Sinclair A. A comparative review of frailty models and a description of the European‐wide FRAILOMIC initiative. Med Res Arch 2018;6. 10.18103/mra.v6i6.1760 [DOI] [Google Scholar]
- 6. Angulo J, El Assar M, Rodriguez‐Manas L. Frailty and sarcopenia as the basis for the phenotypic manifestation of chronic diseases in older adults. Mol Aspects Med 2016;50:1–32. [DOI] [PubMed] [Google Scholar]
- 7. Tieland M, Trouwborst I, Clark BC. Skeletal muscle performance and ageing. J Cachexia Sarcopenia Muscle 2018;9:3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morley JE, von Haehling S, Anker SD, Vellas B. From sarcopenia to frailty: a road less traveled. J Cachexia Sarcopenia Muscle 2014;5:5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wilson D, Jackson T, Sapey E, Lord JM. Frailty and sarcopenia: The potential role of an aged immune system. Ageing Res Rev 2017;36:1–10. [DOI] [PubMed] [Google Scholar]
- 10. Wilkinson DJ, Piasecki M, Atherton PJ. The age‐related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res Rev 2018;47:123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. le Couteur DG, Ribeiro R, Senior A, Hsu B, Hirani V, Blyth FM, et al. Branched chain amino acids, cardiometabolic risk factors and outcomes in older men: The Concord health and ageing in men project. J Gerontol A Biol Sci Med Sci 2020;75:1805–1810. [DOI] [PubMed] [Google Scholar]
- 12. Ter Borg S, Luiking YC, van Helvoort A, Boirie Y, Schols J, de Groot C. Low levels of branched chain amino acids, eicosapentaenoic acid and micronutrients are associated with low muscle mass, strength and function in community‐dwelling older adults. J Nutr Health Aging 2019;23:27–34. [DOI] [PubMed] [Google Scholar]
- 13. Semba RD, Bartali B, Zhou J, Blaum C, Ko CW, Fried LP. Low serum micronutrient concentrations predict frailty among older women living in the community. J Gerontol A Biol Sci Med Sci 2006;61:594–599. [DOI] [PubMed] [Google Scholar]
- 14. Inglés M, Gambini J, Carnicero JA, García‐García FJ, Rodríguez‐Mañas L, Olaso‐González G, et al. Oxidative stress is related to frailty, not to age or sex, in a geriatric population: Lipid and protein oxidation as biomarkers of frailty. J Am Geriatr Soc 2014;62:1324–1328. [DOI] [PubMed] [Google Scholar]
- 15. Kochlik B, Stuetz W, Pérès K, Pilleron S, Féart C, García García FJ, et al. Associations of fat‐soluble micronutrients and redox biomarkers with frailty status in the FRAILOMIC initiative. J Cachexia Sarcopenia Muscle 2019;10:1339–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. FRAILOMIC initiative , Pilleron S, Weber D, Pérès K, Colpo M, Gomez‐Cabrero D, et al. Patterns of circulating fat‐soluble vitamins and carotenoids and risk of frailty in four European cohorts of older adults. Eur J Nutr 2018;58:379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Soysal P, Stubbs B, Lucato P, Luchini C, Solmi M, Peluso R, et al. Inflammation and frailty in the elderly: A systematic review and meta‐analysis. Ageing Res Rev 2016;31:1–8. [DOI] [PubMed] [Google Scholar]
- 18. Kim D, Won CW, Park Y. Association between erythrocyte levels of n‐3 polyunsaturated fatty acids and risk of frailty in community‐dwelling older adults: The Korean frailty and aging cohort study. J Gerontol A Biol Sci Med Sci 2020;76:499–504. [DOI] [PubMed] [Google Scholar]
- 19. Kochlik B, Gerbracht C, Grune T, Weber D. The influence of dietary habits and meat consumption on plasma 3‐methylhistidine‐A potential marker for muscle protein turnover. Mol Nutr Food Res 2018;62:e1701062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Long CL, Haverberg LN, Young VR, Kinney JM, Munro HN, Geiger JW. Metabolism of 3‐methylhistidine in man. Metabolism 1975;24:929–935. [DOI] [PubMed] [Google Scholar]
- 21. Kochlik B, Stuetz W, Pérès K, Féart C, Tegner J, Rodriguez‐Mañas L, et al. Associations of plasma 3‐methylhistidine with frailty status in French cohorts of the FRAILOMIC initiative. J Clin Med 2019;8:1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stuetz W, Weber D, Dollé M, Jansen E, Grubeck‐Loebenstein B, Fiegl S, et al. Plasma carotenoids, tocopherols, and retinol in the age‐stratified (35‐74 years) general population: A cross‐sectional study in six European countries. Nutrients 2016;8:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weber D, Stuetz W, Bernhard W, Franz A, Raith M, Grune T, et al. Oxidative stress markers and micronutrients in maternal and cord blood in relation to neonatal outcome. Eur J Clin Nutr 2014;68:215–222. [DOI] [PubMed] [Google Scholar]
- 25. van den Ouweland JM, Beijers AM, Demacker PN, van Daal H. Measurement of 25‐OH‐vitamin D in human serum using liquid chromatography tandem‐mass spectrometry with comparison to radioimmunoassay and automated immunoassay. J Chromatogr B Analyt Technol Biomed Life Sci 2010;878:1163–1168. [DOI] [PubMed] [Google Scholar]
- 26. Jannasch F, Bedu‐Addo G, Schulze MB, Mockenhaupt FP, Danquah I. Serum phospholipid fatty acids, dietary patterns and type 2 diabetes among urban Ghanaians. Nutr J 2017;16:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weber D, Kneschke N, Grimm S, Bergheim I, Breusing N, Grune T. Rapid and sensitive determination of protein‐nitrotyrosine by ELISA: Application to human plasma. Free Radic Res 2012;46:276–285. [DOI] [PubMed] [Google Scholar]
- 28. Prinsen H, Schiebergen‐Bronkhorst BGM, Roeleveld MW, Jans JJM, de Sain‐van der Velden MGM, Visser G, et al. Rapid quantification of underivatized amino acids in plasma by hydrophilic interaction liquid chromatography (HILIC) coupled with tandem mass‐spectrometry. J Inherit Metab Dis 2016;39:651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rodriguez‐Mañas L, Araujo de Carvalho I, Bhasin S, Bischoff‐Ferrari HA, Cesari M, Evans W, et al. ICFSR task force perspective on biomarkers for sarcopenia and frailty. J Frailty Aging 2020;9:4–8. [DOI] [PubMed] [Google Scholar]
- 30. Marzetti E, Picca A, Marini F, Biancolillo A, Coelho‐Junior HJ, Gervasoni J, et al. Inflammatory signatures in older persons with physical frailty and sarcopenia: The frailty “cytokinome” at its core. Exp Gerontol 2019;122:129–138. [DOI] [PubMed] [Google Scholar]
- 31. Calvani R, Picca A, Marini F, Biancolillo A, Gervasoni J, Persichilli S, et al. A distinct pattern of circulating amino acids characterizes older persons with physical frailty and sarcopenia: Results from the BIOSPHERE study. Nutrients 2018;10:1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Calvani R, Picca A, Marini F, Biancolillo A, Gervasoni J, Persichilli S, et al. Identification of biomarkers for physical frailty and sarcopenia through a new multi‐marker approach: results from the BIOSPHERE study. Geroscience 2021;43:727–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saum KU, Schöttker B, Meid AD, Holleczek B, Haefeli WE, Hauer K, et al. Is polypharmacy associated with frailty in older people? Results from the ESTHER cohort study. J Am Geriatr Soc 2017;65:e27–e32. [DOI] [PubMed] [Google Scholar]
- 34. Bernabeu‐Wittel M, González‐Molina Á, Fernández‐Ojeda R, Díez‐Manglano J, Salgado F, Soto‐Martín M, et al. Impact of sarcopenia and frailty in a multicenter cohort of polypathological patients. J Clin Med 2019;8:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gagesch M, Chocano‐Bedoya PO, Abderhalden LA, Freystaetter G, Sadlon A, Kanis JA, et al. Prevalence of physical frailty: Results from the DO‐HEALTH study. J Frailty Aging 2021;11:1–8. [DOI] [PubMed] [Google Scholar]
- 36. Bauer J, Biolo G, Cederholm T, Cesari M, Cruz‐Jentoft AJ, Morley JE, et al. Evidence‐based recommendations for optimal dietary protein intake in older people: A position paper from the PROT‐AGE study group. J Am Med Dir Assoc 2013;14:542–559. [DOI] [PubMed] [Google Scholar]
- 37. Grosicki GJ, Barrett BB, Englund DA, Liu C, Travison TG, Cederholm T, et al. Circulating interleukin‐6 is associated with skeletal muscle strength, quality, and functional adaptation with exercise training in mobility‐limited older adults. J Frailty Aging 2020;9:57–63. [DOI] [PubMed] [Google Scholar]
- 38. Zupo R, Castellana F, De Nucci S, Sila A, Aresta S, Buscemi C, et al. Role of dietary carotenoids in frailty syndrome: A systematic review. Biomedicine 2022;10:632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Davinelli S, Intrieri M, Corbi G, Scapagnini G. Metabolic indices of polyunsaturated fatty acids: current evidence, research controversies, and clinical utility. Crit Rev Food Sci Nutr 2021;61:259–274. [DOI] [PubMed] [Google Scholar]
- 40. Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 2018;15:505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weber D, Kochlik B, Demuth I, Steinhagen‐Thiessen E, Grune T, Norman K. Plasma carotenoids, tocopherols and retinol ‐ Association with age in the Berlin aging study II. Redox Biol 2020;32:101461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weber D, Kochlik B, Stuetz W, Dollé MET, Jansen E, Grubeck‐Loebenstein B, et al. Medication Intake Is Associated with Lower Plasma Carotenoids and Higher Fat‐Soluble Vitamins in the Cross‐Sectional MARK‐AGE Study in Older Individuals. J Clin Med 2020;9:2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. von Haehling S, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Circulating biomarker concentrations, ratios and percentages by frailty status among 220 patients


