Abstract
Background
Personalized therapy planning remains a significant challenge in advanced colorectal cancer care, despite extensive research on prognostic and predictive markers. A strong correlation of sarcopenia or overall body composition and survival has been described. Here, we explore whether automated assessment of body composition and liver metastases from standard of care CT images can add to clinical parameters in personalized survival risk prognostication.
Methods
We retrospectively analysed clinical imaging data from 85 patients (50.6% female, mean age 58.9 SD 12.2 years) with colorectal cancer and synchronous liver metastases. Pretrained deep learning models were used to assess body composition and liver metastasis geometry from abdominal CT images before the initiation of systemic treatment. Abdominal muscle‐to‐bone ratio (MBR) was calculated by dividing abdominal muscle volume by abdominal bone volume. MBR was compared with body mass index (BMI), abdominal muscle volume, and abdominal muscle volume divided by height squared. Differences in overall survival based on body composition and liver metastasis parameters were compared using Kaplan–Meier survival curves. Results were correlated with clinical and biomarker data to develop a machine learning model for survival risk prognostication.
Results
The MBR, unlike abdominal muscle volume or BMI, was significantly associated with overall survival (HR 0.39, 95% CI: 0.19–0.80, P = 0.009). The MBR (P = 0.022), liver metastasis surface area (P = 0.01) and primary tumour sidedness (P = 0.007) were independently associated with overall survival in multivariate analysis. Body composition parameters did not correlate with KRAS mutational status or primary tumour sidedness. A prediction model based on MBR, liver metastasis surface area and primary tumour sidedness achieved a concordance index of 0.69.
Conclusions
Automated segmentation enables to extract prognostic parameters from routine imaging data for personalized survival modelling in advanced colorectal cancer patients.
Keywords: Body composition, Machine learning, Colorectal cancer, Prognosis, Computed tomography
1. Background
Colorectal cancer (CRC) is the third most common cancer in Western countries. Up to 50% of patients either are initially diagnosed with synchronous metastases or develop metachronous metastases. The predominant organ of metastases is with 77% the liver with 5 year overall survival of only 15% in these patients. 1 , 2 Curative resection of liver metastases is well established in colorectal cancer care, but only about 25% of patients qualify for this procedure. 1 , 3 The personalized identification of colorectal liver metastasis (CRLM) patients with poor prognosis under current treatment standards is of utmost importance. This may prevent futile overtreatment and enable personalized referral for experimental therapies within clinical trials. In this regard, studies have shown that sarcopenia is an independent predictor of prognosis in advanced CRC. 4 , 5 , 6 , 7 However, to date, body mass index (BMI) has been used as a standard measure in routine clinical care, although it is not an appropriate prognostic marker in CRC. Because the BMI fails to distinguish between different tissues, it does not accurately reflect the physical condition of patients. In contrast, CT image‐based markers of body composition have been shown to be more informative than BMI. 8 , 9 By assessing the body composition, the individual distribution of fat, muscle and bone of each patient can be examined. Previous studies calculated the body composition by segmentation of CT sectional images at the level of the L3 vertebra, which is only an approximation of the actual body composition. Moreover, this approach depended to varying degrees on manual processing, which is time‐ and labor‐intensive and is not suited for integration in routine care. Consequently, body composition analysis is still largely neglected in survival risk stratification. Due to recent developments in deep learning and image segmentation, fully automated assessment of body composition and tumour characteristics from 3D CT scans has become possible. 10 , 11 In addition to assessing body compositions, this also enables the accurate geometric analysis of CRLM, which has been shown to be associated with overall survival in advanced CRC patients. 12 , 13 , 14 , 15 As regular CT scans are part of routine care in oncology, automated extraction of in‐depth information could allow more granular prediction of prognosis.
Against this background, we have taken advantage of current technical capabilities to develop a new prediction model that can be incorporated into routine clinical care to enable personalization.
2. Methods
2.1. Study design
We retrospectively evaluated 258 CRC patients treated at the University Hospital Essen and included 85 patients with synchronous CRLM in the final analysis (Figure 1). All patients were treated with systemic cancer therapy between 2004 and 2017. Overall survival time was defined as time from the start of systemic treatment to the date of death from any cause. We censored patients for whom no date of death was available at the date of the last follow‐up. The study was approved by the Ethics Committee of the Medical Faculty of the University Duisburg‐Essen (No. 21‐10347).
Figure 1.
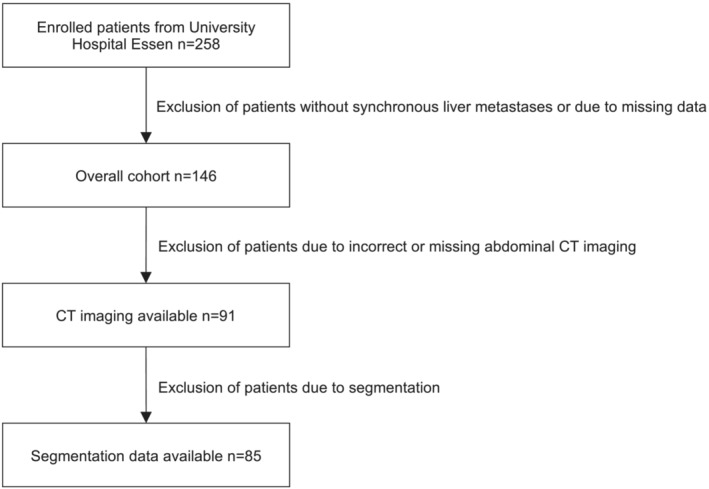
Flowchart depicting the process of patient enrolment.
2.2. Patient characteristics
All included patients were diagnosed with stage IV CRC with synchronous liver metastases. The median age was 59.0 years and 50.6% were female. 63.5% of patients were diagnosed with a left‐sided primary tumour. The median survival time (MST) from start of palliative chemotherapy was 21.2 months (95% CI: 15.6–27.2 months; see Figure S1). The patient characteristics are summarized in Table 1.
Table 1.
Characteristics of the overall cohort
| n = 85 | |
|---|---|
| Age, years | |
| Median | 59.0 |
| Range | 26–83 |
| Gender, N (%) | |
| Male | 42 (49.4) |
| Female | 43 (50.6) |
| AJCC Stage, N (%) | |
| IV | 85 (100) |
| Liver metastasis, N (%) | |
| Synchronous | 85 (100) |
| First‐line chemotherapy, N (%) | |
| FOLFOX | 57 (67.1) |
| FOLFIRI | 18 (21.2) |
| Other | 10 (11.8) |
| First‐line antibody, N (%) | |
| Bevacizumab | 39 (45.9) |
| Cetuximab | 21 (24.7) |
| KRAS status, N (%) | |
| Mutant | 21 (24.7) |
| Wild‐type | 50 (58.8) |
| Unknown | 14 (16.5) |
| Primary tumour sidedness, N (%) | |
| Left | 54 (63.5) |
| Right | 31 (36.5) |
| Abdominal body composition, median (range), L | |
| Muscle | 5.77 (3.21–10.10) |
| Bone | 2.43 (1.66–3.84) |
| TAT | 10.57 (1.22–33.09) |
| SAT | 7.08 (0.50–21.33) |
| VAT | 2.59 (0.25–10.60) |
| IMAT | 0.89 (0.24–3.73) |
| Median survival time, months (95% CI) | 21.2 (15.6–27.2) |
| 1 year mortality rate, % (95% CI) | 23.5 (15.9–34.1) |
| Censored, N (%) | 6 (7.1) |
2.3. Assessments
Baseline abdominal CT images taken before the initiation of systemic treatment were used to segment body composition and liver metastases. Body composition markers of the abdominal cavity were assessed using a fully automated extraction pipeline. 11 The markers analysed comprised the volumes of muscle, bone, total adipose tissue (TAT), intermuscular adipose tissue (IMAT), subcutaneous adipose tissue (SAT), and visceral adipose tissue (VAT). Prior to extraction of body composition markers, all CT images were resampled to 5 mm slice thickness to meet the requirements of our extraction pipeline. To ensure comparability between patients, the collected markers were divided by the number of CT slices of the automatically detected abdominal cavity for further analysis. Abdominal muscle‐to‐bone ratio (MBR) and muscle/height2 were calculated by dividing abdominal muscle volume by abdominal bone volume or by the body height squared (Figure S2). Liver metastases were automatically segmented using the pretrained nnU‐Net architecture. 10 The volume and surface area of metastases were extracted with a triangle mesh approach using an internal feature extraction pipeline including PyRadiomics 3.0.1 and were normalized to the [0, 1] interval. 16 In four cases, the segmented liver metastases were too small for feature extraction and the respective values were set to zero. Further patient information used in this study, including primary tumour sidedness, was obtained from the patients' electronic health records. Tumours originating from the rectum, sigma, descending colon, and left flexure were defined as left‐sided. All tumours located in the transverse colon, right flexure, ascending colon, and caecum were defined as right‐sided. BMI was calculated in 80 patients in whom respective data were available at the initiation of therapy. In a subgroup of 71 patients, data from genomic sequencing of macrodissected tissue samples were available.
2.4. Statistical analysis
All statistical analyses were performed using Python 3.8 and the packages lifelines, scikit‐survival and SciPy. 17 , 18 , 19 Based on a Cox proportional hazards model, we selected the three parameters MBR, liver metastasis surface area, and primary tumour sidedness for further analysis. We formed subgroups based on median MBR, median CRLM surface area or the primary tumour sidedness and compared overall survival using Kaplan–Meier survival curves and a log‐rank test. From these parameters, we then built a prediction model using a component‐wise gradient boosting algorithm with partial likelihood loss of a Cox proportional hazards model. 17 , 20 Predictive performance was assessed calculating the mean concordance index as result of a 5 × 5‐fold cross validation. The correlation between parameters was analysed by calculating the Pearson correlation coefficient (r). P‐values ≤0.05 were regarded statistically significant. To understand the decision‐making of our model, we used SHapley Additive exPlanations (SHAP). 21
3. Results
3.1. MBR is a predictor of prognosis in advanced CRC patients
Body composition parameters extracted from abdominal CT images were analysed (Table 1). Cox proportional hazards analysis adjusted for age and gender revealed that abdominal muscle volume alone did not serve as prognostic parameter (HR 0.98, 95% CI: 0.95–1.00, P = 0.062). However, calculating MBR by dividing abdominal muscle volume by abdominal bone volume provided an independent predictor of prognosis (HR 0.39, 95% CI: 0.19–0.80, P = 0.009). None of the remaining body composition parameters were significantly associated with overall survival (Table S3).
3.2. MBR compared with muscle/height2 and BMI
For 80 patients, data on weight and height at initiation of therapy were available. The median BMI was 24.0 kg/m2 with a range of 14.5 to 38.4 kg/m2. In this subgroup, the MBR (HR 0.41, 95% CI: 0.19–0.87, P = 0.02) and muscle/height2 (HR 0.91, 95% CI: 0.84–0.98, P = 0.013), unlike the BMI (HR 0.98, 95% CI: 0.93–1.03, P = 0.507), were significantly associated with overall survival (Table 2). Further analysis using Kaplan–Meier curves showed that based on the median MBR, two subgroups could be defined that had significantly different overall survival (P = 0.019, Figure 2A). In contrast, subgroups based on median muscle/height2 (P = 0.219, Figure 2B), or median BMI (P = 0.733, Figure 2C) did not show significantly different overall survival. By plotting the relationship between BMI and MBR, we found that a low BMI was related to a lower MBR. With a higher BMI, there was no clear correlation to MBR (Figure S4).
Table 2.
Results of a univariate Cox proportional hazards model adjusted for age and gender in a subgroup where BMI was available
| n = 80 | HR (95% CI) | P‐value |
|---|---|---|
| BMI | 0.98 (0.93–1.03) | 0.507 |
| MBR | 0.41 (0.19–0.87) | 0.020 |
| Muscle/height2 | 0.91 (0.84–0.98) | 0.013 |
Figure 2.
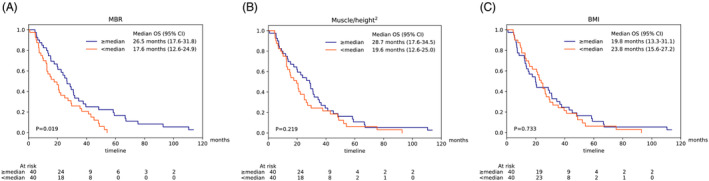
Kaplan–Meier survival curves showing the overall survival according to MBR (A), muscle/height2 (B), or BMI (C) in a subgroup (n = 80) where data on weight and height were available.
3.3. Prognostic relevance of CRLM geometry, primary tumour sidedness and KRAS mutational status
Using automated image segmentation, we confirmed the surface area (P = 0.006) and volume (P = 0.035) of liver metastases being predictors of prognosis (Table 3). Furthermore, primary tumour sidedness significantly associated with overall survival (P = 0.034). KRAS mutational status was available in 71 of our 85 patients (84%). There was no significant association between the KRAS mutational status and overall survival (P = 0.274, Table 3). In a multivariate analysis together with KRAS mutational status, primary tumour sidedness, age, and gender, MBR remained an independent predictor of overall survival (HR 0.32, 95% CI: 0.15–0.69, P < 0.005).
Table 3.
Results of a univariate Cox proportional hazards model adjusted for age and gender
| HR (95% CI) | P‐value | |
|---|---|---|
| CRLM volume (n = 85) | 2.96 (1.08–8.16) | 0.035 |
| CRLM surface area (n = 85) | 4.49 (1.55–13.02) | 0.006 |
| Left‐sided primary tumour (n = 85) | 0.61 (0.38–0.96) | 0.034 |
| KRAS mutation (n = 71) | 1.37 (0.78–2.41) | 0.274 |
3.4. Development of a machine learning model for survival risk prognostication
In a multivariate Cox proportional hazards model together with MBR and primary tumour sidedness, CRLM surface area was an independent predictor (HR 4.52, 95% CI: 1.43–14.3, P = 0.01, see Table S5), whereas CRLM volume showed only borderline significance (HR 3.00, 95% CI: 1.00–9.05, P = 0.051). All three independent parameters could significantly separate the overall cohort in Kaplan–Meier analyses (Figure 3). Using the three predictors MBR, CRLM surface area and primary tumour sidedness, a prediction model was developed. After 5x5 cross validation our model achieved a mean concordance index of 0.69 (95% CI: 0.65–0.72). Our model was able to identify risk groups that showed significantly different overall survival (P < 0.005, Figure S6). Through SHAP, we could confirm that our model uses the three parameters in a reasonable way and in accordance with the hazard ratios calculated by the Cox proportional hazards model (Figure 4). Interestingly, primary tumour sidedness had the highest impact on the model output, followed by CRLM surface area and MBR.
Figure 3.
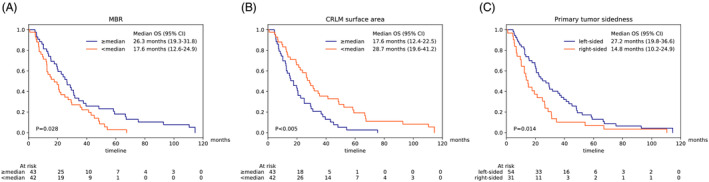
Kaplan–Meier survival curves showing the overall survival according to MBR (A), CRLM surface area (B), or primary tumour sidedness (C) in the overall cohort.
Figure 4.
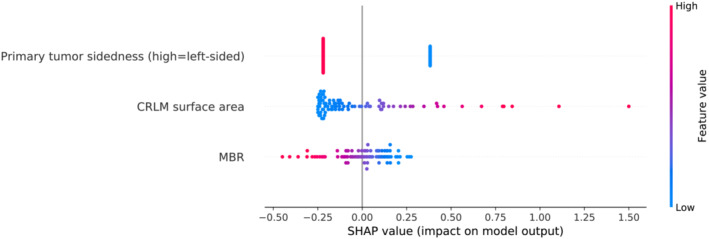
Decision‐making of the prediction model based on SHAP. The parameters are ranked from top to bottom by their responsibility on the model output. Feature values are represented by the colour gradient. Higher SHAP values indicate the responsibility of parameters for higher predicted survival risk.
3.5. Correlation analysis of the selected parameters and KRAS mutational status
In the overall cohort there was a weak negative correlation between both MBR and CRLM volume (r = −0.28, P = 0.009) and between MBR and CRLM surface area (r = −0.27, P = 0.014). We found no significant correlation between MBR and the primary tumour sidedness (P = 0.82) as well as between primary tumour sidedness and CRLM surface area (P = 0.15). In a subgroup with available data (n = 71), there was no significant correlation between KRAS mutational status and MBR (P = 0.57), CRLM surface area (P = 0.37), or primary tumour sidedness (P = 0.87).
4. Discussion
Multiple effective treatment options and strategies are available for patients with CRC. In clinical practice, patients are characterized by clinical and laboratory examination, imaging studies, tumour histology and molecular pathology. Nevertheless, the selection of the best strategy for an individual patient is still based on very few parameters, and thus does not make sufficient use of all available clinical information. Against this background we have demonstrated that it is possible to exploit body composition and liver tumour burden by automated extraction from staging CT images. In contrast to previous studies, we have assessed the body composition of the entire abdomen and not only at the level of the L3 vertebra. We have identified MBR and CRLM surface area in addition to primary tumour sidedness as independent predictors of overall survival. These parameters enabled us to develop a prediction model that can be easily implemented in routine clinical care.
There are conflicting studies on the importance of body mass index in the prognosis of advanced CRC. 22 , 23 , 24 In many other solid tumours, however, a favourable effect of a higher BMI on overall survival has been found, which is referred to as the obesity paradox. 25 In early stage CRC, the negative correlation between BMI and sarcopenia was found to be an underlying factor of this effect. 26 In advanced CRC, sarcopenia was thus demonstrated to be an independent negative predictor of overall survival. 4 , 5 , 6 , 7 Because BMI does not distinguish between fat and muscle tissue, CT image‐derived body composition analyses proved to be a superior approach to assess prognostic parameters such as sarcopenia. 8 , 9 In those studies, body composition was measured from CT sectional images at the level of the L3 vertebra, which due to the limited data source may be prone to imprecision or confounders. In contrast, our study is the first to automatically assess body composition from the entire abdominal cavity and compare resulting markers with BMI. By segmenting the entire abdominal cavity instead of single sectional images, our study seeks to describe body composition more accurately and thus does not rely on approximation. Moreover, previous studies have used the skeletal muscle index, which normalizes muscle area with the square of height, whereas we have normalized muscle volume with bone volume by calculating the MBR. This is based on the assumption that bone volume allows an inference of the general body structure and remains relatively constant regardless of other changing body composition parameters. Our finding that MBR, as well as abdominal muscle volume divided by height squared, are predictors of overall survival in CRC patients independent of age and gender supports this hypothesis. Because only MBR could form subgroups that showed significantly different overall survival and can be automatically extracted from CT images without additional patient information, we propose MBR as a new prognostic marker in this cancer entity.
Treatment decisions in advanced CRC are highly dependent on the tumour genotype. In particular, the presence of RAS mutations in association with the primary tumour sidedness is of major importance for the selection of antibody therapies. In our study, we saw no correlation between the KRAS mutational status and body composition parameters, which is in line with previous studies. 27 Because MBR was a predictor of overall survival independent of KRAS mutational status and the primary tumour sidedness, this suggests high generalizability. In a subgroup with available information at baseline, we could demonstrate that BMI, unlike MBR, did not serve as predictor of overall survival. This finding is consistent with the study by Dell'Aquila et al., which also found no association between BMI and overall survival in metastatic CRC. 22 This underscores the need for a more differentiated analysis of body composition than by using the traditional body mass index, which is still part of routine clinical care.
To obtain additional assessment of individual patients beyond body composition, we performed automated image segmentation to measure liver tumour burden. Previous studies investigating CRLM from CT images have often placed a strong focus on complex radiomic signatures including texture features. 15 , 28 , 29 However, it was previously shown that radiomic parameters are often only a surrogate for tumour volume and are not prognostic on their own. 30 Furthermore, the use of different CT scanners and protocols poses significant difficulties for the analysis of many radiomic features. To overcome this issue, we focused on shape features such as the surface area and tumour volume, which are reproducible across different CT scanners and have shown a plausible association with survival independent of the CT settings. 12 , 31 , 32 We could confirm the surface area and volume of CRLM as predictors of prognosis in advanced CRC patients. Because CRLM surface area, unlike CRLM volume, was shown to be prognostically independent of MBR and primary tumour sidedness, we included it in our prediction model.
Tumour sidedness was previously shown to associate with overall survival in advanced CRC. 33 , 34 As studies have indicated remarkable differences between right‐ and left‐sided tumours and also suggested an impact on the body composition of patients, we included primary tumour sidedness as parameter in our model. 7 , 35 Using SHAP we identified primary tumour sidedness as most relevant parameter for the decision‐making of our model, emphasizing its importance in patient prognostication and treatment planning.
The three parameters included in our model were all independently correlated with overall survival in our study. In contrast to other prognostic tools, our model is based on non‐invasive parameters that do not depend on additional laboratory testing or tumour morphology as determined by histopathology. 36 Furthermore, the included parameters are relatively robust to acute events such as inflammation, which can temporarily affect blood values. This distinguishes our model from established prognostic tools such as the Glasgow prognostic score and allows for future integration of additional parameters. 37 We see great potential of our model in identifying patients who are suitable for curative resection of CRLM or who should be enrolled in clinical trials. Further research is needed to investigate the prognostic value of MBR in patients with earlier stages of CRC and in other cancer types.
Our study has limitations, which are particularly due to its retrospective design and the lack of external validation using an independent patient cohort. As we had to exclude patients for whom no suitable CT image was available or segmentation was not possible, our cohort is only of medium size. We investigated the association of our results with KRAS mutational status but were unable to analyse the influence of mutations or additional proto‐oncogenes such as NRAS or BRAF due to their overall rarity and representation in our cohort. Most patients were treated at a time, when NRAS and BRAF testing were not part of routine diagnostics. Although we performed extensive cross validation to prevent overfitting on our data, external validation will be necessary to confirm the results.
In conclusion, we propose a new pragmatic model for personalized survival risk prognostication in patients with advanced CRC that is based entirely on parameters that can be automatically extracted from routine clinical data. We have demonstrated that the inclusion of body composition and exact metastasis geometry hold great promise in refining current routine decision‐making.
Conflict of interest
We declare the following potential competing interests: M. Schuler reports compensated consultancy from Amgen, AstraZeneca, BIOCAD, Boehringer Ingelheim, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Merck Serono, Novartis, Roche, Sanofi, and Takeda; honoraria for continuing medical education presentations from Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Janssen, and Novartis; and received research funding to his institution from AstraZeneca and Bristol Myers Squibb. S. Kasper reports honoraria from Merck Serono, MSD, Novartis, Bristol Myers Squibb, Amgen, Roche, Sanofi‐Aventis, Servier, Incyte, and Lilly; research funding from Merck Serono, Lilly, Bristol Myers Squibb, and Roche. None of which apply to the current study. All remaining authors declare that they have no competing interests related to this study.
5. Funding
J. Keyl is supported by funding from the German Cancer Consortium Joint Funding Program (DKTK JF RAMTAS).
Supporting information
Figure S1: Kaplan–Meier survival curve depicting the survival of the overall cohort.
Figure S2: Illustration of the acquisition of body composition markers. The abdominal cavity is automatically detected from CT images and body composition markers are extracted. These markers are used together with body size to create combined features.
Table S3: Results of a univariate Cox Proportional Hazards model adjusted for age and gender.
Figure S4: Scatter plot showing the relationship between MBR and BMI.
Table S5: Multivariate analysis of the parameters included in the prediction model.
Figure S6: Kaplan–Meier survival curves showing high‐ and low‐risk subgroups identified by our prediction model. High and low risk groups were defined by the highest and lowest 20% risk score predicted by our model. The figure shows a representative fold after performing a 2‐fold cross validation.
Acknowledgement
The authors certify that they comply with the ethical guidelines for publishing of the Journal of Cachexia, Sarcopenia and Muscle. 38
Open Access funding enabled and organized by Projekt DEAL.
Keyl J., Hosch R., Berger A., Ester O., Greiner T., Bogner S., Treckmann J., Ting S., Schumacher B., Albers D., Markus P., Wiesweg M., Forsting M., Nensa F., Schuler M., Kasper S., and Kleesiek J. (2023) Deep learning‐based assessment of body composition and liver tumour burden for survival modelling in advanced colorectal cancer, Journal of Cachexia, Sarcopenia and Muscle, 14, 545–552, 10.1002/jcsm.13158
Stefan Kasper and Jens Kleesiek contributed equally to this work.
References
- 1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7–33. [DOI] [PubMed] [Google Scholar]
- 2. van der Geest LGM, Lam‐Boer J, Koopman M, Verhoef C, Elferink MAG, de Wilt JHW. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin Exp Metastasis 2015;32:457–465. [DOI] [PubMed] [Google Scholar]
- 3. Hackl C, Neumann P, Gerken M, Loss M, Klinkhammer‐Schalke M, Schlitt HJ. Treatment of colorectal liver metastases in Germany: a ten‐year population‐based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer 2014;14:810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Vledder MG, Levolger S, Ayez N, Verhoef C, Tran TCK, Ijzermans JNM. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg 2012;99:550–557. [DOI] [PubMed] [Google Scholar]
- 5. da Cunha LP, Silveira MN, Mendes MCS, Costa FO, Macedo LT, de Siqueira NS, et al. Sarcopenia as an independent prognostic factor in patients with metastatic colorectal cancer: A retrospective evaluation. Clin Nutr ESPEN 2019;32:107–112. [DOI] [PubMed] [Google Scholar]
- 6. Charette N, Vandeputte C, Ameye L, Bogaert CV, Krygier J, Guiot T, et al. Prognostic value of adipose tissue and muscle mass in advanced colorectal cancer: a post hoc analysis of two non‐randomized phase II trials. BMC Cancer 2019;19:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shirdel M, Andersson F, Myte R, Axelsson J, Rutegård M, Blomqvist L, et al. Body composition measured by computed tomography is associated with colorectal cancer survival, also in early‐stage disease. Acta Oncol 2020;59:799–808. [DOI] [PubMed] [Google Scholar]
- 8. Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539–1547. [DOI] [PubMed] [Google Scholar]
- 9. Choi Y, Oh D‐Y, Kim T‐Y, Lee K‐H, Han S‐W, Im S‐A, et al. Skeletal Muscle Depletion Predicts the Prognosis of Patients with Advanced Pancreatic Cancer Undergoing Palliative Chemotherapy, Independent of Body Mass Index. PLOS ONE 2015;10:e0139749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Isensee F, Jaeger PF, Kohl SAA, Petersen J, Maier‐Hein KH. nnU‐Net: a self‐configuring method for deep learning‐based biomedical image segmentation. Nat Methods 2021;18:203–211. [DOI] [PubMed] [Google Scholar]
- 11. Koitka S, Kroll L, Malamutmann E, Oezcelik A, Nensa F. Fully automated body composition analysis in routine CT imaging using 3D semantic segmentation convolutional neural networks. Eur Radiol 2021;31:1795–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mühlberg A, Holch JW, Heinemann V, Huber T, Moltz J, Maurus S, et al. The relevance of CT‐based geometric and radiomics analysis of whole liver tumor burden to predict survival of patients with metastatic colorectal cancer. Eur Radiol 2021;31:834–846. [DOI] [PubMed] [Google Scholar]
- 13. Dexiang Z, Li R, Ye W, Haifu W, Yunshi Z, Qinghai Y, et al. Outcome of Patients with Colorectal Liver Metastasis: Analysis of 1,613 Consecutive Cases. Ann Surg Oncol 2012;19:2860–2868. [DOI] [PubMed] [Google Scholar]
- 14. Beckers RCJ, Lambregts DMJ, Schnerr RS, Maas M, Rao S‐X, Kessels AGH, et al. Whole liver CT texture analysis to predict the development of colorectal liver metastases—A multicentre study. Eur J Radiol 2017;92:64–71. [DOI] [PubMed] [Google Scholar]
- 15. Lubner MG, Stabo N, Lubner SJ, del Rio AM, Song C, Halberg RB, et al. CT textural analysis of hepatic metastatic colorectal cancer: pre‐treatment tumor heterogeneity correlates with pathology and clinical outcomes. Abdom Imaging 2015;40:2331–2337. [DOI] [PubMed] [Google Scholar]
- 16. van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, et al. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res 2017;77:e104–e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pölsterl S. scikit‐survival: A Library for Time‐to‐Event Analysis Built on Top of scikit‐learn. J Mach Learn Res 2020;21:1–6.34305477 [Google Scholar]
- 18. Davidson‐Pilon C. lifelines, survival analysis in Python 2021.
- 19. Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat Methods 2020;17:261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hothorn T, Bühlmann P, Dudoit S, Molinaro A, Van Der Laan MJ. Survival ensembles. Biostatistics 2006;7:355–373. [DOI] [PubMed] [Google Scholar]
- 21. Lundberg SM, Lee S‐I. A Unified Approach to Interpreting Model Predictions. In Advances in Neural Information Processing Systems, Vol. 30. Curran Associates, Inc.; 2017. [Google Scholar]
- 22. Dell'Aquila E, Rossini D, Galletti A, Stellato M, Boccaccino A, Conca V, et al. Prognostic and Predictive Role of Body Mass Index (BMI) in Metastatic Colorectal Cancer (mCRC): A Pooled Analisys of Tribe and Tribe‐2 Studies by GONO. Clin Colorectal Cancer 2022;0. [DOI] [PubMed] [Google Scholar]
- 23. Renfro LA, Loupakis F, Adams RA, Seymour MT, Heinemann V, Schmoll H‐J, et al. Body Mass Index Is Prognostic in Metastatic Colorectal Cancer: Pooled Analysis of Patients From First‐Line Clinical Trials in the ARCAD Database. J Clin Oncol 2016;34:144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Simkens LHJ, Koopman M, Mol L, Veldhuis GJ, Ten Bokkel HD, Muller EW, et al. Influence of body mass index on outcome in advanced colorectal cancer patients receiving chemotherapy with or without targeted therapy. Eur J Cancer 2011;47:2560–2567. [DOI] [PubMed] [Google Scholar]
- 25. Bhaskaran K, Douglas I, Forbes H, dos‐Santos‐Silva I, Leon DA, Smeeth L. Body‐mass index and risk of 22 specific cancers: a population‐based cohort study of 5·24 million UK adults. Lancet 2014;384:755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Caan BJ, Meyerhardt JA, Kroenke CH, Alexeeff S, Xiao J, Weltzien E, et al. Explaining the Obesity Paradox: The Association between Body Composition and Colorectal Cancer Survival (C‐SCANS Study). Cancer Epidemiol Biomarkers Prev 2017;26:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Best TD, Roeland EJ, Horick NK, Van Seventer EE, El‐Jawahri A, Troschel AS, et al. Muscle Loss Is Associated with Overall Survival in Patients with Metastatic Colorectal Cancer Independent of Tumor Mutational Status and Weight Loss. Oncologist 2021;26:e963–e970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dohan A, Gallix B, Guiu B, Malicot KL, Reinhold C, Soyer P, et al. Early evaluation using a radiomic signature of unresectable hepatic metastases to predict outcome in patients with colorectal cancer treated with FOLFIRI and bevacizumab. Gut 2020;69:531–539. [DOI] [PubMed] [Google Scholar]
- 29. Beckers RCJ, Trebeschi S, Maas M, Schnerr RS, Sijmons JML, Beets GL, et al. CT texture analysis in colorectal liver metastases and the surrounding liver parenchyma and its potential as an imaging biomarker of disease aggressiveness, response and survival. Eur J Radiol 2018;102:15–21. [DOI] [PubMed] [Google Scholar]
- 30. Welch ML, McIntosh C, Haibe‐Kains B, Milosevic MF, Wee L, Dekker A, et al. Vulnerabilities of radiomic signature development: The need for safeguards. Radiother Oncol 2019;130:2–9. [DOI] [PubMed] [Google Scholar]
- 31. van Timmeren JE, Leijenaar RTH, van Elmpt W, Wang J, Zhang Z, Dekker A, et al. Test‐Retest Data for Radiomics Feature Stability Analysis: Generalizable or Study‐Specific? Tomography 2016;2:361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sasaki K, Margonis GA, Andreatos N, Zhang X‐F, Buettner S, Wang J, et al. The prognostic utility of the “Tumor Burden Score” based on preoperative radiographic features of colorectal liver metastases. J Surg Oncol 2017;116:515–523. [DOI] [PubMed] [Google Scholar]
- 33. Modest DP, Schulz C, von Weikersthal LF, Quietzsch D, von Einem JC, Schalhorn A, et al. Outcome of patients with metastatic colorectal cancer depends on the primary tumor site (midgut vs. hindgut): analysis of the FIRE1‐trial (FuFIRI or mIROX as first‐line treatment). Anticancer Drugs 2014;25:212–218. [DOI] [PubMed] [Google Scholar]
- 34. Ulanja MB, Rishi M, Beutler BD, Sharma M, Patterson DR, Gullapalli N, et al. Colon Cancer Sidedness, Presentation, and Survival at Different Stages. J Oncol 2019;2019:4315032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nawa T, Kato J, Kawamoto H, Okada H, Yamamoto H, Kohno H, et al. Differences between right‐ and left‐sided colon cancer in patient characteristics, cancer morphology and histology. J Gastroenterol Hepatol 2008;23:418–423. [DOI] [PubMed] [Google Scholar]
- 36. Sjoquist KM, Renfro LA, Simes RJ, Tebbutt NC, Clarke S, Seymour MT, et al. Personalizing Survival Predictions in Advanced Colorectal Cancer: The ARCAD Nomogram Project. JNCI: Journal of the National Cancer Institute 2018;110:638–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shibutani M, Maeda K, Nagahara H, Ohtani H, Sakurai K, Yamazoe A, et al. Significance of Markers of Systemic Inflammation for Predicting Survival and Chemotherapeutic Outcomes and Monitoring Tumor Progression in Patients with Unresectable Metastatic Colorectal Cancer. Anticancer Res 2015;35:5037–5046. [PubMed] [Google Scholar]
- 38. von Haehling S, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Kaplan–Meier survival curve depicting the survival of the overall cohort.
Figure S2: Illustration of the acquisition of body composition markers. The abdominal cavity is automatically detected from CT images and body composition markers are extracted. These markers are used together with body size to create combined features.
Table S3: Results of a univariate Cox Proportional Hazards model adjusted for age and gender.
Figure S4: Scatter plot showing the relationship between MBR and BMI.
Table S5: Multivariate analysis of the parameters included in the prediction model.
Figure S6: Kaplan–Meier survival curves showing high‐ and low‐risk subgroups identified by our prediction model. High and low risk groups were defined by the highest and lowest 20% risk score predicted by our model. The figure shows a representative fold after performing a 2‐fold cross validation.


