Abstract
Background
It remains unknown why adiponectin levels are associated with poor physical functioning, skeletal muscle mass and increased mortality in older populations.
Methods
In 190 healthy adults (59–86 years, BMI 17–37 kg/m2, 56.8% female), whole body skeletal muscle mass (normalized by height, SMI, kg/m2), muscle and liver fat were determined by magnetic resonance imaging. Bone mineral content (BMC) and density (BMD) were assessed by dual X‐ray absorptiometry (n = 135). Levels of insulin‐like growth factor 1 (IGF‐1), insulin, inflammation markers, leptin and fibroblast growth factor 21 were measured as potential determinants of the relationship between adiponectin and body composition.
Results
Higher adiponectin levels were associated with a lower SMI (r = −0.23, P < 0.01), BMC (r = −0.17, P < 0.05) and liver fat (r = −0.20, P < 0.05) in the total population and with higher muscle fat in women (r = 0.27, P < 0.01). By contrast, IGF‐1 showed positive correlations with SMI (r = 0.33), BMD (r = 0.37) and BMC (r = 0.33) (all P < 0.01) and a negative correlation with muscle fat (r = −0.17, P < 0.05). IGF‐1 was negatively associated with age (r = −0.21, P < 0.01) and with adiponectin (r = −0.15, P < 0.05). Stepwise regression analyses revealed that IGF‐1, insulin and leptin explained 18% of the variance in SMI, and IGF‐1, leptin and age explained 16% of the variance in BMC, whereas adiponectin did not contribute to these models.
Conclusions
Associations between higher adiponectin levels and lower muscle or bone mass in healthy older adults may be explained by a decrease in IGF‐1 with increasing adiponectin levels.
Keywords: Adiponectin paradox, Older adults, Skeletal muscle mass, Muscle quality, Bone, Liver fat
Introduction
Adiponectin is an abundant peptide hormone primarily secreted by adipose tissue (AT), and to a lesser extent by skeletal muscle (SM) and bone marrow adipocytes (for a review, see Fazeli et al. 1 ). It is well‐known for improving insulin sensitivity as well as for anti‐inflammatory and anti‐atherogenic properties (for a review, see Robinson et al. 2 ). Several metabolic and cardiovascular disorders including type 2 diabetes, metabolic syndrome (for a review, see Di Chiara et al. 3 ), atherosclerosis 4 and non‐alcoholic fatty liver disease 5 are thus accompanied by hypoadiponectinaemia. As a myokine, adiponectin acts as a myogenic factor through the participation in muscle differentiation and tissue regeneration, and influencing the behaviour of muscle cells (for a review, see Gamberi et al. 6 ). Accumulating evidence also suggests that adiponectin promotes osteoblastogenesis, while simultaneously inhibiting osteoclastogenesis (for a review, see Lewis et al. 7 ). It is therefore causally enigmatic why adiponectin levels are negatively associated with SM, muscle density, physical functioning 8 or bone mineral density (BMD) 9 among older adults. Adiponectin levels were shown to increase with age 10 and are even associated with higher rates of cardiovascular and all‐cause mortality in older populations. 11 The so‐called adiponectin paradox therefore suggests that adiponectin may not exert beneficial effects in older adults.
Different explanations for the adiponectin paradox in older people have been discussed including adiponectin resistance, 12 compensatory effects of adiponectin to subclinical pathologies, impaired renal function and decreased hepatic clearance of adiponectin (for a review, see Kalkman 13 ). In addition, adiponectin was proposed to be a biomarker of adverse catabolic processes (i.e., a low SM due to sarcopenia related to aging, 8 , 14 or cachexia associated with chronic inflammation and pre‐existing illness 15 , 16 ). It remains unknown if higher adiponectin levels in older adults are a cause of a low muscle mass by mediating catabolic processes or rather a consequence of catabolic processes that lead to a lower SM. The relationship between adiponectin and detailed body composition analysis therefore needs to be investigated in healthy older adults without catabolic disease or impaired renal function.
The aim of the present study was therefore (i) to investigate the association between adiponectin levels and SM (by whole body magnetic resonance imaging, (MRI)), muscle fat and strength or bone mass and bone density in healthy community‐dwelling older adults and (ii) to identify potential endocrine determinants of adiponectin levels.
Methods
Study population
The present study was conducted at the ‘German Reference Center for Body Composition’ (Institute of Human Nutrition and Food Science at the University of Kiel, Germany) between 2019 and 2020. Exclusion criteria were oedema, acute diseases, heart failure, renal failure, intake of diuretics, paralysis (e.g., after a stroke), neurodegenerative diseases, tumours in treatment, amputation of limbs, electrical and metallic implants, current alcohol abuse, not removable piercings and large tattoos on the arms or legs (because of possible interference with MRI examinations) as well as medication, which could influence body composition. Subjects were recruited using notice board postings and local advertisements. Written informed consent was obtained from each participant. The study protocol was approved by the medical ethics committee of the Christian‐Albrechts‐University of Kiel, Germany, and followed the guidelines based on the ‘Declaration of Helsinki’. The primary aim of the study was to validate measures of bioelectrical impedance analysis vs. reference methods in older adults. The trial was registered at ClinicalTrials.gov as NCT04028648. The study population was expanded by a subgroup of 40 healthy Caucasian older participants as described in detail elsewhere. 17 From the included 190 participants, data of 173 adults were analysed (data from 15 participants were excluded because of motion artefacts or incorrect patient positioning in MRI. Further data from two subjects were excluded due to missing endocrine parameters).
Body composition analysis
Body weight was measured to the nearest 0.01 kg by an electronic Tanita scale (Tanita, Tokyo, Japan) coupled to the BOD POD® Body Composition System (Cosmed srl, Rome, Italy) with subjects in underwear. Height was determined without shoes using a stadiometer (SECA, Modell 285, Hamburg, Germany). Fat mass (FM) and fat‐free mass (FFM) were determined via air‐displacement plethysmography (BOD POD® Cosmed srl, Rome, Italy) as previously described. 18 FM was calculated using the equation by Siri et al. 19 FFM was calculated from the difference between body weight and FM. On the basis of FM and FFM, FM‐Index (FMI) and FFM‐Index (FFMI) were calculated as FM (kg)/height (m2) and FFM (kg)/height (m2).
SM and AT were measured using whole body MRI as described in detail elsewhere. 20 Briefly, subjects were examined in a supine position with arms extended above their heads. For scans in abdominal and thoracic regions participants were required to hold their breath. Images were obtained using a 1.5 T scanner (Magnetom Avanto, Siemens Medical Systems, Erlangen, Germany) with a T1‐weighted‐gradient echo sequence (repetition time (TR): 157 ms; echo time (TE): 4 ms for scans of arms, legs and abdominal region). The whole body was scanned from wrist to ankle using continuous axial images with a slice thickness of 8 mm and 2 mm interslice gaps for arms, legs and trunk. Volumes of SM, subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) were manually segmented using SliceOmatic 4.3 software (Tomovision, Montreal, Canada). VAT was evaluated from the top of the liver to femoral heads. Volumes of total SM (excluding head and neck muscles, hands and feet), SAT and VAT were determined from the sum of tissue areas (cm2) multiplied by the slice thickness. Tissue volumes were then converted into masses using the assumed densities of 1.04 g cm−3 for SM and 0.92 g cm−3 for SAT and VAT. 21 SM was normalized to height squared to calculate skeletal muscle mass index (SMI, (kg)/height (m2)).
In a subgroup of 135 subjects, whole body bone mineral content (BMC), BMD and T‐Score were quantified using dual energy X‐ray absorptiometry (DXA) (HOLOGIC Discovery A (S/N 82686), Inc., Bedford, MA, USA). Before daily measurements, a spine phantom calibration was performed. Scans were analysed using manufacturer's software (version 12.6.1:3, Hologic, Inc.). Results were summed up for both arms and legs as well as the head.
Liver fat was determined by MRI (Magnetom Avanto, Siemens Medical Systems, Erlangen, Germany) based on the two‐point Dixon method with a volumetric interpolated breath‐hold examination sequence as described in detail elsewhere. 22 Briefly, a T1‐weighted gradient‐echo sequence with in‐phase and out‐of‐phase imaging was conducted (TR: 10.4 ms; TE: 4.76 (in‐phase) and 7.14 (opposed‐phase) ms; flip angle, 10° matrix, 80 × 128; and field of view, 440 mm; slice thickness/interslice gap: 5/1 mm; total scan time: 19 s). From in‐phase and opposed‐phase images, the water‐only (WO) and fat‐only (FO)‐images were calculated:
| (1) |
| (2) |
WO and FO‐images were than analysed using ImageJ software (US NIH, Bethesda, MD, USA 22 ) to determine hepatic fat fraction (HFF). In each of five adjacent HFF images, a single continuous region of interest (ROI) was defined (20.62 × 20.62) and was placed in the liver parenchyma, avoiding vascular structures. The quantity of liver fat was averaged for the five HFF images and was determined as percentage of the total liver core.
Intermuscular adipose tissue (IMAT) in a single mid‐thigh MRI slice and muscle fat by the two‐point Dixon method were used to assess muscle quality in 173 and 172 subjects, respectively (Figures 1 and 2).
Figure 1.
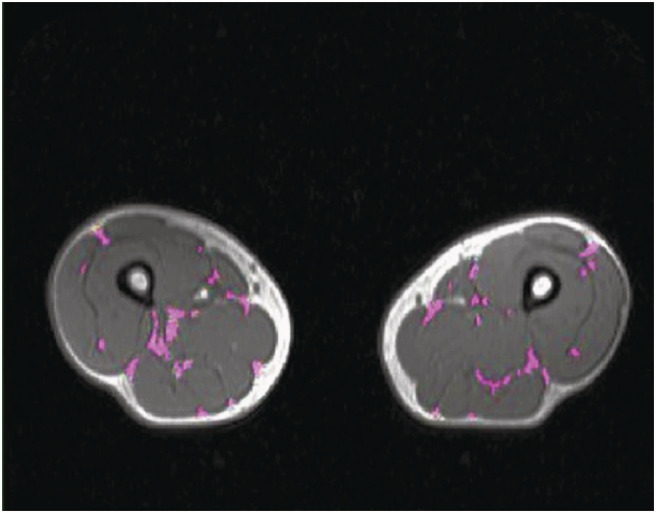
Intermuscular adipose tissue (IMAT) in a single mid‐thigh magnetic resonance imaging (MRI) slice segmented in purple using SliceOmatic software, acquired as axial T1‐weighted gradient‐echo sequence. Result for total IMAT was 9.30 g.
Figure 2.
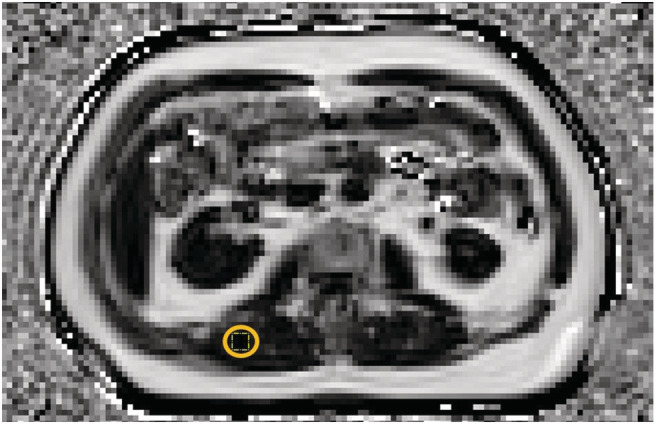
Lumbar muscle fat in the region of multifidus and erector spinae muscles with a yellow 10.75 × 10.75 region of interest (ROI) determined using ImageJ software, acquired as axial T1‐weighted gradient‐echo Dixon sequence. Result for the percentage of muscle fat was 9.54%.
IMAT was defined as visible AT between muscle groups that is located within a muscle and beneath the muscle fascia. It was assessed in single cross‐sectional MRI images at the level of the mid‐thigh. The midpoint of the thigh was defined as half way between the femoral head and the tibial plateau. Masses of IMAT were manually determined by using a semi‐automatic segmentation software (SliceOmatic 4.3, Tomovision, Montreal, Canada).
Muscle fat was determined based on the Dixon method using ImageJ software (US NIH, Bethesda, MD, USA 22 ). In lumbar region of multifidus and erector spinae muscles, a single continuous ROI was defined (10.75 × 10.75) in each of five adjacent muscle fat fraction images and was placed in the same area for all repeated measurements. Muscle fat was quantified as percentage of the total muscle area and was averaged for the five muscle fat fraction images. All procedures were conducted by the same observer.
In 173 subjects, hand grip strength (HGS) was measured using a hydraulic SAEHAN® handgrip dynamometer (SH5001, Masan, South Korea). 135 subjects conducted the test in a standing position and 38 in a sitting position and the elbow was flexed at 90 degrees with the shoulder attached to the torso. In 135 subjects, HGS of the left and right hand was determined three times and the greatest value of the dominant hand was included in the analysis, whereas in 38 subjects, HGS was determined only twice.
Endocrine parameters
Serum and plasma blood samples were taken from an antecubital vein after >10 h overnight fast. The subjects were instructed to refrain from vigorous exercise and alcohol intake for 24 h prior to blood sampling. After collection, serum samples were stored at room temperature in an upright position for 30 min for complete clot formation. Plasma and serum were obtained by centrifugation at 2000 g for 10 min at 20°C and stored at −40°C. Sample analyses were performed at the ‘German Institute of Human Nutrition’, Potsdam‐Rehbrücke, Department of Nutrition and Gerontology, Nuthetal, Germany and a laboratory in Kiel, Germany.
In 172 subjects, leptin (intra‐assay CV: 4.2–7.6%, inter‐assay CV: 4.4–6.7%; BioVendor, Brno, Czech Republic), adiponectin (intra‐assay CV: 2.8–3.9%, inter‐assay CV: 5.9–6.4%; Immundiagnostik AG, Bensheim, Germany) as well as insulin‐like growth factor 1 (IGF‐1) (intra‐assay CV: 5.1–6.7%, inter‐assay CV: 5.5–6.6%; BioVendor, Brno, Czech Republic) were measured by commercial ELISA kits. As adiponectin expression is regulated by the hepatic hormone fibroblast growth factor 21 (FGF‐21), also FGF‐21 concentrations were quantified by ELISA (intra‐assay CV: 1.6–2.4%, inter‐assay CV: 3.1–3.5%; BioVendor, Brno, Czech Republic) in a subgroup of 135 subjects. As inflammatory parameters, interleukin 6 (IL‐6) (intra‐assay CV: 4.2–5.1%, inter‐assay CV: 4.7–5.0%; BioVendor, Brno, Czech Republic) was determined in 135 participants using commercial ELISA kit, and high‐sensitivity C‐reactive protein (hsCRP) (intra‐assay CV: 0.73–5.73%, inter‐assay CV: 1.50–5.76%; BECKMAN COULTER, Brea, CA, USA) was measured using an immuno‐turbidimetric test. Insulin was determined by chemiluminescent microparticle immunoassay (intra‐assay CV: 1.4–2.1%, inter‐assay CV: 1.5–2.2%; Abbott, Wiesbaden, Germany). Inflammation was based on hsCRP >3 mg/L and elevated insulin levels were set at >25.0 mU/L.
Statistical analysis
Statistical analyses were performed using SPSS statistical software (SPSS 28.0, Inc., Chicago, IL, USA). All data are given as means ±SD. Differences between independent samples were analysed using unpaired t‐test. Evaluation of normality was performed using the Shapiro–Wilk test and residual analysis. Pearson's and Spearman's correlation coefficients were calculated to identify bivariate associations between and within body composition, endocrine and functional parameters. Partial correlations were used to adjust for various confounders. Stepwise multiple regression analyses were performed to assess factors independently associated with SMI, BMC and lumbar muscle fat. All tests were two‐sided and level of significance was set at P < 0.05.
Results
In total, 173 older adults (101 women and 72 men) aged 59–86 years with a BMI between 18 and 37 kg/m2 were included in the study. Descriptive characteristics are summarized in Table 1. Men were significantly older and had a higher BMI, FFMI, SM, SMI, IMAT, VAT, HGS as well as BMC, BMD and T‐Score compared with women. According to WHO criteria, 26.6% of women and 30.6% of men were overweight or obese. In a subpopulation of 135 subjects with DXA results, the prevalence for a reduced muscle mass was 7.40% according to the recommended FFMI thresholds of the ‘Global Leadership Initiative on Malnutrition’ (FFMI cut‐offs: <15 and <17 kg/m2 in women and men, respectively 23 ).
Table 1.
Characteristics of the study population
| All subjects | Women | Men | |
|---|---|---|---|
| n | 173 | 101 | 72 |
| Age (years) | 70.7 ± 5.3 | 70.0 ± 4.9* | 71.7 ± 5.7 |
| Height (m) | 1.68 ± 0.10 | 1.62 ± 0.06*** | 1.77 ± 0.1 |
| Weight (kg) | 73.3 ± 15.2 | 65.1 ± 11.0*** | 84.7 ± 12.7 |
| BMI (kg/m2) | 25.7 ± 3.8 | 24.9 ± 4.0*** | 27.0 ± 3.2 |
| FMI (kg/m2) | 9.2 ± 3.4 | 9.9 ± 3.6*** | 8.1 ± 2.7 |
| SAT (kg) | 17.4 ± 6.5 | 18.8 ± 6.8*** | 15.5 ± 5.5 |
| VAT (kg) | 2.2 ± 1.6 | 1.4 ± 0.9*** | 3.3 ± 1.7 |
| FFMI (kg/m2) | 16.6 ± 2.3 | 15.0 ± 1.2*** | 18.8 ± 1.2 |
| SM (kg) | 22.2 ± 5.9 | 18.0 ± 2.5*** | 28.0 ± 4.1 |
| SMI (kg/m2) | 7.7 ± 1.4 | 6.9 ± 0.8*** | 8.9 ± 1.1 |
| BMC (kg) | 2.07 ± 0.54 | 1.71 ± 0.27*** | 2.61 ± 0.35 |
| BMD (g/cm2) | 1.00 ± 0.14 | 0.92 ± 0.10*** | 1.12 ± 0.10 |
| T‐Score | −1.5 ± 1.2 | −2.1 ± 1.1*** | −0.7 ± 1.0 |
| Liver fat (%) | 8.4 ± 4.2 | 8.1 ± 4.5 | 8.8 ± 3.8 |
| Lumbar muscle fat (%) | 9.5 ± 3.7 | 9.3 ± 3.8 | 9.6 ± 3.7 |
| IMAT single mid‐thigh (g) | 4.5 ± 2.3 | 3.4 ± 2.1*** | 5.4 ± 2.3 |
| HGS (kg) | 31.9 ± 10.3 | 25.0 ± 5.0*** | 41.4 ± 7.9 |
Abbreviations: BMC, bone mineral content; BMD, bone mineral density; BMI, body mass index; FFMI, fat‐free mass index; FMI, fat mass index; HGS, hand grip strength; IMAT, intermuscular adipose tissue; SAT, subcutaneous adipose tissue; SM, skeletal muscle; SMI, skeletal muscle mass index; VAT, visceral adipose tissue.
Note: Values are means ± SD; n, no. of subjects.
P < 0.05.
P < 0.001 sex differences by t‐test, two‐sided.
Potential endocrine determinants of adiponectin levels are summarized in Table 2. Adiponectin levels were lower in men compared with women. Insulin and IGF‐1 levels were higher and leptin levels were lower in men compared with women. Prevalence for elevated hsCRP and insulin levels were 20.2% and 2.3%, respectively.
Table 2.
Adiponectin levels and its potential endocrine determinants
| All subjects | Women | Men | |
|---|---|---|---|
| n | 173 | 101 | 72 |
| Adiponectin (mg/L) | 19.8 ± 16.4 | 22.8 ± 19.3** | 15.5 ± 9.8 |
| IGF‐1 (μg/L) | 151.3 ± 56.8 | 140.3 ± 52.5** | 166.7 ± 59.4 |
| FGF‐21 (pg/mL) | 212.2 ± 212.2 | 233.5 ± 255.3 | 180.3 ± 117.3 |
| Leptin (ng/mL) | 11.2 ± 11.0 | 14.2 ± 12.7*** | 6.6 ± 5.5 |
| IL‐6 (pg/mL) | 10.11 ± 24.52 | 6.72 ± 6.47 | 15.20 ± 37.58 |
| hsCRP (mg/L) | 2.31 ± 3.07 | 2.12 ± 2.60 | 2.58 ± 3.63 |
| Insulin (μU/L) | 9.8 ± 6.1 | 8.9 ± 4.3* | 10.9 ± 7.8 |
Abbreviations: FGF‐21, fibroblast growth factor 21; hsCRP, high‐sensitivity C‐reactive protein; IGF‐1, insulin‐like growth factor 1; IL‐6, interleukin 6.
Note: Values are means ± SD; n, no. of subjects.
P < 0.05.
P < 0.01.
P < 0.001 sex differences by t‐test, two‐sided.
Correlations between adiponectin or IGF‐1 levels and body composition parameters are presented in Table 3. In accordance with the adiponectin paradox, higher adiponectin levels were associated with a lower SMI and BMC in the total study population. No correlation was observed between adiponectin and HGS. Lower adiponectin levels were associated with a higher BMI (r = −0.34, P < 0.01) and FMI in men and with greater VAT in the total population. The association between adiponectin and SMI remained significant after adjustment for VAT (r = −0.16, P < 0.04). No relationships between adiponectin and FGF‐21, leptin, IL‐6 or hsCRP and insulin were found.
Table 3.
Correlations between adiponectin or IGF‐1 and body composition parameters
| All subjects | Women | Men | ||||
|---|---|---|---|---|---|---|
| Adiponectin (mg/L) | IGF‐1 (μg/L) | Adiponectin (mg/L) | IGF‐1 (μg/L) | Adiponectin (mg/L) | IGF‐1 (μg/L) | |
| SMI (kg/m2) | −0.23** | 0.33** | ‐ | 0.22* | ‐ | 0.39** |
| BMC (kg) | −0.17* | 0.33** | ‐ | ‐ | ‐ | ‐ |
| BMD (g/cm2) | ‐ | 0.37** | ‐ | 0.22* | ‐ | ‐ |
| T‐Score | ‐ | 0.34** | ‐ | 0.22* | ‐ | ‐ |
| FMI (kg/m2) | ‐ | ‐ | ‐ | ‐ | −0.32** | ‐ |
| VAT (kg) | −0.21** | ‐ | ‐ | ‐ | ‐ | ‐ |
| Liver fat (%) | −0.20* | ‐ | ‐ | ‐ | −0.26* | ‐ |
| Lumbar muscle fat (%) | ‐ | −0.17* | 0.27** | −0.30** | ‐ | ‐ |
| IMAT single mid‐thigh (g) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
Abbreviations: BMC, bone mineral content; BMD, bone mineral density; FMI, fat mass index; IGF‐1, insulin‐like growth factor 1; IMAT, intermuscular adipose tissue; SMI, skeletal muscle mass index; VAT, visceral adipose tissue.
P < 0.05.
P < 0.01.
In contrast to adiponectin, IGF‐1 levels were positively associated with SMI in the total population and in the subgroups of men and women. Furthermore, IGF‐1 concentrations were correlated with BMC, BMD and T‐Score in the total population and BMD and T‐Score in women. IGF‐1 levels were also positively associated with HGS (r = 0.31, P < 0.05, all; r = 0.35, P < 0.01, men) and negatively with age (r = −0.21, P < 0.01, all; r = −0.20, P < 0.05, women; r = −0.33, P < 0.01, men) and adiponectin levels (r = −0.15, P < 0.05). To test, if the paradoxical association between higher adiponectin and lower SMI could be explained by lower IGF‐1 levels with higher age, partial correlation analysis between adiponectin and SMI adjusted for IGF‐1 was performed. The negative correlation between SMI and adiponectin in the total study population persisted, but was slightly weakened (r = −0.20, P = 0.01).
Stepwise regression analyses with SMI or BMC as dependent variables and adiponectin, IGF‐1, insulin, IL‐6, hsCRP, leptin and age (for SMI) and adiponectin, IGF‐1, IL‐6, leptin and age (for BMC), respectively as independent variables were performed (Table 4). Insulin, IGF‐1 and leptin explained 18.4% of the variance in SMI. After considering sex as further covariate, sex and insulin together explained 65.1% of the variance in SMI. IGF‐1, leptin and age explained 16.4% of the variance in BMC. After additional adjustment for sex, only sex was entered in the equation and explained 68.5% of the variance in BMC.
Table 4.
Stepwise multiple regression analyses with SMI, BMC and lumbar muscle fat as dependent variables
| Dependent variables and predictors | β coefficient | R 2 | SEE | P‐value | VIF |
|---|---|---|---|---|---|
| SMI (kg/m 2 ) | |||||
| Model a | |||||
| Step 1: insulin (μU/L) | 0.073 | 0.101 | 0.018 | <0.001 | 1.135 |
| Step 2: IGF‐1 (μg/L) | 0.005 | 0.150 | 0.002 | 0.015 | 1.053 |
| Step 3: leptin (ng/mL) | −0.023 | 0.184 | 0.010 | 0.021 | 1.104 |
| BMC (kg) | |||||
| Model b | |||||
| Step 1: IGF‐1 (μg/L) | 3.232 | 0.098 | 0.794 | <0.001 | 1.044 |
| Step 2: leptin (ng/mL) | −8.500 | 0.135 | 3.715 | 0.024 | 1.009 |
| Step 3: age (y) | 16.918 | 0.164 | 7.949 | 0.035 | 1.040 |
| Lumbar muscle fat (%) | |||||
| Model c | |||||
| Step 1: FMI (kg/m2) | 0.56 | 0.296 | 0.100 | 0.100 | 1.020 |
| Step 2: IGF‐1 (μg/L) | −0.02 | 0.343 | 0.007 | 0.007 | 1.020 |
Abbreviations: BMC, bone mineral content; FMI, fat mass index; IGF‐1, insulin‐like growth factor 1; SEE, standard error of estimation; SMI, skeletal muscle mass index; VIF, variance inflation factor.
Model: independent variables: adiponectin, IGF‐1, insulin, IL‐6, hsCRP, leptin and age.
Model: independent variables: adiponectin, IGF‐1, leptin, IL‐6 and age.
Model: independent variables: adiponectin, IGF‐1, leptin, insulin, IL‐6, hsCRP, FMI and age.
After excluding subjects with elevated hsCRP levels, the negative association between adiponectin concentrations and BMC in the total population was no longer significant (P = 0.08), whereas negative correlations between adiponectin levels and insulin (r = −0.18, P < 0.05), BMI (r = −0.18, P < 0.05) and HGS (r = −0.18, P < 0.05) could be observed. The negative association between IGF‐1 and adiponectin levels in the total population persisted (r = −0.18, P < 0.05).
Concerning ectopic fat, higher adiponectin levels were associated with lower liver fat in the total study population and in the subgroup of men, whereas adiponectin levels were paradoxically positively correlated with lumbar muscle fat in women (Table 3). By contrast, IGF‐1 showed negative correlations with lumbar muscle fat in women and in the total study population. The negative association between adiponectin and lumbar muscle fat in women weakened after adjustment for IGF‐1 (r = 0.19, P = 0.05). Stepwise regression analysis with lumbar muscle fat as the dependent variable and adiponectin, IGF‐1, leptin, insulin, IL‐6, hsCRP, FMI and age as independent variables, revealed that only FMI and IGF‐1 independently explained 34.3% of the variance (Table 4).
Insulin, leptin, IL‐6 and hsCRP were positively correlated with ectopic fat deposition in liver and muscle, whereas higher FGF‐21 levels were positively correlated with liver fat content. Furthermore, ectopic liver and muscle fat showed consistent positive associations with FMI and VAT ranging between r = 0.31 and r = 0.61 (all P < 0.05).
Discussion
Our data confirm the adiponectin paradox in older adults without inflammatory diseases. Higher adiponectin levels were associated with lower SMI and BMC in the total study population and higher lumbar muscle fat in women. These observations may explain the positive association between adiponectin and mortality that was found in previous studies even independent of comorbid conditions like history of cancer, 8 hypertension, diabetes, cardiovascular disease, congestive heart failure 8 , 24 and chronic kidney disease. 24
However, adiponectin may not be causally related to an unhealthy body composition because of the negative association between adiponectin and IGF‐1. IGF‐1 has well‐known anabolic effects on muscle and bone (for a review, see Gomarasca et al. 25 ). In line with our hypothesis, stepwise multiple regression analyses revealed that adiponectin was no significant predictor of SMI, BMC and lumbar muscle fat when considering IGF‐1 as a dependent variable (Table 4). Similar to our results in healthy people, adiponectin levels were negatively related to serum IGF‐1 in acromegaly 26 as well as in men with type 2 diabetes. 27 This correlation was independent of BMI, 26 , 27 renal function and age, 27 suggesting that IGF‐1 might inhibit the expression of adiponectin. In vitro experiments in cultured 3T3‐L1 adipocytes have indeed shown decreased adiponectin mRNA levels by IGF‐1 or insulin 28 and a study in rats demonstrated that infusion of recombinant human IGF‐1 decreased plasma levels of adiponectin. 29 By contrast, IGF‐1 supplementation in patients with growth hormone deficiency did not affect serum adiponectin levels. 30
In our study, IGF‐1 levels were negatively correlated with age and may therefore explain higher adiponectin levels in an older population. The negative association between adiponectin and IGF‐1 in patients with type 2 diabetes 27 may be due to impaired insulin secretion and thus lower IGF‐1 levels in these patients, 31 whereas in patients with acromegaly, overproduction of IGF‐1 may contribute to lower adiponectin concentrations. There was a lack of association between adiponectin and IGF‐1 levels in patients with morbid obesity after weight loss induced by bariatric surgery 32 whereas in young women with non‐diabetic obesity a positive association between IGF‐1 and adiponectin was found. 33 In the latter study, a significant negative correlation between IGF‐1 and CRP was observed indicating that inflammation may be causal for the positive association between IGF‐1 and adiponectin because inflammation may impair the expression of both hormones 33 , 34 , 35 (for a review, see Kirk et al. 36 ).
In contrast to the association between IGF‐1 and muscle mass, muscle fat or bone mass, to the best of our knowledge there is no direct causal effect of IGF‐1 on VAT or liver fat. Therefore, the plausible negative correlations between adiponectin and VAT (total population), FMI and liver fat (especially in men) were evident in our heathy older population (Table 3).
An alternative explanation of the adiponectin paradox is that higher adiponectin levels in people with a lower SM may be interpreted as a starvation signal (i.e. as a consequence of poor nutritional status 37 or history of weight loss among older people 8 ). In line with this argument, weight loss leads to an increase in adiponectin levels not only in people with obesity 32 but also in healthy lean subjects. 38 In the present study, partial correlation between adiponectin and SMI adjusted for IGF‐1 revealed, that the negative correlation between SMI and adiponectin was only slightly weakened. Because our data are cross‐sectional, we cannot exclude the possibility that a low SMI is causal for higher adiponectin levels. Adiponectin is also secreted by myocytes, therefore the impact of energy availability on this effect needs to be investigated. The interpretation of higher adiponectin levels as a starvation signal is however unlikely in a healthy non‐malnourished study population of community‐dwelling older people.
The present study has several strengths. First, the sample of older community‐dwelling Caucasians was healthy, thus confounders caused by disease can be excluded. In addition, the population was well characterized using whole body MRI, which is considered as the gold standard method of assessment of SM and muscle fat. Body composition was complemented with HGS as a functional parameter. Nevertheless, our findings should be considered in the context of some limitations. First, adiponectin circulates in blood in multiple isoforms with different physiologic functions. 39 , 40 As only total adiponectin concentrations were measured, the effects of the different isoforms cannot be examined. Finally, our results need to be confirmed using a longitudinal study design. For example, nutrition interventions might affect adiponectin levels mediated by IGF‐1. It has been demonstrated that IGF‐1 levels decrease in response to fasting and short‐term caloric restriction or protein restriction 41 (for a review, see Thissen et al. 42 ). The effect of protein supplementation in healthy older people on adiponectin levels remains to be investigated.
In conclusion, our results suggest that adiponectin is not causally related to impaired mass and function of the musculoskeletal system in healthy older people but the associations are mediated by an age‐related decline in IGF‐1 levels that contribute to an increase in adiponectin. These results support the hypothesis of a functional interplay between IGF‐1 and adiponectin that had been proposed by Orrù et al. 43
Conflict of interest
Anja Bosy‐Westphal serves a consultant for seca gmbh & co. kg. The other authors declare no conflict of interest.
Acknowledgements
The study protocol was approved by the medical ethics committee of the Christian‐Albrechts‐University of Kiel, Germany, and followed the guidelines based on the ‘Declaration of Helsinki’. Written informed consent was obtained from each participant. The authors certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 44 This work was supported by a grant for seca gmbh & co.kg., Germany (2019/2020) and the Danone Institute‐Nutrition for Health, Germany (2013/14). We thank Britta Jux and the Clinic for Diagnostic Radiology, University medical Center Schleswig‐Holstein, Kiel (Germany) for the help with MRI scanning.
Open Access funding enabled and organized by Projekt DEAL.
Walowski C. O., Herpich C., Enderle J., Braun W., Both M., Hasler M., Müller M. J., Norman K., and Bosy‐Westphal A. (2023) Analysis of the adiponectin paradox in healthy older people, Journal of Cachexia, Sarcopenia and Muscle, 14, 270–278, 10.1002/jcsm.13127
References
- 1. Fazeli PK, Horowitz MC, MacDougald OA, Scheller EL, Rodeheffer MS, Rosen CJ, et al. Marrow fat and bone‐‐new perspectives. J Clin Endocrinol Metab 2013;98:935–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robinson K, Prins J, Venkatesh B. Clinical review: Adiponectin biology and its role in inflammation and critical illness. Crit Care 2011;15:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Di Chiara T, Argano C, Corrao S, Scaglione R, Licata G. Hypoadiponectinemia: A Link between Visceral Obesity and Metabolic Syndrome. J Nutr Metab 2012;2012:175245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hui E, Xu A, Chow W‐S, Lee PCH, Fong CHY, Cheung SCW, et al. Hypoadiponectinemia as an independent predictor for the progression of carotid atherosclerosis: A 5‐year prospective study. Metab Syndr Relat Disord 2014;12:517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Engl J, Sturm W, Sandhofer A, Kaser S, Tschoner A, Tatarczyk T, et al. Effect of pronounced weight loss on visceral fat, liver steatosis and adiponectin isoforms. Eur J Clin Invest 2008;38:238–244. [DOI] [PubMed] [Google Scholar]
- 6. Gamberi T, Magherini F, Fiaschi T. Adiponectin in Myopathies. Int J Mol Sci 2019;20:1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lewis JW, Edwards JR, Naylor AJ, McGettrick HM. Adiponectin signalling in bone homeostasis, with age and in disease. Bone Res 2021;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baker JF, Newman AB, Kanaya A, Leonard MB, Zemel B, Miljkovic I, et al. The Adiponectin Paradox in the Elderly: Associations With Body Composition, Physical Functioning, and Mortality. J Gerontol A Biol Sci Med Sci 2019;74:247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Michaëlsson K, Lind L, Frystyk J, Flyvbjerg A, Gedeborg R, Berne C, et al. Serum adiponectin in elderly men does not correlate with fracture risk. J Clin Endocrinol Metab 2008;93:4041–4047. [DOI] [PubMed] [Google Scholar]
- 10. Obata Y, Yamada Y, Takahi Y, Baden MY, Saisho K, Tamba S, et al. Relationship between serum adiponectin levels and age in healthy subjects and patients with type 2 diabetes. Clin Endocrinol (Oxf) 2013;79:204–210. [DOI] [PubMed] [Google Scholar]
- 11. Menzaghi C, Trischitta V. The Adiponectin Paradox for All‐Cause and Cardiovascular Mortality. Diabetes 2018;67:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Uetani E, Tabara Y, Kawamoto R, Onuma H, Kohara K, Osawa H, et al. CDH13 Genotype–Dependent Association of High–Molecular Weight Adiponectin With All‐Cause Mortality: The J‐SHIPP Study. Diabetes Care 2014;37:396–401. [DOI] [PubMed] [Google Scholar]
- 13. Kalkman HO. An Explanation for the Adiponectin Paradox. Pharmaceuticals (Basel) 2021;14:1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sattar N, Nelson SM. Adiponectin, Diabetes, and Coronary Heart Disease in Older Persons: Unraveling the Paradox. J Clin Endocrinol Metab 2008;93:3299–3301. [DOI] [PubMed] [Google Scholar]
- 15. Szabó T, Scherbakov N, Sandek A, Kung T, von Haehling S, Lainscak M, et al. Plasma adiponectin in heart failure with and without cachexia: Catabolic signal linking catabolism, symptomatic status, and prognosis. Nutr Metab Cardiovasc Dis 2014;24:50–56. [DOI] [PubMed] [Google Scholar]
- 16. Baker JF, von Feldt JM, Mostoufi‐Moab S, Kim W, Taratuta E, Leonard MB. Insulin‐like Growth Factor 1 and Adiponectin and Associations with Muscle Deficits, Disease Characteristics, and Treatments in Rheumatoid Arthritis. J Rheumatol 2015;42:2038–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schweitzer L, Geisler C, Johannsen M, Glüer C‐C, Müller MJ. Associations between body composition, physical capabilities and pulmonary function in healthy older adults. Eur J Clin Nutr 2017;71:389–394. [DOI] [PubMed] [Google Scholar]
- 18. Bosy‐Westphal A, Mast M, Eichhorn C, Becker C, Kutzner D, Heller M, et al. Validation of air‐displacement plethysmography for estimation of body fat mass in healthy elderly subjects. Eur J Nutr 2003;42:207–216. [DOI] [PubMed] [Google Scholar]
- 19. Siri WE. Body composition from fluid spaces and density: Analysis of methods 1961. Nutrition 1993;9:480–491. [PubMed] [Google Scholar]
- 20. Hübers M, Geisler C, Plachta‐Danielzik S, Müller MJ. Association between individual fat depots and cardio‐metabolic traits in normal‐ and overweight children, adolescents and adults. Nutr Diabetes 2017;7:e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Snyder WS, Cook MJ, Nasset ES, Karhausen LR, Howells GP, Tipton IH. Report of the Task Group on Reference Man. (ICRP 23). Oxford: Pergamon Press, 1975. Ann ICRP 1979;3:iii–iii. [DOI] [PubMed] [Google Scholar]
- 22. Ma J. Dixon techniques for water and fat imaging. J Magn Reson Imaging 2008;28:543–558. [DOI] [PubMed] [Google Scholar]
- 23. Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition – A consensus report from the global clinical nutrition community. Clin Nutr 2019;38:1–9. [DOI] [PubMed] [Google Scholar]
- 24. Poehls J, Wassel CL, Harris TB, Havel PJ, Swarbrick MM, Cummings SR, et al. Association of adiponectin with mortality in older adults: The Health, Aging, and Body Composition Study. Diabetologia 2009;52:591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gomarasca M, Banfi G, Lombardi G. Myokines: The endocrine coupling of skeletal muscle and bone. Adv Clin Chem 2020;94:155–218. [DOI] [PubMed] [Google Scholar]
- 26. Fukuda I, Hizuka N, Ishikawa Y, Itoh E, Yasumoto K, Murakami Y, et al. Serum adiponectin levels in adult growth hormone deficiency and acromegaly. Growth Horm IGF Res 2004;14:449–454. [DOI] [PubMed] [Google Scholar]
- 27. Kanazawa I, Yamaguchi T, Sugimoto T. Serum insulin‐like growth factor‐I is negatively associated with serum adiponectin in type 2 diabetes mellitus. Growth Horm IGF Res 2011;21:268–271. [DOI] [PubMed] [Google Scholar]
- 28. Lam KS‐L, Xu A, Tan KC‐B, Wong L‐C, Tiu S‐C, Tam S. Serum adiponectin is reduced in acromegaly and normalized after correction of growth hormone excess. J Clin Endocrinol Metab 2004;89:5448–5453. [DOI] [PubMed] [Google Scholar]
- 29. Yamaza H, Komatsu T, To K, Toyama H, Chiba T, Higami Y, et al. Involvement of Insulin‐Like Growth Factor‐1 in the Effect of Caloric Restriction: Regulation of Plasma Adiponectin and Leptin. J Gerontol A Biol Sci Med Sci 2007;62:27–33. [DOI] [PubMed] [Google Scholar]
- 30. Schmid C, Bianda T, Zwimpfer C, Zapf J, Wiesli P. Changes in insulin sensitivity induced by short‐term growth hormone (GH) and insulin‐like growth factor I (IGF‐I) treatment in GH‐deficient adults are not associated with changes in adiponectin levels. Growth Horm IGF Res 2005;15:300–303. [DOI] [PubMed] [Google Scholar]
- 31. Suda K, Matsumoto R, Fukuoka H, Iguchi G, Hirota Y, Nishizawa H, et al. The influence of type 2 diabetes on serum GH and IGF‐I levels in hospitalized Japanese patients. Growth Horm IGF Res 2016;29:4–10. [DOI] [PubMed] [Google Scholar]
- 32. Pardina E, Ferrer R, Baena‐Fustegueras JA, Lecube A, Fort JM, Vargas V, et al. The relationships between IGF‐1 and CRP, NO, leptin, and adiponectin during weight loss in the morbidly obese. Obes Surg 2010;20:623–632. [DOI] [PubMed] [Google Scholar]
- 33. Sirbu A, Gologan S, Arbanas T, Copaescu C, Martin S, Albu A, et al. Adiponectin, body mass index and hepatic steatosis are independently associated with IGF‐I status in obese non‐diabetic women. Growth Horm IGF Res 2013;23:2–7. [DOI] [PubMed] [Google Scholar]
- 34. Savastano S, Di Somma C, Pizza G, de Rosa A, Nedi V, Rossi A, et al. Liver‐spleen axis, insulin‐like growth factor‐(IGF)‐I axis and fat mass in overweight/obese females. J Transl Med 2011;9:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ouchi N, Kihara S, Funahashi T, Nakamura T, Nishida M, Kumada M, et al. Reciprocal association of C‐reactive protein with adiponectin in blood stream and adipose tissue. Circulation 2003;107:671–674. [DOI] [PubMed] [Google Scholar]
- 36. Kirk B, Feehan J, Lombardi G, Duque G. Muscle, Bone, and Fat Crosstalk: The Biological Role of Myokines, Osteokines, and Adipokines. Curr Osteoporos Rep 2020;18:388–400. [DOI] [PubMed] [Google Scholar]
- 37. Hyun YY, Lee K‐B, Oh K‐H, Ahn C, Park SK, Chae DW, et al. Serum adiponectin and protein‐energy wasting in predialysis chronic kidney disease. Nutrition 2017;33:254–260. [DOI] [PubMed] [Google Scholar]
- 38. Müller MJ, Enderle J, Pourhassan M, Braun W, Eggeling B, Lagerpusch M, et al. Metabolic adaptation to caloric restriction and subsequent refeeding: The Minnesota Starvation Experiment revisited. Am J Clin Nutr 2015;102:807–819. [DOI] [PubMed] [Google Scholar]
- 39. Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem 2003;278:40352–40363. [DOI] [PubMed] [Google Scholar]
- 40. Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A Novel Serum Protein Similar to C1q, Produced Exclusively in Adipocytes. J Biol Chem 1995;270:26746–26749. [DOI] [PubMed] [Google Scholar]
- 41. Smith WJ, Underwood LE, Clemmons DR. Effects of caloric or protein restriction on insulin‐like growth factor‐I (IGF‐I) and IGF‐binding proteins in children and adults. J Clin Endocrinol Metab 1995;80:443–449. [DOI] [PubMed] [Google Scholar]
- 42. Thissen JP, Ketelslegers JM, Underwood LE. Nutritional regulation of the insulin‐like growth factors. Endocr Rev 1994;15:80–101. [DOI] [PubMed] [Google Scholar]
- 43. Orrù S, Nigro E, Mandola A, Alfieri A, Buono P, Daniele A, et al. A Functional Interplay between IGF‐1 and Adiponectin. Int J Mol Sci 2017;18:2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. von Haehling S, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: Update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]


