Abstract
Background
There is lack of data on effect modification by age on the association between body mass index (BMI) or waist circumference (WC) and cardiovascular diseases (CVDs). We aimed to investigate the impact of BMI and WC on incident CVDs in individuals aged 40 and 66 years.
Methods
Overall, 2 430 510 participants who underwent a national health screening for transitional ages provided by the Korean National Health Insurance Service between 2009 and 2012 were included. The adjusted hazard ratios and 95% confidence intervals for myocardial infarction (MI), ischaemic stroke and CVDs as a composite outcome of MI and ischaemic stroke were calculated using multivariable Cox proportional hazard regression analysis.
Results
During a mean follow‐up of 7.7 years, 24 884 MI and 29 415 ischaemic stroke events occurred. Among participants aged 40 years, there was a J‐shaped association of BMI with incident CVDs, MI and ischaemic stroke with nadir at BMI 18.5–22.9 kg/m2 (P for trend < 0.001 for all). Among those aged 66 years, there were significant U‐shaped associations of BMI with CVDs and MI with nadir at a BMI of 23.0–24.9 kg/m2 (P for trend 0.013 and 0.017, respectively). WC was linearly associated with all study outcomes in both age groups (P for trend < 0.001). The impact of general and abdominal obesity on both study outcomes was more prominent in those aged 40 years than in those aged 66 years (P for interaction < 0.001).
Conclusions
To prevent cardiovascular risk, weight loss intervention should be cautiously implemented and individualized according to age. The maintenance of muscle mass may be essential in managing weight loss particularly in older population.
Keywords: body mass index, cardiovascular disease, ischaemic stroke, middle age, myocardial infarction, old age, waist circumference
Introduction
Cardiovascular diseases (CVDs), principally myocardial infarction (MI) and ischaemic stroke, are the leading cause of disease burden, accounting for approximately 32% of all death worldwide, and a major contributor to disability. 1 The prevalence of CVD nearly doubled from 1999 to 2019, and the number of CVD deaths also steadily increased. 2 Obesity is a well‐known risk factor for CVDs and mortality in the general population. 3 Obesity contributes directly to incident cardiovascular risk factors, including lipid profiles, blood pressure and glucose levels. 3 Low grade inflammation caused by obesity may play a significant role in atherosclerotic processes and cardiovascular burden, independently of other conventional cardiovascular risk factors. 3
Although obesity is associated with an increased risk of CVD in even healthy individuals, the risk levels may vary with age. It has been suggested that the effect of body mass index (BMI) on CVD is influenced by age. 4 , 5 A pooled analysis of prospective data showed that the strength of the association between BMI and death caused by coronary heart disease and ischaemic stroke was stronger in younger adults than in older adults. 6 In addition, CVD mortality was higher in younger men than in older men, even at the same BMI. 7 A high BMI was associated with an increased risk of death caused by CVD, but the risk associated with BMI was higher in younger people. 8 The mortality rate caused by CVDs according to BMI showed curvilinear pattern in younger adults: J‐shaped and U‐shaped associations with increasing age and near reverse J‐shaped association in older adults. 9 However, most previous studies mainly focused on the impact of age on the association between BMI and CVD mortality, but not CVD incidence. 5 , 6 , 7 , 8 They also did not compare middle and older age groups but simply dichotomized age, such as <60 and ≥60 years 7 , 8 and <53 and ≥53 years. 6 There is another limitation relative to the study population and cases, such as the largest pooled analysis with 9142 coronary heart diseases and 5771 ischaemic strokes among 1 124 897 Asians. 6
Meanwhile, as BMI is unable to distinguish between lean and fat mass and may not be equally valid across age groups, abdominal obesity measured by waist circumference (WC) might be better than BMI to identify individuals at increased risk of developing CVDs, even in the absence of other metabolic abnormalities. WC showed a better ability to predict CVDs including MI and ischaemic stroke than BMI. 10 However, there is lack of data on effect modification by age on the association between WC and CVDs and how different from the association between BMI and CVDs. Understanding the presence of such effect modification by age is important and prioritizing public health education.
The aim of this study was to evaluate the impact of BMI and WC on the risk of MI and ischaemic stroke incidence in individuals aged 40 and 66 years using the database of the Korean National Health Insurance Service (NHIS) life‐transition health screening program.
Methods
Data source and study setting
The NHIS in Republic of Korea, a single and universal insurance system provided by the South Korean government, covers approximately 97% of the population. The remaining 3% of the population in the lowest income bracket is covered by the government‐financed‐medical aid program, which is also administered by the NHIS. The NHIS recommends all insured individuals, such as all citizens aged 40 years and above and all employees regardless of age, to receive a general health examination at least every 2 years. This national health examination consists of a standard questionnaire (regarding medical history, current medications and lifestyle habits, such as drinking, smoking and exercise), anthropometric measurements (height, weight and WC) and laboratory tests. 11 The serial data of the individuals from health examinations are deposited to the NHIS database. In addition, they can be linked with the information on claimed health care utilization, which has been widely used for epidemiological studies. 12 , 13
Since 2007, the Korean government has launched the National Screening Program for Transitional Ages to promote the national screening program to the more advanced and improve the screening rate. 14 It targeted two age groups, people aged 40 and 66 years; these ages are believed to be important transition periods in terms of health in one's life cycle. Age 40 is regarded as the time of transition to middle age, and the incidence of many chronic diseases begins to increase during this transition, whereas age 66 is regarded as the time of transition to old age when a geriatric approach becomes necessary for comprehensive health promotion.
Study population
We initially identified 2 968 660 participants aged 40 and 66 years who underwent a national health screening for transitional ages provided by the NHIS in 2009–2012. We excluded individuals who had been diagnosed with MI or ischaemic stroke (n = 39 245) and those who had percutaneous coronary intervention or peripheral arterial disease (n = 432 892) before the health screening. To reduce the effect of reverse causality, we applied a 1‐year lag time by excluding participants who were diagnosed with MI or ischaemic stroke and who died within 1 year after the health screening (n = 7989). Those with missing information on the variables used in this study (n = 58 024) were also excluded. Finally, 2 430 510 eligible participants were included in the study.
This study adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of Korea University Anam Hospital (No. 2020AN0268). The requirement for written informed consent was waived because anonymous and de‐identified information was used for the analysis.
Exposure: Body mass index and waist circumference
Height, weight and WC were measured while participants wore lightweight clothing. BMI was calculated as weight (kg) divided by height in metres squared (m2). According to the World Health Organization recommendations for Asian populations, participants were categorized into five BMI groups: (1) <18.5 kg/m2 (underweight), (2) 18.5–22.9 kg/m2 (normal), (3) 23.0–24.9 kg/m2 (overweight), (4) 25.0–29.9 kg/m2 (class I obesity) and (5) ≥30.0 kg/m2 (class II and III obesity). 15 General obesity was defined as BMI ≥ 25 kg/m2. Participants were classified into six groups with 5‐cm intervals of WC as follows: for men, (1) <80.0 cm, (2) 80.0–84.9 cm, (3) 85.0–89.9 cm, (4) 90.0–94.9 cm, (5) 95.0–99.9 cm and (6) ≥100.0 cm; for women, (1) <75.0 cm, (2) 75.0–79.9 cm, (3) 80.0–84.9 cm, (4) 85.0–89.9 cm, (5) 90.0–94.9 cm and (6) ≥95.0 cm. Abdominal obesity was defined as WC ≥ 90.0 cm for men and ≥85.0 cm for women according to the obesity guidelines for the management of obesity in the Korean population. 16
Study outcomes: Myocardial infarction, ischaemic stroke, and cardiovascular diseases
The primary endpoints of this study were newly diagnosed MI and ischaemic stroke. Newly diagnosed MI and ischaemic stroke were identified on the basis of the International Classification of Diseases (ICD)‐10 codes. The ICD‐10 codes for MI were I21 or I22 during hospitalization. The ICD‐10 codes for ischaemic stroke were I63 or I64 during hospitalization, with claims for brain magnetic resonance imaging or brain computed tomography. 17 We also defined CVDs as a composite outcome of MI and ischaemic stroke. The cohort was followed from 1 year after the health screening date to the date of any incident MI, ischaemic stroke, death or the end of the study period (31 December 2019), whichever came first.
Covariates
Household income was categorized into quartiles based on insurance premium levels in which in Korea, insurance premiums are determined by income level, with those covered by medical aid (3% of the poorest) being merged into the lowest income quartile. Smoking status was classified as non‐smoker or current smoker. Heavy drinkers were defined as those who consumed ≥30 g/day of alcohol. Regular exercise was defined as moderate physical activity for more than 30 min and more than 5 days/week during the past week.
Systolic and diastolic blood pressure was measured in a seated position after at least 5‐min rest. Blood sampling, measuring serum glucose, lipid and creatinine levels, was conducted after an overnight fast. These health examinations were performed in hospitals certified by the NHIS under regular quality control. Hypertension was defined as a claim according to ICD‐10 codes for diagnosis (I10–I13 or I15), antihypertensive medication or systolic/diastolic blood pressure of ≥140/90 mmHg. Type 2 diabetes was defined as a claim with E11–E14 codes linked to a history of antidiabetic medication prescription or fasting glucose level ≥ 126 mg/dL. Dyslipidaemia was defined as a history of claims with E78 codes, lipid‐lowering medications or a total cholesterol level ≥ 240 mg/dL. Chronic kidney disease (CKD) was defined as glomerular filtration rate < 60 mL/min/1.73 m2 as estimated using the Modification of Diet in Renal Disease equation.
Statistical analysis
Continuous variables are presented as means ± standard deviation (SD) and categorical variables are presented as numbers and percentages. Hazard ratios (HRs) and 95% confidence intervals (CIs) for CVDs, MI and ischaemic stroke were calculated using the multivariable Cox proportional hazards regression analysis. Model 1 was adjusted for sex, income, smoking status, alcohol consumption, physical activity and comorbidities (hypertension, type 2 diabetes, dyslipidaemia and CKD). Model 2 was additionally adjusted for baseline WC for the association of BMI with outcomes and baseline BMI for the association of WC with outcomes. Stratified analyses were performed to determine the association between general and abdominal obesity and the incidence of CVDs, MI and ischaemic stroke in subgroups according to sex, smoking status, type 2 diabetes, hypertension and CKD. Statistical analyses were performed using SAS Version 9.4 (SAS Institute Inc., Cary, NC, USA), and a P value < 0.05 was considered statistically significant.
Results
Baseline characteristics of study participants
Table 1 shows the baseline characteristics of individuals aged 40 and 66 years. Compared with the individuals aged 66 years, those aged 40 years tended to be current smokers, heavy alcohol drinkers and less likely to engage in regular exercise (P < 0.001 for all). Individuals aged 40 years had lower BMI and WC and fewer comorbidities such as hypertension, type 2 diabetes, dyslipidaemia and CKD than those aged 66 years (P < 0.001 for all).
Table 1.
Baseline characteristics of study population
| Variables | Age group | P value | |
|---|---|---|---|
| 40 years (N = 1 866 591) | 66 years (N = 563 919) | ||
| Sex (male) | 931 010 (49.9) | 276 665 (49.1) | <0.001 |
| Income (lowest quartile) | 295 293 (15.8) | 121 367 (21.5) | <0.001 |
| Current smoker | 517 623 (27.7) | 79 989 (14.2) | <0.001 |
| Heavy alcohol drinker | 150 276 (8.1) | 29 902 (5.3) | <0.001 |
| Regular exerciser | 302 868 (16.2) | 140 455 (24.9) | <0.001 |
| Height (cm) | 165.4 ± 8.4 | 159.3 ± 8.4 | <0.001 |
| Weight (kg) | 64.8 ± 12.3 | 61.3 ± 9.5 | <0.001 |
| Body mass index (kg/m2) | 23.5 ± 3.3 | 24.1 ± 3.0 | <0.001 |
| <18.5 | 68 963 (3.7) | 12 942 (2.3) | <0.001 |
| 18.5–22.9 | 808 667 (43.3) | 186 458 (33.1) | |
| 23–24.9 | 418 738 (22.4) | 156 310 (27.7) | |
| 25–29.9 | 495 819 (26.6) | 189 944 (33.7) | |
| ≥30.0 | 74 404 (4.0) | 18 265 (3.2) | |
| General obesity | 570 223 (30.6) | 208 209 (36.9) | |
| Waist circumference (cm) | 78.7 ± 9.4 | 82.7 ± 8.2 | <0.001 |
| M < 80.0; W < 75.0 | 830 915 (44.5) | 127 141 (22.6) | <0.001 |
| M 80.0–84.9; W 75.0–79.9 | 432 110 (23.2) | 134 141 (23.8) | |
| M 85.0–89.9; W 80.0–84.9 | 314 492 (16.9) | 141 850 (25.2) | |
| M 90.0–94.9; W 85.0–89.9 | 170 975 (9.2) | 93 613 (16.6) | |
| M 95.0–99.9; W 90.0–94.9 | 73 837 (4.0) | 44 317 (7.9) | |
| M ≥ 100.0; W ≥ 95.0 | 44 262 (2.4) | 22 857 (4.1) | |
| Abdominal obesity | 289 074 (15.5) | 16 079 (28.5) | <0.001 |
| Systolic blood pressure (mmHg) | 118.6 ± 13.9 | 128.3 ± 15.6 | <0.001 |
| Diastolic blood pressure (mmHg) | 74.7 ± 10.1 | 77.9 ± 9.9 | <0.001 |
| Fasting glucose (mg/dL) | 94.5 ± 19.2 | 102.4 ± 25.2 | <0.001 |
| Total cholesterol (mg/dL) | 193.0 ± 36.8 | 199.4 ± 40.4 | <0.001 |
| HDL‐C (mg/dL) | 56.8 ± 27.4 | 54.7 ± 26.8 | <0.001 |
| Triglycerides (mg/dL) | 126.8 ± 102.0 | 134.7 ± 87.4 | <0.001 |
| LDL‐C (mg/dL) | 113.1 ± 53.8 | 119.5 ± 51.9 | <0.001 |
| eGFR (mL/min/1.73 m2) | 92.7 ± 38.5 | 83.7 ± 31.9 | <0.001 |
| Comorbidities | |||
| Hypertension | 217 086 (11.6) | 279 215 (49.5) | <0.001 |
| Type 2 diabetes | 65 891 (3.5) | 90 616 (16.1) | <0.001 |
| Dyslipidaemia | 202 581 (10.9) | 162 251 (28.8) | <0.001 |
| Chronic kidney disease | 52 147 (2.8) | 62 587 (11.1) | <0.001 |
Note: Data are expressed as the mean ± standard deviation, or number (percentage). Abbreviations: eGFR, estimated glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; M, men; W, women.
Associations between body mass index and the risk of cardiovascular diseases by age group
During a mean follow‐up of 7.7 years from 1 year after the health screening, there were 52 095 CVD events (16 431 in those aged 40 and 35 664 in those aged 66 years), 24 884 MI events (9964 in those aged 40 and 14 920 in those aged 66 years) and 29 415 ischaemic stroke events (6829 in those aged 40 and 22 586 in those aged 66 years). Table 2 presents the associations of BMI and general obesity with study outcomes in the different age groups. Among individuals aged 40 years, general obesity was associated with an increased risk of CVDs (adjusted HR [aHR] 1.26, 95% CI = 1.22–1.31), MI (aHR 1.26, 95% CI = 1.21–1.32) and ischaemic stroke (aHR 1.25, 95% CI = 1.19–1.32). A J‐shaped association between BMI categories and CVDs, MI and ischaemic stroke was observed among individuals aged 40 years (P for trend < 0.001) (Table 2 , Figure 1A and Figure S1 ). In individuals aged 66 years, general obesity was significantly associated with the risk of CVDs (aHR 1.03, 95% CI = 1.01–1.06) and MI (aHR 1.06, 95% CI = 1.03–1.10), but not with ischaemic stroke (aHR 1.01, 95% CI = 0.99–1.04). There were significant U‐shaped associations between BMI and CVDs and MI with nadir at a BMI of 23.0–24.9 kg/m2 (P for trend 0.013 and 0.017) (Table 2 , Figure 1B and Figure S1 ). The risk of outcomes was increased in individuals aged 66 years with BMI < 18.5 kg/m2 (aHR 1.12, 95% CI = 1.05–1.21 for CVDs; aHR 1.19, 95% CI = 1.07–1.32 for MI; aHR 1.10, 95% CI = 1.00–1.20 for ischaemic stroke) and in those with BMI ≥ 30.0 kg/m2 (aHR 1.17, 95% CI = 1.11–1.24 for CVDs; aHR 1.23, 95% CI = 1.13–1.35 for MI; aHR 1.13, 95% CI = 1.05–1.21 for ischaemic stroke). The association between BMI and study outcomes was more prominent in individuals aged 40 years than in those aged 66 years (P for interaction < 0.001). Similar patterns were maintained after additionally adjusting WC (Model 2) and adjusting low‐density lipoprotein cholesterol and systolic blood pressure instead of dyslipidaemia and hypertension (Table S1 ). After stratification by sex, these associations were consistently observed (Tables S2 and S3 ).
Table 2.
Hazard ratios (95% confidence intervals) of study outcomes according to body mass index categories between individuals aged 40 and 66 years
| Age group | N | Cardiovascular diseases | Myocardial infarction | Ischaemic stroke | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case/IR a | HR (95% CI) b | HR (95% CI) c | Case/IR a | HR (95% CI) b | HR (95% CI) c | Case/IR a | HR (95% CI) b | HR (95% CI) c | ||
| 40 years d | ||||||||||
| BMI (kg/m2) | ||||||||||
| <18.5 | 68 963 | 443/0.8 | 1.07 (0.97–1.18) | 1.11 (1.01–1.23) | 273/0.5 | 1.13 (1.00–1.28) | 1.17 (1.03–1.32) | 176/0.3 | 0.98 (0.84–1.14) | 1.01 (0.87–1.18) |
| 18.5–22.9 | 808 667 | 5276/0.8 | 1 (Ref.) | 1 (Ref.) | 3137/0.5 | 1 (Ref.) | 1 (Ref.) | 2256/0.4 | 1 (Ref.) | 1 (Ref.) |
| 23.0–24.9 | 418 738 | 3676/1.1 | 1.13 (1.08–1.18) | 1.10 (1.05–1.15) | 2258/0.7 | 1.15 (1.08–1.21) | 1.11 (1.05–1.18) | 1500/0.5 | 1.11 (1.04–1.18) | 1.08 (1.01–1.15) |
| 25.0–29.9 | 495 819 | 5824/1.5 | 1.30 (1.25–1.36) | 1.23 (1.18–1.28) | 3553/0.9 | 1.31 (1.24–1.38) | 1.23 (1.16–1.30) | 2398/0.6 | 1.28 (1.20–1.36) | 1.21 (1.13–1.30) |
| ≥30.0 | 74 404 | 1212/2.2 | 1.55 (1.45–1.66) | 1.38 (1.28–1.49) | 743/1.3 | 1.60 (1.47–1.75) | 1.43 (1.30–1.57) | 499/0.9 | 1.45 (1.31–1.60) | 1.29 (1.15–1.45) |
| P for trend | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| General obesity | ||||||||||
| No | 1 296 368 | 9395/0.9 | 1 (Ref.) | 1 (Ref.) | 5668/0.6 | 1 (Ref.) | 1 (Ref.) | 3932/0.4 | 1 (Ref.) | 1 (Ref.) |
| Yes | 570 223 | 7036/1.6 | 1.26 (1.22–1.31) | 1.18 (1.14–1.23) | 4296/1.0 | 1.26 (1.21–1.32) | 1.18 (1.13–1.23) | 2897/0.7 | 1.25 (1.19–1.32) | 1.17 (1.11–1.24) |
| 66 years d | ||||||||||
| BMI (kg/m2) | ||||||||||
| <18.5 | 12 942 | 849/9.2 | 1.12 (1.05–1.21) | 1.19 (1.11–1.27) | 376/4.0 | 1.19 (1.07–1.32) | 1.26 (1.13–1.40) | 525/5.6 | 1.10 (1.00–1.20) | 1.16 (1.06–1.27) |
| 18.5–22.9 | 186 458 | 11 313/8.1 | 1 (Ref.) | 1 (Ref.) | 4734/3.4 | 1 (Ref.) | 1 (Ref.) | 7182/5.1 | 1 (Ref.) | 1 (Ref.) |
| 23.0–24.9 | 156 310 | 9685/8.2 | 0.99 (0.97–1.02) | 0.96 (0.93–0.98) | 3964/3.3 | 0.97 (0.93–1.02) | 0.94 (0.90–0.98) | 6187/5.2 | 1.00 (0.96–1.03) | 0.96 (0.93–0.99) |
| 25.0–29.9 | 189 944 | 12 419/8.7 | 1.02 (1.00–1.05) | 0.95 (0.92–0.98) | 5237/3.6 | 1.04 (1.00–1.08) | 0.97 (0.93–1.01) | 7821/5.4 | 1.00 (0.97–1.04) | 0.93 (0.90–0.97) |
| ≥30.0 | 18 265 | 1398/10.2 | 1.17 (1.11–1.24) | 1.03 (0.97–1.09) | 609/4.4 | 1.23 (1.13–1.35) | 1.08 (0.99–1.18) | 871/6.3 | 1.13 (1.05–1.21) | 0.99 (0.92–1.06) |
| P for trend | 0.013 | <0.001 | 0.017 | 0.088 | 0.441 | <0.001 | ||||
| General obesity | ||||||||||
| No | 355 710 | 21 847/8.2 | 1 (Ref.) | 1 (Ref.) | 9074/3.4 | 1 (Ref.) | 1 (Ref.) | 13 894/5.2 | 1 (Ref.) | 1 (Ref.) |
| Yes | 208 209 | 13 817/8.8 | 1.03 (1.01–1.06) | 0.98 (0.95–1.00) | 5846/3.7 | 1.06 (1.03–1.10) | 1.00 (0.97–1.04) | 8692/5.5 | 1.01 (0.99–1.04) | 0.96 (0.93–0.98) |
Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio; IR, incidence rate.
Number of cases per 1000 person‐years.
Model 1: Adjusted for sex, income, smoking status, alcohol consumption, physical activity, hypertension, type 2 diabetes, dyslipidaemia and chronic kidney disease.
Model 2: Adjusted for sex, income, smoking status, alcohol consumption, physical activity, hypertension, type 2 diabetes, dyslipidaemia, chronic kidney disease and waist circumference.
All Ps for interaction for the associations between body mass index categories and study outcomes by age group were <0.001.
Figure 1.
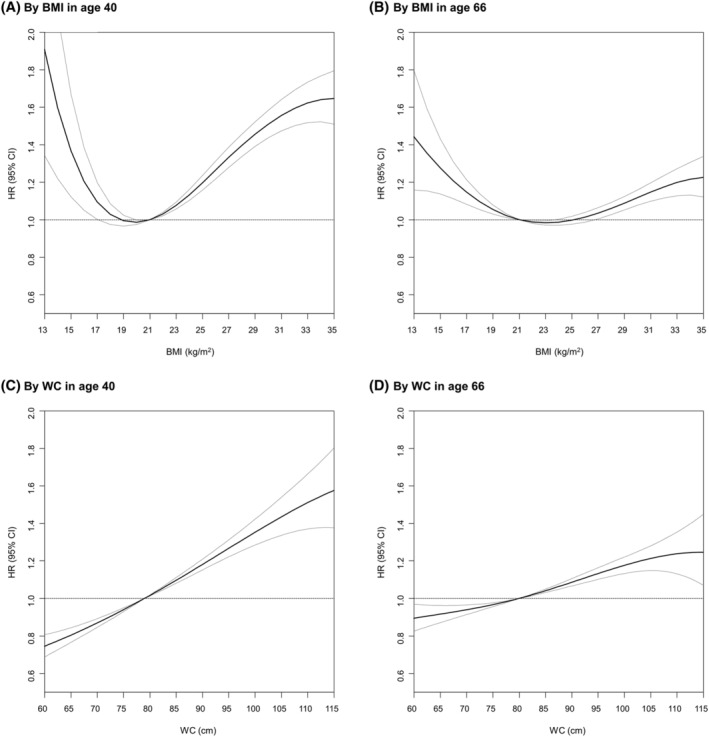
Association between body mass index (BMI) and waist circumference (WC) and cardiovascular diseases according to ages (40 and 66 years). (A) By BMI in age 40; (B) by BMI in age 66; (C) by WC in age 40; (D) by WC in age 66. Adjusted for sex, income, smoking status, alcohol consumption, physical activity, hypertension, type 2 diabetes, dyslipidaemia and chronic kidney disease. CI, confidence interval; HR, hazard ratio
Associations between waist circumference and risk of cardiovascular diseases by age group
Among individuals aged 40 years, abdominal obesity was associated with an increased risk of CVDs (aHR 1.27, 95% CI = 1.22–1.32), MI (aHR 1.27, 95% CI = 1.21–1.34) and ischaemic stroke (aHR 1.26, 95% CI = 1.19–1.33) (Table 3 ). The risk of study outcomes in individuals aged 40 years was increased with WC in a dose‐dependent manner: from individuals with WC < 80.0 cm for men and <75.0 cm for women (aHR 0.84, 95% CI = 0.80–0.88 for CVDs; aHR 0.82, 95% CI = 0.78–0.87 for MI; aHR 0.87, 95% CI = 0.81–0.94 for ischaemic stroke) to those with ≥100.0 cm for men and ≥95.0 cm for women (aHR 1.28, 95% CI = 1.18–1.39 for CVDs; aHR 1.28, 95% CI = 1.15–1.42 for MI; aHR 1.27, 95% CI = 1.12–1.44 for ischaemic stroke) (P for trend < 0.001) (Table 3 , Figure 1C and Figure S1 ). Even to a lesser degree than in the younger age group, abdominal obesity was associated with an increased risk of CVDs (aHR 1.11, 95% CI = 1.09–1.14), MI (aHR 1.14, 95% CI = 1.10–1.18) and ischaemic stroke (aHR 1.09, 95% CI = 1.06–1.12) among individuals aged 66 years. The risk of study outcomes in groups aged 66 years also linearly increased with WC from <80.0 cm for men and <75.0 cm for women (aHR 0.93, 95% CI = 0.90–0.96 for CVDs; aHR 0.95, 95% CI = 0.90–1.00 for MI; aHR 0.93, 95% CI = 0.89–0.96 for ischaemic stroke) to ≥100.0 cm for men and ≥95.0 cm for women (aHR 1.18, 95% CI = 1.12–1.24 for CVDs; aHR 1.20, 95% CI = 1.11–1.30 for MI; aHR 1.17, 95% CI = 1.10–1.25 for ischaemic stroke) (P for trend < 0.001) (Table 3 , Figure 1D and Figure S1 ). More prominent associations between WC and study outcomes were observed in individuals aged 40 years than in individuals aged 66 years (P for interaction < 0.001). Similar patterns were maintained after additionally adjusting BMI (Model 2) and adjusting low‐density lipoprotein cholesterol and systolic blood pressure instead of dyslipidaemia and hypertension (Table S4 ). After stratification by sex, these associations were consistently observed (Tables S5 and S6 ).
Table 3.
Hazard ratios (95% confidence intervals) of study outcomes according to waist circumference categories between individuals aged 40 and 66 years
| Age group | N | Cardiovascular diseases | Myocardial infarction | Ischaemic stroke | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case/IR a | HR (95% CI) b | HR (95% CI) c | Case/IR a | HR (95% CI) b | HR (95% CI) c | Case/IR a | HR (95% CI) b | HR (95% CI) c | ||
| 40 years d | ||||||||||
| WC (cm) | ||||||||||
| M < 80.0; W < 75.0 | 830 915 | 5386/0.8 | 0.84 (0.80–0.88) | 0.92 (0.87–0.97) | 3219/0.5 | 0.82 (0.78–0.87) | 0.91 (0.85–0.97) | 2283/0.4 | 0.87 (0.81–0.94) | 0.95 (0.87–1.03) |
| M 80.0–84.9; W 75.0–79.9 | 432 110 | 3850/1.2 | 0.96 (0.91–1.00) | 0.99 (0.95–1.04) | 2308/0.7 | 0.92 (0.87–0.98) | 0.96 (0.90–1.02) | 1623/0.5 | 1.02 (0.95–1.10) | 1.05 (0.98–1.14) |
| M 85.0–89.9; W 80.0–84.9 | 314 492 | 3222/1.3 | 1 (Ref.) | 1 (Ref.) | 2013/0.8 | 1 (Ref.) | 1 (Ref.) | 1275/0.5 | 1 (Ref.) | 1 (Ref.) |
| M 90.0–94.9; W 85.0–89.9 | 170 975 | 2133/1.6 | 1.11 (1.05–1.18) | 1.07 (1.01–1.13) | 1301/1.0 | 1.09 (1.02–1.17) | 1.05 (0.97–1.12) | 892/0.7 | 1.17 (1.07–1.27) | 1.13 (1.03–1.23) |
| M 95.0–99.9; W 90.0–94.9 | 73 837 | 1095/1.9 | 1.24 (1.15–1.33) | 1.14 (1.06–1.23) | 670/1.2 | 1.22 (1.12–1.34) | 1.13 (1.03–1.24) | 445/0.8 | 1.24 (1.11–1.38) | 1.15 (1.03–1.29) |
| M ≥ 100.0; W ≥ 95.0 | 44 262 | 745/2.2 | 1.28 (1.18–1.39) | 1.10 (1.00–1.21) | 453/1.4 | 1.28 (1.15–1.42) | 1.09 (0.97–1.23) | 311/0.9 | 1.27 (1.12–1.44) | 1.11 (0.96–1.28) |
| P for trend | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.007 | ||||
| Abdominal obesity | ||||||||||
| No | 1 577 517 | 12 458/1.0 | 1 (Ref.) | 1 (Ref.) | 7540/0.6 | 1 (Ref.) | 1 (Ref.) | 5181/0.4 | 1 (Ref.) | 1 (Ref.) |
| Yes | 289 074 | 3973/1.8 | 1.27 (1.22–1.32) | 1.09 (1.04–1.15) | 2424/1.1 | 1.27 (1.21–1.34) | 1.09 (1.02–1.16) | 1648/0.7 | 1.26 (1.19–1.33) | 1.11 (1.03–1.19) |
| 66 years d | ||||||||||
| WC (cm) | ||||||||||
| M < 80.0; W < 75.0 | 127 141 | 7044/7.4 | 0.93 (0.90–0.96) | 0.87 (0.84–0.90) | 2962/3.1 | 0.95 (0.90–1.00) | 0.89 (0.84–0.94) | 4430/4.6 | 0.93 (0.89–0.96) | 0.86 (0.82–0.90) |
| M 80.0–84.9; W 75.0–79.9 | 134 141 | 8187/8.1 | 0.98 (0.95–1.01) | 0.96 (0.93–0.99) | 3425/3.4 | 1.00 (0.96–1.05) | 0.98 (0.93–1.02) | 5179/5.1 | 0.98 (0.95–1.02) | 0.95 (0.91–0.99) |
| M 85.0–89.9; W 80.0–84.9 | 141 850 | 8982/8.4 | 1 (Ref.) | 1 (Ref.) | 3684/3.4 | 1 (Ref.) | 1 (Ref.) | 5735/5.3 | 1 (Ref.) | 1 (Ref.) |
| M 90.0–94.9; W 85.0–89.9 | 93 613 | 6309/8.9 | 1.03 (1.00–1.07) | 1.06 (1.03–1.10) | 2678/3.7 | 1.07 (1.02–1.13) | 1.10 (1.05–1.16) | 3983/5.6 | 1.02 (0.98–1.06) | 1.05 (1.01–1.09) |
| M 95.0–99.9; W 90.0–94.9 | 44 317 | 3336/10.0 | 1.14(1.10–1.19) | 1.21 (1.16–1.26) | 1422/4.2 | 1.19 (1.12–1.27) | 1.25 (1.17–1.34) | 2074/6.2 | 1.10 (1.05–1.16) | 1.17 (1.11–1.24) |
| M ≥ 100.0; W ≥ 95.0 | 22 857 | 1806/10.6 | 1.18 (1.12–1.24) | 1.30 (1.23–1.38) | 749/4.3 | 1.20 (1.11–1.30) | 1.31 (1.20–1.44) | 1167/6.8 | 1.17 (1.10–1.25) | 1.32 (1.23–1.42) |
| P for trend | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Abdominal obesity | ||||||||||
| No | 403 132 | 24 213/8.0 | 1 (Ref.) | 1 (Ref.) | 10 071/3.3 | 1 (Ref.) | 1 (Ref.) | 15 362/5.0 | 1 (Ref.) | 1 (Ref.) |
| Yes | 160 787 | 11 451/9.5 | 1.11 (1.09–1.14) | 1.13 (1.10–1.16) | 4849/3.9 | 1.14 (1.10–1.18) | 1.16 (1.11–1.21) | 7224/5.9 | 1.09 (1.06–1.12) | 1.12 (1.08–1.16) |
Abbreviations: CI, confidence interval; HR, hazard ratio; IR, incidence rate; M, men; W, women; WC, waist circumference.
Number of cases per 1000 person‐years.
Model 1: Adjusted for sex, income, smoking status, alcohol consumption, physical activity, hypertension, type 2 diabetes, dyslipidaemia and chronic kidney disease.
Model 2: Adjusted for sex, income, smoking status, alcohol consumption, physical activity, hypertension, type 2 diabetes, dyslipidaemia, chronic kidney disease and body mass index.
All Ps for interaction for the associations between waist circumference categories and study outcomes by age group were <0.001.
Stratified analyses
Stratified analyses according to sex, smoking status, type 2 diabetes, hypertension, dyslipidaemia and CKD were conducted (Table 4 ). In individuals aged 40 years, the associations of general and abdominal obesity with CVDs and ischaemic stroke were prominent in women (P for interaction < 0.05 for all). The associations between general and abdominal obesity and study outcomes were prominent in those without type 2 diabetes and hypertension (P for interaction < 0.05 for all). In individuals aged 66 years, the associations of general and abdominal obesity with study outcomes were prominent for women, non‐current smokers and those without type 2 diabetes and hypertension (P for interaction < 0.05 for all).
Table 4.
Subgroup analysis of the association between general and abdominal obesity and study outcomes
| Subgroup | Cardiovascular diseases | Myocardial infarction | Ischaemic stroke | ||||
|---|---|---|---|---|---|---|---|
| General obesity a | Abdominal obesity b | General obesity a | Abdominal obesity b | General obesity a | Abdominal obesity b | ||
| HR (95% CI) c | HR (95% CI) c | HR (95% CI) c | HR (95% CI) c | HR (95% CI) c | HR (95% CI) c | ||
| 40 years | |||||||
| Sex | Male | 1.24 (1.19–1.29) | 1.24 (1.18–1.29) | 1.26 (1.19–1.32) | 1.26 (1.19–1.33) | 1.20 (1.13–1.28) | 1.21 (1.12–1.29) |
| Female | 1.32 (1.24–1.40) | 1.36 (1.27–1.47) | 1.29 (1.19–1.40) | 1.32 (1.20–1.47) | 1.34 (1.23–1.47) | 1.39 (1.25–1.55) | |
| P for interaction | 0.030 | 0.005 | 0.949 | 0.650 | 0.001 | <0.001 | |
| Current smoker | No | 1.29 (1.23–1.35) | 1.27 (1.20–1.34) | 1.28 (1.21–1.36) | 1.23 (1.15–1.32) | 1.27 (1.19–1.36) | 1.31 (1.21–1.42) |
| Yes | 1.23 (1.18–1.30) | 1.27 (1.20–1.34) | 1.24 (1.17–1.32) | 1.31 (1.22–1.39) | 1.22 (1.13–1.32) | 1.20 (1.10–1.31) | |
| P for interaction | 0.225 | 0.957 | 0.758 | 0.120 | 0.210 | 0.068 | |
| Type 2 diabetes | No | 1.29 (1.25–1.34) | 1.29 (1.24–1.34) | 1.29 (1.23–1.35) | 1.29 (1.23–1.36) | 1.28 (1.22–1.36) | 1.28 (1.21–1.37) |
| Yes | 0.99 (0.89–1.10) | 1.12 (1.00–1.24) | 1.01 (0.87–1.16) | 1.12 (0.97–1.28) | 0.94 (0.80–1.10) | 1.08 (0.92–1.26) | |
| P for interaction | <0.001 | <0.001 | <0.001 | 0.011 | <0.001 | 0.011 | |
| Hypertension | No | 1.29 (1.24–1.34) | 1.31 (1.25–1.37) | 1.29 (1.23–1.35) | 1.31 (1.24–1.39) | 1.29 (1.21–1.37) | 1.29 (1.20–1.39) |
| Yes | 1.16 (1.09–1.24) | 1.20 (1.12–1.28) | 1.16 (1.07–1.27) | 1.19 (1.09–1.30) | 1.13 (1.02–1.24) | 1.20 (1.09–1.32) | |
| P for interaction | <0.001 | <0.001 | 0.010 | 0.016 | <0.001 | 0.026 | |
| Dyslipidaemia | No | 1.27 (1.22–1.31) | 1.27 (1.22–1.33) | 1.27 (1.21–1.33) | 1.28 (1.21–1.36) | 1.25 (1.18–1.32) | 1.26 (1.17–1.34) |
| Yes | 1.24 (1.16–1.34) | 1.26 (1.17–1.35) | 1.23 (1.12–1.34) | 1.25 (1.14–1.36) | 1.24 (1.10–1.41) | 1.27 (1.12–1.43) | |
| P for interaction | 0.173 | 0.149 | 0.280 | 0.206 | 0.418 | 0.567 | |
| CKD | No | 1.27 (1.23–1.31) | 1.27 (1.22–1.32) | 1.27 (1.21–1.32) | 1.27 (1.21–1.34) | 1.26 (1.19–1.32) | 1.25 (1.18–1.33) |
| Yes | 1.20 (1.01–1.43) | 1.38 (1.14–1.68) | 1.22 (0.97–1.53) | 1.23 (0.95–1.59) | 1.15 (0.87–1.51) | 1.53 (1.14–2.05) | |
| P for interaction | 0.897 | 0.128 | 0.713 | 0.748 | 0.740 | 0.084 | |
| 66 years | |||||||
| Sex | Male | 0.99 (0.96–1.02) | 1.05 (1.02–1.09) | 1.00 (0.96–1.05) | 1.08 (1.03–1.14) | 0.98 (0.95–1.02) | 1.04 (1.00–1.08) |
| Female | 1.09 (1.05–1.12) | 1.18 (1.14–1.22) | 1.13 (1.07–1.18) | 1.20 (1.14–1.26) | 1.05 (1.01–1.09) | 1.16 (1.11–1.21) | |
| P for interaction | <0.001 | <0.001 | <0.001 | 0.002 | 0.003 | <0.001 | |
| Current smoker | No | 1.06 (1.03–1.08) | 1.14 (1.11–1.17) | 1.09 (1.05–1.13) | 1.17 (1.12–1.21) | 1.03 (1.00–1.06) | 1.12 (1.08–1.15) |
| Yes | 0.93 (0.89–0.98) | 1.00 (0.95–1.06) | 0.93 (0.86–1.01) | 1.02 (0.94–1.10) | 0.93 (0.88–0.99) | 0.98 (0.92–1.05) | |
| P for interaction | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Type 2 diabetes | No | 1.07 (1.04–1.09) | 1.14 (1.11–1.17) | 1.10 (1.05–1.14) | 1.18 (1.13–1.23) | 1.05 (1.01–1.08) | 1.11 (1.07–1.15) |
| Yes | 0.95 (0.90–0.99) | 1.04 (1.00–1.09) | 0.96 (0.90–1.03) | 1.04 (0.97–1.11) | 0.92 (0.87–0.97) | 1.05 (0.99–1.11) | |
| P for interaction | <0.001 | 0.003 | 0.007 | 0.008 | <0.001 | 0.142 | |
| Hypertension | No | 1.10 (1.06–1.14) | 1.20 (1.15–1.24) | 1.11 (1.06–1.17) | 1.22 (1.15–1.29) | 1.09 (1.04–1.14) | 1.18 (1.12–1.23) |
| Yes | 0.99 (0.97–1.02) | 1.07 (1.04–1.10) | 1.03 (0.98–1.07) | 1.10 (1.05–1.15) | 0.97 (0.94–1.01) | 1.05 (1.01–1.09) | |
| P for interaction | <0.001 | <0.001 | 0.046 | 0.008 | <0.001 | <0.001 | |
| Dyslipidaemia | No | 1.04 (1.01–1.07) | 1.11 (1.08–1.14) | 1.06 (1.02–1.11) | 1.13 (1.08–1.18) | 1.02 (0.99–1.05) | 1.09 (1.05–1.12) |
| Yes | 1.02 (0.98–1.06) | 1.12 (1.08–1.17) | 1.06 (1.00–1.12) | 1.16 (1.09–1.23) | 1.00 (0.95–1.05) | 1.11 (1.05–1.16) | |
| P for interaction | 0.071 | 0.809 | 0.500 | 0.941 | 0.224 | 0.976 | |
| CKD | No | 1.04 (1.01–1.06) | 1.11 (1.08–1.14) | 1.06 (1.02–1.10) | 1.13 (1.09–1.17) | 1.02 (0.99–1.05) | 1.09 (1.06–1.13) |
| Yes | 1.02 (0.96–1.08) | 1.14 (1.07–1.20) | 1.06 (0.97–1.16) | 1.21 (1.10–1.32) | 1.00 (0.93–1.07) | 1.10 (1.02–1.18) | |
| P for interaction | 0.663 | 0.067 | 0.535 | 0.037 | 0.801 | 0.399 | |
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; HR, hazard ratio.
General obesity was defined as body mass index ≥ 25.0 kg/m2.
Abdominal obesity was defined as waist circumference ≥ 90.0 cm for men and ≥85.0 cm for women.
Adjusted for sex, income, smoking status, alcohol consumption, physical activity, hypertension, type 2 diabetes, dyslipidaemia and chronic kidney disease.
Associations of combined status of general and abdominal obesity with the risk of cardiovascular diseases by age group
In individuals aged 40 years, the aHR of CVDs, MI and ischaemic stroke was (1) 1.25 (95% CI = 1.11–1.40), 1.16 (95% CI = 0.99–1.35) and 1.40 (95% CI = 1.18–1.65) for having abdominal obesity only; (2) 1.20 (95% CI = 1.16–1.25), 1.19 (95% CI = 1.13–1.25) and 1.21 (95% CI = 1.13–1.29) for having general obesity only; and (3) 1.35 (95% CI = 1.30–1.41), 1.36 (95% CI = 1.29–1.44) and 1.33 (95% CI = 1.25–1.42) for having both, compared with those without both (Table 5 ). On the other hand, in individuals aged 66 years, compared with those without both, those who had only general obesity had the lowest risk of CVDs (aHR 0.97, 95% CI = 0.94–1.00), MI (aHR 0.98, 95% CI = 0.93–1.03) and ischaemic stroke (aHR 0.96, 95% CI = 0.92–1.00), which were higher among those who had only abdominal obesity (CVDs: aHR 1.13, 95% CI = 1.09–1.18; MI: aHR 1.13, 95% CI = 1.06–1.21; ischaemic stroke: aHR 1.13, 95% CI = 1.07–1.19). The association between the combined status of general and abdominal obesity and study outcomes significantly differed between the two age groups (P for interaction < 0.001). After stratification by sex, these associations were consistently observed. In addition, analyses combining both BMI and WC showed similar patterns (Table S7 ).
Table 5.
Hazard ratios (95% confidence intervals) a of study outcomes according to combined presence of general obesity and abdominal obesity between individuals aged 40 and 66 years
| General obesity b | Abdominal obesity c | Cardiovascular diseases | Myocardial infarction | Ischaemic stroke | |||
|---|---|---|---|---|---|---|---|
| 40 years | 66 years | 40 years | 66 years | 40 years | 66 years | ||
| Total | |||||||
| No | No | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| No | Yes | 1.25 (1.11–1.40) | 1.13 (1.09–1.18) | 1.16 (0.99–1.35) | 1.13 (1.06–1.21) | 1.40 (1.18–1.65) | 1.13 (1.07–1.19) |
| Yes | No | 1.20 (1.16–1.25) | 0.97 (0.94–1.00) | 1.19 (1.13–1.25) | 0.98 (0.93–1.03) | 1.21 (1.13–1.29) | 0.96 (0.92–1.00) |
| Yes | Yes | 1.35 (1.30–1.41) | 1.10 (1.07–1.13) | 1.36 (1.29–1.44) | 1.14 (1.09–1.18) | 1.33 (1.25–1.42) | 1.07 (1.04–1.10) |
| P for interaction | <0.001 | <0.001 | <0.001 | ||||
| Men | |||||||
| No | No | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| No | Yes | 1.21 (1.04–1.39) | 1.07 (1.01–1.14) | 1.13 (0.93–1.36) | 1.06 (0.97–1.16) | 1.35 (1.09–1.68) | 1.06 (0.99–1.14) |
| Yes | No | 1.18 (1.13–1.24) | 0.94 (0.90–0.99) | 1.18 (1.11–1.25) | 0.92 (0.86–0.98) | 1.17 (1.08–1.26) | 0.95 (0.90–1.00) |
| Yes | Yes | 1.32 (1.26–1.39) | 1.03 (1.00–1.07) | 1.35 (1.27–1.43) | 1.07 (1.01–1.13) | 1.27 (1.17–1.37) | 1.01 (0.97–1.06) |
| P for interaction | <0.001 | <0.001 | <0.001 | ||||
| Women | |||||||
| No | No | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| No | Yes | 1.31 (1.08–1.59) | 1.22 (1.14–1.30) | 1.21 (0.93–1.58) | 1.22 (1.11–1.35) | 1.43 (1.09–1.89) | 1.22 (1.12–1.32) |
| Yes | No | 1.24 (1.15–1.34) | 1.01 (0.96–1.06) | 1.21 (1.09–1.35) | 1.06 (0.99–1.14) | 1.27 (1.13–1.42) | 0.97 (0.91–1.03) |
| Yes | Yes | 1.44 (1.33–1.56) | 1.18 (1.13–1.22) | 1.40 (1.25–1.56) | 1.22 (1.15–1.29) | 1.46 (1.30–1.65) | 1.14 (1.08–1.19) |
| P for interaction | <0.001 | <0.001 | <0.001 | ||||
Adjusted for sex, income, smoking status, alcohol consumption, physical activity, hypertension, type 2 diabetes, dyslipidaemia and chronic kidney disease.
General obesity was defined as body mass index ≥ 25.0 kg/m2.
Abdominal obesity was defined as waist circumference ≥ 90.0 cm for men and ≥85.0 cm for women.
Discussion
We demonstrated a J‐shaped association of BMI with CVDs, MI and ischaemic stroke in aged 40 years, whereas there was U‐shaped association between BMI and study outcomes with nadir at BMI 23.0–24.9 kg/m2 in aged 66 years. The increased risks of study outcomes in those who had general obesity were robust in individuals aged 40 years. WC was linearly associated with study outcomes in both age groups, but the impact of WC and abdominal obesity on study outcomes was more prominent in individuals aged 40 years than in those aged 66 years.
In the present study, the J‐shaped association between BMI and CVDs in the younger age group changed into a gentle U‐shaped association as age increased. The nadir of BMI for incident CVDs was 18.5–22.9 kg/m2 for those aged 40 years and 23.0–24.9 kg/m2 for those aged 66 years. In line with our findings, previous studies reported similar modifying effects of age on the association between BMI and obesity‐related metabolic conditions, including CVD, dyslipidaemia and hypertension, 18 as well as all‐cause and CVD mortality. 7 , 9 , 19 There are several possible explanations for this phenomenon. First, aging is associated with progressive fat distribution. 20 The subcutaneous fat is located away from the truncal region and redistributed to the abdominal areas, which poses a great risk for insulin resistance and CVDs. 20 The increase in the abdominal adipose tissue is not entirely captured by BMI and may explain the weakened association among older populations. 18 Second, an additional process associated with aging is age‐related losses in height that inflates the measured BMI. Third, it is also possible that older participants, even at a normal BMI, have less lean mass and more adiposity, which raises their CVD risk closer to levels of obesity. Together, these factors may weaken the association between BMI and CVDs among older populations, and our findings further support the idea that a high optimal BMI for older adults should be considered regarding the risk of CVD development. 21
In addition, our study demonstrated that abdominal obesity without general obesity had a higher association with CVDs than general obesity without abdominal obesity. This finding supports the idea that the measurement of BMI alone may be a suboptimal marker for adiposity. 22 In the younger age group, abdominal obesity presented higher impact on ischaemic stroke than MI, regardless of sex. Abdominal obesity may play a different role in ischaemic stroke compared with MI, even though both are considered as CVDs. In line with our study, previous study reported that the nadir for BMI varied depending on the mortality outcome. 23
We showed that the increase in CVD risk, representing MI and ischaemic stroke, associated with a higher BMI and WC, tended to be greater among younger participants. Although the mechanisms responsible for the influence of age on the relationship between obesity and CVD are unclear, possible mechanisms have been suggested. First, young‐onset obesity has been reported to have more genetic predisposition to metabolic disorders 24 , 25 and resulted in chronically increased levels of circulating free fatty acid, adipokines and reactive oxygen species. 25 Combined with local and systemic inflammation, this leads the process of atherosclerosis by influencing the function of the endothelial cells, arterial smooth muscle cells and macrophages in vessel walls. 26 Second, patients with young‐onset obesity might have an early life exposure to maternal undernutrition or overnutrition, which is associated with an increased risk of type 2 diabetes. 27 Third, patients with young‐onset obesity tend to have a detrimental or inactive lifestyle, which might also contribute to the development of adiposity, insulin resistance, hyperglycaemia and other cardiovascular risk factors. 28 These multiple mechanisms converge to cause CVD.
Being underweight below BMI 18.5 kg/m2 was associated with an increased risk of MI and ischaemic stroke in both age groups. In line with our study, previous studies indicated that underweight and lower lean body mass increased the risk of CVD and mortality. 29 Sarcopenia can be a possible explanation, because loss of muscle mass is known to be associated with a hyperinflammatory status, 30 insulin resistance and multiple metabolic disorders, 31 which could lead to MI and ischaemic stroke. Greater muscle mass is associated with better exercise capacity and cardiorespiratory fitness and could in turn lead to decreased CVD risk. 32 , 33 Another explanation can be that being underweight can be a surrogate marker for patients' frail conditions including malnutrition and combined non‐cardiovascular comorbidities, and frailty itself can also increase the risk of CVD. 34 , 35
General and abdominal obesity were associated with an increased risk of CVD even after various stratifications. Interestingly, these associations were more prominent in women, non‐smokers and patients without type 2 diabetes and hypertension, who were considered to have a low risk of CVD. Obesity presents a significant CVD risk in healthy individuals; however, these effects may differ as they age and have chronic conditions. This is the phenomenon called obesity paradox. These findings suggest that even in populations with low CVD risk, attention must be paid to the prevention and management of obesity.
Current guidelines on the primary prevention of CVD recommend that adults who are overweight and obese reduce weight and improve their CVD risk factor profile. 36 , 37 They provide clinically meaningful weight loss as ≥5% of the initial weight, which was shown to be associated with moderate improvement in CVD risk factors, and mention that it was reasonable to measure WC to identify those at high cardiometabolic risk factors. In addition, our study highlights that, depending on age, individualized approaches are needed in obesity management to prevent CVD. Weight loss interventions should be cautiously implemented, especially in older adults, considering the loss of lean body or muscle mass.
This study had several limitations. First, although WC is a convenient and common method to assess abdominal obesity, WC measurement could have bias and the accuracy could depend on the measurer's experience, which is relatively subjective compared with BMI. Second, although we used the operational definition of study outcomes, which has been widely used for epidemiological studies with claims data, 10 , 38 this may have led to misdiagnosis. Third, due to the retrospective design of this study, the findings should be interpreted with caution. To overcome reverse causality, we excluded participants with outcomes that occurred in the first 1 year of follow‐up. Lastly, our study included only Korean participants and may not be generalizable to other ethnicities, who could have different associations between obesity and CVD risk.
Despite these limitations, our study used a significantly large sample to assess the association between obesity measures and CVD. To the best of our knowledge, this is the first study to compare the association between two transitional homogenous age groups, 40 and 66 years. Another strength of the present study is that the NHIS database includes data from the entire Korean population, which results in a near‐complete follow‐up.
In conclusion, we demonstrated a J‐shaped association of BMI with CVDs, MI and ischaemic stroke in young adults, whereas there was U‐shaped association between BMI and study outcomes with nadir at BMI 23.0–24.9 kg/m2 in those aged 66 years. WC was linearly associated with study outcomes in both age groups. The impact of general and abdominal obesity on study outcomes was more prominent in those aged 40 years than those aged 66 years. To prevent CVD risk, weight loss interventions should be cautiously implemented and individualized according to age.
Funding
This work was supported by the Korean Society for the Study of Obesity and Korea University.
Conflict of interest
Jung Eun Yoo, Kyungdo Han, Jin‐Hyung Jung, Yang‐Im Hur, Yang Hyun Kim, Eun Sook Kim, Jang Won Son, Eun‐Jung Rhee, Won‐Young Lee and Ga Eun Nam declare that they have no conflict of interest.
Supporting information
Table S1 Hazard ratios (95% confidence intervals) of study outcomes according to body mass index categories between individuals aged 40 years and 66 years
Table S2 Hazard ratios (95% confidence intervals) of study outcomes according to body mass index categories between individuals aged 40 years and 66 years (Men)
Table S3 Hazard ratios (95% confidence intervals) of study outcomes according to body mass index categories between individuals aged 40 years and 66 years (Women)
Table S4 Hazard ratios (95% confidence intervals) of study outcomes according to waist circumference categories between individuals aged 40 years and 66 years
Table S5 Hazard ratios (95% confidence intervals) of study outcomes according to waist circumference categories between individuals aged 40 years and 66 years (Men)
Table S6 Hazard ratios (95% confidence intervals) of study outcomes according to waist circumference categories between individuals aged 40 years and 66 years (Women)
Table S7 Hazard ratios (95% confidence intervals)* of study outcomes according to combined body mass index and waist circumference between individuals aged 40 years and 66 years
Figure S1. Association between body mass index and waist circumference and cardiovascular disease according to ages (40 and 66 years)
Acknowledgements
The data from the Korean National Health Insurance Service (NHIS) can be accessed via the Health Insurance Data Service website (http://nhiss.nhis.or.kr). However, researchers should submit a study proposal for approval from each institutional review board, which is reviewed by the NHIS review committee, to access the database. The raw data cannot be retrieved from the NHIS server. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 39
Yoo J. E., Han K., Jung J.‐H., Hur Y.‐I., Kim Y. H., Kim E. S., Son J. W., Rhee E.‐J., Lee W.‐Y., and Nam G. E. (2023) Body mass index, waist circumference and cardiovascular diseases in transitional ages (40 and 66years), Journal of Cachexia, Sarcopenia and Muscle, 14, 369–381, 10.1002/jcsm.13138
Won‐Young Lee and Ga Eun Nam contributed equally as co‐corresponding authors.
Contributor Information
Won‐Young Lee, Email: drlwy@hanmail.net.
Ga Eun Nam, Email: namgaaa@daum.net, Email: silver79@korea.ac.kr.
References
- 1. Choi S, Chang J, Kim K, Park SM, Lee K. Effect of smoking cessation and reduction on the risk of cancer in Korean men: a population based study. Cancer Res Treat 2018;50:1114–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol 2020;76:2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Powell‐Wiley TM, Poirier P, Burke LE, Després JP, Gordon‐Larsen P, Lavie CJ, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2021;143:e984–e1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang Z, Hoy WE. Age‐dependent decline of association between obesity and coronary heart disease: a cohort study in a remote Australian Aboriginal community. BMJ Open 2013;3:e004042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body‐mass index and mortality. N Engl J Med 1998;338:1–7. [DOI] [PubMed] [Google Scholar]
- 6. Chen Y, Copeland WK, Vedanthan R, Grant E, Lee JE, Gu D, et al. Association between body mass index and cardiovascular disease mortality in east Asians and south Asians: pooled analysis of prospective data from the Asia Cohort Consortium. BMJ 2013;347:f5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park HS, Song YM, Cho SI. Obesity has a greater impact on cardiovascular mortality in younger men than in older men among non‐smoking Koreans. Int J Epidemiol 2006;35:181–187. [DOI] [PubMed] [Google Scholar]
- 8. Abell JE, Egan BM, Wilson PW, Lipsitz S, Woolson RF, Lackland DT. Age and race impact the association between BMI and CVD mortality in women. Public Health Rep 2007;122:507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Childers DK, Allison DB. The ‘obesity paradox’: a parsimonious explanation for relations among obesity, mortality rate and aging? Int J Obes (Lond) 2010;34:1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cho JH, Rhee EJ, Park SE, Kwon H, Jung JH, Han KD, et al. The risk of myocardial infarction and ischemic stroke according to waist circumference in 21,749,261 Korean adults: a nationwide population‐based study. Diabetes Metab J 2019;43:206–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Song SO, Jung CH, Song YD, Park CY, Kwon HS, Cha BS, et al. Background and data configuration process of a nationwide population‐based study using the Korean National Health Insurance system. Diabetes Metab J 2014;38:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jung CH, Chung JO, Han K, Ko SH, Ko KS, Park JY. Improved trends in cardiovascular complications among subjects with type 2 diabetes in Korea: a nationwide study (2006–2013). Cardiovasc Diabetol 2017;16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park JH, Kim DH, Park YG, Kwon DY, Choi M, Jung JH, et al. Association of Parkinson disease with risk of cardiovascular disease and all‐cause mortality: a nationwide, population‐based cohort study. Circulation 2020;141:1205–1207. [DOI] [PubMed] [Google Scholar]
- 14. Kim HS, Shin DW, Lee WC, Kim YT, Cho B. National screening program for transitional ages in Korea: a new screening for strengthening primary prevention and follow‐up care. J Korean Med Sci 2012;27:S70–S75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–163. [DOI] [PubMed] [Google Scholar]
- 16. Kim BY, Kang SM, Kang JH, Kang SY, Kim KK, Kim KB, et al. 2020 Korean Society for the Study of Obesity guidelines for the management of obesity in Korea. J Obes Metab Syndr 2021;30:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim MK, Han K, Park YM, Kwon HS, Kang G, Yoon KH, et al. Associations of variability in blood pressure, glucose and cholesterol concentrations, and body mass index with mortality and cardiovascular outcomes in the general population. Circulation 2018;138:2627–2637. [DOI] [PubMed] [Google Scholar]
- 18. Canning KL, Brown RE, Jamnik VK, Kuk JL. Relationship between obesity and obesity‐related morbidities weakens with aging. J Gerontol A Biol Sci Med Sci 2014;69:87–92. [DOI] [PubMed] [Google Scholar]
- 19. di Angelantonio E, Bhupathiraju SN, Wormser D, Gao P, Kaptoge S, de Gonzalez AB, et al. Body‐mass index and all‐cause mortality: individual‐participant‐data meta‐analysis of 239 prospective studies in four continents. Lancet 2016;388:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuk JL, Saunders TJ, Davidson LE, Ross R. Age‐related changes in total and regional fat distribution. Ageing Res Rev 2009;8:339–348. [DOI] [PubMed] [Google Scholar]
- 21. Nam GE, Park HS. Perspective on diagnostic criteria for obesity and abdominal obesity in Korean adults. J Obes Metab Syndr 2018;27:134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Batsis JA, Mackenzie TA, Bartels SJ, Sahakyan KR, Somers VK, Lopez‐Jimenez F. Diagnostic accuracy of body mass index to identify obesity in older adults: NHANES 1999–2004. Int J Obes (Lond) 2016;40:761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin YK, Wang CC, Yen YF, Chen LJ, Ku PW, Chen CC, et al. Association of body mass index with all‐cause mortality in the elderly population of Taiwan: a prospective cohort study. Nutr Metab Cardiovasc Dis 2021;31:110–118. [DOI] [PubMed] [Google Scholar]
- 24. Choquet H, Meyre D. Genomic insights into early‐onset obesity. Genome Med 2010;2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wallis N, Raffan E. The genetic basis of obesity and related metabolic diseases in humans and companion animals. Genes (Basel) 2020;11:1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yoo HJ, Choi KM. Adipokines as a novel link between obesity and atherosclerosis. World J Diabetes 2014;5:357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vrachnis N, Antonakopoulos N, Iliodromiti Z, Dafopoulos K, Siristatidis C, Pappa KI, et al. Impact of maternal diabetes on epigenetic modifications leading to diseases in the offspring. Exp Diabetes Res 2012;2012:538474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gustat J, Srinivasan SR, Elkasabany A, Berenson GS. Relation of self‐rated measures of physical activity to multiple risk factors of insulin resistance syndrome in young adults: the Bogalusa Heart Study. J Clin Epidemiol 2002;55:997–1006. [DOI] [PubMed] [Google Scholar]
- 29. Kwon H, Yun JM, Park JH, Cho BL, Han K, Joh HK, et al. Incidence of cardiovascular disease and mortality in underweight individuals. J Cachexia Sarcopenia Muscle 2021;12:331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li CW, Yu K, Shyh‐Chang N, Li GX, Jiang LJ, Yu SL, et al. Circulating factors associated with sarcopenia during ageing and after intensive lifestyle intervention. J Cachexia Sarcopenia Muscle 2019;10:586–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Du Y, Oh C, No J. Associations between sarcopenia and metabolic risk factors: a systematic review and meta‐analysis. J Obes Metab Syndr 2018;27:175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. O'Donovan G, Owen A, Kearney EM, Jones DW, Nevill AM, Woolf‐May K, et al. Cardiovascular disease risk factors in habitual exercisers, lean sedentary men and abdominally obese sedentary men. Int J Obes (Lond) 2005;29:1063–1069. [DOI] [PubMed] [Google Scholar]
- 33. Lee DC, Sui X, Artero EG, Lee IM, Church TS, McAuley PA, et al. Long‐term effects of changes in cardiorespiratory fitness and body mass index on all‐cause and cardiovascular disease mortality in men: the Aerobics Center Longitudinal Study. Circulation 2011;124:2483–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Adabag S, Vo TN, Langsetmo L, Schousboe JT, Cawthon PM, Stone KL, et al. Frailty as a risk factor for cardiovascular versus noncardiovascular mortality in older men: results from the MrOS sleep (outcomes of sleep disorders in older men) study. J Am Heart Assoc 2018;7:e008974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Veronese N, Sigeirsdottir K, Eiriksdottir G, Marques EA, Chalhoub D, Phillips CL, et al. Frailty and risk of cardiovascular diseases in older persons: the age. Gene/Environ Susceptibility‐Reykjavik Study Rejuvenation Res 2017;20:517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;140:e596–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC). Eur Heart J 2021;42:3227–3337.34458905 [Google Scholar]
- 38. Jeong SM, Jeon KH, Shin DW, Han K, Kim D, Park SH, et al. Smoking cessation, but not reduction, reduces cardiovascular disease incidence. Eur Heart J 2021;42:4141–4153. [DOI] [PubMed] [Google Scholar]
- 39. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Hazard ratios (95% confidence intervals) of study outcomes according to body mass index categories between individuals aged 40 years and 66 years
Table S2 Hazard ratios (95% confidence intervals) of study outcomes according to body mass index categories between individuals aged 40 years and 66 years (Men)
Table S3 Hazard ratios (95% confidence intervals) of study outcomes according to body mass index categories between individuals aged 40 years and 66 years (Women)
Table S4 Hazard ratios (95% confidence intervals) of study outcomes according to waist circumference categories between individuals aged 40 years and 66 years
Table S5 Hazard ratios (95% confidence intervals) of study outcomes according to waist circumference categories between individuals aged 40 years and 66 years (Men)
Table S6 Hazard ratios (95% confidence intervals) of study outcomes according to waist circumference categories between individuals aged 40 years and 66 years (Women)
Table S7 Hazard ratios (95% confidence intervals)* of study outcomes according to combined body mass index and waist circumference between individuals aged 40 years and 66 years
Figure S1. Association between body mass index and waist circumference and cardiovascular disease according to ages (40 and 66 years)


