Abstract
Background
The associations between body flexibility and sarcopenia were not well understood. This study aimed to explore the cross‐sectional and longitudinal associations of flexibility with sarcopenia.
Methods
Our study selected participants aged 50–80 from the WELL‐China cohort and the Lanxi cohort. Participants from the urban area of the Lanxi cohort were followed up 4 years later. Body flexibility was measured by the sit‐and‐reach test. Muscle mass was evaluated by dual‐energy X‐ray absorptiometry. Muscle strength was evaluated using handgrip strength. Sarcopenia was defined as having both low muscle mass and low muscle strength. We used multivariable logistic regressions to assess the cross‐sectional associations of body flexibility with low muscle mass, low muscle strength and sarcopenia. We also used multivariable logistic regressions to explore the associations of baseline flexibility and 4‐year changes in flexibility with incident low muscle mass, low muscle strength and sarcopenia.
Results
A total of 9453 participants were enrolled in the cross‐sectional study, and 1233 participants were included in the longitudinal analyses. In the cross‐sectional analyses, compared with low body flexibility, high body flexibility was inversely associated with low muscle mass (odds ratio [OR], 0.58; 95% confidence interval [CI], 0.50–0.68; P < 0.001), low muscle strength (OR, 0.62; 95% CI, 0.55–0.69; P < 0.001) and sarcopenia (OR, 0.52; 95% CI, 0.41–0.65; P < 0.001), and these associations did not differ in different age groups, sex or physical activity levels. In the longitudinal analyses, compared with participants with low body flexibility, participants with high body flexibility had lower risk of the incident low muscle strength (OR, 0.53; 95% CI, 0.38–0.74; P < 0.001) and sarcopenia (OR, 0.36; 95% CI, 0.21–0.61; P < 0.001), but not incident low muscle mass (OR, 0.59; 95% CI, 0.33–1.06; P = 0.076). Every 1‐cm increase in flexibility during 4 years was associated with reduced risk of incident low muscle mass (OR, 0.96; 95% CI, 0.93–1.00; P = 0.025), low muscle strength (OR, 0.96; 95% CI, 0.94–0.98; P = 0.002) and sarcopenia (OR, 0.96; 95% CI, 0.93–0.99; P = 0.007).
Conclusions
High flexibility was associated with reduced risk of incident low muscle strength and sarcopenia. Increases in flexibility were associated with reduced risk of incident low muscle mass, low muscle strength and sarcopenia. Flexibility exercises and monitoring the dynamic change of flexibility might be helpful in preventing sarcopenia among adults aged 50 years or over.
Keywords: change in flexibility, change in muscle mass and muscle strength, muscle mass, muscle strength, sit‐and‐reach test
Introduction
Sarcopenia is characterized as progressive loss of muscle mass and strength and is recognized as an important health problem. 1 Sarcopenia increases the risk of fractures, loss of mobility and independence, and death. 2 The prevalence of sarcopenia ranged from 9.9% to 40.4% among older adults in the world. 3 Due to the rapid aging worldwide, the burden of sarcopenia has increased significantly, which suggests the urgency of exploring effective strategies to prevent sarcopenia. 4
Exercise is the cornerstone of the prevention and rehabilitation treatment for sarcopenia. 5 Although some consensus and researches for the treatment of sarcopenia emphasize flexibility exercise programmes, 5 , 6 , 7 the evidence to support the inclusion of flexibility training in exercise programmes for older adults with sarcopenia is limited. Flexibility is the ability to move a joint through its complete range of motion and is one of the components of health‐related physical fitness. 8 Emerging evidence found that increasing flexibility by stretching enhances blood flow and capillary growth in skeletal muscle, 9 , 10 which might influence the growth and loss of muscle. 11 , 12 In addition, flexibility is necessary for the elderly to be physically active, 13 , 14 , 15 which is important for maintaining muscle health and preventing sarcopenia. However, the associations between flexibility and sarcopenia are not well understood.
Only a few studies have investigated the associations between flexibility and sarcopenia. Studies found that participants with sarcopenia had lower flexibility than those without, 16 and flexibility was reported to be associated with muscle mass and muscle strength. 17 , 18 However, the study designs of these studies were cross‐sectional and the sample size was small. The causal relationship between flexibility and sarcopenia was unknown.
In addition, it is unclear whether we could identify the risk of sarcopenia by monitoring the change of flexibility. Compared with the measurement for evaluating sarcopenia, the assessment of flexibility is simple and easy. Therefore, the dynamic measurement of flexibility might be a useful way in evaluating the risk of sarcopenia if the associations between changes in flexibility and sarcopenia existed. However, there is no study exploring the associations of changes in flexibility with sarcopenia.
Thus, in the present study, we carried out cross‐sectional and longitudinal analyses to investigate the associations of flexibility with low muscle mass, low muscle strength and sarcopenia, and whether these associations are modified by age, sex and physical activity. In addition, we explored the associations of changes in flexibility with low muscle mass, low muscle strength and sarcopenia.
Methods
Participants
The study participants were selected from two prospective cohorts: the WELL‐China cohort and the Lanxi cohort. The WELL‐China cohort is located in Hangzhou, the capital of Zhejiang Province, China, and was jointly established by Zhejiang University and Stanford University. The baseline survey was completed from 2016 to 2019, and a total of 10 268 residents were enrolled. 19
The Lanxi cohort is located in Lanxi City, Zhejiang Province, China. The Lanxi cohort completed a baseline survey from 2015 to 2017. A total of 5132 residents were recruited, including 3327 urban residents (underwent a baseline survey from June to August 2015) and 1805 rural residents (underwent a baseline survey from June to August 2017). 20 All participants from both cohorts were invited to undergo general physical examinations, blood testing, face‐to‐face questionnaire surveys and dual‐energy X‐ray absorptiometry (DXA) scans.
During June and August 2019, participants from the urban area of the Lanxi cohort were invited to undergo a second wave survey. During this survey, we repeated measures for most items in the baseline, including general physical examinations, blood testing, face‐to‐face questionnaire surveys and DXA scans. A total of 2468 participants were followed up in this survey.
In the cross‐sectional analyses, we included participants aged 50 years and above from both the WELL‐China cohort and the Lanxi cohort, and without missing information on flexibility, anthropometric measurements, DXA scan and handgrip strength (HGS).
In the longitudinal analyses, we included participants from the urban area of the Lanxi cohort who were aged 50 years and above. Participants who satisfied the following criteria were included in the final longitudinal analyses: (1) without missing data in flexibility, anthropometric measurements, DXA scan and HGS at baseline; (2) without sarcopenia at baseline; (3) were followed up 4 years later; and (4) without missing data in the development of sarcopenia 4 years later. The selection flow of participants for cross‐sectional and longitudinal analyses is presented in Figure 1 .
Figure 1.
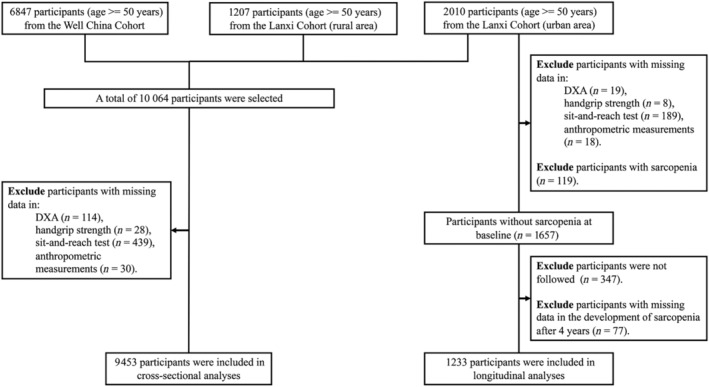
The selection flow of participants for cross‐sectional and longitudinal analyses in the study. DXA, dual‐energy X‐ray absorptiometry scans
Measurement of flexibility
Flexibility was assessed using the sit‐and‐reach test (SRT) in this study. The SRT results were measured using a digital device (TQQ‐II, Beijing Xindong Huateng Sports Equipment Co., Ltd, China). All participants underwent two measurements, with the higher value used for analysis. According to the sex‐specific tertiles of the SRT, we divided flexibility into three groups: low flexibility, middle flexibility and high flexibility. Change in flexibility was calculated by subtracting the SRT at baseline from the SRT at the 4‐year follow‐up.
Assessments of muscle mass and muscle strength
Whole‐body DXA scans were performed for all participants in both cohorts to assess the total and regional lean mass at baseline and follow‐up (Software Version 11.40.004, GE Lunar Prodigy; GE Healthcare, Milwaukie, WI, USA). Appendicular lean mass (ALM) was calculated as the sum of the lean mass of the legs and arms. Muscle mass was evaluated using the skeletal muscle mass index (SMI): SMI (kg/m2) = ALM/height2. 21
Muscle strength was assessed using HGS. HGS was measured using a hand dynamometer. Every participant was measured twice on their dominant hand, and the higher value was used for analysis.
Assessment of sarcopenia
Based on the criterion developed by the Asian Working Group of Sarcopenia (AWGS2, 2019 Consensus Report), participants were defined as having low muscle mass if SMI < 7.0 kg/m2 in men and SMI < 5.4 kg/m2 in women. 21 Participants were defined as having low muscle strength if HGS < 28 kg in men and HGS < 18 kg in women. 21 Participants who had both low muscle mass and low muscle strength were considered to have sarcopenia. 21
Participants who were without sarcopenia at baseline and developed sarcopenia at the 4‐year follow‐up were considered as having incident sarcopenia. Incident low muscle mass and low muscle strength were defined in the same way.
Covariates
Age was categorized into three groups, that is, 50–59, 60–69 and 70–80. Total fat mass was measured by the DXA scan and the fat mass index (FMI; kg/m2) was calculated by dividing the total fat mass by the height squared. FMI was classified as fat deficit (<3 kg/m2 in men and <5 kg/m2 in women), normal (3–6 kg/m2 in men and 5–9 kg/m2 in women), excess fat (6.1–9 kg/m2 in men and 9.1–13 kg/m2 in women) and obese (>9 kg/m2 in men and >13 kg/m2 in women). 22
Through face‐to‐face questionnaire surveys, information on sex, age, drinking status, smoking status, education level, physical activity status and disease history were collected. Drinking and smoking status were classified as current, ever or never, respectively. Education level was classified as elementary (illiterate or elementary school), secondary (middle school or high school/vocational school) or higher (college or above). Physical activity level was assessed using the International Physical Activity Questionnaire‐Short Form, and participants were categorized into low or high physical activity groups based on the median value of metabolic equivalence tasks (METs–h/week). 23
Participants with self‐reported hypertension, anti‐hypertensive medication, systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg at baseline were classified as having hypertension. Overnight fasting blood samples (12 h) were collected from participants and were tested for fasting plasma triglyceride, high‐density lipoprotein, glucose (FPG) and glycosylated haemoglobin (HbA1c). Participants with triglyceride ≥ 1.7 mmol/L were classified as having hypertriglyceridaemia and participants with high‐density lipoprotein < 1.03 mmol/L (male) or <1.3 mmol/L (female) were classified as having low high‐density lipoprotein. 24 Participants with self‐reported diabetes, anti‐diabetic medication, FPG ≥ 7.0 mmol/L or HbA1c ≥ 6.5 were classified as having diabetes.
Statistical analyses
Comparisons between participants with different levels of flexibility were performed using chi‐squared tests for categorical variables and one‐way analysis of variance for continuous variables. We used chi‐squared tests to compare the prevalence of low muscle mass, low muscle strength and sarcopenia in participants with different levels of baseline flexibility, and the Benjamini‐Hochberg method was used to adjust the P value. We used multivariate logistic regressions to examine the cross‐sectional associations of flexibility with low muscle mass, low muscle strength and sarcopenia, and whether these associations differed by age, sex and physical activity level groups.
In the longitudinal analyses, we performed multivariate logistic regressions to investigate the associations of baseline flexibility with incident low muscle mass, low muscle strength and sarcopenia. We further used multivariate logistic regressions to explore the associations of 4‐year changes in flexibility with incident low muscle mass, low muscle strength and sarcopenia.
In the above analyses, age groups, sex, FMI categories, residential area, education level, physical activity level, drinking status, smoking status, diabetes and hypertension were adjusted in regression models. In addition, baseline flexibility categories were further adjusted in regression models when exploring the associations of 4‐year changes in flexibility with incident low muscle mass, low muscle strength and sarcopenia. All statistical analyses were performed using R (Version 4.1.0). Statistical significance was set as P < 0.05 (two‐tailed).
Sensitivity analyses
We reanalysed the cross‐sectional associations of flexibility with low muscle mass, low muscle strength and sarcopenia in participants from the urban areas of the Lanxi cohort. Additionally, to explore whether the cross‐sectional and longitudinal associations of flexibility with sarcopenia could be influenced by the classification method of flexibility, we categorized flexibility into quartiles and reanalysed these associations.
We also used multivariable linear regression models to examine the associations of (1) baseline flexibility with ALM, SMI and HGS; (2) baseline flexibility with 4‐year changes in ALM, SMI and HGS; and (3) changes in flexibility with changes in ALM, SMI and HGS.
To determine whether the changes in physical activity level were different in participants with different flexibility levels after 4 years, we used multivariable linear regression models to examine the associations between baseline flexibility and 4‐year changes in METs consumed by physical activity.
Results
Baseline characteristics of participants in the cross‐sectional analyses
A total of 9453 participants were included in the cross‐sectional analyses. Table 1 presents the participants' baseline characteristics by baseline flexibility categories. Compared with participants with high flexibility, participants with low flexibility were older; had a higher level of FMI and a lower level of physical activity; and had a higher prevalence of hypertension, low high‐density lipoprotein, hypertriglyceridaemia and diabetes. In addition, participants were divided into different groups based on study design and sex, and the baseline characteristics of each group were described in Table S1 .
Table 1.
Baseline characteristics of participants with different categories of flexibility (n = 9453)
| Characteristics | Low flexibility | Middle flexibility | High flexibility | P value |
|---|---|---|---|---|
| Total participants | 3099 | 3181 | 3173 | |
| Age (years) | 62.4 ± 7.6 | 61.6 ± 7.3 | 60.9 ± 6.9 | <0.001 |
| Age groups, n (%) | <0.001 | |||
| 50–59 | 1200 (38.7) | 1331 (41.8) | 1379 (43.5) | |
| 60–69 | 1282 (41.4) | 1340 (42.1) | 1410 (44.4) | |
| 70–80 | 617 (20.0) | 510 (16.0) | 384 (12.1) | |
| Sex, n (%) | 0.999 | |||
| Male | 1274 (41.1) | 1306 (41.1) | 1304 (41.1) | |
| Female | 1825 (58.9) | 1875 (58.9) | 1869 (59.0) | |
| Fat mass index (kg/m2) | 7.2 ± 2.4 | 6.8 ± 2.5 | 6.5 ± 2.3 | <0.001 |
| Fat mass index category, n (%) | <0.001 | |||
| Fat deficit | 244 (7.9) | 356 (11.2) | 356 (11.2) | |
| Normal | 1716 (55.4) | 1841 (57.9) | 1993 (62.8) | |
| Excess fat | 1017 (32.8) | 893 (28.1) | 767 (24.2) | |
| Obese | 122 (3.9) | 91 (2.9) | 57 (1.8) | |
| Residential area, n (%) | ||||
| Lanxi rural | 348 (11.2) | 384 (12.1) | 411 (13.0) | 0.048 |
| Lanxi urban | 550 (17.8) | 628 (19.7) | 598 (18.9) | |
| WELL‐China | 2201 (71.0) | 2169 (68.2) | 2164 (68.2) | |
| Smoking status, n (%) | ||||
| Never | 2231 (72.0) | 2257 (71.0) | 2284 (72.0) | 0.417 |
| Ever | 260 (8.4) | 250 (7.9) | 247 (7.8) | |
| Current | 526 (17.0) | 604 (19.0) | 567 (17.9) | |
| Unknown | 82 (2.7) | 70 (2.2) | 75 (2.4) | |
| Drinking status, n (%) | 0.465 | |||
| Never | 1679 (54.2) | 1676 (52.7) | 1644 (51.8) | |
| Ever | 81 (2.6) | 79 (2.5) | 82 (2.6) | |
| Current | 1262 (40.7) | 1359 (42.7) | 1376 (43.4) | |
| Unknown | 77 (2.5) | 67 (2.1) | 71 (2.2) | |
| Physical activity, n (%) | <0.001 | |||
| Low | 1575 (50.8) | 1585 (49.8) | 1405 (44.3) | |
| High | 1415 (45.7) | 1507 (47.4) | 1647 (51.9) | |
| Unknown | 109 (3.5) | 89 (2.8) | 121 (3.8) | |
| Education levels, n (%) | 0.439 | |||
| Elementary | 1045 (33.7) | 1146 (36.0) | 1101 (34.7) | |
| Secondary | 1683 (54.3) | 1692 (53.2) | 1726 (54.4) | |
| Higher | 291 (9.4) | 276 (8.7) | 272 (8.6) | |
| Unknown | 80 (2.6) | 67 (2.1) | 74 (2.3) | |
| Comorbidity | ||||
| Hypertension, n (%) | 0.002 | |||
| No | 1557 (50.2) | 1695 (53.3) | 1755 (55.3) | |
| Yes | 1481 (47.8) | 1423 (44.7) | 1357 (42.8) | |
| Unknown | 61 (2.0) | 63 (2.0) | 61 (1.9) | |
| Low high‐density lipoprotein, n (%) | <0.001 | |||
| No | 2138 (69.0) | 2263 (71.1) | 2374 (74.8) | |
| Yes | 954 (30.8) | 908 (28.5) | 790 (24.9) | |
| Unknown | 7 (0.2) | 10 (0.3) | 9 (0.3) | |
| Hypertriglyceridaemia, n (%) | 0.005 | |||
| No | 2059 (66.4) | 2148 (67.5) | 2244 (70.7) | |
| Yes | 1033 (33.3) | 1023 (32.2) | 921 (29.0) | |
| Unknown | 7 (0.2) | 10 (0.3) | 8 (0.3) | |
| Diabetes, n (%) | <0.001 | |||
| No | 2486 (80.2) | 2605 (81.9) | 2723 (85.8) | |
| Yes | 524 (16.9) | 476 (15.0) | 339 (10.7) | |
| Unknown | 89 (2.9) | 100 (3.1) | 111 (3.5) |
Cross‐sectional associations of baseline flexibility with low muscle mass, low muscle strength and sarcopenia
Among the 9453 participants at baseline, 1436 had low muscle mass, 2164 had low muscle strength and 577 had sarcopenia. Figure 2 shows the prevalence of low muscle mass, low muscle strength and sarcopenia by baseline flexibility level. Compared with participants with low or middle flexibility, participants with high flexibility had a lower prevalence of low muscle mass, low muscle strength and sarcopenia.
Figure 2.
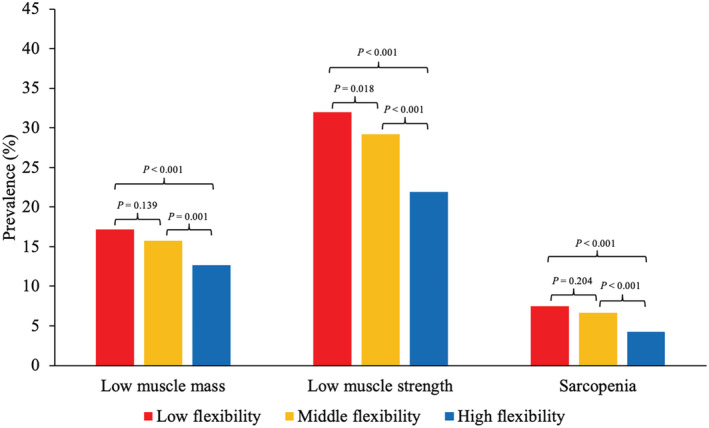
Prevalence of low muscle mass, low muscle strength and sarcopenia in participants with different categories of flexibility at baseline (n = 9453). P values were adjusted using the Benjamini‐Hochberg method.
Table 2 shows the cross‐sectional associations between flexibility and low muscle mass, low muscle strength and sarcopenia. Compared with participants with low flexibility, participants with high flexibility had a 42% decreased risk of low muscle mass, 38% decreased risk of low muscle strength and 48% decreased risk of sarcopenia (all P < 0.001). Compared with participants with low flexibility, participants with middle flexibility only had a significantly decreased risk of low muscle mass (odds ratio [OR], 0.79; 95% confidence interval [CI], 0.69–0.92; P = 0.002).
Table 2.
Cross‐sectional associations of flexibility with low muscle mass, low muscle strength and sarcopenia (n = 9453)
| Outcomes | Per SD increase in flexibility | Low flexibility | Middle flexibility | High flexibility | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Low muscle mass | 0.77 (0.73, 0.82) | <0.001 | 1.00 (reference) | / | 0.79 (0.69, 0.92) | 0.002 | 0.58 (0.50, 0.68) | <0.001 |
| Low muscle strength | 0.80 (0.77, 0.84) | <0.001 | 1.00 (reference) | / | 0.91 (0.81, 1.01) | 0.084 | 0.62 (0.55, 0.69) | <0.001 |
| Sarcopenia | 0.74 (0.67, 0.80) | <0.001 | 1.00 (reference) | / | 0.82 (0.67, 1.00) | 0.051 | 0.52 (0.41, 0.65) | <0.001 |
Abbreviations: CI, confidence interval; OR, odds ratio; SD, standard deviation.
Note: Adjusted for age groups, sex, residential area, fat mass index categories, education levels, physical activity, drinking status, smoking status, diabetes, hypertension, hypertriglyceridaemia and low high‐density lipoprotein.
Figure 3 shows the cross‐sectional associations of flexibility with low muscle mass, low muscle strength and sarcopenia in different subgroups. The inverse associations of high flexibility with low muscle mass, low muscle strength and sarcopenia remained consistent across different subgroups.
Figure 3.
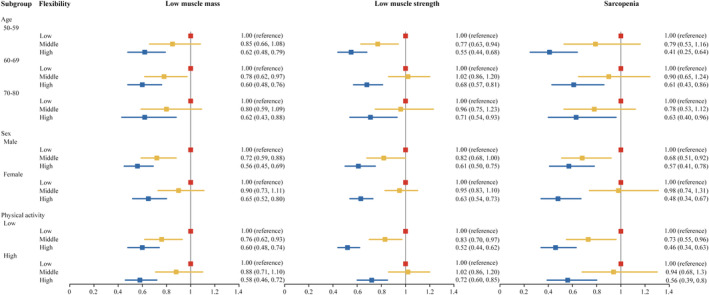
Subgroup analyses of the cross‐sectional associations of flexibility with low muscle mass, low muscle strength and sarcopenia (n = 9453). Adjusted for age groups, sex, residential area, fat mass index categories, education levels, physical activity, drinking status, smoking status, diabetes, hypertension, hypertriglyceridaemia and low high‐density lipoprotein. Continuous age was included as a covariate in the regression models when stratified analyses by age group were performed.
Associations of baseline flexibility with incident low muscle mass, low muscle strength and sarcopenia
A total of 1657 participants without sarcopenia at baseline from the Lanxi cohort (urban area) were included in the longitudinal analyses. We further excluded 424 participants without information on the development of sarcopenia, resulting in an analytic sample of 1233 participants. The baseline characteristics of participants from the Lanxi cohort (urban area) who were included or not included in the longitudinal analysis were presented in Table S2 . Except for a minor difference in age, no significant differences were observed in baseline characteristics between participants who were included and not included in the longitudinal analysis.
After 4 years, 89 participants developed low muscle mass, 364 participants developed low muscle strength and 111 participants developed sarcopenia. Table 3 shows the associations of baseline flexibility with incident low muscle mass, low muscle strength and sarcopenia. Compared with low flexibility, high flexibility was associated with decreased risks of incident low muscle strength (OR, 0.53; 95% CI, 0.38–0.74; P < 0.001) and sarcopenia (OR, 0.36; 95% CI, 0.21–0.61; P < 0.001), but not incident low muscle mass (OR, 0.59; 95% CI, 0.33–1.06; P = 0.076). Compared with low flexibility, middle flexibility was only associated with decreased risk of incident sarcopenia (OR, 0.61; 95% CI, 0.38–0.99; P = 0.046).
Table 3.
Associations of baseline flexibility with incident low muscle mass, low muscle strength and sarcopenia after 4 years
| New‐onset outcomes | Flexibility, per SD | Low flexibility | Middle flexibility | High flexibility | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Low muscle mass a | 0.83 (0.66, 1.05) | 0.128 | 1.00 (reference) | / | 0.75 (0.43, 1.31) | 0.308 | 0.59 (0.33, 1.06) | 0.076 |
| Low muscle strength b | 0.75 (0.65, 0.85) | <0.001 | 1.00 (reference) | / | 0.88 (0.63, 1.22) | 0.447 | 0.53 (0.38, 0.74) | <0.001 |
| Sarcopenia c | 0.66 (0.53, 0.81) | <0.001 | 1.00 (reference) | / | 0.61 (0.38, 0.99) | 0.046 | 0.36 (0.21, 0.61) | <0.001 |
Abbreviations: CI, confidence interval; OR, odds ratio; SD, standard deviation.
Note: Adjusted for age groups, sex, fat mass index categories, education levels, physical activity, drinking status, smoking status, diabetes, hypertension, hypertriglyceridaemia and low high‐density lipoprotein.
Participants (n = 1030) without low muscle mass at baseline and having information on the development of low muscle mass were included in the analysis.
Participants (n = 1088) without low muscle strength at baseline and having information on the development of low muscle strength were included in the analysis.
Participants (n = 1233) without sarcopenia at baseline and having information on the development of sarcopenia were included in the analysis.
Associations of changes in flexibility with incident low muscle mass, low muscle strength and sarcopenia
Table 4 shows the associations of 4‐year changes in flexibility with incident low muscle mass, low muscle strength and sarcopenia. The mean change in flexibility is −1.0 ± 6.6 cm after 4 years. Every 1‐cm increase in flexibility was associated with a 4% decrease in the risk of incident low muscle mass (P = 0.025), a 4% decrease in the risk of incident low muscle strength (P = 0.002) and a 4% decrease in the risk of incident sarcopenia (P = 0.007).
Table 4.
Associations of 4‐year changes in flexibility with incident low muscle mass, low muscle strength and sarcopenia
| New‐onset outcomes | Every 1‐cm increase in flexibility | |
|---|---|---|
| OR (95% CI) | P | |
| Low muscle mass a | 0.96 (0.93, 1.00) | 0.025 |
| Low muscle strength b | 0.96 (0.94, 0.98) | 0.002 |
| Sarcopenia c | 0.96 (0.93, 0.99) | 0.007 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Note: Adjusted for age groups, sex, fat mass index categories, education levels, physical activity, drinking status, smoking status, diabetes, hypertension, hypertriglyceridaemia, low high‐density lipoprotein and baseline body flexibility categories.
Participants (n = 992) without low muscle mass at baseline and having information on 4‐year changes in flexibility and the development of low muscle mass were included in the analysis.
Participants (n = 1054) without low muscle strength at baseline and having information on 4‐year changes in flexibility and the development of low muscle strength were included in the analysis.
Participants (n = 1191) without sarcopenia at baseline and having information on 4‐year changes in flexibility and the development of sarcopenia were included in the analysis.
Sensitivity analyses
We performed a sensitivity analysis that only participants from the urban area of the Lanxi cohort were included in the cross‐sectional analysis. We found that compared with low flexibility, high flexibility was associated with a lower risk of low muscle mass (OR, 0.49; 95% CI, 0.36–0.68; P < 0.001), low muscle strength (OR, 0.40; 95% CI, 0.28–0.55; P < 0.001) and sarcopenia (OR, 0.25; 95% CI, 0.13–0.44; P < 0.001) (Table S3 ). These results were consistent with the previous analyses, which included participants from both the Lanxi cohort and the WELL‐China cohort.
We categorized the flexibility into quartiles and reanalysed the associations of flexibility quartiles with low muscle mass, low muscle strength and sarcopenia. In cross‐sectional analyses, compared with the lowest flexibility quartile, the highest flexibility quartile was associated with low muscle mass (OR, 0.46; 95% CI, 0.38–0.55; P < 0.001), low muscle strength (OR, 0.56; 95% CI, 0.49–0.65; P < 0.001) and sarcopenia (OR, 0.43; 95% CI, 0.33–0.56; P < 0.001) (Table S4 ). In longitudinal analyses, compared with the lowest flexibility quartile, the highest flexibility quartile was associated with incident low muscle strength (OR, 0.52; 95% CI, 0.35–0.76; P = 0.001) and sarcopenia (OR, 0.35; 95% CI, 0.19–0.66; P = 0.001), but not incident low muscle mass (OR, 0.94; 95% CI, 0.47–1.93; P = 0.873) (Table S5 ). These results were consistent with previous results, which categorized flexibility into tertile.
The results of linear regression showed that each standard deviation increase in baseline flexibility was positively associated with ALM (β, 0.14; 95% CI, 0.10–0.17; P < 0.001), SMI (β, 0.05; 95% CI, 0.04–0.06; P < 0.001) and HGS (β, 0.86; 95% CI, 0.73–0.99; P < 0.001) (Table S6 ); each standard deviation increase in baseline flexibility was positively associated with 4‐year changes in ALM (β, 0.06; 95% CI, 0.02–0.09; P = 0.005), 4‐year changes in SMI (β, 0.02; 95% CI, 0.00–0.03; P = 0.013) and 4‐year changes in HGS (β, 0.34; 95% CI, 0.07–0.61; P = 0.013) (Table S7 ); every 1‐cm increase in flexibility was positively associated with 4‐year changes in HGS (β, 0.12; 95% CI, 0.08–0.16; P < 0.001), but not 4‐year changes in ALM (β, 0.00; 95% CI, 0.00–0.01; P = 0.595) and 4‐year changes in SMI (β, 0.001; 95% CI, −0.002 to 0.003; P = 0.619) (Table S8 ).
Table S9 shows the associations between baseline flexibility and 4‐year changes in METs consumed by physical activity. Compared with low flexibility, high flexibility was positively associated with 4‐year changes in METs (β, 5.70; 95% CI, 0.08–11.33; P = 0.047).
Discussion
To the best of our knowledge, this was the first study to examine the associations of flexibility and changes in flexibility with the development of low muscle mass, low muscle strength and sarcopenia. In the cross‐sectional analyses, we found that compared with low flexibility, high flexibility was inversely associated with low muscle mass, low muscle strength and sarcopenia, and these associations were consistent across different age groups, sex and physical activity levels. In the longitudinal analyses, we found that participants with high baseline flexibility had lower risk of incident low muscle strength and sarcopenia, but not incident low muscle mass. Moreover, every 1‐cm increase in flexibility during 4 years was associated with reduced risk of the development of low muscle mass, low muscle strength and sarcopenia.
Only a few studies have investigated the associations between flexibility and sarcopenia, and these studies were cross‐sectional designs. 16 , 17 , 18 Han et al. found that flexibility was positively associated with SMI in 878 individuals aged over 65. 17 Wiśniowska‐Szurlej et al. reported that flexibility was positively associated with HGS in 209 older participants aged over 65. 18 Meng et al. found that flexibility was inversely associated with sarcopenia in 857 community residents aged over 65. 16 Our cross‐sectional analyses confirmed these previous findings in a relatively large sample. We further proved that these associations were maintained in different subgroups of age, sex and physical activity level. These findings suggested that the cross‐sectional associations between flexibility and sarcopenia were not affected by age, sex and physical activity.
Furthermore, our longitudinal analyses found that participants with high baseline flexibility were less likely to have incident low muscle strength and sarcopenia than participants with low flexibility after 4 years, which suggested that the connections between flexibility and sarcopenia were likely to be causal. It highlighted the value of cross‐sectional assessment of flexibility for preventing incident low muscle strength and sarcopenia in old people. The reason why baseline flexibility was associated with incident low muscle strength but not incident low muscle mass might be that the decline rate of muscle strength was faster than the decline rate of muscle mass during the aging process. Studies reported that annualized rates of leg strength decline were about three times greater than the rates of loss of leg lean mass. 25 Our data also showed that 364 participants developed low muscle strength but only 89 participants developed low muscle mass after 4 years. These findings suggested that the effect size of risk factors on incident low muscle strength might be larger than on incident low muscle mass. In addition, the sample size of the longitudinal analyses was relatively small, and the P value for the prospective associations between baseline flexibility and low muscle mass was nearly significant (P = 0.076). These non‐significant associations might attribute to insufficient statistical power. In addition, we found that baseline flexibility was associated with 4‐year changes in SMI and HGS in the sensitivity analyses. A recent study also revealed that 2 weeks of passive stretching (with a frequency of 15 min/day for 5 days/week) prevented muscle loss in senescence‐accelerated mouse models. 26
The current results also found that increased flexibility during 4 years was associated with incident low muscle mass, low muscle strength and sarcopenia. Our findings suggested that maintaining high flexibility and monitoring the dynamic change of flexibility are both helpful in preventing sarcopenia.
The mechanisms underlying the protective effects of high flexibility against sarcopenia remain unclear. Physical inactivity might link flexibility with sarcopenia. Flexibility is necessary for moving and performing physical activities, 13 , 14 , 15 and participants with low flexibility were likely to be physically inactive. 27 , 28 Our data also supported these views. We found that participants with high flexibility were more likely to have a high level of physical activity and had less decrease in METs consumed by physical activity after 4 years than participants with low flexibility. To further determine whether the associations between flexibility and low muscle mass, low muscle strength and sarcopenia were influenced by physical activity, we performed stratified analyses by the levels of physical activity. Compared with low flexibility, high flexibility was inversely associated with low muscle mass, low muscle strength and sarcopenia in different levels of physical activity. These findings suggested that the associations between flexibility and sarcopenia were independent of the physical activity level.
Other factors such as blood flow might link flexibility to sarcopenia. Recent studies proposed that reduced blood flow with aging might contribute to the development of sarcopenia. 11 , 12 , 29 , 30 , 31 Reduced blood flow with aging impaired the delivery of oxygen and nutrients to skeletal muscle, which could accelerate the loss of muscle mass and strength. Vascular dysfunction and decreased capillarity were key factors that restrict blood flow and muscle perfusion. 11 , 12 , 30 , 31 Population‐based studies had observed that flexibility was associated with vascular dysfunction, 32 , 33 and human trials reported that increasing flexibility enhanced blood flow and capillary growth in skeletal muscle and improved vascular function. 9 , 10 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 Animal‐based studies also found that muscle stretching enhanced blood flow, improved vessel function and induced capillarization of aged skeletal muscle. 40 These findings indicated that flexibility exercises might be helpful in preventing the development of sarcopenia by improving blood supply in muscles.
In the present study, the average age of participants in the cross‐sectional study was nearly 2 years older than those in the longitudinal study. It is necessary to elucidate whether the age difference could affect the interpretation of cross‐sectional versus longitudinal results. The age‐stratified analyses in the cross‐sectional study indicated that the associations of flexibility with low muscle mass, low muscle strength and sarcopenia were consistent across age groups. We further investigated the cross‐sectional associations of flexibility with SMI and HGS only among participants who were included in the longitudinal analyses and found that the results remained similar (data not shown). Besides, age was adjusted in all regression models. These findings suggested that the associations of flexibility with muscle mass and muscle strength might be independent of age.
Evidence suggested that body fat mass had both anabolic and catabolic effects on muscle, 41 , 42 and excess adiposity depressed the anabolic action of insulin in stimulating protein synthesis, 43 which might contribute to progressive loss of muscle mass, strength and quality. Population‐based studies also observed that body fat mass was positively associated with muscle strength, and greater fat mass was associated with a greater decline in leg lean mass. 44 Considering the important role of fat in muscle mass and muscle strength, our study adjusted for the FMI category in the regression models to remove the possible influence of body fat mass on associations of flexibility with low muscle mass, low muscle strength and sarcopenia.
Implications for sarcopenia prevention and research
To the best of our knowledge, our study first found that maintaining high flexibility could reduce the risk of incident sarcopenia. Flexibility exercise is easy to perform and can be adopted by people of different ages and health statuses. Our study suggested that performing flexibility exercises such as stretching, yoga and taichi could slow the speed of muscle loss during the aging process. In addition, we found that the associations between flexibility and sarcopenia were independent of physical activity level. This finding suggested that incorporating flexibility exercise into regular exercise programmes could bring additional improvement in preventing sarcopenia. Human‐based intervention studies are further needed to determine the effectiveness of flexibility exercises in preventing sarcopenia.
Strengths
Several strengths existed in this study. First, our study explored the cross‐sectional and longitudinal associations of flexibility with low muscle mass, low muscle strength and sarcopenia. Second, the sample size of our study was large, which made our findings convincing and enabled us to conduct the stratified analysis. Furthermore, our study was strengthened by using DXA to measure muscle mass.
Limitations
Our study also had some limitations. First, the measurement of flexibility was joint specific. 8 Although the SRT was commonly used to assess flexibility, this test mainly reflected the flexibility of the low back and hamstrings. 8 We did not measure the flexibility of other regions such as the neck or upper extremities. Second, because physical performance data were unavailable, we were unable to define severe sarcopenia. Third, all participants in our study were Chinese, which might limit the generalizability of our findings. Fourth, data on socioeconomic statuses, such as employment and income status, and subjective measures of physical activity were unavailable in our study. Fifth, because DXA was unable to measure the muscle mass of the back, shoulders and lumbar regions, we did not take these components into consideration in exploring the associations between flexibility and muscle mass. Sixth, in the longitudinal analyses, we did not adjust for the changes in metabolic variables, which might influence body composition in the regression models.
Conclusions
High body flexibility was associated with reduced risk of incident low muscle strength and sarcopenia. Monitoring the dynamic change of flexibility was helpful in identifying the risk of low muscle mass, low muscle strength and sarcopenia. Body flexibility exercises and continuous measurement of flexibility might be worth recommending for older adults to prevent sarcopenia. More prospective studies with comprehensive data are needed to further explore the associations of flexibility and changes in flexibility with sarcopenia.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This work was funded by the Nutrilite Health Institute Wellness Fund, the Cyrus Tang Foundation, the China Medical Board (CMB) and the Hsun K. Chou Fund of Zhejiang University Education Foundation. The funding sources had no role in the study design, collection, analysis or interpretation of the data; the writing of the manuscript; or the decision to approve the publication of the finished manuscript.
Supporting information
Table S1. Baseline characteristics of participants
Table S2. Baseline characteristics of participants from urban area of the Lanxi cohort who were included or not included in the longitudinal analysis (n = 1,657)
Table S3. Cross‐sectional associations of flexibility with low muscle mass, low muscle strength and sarcopenia in participants from the urban area of the Lanxi cohort (n = 1,776)
Table S4. Cross‐sectional associations of flexibility quartiles with low muscle mass, low muscle strength and sarcopenia (n = 9,453)
Table S5. Associations of flexibility quartiles at baseline with incident low muscle mass, low muscle strength and sarcopenia after 4 years
Table S6. Cross‐sectional associations of flexibility with appendicular lean mass, skeletal muscle mass index and handgrip strength (n = 9,453)
Table S7. Associations of baseline flexibility with 4‐year changes in appendicular lean mass, skeletal muscle mass index and handgrip strength (n = 1,312)†
Table S8. Associations of 4‐year changes in flexibility with 4‐year changes in appendicular lean mass, skeletal muscle mass index and handgrip strength (n = 1,263)†
Table S9. Associations between baseline flexibility and 4‐year changes in metabolic equivalence tasks consumed by physical activity (n = 1,249)†
Acknowledgements
We would like to thank all participants in the WELL‐China cohort and the Lanxi cohort for their highly valued contributions. The authors of this manuscript certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle. 45
Gao P., Gan D., Li S., Kang Q., Wang X., Zheng W., Xu X., Zhao X., He W., Wu J., Lu Y., Hsing A. W., and Zhu S. (2023) Cross‐sectional and longitudinal associations between body flexibility and sarcopenia, Journal of Cachexia, Sarcopenia and Muscle, 14, 534–544, 10.1002/jcsm.13157
Peng Gao and Da Gan have contributed equally to this work and share first authorship.
References
- 1. Cruz‐Jentoft AJ, Sayer AA. Sarcopenia. Lancet 2019;393:2636–2646. [DOI] [PubMed] [Google Scholar]
- 2. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mayhew AJ, Amog K, Phillips S, Parise G, McNicholas PD, de Souza RJ, et al. The prevalence of sarcopenia in community‐dwelling older adults, an exploration of differences between studies and within definitions: a systematic review and meta‐analyses. Age Ageing 2019;48:48–56. [DOI] [PubMed] [Google Scholar]
- 4. Ageing and health. https://www.who.int/news‐room/fact‐sheets/detail/ageing‐and‐health. Accessed 12 January 2022.
- 5. Agostini F, Bernetti A, di Giacomo G, Viva MG, Paoloni M, Mangone M, et al. Rehabilitative good practices in the treatment of sarcopenia: a narrative review. Am J Phys Med Rehabil 2021;100:280–287. [DOI] [PubMed] [Google Scholar]
- 6. Eckstrom E, Neukam S, Kalin L, Wright J. Physical activity and healthy aging. Clin Geriatr Med 2020;36:671–683. [DOI] [PubMed] [Google Scholar]
- 7. Montero‐Fernández N, Serra‐Rexach JA. Role of exercise on sarcopenia in the elderly. Eur J Phys Rehabil Med 2013;49:131–143, PMID: 23575207. [PubMed] [Google Scholar]
- 8. Riebe D. ACSM's Guidelines for Exercise Testing and Prescription, 10th ed. Wolters Kluwer; 2018. [DOI] [PubMed] [Google Scholar]
- 9. Hellsten Y, Rufener N, Nielsen JJ, Høier B, Krustrup P, Bangsbo J. Passive leg movement enhances interstitial VEGF protein, endothelial cell proliferation, and eNOS mRNA content in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 2008;294:R975–R982. [DOI] [PubMed] [Google Scholar]
- 10. Hotta K, Batchelor WB, Graven J, Dahya V, Noel TE, Ghai A, et al. Daily passive muscle stretching improves flow‐mediated dilation of popliteal artery and 6‐minute walk test in elderly patients with stable symptomatic peripheral artery disease. Cardiovasc Revasc Med 2019;20:642–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dvoretskiy S, Lieblein‐Boff JC, Jonnalagadda S, Atherton PJ, Phillips BE, Pereira SL. Exploring the association between vascular dysfunction and skeletal muscle mass, strength and function in healthy adults: a systematic review. Nutrients 2020;12:715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jeon YK, Shin MJ, Saini SK, Custodero C, Aggarwal M, Anton SD, et al. Vascular dysfunction as a potential culprit of sarcopenia. Exp Gerontol 2021;145:111220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holland GJ, Tanaka K, Shigematsu R, Nakagaichi M. Flexibility and physical functions of older adults: a review. J Aging Phys Act 2002;10:169–206. [Google Scholar]
- 14. Stenholm S, Shardell M, Bandinelli S, Guralnik JM, Ferrucci L. Physiological factors contributing to mobility loss over 9 years of follow‐up—results from the InCHIANTI study. J Gerontol Ser A 2015;70:591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kell RT, Bell G, Quinney A. Musculoskeletal fitness, health outcomes and quality of life. Sports Med 2001;31:863–873. [DOI] [PubMed] [Google Scholar]
- 16. Meng N‐H, Li C‐I, Liu C‐S, Lin W‐Y, Lin C‐H, Chang C‐K, et al. Sarcopenia defined by combining height‐ and weight‐adjusted skeletal muscle indices is closely associated with poor physical performance. J Aging Phys Act 2015;23:597–606. [DOI] [PubMed] [Google Scholar]
- 17. Han D‐S, Chang K‐V, Li C‐M, Lin Y‐H, Kao T‐W, Tsai K‐S, et al. Skeletal muscle mass adjusted by height correlated better with muscular functions than that adjusted by body weight in defining sarcopenia. Sci Rep 2016;6:19457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wiśniowska‐Szurlej A, Ćwirlej‐Sozańska A, Wołoszyn N, Sozański B, Wilmowska‐Pietruszyńska A. Association between handgrip strength, mobility, leg strength, flexibility, and postural balance in older adults under long‐term care facilities. Biomed Res Int 2019;2019:1042834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Min Y, Zhao X, Stafford RS, Ma X, Chen S‐H, Gan D, et al. Cohort profile: WELL Living Laboratory in China (WELL‐China). Int J Epidemiol 2021;50:1432–1443. [DOI] [PubMed] [Google Scholar]
- 20. Wei C, Ye S, Ru Y, Gan D, Zheng W, Huang C, et al. Cohort profile: the Lanxi Cohort study on obesity and obesity‐related non‐communicable diseases in China. BMJ Open 2019;9:e025257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen L‐K, Woo J, Assantachai P, Auyeung T‐W, Chou M‐Y, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 2020;21:300–307.e2. [DOI] [PubMed] [Google Scholar]
- 22. Kelly TL, Wilson KE, Heymsfield SB. Dual energy X‐ray absorptiometry body composition reference values from NHANES. PLoS ONE 2009;4:e7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12‐country reliability and validity. Med Sci Sports Exerc 2003;35:1381–1395. [DOI] [PubMed] [Google Scholar]
- 24. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005;112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 25. Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci 2006;61:1059–1064. [DOI] [PubMed] [Google Scholar]
- 26. Wang Y, Ikeda S, Ikoma K. Passive repetitive stretching is associated with greater muscle mass and cross‐sectional area in the sarcopenic muscle. Sci Rep 2021;11:15302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Knaeps S, Bourgois JG, Charlier R, Mertens E, Lefevre J. Associations between physical activity and health‐related fitness—volume versus pattern. J Sports Sci 2017;35:539–546. [DOI] [PubMed] [Google Scholar]
- 28. Park HY, Jung WS, Kim SW, Lim K. Relationship between sarcopenia, obesity, osteoporosis, and cardiometabolic health conditions and physical activity levels in Korean older adults. Front Physiol 2021;12:706259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zempo H, Isobe M, Naito H. Link between blood flow and muscle protein metabolism in elderly adults. J Phys Fit Sports Med 2017;6:25–31. [Google Scholar]
- 30. Aminuddin A, Noor Hashim MF, Mohd Zaberi NAS, Zheng Wei L, Ching Chu B, Jamaludin NA, et al. The association between arterial stiffness and muscle indices among healthy subjects and subjects with cardiovascular risk factors: an evidence‐based review. Front Physiol 2021;12:742338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Banks NF, Rogers EM, Church DD, Ferrando AA, Jenkins NDM. The contributory role of vascular health in age‐related anabolic resistance. J Cachexia Sarcopenia Muscle 2022;13:114–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yamamoto K, Kawano H, Gando Y, Iemitsu M, Murakami H, Sanada K, et al. Poor trunk flexibility is associated with arterial stiffening. Am J Physiol Heart Circ Physiol 2009;297:H1314–H1318. [DOI] [PubMed] [Google Scholar]
- 33. Gando Y, Murakami H, Yamamoto K, Kawakami R, Ohno H, Sawada SS, et al. Greater progression of age‐related aortic stiffening in adults with poor trunk flexibility: a 5‐year longitudinal study. Front Physiol 2017;8:454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cortez‐Cooper MY, Anton MM, DeVan AE, Neidre DB, Cook JN, Tanaka H. The effects of strength training on central arterial compliance in middle‐aged and older adults. Eur J Cardiovasc Prev Rehabil 2008;15:149–155. [DOI] [PubMed] [Google Scholar]
- 35. McDaniel J, Ives SJ, Richardson RS. Human muscle length‐dependent changes in blood flow. J Appl Physiol (1985) 2012;112:560–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kruse NT, Silette CR, Scheuermann BW. Influence of passive stretch on muscle blood flow, oxygenation and central cardiovascular responses in healthy young males. Am J Physiol Heart Circ Physiol 2016;310:H1210–H1221. [DOI] [PubMed] [Google Scholar]
- 37. Kruse NT, Scheuermann BW. Cardiovascular responses to skeletal muscle stretching: “stretching” the truth or a new exercise paradigm for cardiovascular medicine? Sports Med 2017;47:2507–2520. [DOI] [PubMed] [Google Scholar]
- 38. Yamato Y, Hasegawa N, Fujie S, Ogoh S, Iemitsu M. Acute effect of stretching one leg on regional arterial stiffness in young men. Eur J Appl Physiol 2017;117:1227–1232. [DOI] [PubMed] [Google Scholar]
- 39. Yamato Y, Hasegawa N, Sato K, Hamaoka T, Ogoh S, Iemitsu M. Acute effect of static stretching exercise on arterial stiffness in healthy young adults. Am J Phys Med Rehabil 2016;95:764–770. [DOI] [PubMed] [Google Scholar]
- 40. Hotta K, Behnke BJ, Arjmandi B, Ghosh P, Chen B, Brooks R, et al. Daily muscle stretching enhances blood flow, endothelial function, capillarity, vascular volume and connectivity in aged skeletal muscle. J Physiol 2018;596:1903–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cooper R, Tomlinson D, Hamer M, Pinto Pereira SM. Lifetime body mass index and grip strength at age 46 years: the 1970 British Cohort Study. J Cachexia Sarcopenia Muscle 2022;13:1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tomlinson DJ, Erskine RM, Morse CI, Winwood K, Onambélé‐Pearson G. The impact of obesity on skeletal muscle strength and structure through adolescence to old age. Biogerontology 2016;17:467–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chevalier S, Gougeon R, Choong N, Lamarche M, Morais JA. Influence of adiposity in the blunted whole‐body protein anabolic response to insulin with aging. J Gerontol A Biol Sci Med Sci 2006;61:156–164. [DOI] [PubMed] [Google Scholar]
- 44. Koster A, Ding J, Stenholm S, Caserotti P, Houston DK, Nicklas BJ, et al. Does the amount of fat mass predict age‐related loss of lean mass, muscle strength, and muscle quality in older adults? J Gerontol A Biol Sci Med Sci 2011;66:888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics of participants
Table S2. Baseline characteristics of participants from urban area of the Lanxi cohort who were included or not included in the longitudinal analysis (n = 1,657)
Table S3. Cross‐sectional associations of flexibility with low muscle mass, low muscle strength and sarcopenia in participants from the urban area of the Lanxi cohort (n = 1,776)
Table S4. Cross‐sectional associations of flexibility quartiles with low muscle mass, low muscle strength and sarcopenia (n = 9,453)
Table S5. Associations of flexibility quartiles at baseline with incident low muscle mass, low muscle strength and sarcopenia after 4 years
Table S6. Cross‐sectional associations of flexibility with appendicular lean mass, skeletal muscle mass index and handgrip strength (n = 9,453)
Table S7. Associations of baseline flexibility with 4‐year changes in appendicular lean mass, skeletal muscle mass index and handgrip strength (n = 1,312)†
Table S8. Associations of 4‐year changes in flexibility with 4‐year changes in appendicular lean mass, skeletal muscle mass index and handgrip strength (n = 1,263)†
Table S9. Associations between baseline flexibility and 4‐year changes in metabolic equivalence tasks consumed by physical activity (n = 1,249)†


