Abstract
Background
There are several mechanisms via which increased protein intake might maintain or improve bone mineral density (BMD), but current evidence for an association or effect is inconclusive. The objectives of this study were to investigate the association between dietary protein intake (total, plant and animal) with BMD (spine and total body) and the effects of protein supplementation on BMD.
Methods
Individual data from four trials that included either (pre‐)frail, undernourished or healthy older adults (aged ≥65 years) were combined. Dietary intake was assessed with food records (2, 3 or 7 days) and BMD with dual‐energy X‐ray absorptiometry (DXA). Associations and effects were assessed by adjusted linear mixed models.
Results
A total of 1570 participants [57% women, median (inter‐quartile range): age 71 (68–75) years] for which at least total protein intake and total body BMD were known were included in cross‐sectional analyses. In fully adjusted models, total protein intake was associated with higher total body and spine BMD [beta (95% confidence interval): 0.0011 (0.0006–0.0015) and 0.0015 (0.0007–0.0023) g/cm2, respectively]. Animal protein intake was associated with higher total body and spine BMD as well [0.0011 (0.0007–0.0016) and 0.0017 (0.0010–0.0024) g/cm2, respectively]. Plant protein intake was associated with a lower total body and spine BMD [−0.0010 (−0.0020 to −0.0001) and −0.0019 (−0.0034 to −0.0004) g/cm2, respectively]. Associations were similar between sexes. Participants with a high ratio of animal to plant protein intake had higher BMD. In participants with an adequate calcium intake and sufficient serum 25(OH)D concentrations, the association between total protein intake with total body and spine BMD became stronger. Likewise, the association between animal protein intake with total body BMD was stronger. In the longitudinal analyses, 340 participants [58% women, median (inter‐quartile range): age 75 (70–81) years] were included. Interventions of 12 or 24 weeks with protein supplementation or protein supplementation combined with resistance exercise did not lead to significant improvements in BMD.
Conclusions
An association between total and animal protein intake with higher BMD was found. In contrast, plant protein intake was associated with lower BMD. Research is warranted to further investigate the added value of dietary protein alongside calcium and vitamin D for BMD improvement, especially in osteopenic or osteoporotic individuals. Moreover, more research on the impact of a plant‐based diet on bone health is needed.
Keywords: Protein, Bone, Osteoporosis, Ageing, Older adults
Introduction
Osteoporosis is a public health problem affecting the quality of life of 20 million older adults in Europe. 1 Calcium and vitamin D are well known to be key bone nutrients, and relatively high combined intakes can lead to a modest fracture risk reduction, especially in individuals with an insufficiency for these nutrients. 2 Dietary protein is also believed to play a role in combatting osteoporosis. 3 Protein intake is important for bone health via the up‐regulation of anabolic hormones, improvements in intestinal calcium absorption and maintaining muscle mass and muscle strength. 4 However, evidence from studies investigating the association between protein intake and bone mineral density (BMD) or the effects of increasing protein intake on BMD is inconsistent. 3 For example, observational studies in older adults showed a positive trend between higher protein intakes and higher femoral neck and total hip BMD, 3 although at the same time, no associations were observed between protein intake and lumbar spine BMD or total body BMD. 3 One large cohort from 1988 showed positive associations between animal protein intake with BMD at different sites, whereas plant‐based protein was found to be negatively associated with BMD. 5 However, they did not adjust for vitamin D status.
Evidence from randomized controlled trials (RCTs) in older adults regarding the effect of protein on BMD is limited. One trial investigated the effect of consuming a high‐protein drink containing 30 g of protein compared with a placebo drink with 2.1 g of protein for 2 years in healthy ambulant postmenopausal women. 6 No significant differences in hip and femoral neck BMD were found between females in the protein or placebo group after 1 and 2 years of protein supplementation. 6 In contrast, the PROVIDE study showed that 13‐week vitamin D, calcium and leucine‐enriched whey protein supplementation increased total body BMD (0.02 g/cm2; ~2%) in sarcopenic non‐malnourished older adults (intervention n = 184, control n = 196), possibly via suppression of parathyroid hormone (PTH) concentrations. 7
There are several potential mechanisms through which increased protein intake can maintain or improve BMD, but current evidence of an association or effect is inconclusive, and large trials are scarce. We therefore integrated data from four trials that included either (pre‐)frail, undernourished or healthy older adults 8 , 9 , 10 , 11 to investigate the cross‐sectional association between dietary protein and BMD and the effects of protein supplementation for 12–24 weeks on BMD. A further aim was to investigate if there were any differences between intakes of total, plant and animal protein in relation to BMD.
Methods
This study included data from participants of four previously published trials under the acronyms NU‐AGE (New dietary strategies addressing the specific needs of elderly population for healthy ageing in Europe), ProMO (Evaluating the Efficacy of a Novel Oral Supplement in Tackling Malnutrition in the Elderly), ProMuscle (Protein Supplementation and Exercise Strategy to Promote Muscle Protein Anabolism in Frail Elderly People) and PiP (ProMuscle in Practice). 8 , 9 , 10 , 11 Table 1 presents their inclusion and exclusion criteria and methods of dietary and BMD assessment. All four studies have been performed in accordance with the 1964 Declaration of Helsinki ethical standards and obtained medical and ethical approval from the South‐East 6 Person Protection Committee (France), Independent Ethics Committee of the S. Orsola‐Malpighi Hospital Bologna (Italy), the Wageningen University Medical Ethical Committee (Netherlands), the National Research Ethics Committee—East of England (UK) and the Bioethics Committee of the Polish National Food and Nutrition Institute (Poland). Informed consent of all participants was obtained prior to their inclusion in the study.
Table 1.
Overview of the four included trials
| NU‐AGE (n = 1296) | ProMO (n = 82) | ProMuscle (n = 127) | PiP (n = 168) | |
|---|---|---|---|---|
| Trial registration number a | NCT01754012 | NCT02683720 | NTR6038 | |
| Years of enrolment | 2012–2014 | 2016–2017 | 2009–2010 | 2016–2018 |
| Inclusion criteria | 65–79 years | ≥65 years; Undernutrition (MNA‐SF score <12) | ≥65 years; frail or pre‐frail according to Fried criteria | ≥65 years; frail or pre‐frail according to Fried criteria or physical inactive and experiencing difficulties in daily activities |
| Exclusion criteria | Frail according to Fried criteria; malnutrition; dementia; major chronic diseases; severe heart disease; insulin‐treated diabetes | Resistance exercise >2 h/week; life expectancy <12 months; eGFR <30 mL min−1 (1.73 m2)−1; use of diabetes medication; use of >21 alcohol units per week | Participation in resistance‐type exercise programmes in 2 years prior to study; eGFR <60 mL min−1 (1.73 m2)−1; any present form of cancer; COPD; diabetes | Recent surgery (<3 months); receiving terminal care; eGFR <30 mL min−1 (1.73 m2)−1; any present form of cancer; COPD; unregulated diabetes or hypertension |
| Dietary assessment | 7‐day food records | 2‐day food records | 3‐day food records | 3‐day food records |
| BMD assessment | DXA (Lunar Prodigy, Lunar iDXA, Discovery Wi, Discovery QDR) | DXA (Lunar Prodigy Advance) | DXA (Lunar Prodigy Advance) | DXA (Lunar Prodigy Advance) |
| Intervention | Two arms: dietary intervention based on dietary recommendations for older adults and a vitamin D supplement (10 μg/d) versus control | Two arms: supplementation with A (24 g casein, 4 μg vitamin D, 364 mg calcium), or B (11 g casein, 11 g whey, 7 g free BCAA, 11 μg vitamin D, 296 mg calcium) | Four arms: supplementation with 31 g of milk protein concentrate or placebo, with or without resistance exercise training | Two arms: increased protein intake of 25 g per main meal via conventional and enriched products in combination with resistance exercise training versus control |
| Duration | 1 year | 12 weeks | 24 weeks | 24 weeks |
| Primary outcome | C‐reactive protein | Lean body mass | Lean body mass | Short Physical Performance Battery |
BCAA, branched‐chain amino acids; BMD, bone mineral density; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; MNA‐SF, Mini Nutritional Assessment Short Form; NU‐AGE, New dietary strategies addressing the specific needs of elderly population for healthy ageing in Europe; PiP, ProMuscle in Practice; ProMO, Evaluating the Efficacy of a Novel Oral Supplement in Tackling Malnutrition in the Elderly; ProMuscle, Protein Supplementation and Exercise Strategy to Promote Muscle Protein Anabolism in Frail Elderly People.
Trial registration numbers starting with NCT are registered at clinicaltrials.gov and numbers starting with NTR are registered at the Dutch Trial Register.
NU‐AGE
The NU‐AGE trial was conducted in five European study centres (Clermont Ferrand in France, Bologna in Italy, Wageningen in the Netherlands, Warsaw in Poland and Norwich in the UK). In total, 1296 participants were included, of which n = 1245 had sufficient data to be included in the current analysis. A complete overview of the study protocol is presented in previous papers. 8 , 12 In brief, NU‐AGE included adults aged 65–79 years who were living independently, were non‐frail and non‐malnourished and were free of dementia, major chronic diseases and diabetes. The NU‐AGE trial was a 1‐year intervention with a Mediterranean‐style diet designed to meet the nutritional needs of older adults. As this intervention was not aiming at increasing protein intake, we only used the baseline data for the analyses presented in this paper.
Dietary intake was assessed via 7‐day food records. Participants were trained in describing foods, portion sizes, preparation methods and complex recipes. During home visits or university visits, the food records were discussed and checked for missing data by trained dietitians or nutritionists. Dietary intake was coded via standardized coding protocols, and nutrient values were calculated by using country‐specific food composition tables. 13 , 14 , 15 , 16 , 17 , 18 BMD was assessed via DXA [Discovery Wi; software version 2.3.1 Hologic, Inc. (Norwich, UK); Lunar iDXA; GE Health Care; enCORE 2011 software version 13.6 (Bologna, Italy); Discovery QDR, software version 3; Hologic, Inc. (Clermont‐Ferrand, France); Lunar Prodigy; GE Health Care; enCORE 2011 software version 13.6 (Wageningen, Netherlands and Warsaw, Poland)] operated by trained nurses or researchers.
ProMO
The intervention trial ProMO (n = 82) included adults aged 65 years and above who were (at risk of being) malnourished. The full protocol of the trial has been described elsewhere. 9 The trial excluded those with an expected life expectancy of <12 months, performing over 2 h/week of resistance exercise, impaired kidney function, lactose intolerance or milk protein allergy and those who used corticosteroids (unless administered via inhaler or topically) or diabetes medications.
Nutritional intake was assessed by 2‐day food records on consecutive days. Trained dietitians interviewed participants to maximize record completeness and to estimate portion sizes by using household measures. Food records were calculated into nutrients by using the Dutch Food Consumption Database 2011. 13 DXA, performed by trained research assistants, was used to assess BMD (Lunar Prodigy Advance; GE Health Care, Madison, WI).
The participants in ProMO received 12 weeks of oral nutritional supplementation from two different brands. Brand 1 delivered, per day, 600 kcal, 23 g of fat, 74 g of carbohydrates, 24 g of protein (casein), 4.4 μg vitamin D3 and 364 mg calcium. The daily nutrient content of brand 2 was 586 kcal, 23 g of fat, 65 g of carbohydrates, 22 g of protein (whey and casein 1:1), 7 g of free branched‐chain amino acids, 10.8 μg vitamin D3 and 296 mg calcium. The intervention did not contain any concurrent exercise programme, and all participants are coded as receiving a protein intervention in our analyses, regardless of the brand they received.
ProMuscle
The ProMuscle intervention included 127 frail or pre‐frail adults aged 65 years and above. 10 , 19 The trial excluded participants who were involved in resistance exercise training programmes in the 2 years prior to screening, who were diagnosed with chronic obstructive pulmonary disease (COPD), renal insufficiency, diabetes or cancer.
Dietary intake assessment was carried out via 3‐day food records (one random weekday plus both weekend days). Trained dietitians discussed food records with participants and made use of household measures to estimate portion sizes. Foods were calculated into nutrients by using the Dutch Food Consumption Database of 2006. 20 BMD was assessed by DXA (Lunar Prodigy Advance; GE Health Care, Madison, WI).
The ProMuscle intervention consisted of four arms: placebo, protein, exercise and protein + exercise. We included all participants for the cross‐sectional analyses and all participants except those admitted to solely exercise for the longitudinal analyses. The exercise intervention consisted of 24 weeks of supervised progressive resistance exercise twice per week. The protein intervention consisted of 24‐week supplementation with two 250 mL beverages, which contained (per daily dose) 30 g of protein (milk protein concentrate), 14.2 g of lactose, 1 g of fat and 800 mg calcium. Beverages were consumed after breakfast and after lunch or after resistance exercise training. The control group received matched beverages with similar calcium and lactose content but without proteins. Protein and placebo beverages were matched on appearance and taste.
PiP
The intervention trial ProMuscle in Practice, or PiP, was a practice‐based sequel to the more lab‐based ProMuscle intervention. The protocol of PiP has been published before. 21 In brief, 168 community‐dwelling older adults (aged 65 years and above) were recruited from five Dutch municipalities within the province of Gelderland. Participants were included when they were (pre‐)frail or when they experienced difficulties in daily activities combined with physical inactivity. Excluded were those with COPD, cancer, unstable diabetes, unregulated hypertension, physical impairments or cognitive impairments.
Dietary intake was assessed via 3‐day food records (three random days, of which one weekend day) for which participants received written and verbal instructions. Participants were visited by a trained research dietitian, who checked the records for completeness and used household measures to assess portion sizes. Nutrient intake was calculated by using the Dutch Food Consumption Database 2011. 13 BMD was assessed via DXA (Lunar Prodigy Advance, GE Health Care, Madison, WI), which was operated by trained research assistants.
The PiP intervention consisted of protein + exercise. The protein intervention aimed to increase protein intake during each main meal moment to at least 25 g. Based on the food record, a dietitian gave tailored advice to each participant in the intervention group. No protein supplements were used—the protein intake was increased via protein‐rich dairy products, such as yoghurt, quark and cheese. Progressive resistance exercise sessions (twice a week, 1 h per session) were supervised by physiotherapists. During the first 12 weeks of the intervention, participants were supervised intensively and received protein‐rich products for free. During the second 12 weeks, the supervision was less intense: The exercise had to be done without supervision at local gyms, and the participants did not receive free protein‐rich products and dietary advice but could attend nutritional workshops. Participants assigned to the control group received no intervention and were asked to stick to their regular diet and exercise habits.
Other measurements
Physical activity was measured differently in each study: Physical Activity Scale for Elderly (PASE) questionnaire was used in NU‐AGE, an accelerometer (ActiGraph GTX3, 2009, Pensacola, FL, USA) with data expressed in counts per minute in ProMO and ProMuscle, and total activity in min/day in PiP. In order to combine the physical activity variables, z‐scores were constructed as follows: . Smoking status was classified as never, former or current smoker, and alcohol intake was calculated in g/d. The Mini Nutritional Assessment (MNA) was used in NU‐AGE and ProMO to evaluate nutritional status. Details about fracture history were asked in PiP and NU‐AGE and fall history in NU‐AGE only. Serum 25(OH)D concentrations, assessed by liquid chromatography–mass spectrometry, were measured in all studies except PiP. Vitamin B12 concentrations were only measured in NU‐AGE by chemiluminescence.
Statistical methods
Descriptive statistics are presented as median (interquartile range) or as frequency (%). Linear mixed models were used to test for cross‐sectional associations between protein and BMD while adjusting for covariance between participants within the same study cohort in a random intercept model. Three models of increasing complexity were built to adjust for confounding factors. The first model represented the crude association between protein intake and BMD. The second model was adjusted for age, sex, physical activity level, smoking status (never, former, current) and alcohol intake. The third model additionally adjusted for calcium intake, vitamin D intake and energy intake. Models 1–3 were also performed for males and females separately. In addition, we performed a subgroup analysis in which only participants with an adequate calcium intake [>950 mg, based on EFSA Population Reference Intake (PRI) 22 ] and sufficient serum 25(OH)D concentrations (>50 nmol/L 23 ) were included. For this analysis, we applied a variant on the third model in which we omit adjustment for calcium intake and vitamin D intake. Lastly, the ratio of animal protein to plant protein intake was divided in tertiles and evaluated with the third model as well. All cross‐sectional analyses were carried out in SPSS 25 (IBM Corp., Armonk, NY, USA), and graphs and figures were created using GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA, USA). Alpha was set at 5%.
The effects of protein or protein and exercise interventions versus control were analysed by using linear mixed models. Models were built with fixed effects for time, treatment, time*treatment, sex and age. Random effects were used to model subject‐specific intercepts nested within the study these subjects participated in. Post hoc testing for differences between fixed effects was adjusted by Bonferroni correction. All longitudinal analyses were carried out in SAS 9.4 (SAS Institute, Cary, NC, USA), and graphs and figures were created using GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA, USA). Alpha was set at 5%.
Results
In total, 1570 participants were included in the analyses (Table 2 and Figure S1). Participants had a median age of 71 (IQR 68–75) years, and 56% were female. Median protein intake was 1.03 g/kg/d (IQR 0.88–1.22) The percentage of participants with a protein intake below the recommended dietary allowance (RDA) of 0.8 and below the recommendation from the ESPEN Expert Group for healthy older adults of 1.0 and 1.2 g/kg/day 24 amounted to 17, 45 and 73%, respectively. Median total body BMD was 1.10 g/cm2 (IQR 1.01–1.20), and 12% of the participants was diagnosed with osteoporosis. A calcium intake below the PRI was observed in 56% of the participants, 98% did not reach the estimated average requirement (EAR) of 10 μg vitamin D, 25 and 31% had serum 25(OH)D concentrations below the recommendation for prevention of osteoporosis in postmenopausal women (>50 nmol/L) 23 and 64% below the suggested optimal concentration for a lower fracture risk and to support the skeleton (70–80 nmol/L). 26 Characteristics of the study sample per study centre can be found in Table S1.
Table 2.
Characteristics of the total study sample
| n | Median [IQR] | Freq. (%) | |
|---|---|---|---|
| Age, year | 1569 | 71 [68–75] | |
| Women | 882 (56.2) | ||
| BMI, kg/m2 | 1530 | 26.1 [23.8–28.9] | |
| Smoking | 1565 | ||
| Never | 827 (52.8) | ||
| Former | 663 (42.4) | ||
| Current | 75 (4.8) | ||
| Alcohol intake, g/d | 1543 | 5.3 [0.2–13] | |
| Energy intake, kcal/d | 1570 | 1852 [1578–2168] | |
| Calcium intake, mg/d | 1569 | 901 [702–1153] | |
| Vitamin D intake from food, μg/d | 1408 | 2.9 [1.8–4.1] | |
| Vitamin D intake from food + supplements, μg/d | 1569 | 3.2 [2.0–4.8] | |
| Serum 25(OH)D concentrations, nmol/L | 1363 | 61.0 [45.2–78.0] | |
| Vitamin B‐12 intake, μg/d | 1366 | 4.3 [3.0–6.2] | |
| Serum vitamin B‐12 concentrations, pmol/L | 1215 | 357 [281–443] | |
| Hip fracture past 12 months | 1405 | 25 (1.8) | |
| Fracture other than hip past 12 months | 163 | 7 (4.3) | |
| One or more falls past 12 months | 1244 | 172 (12.3) | |
| Diagnosis of osteoporosis | 1401 | 172 (12.3) | |
| MNA | 1312 | 14 [12–14] | |
| Malnourished | 13 (1.0) | ||
| At risk of malnutrition | 184 (14.0) | ||
| Normal nutritional status | 1115 (85.0) | ||
| Total protein intake, g/d | 1570 | 74.5 [64.1–87.0] | |
| Total protein intake, g/kg/d | 1566 | 1.03 [0.88–1.22] | |
| Plant protein intake, g/d | 1570 | 26.5 [21.5–32.2] | |
| Animal protein intake, g/d | 1570 | 45.7 [36.1–55.3] | |
| Total body BMD, g/cm2 | 1570 | 1.10 [1.01–1.20] | |
| Spine BMD, g/cm2 | 1369 | 1.04 [0.92–1.18] |
25(OH)D, 25‐hydroxyvitamin D; BMD, bone mineral density; BMI, body mass index; IQR, interquartile range; MNA, Mini Nutritional Assessment.
Cross‐sectional
A total of 1570 participants for which at least total protein intake and total body BMD were known were included in cross‐sectional analyses (Table 3 and Figures S2 and S3). In fully adjusted models, total protein intake and animal protein intake were associated with higher BMD in the total body and spine (beta ranging from 0.0011 to 0.0017 g/cm2). In contrast, plant protein intake was associated with a lower total body and spine BMD (beta ranging from −0.0019 to −0.0010 g/cm2). Sex‐stratified fully adjusted models showed a stronger association between total protein intake and spine BMD in females (0.0022 g/cm2; 95% CI: 0.0011 to 0.0032 g/cm2) than in males (0.012 g/cm2; 95% CI: −0.00006 to 0.0025 g/cm2). However, plant protein intake had a stronger association with spine BMD in males (−0.0024 g/cm2; 95% CI: −0.0047 to −0.00015 g/cm2) compared with females (−0.0016 g/cm2; 95% CI: −0.0036 to 0.0003 g/cm2). In subgroup analysis of participants with an adequate calcium intake and sufficient serum 25(OH)D concentrations, the association between total protein intake with total body and spine BMD became stronger (Table 4). Likewise, the association between animal protein intake with total body BMD was stronger. Associations with plant protein intake became non‐significant. Furthermore, older adults with a high ratio of animal to plant protein intake had a higher total body and spine BMD compared with those with a low ratio, while total protein intake was similar between the groups (Figure S4). Lastly, results were similar to the total sample for persons with a low BMI, defined as lower than 24 kg/m2 27 (data not shown).
Table 3.
Associations between total, plant and animal protein intake with total and spine BMD a
| All | Males | Females | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure | BMD (g/cm2) | Model | B | SE | P value | B | SE | P value | B | SE | P value |
| Total protein, g/d | Total body | 1 | 0.0024 | 0.0002 | <0.001 | 0.0009 | 0.0002 | <0.001 | 0.0009 | 0.0002 | <0.001 |
| F: n = 843 | 2 | 0.0008 | 0.0002 | <0.001 | 0.0009 | 0.0002 | <0.001 | 0.0008 | 0.0002 | <0.001 | |
| M: n = 649 | 3 | 0.0011 | 0.0002 | <0.001 | 0.0009 | 0.0004 | 0.013 | 0.0014 | 0.0003 | <0.001 | |
| Spine | 1 | 0.0025 | 0.0003 | <0.001 | 0.0010 | 0.0004 | 0.013 | 0.0012 | 0.0003 | 0.001 | |
| F: n = 743 | 2 | 0.0010 | 0.0003 | <0.001 | 0.0010 | 0.0004 | 0.013 | 0.0012 | 0.0004 | 0.001 | |
| M: n = 549 | 3 | 0.0015 | 0.0004 | <0.001 | 0.0012 | 0.0006 | 0.062 | 0.0022 | 0.0005 | <0.001 | |
| Plant protein, g/d | Total body | 1 | 0.0032 | 0.0004 | <0.001 | 0.0003 | 0.0004 | 0.52 | −0.0002 | 0.0005 | 0.73 |
| F: n = 843 | 2 | −0.0002 | 0.0004 | 0.582 | 0.00002 | 0.0005 | 0.97 | −0.0004 | 0.0005 | 0.50 | |
| M: n = 649 | 3 | −0.0010 | 0.0005 | 0.026 | −0.0013 | 0.0007 | 0.047 | −0.0009 | 0.0007 | 0.18 | |
| Spine | 1 | 0.0026 | 0.0006 | <0.001 | −0.0003 | 0.0008 | 0.68 | −0.0010 | 0.0007 | 0.16 | |
| F: n = 743 | 2 | −0.0006 | 0.0006 | 0.319 | −0.0005 | 0.0009 | 0.59 | −0.0007 | 0.0008 | 0.38 | |
| M: n = 549 | 3 | −0.0019 | 0.0008 | 0.013 | −0.0024 | 0.0012 | 0.037 | −0.0016 | 0.0010 | 0.10 | |
| Animal protein, g/d | Total body | 1 | 0.0025 | 0.0002 | <0.001 | 0.0012 | 0.0003 | <0.001 | 0.0013 | 0.0003 | <0.001 |
| F: n = 843 | 2 | 0.0012 | 0.0002 | <0.001 | 0.0012 | 0.0003 | <0.001 | 0.0012 | 0.0003 | <0.001 | |
| M: n = 649 | 3 | 0.0011 | 0.0002 | <0.001 | 0.0011 | 0.0003 | 0.001 | 0.0014 | 0.0003 | <0.001 | |
| Spine | 1 | 0.0029 | 0.0003 | <0.001 | 0.0015 | 0.0005 | 0.001 | 0.0019 | 0.0004 | <0.001 | |
| F: n = 743 | 2 | 0.0016 | 0.0003 | <0.001 | 0.0016 | 0.0005 | 0.001 | 0.0018 | 0.0004 | <0.001 | |
| M: n = 549 | 3 | 0.0017 | 0.0004 | <0.001 | 0.0017 | 0.0006 | 0.005 | 0.0022 | 0.0005 | <0.001 | |
Model 1: crude association. Model 2: adjusted for age, sex, physical activity level, smoking status and alcohol intake. Model 3: additionally adjusted for calcium intake, vitamin D intake and energy intake. Presented numbers of participants are based on Model 3.
Table 4.
Associations between total, plant and animal protein intake with total and spine BMD in participants with adequate calcium intakes and sufficient 25(OH)D concentrations a
| Exposure | BMD | Model | B | SE | P value |
|---|---|---|---|---|---|
| Total protein, g/d | Total body | 4 | 0.0016 | 0.0005 | 0.001 |
| Spine | 4 | 0.0021 | 0.0008 | 0.012 | |
| Plant protein, g/d | Total body | 4 | −0.0007 | 0.0009 | 0.45 |
| Spine | 4 | 0.0010 | 0.0016 | 0.56 | |
| Animal protein, g/d | Total body | 4 | 0.0015 | 0.0004 | <0.001 |
| Spine | 4 | 0.0014 | 0.0007 | 0.049 |
n = 355 for associations with total body BMD, n = 258 for associations with spine BMD. Model 4: adjusted for age, sex, physical activity level, smoking status, alcohol intake and energy intake.
Longitudinal
In total, 340 participants were included in longitudinal analyses. Interventions of 12 or 24 weeks with protein or protein and resistance exercise did not lead to significant improvements in total body BMD (time*treatment interaction 0.66; Figure 1) or spine BMD (time*treatment interaction 0.67; Figure 2). Also within groups, post hoc contrasts did not reveal any significant changes. Notable was the 24‐week increase in total body BMD after protein plus resistance exercise (0.006 g/cm2; 95% CI: 0.001 to 0.011 g/cm2), but this contrast lost significance after Bonferroni correction.
Figure 1.
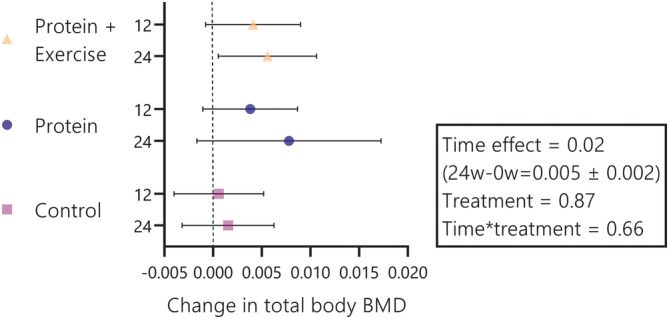
Change in total body bone mineral density (BMD) per treatment group and per time (after 12 and 24 weeks). Wicked bars represent 95% confidence intervals. The wide confidence interval at the 24‐week time point in the protein group is a consequence of lower sample size due to the 12‐week duration of the ProMO‐trial. The 24‐week change in total body BMD after protein and exercise lost significance after Bonferroni correction. For protein + exercise, protein and control, sample sizes were n = 112, n = 113 and n = 115 at week 12 and n = 111, n = 31 and n = 115 at week 24, respectively.
Figure 2.
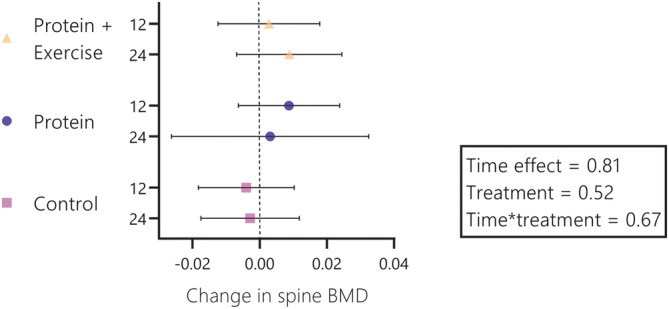
Change in spine bone mineral density (BMD) per treatment group and per time (after 12 and 24 weeks). Wicked bars represent 95% confidence intervals. The wide confidence interval at the 24‐week time point in the protein group is a consequence of lower sample‐size due to the 12‐week duration of the ProMO‐trial. For protein + exercise, protein and control, sample sizes were n = 112, n = 113 and n = 115 at week 12 and n = 111, n = 31 and n = 115 at week 24, respectively.
Sex‐stratified analyses suggest that female participants responded better to the protein + exercise treatment on both total body and spine level (Figures S5 and S6). However, no statistically significant differences were observed. After 24 weeks of protein plus resistance exercise in female participants, their total body BMD had increased by 0.010 g/cm2 (95% CI: 0.002 to 0.017 g/cm2) and their spine BMD by 0.011 g/cm2 (95% CI: −0.003 to 0.025 g/cm2).
Discussion
This study included data from (pre‐)frail, undernourished or healthy older adults 8 , 9 , 10 , 11 and showed that total and animal protein intakes were associated with higher BMD in the total body and spine. In contrast, plant protein intake was associated with a lower total body and spine BMD. We observed no significant improvements in total body or spine BMD after protein supplementation for 12–24 weeks, with or without a resistance exercise programme.
The possible explanation for our observation that only total protein and animal protein, but not plant protein, are associated with BMD is as follows. First, animal protein has a greater digestibility and a more complete amino acid profile than plant protein. 28 Second, animal foods typically contain calcium, vitamin D and/or vitamin B12, nutrients that have been linked to improved BMD. 2 , 29 Indeed, earlier studies have linked vegetarianism and veganism to increased fracture risk. 30 , 31 However, there are also reasons to argue that plant protein would lead to increased BMD. Consumption of plant protein sources typically result in a more bone‐sparing alkaline metabolic environment, which may increase BMD. 32 In addition, animal foods have typically a higher sodium chloride (NaCl) content, 33 and a high NaCl intake combined with a low calcium intake causes high calcium excretion, leading to bone resorption. 34 Previous cross‐sectional research in older adults also found positive associations between animal protein 5 , 35 , 36 and plant protein 5 , 35 with total body and spine BMD. A cohort from 1988 showed similar results in terms of direction and magnitude, for both plant and animal protein intake on spine and total body BMD. 5 On the contrary, one study observed a positive relationship between plant protein and spine BMD, 37 but only in White women and not in any other sexes or ethnicities. Our finding that older adults with a high ratio of animal to plant protein intake had higher BMD conflicts with a study from Sellmeyer et al., 38 who found that older females with a high animal–plant protein ratio had lower BMD (not significant) and had a higher rate of bone loss over the year. However, total protein intake of the participants in this study from 1986 was much lower than in our study and below the recommendation. In addition, no adjustments for vitamin D status were made.
Comparing the sexes, we found a stronger association between total protein intake and spine BMD in females, but a weaker association for plant protein intake with total body and spine BMD. The other associations were similar, suggesting that the association between protein intake and BMD is not sex specific. This was previously also found for hip fracture risk. 3
Subgroup analysis of participants with an adequate calcium intake (>950 mg) 22 and sufficient serum 25(OH)D concentrations (>50 nmol/L) 23 showed a stronger association between total protein intake with total body and spine BMD compared with all participants. Likewise, the association between animal protein intake with total body BMD was stronger. This suggests that animal protein has added value alongside calcium and vitamin D. Interestingly, the negative association between plant‐based protein intake and BMD diminished in this subgroup analysis. Although the subgroup had a slightly higher median protein intake compared with the total sample, the proportion of protein from plant‐based sources was equal (35.6% in both groups). A reason for the diminished associations could be inadequate power, as only 23% had both an adequate calcium intake and sufficient serum 25(OH)D concentrations.
Food products high in protein are generally also high in vitamin B6 and B12, which could play some role in bone health, 29 but for which allowances have not been made. However, B12 concentrations were adequate (≥150 pmol/L) in >99% of our participants. Still, further analyses using other ways to control for nutrient–nutrient correlations in the light of bone health, such as network analyses, are warranted.
The advantage of the current study is that data from different trials were pooled, which increases the power and leads to a higher generalizability of the results. On the other hand, pooling of different studies has the disadvantage that data collection is often not unified. This limitation is mitigated by pooling studies performed by the same lab, with similar equipment and standard operating procedures. Also, correlated errors between participants from the same study have been modelled via a random factor in all models. In light of BMD, cross‐sectional models do give valuable information despite their limitations regarding causality and temporal orders of exposure and outcome. BMD in older adults is affected by environmental factors, for example, diet, smoking and exercise. If dietary intake habits do not alter drastically over the life course, the cross‐sectional association between protein intake and BMD could very well represent the impact of lifelong high versus low protein intake on BMD. However, there are reasons to assume that in our cohort protein intake may have changed significantly during the life course. For instance, participants with malnutrition might have been advised to increase their intake of protein‐rich products such as dairy. Alternatively, participants with poor mouth health, decreased appetite or anosmia might have lowered their intake of meat products. Therefore, in some participants, the protein intake assessed over the 2 or 3 days will not have been a good representation of their past habits.
To address the limitations of the cross‐sectional analysis, we carried out analyses regarding changed protein intake over time. These longitudinal analyses still present limitations, as the duration of exposure (12–24 weeks) might be too short to induce changes in BMD. For the interpretation of our results, this short timeframe means that the direction of results might be more meaningful than the magnitude of the effects. We found no significant time*treatment effects in the models for total body BMD and spine BMD in the whole population and in the sex strata. Protein and exercise for 24 weeks seemed to increase total BMD in the whole population and in female participants, but in both cases, the significance of the post hoc comparison did not hold after Bonferroni correction. It is likely that a larger sample size or a longer exposure to a protein and exercise intervention would increase the magnitude of effect, but this hypothesis has to be tested in trials.
So far, well‐designed studies on the effects of protein plus exercise on BMD in older adults are scarce. In general, BMD is only reported as a secondary outcome of protein and exercise interventions. One RCT by Kemmler et al. did look into the effects of 18 months of protein and exercise on bone health in older males. 39 In their study, the intervention group received high‐intensity resistance exercise training combined with a protein intake increased by whey protein supplementation to 1.5–1.6 g/kg/d. The control group did not follow any exercise programmes, but did receive whey protein supplements to achieve a total protein intake of 1.2 g/kg/d. The authors reported significant between‐group differences in lumbar spine BMD (MD = 0.012 mg/cm2) and total hip BMD (MD = 0.013 mg/cm2) in favour of the intervention group, fuelling our hypothesis that a longer training regimen is needed to observe effects from protein plus resistance exercise interventions on BMD.
To effectively increase BMD, mechanical loading is needed. 40 In short, impact and muscle forces cause strains on bones, thereby activating osteocytes, which in turn signal osteoblasts and osteoclasts to adapt to the load. 40 The mechanical load need to be strong enough, and combined resistance and impact exercise training are suggested to be the most effective. 41 Resistance exercise programmes that are progressive in nature could therefore stimulate BMD improvement for a longer period of time, as the mechanical loading keeps increasing throughout the programmes.
In the longitudinal analyses, only total body and spine BMD were available. Total body BMD may be incapable of capturing the effects of bone‐loading activities. Exercise probably has the largest impact on femoral neck BMD, because the femoral neck is part of a weight‐bearing joint. Furthermore, cancellous bone, which is found in the spine and femoral neck, is often more responsive to stimuli than cortical bone. 42 In addition, the BMD values were already at a sufficient level at baseline (median total body BMD = 1.10 g/cm2, median spine BMD = 1.04 g/cm2). A protein and exercise intervention may have a larger impact in osteopenic or osteoporotic individuals.
In conclusion, we found an association between higher total and animal protein intake with higher total body and spine BMD. In contrast, higher plant protein intake was associated with a lower total body and spine BMD. Research is warranted to investigate further the added value of dietary protein alongside calcium and vitamin D for BMD improvement, especially in osteopenic or osteoporotic individuals. Furthermore, more research on the impact of a plant‐based diet on bone health is needed.
Conflict of interest
All authors declare no conflicts of interest.
Supporting information
Figure S1 Flow‐chart of the included data in the cross‐sectional and longitudinal analyses from four previously published trials under the acronyms NU‐AGE, ProMO, ProMuscle and PiP. 25(OH)D, 25‐hydroxyvitamin D; NU‐AGE, New dietary strategies addressing the specific needs of elderly population for healthy ageing in Europe; PiP, ProMuscle in Practice; ProMO, Evaluating the Efficacy of a Novel Oral Supplement in Tackling Malnutrition in the Elderly; ProMuscle, Protein Supplementation and Exercise Strategy to Promote Muscle Protein Anabolism in Frail Elderly People.
Figure S2 Associations between protein intake and total body bone mineral density (BMD). Markers represent betas and wicked lines represent 95% confidence intervals. All presented data stems from fully adjusted models (adjustment for age, sex, physical activity, smoking, alcohol, intake of calcium, vitamin D and energy). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Figure S3 Associations between protein intake and spine bone mineral density (BMD). Markers represent betas and wicked lines represent 95% confidence intervals. All presented data stems from fully adjusted models (adjustment for age, sex, physical activity, smoking, alcohol, intake of calcium, vitamin D and energy). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Figure S4 Mean (+SE) bone mineral density per tertile of animal protein:plant protein ratio. All presented data stems from fully adjusted models (adjustment for age, sex, physical activity, smoking, alcohol, intake of calcium, vitamin D and energy). *, P < 0.05; **, P < 0.01; ***. P < 0.001.
Figure S5 Change in total body bone mineral density (BMD) per treatment group and per time (after 12 and 24 weeks) in [A] female and [B] male participants. Wicked bars represent 95% confidence intervals.
Figure S6 Change in spine bone mineral density (BMD) per treatment group and per time (after 12 and 24 weeks) in [A] female and [B] participants. Wicked bars represent 95% confidence intervals.
Table S1. Characteristics of the study sample per study center1.
Acknowledgement
This work is funded by the Jaap Schouten Foundation, the Netherlands. The funder had no role in study design; collection, analysis and interpretation of data or writing of this article. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 43
Groenendijk I., Grootswagers P., Santoro A., Franceschi C., Bazzocchi A., Meunier N., Caille A., Malpuech‐Brugere C., Bialecka‐Debek A., Pietruszka B., Fairweather‐Tait S., Jennings A., and de Groot L. C. P. G. M. (2023) Protein intake and bone mineral density: Cross‐sectional relationship and longitudinal effects in older adults, Journal of Cachexia, Sarcopenia and Muscle, 14, 116–125, 10.1002/jcsm.13111
References
- 1. International Osteoporosis Foundation . Broken bones, broken lives: A roadmap to solve the fragility fracture crisis in Europe. 2018.
- 2. Harvey NC, Biver E, Kaufman JM, Bauer J, Branco J, Brandi ML, et al. The role of calcium supplementation in healthy musculoskeletal ageing: An expert consensus meeting of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the International Foundation for Osteoporosis (IOF). Osteoporos Int 2017;28:447–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Groenendijk I, den Boeft L, van Loon LJC, de Groot L. High versus low dietary protein intake and bone health in older adults: A systematic review and meta‐analysis. Comput Struct Biotechnol J 2019;17:1101–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonjour JP. The dietary protein, IGF‐I, skeletal health axis. Horm Mol Biol Clin Invest 2016;28:39–53. [DOI] [PubMed] [Google Scholar]
- 5. Promislow JHE, Goodman‐Gruen D, Slymen DJ, Barrett‐Connor E. Protein consumption and bone mineral density in the elderly: The Rancho Bernardo study. Am J Epidemiol 2002;155:636–644. [DOI] [PubMed] [Google Scholar]
- 6. Zhu K, Meng X, Kerr DA, Devine A, Solah V, Binns CW, et al. The effects of a two‐year randomized, controlled trial of whey protein supplementation on bone structure, IGF‐1, and urinary calcium excretion in older postmenopausal women. J Bone Miner Res 2011;26:2298–2306. [DOI] [PubMed] [Google Scholar]
- 7. Hill TR, Verlaan S, Biesheuvel E, Eastell R, Bauer JM, Bautmans I, et al. A vitamin D, calcium and leucine‐enriched whey protein nutritional supplement improves measures of bone health in sarcopenic non‐malnourished older adults: The PROVIDE study. Calcif Tissue Int 2019;105:383–391. [DOI] [PubMed] [Google Scholar]
- 8. Berendsen A, Santoro A, Pini E, Cevenini E, Ostan R, Pietruszka B, et al. Reprint of: A parallel randomized trial on the effect of a healthful diet on inflammageing and its consequences in European elderly people: Design of the NU‐AGE dietary intervention study. Mech Ageing Dev 2014;136‐137:14–21. [DOI] [PubMed] [Google Scholar]
- 9. Grootswagers P, Smeets E, Oteng A‐B, Groot L. A novel oral nutritional supplement improves gait speed and mitochondrial functioning compared to standard care in older adults with (or at risk of) undernutrition: Results from a randomized controlled trial. Aging 2021;13:9398–9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tieland M, Dirks ML, van der Zwaluw N, Verdijk LB, van de Rest O, de Groot LC, et al. Protein supplementation increases muscle mass gain during prolonged resistance‐type exercise training in frail elderly people: A randomized, double‐blind, placebo‐controlled trial. J Am Med Dir Assoc 2012;13:713–719. [DOI] [PubMed] [Google Scholar]
- 11. van Dongen EJI, Haveman‐Nies A, Doets EL, Dorhout BG, de Groot LCPGM. Effectiveness of a diet and resistance exercise intervention on muscle health in older adults: ProMuscle in practice. J Am Med Dir Assoc 2020;21:1065–72.e3. [DOI] [PubMed] [Google Scholar]
- 12. Santoro A, Pini E, Scurti M, Palmas G, Berendsen A, Brzozowska A, et al. Combating inflammaging through a Mediterranean whole diet approach: The NU‐AGE project's conceptual framework and design. Mech Ageing Dev 2014;136‐137:3–13. [DOI] [PubMed] [Google Scholar]
- 13. RIVM/Voedingscentrum . Nevo‐Tabel; Nederlands Voedingsstoffenbestand 2011. Den Haag, The Netherlands; 2011.
- 14. Food Standards Agency . McCance and Widdowson's the composition of foods. Royal Society of Chemistry Cambridge; 2002. [Google Scholar]
- 15. Tabelle INRAN di Composizione degli Alimenti Banca dati di Composizione degli Alimenti per Studi Epidemiologici in Italia. 2011.
- 16. Food composition database for epidemiological studies in Italy (Banca Dati di Composizione degli Alimenti per Studi Epidemiologici in Italia). 2011.
- 17. Kunachowicz H, Przygoda B, Nadolna I, Iwanow K. Tabele składu i wartości odżywczej żywności. Wydawnictwo Lekarskie PZWL; 2019.
- 18. ANSES . CIQUAL French food composition table; 2017.
- 19. Tieland M, van de Rest O, Dirks ML, van der Zwaluw N, Mensink M, van Loon LJ, et al. Protein supplementation improves physical performance in frail elderly people: A randomized, double‐blind, placebo‐controlled trial. J Am Med Dir Assoc 2012;13:720–726. [DOI] [PubMed] [Google Scholar]
- 20. RIVM/Voedingscentrum . Nevo‐tabel: Nederlands voedingsstoffenbestand 2006. Voedingscentrum: Den Haag, The Netherlands. 2006.
- 21. van Dongen EJI, Haveman‐Nies A, Wezenbeek NLW, Dorhout BG, Doets EL, de Groot L. Effect, process, and economic evaluation of a combined resistance exercise and diet intervention (ProMuscle in practice) for community‐dwelling older adults: Design and methods of a randomised controlled trial. BMC Public Health 2018;18:877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. EFSA Panel on Dietetic Products, Nutrition and Allergies . Scientific opinion on dietary reference values for calcium. EFSA J 2015;13:4101. [Google Scholar]
- 23. Rizzoli R, Stevenson JC, Bauer JM, van Loon LJC, Walrand S, Kanis JA, et al. The role of dietary protein and vitamin D in maintaining musculoskeletal health in postmenopausal women: A consensus statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Maturitas 2014;79:122–132. [DOI] [PubMed] [Google Scholar]
- 24. Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy‐Westphal A, et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin Nutr 2014;33:929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Health Council of the Netherlands . Voedingsnormen Voor Vitamines en Mineralen Voor Volwassenen. The Hague, The Netherlands: Health Council of the Netherlands; 2018.
- 26. Dawson‐Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int 2005;16:713–716. [DOI] [PubMed] [Google Scholar]
- 27. Estrella‐Castillo DF, Gómez‐de‐Regil L. Comparison of body mass index range criteria and their association with cognition, functioning and depression: A cross‐sectional study in Mexican older adults. BMC Geriatr 2019;19:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Berrazaga I, Micard V, Gueugneau M, Walrand S. The role of the anabolic properties of plant‐ versus animal‐based protein sources in supporting muscle mass maintenance: A critical review. Nutrients 2019;11:1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dai Z, Koh W‐P. B‐vitamins and bone health‐‐A review of the current evidence. Nutrients 2015;7:3322–3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tong TYN, Appleby PN, Armstrong MEG, Fensom GK, Knuppel A, Papier K, et al. Vegetarian and vegan diets and risks of total and site‐specific fractures: Results from the prospective EPIC‐Oxford study. BMC Med 2020;18:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iguacel I, Miguel‐Berges ML, Gómez‐Bruton A, Moreno LA, Julián C. Veganism, vegetarianism, bone mineral density, and fracture risk: A systematic review and meta‐analysis. Nutr Rev 2019;77:1–18. [DOI] [PubMed] [Google Scholar]
- 32. Han Y, An M, Yang L, Li L, Rao S, Cheng Y. Effect of acid or base interventions on bone health: A systematic review, meta‐analysis, and meta‐regression. Adv Nutr 2021;12:1540–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) , Turck D, Castenmiller J, de Henauw S, Hirsch‐Ernst K‐I, Kearney J, et al. Dietary reference values for sodium. EFSA J 2019;17:e05778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heaney RP. Role of dietary sodium in osteoporosis. J Am Coll Nutr 2006;25:271S–276S. [DOI] [PubMed] [Google Scholar]
- 35. Langsetmo L, Barr SI, Berger C, Kreiger N, Rahme E, Adachi JD, et al. Associations of protein intake and protein source with bone mineral density and fracture risk: A population‐based cohort study. J Nutr Health Aging 2015;19:861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weaver AA, Tooze JA, Cauley JA, Bauer DC, Tylavsky FA, Kritchevsky SB, et al. Effect of dietary protein intake on bone mineral density and fracture incidence in older adults in the health, aging, and body composition study. J Gerontol A Biol Sci Med Sci 2021;76:2213–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hu T, Rianon NJ, Nettleton JA, Hyder JA, He J, Steffen LM, et al. Protein intake and lumbar bone density: The multi‐ethnic study of atherosclerosis (MESA). Br J Nutr 2014;112:1384–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sellmeyer DE, Stone KL, Sebastian A, Cummings SR. A high ratio of dietary animal to vegetable protein increases the rate of bone loss and the risk of fracture in postmenopausal women. Study of Osteoporotic Fractures Research Group. Am J Clin Nutr 2001;73:118–122. [DOI] [PubMed] [Google Scholar]
- 39. Kemmler W, Kohl M, Jakob F, Engelke K, von Stengel S. Effects of high intensity dynamic resistance exercise and whey protein supplements on osteosarcopenia in older men with low bone and muscle mass. Final results of the randomized controlled FrOST study. Nutrients 2020;12:2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Florencio‐Silva R, Sasso GRS, Sasso‐Cerri E, Simões MJ, Cerri PS. Biology of bone tissue: Structure, function, and factors that influence bone cells. Biomed Res Int 2015;2015:421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kistler‐Fischbacher M, Weeks BK, Beck BR. The effect of exercise intensity on bone in postmenopausal women (part 2): A meta‐analysis. Bone 2021;143:115697. [DOI] [PubMed] [Google Scholar]
- 42. Kanis JA. Assessment of osteoporosis at the primary health‐care level. Technical Report; 2008. http://wwwshefacuk/FRAX.
- 43. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: Update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Flow‐chart of the included data in the cross‐sectional and longitudinal analyses from four previously published trials under the acronyms NU‐AGE, ProMO, ProMuscle and PiP. 25(OH)D, 25‐hydroxyvitamin D; NU‐AGE, New dietary strategies addressing the specific needs of elderly population for healthy ageing in Europe; PiP, ProMuscle in Practice; ProMO, Evaluating the Efficacy of a Novel Oral Supplement in Tackling Malnutrition in the Elderly; ProMuscle, Protein Supplementation and Exercise Strategy to Promote Muscle Protein Anabolism in Frail Elderly People.
Figure S2 Associations between protein intake and total body bone mineral density (BMD). Markers represent betas and wicked lines represent 95% confidence intervals. All presented data stems from fully adjusted models (adjustment for age, sex, physical activity, smoking, alcohol, intake of calcium, vitamin D and energy). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Figure S3 Associations between protein intake and spine bone mineral density (BMD). Markers represent betas and wicked lines represent 95% confidence intervals. All presented data stems from fully adjusted models (adjustment for age, sex, physical activity, smoking, alcohol, intake of calcium, vitamin D and energy). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Figure S4 Mean (+SE) bone mineral density per tertile of animal protein:plant protein ratio. All presented data stems from fully adjusted models (adjustment for age, sex, physical activity, smoking, alcohol, intake of calcium, vitamin D and energy). *, P < 0.05; **, P < 0.01; ***. P < 0.001.
Figure S5 Change in total body bone mineral density (BMD) per treatment group and per time (after 12 and 24 weeks) in [A] female and [B] male participants. Wicked bars represent 95% confidence intervals.
Figure S6 Change in spine bone mineral density (BMD) per treatment group and per time (after 12 and 24 weeks) in [A] female and [B] participants. Wicked bars represent 95% confidence intervals.
Table S1. Characteristics of the study sample per study center1.


