Abstract
Objectives:
People with HIV (PWH) are at increased risk for premature cardiovascular disease (CVD). Clonal hematopoiesis is a common age-related condition that may be associated with increased CVD risk. The goal of this study was to determine the prevalence of clonal hematopoiesis and its association with chronic inflammation and CVD in PWH.
Design:
Cross-sectional study utilizing archived specimens and data from 118 men (86 PWH and 32 HIV-uninfected) from the Baltimore-Washington DC center of the Multicenter AIDS Cohort Study (MACS) who had had coronary computed tomography angiography (CTA) and measurement of 34 serologic inflammatory biomarkers.
Methods:
Clonal hematopoiesis was assessed on peripheral blood mononuclear cells utilizing targeted error-corrected next generation sequencing (NGS) focused on 92 genes frequently mutated in hematologic malignancies. Clinical and laboratory data were obtained from the MACS database.
Results:
Clonal hematopoiesis with a variant allele frequency (VAF) greater than 1% was significantly more common in PWH [20/86 (23.3%)] than in HIV-uninfected men [2/32 (6.3%)] (P = 0.035). PWH with clonal hematopoiesis (VAF > 1%) were more likely to have coronary artery stenosis of at least 50% than those without clonal hematopoiesis [6/20 (30%) vs. 6/64 (9%); P = 0.021]. Presence of clonal hematopoiesis was not significantly associated with serological inflammatory markers, except for significantly lower serum leptin levels; this was not significant after adjustment for abdominal or thigh subcutaneous fat area.
Conclusion:
Clonal hematopoiesis was more common in PWH and among PWH was associated with the extent of coronary artery disease. Larger studies are needed to further examine the biological and clinical consequences of clonal hematopoiesis in PWH.
Keywords: clonal hematopoiesis, cross-sectional study, HIV, inflammation, subclinical atherosclerosis
Introduction
In the era of highly effective antiretroviral therapy (ART), HIV infection has become a chronic disease. As the life expectancy of people with HIV (PWH) continues to improve, non-AIDS-related comorbidities, such as cardiovascular disease (CVD), have become the leading causes of morbidity and mortality in this population [1]. The frequency of acute myocardial infarction (AMI) and coronary heart disease (CHD) is 1.2–2-fold higher in PWH compared with the HIV-uninfected population [2–5]. The difference remains significant in demographically and behaviorally similar populations; for example, in men who have sex with men (MSM) subclinical CVD was more frequent in PWH [6]. Likewise, the risk of CVD in virologically suppressed PWH remained significantly higher even after controlling for known sociodemographic and cardiovascular confounders [7], suggesting that other factors also contribute to this risk. Rapidly evolving knowledge regarding the mechanism of HIV-related CVD implicates immune system activation and inflammation as such a factor. Of note, chronic low-level inflammation persists in virologically suppressed PWH [8–11], and may activate monocytes, which is hypothesized to accelerate atherogenesis [12]. Although many factors, such asbacterial translocation, viral infections, low-level HIV replication, and altered balance of T-cell subsets, have been proposed to fuel chronic inflammation, the exact mechanism of this persistent immune activation in PWH remains unknown [13,14]. Additionally, the use of certain ART medications, particularly protease inhibitors, and the interaction between certain ART medications and altered immune function have been implicated in the accelerated CVD in PWH [15,16].
Somatic mutations accumulate in all tissues with time. The vast majority of these mutations are functionally inconsequential, but some affect genes critical to the fitness of hematopoietic stem cells (HSC). HSC with such mutations may gain a growth advantage over unmutated counterparts, resulting in their relative expansion. In hematopoietic tissue, this process is known as clonal hematopoiesis, which has been defined as the dominance of a clonal population of cells arising from a single HSC [17,18]. In the general population, clonal hematopoiesis is common in older people, with a prevalence exceeding 15% in individuals older than 70 years [19,20], and has been associated with adverse outcomes including a higher incidence of atherosclerosis and CVD [21,22]. This appears to be mediated, at least in part, by activation of the innate immune system and chronic inflammation [20]. Somatic mutations in the epigenetic modifiers DNMT3A and TET2 can lead to preferential monocytic differentiation of hematopoietic stem cells and altered gene expression in mature monocytes/macrophages, resulting in significantly augmented production of proinflammatory cytokines, such as IL-1β and IL-6 [21]. Although the prevalence and genetic characteristics of clonal hematopoiesis have been well characterized in the general population, data on the prevalence of clonal hematopoiesis in PWH have just started to emerge and indicate that clonal hematopoiesis is more common in PWH [23,24].
The primary aim of the current study was to determine the prevalence of clonal hematopoiesis in PWH and its association with subclinical atherosclerosis. We also aimed to determine the association between clonal hematopoiesis and inflammatory markers in PWH.
Methods
Participants
Participants were selected from the MACS, a prospective study of MSM who are HIV-infected or at risk for HIV infection. As described in detail elsewhere [25–27]. MACS participants have been followed semiannually with monitoring of HIV viral load and T-cell counts, and storage of peripheral mononuclear blood cells (PBMC) both viably and as cell pellets. The institutional review board of the Baltimore-DC MACS site approved the study protocol, and all participants provided informed consent. The study was performed in accordance with the Declaration of Helsinki.
For the current pilot study, we selected participants who had had computed tomography angiography (CTA) per MACS protocols in 2010–2013 [6]. CTA was performed on active MACS participants aged 40–70 years, weighing less than 300 lb (136 kg), and with no history of cardiac surgery or percutaneous coronary intervention (procedures which would interfere with CTA). Exclusion criteria included atrial fibrillation, chronic kidney disease [estimated glomerular filtration rate (GFR) <60 ml/min per 1.73 m2 within 1 month of CTA], and a history of allergy to intravenous contrast material. Of the 206 (110 PWH and 95 HIV-uninfected) participants at the Baltimore-Washington DC MACS site who had undergone CTA, we prioritized HIV-infected participants as our primary objective was to determine the impact of clonal hematopoiesis on subclinical coronary artery disease (CAD) in PWH. Given available funding, we selected 120 men who had stored specimens available for analysis. High-quality DNA was obtained from 118 men (86 PWH, 32 HIV-uninfected). The minimum age was 42 years in both PWH and HIV-uninfected men.
Targeted gene panel sequencing
Genomic DNA was extracted (Zymo Quick-DNA kit) from PBMC pellets or viably frozen PBMC previously collected at the MACS study visit closest to the CTA. Sequencing and sequence analysis were implemented as described [28,29], covering the 92 genes most commonly mutated in hematologic malignancies (listed in Supplementary Table 1, http://links.lww.com/QAD/C548) [30]. All reads were aligned to the human genome (GRCh37/hg19) using the Burrows–Wheeler alignment algorithm. Variants were identified using a haplotype caller and our custom variant caller pipeline with a 0.5% variant allele frequency (VAF) filter, a filter of greater than four mutant reads in both directions, and a strand bias filter [28,29]. Variants with VAF 40–60% (heterozygous calls) and more than 90% (homozygous calls) were excluded as likely germline polymorphisms. Recurrent technical artifacts were excluded using a panel of normal samples derived from clinical validation samples using the same platform.
Computed tomography angiography and assessment of coronary artery stenosis
CTA scans were generated and interpreted previously as described [31]. Briefly, participants received a beta-blocker or calcium channel blocker if needed for heart rate control just before the time of scanning, followed by sublingual nitroglycerin before administration of intravenous contrast unless contraindicated. Coronary CTA was performed with electrocardiogram-triggered protocols. Coronary CTA images were analyzed at the core CT reading center (Lundquist Institute at Harbor-UCLA) by trained, experienced readers to characterize the degree of coronary stenosis (normal, <30, 30–49, 50–69, and ≥70%) in 15 arterial segments following the modified 15-segment model of the American Heart Association [32]. A person was considered to have at least 50% stenosis if this degree of stenosis was present in any of the 15 segments analyzed. Readers were blinded to participants’ clinical information. Quantitative CT was used to measure visceral and subcutaneous adipose tissue areas as described [33].
Biomarker assays
Serum levels of 34 cytokines, chemokines, and other inflammatory markers were available from previous studies. Fifteen were previously analyzed by multiplex methods or single ELISAs at the University of Vermont from samples collected at the time of the CTA, as described [11,12,34,35]: C-reactive protein (CRP), fibrinogen, d-dimer, IL-6, adiponectin, leptin, ICAM-1, RANKL, osteoprotegerin, CCL3, TNF-αRI, TNF-αRII, sCD163, sCD14, and cystatin-c. An additional 19 had been assessed by multiplex methods at Johns Hopkins School of Public Health or University of California (Los Angeles) as described [8,9] using samples obtained at MACS study visits closest to the CTA [median days 713 (Q1 499, Q3 962)]: GM-CSF, IFNγ, IL-10, IL-12p70, IL-1β, IL-2, TNF-α, CCL11, IL-8, CXCL10, CCL13, CCL4, CCL17, BAFF, CXCL13, sCD27, sgp130, sIL-2Rα, sIL-6R.
Clinical data
Diabetes was defined as a fasting blood glucose concentration of at least 126 mg/dl (7 mmol/l), self-reported diagnosis of diabetes, or self-reported use of antidiabetic medication [36]. Hypertension was defined as either a systolic blood pressure greater than 140 mmHg, a diastolic blood pressure greater than 90 mmHg, use of antihypertensive medication, or a previous hypertension diagnosis [37]. Current ART and cumulative years of ART with specific classes of medications until the visit at which clonal hematopoiesis was assessed were classified for each study participant, using the following classes: nucleoside reverse transcriptase inhibitors (NRTI), protease inhibitors, nonnucleoside reverse transcriptase inhibitors (NNRTI), and integrase inhibitors [36].
Data analysis
Data analysis was performed using R 4.0.1 and ggplot2 [38]. Contingency tables were analyzed using the chi-square test. All continuous variables were nonnormally distributed as assessed by Shapiro–Wilk’s test and histogram visualization; thus, these variables are summarized as median [quartile 1(Q1), quartile 3 (Q3)] and were log10-transformed before linear regression analyses described below.
Significance of differences between two nonnormally distributed groups was assessed using the Mann–Whitney–Wilcoxon rank sum test. To determine if the association between HIV status and clonal hematopoiesis was independent of ethnic background and BMI, we used a multivariable logistic regression model using clonal hematopoiesis as a response variable and HIV status, ethnic background, and BMI as independent variables. To determine if the association between clonal hematopoiesis and subclinical atherosclerosis was independent of known cardiovascular risk factors, we used a multivariable logistic regression model to adjust for the ACC-AHA Pooled Cohort Equation, which incorporates several variables important for cardiovascular disease (age, total cholesterol, high-density lipoprotein cholesterol, SBP, receiving treatment for hypertension, race, diabetes, sex, and current smoking status) [39]. Cases with missing data for any of the variables used in multivariable analysis were excluded from those analyses.
To determine if the association between clonal hematopoiesis and leptin was independent of thigh and abdominal subcutaneous fat area, we used two multivariable linear regression models, which adjusted for thigh and abdominal subcutaneous subcutaneous fat area.
A P value less than 0.05 was considered statistically significant.
Results
Study participants
Characteristics of the participants at the time of angiography and assessment of clonal hematopoiesis are provided in Table 1. PWH and HIV-uninfected men did not differ significantly in age and smoking status, both known risk factors for clonal hematopoiesis. However, PWH were significantly less likely to be Caucasian and had significantly lower BMI, cholesterol levels, and prevalence of hypertension. The vast majority of PWH were receiving ART [80 (93.0%)] and 77 (89.5%) had an HIV viral load <400 copies/ml, the sensitivity of the assay used at the time. The prevalence of coronary artery stenosis of at least 50% was similar in PWH and in HIV-uninfected men [12/84 (14.3%) vs. 5/31 (16.1%), respectively; P = 0.80].
Table 1.
Demographics and laboratory characteristics of the cohort.
| Variable | Overall (n = 118) | PWH (n = 86) | HIV-uninfected (n = 32) | P value |
|---|---|---|---|---|
| Age (years), median (Q1, Q3) | 54 (50, 60) | 53 (49, 58) | 54 (51, 64) | 0.15 |
| Tobacco use | ||||
| Never | 39 (33.1%) | 29 (33.7%) | 10 (31.3%) | 0.101 |
| Former | 48 (40.7%) | 30 (34.9%) | 18 (56.3%) | |
| Current | 27 (22.9%) | 23 (26.7%) | 4 (12.5%) | |
| Unknown | 4 (3.4%) | 4 (4.7%) | 0 (0%) | |
| Race | ||||
| White, non-Hispanic | 58 (49.2%) | 37 (43.0%) | 21 (65.6%) | 0.01 |
| Black, non-Hispanic | 52 (44.1%) | 44 (51.2%) | 8 (25.0%) | |
| White, Hispanic | 4 (3.4%) | 3 (3.5%) | 1 (3.1%) | |
| Black, Hispanic | 1 (0.8%) | 1 (1.2%) | 0 (0%) | |
| American Indian or Alaskan Native | 1 (0.8%) | 0 (0%) | 1 (3.1%) | |
| Asian or Pacific Islander | 1 (0.8%) | 0 (0%) | 1 (3.1%) | |
| Other | 1 (0.8%) | 1 (1.2%) | 0 (0%) | |
| BMI (kg/m2), median (Q1, Q3) | 24.8 (22.85, 28.15) | 23.9 (22, 27.5) | 27.2 (24.4, 32.2) | <0.001 |
| Diabetes | ||||
| Yes | 12 (10.2%) | 9 (10.5%) | 3 (9.4%) | 0.89 |
| No | 97 (82.2%) | 71 (82.6%) | 26 (81.3%) | |
| Unknown | 9 (7.6%) | 6 (7.0%) | 3 (9.4%) | |
| Hypertension | ||||
| Yes | 54 (45.8%) | 34 (39.5%) | 20 (62.5%) | 0.02 |
| No | 61 (51.7%) | 50 (58.1%) | 11 (34.4%) | |
| Unknown | 3 (2.5%) | 2 (2.3%) | 1 (3.1%) | |
| HDL cholesterol (mg/dl), median (Q1, Q3) | 49.1 (40.3, 60.5) | 47.6 (38.8, 57.7) | 58.9 (46.7, 65.9) | 0.01 |
| Total cholesterol (mg/dl), median (Q1, Q3) | 184 (158, 206) | 179 (154, 203) | 196 (177, 216) | 0.03 |
| CD4+ cells/μl, median (Q1, Q3) | 585 (397, 745) | |||
| CD4+ nadir before ART, median (Q1, Q3) | 296 (163, 426) | |||
| Viral load less than 400 copies/ml | 77 (89.5%) | |||
| ART | ||||
| PI-based regimen | 41 (47.7%) | |||
| NNRTI-based regimen | 31 (36.0%) | |||
| II-based regimen | 1 (1.2%) | |||
| Other | 7 (8.1%) |
The statistically significant (P < 0.05) P values are bolded. ART, antiretroviral therapy; II, integrase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; PWH, people with HIV.
Prevalence of clonal hematopoiesis in people with HIV and HIV-negative men
In the entire study population, 105 mutations in 42 clonal hematopoiesis genes had VAFs over 0.5%: 79 with VAF between 0.5 and 1%, and 26 with VAF greater than 1% (raw data in Supplementary Figure 1, http://links.lww.com/QAD/C547). Using a VAF cutoff of 0.5%, clonal hematopoiesis was significantly more common in PWH [55/86 (64%)] than in HIV-uninfected men [12/32 (37.5%); P < 0.01] (Fig. 1a, details in Supplementary Table 2, http://links.lww.com/QAD/C548). This difference remained statistically significant when adjusting for BMI and ethnic background [OR 2.9 (1.1, 8.0), P = 0.03, Supplementary Table 3, http://links.lww.com/QAD/C548]. Using a VAF cutoff of 1%, this difference persisted [for PWH 20/86 (23.3%) and for HIV-uninfected men 2/32 (6.3%); P = 0.035; Fig. 1a]. DNMT3A was the most commonly mutated gene [in 17(19.8%) PWH and 2 (6.3%) HIV-uninfected men; P = 0.076]. TET2 mutations were observed in three (3.5%) PWH and 1 (3.1%) HIV-uninfected man (P = 0.92; Fig. 1b and Supplementary Figure 1, http://links.lww.com/QAD/C547). The distribution of less commonly mutated genes was different between the groups. For example, mutations in ARID1A, TP53, STAT3, PTPN11, KIT, and ALK were found exclusively in PWH (Fig. 1b).
Fig. 1. Prevalence of clonal hematopoiesis and the most common clonal hematopoiesis mutations in people with HIV and HIV-uninfected men.
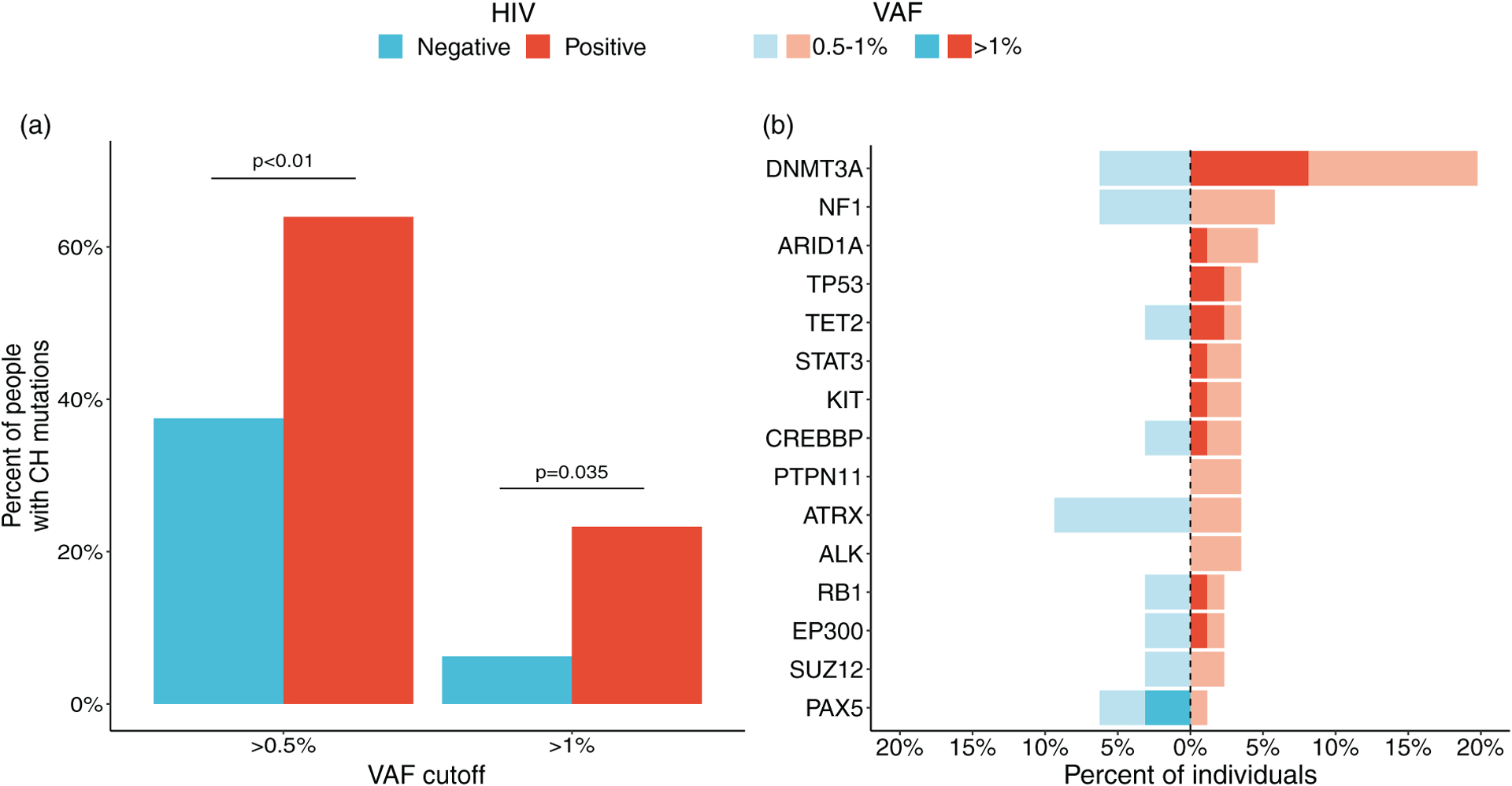
(a) Frequencies of CH with VAF cutoffs of greater than 0.5% (left) and >1% (right). The P values for differences between PWH and HIV-uninfected men are shown above the relevant bar pairs. (b) Frequencies for the most commonly mutated genes in PWH and HIV-uninfected men, expressed as the percentage of individuals tested. Only genes mutated in more than two individuals are included. Solid bars represent frequencies using a cutoff of VAF greater than 1%, and transparent bars represent frequencies using VAF 0.5–1%. CH, clonal hematopoiesis; PWH, people with HIV; VAF, variant allele frequency.
Among men with clonal hematopoiesis, numbers of mutations per participant were similar for PWH and HIV-uninfected men (Fig. 2). The size of clonal hematopoiesis clones was greater in PWH than in HIV-uninfected men [median (Q1, Q3) = 0.79% (0.56, 1.4) vs. 0.69% (0.58, 0.77), respectively] (Fig. 3), but this difference was not significant. The proportion of clonal hematopoiesis clones with VAF greater than 1% was 24/84 (28%) in PWH and 2/21 (9%) in HIV-uninfected men, a difference that was of borderline significance (P = 0.07).
Fig. 2. Numbers of clonal hematopoiesis mutations in people with HIV and HIV-uninfected men who had such mutations, at variant allele frequency cutoffs of greater than 0.5% and greater than 1%.
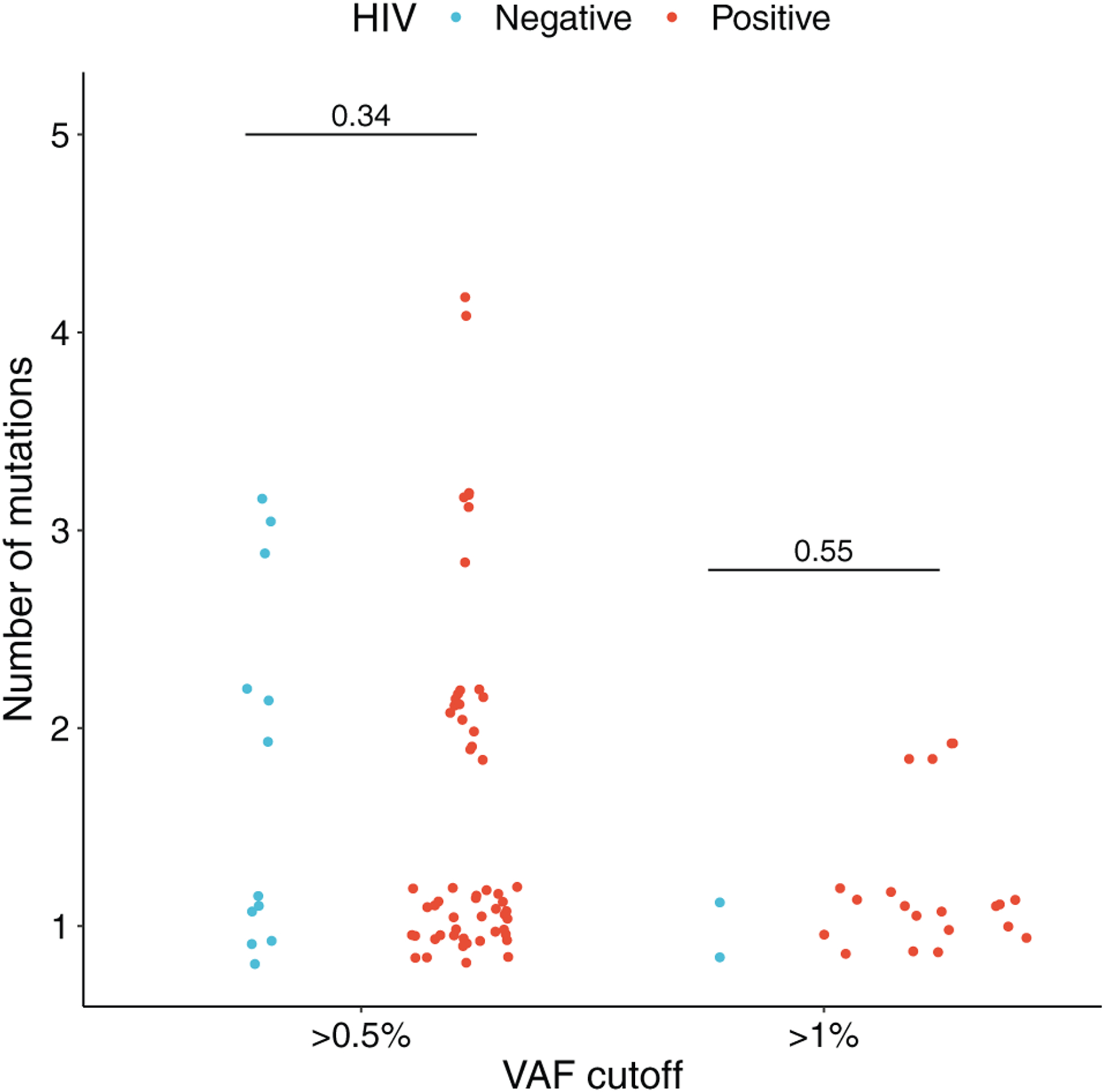
Dots represent individuals with CH. Dots are jittered for clarity. The p-values for differences between PWH (red) and HIV-uninfected men (blue) are shown above the comparisons. CH, clonal hematopoiesis; PWH, people with HIV.
Fig. 3. Variant allele frequencies of the 15 most common clonal hematopoiesis mutations observed in people with HIV and HIV-uninfected men who had clonal hematopoiesis mutations.
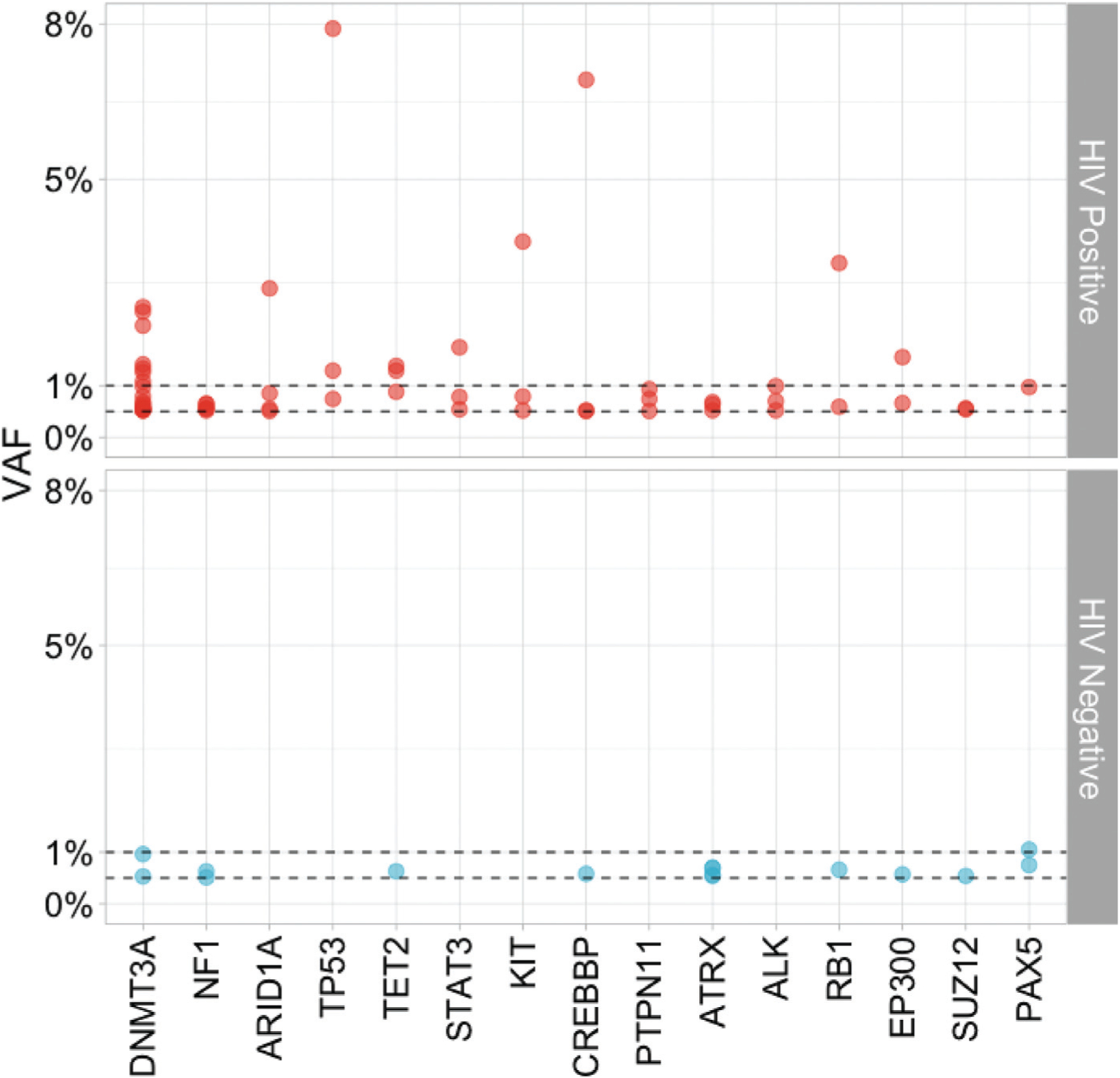
Each dot represents the VAF of a CH mutation in the indicated gene in one study participant; red dots show data from PWH and blue dots data from HIV-uninfected men. Horizontal dashed lines indicate VAFs of 1 and 0.5%. CH, clonal hematopoiesis; PWH, people with HIV; VAF, variant allele frequencies.
The association of clonal hematopoiesis with cardiovascular disease, inflammation, and antiretroviral therapy in people with HIV
In order to determine the association between clonal hematopoiesis and subclinical CVD, we focused on clonal hematopoiesis with VAF greater than 1%. This decision was based on previous reports that clinically relevant outcomes were limited to clonal hematopoiesis with larger clones [40]. The association between any degree of coronary artery stenosis with clonal hematopoiesis in PWH was of borderline significance (P = 0.06, Fig. 4a). However, a moderate-to-severe coronary artery stenosis (≥50% stenosis), which is considered clinically significant, was significantly more common in PWH with clonal hematopoiesis compared with those without clonal hematopoiesis [6/20 (30%) vs. 6/64 (9%), respectively, P = 0.021, Fig. 4b]. This difference remained significant after adjustment for the ACC-AHA Pooled Cohort Equation [odds ratio (OR) = 5.4, 95% confidence interval (CI) 1.4–22.2, P = 0.015]. The presence of clonal hematopoiesis with VAF greater than 0.5% was not significantly associated with subclinical CVD in PWH (OR = 1.1, 95% CI 0.3–4.3, P = 0.92). Also, among those with stenosis, the number of coronary arterial segments involved did not differ between PWH with clonal hematopoiesis and PWH without clonal hematopoiesis [median 2 (1, 4) vs. 2 (1, 4), respectively, P = 0.85]. As certain ARTs, particularly protease inhibitors, may contribute to increased CVD risk, we examined whether the association between clonal hematopoiesis and stenosis at least 50% was influenced by current or past use of protease inhibitors. Although the numbers were too small to be definitive, there was no evidence that the association of clonal hematopoiesis with stenosis differed by protease inhibitor use (Supplementary Table 4, http://links.lww.com/QAD/C548).
Fig. 4. Frequency of subclinical coronary artery disease in people with HIV, stratified by presence or absence of clonal hematopoiesis.
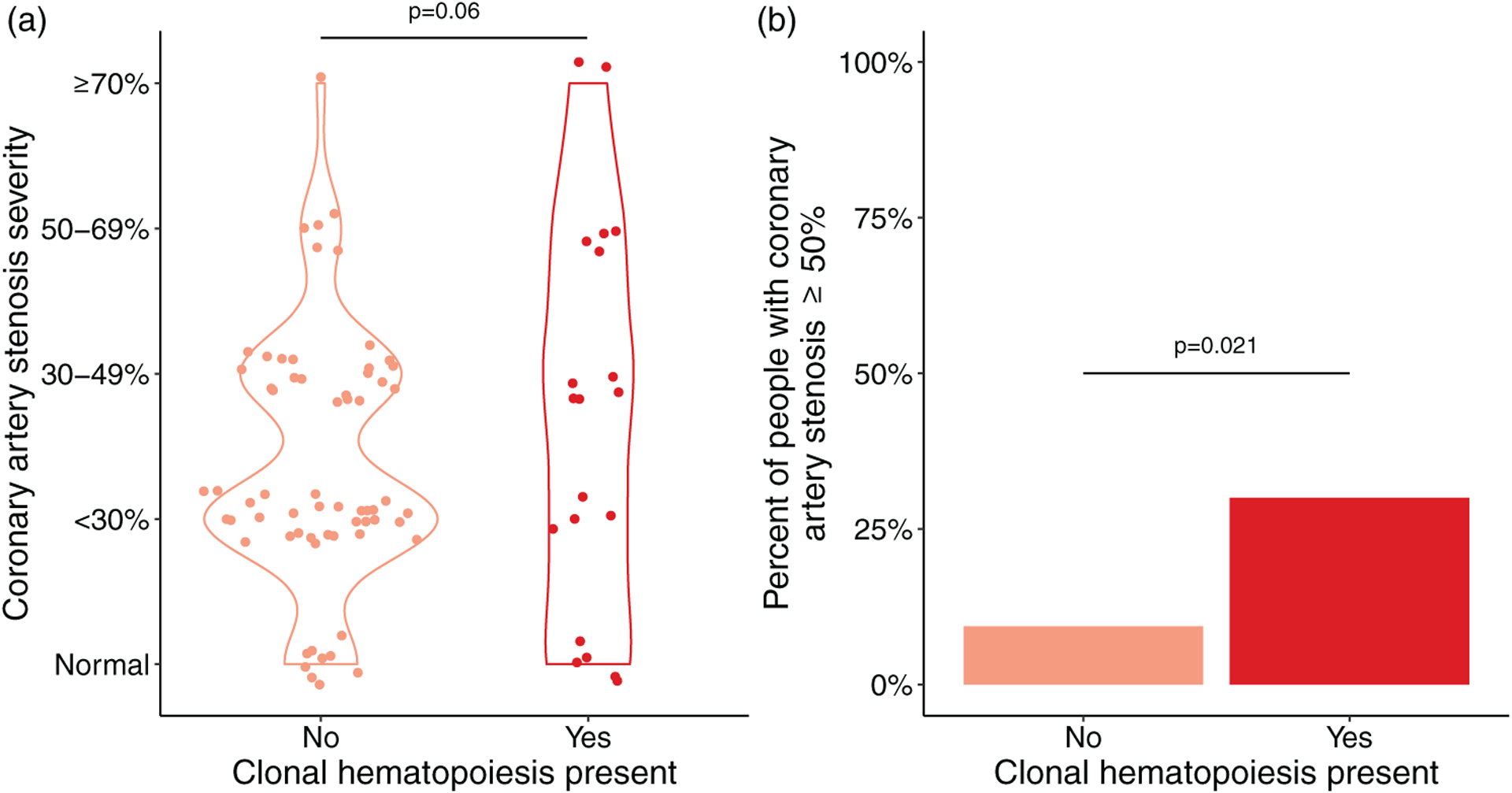
(a) Severity of CAD in PWH. Each dot represents data from one participant. Dots are jittered for clarity. (b) Bar representation of the prevalence of subclinical CAD in PWH. Subclinical CAD was defined as stenosis at least 50% in any of 15 coronary artery segments analyzed per study participant. Clonal hematopoiesis was defined as the presence of any CH mutation with a VAF greater than 1%. The P value comparing PWH with and without CH is shown above the bars. CAD, coronary artery disease; PWH, people with HIV; VAF, variant allele frequency.
As clonal hematopoiesis is a well recognized myeloid neoplasm precursor state, we examined the association between clonal hematopoiesis and various peripheral blood parameters. White blood cell count and both absolute and relative counts of neutrophils and lymphocytes did not differ significantly between men with and without clonal hematopoiesis in PWH (Supplementary Table 5, http://links.lww.com/QAD/C548).
Regarding the relationship of clonal hematopoiesis with chronic inflammation among PWH, there was no significant association between clonal hematopoiesis and serum concentrations of most of the inflammatory biomarkers studied, including cytokines implicated in the pathogenesis of CVD, such as CRP, IL-1β, IL-6. Interestingly, the presence of clonal hematopoiesis was significantly associated with lower serum leptin levels (Supplementary Table 6, http://links.lww.com/QAD/C548). However, this association was no longer significant after adjustment for abdominal or thigh subcutaneous fat area (P = 0.27 and P = 0.11, respectively).
Given the potential genotoxicity of certain ART agents, we examined the association between clonal hematopoiesis and the receipt of receiving different classes of ART (Supplementary Table 7, http://links.lww.com/QAD/C548). No significant associations were observed.
Discussion
In this study of PWH and HIV-uninfected men in the MACS, PWH had a significantly higher prevalence of clonal hematopoiesis, by a factor of nearly 4 if a VAF cutoff of 1% was used to define presence of clonal hematopoiesis, and nearly 2 if a cutoff of 0.5% was used. This finding is consistent with results from two recent studies that have examined this question [23,24]. In the first study, Bick et al. [24] found a significantly higher prevalence of clonal hematopoiesis mutations in members of the Swiss HIV Cohort Study (SHCS) than in HIV-uninfected participants in the Atherosclerosis Risk in the Communities (ARIC) study, even after accounting for demographic differences between the cohorts (7 vs. 3%, respectively, P = 0.004). In the second report, Dharan et al., studying people older than 55 years in the ARCHIVE cohort, found a higher prevalence of clonal hematopoiesis mutations among PWH than among HIV-uninfected people (28.2 vs. 16.8%, respectively; P = 0.004); both populations were predominantly, though not exclusively, MSM). It is worth noting that those studies defined clonal hematopoiesis using a VAF greater than 1% or utilized whole exome sequencing that frequently cannot detect clones with VAF less than 2%. The present study utilized an error-corrected sequencing approach that identified clones with VAF as low as 0.5%, extending the range of VAF at which clonal hematopoiesis has been found to be significantly more frequent in PWH than in HIV-uninfected men. This is consistent with previous reports that the prevalence of clonal hematopoiesis is directly proportional to the sequencing depth [40,41]. Notably, 35 of 86 (40%) PWH and 10 of 32 (31%) of HIV-uninfected men had clonal hematopoiesis with VAF between 0.5% and 1%, and thus would have not been identified in previously published studies. This is important, as certain clinical consequences, such as the propensity for myeloid malignancy, are determined not only by VAF but also by the specific genes mutated regardless of the clone size [28,40,42,43].
Interestingly, the frequency of CH with VAF greater than 1% in PWH was similar to that of Dharan et al. who used the same VAF cutoff (frequencies of 23.3 and 28.2%, respectively). However, we found a lower frequency of clonal hematopoiesis in HIV-uninfected individuals than Dharan et al. did (6.3 vs. 16.8%, respectively) [23]. This may reflect the fact that the current study population was significantly younger than that analyzed by Dharan et al. Additionally, the relatively small number of HIV-uninfected participants in the present study may have limited the precision of the observed frequency. In addition, the HIV-uninfected comparison group in the present study was composed exclusively of men who were similar to the PWH in demographics and HIV risk factors, thus providing a comparison group optimally balanced in biological, socioeconomic, and environmental risk factors as well as unknown variables associated with high-risk behaviors. For these reasons, the present study adds substantially to the evidence that HIV infection is associated with clonal hematopoiesis.
The most common clonal hematopoiesis mutations in the study by Bick et al. were ASXL1, TET2, and DNMT3A in the SHCS and DNMT3A, TET2, and ASXL1 in ARIC. In the ARCHIVE study, the most common clonal hematopoiesis mutations in both PWH and the HIV-uninfected population were the same as in the HIV-uninfected ARIC cohort in the Bick et al. study. In contrast with these studies, in which ASXL1 was among the most commonly mutated gene in PWH, this mutation was not present in either PWH or HIV-uninfected men in the present study. We also found that somatic mutations in certain genes, such as TP53 and ARIDIA, were present only in PWH, and that DNMT3A mutations had a tendency to occur more frequently in PWH than in HIV-uninfected men, a fact not observed previously [23]. These discrepancies between the current and previously published studies may be because of a relatively limited cohort and exclusive enrollment of men from a high-risk population in the current study. Moreover, our analysis included small clones with VAF greater than 0.5% but less than 1.0%, and these constituted the vast majority of the clonal hematopoiesis mutations detected. Further studies are needed to examine the association between treated HIV infection and specific clonal hematopoiesis mutations.
In the non-HIV setting, clonal hematopoiesis has been associated with increased mortality, mostly because of cardiovascular causes [19,44,45]. In one study, the risk of cardiovascular events, such as ischemic stroke and myocardial infarction, was twice as high in clonal hematopoiesis carriers compared with clonal hematopoiesis noncarriers [21]. The most commonly mutated clonal hematopoiesis genes, that is, DNMT3A and TET2, influence monocyte activation, and this is presumed to be the mechanism for their effect on CAD and aging [21,22]. Other commonly mutated clonal hematopoiesis genes, such as ASXL1 and PPMID, also influence chronic inflammation, but the exact mechanism by which they may contribute to development of CAD remains to be determined [46,47]. In the present study, PWH who had clonal hematopoiesis had a significantly higher prevalence of moderate/severe coronary artery stenosis than those who did not, and this remained significant after adjusting for the ACC-AHA Pooled Cohort Equation. This finding is consistent with the report by Bick et al. [24], who found a trend toward greater coronary artery disease in those with clonal hematopoiesis, though details on how this was assessed were not provided, and may explain, at least partially, the higher incidence of CVD in PWH even after adjusting for traditional cardiovascular risk factors. The exact contribution of individual mutations to this risk could not be established in this study because of the limited number of PWH with specific clonal hematopoiesis mutations.
In animal models, clonal hematopoiesis was demonstrated to accelerate atherosclerosis mostly thorough activation of IL-1β and IL-6 signaling [21,22]. In people with clonal hematopoiesis, both pharmacological inhibition of IL-1β and genetic deficiency in IL-6 signaling attenuated CVD [48,49], and clonal hematopoiesis was also associated with higher serum concentration of hs-CRP [50]. Additionally, in PWH, elevated circulating levels of sCD14 and sCD163 have been associated with CVD, rupture-prone plaques, and vascular inflammation [51–53]. In the present study, although PWH had significantly higher levels of sCD14 and sCD163 than HIV-negative men (data not shown), PWH with and without clonal hematopoiesis had similar levels of these markers. Reasons why these markers did not differ by clonal hematopoiesis, when they have been reported to differ by CAD, are unclear, but this finding could suggest that other mediators are involved in the development of CAD.
In the present study, PWH with and without clonal hematopoiesis did not differ significantly in serum levels of 34 cytokines and other inflammatory markers, except that leptin concentrations were significantly lower in PWH with clonal hematopoiesis. The mechanism of this association is unclear and deserves further study. It is noteworthy that the significance of this association was abrogated after adjusting for amount of fat in abdomen or thigh. Leptin signaling has been shown to play a significant role in myelopoiesis, lymphopoiesis, and HSC function in mice [54–56]. In humans, low leptin levels have been found in HIV lipodystrophy syndrome [45].
Van der Heijden et al. [57] recently reported that clonal hematopoiesis was associated with low CD4+ cell nadir, increased viral transcriptional activity, and elevated levels of d-dimer and von Willebrand factor (vWF). In the current study, CD4+ cell nadir was lower in PWH with clonal hematopoiesis than those without, but the difference was not significant (Table 1). The level of D-dimer did not differ between these two groups (Supplementary Table 6, http://links.lww.com/QAD/C548). Neither viral transcription nor vWF was measured in our study. There are several possible explanations for the lack of significant association between clonal hematopoiesis and CD4+ cell nadir and D-dimer in the present study, as opposed to the observations by Van der Heijden et al.: those authors used a targeted 24-gene assay, rather than the broader, 92 genes evaluated in the present study; those authors used a VAF cut-off as low as 0.02% to define clonal hematopoiesis, whereas we used cutoffs of 0.5 and 1%, so different populations of mutations were compared; if very low VAFs of clonal hematopoiesis mutations are associated with coagulation and CD4+ cell nadir, our results may have been biased toward the null. In addition, our study was somewhat smaller, and thus may have had lower power to find such associations.
The cause of clonal hematopoiesis in PWH is not entirely clear. In the general population, clonal hematopoiesis is significantly more prevalent in older individuals [19,20]. In addition to random acquisition of somatic mutations during HSC aging [58], increased prevalence of clonal hematopoiesis has been attributed to smoking, ionizing radiation, genotoxic agents, infection, and inflammation [59–62]. HIV infection could promote clonal hematopoiesis in several ways, including by the chronic inflammation associated with virologically suppressed HIV infection [63,64], or indirectly as a result of immunodeficiency [65]. Reactivation of cytomegalovirus infection [66] and microbial translocation in the gastrointestinal tract [67] can further potentiate chronic inflammation and promote expansion of mutated hematopoietic clones [61]. It is possible that the chronic inflammatory environment in PWH might cause and/or select for hematopoietic stem cell clones harboring certain advantageous mutations. Another potential mechanism could be direct or indirect genotoxicity of ART [68–71]. Mutations in DNA-damage response pathway genes, for example, TP53, though rare in otherwise healthy individuals, may provide growth advantage in the setting of anticancer therapies [62]. It is also possible that ART may further select for clones that are inherently resistant to cytotoxic therapies, such as those carrying TP53 mutations. This may partially explain why PWH are at higher risk for myeloid malignancies [72]. In the current study, cumulative exposure to ART was not significantly associated with clonal hematopoiesis in PWH, but the study may not have been sufficiently powered to detect such an association, as discussed above.
A major strength of the present study is the analysis of both PWH and HIV-uninfected men from the MACS cohort, thus minimizing the potential effect of unmeasured risk factors for clonal hematopoiesis to which MSM are exposed. Moreover, using an error-corrected approach, we were able to determine the prevalence of clonal hematopoiesis with VAF greater than 0.5%. Limitations of the current study include a relatively modest number of subjects studied and the cross-sectional study design, as well as the significant difference in ethnic background between PWH and HIV-negative men. The data did not show an effect of ethnicity on clonal hematopoiesis but did not rule out such an effect. Additional limitations are that ART received by men in this study did not reflect modern ART regimens, and this study did not include women.
This study is the first report of frequency of clonal hematopoiesis in PWH and HIV-uninfected MSM in the United States. Even though we observed a higher prevalence of clonal hematopoiesis in PWH and an association between clonal hematopoiesis and subclinical atherosclerosis, we recognize that analyses of well-characterized large prospective cohorts are needed to better define the incidence, biology and clinical consequences of clonal hematopoiesis in PWH. The results from such studies will provide a more precise CVD risk stratification and may lay the foundation for new preventive strategies in PWH.
Supplementary Material
Acknowledgements
The study was supported by the National Institutes of Health (K08HL136894, R01HL156144, R01 HL095129, P30CA006973, P30AI094189, U01AI035 042, UM1AI035043, UM1AI068613, U01HL146201), the Center for AIDS Research Career Development Award, and the Johns Hopkins Institute for Clinical and Translational Research (ICTR), which is funded in part by Grant Number UL1 TR003098 from the National Center for Advancing Translational Sciences (NCATS). We also thank SHARE staff for accessing data and men participating in the SHARE study for their contributions to the study.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lazarus JV, Safreed-Harmon K, Kamarulzaman A, Anderson J, Leite RB, Behrens G, et al. Consensus statement on the role of health systems in advancing the long-term well being of people living with HIV. Nat Commun 2021; 12:4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Currier JS, Taylor A, Boyd F, Dezii CM, Kawabata H, Burtcel B, et al. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr 19992003; 33: 506–512. [DOI] [PubMed] [Google Scholar]
- 3.Durand M, Sheehy O, Baril J-G, Lelorier J, Tremblay CL. Association Between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: a cohort and nested case–control study using Québec’s Public Health Insurance Database. JAIDS J Acquir Immune Defic Syndr 2011; 57:245–253. [DOI] [PubMed] [Google Scholar]
- 4.Lang S, Mary-Krause M, Cotte L, Gilquin J, Partisani M, Simon A, et al. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS 2010; 24:1228–1230. [DOI] [PubMed] [Google Scholar]
- 5.Obel N, Thomsen HF, Kronborg G, Larsen CS, Hildebrandt PR, Sørensen HT, et al. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: a population-based cohort study. Clin Infect Dis 2007; 44:1625–1631. [DOI] [PubMed] [Google Scholar]
- 6.Post WS, Budoff M, Kingsley L, Palella FJ, Witt MD, Li X, et al. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med 2014; 160:458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freiberg MS, Chang C-CH, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013; 173:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wada NI, Bream JH, Martínez-Maza O, Macatangay B, Galvin SR, Margolick JB, et al. Inflammatory biomarkers and mortality risk among HIV-suppressed men: a multisite prospective cohort study. Clin Infect Dis 2016; 63:984–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wada NI, Jacobson LP, Margolick JB, Breen EC, Macatangay B, Penugonda S, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015; 29:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castillo-Mancilla JR, Brown TT, Erlandson KM, Palella FJ, Gardner EM, Macatangay BJC, et al. Suboptimal adherence to combination antiretroviral therapy is associated with higher levels of inflammation despite HIV suppression. Clin Infect Dis 2016; 63:1661–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bahrami H, Budoff M, Haberlen SA, Rezaeian P, Ketlogetswe K, Tracy R, et al. Inflammatory markers associated with subclinical coronary artery disease: the Multicenter AIDS Cohort Study. JAHA 2016; 5:e003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKibben RA, Margolick JB, Grinspoon S, Li X, Palella FJ, Kingsley LA, et al. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. J Infect Dis 2015; 211:1219–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klatt NR, Chomont N, Douek DC, Deeks SG. Immune activation and HIV persistence: implications for curative approaches to HIV infection. Immunol Rev 2013; 254:326–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paiardini M, Müller-Trutwin M. HIV-associated chronic immune activation. Immunol Rev 2013; 254:78–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvi RM, Neilan AM, Tariq N, Awadalla M, Afshar M, Banerji D, et al. Protease inhibitors and cardiovascular outcomes in patients with HIV and heart failure. J Am Coll Cardiol 2018; 72:518–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laurence J, Elhadad S, Ahamed J. HIV-associated cardiovascular disease: importance of platelet activation and cardiac fibrosis in the setting of specific antiretroviral therapies. Open Heart 2018; 5:e000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gondek LP, DeZern AE. Assessing clonal haematopoiesis: clinical burdens and benefits of diagnosing myelodysplastic syndrome precursor states. Lancet Haematol 2020; 7:e73–e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasca S, Gondek LP. Clonal hematopoiesis and bone marrow failure syndromes. Best Pract Res Clin Haematol 2021;34:101273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. New Engl J Med 2014; 371:2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frick M, Chan W, Arends CM, Hablesreiter R, Halik A, Heuser M, et al. Role of donor clonal hematopoiesis in allogeneic hematopoietic stem-cell transplantation. J Clin Oncol 2019; 37:375–385. [DOI] [PubMed] [Google Scholar]
- 21.Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. New Engl J Med 2017; 377:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 2017; 355:842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dharan NJ, Yeh P, Bloch M, Yeung MM, Baker D, Guinto J, et al. HIV is associated with an increased risk of age-related clonal hematopoiesis among older adults. Nat Med 2021;27:1006–1011. [DOI] [PubMed] [Google Scholar]
- 24.Bick AG, Popadin K, Thorball CW, Uddin MM, Zanni M, Yu B, et al. Increased prevalence of clonal hematopoiesis of indeterminate potential amongst people living with HIV. Sci Rep 2020; 12:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol 1987; 126:310–318. [DOI] [PubMed] [Google Scholar]
- 26.Kaslow RA, Phair JP, Friedman HB, Lyter D, Solomon RE, Dudley J, et al. Infection with the human immunodeficiency virus: clinical manifestations and their relationship to immune deficiency. A report from the Multicenter AIDS Cohort Study. Ann Intern Med 1987; 107:474–480. [DOI] [PubMed] [Google Scholar]
- 27.Kingsley LA, Detels R, Kaslow R, Polk BF, Rinaldo CR, Chmiel J, et al. Risk factors for seroconversion to human immunodeficiency virus among male homosexuals. Results from the Multicenter AIDS Cohort Study. Lancet 1987; 1:345–349. [DOI] [PubMed] [Google Scholar]
- 28.Gondek LP, Zheng G, Ghiaur G, DeZern AE, Matsui W, Yegnasubramanian S, et al. Donor cell leukemia arising from clonal hematopoiesis after bone marrow transplantation. Leukemia 2016; 30:1916–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng G, Chen P, Pallavajjalla A, Haley L, Gondek L, Dezern A, et al. The diagnostic utility of targeted gene panel sequencing in discriminating etiologies of cytopenia. Am J Hematol 2019; 94:1141–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tate JG, Bamford S, Jubb HC, Sondka Z, Beare DM, Bindal N, et al. COSMIC: the Catalogue Of Somatic Mutations In Cancer. Nucleic Acids Res 2019; 47:D941–D947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hacøoğlu Y, Gupta M, Choi TY, George RT, Deible CR, Jacobson LP, et al. Use of cardiac CT angiography imaging in an epidemiology study - the Methodology of the Multicenter AIDS Cohort Study cardiovascular disease substudy. Anadolu Kardiyol Derg 2013; 13:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 1975; 51:5–40. [DOI] [PubMed] [Google Scholar]
- 33.Brown TT, Xu X, John M, Singh J, Kingsley LA, Palella FJ, et al. Fat distribution and longitudinal anthropometric changes in HIV-infected men with and without clinical evidence of lipodystrophy and HIV-uninfected controls: a substudy of the Multicenter AIDS Cohort Study. AIDS Res Ther 2009; 6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ketlogetswe KS, Post WS, Li X, Palella FJ, Jacobson LP, Margolick JB, et al. Lower adiponectin is associated with subclinical cardiovascular disease among HIV-infected men. AIDS 2014; 28:901–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ketlogetswe KS, McKibben R, Jacobson LP, Li X, Dobs AS, Budoff M, et al. Osteoprotegerin, but not receptor activator for nuclear factor-κB ligand, is associated with subclinical coronary atherosclerosis in HIV-infected men. J Acquir Immune Defic Syndr 2015; 70:362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med 2005; 165:1179–1184. [DOI] [PubMed] [Google Scholar]
- 37.Seaberg EC, Muñoz A, Lu M, Detels R, Margolick JB, Riddler SA, et al. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS 2005; 19:953–960. [DOI] [PubMed] [Google Scholar]
- 38.Wickham H ggplot2: elegant graphics for data analysis. 2nd ed. Cham: Springer International Publishing: Imprint: Springer; 2016. [Google Scholar]
- 39.Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129:S49–73. [DOI] [PubMed] [Google Scholar]
- 40.Gibson CJ, Kim HT, Zhao L, Murdock HM, Hambley B, Ogata A, et al. Donor clonal hematopoiesis and recipient outcomes after transplantation. JCO 2021; 40:189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Young AL, Challen GA, Birmann BM, Druley TE. Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat Commun 2016; 7:12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abelson S, Collord G, Ng SWK, Weissbrod O, Mendelson Cohen N, Niemeyer E, et al. Prediction of acute myeloid leukaemia risk in healthy individuals. Nature 2018; 559:400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desai P, Mencia-Trinchant N, Savenkov O, Simon MS, Cheang G, Lee S, et al. Somatic mutations precede acute myeloid leukemia years before diagnosis. Nat Med 2018; 24:1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Genovese G, Kähler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, et al. Clonal Hematopoiesis and Blood-Cancer Risk Inferred from Blood DNA Sequence. N Engl J Med 2014; 371:2477–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biernaux C, Loos M, Sels A, Huez G, Stryckmans P. Detection of major bcr-abl gene expression at a very low level in blood cells of some healthy individuals. Blood 1995; 86:3118–3122. [PubMed] [Google Scholar]
- 46.Yura Y, Miura-Yura E, Katanasaka Y, Min K-D, Chavkin N, Polizio AH, et al. The cancer therapy-related clonal hematopoiesis driver gene Ppm1d promotes inflammation and non-ischemic heart failure in mice. Circ Res 2021; 129:684–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujino T, Kitamura T. ASXL1 mutation in clonal hematopoiesis. Exp Hematol 2020; 83:74–84. [DOI] [PubMed] [Google Scholar]
- 48.Bick AG, Pirruccello JP, Griffin GK, Gupta N, Gabriel S, Saleheen D, et al. Genetic interleukin 6 signaling deficiency attenuates cardiovascular risk in clonal hematopoiesis. Circulation 2020; 141:124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 50.Busque L, Sun M, Buscarlet M, Ayachi S, Feroz Zada Y, Provost S, et al. High-sensitivity C-reactive protein is associated with clonal hematopoiesis of indeterminate potential. Blood Adv 2020; 4:2430–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis 2012; 206:1558–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Longenecker CT, Jiang Y, Orringer CE, Gilkeson RC, Debanne S, Funderburg NT, et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS 2014; 28:969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hanna DB, Lin J, Post WS, Hodis HN, Xue X, Anastos K, et al. Association of macrophage inflammation biomarkers with progression of subclinical carotid artery atherosclerosis in HIV-infected women and men. J Infect Dis 2017; 215:1352–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bennett BD, Solar GP, Yuan JQ, Mathias J, Thomas GR, Matthews W. A role for leptin and its cognate receptor in hematopoiesis. Curr Biol 1996; 6:1170–1180. [DOI] [PubMed] [Google Scholar]
- 55.Trinh T, Ropa J, Aljoufi A, Cooper S, Sinn A, Srour EF, et al. Leptin receptor, a surface marker for a subset of highly en-grafting long-term functional hematopoietic stem cells. Leukemia 2021; 35:2064–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frodermann V, Rohde D, Courties G, Severe N, Schloss MJ, Amatullah H, et al. Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat Med 2019; 25:1761–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van der Heijden WA, van Deuren RC, van de Wijer L, van den Munckhof ICL, Steehouwer M, Riksen NP, et al. Clonal hematopoiesis is associated with low CD4 nadir and increased residual HIV transcriptional activity in virally suppressed individuals with HIV. J Infect Dis 2021; 225:1339–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watson CJ, Papula AL, Poon GYP, Wong WH, Young AL, Druley TE, et al. The evolutionary dynamics and fitness landscape of clonal hematopoiesis. Science 2020; 367:1449–1454. [DOI] [PubMed] [Google Scholar]
- 59.Cook EK, Luo M, Rauh MJ. Clonal hematopoiesis and inflammation: partners in leukemogenesis and comorbidity. Exp Hematol 2020; 83:85–94. [DOI] [PubMed] [Google Scholar]
- 60.Hormaechea-Agulla D, Matatall KA, Le DT, Kain B, Long X, Kus P, et al. Chronic infection drives Dnmt3a-loss-of-function clonal hematopoiesis via IFNγ signaling. Cell Stem Cell 2021; 28:1428.e6–1442.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meisel M, Hinterleitner R, Pacis A, Chen L, Earley ZM, Mayassi T, et al. Microbial signals drive preleukaemic myeloproliferation in a Tet2-deficient host. Nature 2018; 557:580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bolton KL, Ptashkin RN, Gao T, Braunstein L, Devlin SM, Kelly D, et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat Genet 2020; 52:1219–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013; 39:633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hsue PY, Waters DD. HIV infection and coronary heart disease: mechanisms and management. Nat Rev Cardiol 2019; 16:745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Akase IE, Musa BOP, Obiako RO, Ahmad Elfulatiy A, Mohammed AA. Immune dysfunction in HIV: a possible role for pro- and anti-inflammatory cytokines in HIV staging. J Immunol Res 2017; 2017:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Christensen-Quick A, Vanpouille C, Lisco A, Gianella S. Cytomegalovirus and HIV persistence: pouring gas on the fire. AIDS Res Hum Retrovirus 2017; 33:S23–S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tincati C, Douek DC, Marchetti G. Gut barrier structure, mucosal immunity and intestinal microbiota in the pathogenesis and treatment of HIV infection. AIDS Res Ther 2016; 13:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hileman CO, Funderburg NT. Inflammation, immune activation, and antiretroviral therapy in HIV. Curr HIV/AIDS Rep 2017; 14:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olivero OA. Mechanisms of genotoxicity of nucleoside reverse transcriptase inhibitors. Environ Mol Mutagen 2007; 48:215–223. [DOI] [PubMed] [Google Scholar]
- 70.Smith RL, de Boer R, Brul S, Budovskaya Y, van Spek H. Premature and accelerated aging: HIV or HAART? Front Genet 2012; 3:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.André-Schmutz I, Dal-Cortivo L, Six E, Kaltenbach S, Cocchiarella F, Le Chenadec J, et al. Genotoxic signature in cord blood cells of newborns exposed in utero to a Zidovudine-based antiretroviral combination. J Infect Dis 2013; 208:235–243. [DOI] [PubMed] [Google Scholar]
- 72.Williamson BT, Leitch HA. Higher risk myelodysplastic syndromes in patients with well controlled HIV infection: clinical features, treatment, and outcome. Case Rep Hematol 2016; 2016:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


