Abstract
It has been shown that innate immune responses can adopt adaptive properties such as memory. Whether T cells utilize innate immune signaling pathways to diversify their repertoire of effector functions is unknown. Gasdermin E (GSDME) is a membrane pore-forming molecule that has been shown to execute pyroptotic cell death and thus to serve as a potential cancer checkpoint. In the present study, we show that human T cells express GSDME and, surprisingly, that this expression is associated with durable viability and repurposed for the release of the alarmin interleukin (IL)-1α. This property was restricted to a subset of human helper type 17 T cells with specificity for Candida albicans and regulated by a T cell-intrinsic NLRP3 inflammasome, and its engagement of a proteolytic cascade of successive caspase-8, caspase-3 and GSDME cleavage after T cell receptor stimulation and calcium-licensed calpain maturation of the pro-IL-1α form. Our results indicate that GSDME pore formation in T cells is a mechanism of unconventional cytokine release. This finding diversifies our understanding of the functional repertoire and mechanistic equipment of T cells and has implications for antifungal immunity.
Subject terms: Inflammasome, Fungal infection, Lymphocyte differentiation, Autoimmunity
Gasdermin E pore formation has been associated with pyroptotic cell death. Here the authors identify gasdermin E pores in a subset of human TH17 cells and show that rather than killing these cells the pores enable the release of IL-1α on NLRP3 inflammasome activation as an antifungal immune response.
Main
Helper T cells (TH cells) are important enactors of antigen-specific effector responses via their secretion of distinct cytokines. Helper type 17 T cells (TH17 cells), in particular, are recognized for their antifungal functions through the secretion of their signature cytokine IL-17A, which is regulated by the transcription factor RAR-related orphan receptor (ROR)-γt1. They are also the main culprits in the pathogenesis of autoimmune diseases2. TH17 cells have previously been recognized to display functional heterogeneity3. Pro- or anti-inflammatory functions are exerted via the differential coexpression of IL-17 with either interferon (IFN)-γ or IL-10, respectively4–7. Overall, this has shaped the concept of a TH17 cell dualism and has stimulated investigation into the signals and molecular targets that control the dichotomy between the two functional TH17 cell outcomes for therapeutic applications3,4,8,9. However, a deep understanding of the identity and mechanistic basis of pathogenic versus immunoregulatory TH17 cell fates remains elusive. Additional, yet-to-be-found effector mechanisms that go beyond IL-17 production might also operate in TH17 cells with antifungal or antibacterial target specificities.
IL-1 cytokines, of which IL-1α and IL-1β represent the most prominent members, exert profound inflammatory effects. On release from antigen-presenting cells (APCs), they not only induce rapid innate inflammatory responses, but also orchestrate adaptive immunity by promoting TH17 cell polarization and T cell pathogenicity on binding to their shared IL-1R1 receptor4,10,11. IL-1-independent TH17 cell priming, which has also been previously described, results in the production of anti-inflammatory TH17 cells4. IL-1 from innate cellular sources therefore serves as a switch factor for the dichotomy of pro- versus anti-inflammatory TH17 cell fates. Unlike most other cytokines, IL-1 cytokines lack a signal peptide and are therefore secreted by an unconventional, endoplasmic reticulum (ER)–Golgi-independent mechanism. Pro-IL-1β requires enzymatic cleavage before release into the extracellular space and engagement of its receptor. The NLRP3 inflammasome is a multimeric cytosolic protein complex that assembles on microbial infection and cellular damage and recruits caspase-1 for subsequent pro-IL-1β cleavage12. IL-1β exit also requires caspase-1-mediated gasdermin D (GSDMD) cleavage and pore formation in a process called pyroptosis, an inflammatory form of cell death13,14. IL-1α, on the other hand, is thought to be processed independently of the NLRP3 inflammasome through regulatory checkpoints that are still poorly understood10. Despite these completely distinct pathways for the maturation and release of IL-1β and IL-1α, both cytokines are jointly produced by cells of the innate immune system, pointing to the existence of yet-to-be-identified co-regulatory routes.
In the present study, we show that a subset of human TH17 cells engages an NLRP3-dependent signaling cascade to induce membrane pore formation by GSDME, which serves the autocrine release of proinflammatory IL-1α. This finding reveals an unconventional mode of cytokine secretion by human T cells and thus diversifies the T cell functional and mechanistic repertoire.
Results
Production of IL-1α is a characteristic of human TH cells
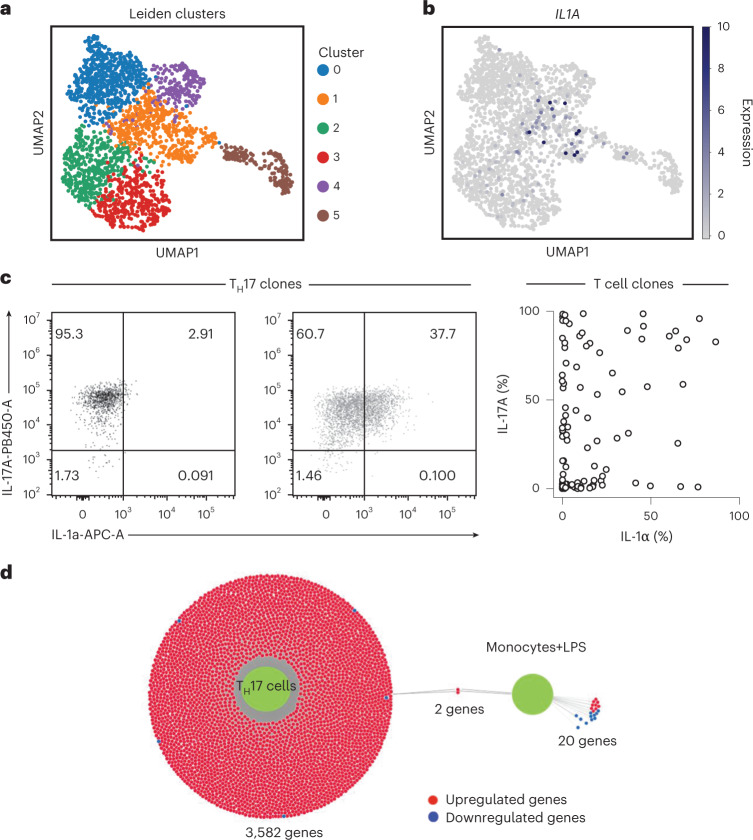
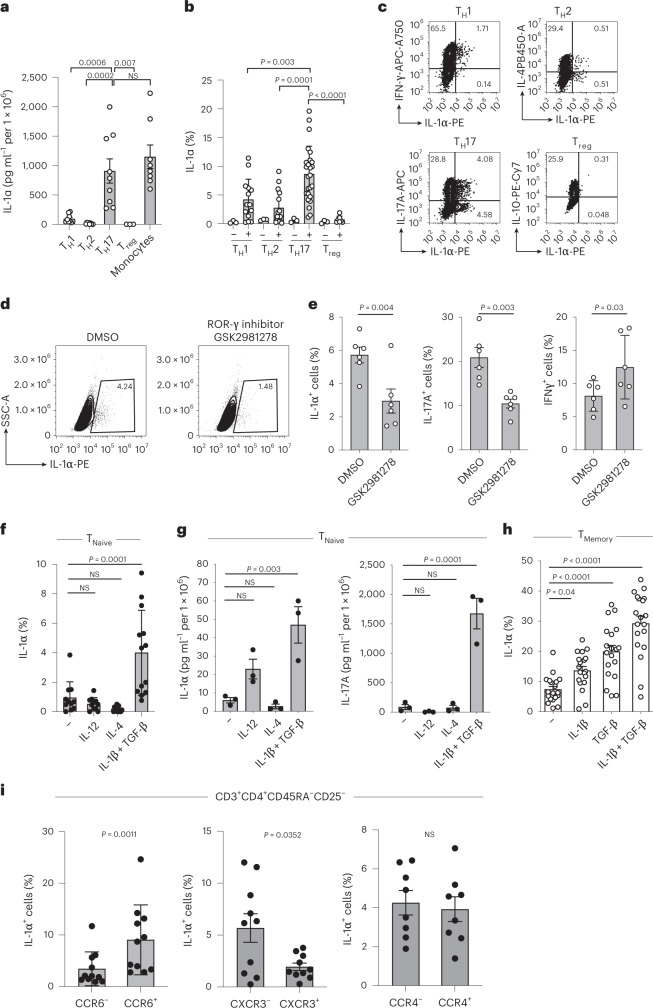
To investigate the heterogeneity of the human TH17 cell subset and to reveal distinct functions and their molecular control, we performed single-cell RNA-sequencing (scRNA-seq) of activated human TH17 cells, which had been isolated ex vivo from peripheral blood according to their unique expression of chemokine receptor surface markers15. Exploratory analysis by uniform manifold approximation and projection (UMAP) and Leiden clustering of all TH17 cells identified six individual clusters (Fig. 1a). A distinct and rare (6%) population of IL1A-expressing TH17 cells was selectively enriched in cluster 1 (Fig. 1a,b). Comparison of all genes in cluster 1 with all other clusters revealed IL1A to be significantly upregulated (Supplementary Table 1). This was unexpected given that IL-1α is not considered to belong to the canonical effector cytokine repertoire of T cells, but instead represents an innate danger signal16. IL1A was not, however, among the top differentially expressed genes (DEGs) in cluster 1, which necessitated a deeper search strategy to unmask its significant upregulation in a subpopulation of TH17 cells (Supplementary Fig. 1). At the protein level, TH17 cell clones also segregated into distinct IL-1α+ and IL-1α– T cell clones, thus supporting the heterogeneity of IL-1α protein expression at the single-cell level within the TH17 cell population (Fig. 1c).
Fig. 1. A distinct subset of human TH17 cells can express IL-1α.
a, ScRNA-seq and Leiden clustering of human TH17 cells after 5 d of stimulation with anti-CD3 and anti-CD28 monoclonal antibodies. b, IL1A expression in TH17 cells visualized in UMAP. c, Intracellular cytokine staining and flow cytometry of T cell clones generated from TH17 cells that were isolated ex vivo according to their differential expression of chemokine receptors. Left, representative flow cytometric analysis of one TH17 cell clone. Right, cumulative data from the blood of three healthy donors. d, DiVenn plot of DEGs obtained from IL1A+ versus IL1A– human TH17 cells stimulated as described in a (shown as left green circle) and compared with IL1A+ versus IL1A– human LPS-stimulated monocytes (GEO, accession no. GSE159113) (shown as right green circle). Upregulated genes (red circles) and downregulated genes (blue circles) are connected via a gray line to either green circle, indicating its dataset of origin. Gray lines connecting both green circles depict common DEGs between both datasets.
To compare IL1A expression by TH17 cells with that in other immune cell types, particularly previously reported bona fide producers of IL-1α, we interrogated multiple public scRNA-seq datasets of human peripheral blood mononuclear cells (PBMCs). Surprisingly, this did not reveal any IL1A expression in various immune cell types of resting PBMCs, including T cells, B cells, natural killer (NK) cells, NKT cells, monocytes and dendritic cells (Supplementary Fig. 2a,b). Even monocytes did not display any IL1A expression at the single-cell level, unless a specific IL1A-inducing stimulus, specifically lipopolysaccharide (LPS), was applied to these cells (Supplementary Fig. 2c,d), which unmasked their IL1A-producing ability and the association of IL1A expression with an ongoing inflammatory innate immune response (Supplementary Fig. 2e). It is interesting that a single-cell transcriptomic comparison of the DEGs between IL1A+ and IL1A– cells from TH17 cells versus monocytes demonstrated hardly any overlap in gene coexpression (IL1A and CCL3), which was highly suggestive of a different mode of IL1A regulation in T cells versus monocytes (Fig. 1d). Taken together, these results reveal the existence of a distinct subpopulation of IL-1α-expressing cells within the TH17 cell subset.
The IL-1α-producing subset of TH17 cells is proinflammatory
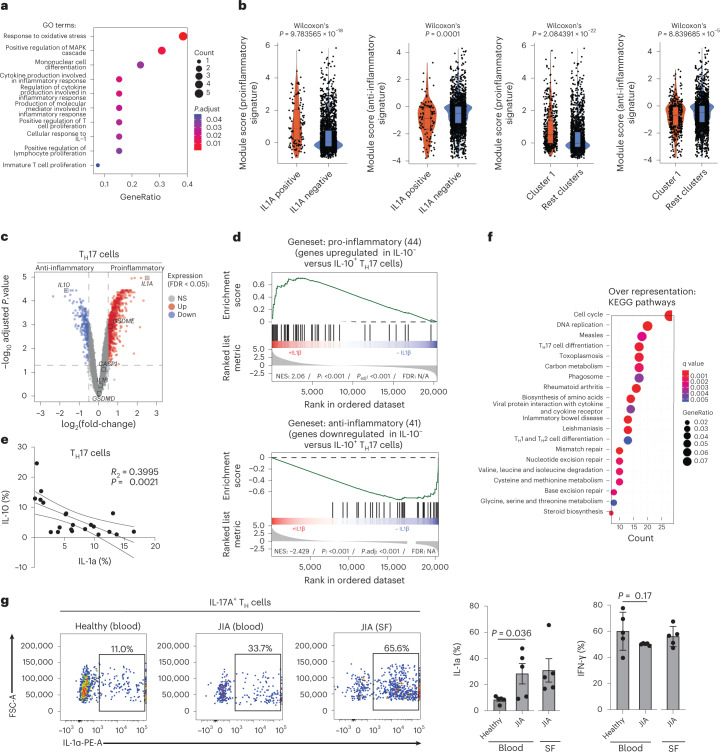
To explore the physiological relevance of IL1A expression in human TH17 cells, we performed an unbiased transcriptomic comparison of IL1A+ and IL1A– TH17 cells after scRNA-seq. Gene set enrichment analysis (GSEA) for genes coexpressed with IL1A in TH17 cells, as well as enrichment analysis using DEGs, revealed a striking association of IL1A with T cell activation and proliferation after an unbiased interrogation of all available gene ontology (GO) terms (Fig. 2a and Supplementary Fig. 3). This finding challenged the previously assigned role of IL-1α in senescence and cell death in the new context of T cells17. The enrichment analysis also revealed a strong overrepresentation for several GO terms related to ‘inflammation’, suggesting that IL1A expression by TH17 cells contributed to a pathogenic T cell identity with roles in inflammatory diseases (Fig. 2a). This idea was supported by the upregulation of genes annotated with the GO term ‘cellular response to interleukin-1’, considering the previously reported proinflammatory switch effect of IL-1β on the overall TH17 cell functionality4 and the suppressive effect of autocrine IL-1α on IL-10 expression (Extended Data Fig. 1a–c). A direct comparison of IL1A+ versus IL1A– TH17 cells across all clusters demonstrated that IL1A+ TH17 cells displayed significantly enhanced proinflammatory, but reduced anti-inflammatory, signatures (Fig. 2b)7. Furthermore, cluster 1, which enriched for IL1A+ TH17 cells, was significantly more proinflammatory and less anti-inflammatory than all other five clusters, as indicated by GSEA (Fig. 2b). It is of interest that a bulk transcriptomic comparison of pro- versus anti-inflammatory TH17 cell subsets revealed IL1A to even be among the top upregulated genes in the proinflammatory TH17 cell subset (Fig. 2c,d and Extended Data Fig. 2). IL10, instead, was highly downregulated, as expected according to previous reports4,7. This reciprocal correlation of IL-1α and IL-10 expression by TH17 cells was also observed at the protein level by flow cytometry (Fig. 2e). Enrichment analysis with the DEGs demonstrated overrepresentation of KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways for autoimmune diseases such as ‘rheumatoid arthritis’ and ‘inflammatory bowel disease’ (Fig. 2f), which supported the proinflammatory nature of the IL1A-expressing TH17 cell subset. In fact, patients suffering from juvenile idiopathic arthritis (JIA), a highly inflammatory form of rheumatoid arthritis in children whose pathogenesis has previously been linked to innate IL-1β and IL-1α18,19, revealed significantly and strongly elevated IL-1α expression by IL-17+ TH cells compared with IL-17+ TH cells from healthy control blood (Fig. 2g). No IFN-γ increase was observed within IL-17+ TH cells from the blood of patients with JIA compared with control donor blood, instead, despite the previously reported association of IFN-γ with TH17 cell pathogenicity (Fig. 2g, right panel)4. Furthermore, the analysis of blood-matched synovial fluid demonstrated very high frequencies of IL-1α+ TH cells, suggesting that T cells, beyond innate cells, could also represent a relevant cellular source of the disease-associated IL-1α at the site of inflammation.
Fig. 2. IL-1α producing TH17 cells are proinflammatory.
a, Enrichment analysis using clusterprofiler with genes coexpressed with IL1A as determined in Extended Data Fig. 3a,b. The top 10 GO terms out of 150 significant GO terms are shown. b, Expression of pro- and anti-inflammatory gene sets obtained from public data7 in TH17 cells analyzed by scRNA-seq after grouping single cells into IL1A+ and IL1A− TH17 cells and after Leiden clustering (Wilcoxon’s rank-sum test). c, Transcriptome analysis showing DEGs (red, upregulated; blue, downregulated; gray, nonsignificant genes) of pro- versus anti-inflammatory TH17 cells after 5 d of polyclonal stimulation in the presence or absence of IL-1β, respectively. d, GSEA of TH17 cells from c. The gene sets were established from a public dataset7 after transcriptomic comparison of IL-10– versus IL-10+ TH17 cell clones. N/S, not significant; NES, normalized enrichment score. e, Intracellular cytokine staining and flow cytometric analysis of TH17 cells stimulated for 5 d with anti-CD3 and anti-CD28 monoclonal antibodies. f, Overrepresentation of KEGG pathways within the DEGs from the transcriptomic comparison of pro- versus anti-inflammatory TH17 cells. g, Intracellular cytokine staining and flow cytometry (left, representative experiment; right, cumulative data) of T cells (from blood and synovial fluid (SF) of patients suffering from JIA and healthy control donors). IL-17A+ gated TH cells are shown (paired Student’s t-test; n = 5 independent patients and healthy donors).
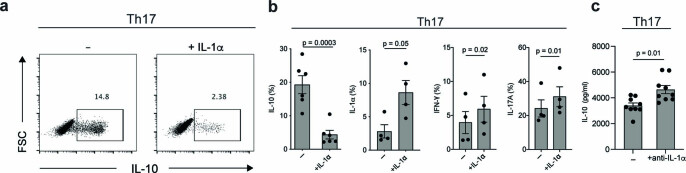
Extended Data Fig. 1. Autocrine IL-1α production by Th17 cells enforces a pathogenic Th17 cell identity.
a, Th17 cells were stimulated with anti-CD3 and anti-CD28 mAbs for 5 days before intracellular cytokine staining and flow cytometry following PMA and ionomycin restimulation. Representative experiment. b, Cumulative data for (a). Two-sided paired t-test. c, ELISA of supernatants from Th17 cells stimulated for 5 days with anti-CD3 and anti-CD28 mAbs. Two-sided paired t-test.
Extended Data Fig. 2. Differential gene expression between pro- and anti-inflammatory human Th17 cells.
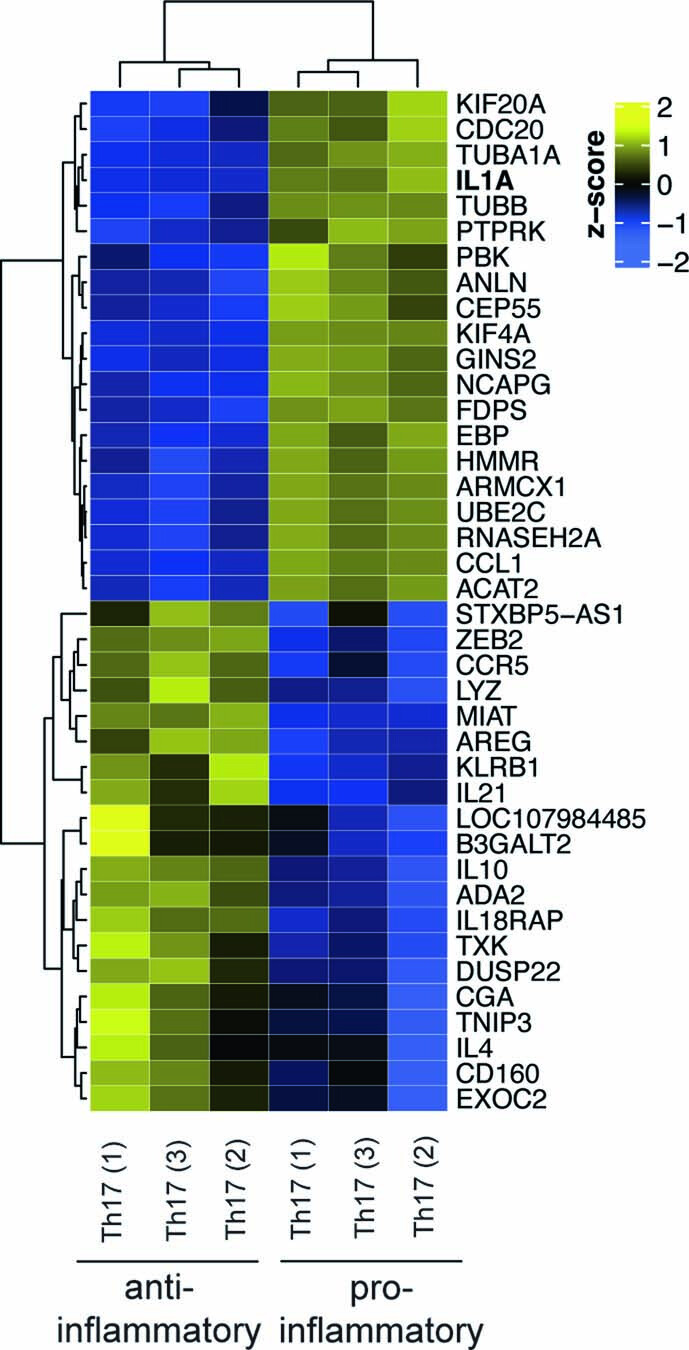
Shown is a heatmap displaying the top 20 up- and downregulated genes (by order of significance) of the transcriptome following stimulation of human Th17 cells with anti-CD3 and anti-CD28mAbs for 5 days in the presence (proinflammatory) or absence (anti-inflammatory) of exogenous IL-1β. The samples and genes were clustered according to the pattern of expression using k-means clustering.
IL-1α expression is regulated by a TH17 program
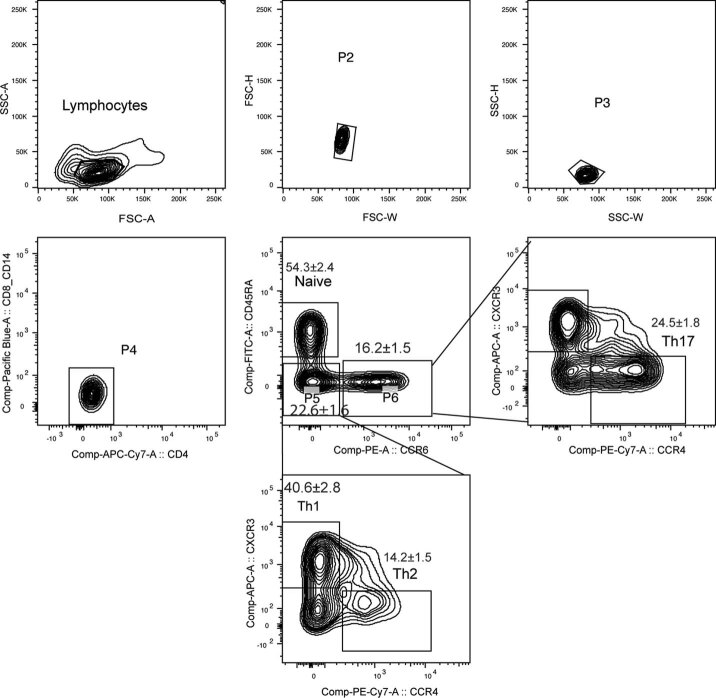
To investigate whether IL-1α expression is a general property of T cells, we enriched individual TH cell subsets from PBMCs according to their differential expression of chemokine receptors (Extended Data Fig. 3) and compared their IL-1α secretion. IL-1α was specifically produced by the TH17 cell subset but not the TH1 cell subset, TH2 cell subset or regulatory T cells (Treg cells) (Fig. 3a–c). Strikingly, its secretion level on stimulation with anti-CD3 and anti-CD28 monoclonal antibodies matched that of human monocytes stimulated with LPS and nigericin, indicating that human TH17 cells, notwithstanding their adaptive immune identity, serve as a major source of the danger signal IL-1α (Fig. 3a). Intracellular IL-1α protein expression was absent in freshly isolated resting TH cells but inducible on T cell receptor (TCR) activation, with significant enrichment in TH17 cells compared with TH1, TH2 and Treg cell-enriched TH cells (Fig. 3b,c). These findings seem to be specific for the human immune system, because previous reports excluded IL-1α production by mouse T cells16.
Extended Data Fig. 3. Gating strategy for the isolation of Th cell subsets from the blood.
Fresh PBMCs from healthy donors were enriched for CD4 expression by MACS before FACS for CD45RA– Th cell subsets according to their differential expression of the chemokine receptor markers CCR6, CCR4 and CXCR3. A representative gating strategy and the percentage of Th-cell subsets in the respective gates for 33 healthy donors are shown (mean ± SEM).
Fig. 3. IL-1α production by T cells is restricted to the TH17 cell fate.
a, ELISA of cell culture supernatants of TH cell subsets after stimulation with anti-CD3 and anti-CD28 monoclonal antibodies for 5 d. Monocytes were stimulated with LPS for 24 h and nigericin for the last 30 min (one-way analysis of variance (ANOVA) with Dunnett’s multiple-comparison test). b–f,h, Intracellular cytokine staining and flow cytometric analysis of cells stimulated as in a (one-way ANOVA with Dunnett’s multiple-comparison test (b, f and h), two-tailed paired Student’s t-test (e)). DMSO, Dimethysulfoxide; NS, not significant. g, ELISA of cell culture supernatants from cells stimulated as in f (one-way ANOVA with Dunnett’s multiple-comparison test). i, Intracellular cytokine staining and flow cytometry of memory TH cells sorted positively and negatively for specific chemokine receptors. The analysis was performed after 5 d of stimulation with anti-CD3 and anti-CD28 monoclonal antibodies (two-tailed, paired Student’s t-test). Data are presented as mean ± s.e.m. Each circle indicates an independent biological sample representing a blood donor.
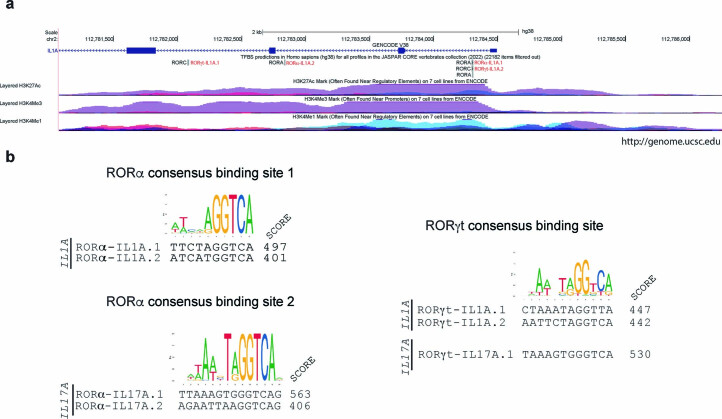
The unique association of IL-1α with the TH17 cell subset prompted us to mechanistically dissect the regulation of this cytokine. It is interesting that IL-1α expression was reduced on specific inhibition of ROR-γt, the master transcription factor of TH17 cells (Fig. 3d,e)1. These data are in line with the presence of putative binding sites for ROR-γt and ROR-α in the IL1A promotor and enhancer regions (Extended Data Fig. 4a,b).
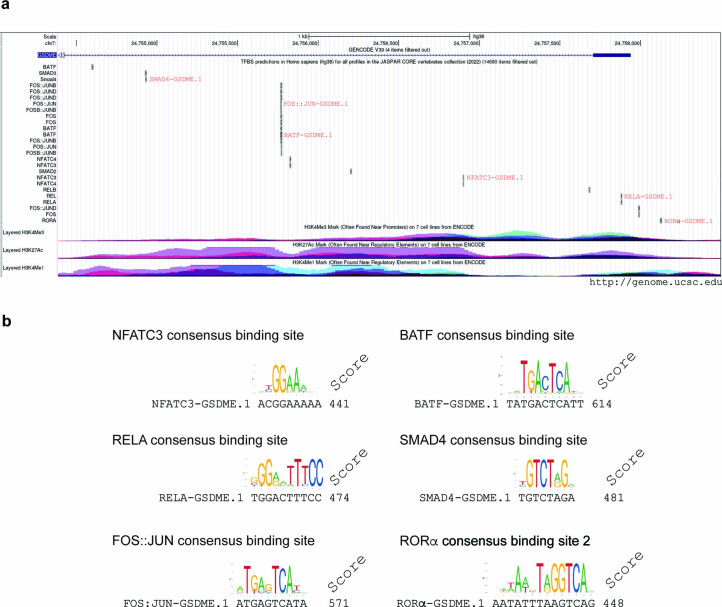
Extended Data Fig. 4. Consensus binding sites for the Th17 master transcription factors ROR-γt and RORα in the IL1A intronic enhancer region.
a, Locations of RORα and ROR-γt consensus binding sites in the regulatory region of IL1A. Binding sites were predicted using the JASPAR2022 database (Castro-Mondragon JA et al., Nucleic Acids Res 2022) and annotated with the UCSC genome browser (assembly hg38) (Kent WJ et al., Genome Res 2002). The quality cutoff was set at 400 (this score indicates a p value of p = 1*10- (score/100)). ENCODE tracks for histone marks were used to indicate the bona fide IL1A regulatory region (chr2:112,781,072-112,786,251) (Rosenbloom KR et al., Nucleic Acids Res 2013). b, Alignment of the RORα and RORγt consensus binding motif with the predicted binding sites in the IL1A enhancer RORα-IL1A.1 (chr2:112784300-112784309), RORα-IL1A.2 (chr2:112782834-112782843); RORγt-IL1A.1 (chr2:112,782,077-112,782,088); RORγt-IL1A.2 (chr2:112,784,298-112,784,309) and established binding sites in the IL17A enhancer RORα-IL17A.1 (chr6:52181117-52181130), RORα-IL17A.2 (chr6:52180979-52180992) and RORγt-IL17A.1 (chr6:52181118-52181129) (Wang X et al., Immunity. 2012). Scores for individual sites are indicated.
The fate of a particular TH cell subset is determined by the distinct polarizing cytokine microenvironment during naive T cell stimulation. We observed the highest intracellular expression and secretion of IL-1α on naive T cell priming in TH17 cell-polarizing conditions (IL-1β and transforming growth factor (TGF)-β) (Fig. 3f,g). Single treatments with IL-1β or TGF-β alone did not, however, lead to significant IL-1α expression in naive T cells, stressing the synergistic effect of the combinatorial TH17 cell-priming cytokines on de novo induction of IL-1α (Supplementary Fig. 4). TH1 (IL-12) and TH2 (IL-4) cell-priming conditions, in contrast, did not significantly alter IL-1α secretion compared with that under stimulation in the absence of polarizing cytokines. Together, these findings demonstrate that naive T cells acquire the capacity to produce IL-1α through TH17 cell-polarizing cytokines. Memory TH cells also displayed a significant further upregulation of their IL-1α effector cytokine in IL-1β and TGF-β microenvironments (Fig. 3h).
The identity of TH cell subsets is also characterized by distinct migration properties, which are associated with the differential expression of chemokine receptors20. We observed that IL-1α expression was enriched in CCR6+ but not CCR6– T cells and was reduced in CXCR3– T cells compared with CXCR3+ T cells, although there was no difference in IL-1α expression between CCR4+ and CCR4– T cells (Fig. 3i). These data demonstrate that IL-1α-producing cells display the migration pattern previously assigned to IL-17-producing cells15. Taken together, these data consistently show that IL-1α is a unique function of human TH17 cells.
Calpain is a prerequisite for IL-1α secretion by TH17 cells
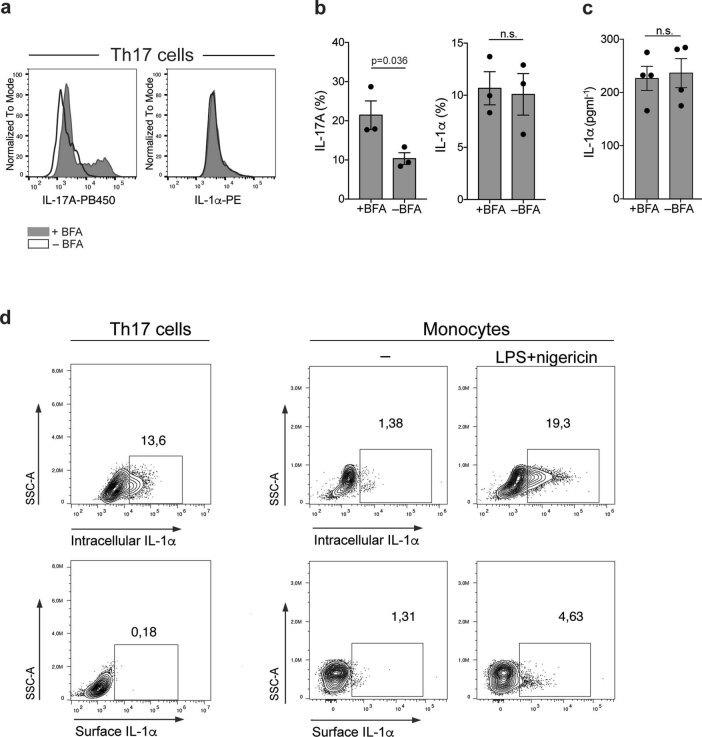
To explore the mechanism of IL-1α secretion, we treated TH17 cells with the protein export inhibitor brefeldin A (BFA) and found that the secretion of IL-1α was not affected, unlike that of conventional cytokines, such as IL-17A (Extended Data Fig. 5a–c). This supports the existence of an unconventional ER–Golgi-independent pathway for IL-1α secretion by T cells and is in line with previous reports for innate cell types21,22.
Extended Data Fig. 5. IL-1α is secreted by an unconventional pathway in human Th17 cells and is not expressed on the cell surface.
a, Th17 cells were stimulated for 5 days with anti-CD3 and anti-CD28 mAbs (48 h plate-bound) before intracellular cytokine staining and flow cytometric analysis on day 5 following PMA and ionomycin restimulation. Representative experiment. b, Cumulative data, Two-sided paired t-test. Each circle indicates an individual healthy blood donor (n = 3 examined over 2 independent experiments). Data are presented as mean ± SEM. c, ELISA of 24 h cumulative supernatants from day 4 to day 5 in the presence or absence of BFA. n.s., not significant. Two-sided paired t-test. Each circle indicates an individual healthy blood donor (n = 4 biologically independent samples examined over 2 independent experiments). Data are presented as mean ± SEM. d, Th17 cells were stimulated and analyzed as in (a). Monocytes were stimulated in the presence and absence of LPS (24 h) and nigericin (last 30 min) and analyzed by intracellular and surface staining and flow cytometry. Representative experiment (n = 3 biologically independent samples).
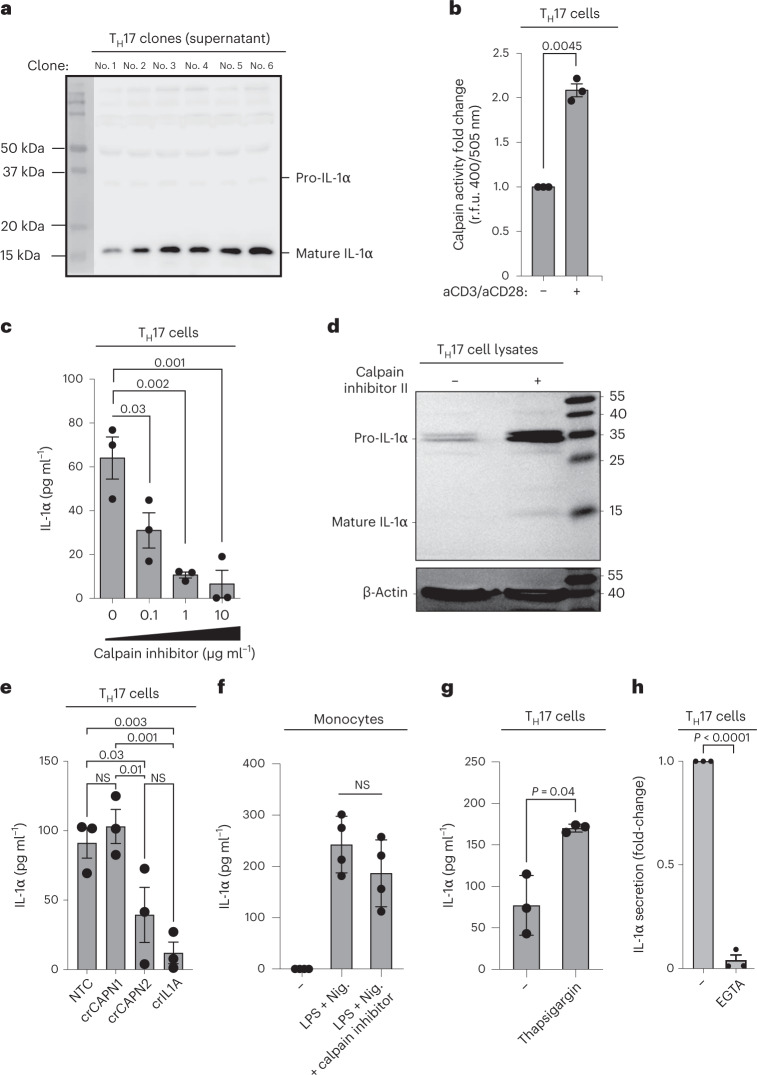
A unique property that has previously been assigned to IL-1α is its simultaneous localization in the cytoplasm and the plasma membrane23. We found, however, that TH17 cells, in contrast to monocytes, did not display membrane-bound IL-1α (Extended Data Fig. 5d). Although cleavage of pro-IL-1β is required to generate bioactive extracellular IL-1β, IL-1α is known to be passively released on cell death and to exert its bioactive potential after binding to IL-1RI in its uncleaved or cleaved form24. To determine whether pro-IL-1α undergoes intracellular processing for controlled release by human T cells, we evaluated the full-length and mature forms of IL-1α in the supernatant of activated TH17 cells. To exclude any contaminating monocytes as a potential source of secreted IL-1α, we generated TH17 cell clones over a period of 2 weeks and restimulated them with anti-CD3 and anti-CD28 monoclonal antibodies for another 5 d before immunoblotting. In all six tested TH17 cell clones, we found preferential enrichment of the cleaved form of IL-1α in the culture supernatants (Fig. 4a). TH17 cell lysates, in contrast, showed preferential enrichment of the uncleaved pro-IL-1α form, as expected (Supplementary Fig. 5a). These results exclude passive release of pro-IL-1α during cell necrosis as the default IL-1α exit modality in T cells and, instead, suggest that human TH17 cells must possess a molecular machinery for pro-IL-1α cleavage to enable the controlled extracellular release of this potent bioactive molecule by viable T cells, in contrast to innate cells (Supplementary Fig. 5b). This does not, however, exclude an additional contribution of T cell necrosis to the liberation of the bioactive pro-form of IL-1α (Supplementary Fig. 5a).
Fig. 4. Calpain is a prerequisite for the release of cleaved IL-1α by human TH17 cells.
a, Immunoblot analysis of cell culture supernatants derived from TH17 cell clones that were restimulated with anti-CD3 and anti-CD28 monoclonal antibodies for 5 d. b, Fold-change in relative fluorescence units (r.f.u.) after 1 h of incubation of TH17 cells with the calpain substrate Ac-LLY-AFC. TH17 cells were stimulated for 3 d with anti-CD3 and anti-CD28 monoclonal antibodies. c,e,g,h, ELISA of cell culture supernatants after stimulation of TH17 cells (c and e–g) with anti-CD3 and anti-CD28 monoclonal antibodies for 5 d and of monocytes (f) with LPS (24 h) and nigericin (30 min). Thapsigargin (g), 1 μM, was added on days 2 and 3 and EGTA (h) on day 0. d, Immunoblot analysis of human TH17 cell lysates. Human TH17 cells were stimulated with anti-CD3 and anti-CD28 monoclonal antibodies and IL-1β in the presence or absence of calpain inhibitor II (10 mM) and analyzed on day 5. The data represent two experiments with two donors. e, TH17 cells were stimulated with anti-CD3 and anti-CD28 monoclonal antibodies for 5 d after genetic depletion of CAPN1 or CAPN2 with CRISPR–Cas9 technology. Each circle indicates an independent blood donor. The data represent three independent experiments (b, c and e–h). P values were calculated using one-way ANOVA with Dunnett’s multiple-comparison test (c) or Fisher’s least significance difference test (e) or two-tailed, paired Student’s t-test (b and f–h).
Pro-IL-1α processing at distinct cleavage sites can be catalyzed by several proteases17,25,26. Calpain is a calcium-dependent cysteine protease that can give rise to the mature IL-1α p17 fragment26, which we identified herein. We detected calpain activity in TH17 cells. It increased on activation with anti-CD3 and anti-CD28 monoclonal antibodies (Fig. 4b). IL-1α secretion by TH17 cells was dependent on T cell-intrinsic calpain activity because pharmacological calpain inhibition reduced IL-1α secretion by activated TH17 cells into the extracellular space in a dose-dependent manner (Fig. 4c). Correspondingly, calpain inhibition resulted in intracellular accumulation of pro-IL-1α (Fig. 4d). To corroborate the dependence of IL-1α secretion on calpain, we also genetically knocked out calpain in human TH17 cells using clustered regularly interspaced short palindromic repeats (CRISPR)–Cas9. This revealed a role for CAPN2, but not CAPN1, in IL-1α secretion by human TH17 cells (Fig. 4e). In contrast, LPS-/nigericin-induced IL-1α secretion by monocytes was not dependent on calpain (Fig. 4f). These data were consistent with the preferential expression of CAPN2 but not CAPN1 by human T cell subsets in contrast to dendritic cells, which displayed a reversed calpain gene expression pattern (Supplementary Fig. 6). IL-1α secretion in TH17 cells increased on intracellular accumulation of calcium and inhibition of the sarco-/ER Ca2+ ATPase with thapsigargin (Fig. 4g) and decreased on extracellular calcium chelation with (ethylenebis(oxonitrilo))tetra-acetate (EGTA) (Fig. 4h), consistent with the calcium-dependent function of calpain and TCR-dependent IL-1α secretion26. Together, these data demonstrated that the proteolytic activity of the calcium-dependent protease calpain is a prerequisite for unconventional IL-1α secretion by TCR-activated human TH17 cells.
IL-1α secretion by TH17 cells is regulated by NLRP3 inflammasome activation
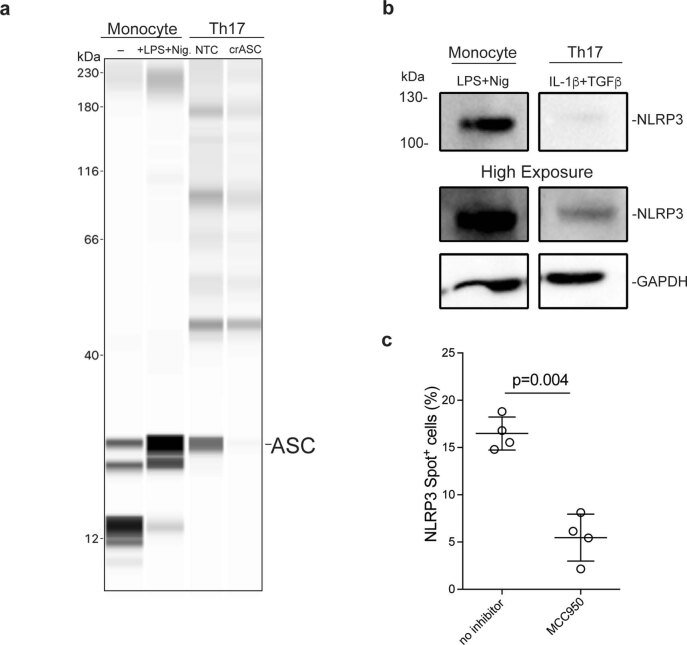
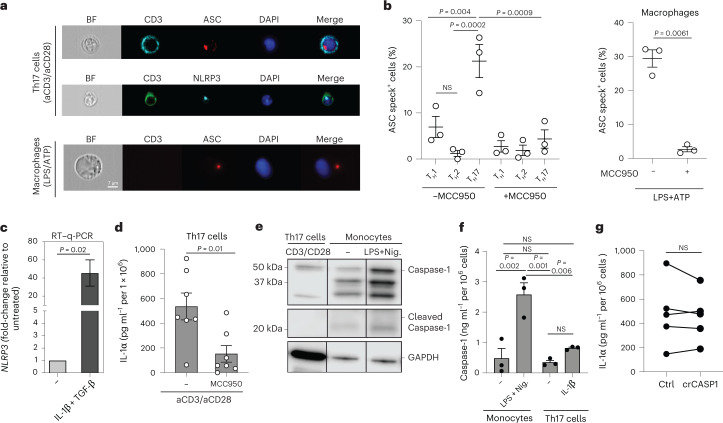
Despite the essential role of calpain in pro-IL-1α maturation, the mechanism leading to the extracellular exit of cleaved IL-1α remained unknown and was therefore addressed next. Extracellular IL-1α release by myeloid cells has previously been associated with NLRP3 inflammasome activation, nonenzymatic activity of caspase-1 and release of IL-1β16,27. We tested whether human TH17 cells possessed the molecular scaffold of the NLRP3 inflammasome and found protein expression of NLRP3 and the adapter molecule, apoptosis-associated speck-like protein containing a CARD (ASC), in human TH17 cells (Extended Data Fig. 6a,b). We observed ongoing inflammasome activation in TCR-activated TH17 cells by identification of ASC specks using ImageStream technology (Fig. 5a,b)28,29. Strikingly, increased frequencies of ASC specks were uniquely confined to the TH17 cell subset (Fig. 5b, left). TH17 cells even approximated the ASC-speck formation of LPS- and ATP-stimulated macrophages (Fig. 5b, right). TH1 and TH2 cell subsets, in contrast, displayed background ASC-speck levels (Fig. 5b, left). Moreover, ASC-speck and NLRP3-speck formation were completely abrogated in the presence of the specific NLRP3 inflammasome inhibitor MCC950, substantiating the existence of ongoing NLRP3 inflammasome activation in TH17 cells (Fig. 5b, left and Extended Data Fig. 6c). The selective engagement of the NLRP3 inflammasome by TH17 cells was further supported by the strong induction of NLRP3 transcripts on T cell stimulation in the presence of the TH17 cell-polarizing cytokines IL-1β and TGF-β (Fig. 5c). Importantly, IL-1α secretion by TH17 cells was significantly reduced by specific inhibition of the NLRP3 inflammasome with MCC950 (Fig. 5d), thus demonstrating the critical role of the NLRP3 inflammasome in the secretion of IL-1α by human TH17 cells.
Extended Data Fig. 6. Human Th17 cells express the inflammasome components ASC and NLRP3.
a, Lane views of electropherograms obtained with the Jess Simple Western System (ProteinSimple). Monocytes were MACS-isolated with CD14 beads and stimulated in the presence or absence of LPS (24 h) and nigericin (Nig.) for 30 min before cell lysis. Th17 cells were FACS-sorted ex vivo from the blood of healthy donors, stimulated for 48 h and transfected with RNP containing nontargeted control (NTC) or crASC. Cell lysates were collected after 7 days. The experiment was repeated independently 3 times with similar results. b, Western blot analysis. Cell culture lysates derived from Th17 cells and monocytes. Th17 cells were sorted as in (a) and stimulated with anti-CD3 and anti-CD28 mAbs (48 h plate-bound) for 5 days. Monocytes were isolated and stimulated as in (a). The experiment was repeated independently 2 times with similar results. c, NLRP3-speck formation assessed by ImageStream in Th17 cells stimulated for 5 days with anti-CD3 and anti-CD28 mAbs. Each circle indicates an individual healthy blood donor and experiment. Two-sided paired t-test. Data are presented as mean ± SEM. n = 4 biologically independent samples examined over 2 independent experiments.
Fig. 5. Unconventional NLRP3 inflammasome activation regulates IL-1α production by human TH17 cells.
a,b, Imaging flow cytometry with TH17 cells on day 5 after stimulation with plate-bound anti-CD3 and anti-CD28 monoclonal antibodies and macrophages after 24 h of stimulation with LPS and ATP for the last 30 min. a, Representative experiment. BF, bright-field. b, Cumulative data with n = 3 biological samples, presented as mean ± s.e.m. Left, P values calculated using one-way ANOVA with Tukey’s multiple-comparison test. Right, P values calculated using two-tailed, paired Student’s t-test. c, RT–qPCR analysis of TH17 cells stimulated as in a and restimulated with PMA and ionomycin for 3 h. Data represent three independent experiments with n = 9 biological replicates (two-tailed, paired Student’s t-test). d, ELISA of cell culture supernatants after stimulation of TH17 cells for 5 d with anti-CD3 and anti-CD28 monoclonal antibodies. Data represent three experiments with n = 7 biological replicates (two-tailed, paired Student’s t-test). e, Immunoblot analysis of cell lysates from TH17 cells after 5 d of stimulation with anti-CD3 and anti-CD28 monoclonal antibodies and of monocyte lysates after stimulation with LPS for 24 h and nigericin (Nig.) for the last 30 min. The conditions from the same blot after removal of irrelevant conditions or replicates are shown. f, ELISA of cell culture supernatants from anti-CD3- and anti-CD28-activated TH17 cells (5 d) and LPS (24 h)- and nigericin (30 min)-stimulated monocytes (n = 3 biological samples presented as mean ± s.e.m.; one-way ANOVA with Tukey’s multiple-comparison test). g, ELISA of cell culture supernatants from anti-CD3- and anti-CD28-activated TH17 cells after depletion of CASP1 by CRISPR–Cas9 gene editing. Data represent five independent experiments. P values were calculated using two-tailed, paired Student’s t-test. Each circle indicates an independent blood donor.
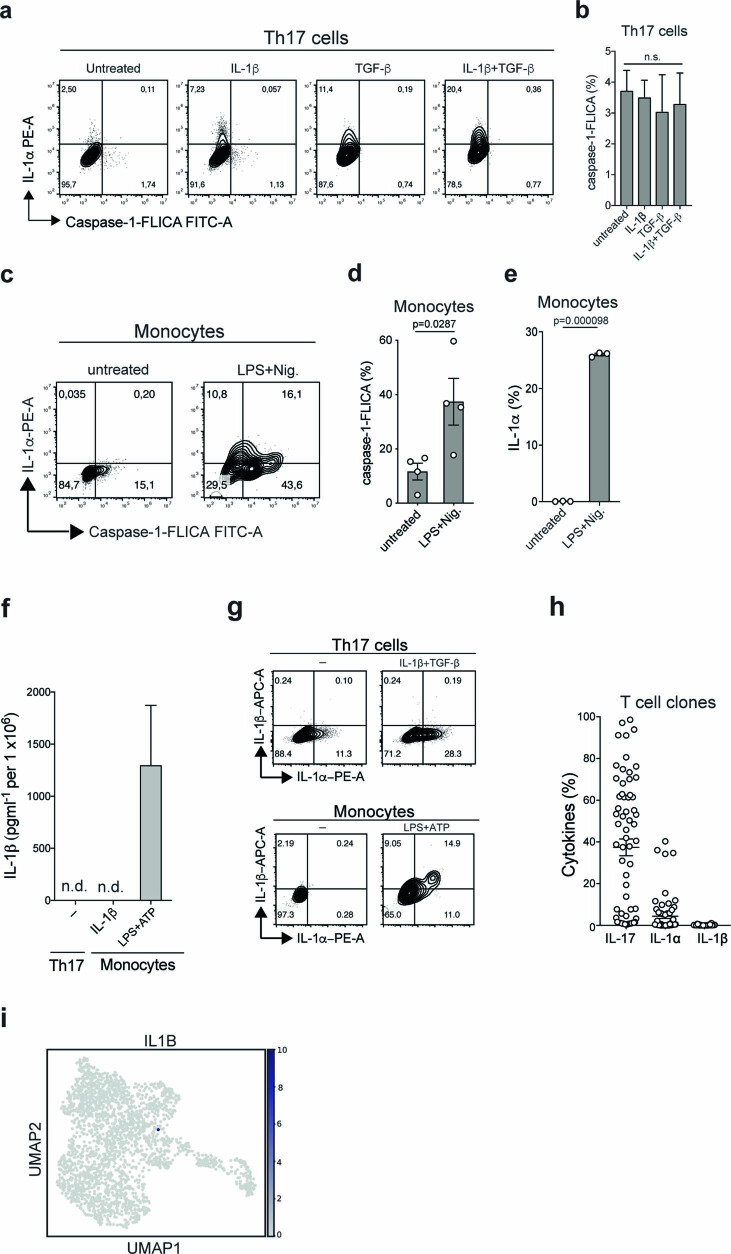
Caspase-1 is the canonical effector protein in the NLRP3 inflammasome complex30. We observed pro-caspase-1 expression in activated human TH17 cells (Fig. 5e). However, in contrast to the findings in LPS- and nigericin-stimulated monocytes, no cleaved caspase-1 was detected in human TH17 cell lysates (Fig. 5e). This observation was corroborated by the absence of caspase-1 FLICA staining in TH17 cells that were stimulated in the presence or absence of IL-1α promoting cytokine stimuli (Extended Data Fig. 7a–e). We further excluded extracellular release of caspase-1 by ELISA after stimulation of TH17 cells with anti-CD3 and anti-CD28 monoclonal antibodies for 5 d (Fig. 5f). Even in the presence of the IL-1α stimulus IL-1β, caspase-1 secretion was not inducible and remained as low as that in resting monocytes (Fig. 5f). Pharmacological inhibition of caspase-1 with Ac-YVAD-CMK did not reduce IL-1α secretion in the presence or absence of IL-1α induction by IL-1β. Rather, we observed slightly elevated IL-1α secretion on caspase-1 inhibition (Supplementary Fig. 7a,b). Importantly, TH17 cells did not show any alteration in IL-1α secretion on CRISPR–Cas9-engineered depletion of CASP1 expression (Fig. 5g). In line with the absence of bioactive caspase-1, we did not observe any secretion of IL-1β by human TH17 cells. This was in contrast to the case in monocytes, which demonstrated caspase-1-dependent IL-1β secretion on stimulation with LPS and ATP (Extended Data Fig. 7f). Furthermore, no intracellular IL-1β was detectable or inducible by IL-1α-polarizing cytokines in TH17 cells or TH17 cell clones or detectable in activated TH17 cells by scRNA-seq analysis (Extended Data Fig. 7g,h,i). This was in contrast to the finding for monocytes, which coexpressed IL-1β and IL-1α at the single-cell level, consistent with the previously suggested cosecretion and putative coregulation pattern of both IL-1 cytokines (Extended Data Fig. 7g)16,27. Cumulatively, these data demonstrate that human TH17 cells produce IL-1α independently of caspase-1 and IL-1β, unlike monocytes and presumably other innate immune cells, despite clear involvement of the NLRP3 inflammasome.
Extended Data Fig. 7. IL-1α expression is neither regulated by active caspase-1 in human Th17 cells nor coregulated with IL-1β, in contrast to the situation in monocytes.
a, Th17 cells were isolated ex vivo by FACS-sorting and stimulated for 5 days with anti-CD3 and anti-CD28 mAbs (48 h plate-bound) in the presence or absence of the indicated cytokines before flow cytometric analysis of intracellular IL-1α and active caspase-1 after PMA and ionomycin restimulation. The results of one representative experiment are shown. b, Cumulative data for active caspase-1 as shown in (a). One-way ANOVA. n = 3 biologically independent samples examined over 3 independent experiments. Data are presented as mean ± SEM. c, Intracellular staining and flow cytometry of monocytes following 24 h stimulation with LPS and 30 min with nigericin (Nig.). The results shown are from one representative experiment. d, e, cumulative data of (d), Two-sided paired t-test. Each circle indicates an individual healthy blood donor (n = 3-4 biologically independent samples examined over 3 independent experiment). Data are presented as mean ± SEM. f, ELISA of Th17 cells, which were stimulated as in (a) and of monocytes were stimulated with LPS (24 h) and ATP (30 min). n = 3 biologically independent samples examined over 2 independent experiments. Data are presented as mean ± SEM. g, Flow cytometric analysis of cells as in (g).h, Intracellular cytokine staining and flow cytometric analysis of T-cell clones generated from the Th17-cell subset. Each circle indicates an individual T cell clone (n = 64). Data are presented as mean ± SEM. i, scRNA-seq and UMAP showing IL-1β expression in human Th17 cells stimulated for 5 days with anti-CD3 and anti-CD28 mAbs.
IL-1a exits TH17 cells via GSDME pores
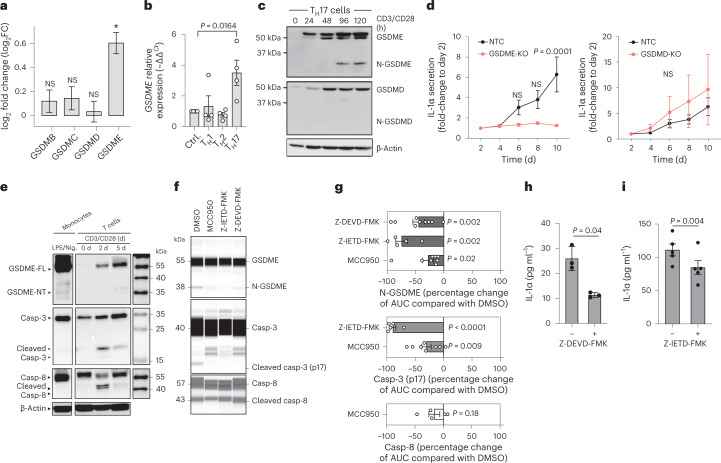
Gasdermins belong to a family of recently identified pore-forming effector molecules that enable the release of inflammatory mediators31. Our transcriptome analysis revealed a selective upregulation of GSDME expression in the proinflammatory IL-1β-stimulated TH17 cell subset, but no regulation of any other member of the gasdermin family (Fig. 6a). This was surprising considering that GSDME expression has never before been reported in primary T cells. In contrast, GSDMD, which is known to be regulated by the NLRP3 inflammasome and to be a target of caspase-1 (ref. 31), was not upregulated, supporting the idea of noncanonical NLRP3 inflammasome signaling. GSDME has previously been shown to form membrane pores in innate immune cells that may serve as conduits for the extracellular release of alarmins and initiate pyroptotic cell death similar to GSDMD32. To test the association of GSDME with the TH17 cell subset, we assessed whether TH17 cell-polarizing cytokines coregulate GSDME. Only the combination of TGF-β with IL-1β (TH17), but not IL-12 (TH1) or IL-4 (TH2), increased GSDME transcript levels as assessed by reverse transcription–quantitative PCR (RT–qPCR) (Fig. 6b). This was supported by the existence of putative binding sites for the TH17 cell-associated transcription factors ROR-α and BATF (basic leucine zipper transcription factor, ATF-like) in the promotor regions of GSDME (Extended Data Fig. 8a,b)33. GSDMD expression, in contrast, was not regulated by T cell-polarizing cytokines (Supplementary Fig. 8).
Fig. 6. The NLRP3–casp8/3 cleavage cascade leads to GSDME pores for IL-1α release.
a, Differential gene expression determined by transcriptome analysis of TH17 cells treated as in Fig. 2c (n = 3 individual healthy blood donors). b, RT–qPCR analysis of anti-CD3 and anti-CD28 monoclonal antibody-stimulated, naive T cells in polarizing cytokine conditions (n = 4, one-way ANOVA with Dunnett’s multiple-comparison test). c, Immunoblot analysis of cell lysates from TH17 cells stimulated with anti-CD3 and anti-CD28 monoclonal antibodies for different durations. The data represent three experiments. d, ELISA of cell culture supernatants from TH17 cells with and without deletion of GSDME (left) or GSDMD (right) by CRISPR–Cas9 technology. Individual experiments were normalized to the first time point of analysis on day 2 (n = 3 individual biological samples, two-way ANOVA with Bonferroni’s multiple-comparison test). e, Immunoblot analysis of cell lysates from TH17 cells stimulated with anti-CD3 and anti-CD28 monoclonal antibodies for the indicated time points and of CD14+ monocytes stimulated for 24 h with LPS and 30 min with nigericin. Casp, Caspase. f, Lane view of electropherograms obtained with a Jess Simple Western System for cell lysates of TH17 cells stimulated for 5 d as in e in the presence or absence of the indicated inhibitors. It is a representative experiment. g, Cumulative data of f (one-sample Student’s t-test). AUC, area under the curve. h, Luminex assay of the supernatants of TH17 cells stimulated with plate-bound anti-CD3 (1 μg ml−1, TR66) and phorbol-12,13-dibutyrate for 8 h on day 4 of culture (n = 3 individual biological samples, two-tailed, paired Student’s t-test). i, ELISA of supernatants of TH17 cells stimulated as in f. Each circle indicates an independent blood donor in h and i (n = 4 individual biological samples; two-tailed, paired Student’s t-test).
Extended Data Fig. 8. The GSDME promoter and intronic enhancer region display consensus binding sites for TCR-induced transcription factors as well as for Th17 master regulators.
a, Locations of consensus binding sites of TCR-induced (NFATC-family, NF-κB-family, AP1-family and BATF) and TGFβ-induced (SMAD 2,3,4) transcription factors as well as the Th17 transcription factors RORα and BATF in the regulatory region of GSDME (chr7:24,754,387-24,758,496). The regulatory region and the consensus binding sites were defined and annotated as in Extended Data Fig.6. b, The consensus binding motives were aligned with predicted binding sites in the GSDME promotor/enhancer region. The binding sites with the highest score for each family are indicated. NFATC3-GSDME.1 (chr7:24756897-24756905), RELA-GSDME.1 (chr7:24757878-24757887), FOS::JUN (chr7:24755766-24755775), BATF-GSDME.1 (chr7:24755766-24755776), SMAD4 (chr7:24754928-24754935), RORα (chr7:24758120-24758133).
This result prompted us to evaluate GSDME expression at the protein level in human TH17 cells. The GSDME pro-form was inducible on TCR activation. GSDME protein induction in response to this adaptive immune signal was unexpected, but further supported by the existence of putative binding sites of TCR signaling-responsive transcription factors such as NFAT, FOS, JUN and RELA in GSDME promotor regions (Extended Data Fig. 8a,b). GSDME was expressed as early as 24 h after polyclonal stimulation. The cleaved N-terminal pore-forming GSDME was detectable at late time points, 3–4 d after TCR stimulation of TH17 cells (Fig. 6c). Full-length GSDMD was concomitantly induced on T cell activation. In contrast, no GSDMD cleavage was observed, as predicted from the absence of caspase-1 and IL-1β secretion, leaving the role of GSDMD in TH17 cells open for further analysis (Fig. 6c).
We next aimed to explore whether GSDME pores serve as conduits for the extracellular release of IL-1α in TH17 cells. We therefore knocked out GSDME with CRISPR–Cas9 technology and monitored IL-1α release into the supernatant over time by ELISA. The absence of GSDME, but not GSDMD, significantly inhibited the release of IL-1α by TH17 cells (Fig. 6d). This clearly demonstrates that GSDME pore formation serves as the mechanism for unconventional IL-1α release by human TH17 cells.
The caspase-8/3 GSDME cleavage cascade enables NLRP3-dependent IL-1α secretion
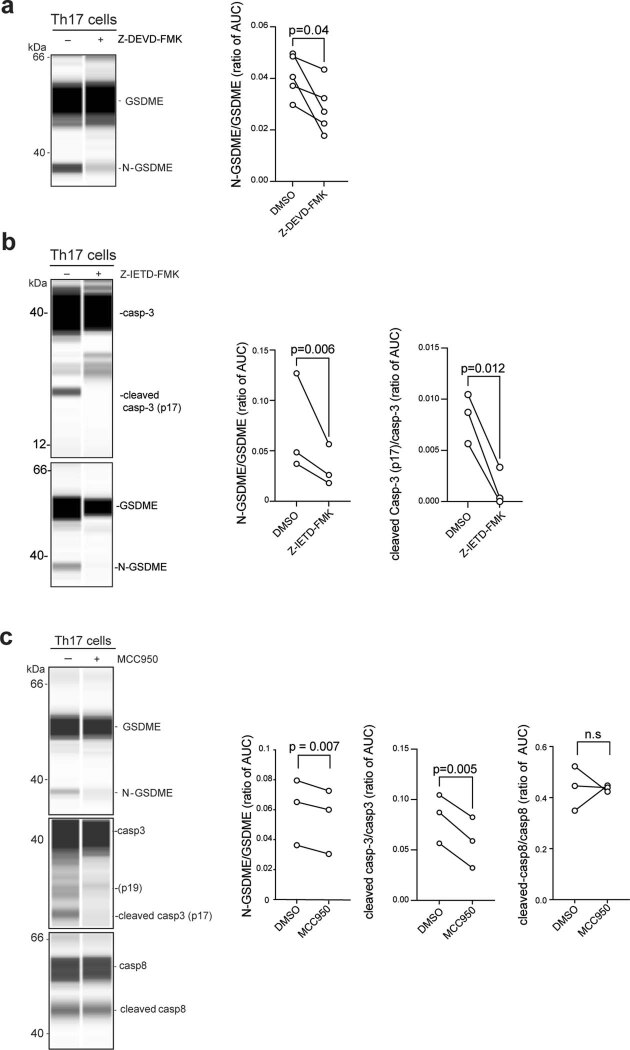
We next explored the possibility of mechanistic crosstalk between NLRP3 inflammasome activation and GSDME cleavage in human TH17 cells. Caspase-3 has recently been shown to cleave GSDME, which in turn is a target of the NLRP3 inflammasome interactor caspase-8 (refs. 34, 35). Indeed, both pro-caspase-8 and pro-caspase-3 were detected in TH17 cells. We found that cleavage of both caspases occurred on TCR stimulation and preceded GSDME cleavage (Fig. 6e). In contrast, no cleaved products of caspase-8 and caspase-3 were detected in nigericin- and LPS-stimulated monocytes (Fig. 6e). To establish a causative role for these caspases in the cleavage of GSDME and the secretion of IL-1α by T cells, we pharmacologically blocked caspase-3 or caspase-8 activity with the inhibitor Z-DEVD-FMK or Z-IETD-FMK, respectively. Inhibition of caspase-8 activity with Z-IETD-FMK abrogated the cleavage of the downstream target caspase-3, in line with previous reports on other cell types36. Both treatments reduced GSDME cleavage while also abrogating IL-1α secretion (Fig. 6f–i and Extended Data Fig. 9a,b). Inhibition of caspase-1, instead, did not affect caspase-3 or GSDME cleavage (Supplementary Fig. 9). These data clearly demonstrated that the caspase-8–caspase-3–GSDME axis was operating in human TH17 cells on TCR activation and that it regulated IL-1α secretion in these cells.
Extended Data Fig. 9. Pharmacological inhibition shows that GSDME pore formation is regulated by each component of the NLRP3 inflammasome – casp8 – casp3 cleavage cascade.
The lane view of electropherograms obtained with the Jess Simple Western System (ProteinSimple) is shown. Th17 cells were sorted according to the differential expression of chemokine receptors and stimulated for 5 days with anti-CD3 and anti-CD28 mAbs (48 h plate-bound) in the presence or absence of specific inhibitors for caspase-3 (a), caspase-8 (b) or the NLRP3 inflammasome (c). The inhibitors were added on day 3 of the 5-day culture period. The AUC was calculated with the Jess software Compass for SW as the ratio of the cleaved versus noncleaved inhibitor target proteins after normalization to total protein. Two-sided paired t-tests.
To finally establish the link between this proteolytic cleavage cascade and the NLRP3 inflammasome, we applied MCC950 to stimulated TH17 cells, which, indeed, produced a significant reduction in caspase-3 and GSDME cleavage on day 5 (Fig. 6f,g and Extended Data Fig. 9c). A reduction in caspase-8 cleavage was, however, less pronounced at the same time point of analysis, which was in line with its earlier activation time window (Fig. 6e,f). In summary, targeted inhibition of each individual molecular player established the NLRP3 inflammasome–caspase-8–caspase-3–GSDME cascade as the proteolytic pathway involved in the extracellular release of bioactive IL-1α by human TH17 cells.
TH17 cells are resilient to pyroptosis despite GSDME pores
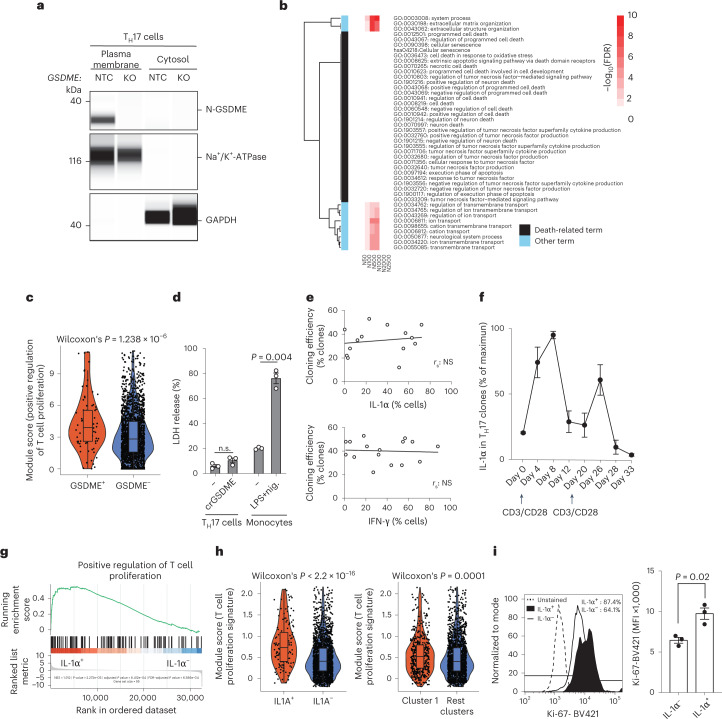
Gasdermin pore formation has previously been associated with pyroptotic cell death in a variety of cell types13,14,37. We found expression of the cleaved pore-forming N-GSDME unit in the plasma membrane but not the cytosol, which supported the pore-forming function of GSDME in T cells (Fig. 7a). Surprisingly, a transcriptomic comparison of GSDME-intact and CRISPR–Cas9 gene-edited, GSDME-deficient bulk human TH17 cells by GSEA excluded differences in various forms of cell death; however, notably, it revealed that the affected processes and pathways in GSDME-deficient TH17 cells were mainly related to genes controlling transmembrane transport, in line with the pore-forming conduits formed by N-GSDME (Fig. 7b and Supplementary Fig. 10). A single-cell transcriptomic comparison of individual TH17 cells selected for the presence versus the absence of GSDME expression supported the viability of GSDME-expressing TH17 cells by their gene set enrichment for proliferation (Fig. 7c). We finally validated the resilience of human GSDME-expressing TH17 cells to pyroptosis by demonstrating the absence of any difference in lactate dehydrogenase (LDH) release across CRISPR–Cas9 gene-edited, GSDME-deficient and GSDME-intact TH17 cells on TCR stimulation (Fig. 7d).
Fig. 7. TH17 cells are resilient to pyroptosis despite GSDME plasma membrane pores.
a, Representative electropherogram obtained with a Jess Simple Western System after normalization to total protein. TH17 cells were stimulated with anti-CD3 and anti-CD28 monoclonal antibodies for 48 h and then transfected with RNPs containing an NTC or crGSDME (KO). The TH17 cells were then expanded for another 7 d. The data represent three experiments. b, Heatmap with gene sets constructed based on the fold-changes of the genes (see Supplementary Fig. 10). All annotation terms significant in at least three gene sets (FDR ≤ 5%) are shown. The observed −log10(FDR) values were capped at 10 for ease of visualization. In addition, all cell death-associated annotation terms are shown. c, Gene set expression comparison using a GO term in TH17 cells analyzed by scRNA-seq after grouping into GSDME+ and GSDME– TH17 cells (Wilcoxon’s rank-sum test). d, CytoTox 96 Non-Radioactive Cytotoxicity Assay from TH17 cells with and without CRISPR–Cas9 gene editing for GSDME stimulated with anti-CD3 and anti-CD28 monoclonal antibodies or from monocytes stimulated with or without LPS and nigericin (24 h). Supernatants from washed TH17 cell cultures were collected between days 4 and 5 of stimulation or from monocytes 24 h after stimulation (paired Student’s t-test). e, Cloning efficiency of TH17 cell clones with varying degrees of IL-1α expression (top) and of control TH cell clones with varying degrees of IFN-γ expression, but lacking IL-1α coexpression (bottom) as assessed by intracellular cytokine staining. f, Intracellular staining and flow cytometric analysis of TH17 cell clones after repetitive restimulation with anti-CD3 and anti-CD28 monoclonal antibodies (n = 5 individual TH17 cell clones). g, GSEA of IL-1α+ compared with IL-1α– TH17 cell clones. h, Gene set expression comparison after scRNA-seq as in c. i, Flow cytometric analysis of TH17 cells stimulated for 5 d with anti-CD3 and anti-CD28 monoclonal antibodies. Left, representative experiment. Right, cumulative data (n = 3, two-tailed, paired Student’s t-test). Each circle indicates an independent blood donor.
We then compared IL-1α+ and IL-1α– TH17 cells with respect to their viability and proliferation potential, because TH17 cells did not display any IL-1α surface expression in contrast to IL-1α-producing cells of other cellular lineages, such as monocytes (Extended Data Fig. 5d). This comparison of IL-1α+ and IL-1α– TH17 cells necessitated the establishment of a homemade IL-1α-secretion assay, enabling isolation of IL-1α+-viable TH17 cells by capture of secreted autocrine IL-1α to the cell surface after phorbol-12-myristate-13-acetate (PMA) and ionomycin stimulation. No difference in the cloning efficiency of TH17 cells that were deposited as single cells after sorting for the presence or absence of surface IL-1α was observed (Supplementary Fig. 11). This finding excluded differences in the viability and expansion of IL-1α+ and IL-1α– TH17 cells at the single-cell level.
We further recloned TH17 clones that were screened, based on varying degrees of intracellular IL-1α expression, and monitored their respective cloning efficiency. If GSDME-enabled IL-1α release was associated with pyroptotic cell death, then an inverse correlation between IL-1α expression in T cell clones and the frequency of growing clones on their individual recloning (cloning efficiency) was to be expected. However, the T cell recloning efficiency was independent of IL-1α expression levels in the TH17 cell clones and was instead similar to that of control T cell clones, selected on the basis of varying expression levels of IFN-γ in the absence of IL-1α coexpression (Fig. 7e). Importantly, IL-1α+ TH17 cell clones continued to re-express IL-1α on repetitive TCR restimulation cycles (Fig. 7f). Thus, a cell death-associated loss of IL-1α-producing cells from their respective clonal T cell population on restimulation was excluded. Notably, this finding indicates a T cell cytokine memory for reinducible IL-1α.
We performed a transcriptomic comparison between bulk IL-1α+ and IL-1α– TH17 cells and observed even greater enrichment for proliferation gene sets in IL-1α+ compared with IL-1α– TH17 cell clones (Fig. 7g). Single-cell transcriptomic comparison of IL1A+ versus IL1A– TH17 cells corroborated the transcriptomic enrichment for proliferation. This was also the case for the comparison of Leiden cluster 1, which was enriched for IL1A-expressing TH17 cells and inflammatory signatures, with all other clusters (Fig. 7h). IL-1α+ TH17 cells displayed higher Ki67 expression according to flow cytometric analysis than their IL-1α– counterparts after 5 d of polyclonal stimulation (Fig. 7i), again supporting the idea that in human TH17 cells IL-1α exit is not associated with cell death, unlike in innate cells.
Cumulatively, these data exclude an association of IL-1α production with (pyroptotic) cell death even at the single-cell level and demonstrate that human T cells have a cytokine memory for IL-1α production on repetitive TCR restimulation.
T cell-derived IL-1α contributes to antifungal host defense
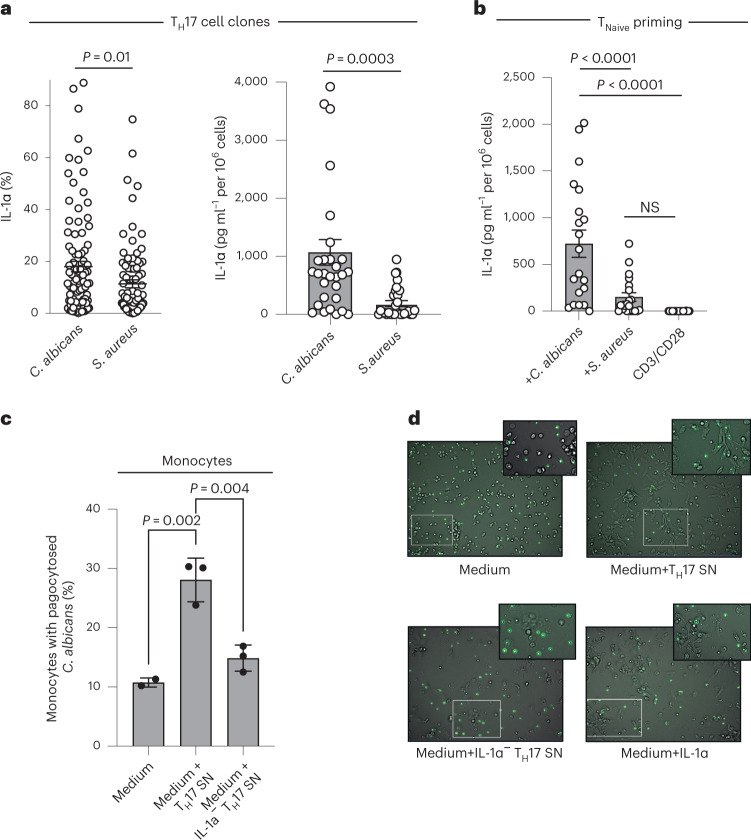
Our finding that human TH17 cells produce the innate danger signal IL-1α and repurpose an innate signaling machinery for its extracellular release blurs the distinction of adaptive versus innate immune responses and thus extends the overall functional repertoire of T cells. A critical feature that remains characteristic for adaptive memory responses is TCR-endowed antigen specificity. We therefore investigated whether the ability of human TH17 cells to produce IL-1α is restricted to specific antigen specificities. TH17 cells have previously been shown to be highly enriched within cells specific for C. albicans and Staphylococcus aureus antigens15. It is interesting that we observed significantly greater IL-1α expression and secretion by C. albicans-specific than by S. aureus-specific TH17 cell clones (Fig. 8a). We next investigated whether naive T cells recognizing C. albicans rather than S. aureus would be primed by their cognate antigen to selectively produce IL-1α. Naive (CD45RA+CCR7+) TH cells were cocultured with autologous monocytes pulsed with heat-inactivated C. albicans or S. aureus antigens or stimulated polyclonally with anti-CD3 and anti-CD28 monoclonal antibodies, and then cloned on day 7 with allogeneic feeder cells. All clones were restimulated on day 14 with anti-CD3 and anti-CD28 monoclonal antibodies for 5 d for ELISA of their supernatants. Strikingly, we found that C. albicans, but not S. aureus or polyclonal TCR activation, induced de novo IL-1α production in human differentiating TH17 cells (Fig. 8b). Our combined ex vivo recall and in vitro priming approach therefore establishes that the ability to produce IL-1α is confined to T cells with TCR specificity for C. albicans.
Fig. 8. TCR specificity controls IL-1α production contributing to C. albicans clearance.
a, Intracellular cytokine staining and flow cytometry (left) and ELISA (right) of C. albicans- versus S. aureus-specific TH17 cell clones from three individual blood donors 14 d after single-cell TH17 cell cloning with irradiated feeder cells and restimulation for 5 d with anti-CD3 and anti-CD28 monoclonal antibodies. The microbial antigen-specific TH17 cells were isolated for subsequent cloning as a carboxyfluorescein succinimidyl ester (CFSE)-negative population after their restimulation with microbe-pulsed autologous monocytes. Each circle indicates an individual T cell clone (n = 30 TH cell clones, 10 clones per healthy blood donor). Data are presented as mean ± s.e.m. (two-tailed, unpaired Student’s t-test). b, Naive TH cells were primed by either C. albicans- or S. aureus-pulsed monocytes. Each circle indicates an individual T cell clone. The ELISA analysis of supernatants after restimulation of each clone with anti-CD3 and anti-CD28 monoclonal antibodies for 5 d is shown (n = 19, 4–5 clones per healthy blood donor; one-way ANOVA with Tukey’s multiple-comparison test). c, Flow cytometric analysis of phagocytosis of FITC-labeled, heat-inactivated C. albicans yeast by monocytes preincubated for 18 h with IL-1α replete or depleted (immunoabsorption or CRISPR–Cas9 KO) TH17 cell supernatants (n = 3 independent biological samples; one-way ANOVA with Tukey’s multiple-comparison test). Each circle indicates an independent blood donor. d, Real-time live cell in vitro imaging (videos in Supplementary Video 1, time points) of monocytes in coculture with FITC-labeled C. albicans as in c. Representative snap shots with magnifications of the videos are shown at a time point 2 h after addition of C. albicans.
We finally tested whether the distinctive ability of C. albicans-specific TH17 cells to produce IL-1α is associated with a physiological role in antifungal host defense. For this, we cocultured human monocytes with supernatants from human TH17 cells after their polyclonal restimulation with anti-CD3 and anti-CD28 monoclonal antibodies for 5 d. We observed significantly increased phagocytosis of FITC-labeled C. albicans by monocytes using flow cytometry. Importantly, the increased C. albicans phagocytosis by TH17 cell supernatants was IL-1α dependent as shown by significant abrogation of C. albicans phagocytosis if TH17 cell supernatants were devoid of IL-1α after immunoabsorption or CRISPR–Cas9-targeted IL-1α depletion in TH17 cells (Fig. 8c). Live cell in vitro imaging further corroborated the increased uptake and elimination of C. albicans by monocytes in the presence of IL-1α-containing TH17 cell supernatants (Fig. 8d, video data). Taken together, these findings strongly suggest that TH17 cells clear C. albicans infections not only via their production of IL-17, as previously thought38, but also, to a significant extent, via the ability of a unique TH17 cell subset to produce IL-1α.
Cumulatively, the findings identifying GSDME pore formation in T cells as an exit strategy for proinflammatory IL-1α and the regulation of GSDME by the NLRP3 inflammasome–caspase-8–caspase-3 axis reveal a new mode of T cell cytokine secretion that is associated with a proinflammatory subset of TH17 cells with antifungal TCR specificities. This provides new therapeutic targets for the modulation of human TH17 cells that are relevant for antifungal host defense and might also participate in the pathogenesis of chronic inflammatory diseases.
Discussion
In summary, our findings reveal a previously unknown biological pathway and cytokine secretion modality in human T cells that diversifies the overall functionality of the T cell population.
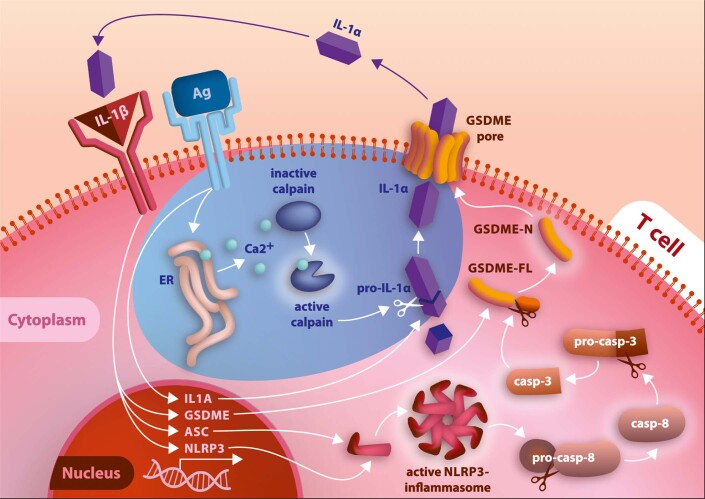
We found that IL-1α expression was uniquely confined to the TH17 cell fate, as evidenced by its coexpression with IL-17A, regulation by ROR-γt, induction by the TH17 cell-priming cytokines IL-1β and TGF-β and by its TH17 cell-associated chemokine receptor expression profile. These findings are consistent with the existence of binding sites for ROR-γt and ROR-α in the IL1Α enhancer and promoter regions as described in the present study and with the previously reported ROR-γt-binding sites in the NLRP3 promotor region39. We further observed that TH17 cell-priming cytokines increased NLRP3 and GSDME expression. Accordingly, master regulators of the TH17 cell fate, such as ROR-α and BATF, as well as multiple TCR-inducible transcription factors, displayed putative binding sites in the GSDME enhancer regions. TH17 cell polarization therefore promoted not only IL-1α expression but also its extracellular exit (Extended Data Fig. 10, graphic summary).
Extended Data Fig. 10. Mechanism of IL-1α production by human Th17 cells: Graphical summary.
The graphic was created with a commercial license from Adobe.
Unexpectedly, we found the NLRP3 inflammasome to be active and repurposed for the release of IL-1α instead of IL-1β in TCR-activated TH17 cells. Unlike innate cells, T cells are not specialized for innate danger sensing, which is known to trigger the assembly of NLRP3 inflammasome components. However, elevation of cytoplasmic Ca2+ has previously been shown to bypass innate danger signaling for NLRP3 inflammasome activation16,40. This is consistent with our finding that TCR activation, which is accompanied by calcium flux, is a requirement for IL-1α release by human TH17 cells.
We observed that TH17 cells engaged an alternative NLRP3 downstream signaling cascade via engagement of caspase-8. This might have been facilitated by the absence of caspase-1 cleavage, because competitive caspase-1 versus caspase-8 inflammasome recruitment has been demonstrated previously34,41. We also found pro-caspase-1 and uncleaved GSDMD in human TH17 cells, but no evidence for their NLRP3 inflammasome-regulated cleavage or for IL-1β production. The expression of their precursors raises the question of whether classic NLRP3 inflammasome signaling and IL-1β release might also operate in human TH17 cells if alternative yet-to-be-identified stimuli are applied. This implies that caspase-8- versus caspase-1-dependent counterregulatory mechanisms might control a dichotomy of IL-1α versus IL-1β production by T cells, consolidating previously suggested roles for the NLRP3 inflammasome in the release of IL-1β in human TH1 cells42 and murine TH17 cells43.
An intriguing observation of our study was identification of GSDME expression and cleavage in T cells. This revealed that unconventional cytokine secretion via membrane pores can occur in T cells. Several of the functions recently assigned to GSDME have been associated with pyroptosis and subsequent enhancement of tumor cell death and an inflammatory microenvironment32,44. Surprisingly, we found that GSDME-expressing TH17 cells instead displayed preserved viability and continued proliferation on repetitive TCR stimulation compared with GSDME-deficient T cells. The same results were observed for IL-1α+ compared with IL-1α– T cells. These findings were unexpected, considering that IL-1α production has thus far been considered a hallmark of senescence and thus of replication-arrested or dying cells45. This evokes the idea that the danger signal IL-1α can be part of a T cell-associated cytokine memory that is re-excitable on cognate antigen recognition46. Furthermore, the GSDME pores might serve a physiological function to enable TH17 cells to release additional as-yet-unidentified molecules beyond IL-1α that are defined by their size or charge47. This would be consistent with the results of our unbiased transcriptomic comparison of GSDME-intact or CRISPR–Cas9-engineered, GSDME-deficient TH17 cells, which revealed multiple roles for GSDME in transmembrane transport but not cell death. The mechanism, by which the viability and long-term IL-1α cytokine memory in T cells with GSDME pores is preserved, remains to be explored in the future.
The availability of IL-1α from different cellular sources, particularly from innate APCs, raises the question about the relative contribution of T cell-derived IL-1α to human health and disease. Although IL-1α+ T cells constitute only a small subset within the T cell lineage, their ability to produce IL-1α was quantitatively comparable to that of LPS-stimulated monocytes, supporting the new concept that T cells can serve as a relevant source of inflammatory IL-1α. Our findings from transcriptomic and functional analyses reveal IL-1α to be associated with the proinflammatory fate of TH17 cells. The IL-1α+ subpopulation of human TH17 cells displayed transcriptomic signatures of enhanced inflammatory pathogenicity and associations with chronic inflammatory diseases. The significantly enhanced expression of IL-1α by circulating TH17 cells from patients with JIA and their abundant localization in the inflamed synovial fluid support this pathogenic potential of the IL-1α+ subset of TH17 cells. A rigorous causal relationship between IL-1α-producing TH17 cells and the pathogenesis of JIA still remains to be established and the relative impact of IL-1α+ TH17 cells validated in future clinical trials.
A striking observation was that IL-1α production by T cells is hard-wired through their TCR specificity. Although IL-1α production by innate cellular sources is triggered by nonspecific stress stimuli16, we found IL-1α production by human TH17 cells to be associated with a TCR specificity for C. albicans. S. aureus-specific TH17 cells, instead, displayed significantly reduced IL-1α production. These findings are consistent with the differential requirement of IL-1β for the generation of C. albicans- but not S. aureus-specific TH17 cells, as previously reported4 and, accordingly, with the critical role of IL-1β for the induction of IL-1α expression, as reported here. In addition, we found IL-1α secretion to be dependent on TCR stimulation and calcium signals, stressing its tight association with specific adaptive immune signaling via the TCR.
TH17 cells are known to be the protagonists for the clearance of C. albicans infections through their secretion of IL-17, which is exemplified by C. albicans dysbiosis in settings of genetic or therapeutic IL-17 deficiencies38. We found the TH17 cell product IL-1α to be involved in C. albicans clearance because its absence in TH17 cell supernatants significantly reduced C. albicans phagocytosis by monocytes. This suggests that antifungal TH17 cell effector functions are exerted not only through IL-17A/F, as previously suggested, but also through IL-1α in a TCR-specific manner. Whether aberrant regulation of the molecular pathway leading to IL-1α production by TH17 cells could predispose to compromised antifungal host defense will therefore need to be tested in the future.
Cumulatively, our findings pave the way for a systematic investigation of the contributions of IL-1α-producing TH17 cells in various inflammatory diseases and antifungal host defense. The TCR–NLRP3 inflammasome–caspase-8–caspase-3–GSDME axis not only represents a previously overlooked mode of immune signaling and fate instruction in TH cells but also provides molecular targets to either disrupt a pathogenic TH17 cell identity or to harness it for host defense.
Methods
Cell purification and sorting
PBMCs were isolated by density gradient centrifugation using Ficoll-Paque Plus (GE Healthcare). CD4+ T cells were isolated from fresh PBMCs by positive selection with CD4-specific MicroBeads (Miltenyi Biotec) using an autoMACS Pro Separator. TH cell subsets were sorted to at least 98% purity as follows: TH1 cell subset, CXCR3+CCR4–CCR6–CD45RA–CD25–CD14–; TH2 cell subset, CXCR3–CCR4+CCR6–CD45RA–CD25–CD14–; and TH17 cell subset, CXCR3–CCR4+CCR6+CD45RA–CD25–CD14–, as described previously4,6,48. Memory TH cells were isolated as CD3+CD14–CD4+CD45RA– lymphocytes, and naive T cells were isolated as CD3+CD14–CD4+CD45RA+CD45RO–CCR7+ lymphocytes to a purity >98%. Cells were stained with the following fluorochrome-conjugated antibodies: CCR4-PE/Cy7 (1:200), CCR6-BV421 (1:100), CD14-PacificBlue (1:200–1:400), CD3-FITC (1:150), CD3-APC (1:100), CD4-APC/Cy7 (1:300), CD45RA-FITC (1:200), CD8-PacificBlue (1:100), CCR7-PE (1:50) and CD25-BV421 (1:100) (all from BioLegend); CCR6-PE (1:50) and CXCR3-APC (1:10) (both from BD). TH cells were sorted with a BD FACSAria III (BD Biosciences) and a BD FACSAria Fusion (BD Biosciences) or an Aurora CS Sorter. Ethical approval for the use of healthy control and patient PBMCs was obtained from the Institutional Review Board of the Technical University of Munich (195/15s, 491/16S, 146/17S), the Charité-Universitätsmedizin Berlin (EA1/221/11) and the Friedrich Schiller University Jena (2020-1984_1). Synovial fluid was obtained from patients with JIA and active disease (oligoarthritis) undergoing therapeutic joint aspiration, with approval of the local ethics committee of the University Medical Center Utrecht. All experiments involving humans were carried out in accordance with the Declaration of Helsinki.
Cell culture
Human T cells were cultured as described previously48. In some experiments, T cell culture was performed in the presence of recombinant cytokines (IL-6, 50 ng ml−1; IL-12, 10 ng ml−1; IL-4, 10 ng ml−1; TGF-β, 10 ng ml−1; IL-1β, 20 ng ml−1; all from R&D Systems) or neutralizing antibodies (anti-IL-1α, 10 μg ml−1, BD Biosciences). The cell cultures were supplemented with the following pharmacological inhibitors where indicated: Z-IETD-FMK (40 µM, R&D Systems), Z-DEVD-FMK (40 µM, R&D Systems), MCC950 (10 µM, R&D Systems), calpain inhibitor II N-acetyl-l-leucyl-l-leucyl-l-methionine (0.1–10 µg ml−1, R&D Systems), thapsigargin (1 mM, EMD Millipore), Ac-YVAD-CMK (50 µM, R&D Systems) and GSK2981278 (10 µM, Cayman Chemical). T cells were stimulated with plate-bound anti-CD3 (2 μg ml−1, clone TR66) and anti-CD28 monoclonal antibodies (2 μg ml−1, clone CD28.2; both from BD Biosciences) for 48 h before transfer into uncoated wells for another 3 d for a total culture period of 5 d, unless indicated otherwise in the legends. Supernatants from these 5-d cultures were used for phagocytosis assays with monocytes and heat-killed, FITC-labeled C. albicans yeast. T cell clones were generated in nonpolarizing conditions as described previously after single-cell deposition with FACS or by limiting dilution cloning (Messi, 2003 no. 70). Human monocytes were isolated from PBMCs by positive selection with CD14-specific MicroBeads (Miltenyi Biotec). Cells were stimulated with or without 1 μg ml−1 of ultrapure LPS-EB (catalog no. tlrl-3pelps, InvivoGen) for 24 h and nigericin (10 μg ml−1, InvivoGen) or ATP (5 mM, Thermo Fisher Scientific) for the last 30 min. In some experiments, CD14+ magnetic activated cell sorting (MACS)-sorted monocytes were differentiated into macrophages for 7 d in the presence of granulocyte–macrophage colony-stimulating factor (R&D Systems).
Pathogen-specific assays
C. albicans and S. aureus lysates were prepared as described previously9,49. Autologous monocytes were isolated by positive selection with CD14-specific microbeads (Miltenyi Biotec) and pulsed with the pathogen lysates for 3 h. TH17 cells or naive CD4+ T cells were isolated as described above and labeled with CellTrace Violet (CTV; Invitrogen) according to the manufacturer’s recommendations and cocultured with pathogen-pulsed monocytes at a ratio of 2:1 for 7 d. CTV− TH17 cells were FACS sorted and cloned by limiting dilution as described previously4,50. Intracellular cytokine staining of TH17 cell clones and flow cytometry with a CytoFLEX (Beckman Coulter) were performed on day 14. CTV− microbe-primed TH cells originating from seeded naive T cells were FACS sorted on day 7, cloned and restimulated on day 14 with anti-CD3 and anti-CD28 monoclonal antibodies for 5 d to harvest their supernatant.
C. albicans killing and phagocytosis assay
FITC-labeled, heat-killed C. albicans yeast was prepared as described before and cocultured at a 1:3 ratio with CD14+ monocytes for 2 h after preincubation of monocytes for 18 h in the presence or absence of TH17 cell supernatants, which were selectively depleted for IL-1α by immunoabsorption (R&D Systems, Human IL-1 alpha/IL-1F1 DuoSet ELISA) or by CRISPR–Cas9 engineering of TH17 cells51. Phagocytosis by monocytes was determined by the fraction of FITC-positive staining among CD14+ monocytes using flow cytometry. In addition, live cell imaging was performed using the same experimental conditions with the Celldiscoverer 7 Live Cell Imaging System (Zeiss) and an integrated AxioCam 506 using Zeiss Zen Blue software at a constant temperature set to 37 °C and 5% CO2. Four indepe ndent fields per well were imaged in 10-min intervals at ×10 (numerical aperture 0.35) magnification for a period of 5 h using the bright-field channel and green fluorescence filter (full videos provided as Supplementary Video 1).
LDH assay
LDH activity was determined with a CytoTox 96 Non-Radioactive Cytotoxicity Assay (catalog no. G1780, Promega). In short, the supernatants were collected from cells stimulated for 24 h in RPMI-1640 medium without phenol red (Gibco). Relative LDH release was calculated as follows: LDH release (%) = 100 × (Experimental LDH release (OD490) − Unstimulated control (OD490))/(Lysis control (OD490) − Unstimulated control (OD490)) where OD490 is the optical density at 490 nm.
CRISPR–Cas9 KO cells
Candidate genes were depleted in sorted cells by using the Alt-R CRISPR–Cas9 system (Integrated DNA Technologies (IDT)) after activation with plate-bound anti-CD3 and anti-CD28 for 3 d. In brief, cripsr (cr)RNA and trans-activating crRNA (tracrRNA; both from IDT) were mixed at a 1:1 ratio, heated at 95 °C for 5 min and cooled to room temperature. Then, 44 μM crRNA:tracrRNA duplex was incubated at a 1:1 ratio with 36 μM Cas9 protein (IDT) for 20 min at room temperature to form a ribonucleoprotein (RNP) complex. A total of (5–10) × 106 activated T cells were washed with phosphate-buffered saline (PBS) and resuspended in 10 μl of R buffer (Neon transfection kit, Invitrogen). The RNP complex was delivered into cells with a Neon transfection system (10 μl of sample, 1,600 V, 10-ms pulse width, 3 pulses) (Thermo Fisher Scientific). The electroporated cells were then immediately incubated with RPMI-1640 complete medium with IL-2 (500 IU). The following crRNAs were used: GTCGGACTTTGTGAAATACG (GSDME), ACGCGCACCCACAAGCGGGA (GSDMD), GTCGGAGGAGATCATCACGC (CAPN1), GGCTTCGAAGACTTCACCGG (CAPN2), GGTAGTAGCAACCAACGGGA (IL1A), CGGCTTGACTTGTCCATTAT (CASP1) and GTATTACTGATATTGGTGGG (control sequence, nontargeted control (NTC)). Knockout (KO) efficiency was evaluated on day 7 after electroporation by immunoblotting or ELISA.
Cytokine and transcription factor analyses
Intracellular cytokine and transcription factor staining was performed as described before with PMA and ionomycin restimulation in the presence of BFA48. Cells were stained with the following antibodies: anti-IL-1α−phycoerythrin (PE) (catalog no. 364−3Β3−14, 1ː50), anti-IL-1β-Alexa Fluor-647 (catalog no. JK1B-1, 1:50), anti-IL-4-BV421 (catalog no. MP4-25D2 5, 1:200), anti-IL-17A-PacificBlue (catalog no. BL168, 1:100), anti-IFN-γ−APC-Cy7 (catalog no. 4 S.Β3, 1ː300) and anti-IL-10-PE-Cy7 (catalog no. JES3-9D7, 1:50) (all from BioLegend); anti-ROR-γt–APC (catalog no. AFKJS-9, eBioscience, 1:10), anti-Ki67-BV421 (BioLegend, 1:10) and anti-IL-1R1-PE (catalog no. FAB269P, R&D Systems, 1:20,). Then, they were analyzed with a BD LSRFortessa (BD Biosciences), a CytoFLEX Flow Cytometer (Beckman Coulter) or a MACSQuant analyzer (Miltenyi Biotec). Flow cytometry data were analyzed with FlowJo software (TreeStar) or Cytobank (Cytobank Inc.). The concentrations of cytokines in cell culture supernatants were measured by ELISA (Duoset ELISA kits from R&D Systems), Human Caspase-1 SimpleStep ELISA Kit (Abcam) or Luminex assays (eBioscience) according to standard protocols as indicated in the corresponding figure legends. Counting beads (CountBright Absolute Counting Beads, Thermo Fisher Scientific) were used to normalize for cell numbers if analysis of cumulative supernatants obtained from 5-d cell cultures was performed.
IL-1α secretion assay
The design of the IL-1α secretion assay was adapted based on a previous report50. TH17 cells (1 × 106) were stained with 1 mg ml−1 of sulfo-NHS-LC-biotin (catalog no. ab145611, Abcam), incubated for 30 min at room temperature and then washed 3× with PBS (pH 8) supplemented with 100 mM glycine. The final washing of cells was performed with PBS supplemented with 0.5% bovine serum albumin. Cell surface biotinylation was validated with PE-labeled streptavidin (catalog no. 554061, BD Pharmingen). Purified anti-human IL-1α antibodies (AF-200-NA, R&D) were labeled with streptavidin using a Lightning-Link Streptavidin Conjugation kit (catalog no. ab102921, Abcam). For cytokine secretion, cells were stimulated with anti-CD3 and anti-CD28 for 72 h. The cells were collected and labeled with streptavidin-IL-1α and incubated for 24 h on the MACSmix tube rotator (Miltenyi Biotec). Recombinant IL-1α (Miltenyi Biotec) was added as a positive control. The cells were then stained with a PE-labeled IL-1α antibody (clone 364-3B3-14, BioLegend, 1:50).
Imaging flow cytometry
Data acquisition was performed using an ImageStream X Mk II imaging flow cytometer (AMNIS, MERCK Millipore) equipped with INSPIRE software. Briefly, a ×60 magnification was used to acquire images with a minimum of 5,000 cells per sample. The following antibodies were used: anti-ASC-PE (catalog no. HASC-71, BioLegend, 1:50), anti-CD3-APC (1:100) or anti-CD3-FITC (catalog no. UCTH1, BioLegend, 1:150) and anti-NLRP3-APC (catalog no. REA668, Miltenyi Biotec, 1:50). Data analysis was performed using IDEAS 6.0 software. A compensation matrix was generated using single-stained cells. Cells that were not in the field of focus, clumped cells and debris were excluded. IDEAS software was used to design masks to define the properties of the spots. For ASC spots, a size of 1–4 µm and a signal:background ratio of 3.0–5.0 were chosen. The mask was trained on at least ten different images with spot-like structures clearly visible to refine the cutoff for the signal:background ratio. From this ‘spot mask’, the diameter of the mask was measured and ASC spots in the range of 1–4 µm were considered to be true spots.
Gene expression analysis
For analysis of individual gene expression, a high-capacity complementary DNA reverse transcription kit (Applied Biosystems) was used for cDNA synthesis according to the manufacturer’s protocol. The transcripts were quantified by RT–qPCR with predesigned TaqMan Gene Expression Assays (IL1A, catalog no. HS00174092-m1; IL1B, catalog no. Hs01555410_m1; NLRP3, catalog no. Hs00918082_m1; CASP1, catalog no. Hs00354836_m1, CAPN2, catalog no. Hs00965097_m1; GSDMD, catalog no. Hs00986739_g1; DFNA5, catalog no. Hs00903185_m1; and 18 S, catalog no. Hs03928990_g1) and reagents (Applied Biosystems). The mRNA abundance was normalized to the amount of 18S ribosomal RNA and expressed as arbitrary units (a.u.).
For microarray analysis (Gene Expression Omnibus (GEO), accession no. GSE214519), total RNA was extracted from pro- and anti-inflammatory TH17 cells that were obtained on restimulation with and without IL-1β, respectively, as described previously4,6, using an RNA MiniPrep kit (Zymo Research), and hybridized to the Human Genome U133 Plus 2 platform (Affymetrix) according to a whole-transcriptome Pico Kit. The raw signals were processed with the affy R package52 and normalized using the robust multiarray average expression measure with background correction and crosschip quantile normalization. The limma R package53 was applied to identify DEGs using linear model fitting, with adjustment for differences between biological replicates. Empirical Bayes statistics was used for the moderation of s.e.m. and P values were adjusted using the Benjamini–Hochberg method. A false discovery rate (FDR) <0.05 and a fold-change cutoff of 2 were used to define the DEGs. For GSEA, the top 50 upregulated genes (proinflammatory, 44 significant DEGs) and the top 50 downregulated genes (anti-inflammatory, 41 significant DEGs) genes from a transcriptomic comparison of IL-10+ and IL-10– TH17 cell clones from a public dataset7 were selected as gene sets and utilized to interrogate the TH17 cell transcriptomes (microarray) after cell stimulation in the presence or absence of IL-1β.
For next-generation mRNA-seq, resting T cell clones categorized as IL-1α+ (>30% IL-1α expression) and IL-1α– (0% IL-1α expression) were restimulated with PMA and ionomycin (both from Sigma-Aldrich) for 3 h (GEO accession no. GSE214475). A total amount of 1 µg of RNA per sample was used as the input material for the RNA sample preparations. Sequencing libraries were generated using an NEBNext Ultra RNA Library Prep Kit for Illumina (New England Biolabs (NEB)) following the manufacturer’s recommendations and index codes were added to attribute sequences to each sample. The mRNA was purified from total RNA using poly(T) oligo-attached magnetic beads. Fragmentation was carried out by using divalent cations under an elevated temperature in NEB Next First Strand Synthesis Reaction Buffer (5×) or sonication with a Diagenode Bioruptor Pico for fragmentation of RNA strands. First-strand cDNA was synthesized using random hexamer primers and M-MuL V Reverse Transcriptase (RNase H-). Second-strand cDNA synthesis was subsequently performed using DNA polymerase I and RNase H. The remaining overhangs were converted into blunt ends via exonuclease/polymerase activity. After adenylation of the 3′-ends of the DNA fragments, NEBNext adapters with a hairpin loop structure were ligated to prepare the fragments for hybridization. To preferentially select cDNA fragments 150–200 bp in length, the library fragments were purified with an AMPure XP system (Beckman Coulter). Then, 3 µI of USER Enzyme (NEB) was used with size-selected, adapter-ligated cDNA at 37 °C for 15 min followed by 5 min at 95 °C before PCR. Then PCR was performed with Phusion High-Fidelity DNA Polymerase, Universal PCR primers and Index (X) Primer. Finally, the PCR products were purified (AMPure XP system) and library quality was assessed on an Agilent Bioanalyzer 2100 system. Clustering of the index-coded samples was performed on a cBot Cluster Generation System using a PE Cluster Kit cBot-HS (Illumina) according to the manufacturer’s instructions. After cluster generation, the libraries were sequenced on an Illumina platform and paired-end reads were generated (Novogene).
For comparison of GSDME-intact (NTC) and CRISPR–Cas9-deficient (KO) TH17 cells (GEO accession no. GSE214292), three matched blood samples were analyzed by mRNA-seq. Quality control was performed using FastQC (v.0.11.9)54. STAR (v.2.7.5a) was used with the ‘quantMode GeneCounts’ option and the other parameters set to the default values to map the reads to the human reference genome (GRCh38; GenBank patch release 13) and count the reads mapped to each gene. Transcriptome annotation was obtained from GENCODE (v.34)55. Differential expression analysis was performed for protein-coding genes (retrieved from BioMart Ensembl Genes release 104)56 using the DESeq2 (v.1.28.1) R package57. Specifically, the DESeq() function was applied with the default parameters to compare the expression levels between KO and NTC samples while controlling for the donor. Significant DEGs were defined as genes with an FDR-adjusted P value ≤0.05 and fold-change ≥2 or ≤0.5. To investigate whether differences in expression were associated with cell death, genes (n = 19,622) were sorted in decreasing order according to the absolute value of their log2(fold-change). Different numbers of genes (50, 100, 500, 1,000, 2,000 and 2,500) from the top of this list were then selected to carry out functional analysis with the DAVID API (v.2021)58, focusing on the categories ‘KEGG_PATHWAY’ and ‘GO_TERM_BP_FAT’, with an FDR cutoff of 100%, a count threshold (minimum gene counts belonging to an annotation term) of 0 and an EASE score threshold of 1. Annotation terms with a minimum gene count of at least 5 and an FDR of 5% in ≥3 of the gene sets were deemed to be associated with expression differences between KO and NTC samples. Gene expression values were obtained using the rlogTransformation() function of the DESeq object. Principal component analysis was performed on gene expression values with the prcomp() R function.
For scRNA-seq, a library of human TH17 cells that were sorted ex vivo as CCR6+CCR4+CXCR3– memory TH cells using FACS and then stimulated with anti-CD3 and anti-CD28 monoclonal antibodies for 4 d (2 d plate-bound) was constructed with Chromium Next GEM Single Cell 5′ Reagents v.2 (Dual Index) (10x Genomics, Inc.) (GEO accession no. GSE214444). The library was sequenced on an Illumina NovaSeq 6000 Sequencing System according to the manufacturer’s instructions, with 150-bp, paired-end, dual-indexing sequencing (sequencing depth: 20,000 read pairs per cell). Read alignment and gene counting of the single-cell datasets were performed with CellRanger v.6.1.1 (10x Genomics, Inc.), using the default parameters and the prebuilt human reference 2020-A (10x Genomics, Inc.) based on Ensembl GRCh38 release 98. The output filtered data were first processed with the Python package scanpy v.1.7.2 and also analyzed with the R package Seurat v.4.0.4. The total count was normalized to 10,000 reads per cell. Each gene was scaled to unit variance and values exceeding the s.d. by ten were clipped. A KNN nearest neighbor graph was constructed with a size of ten local neighboring data points. UMAP with the default settings was applied for dimensionality reduction. Clusters were identified by running the Leiden algorithm with a cluster resolution of 0.4. Differential gene expression analysis was performed using the FindAllMarkers function with the nonparametric Wilcoxon’s rank-sum test from the R package Seurat v.4.0.4.
Pro- and anti-inflammatory gene sets were established from a public dataset after transcriptomic comparison of IL-10– versus IL-10+ TH17 cell clones7. For both gene sets an average expression score was calculated for each individual cell using the addModuleScore method from the R package Seurat. To compare the scores between groups of cells, Wilcoxon’s rank-sum test as implemented in the R package stats was used. Similar comparisons were performed with GO terms taken from the Molecular Signatures Database (MSigDB).
Immunoblotting
Cells were lysed in radioimmunoprecipitation buffer (50 mM Tris, 150 mM NaCl, 1 mM EDTA, 0.1% NP-40, pH 7.5) containing protease inhibitor (Roche) and PhosphoSTOP Easypack (Roche). The protein concentrations of cell lysates were determined with a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Total protein (20-40 mg) was boiled with 4× Laemmli sample buffer (BioRad Laboratories) containing 355 mM 2-mercaptoethanol (Thermo Fisher Scientific) at 99 °C for 10 min. The supernatants and lysates were separated by sodium dodecylsulfate–polyacrylamide gel electrophoresis and transferred to a poly(vinylidene) membrane (BioRad Laboratories) by using a Mini-Protean system (BioRad Laboratories) according to the manufacturer’s protocol. The following primary antibodies were used for immunoblotting: mouse anti-human caspase-8 (Cell Signaling Technology), rabbit anti-human caspase-1 (Cell Signaling Technology), rabbit anti-human IL-1α (Abcam), mouse anti-human glyceraldehyde-2-phosphate dehydrogenase (GAPDH; Merck Millipore), mouse anti-human β-actin (Cell Signaling Technology) and rabbit anti-human GSDME (Abcam), rabbit anti-human caspase-3 (Cell Signaling Technology), mouse anti-human caspase-8 (Cell Signaling Technology), rabbit anti-human GSDMD (Cell Signaling Technology), rabbit anti-human cleaved GSDMD (Cell Signaling Technology) and rabbit anti-NLRP3 (Cell Signaling Technology). Horseradish peroxidase (HRP)-conjugated anti-mouse and anti-rabbit immunoglobulin G antibodies (Cell Signaling Technology) were used as secondary antibodies. The immunoreactive bands were detected by Pierce ECL Western Blotting Substrate or SuperSignal West Femto Maximum Sensitivity Substrate (both from Thermo Fisher Scientific). The chemiluminescence signals were recorded with an Odyssey Imaging system (LI-COR Biosciences) and analyzed on Image Studio Lite (LI-COR Biosciences). Image contrast was enhanced in a linear fashion when necessary. Protein lysates were also prepared for automated immunoblotting using a Jess System (ProteinSimple) according to the manufacturer’s instructions. The following primary and secondary antibodies were used: recombinant rabbit anti-GSDME-N-terminal (Abcam), rabbit anti-GSDMD (Cell Signaling Technology), rabbit anti-caspase-3 (Cell Signaling Technology), mouse anti-caspase-8 (Cell Signaling Technology), mouse anti-ASC (Santa Cruz Biotechnology, B-3), mouse anti-NLRP3 (Novus Biologicals, catalog no. 25N10E9), rabbit recombinant anti-sodium potassium ATPase antibody (Abcam), mouse anti-GAPDH (Sigma-Aldrich) and mouse anti-β-actin (Cell Signaling Technology) primary antibodies, an anti-mouse HRP-linked secondary antibody (ProteinSimple) and an anti-rabbit HRP-linked secondary antibody (ProteinSimple).
Extraction of plasma membrane proteins
Plasma membrane proteins were fractionated with a plasma membrane protein kit (Abcam) according to the manufacturer’s protocol. In short, (0.5–1) × 107 cells were collected, homogenized in an ice-cold Dounce homogenizer (Bellco Glass Inc.) and centrifuged at 700g for 10 min. The supernatants were collected and centrifuged at 10,000g for 30 min. The supernatants were collected as the cytosol fraction. The pellets were used for further extraction of plasma membrane proteins. The purified plasma membrane proteins were enriched in the upper phase solution (Abcam), whereas the lower phase solution contained the cellular organelle membranes. The cytosolic fraction was concentrated with Amicon Ultra Centrifugal Filters (10k). The lysates generated from different fractions were boiled with 4× Laemmli sample buffer (BioRad Laboratories) and subjected to immunoblotting. A rabbit anti-sodium–potassium ATPase antibody (Abcam) was used as a positive control for plasma membrane proteins.
Calpain activity assay
Cells were harvested and washed with cold PBS. Cells were then resuspended in Extraction Buffer (Abcam) and centrifuged at 13,000g for 5 min. The protein concentration in the supernatants was measured with a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Total lysate protein, 40 μg, was used to perform a calpain activity assay (Abcam) following the manufacturer’s instructions. A total of 1–2 μl of active calpain (Abcam) was used as a positive control. Calpain inhibitor Z-LLY-FMK (Abcam), 1 μl, was used as a negative control. The lysates and calpain substrate were incubated at 37 °C for 60 min. The fluorometric signal was detected at excitation/emission wavelengths of 400/505 nm with a CLARIOstar plate reader (BMG-Labtech).
FLICA assays
A FAM-FLICA Caspase-1 Assay Kit and Caspase-8 Assay Kit (ImmunoChemistry Technologies, LLC) were used to evaluate the presence of catalytically active forms of caspase-1 p10 and p12 and caspase-8 according to the manufacturer’s instructions. Cells were incubated with 30× FAM-VAD-FMK for 30 min at 37 °C, then washed twice with the apoptosis wash buffer (ImmunoChemistry Technologies, LLC) and analyzed by flow cytometry on a Cytoflex instrument.
Statistical analysis
The use of the statistical tests is indicated in the respective figure legends, with the error bars indicating the mean ± s.e.m. P values ≤0.05 were considered to indicate significance. Analyses were performed using GraphPad Prism v.9 or R v.4.1. No statistical methods were used to predetermine sample sizes but our sample sizes are similar to those reported in previous publications7. Data distribution was assumed to be normal, but this was not formally tested. No randomization was performed. No data points were excluded.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41590-022-01386-w.
Supplementary information
Supplementary Figs. 1–11 with legends.
Unprocessed immunoblot scans.
Supplementary Table 1 DEGs in cluster 1 versus rest clusters within TH17 cells. DEGs resulting from comparing cluster 1 versus rest clusters in one healthy donor are shown. Differential expression of IL1A in cluster 1 versus rest clusters, log(fold-change) 25.39236, P = 2.407359 × 10−3; Wilcoxon’s rank-sum test as implemented in Seurat’s function FindAllMarkers.
Coculture of monocytes with FITC-labelled C. albicans in medium control conditions. Real-time live cell in vitro imaging for 5 h. Monocytes were cultured in medium control conditions for 18 h before addition of C. albicans for another 5 h.
Coculture of monocytes with FITC-labelled C. albicans in the presence of Th17 cell supernatants. Real-time live cell in vitro imaging for 5 h. Th17 cell supernatants were harvested after 5 days of Th17 cell stimulation with anti-CD3 and anti-CD28 mAbs. Monocytes were cultured for 18 h with Th17 cell supernatants before addition of C. albicans for another 5 h.
Coculture of monocytes with FITC-labelled C. albicans in the presence of IL-1α depleted Th17 cell supernatants. Real-time live cell in vitro imaging for 5 h. Th17 cell supernatants were harvested after 5 days of Th17 cell stimulation with anti-CD3 and anti-CD28 mAbs. IL-1α was depleted by immune absorption from Th17 cell supernatants. Monocytes were cultured for 18 h with IL-1α-depleted Th17 cell supernatants before addition of C. albicans for another 5 h.
Coculture of monocytes with FITC-labelled C. albicans in the presence of recombinant IL-1α. Real-time live cell in vitro imaging for 5 h. Monocytes were cultured for 18 h with recombinant IL-1α before addition of C. albicans for another 5 h.
Acknowledgements
We thank all past and present members of the Zielinski laboratory for fruitful discussions and technical support, in particular R. Noster, A. Burrell, F. Laudisi and S.-H. Park for technical and experimental help. We thank L. Richter and J. Klein (Core Facility Flow Cytometry, Biomedical Center, Munich) for support with imaging flow cytometry, M. Schmidt-Supprian for support with CRISPR–Cas9 gene editing and P. Wehner for support with live cell imaging analysis. This work was funded by the Deutsche Forschungsgemeinschaft (German Research Foundation) through grant no. SFB 1054 (project ID 210592381, to C.E.Z.), TRR/SFB 124 (project ID 210879364, to C.E.Z. and M.S.G.), Leibniz Center for Photonics in Infection Research (grant no. LPI-BT6, to C.E.Z.), GRK 2606 (project ID 423813989, to O.G.), Germany’s Excellence Strategy (Balance of the Microverse, to C.E.Z), Emmy Noether Program (project no. 434385622/GR 5617/1-1, to M.S.G.), the German Center of Infection Research (to C.E.Z.), Carl-Zeiss Stiftung (to C.E.Z.) and by the European Research Council (grant nos. 337689 and 966687, to O.G.).
Extended data
Source data
Statistical source data table for main figures (>10 data points).
Statistical source data table for main figures (>10 data points).
Statistical source data table for main figures (>10 data points).
Unprocessed immunoblot.
Unprocessed immunoblot.
Unprocessed immunoblot.
Statistical source data table for main figures (>10 data points).
Statistical source data table for main figures (>10 data points).
Unprocessed immunoblot.
Statistical source data table for main figures (>10 data points).
Author contributions
Y.Y.C., A.P., D.F., T.S. and T.C. designed and performed the experiments and analyzed the data. A.G.P., M.A., S.F.S. and L.L. performed bioinformatic analyses. G.P. and L.T. supervised the bioinformatic analyses. D.K. and O.G. supervised experiments and helped to write the manuscript. M.G. provided FITC-labeled C. albicans and helped with live cell imaging using CellDiscoverer 7. M.W. and F.W. designed and performed experiments. S.J.V. provided JIA patient samples. A.P. analyzed the samples. C.E.Z. designed and supervised the project, analyzed the data and wrote the manuscript.
Peer review
Peer review information
Nature Immunology thanks Seth Masters and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: N. Bernard, in collaboration with the Nature Immunology team.
Data availability
Transcriptomic datasets for scRNA-seq (raw scRNA-seq fastq files, count matrix, gene and barcode files) and bulk RNA-seq (raw fastq files, raw and MRN count matrices) have been deposited in the GEO under the accession nos. GSE214519, GSE214292, GSE214475 and GSE214444. Source data are provided with this paper.
Code availability
The original code used to analyze the presented data are available at GitHub (https://github.com/thisisalbert/christina_zielinski_code_repository) and Zenodo (10.5281/zenodo.7257536).
Competing interests
A patent application has been filed by the authors. Otherwise, the authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
is available for this paper at 10.1038/s41590-022-01386-w.
Supplementary information
The online version contains supplementary material available at 10.1038/s41590-022-01386-w.
References
- 1.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 2.Zwicky P, Unger S, Becher B. Targeting interleukin-17 in chronic inflammatory disease: a clinical perspective. J. Exp. Med. 2020;217:e20191123. doi: 10.1084/jem.20191123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stockinger B, Omenetti S. The dichotomous nature of T helper 17 cells. Nat. Rev. Immunol. 2017;17:535–544. doi: 10.1038/nri.2017.50. [DOI] [PubMed] [Google Scholar]
- 4.Zielinski CE, et al. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature. 2012;484:514–518. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- 5.Ghoreschi K, et al. Generation of pathogenic TH17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noster R, et al. Dysregulation of proinflammatory versus anti-inflammatory human TH17 cell functionalities in the autoinflammatory Schnitzler syndrome. J. Allergy Clin. Immunol. 2016;138:1161–1169.e1166. doi: 10.1016/j.jaci.2015.12.1338. [DOI] [PubMed] [Google Scholar]
- 7.Aschenbrenner D, et al. An immunoregulatory and tissue-residency program modulated by c-MAF in human TH17 cells. Nat. Immunol. 2018;19:1126–1136. doi: 10.1038/s41590-018-0200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu C, et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496:513–517. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthias J, et al. Salt generates antiinflammatory Th17 cells but amplifies pathogenicity in proinflammatory cytokine microenvironments. J. Clin. Invest. 2020;130:4587–4600. doi: 10.1172/JCI137786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavalli G, et al. Interleukin 1alpha: a comprehensive review on the role of IL-1alpha in the pathogenesis and treatment of autoimmune and inflammatory diseases. Autoimmun. Rev. 2021;20:102763. doi: 10.1016/j.autrev.2021.102763. [DOI] [PubMed] [Google Scholar]
- 11.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 12.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi J, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acosta-Rodriguez EV, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 16.Gross O, et al. Inflammasome activators induce interleukin-1alpha secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity. 2012;36:388–400. doi: 10.1016/j.immuni.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Malik A, Kanneganti TD. Function and regulation of IL-1alpha in inflammatory diseases and cancer. Immunol. Rev. 2018;281:124–137. doi: 10.1111/imr.12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cimaz R. Systemic-onset juvenile idiopathic arthritis. Autoimmun. Rev. 2016;15:931–934. doi: 10.1016/j.autrev.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Fong KY, Boey ML, Koh WH, Feng PH. Cytokine concentrations in the synovial fluid and plasma of rheumatoid arthritis patients: correlation with bony erosions. Clin. Exp. Rheumatol. 1994;12:55–58. [PubMed] [Google Scholar]
- 20.Eyerich S, Zielinski CE. Defining Th-cell subsets in a classical and tissue-specific manner: examples from the skin. Eur. J. Immunol. 2014;44:3475–3483. doi: 10.1002/eji.201444891. [DOI] [PubMed] [Google Scholar]
- 21.Monteleone M, Stow JL, Schroder K. Mechanisms of unconventional secretion of IL-1 family cytokines. Cytokine. 2015;74:213–218. doi: 10.1016/j.cyto.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 22.Keller M, Ruegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 23.Kurt-Jones EA, Beller DI, Mizel SB, Unanue ER. Identification of a membrane-associated interleukin 1 in macrophages. Proc. Natl Acad. Sci. USA. 1985;82:1204–1208. doi: 10.1073/pnas.82.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mosley B, et al. The interleukin-1 receptor binds the human interleukin-1 alpha precursor but not the interleukin-1 beta precursor. J. Biol. Chem. 1987;262:2941–2944. [PubMed] [Google Scholar]
- 25.Carruth LM, Demczuk S, Mizel SB. Involvement of a calpain-like protease in the processing of the murine interleukin 1alpha precursor. J. Biol. Chem. 1991;266:12162–12167. [PubMed] [Google Scholar]
- 26.Kobayashi Y, et al. Identification of calcium-activated neutral protease as a processing enzyme of human interleukin 1 alpha. Proc. Natl Acad. Sci. USA. 1990;87:5548–5552. doi: 10.1073/pnas.87.14.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fettelschoss A, et al. Inflammasome activation and IL-1beta target IL-1alpha for secretion as opposed to surface expression. Proc. Natl Acad. Sci. USA. 2011;108:18055–18060. doi: 10.1073/pnas.1109176108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franklin BS, Latz E, Schmidt FI. The intra- and extracellular functions of ASC specks. Immunol. Rev. 2018;281:74–87. doi: 10.1111/imr.12611. [DOI] [PubMed] [Google Scholar]
- 29.Nagar A, DeMarco RA, Harton JA. Inflammasome and caspase-1 activity characterization and evaluation: an imaging flow cytometer-based detection and assessment of inflammasome specks and caspase-1 activation. J. Immunol. 2019;202:1003–1015. doi: 10.4049/jimmunol.1800973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rathinam VA, Fitzgerald KA. Inflammasome complexes: emerging mechanisms and effector functions. Cell. 2016;165:792–800. doi: 10.1016/j.cell.2016.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Broz P, Pelegrin P, Shao F. The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 2020;20:143–157. doi: 10.1038/s41577-019-0228-2. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z, et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature. 2020;579:415–420. doi: 10.1038/s41586-020-2071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schraml BU, et al. The AP-1 transcription factor Batf controls TH17 differentiation. Nature. 2009;460:405–409. doi: 10.1038/nature08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antonopoulos C, et al. Caspase-8 as an effector and regulator of NLRP3 inflammasome signaling. J. Biol. Chem. 2015;290:20167–20184. doi: 10.1074/jbc.M115.652321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vajjhala PR, et al. The inflammasome adaptor ASC induces procaspase-8 death effector domain filaments. J. Biol. Chem. 2015;290:29217–29230. doi: 10.1074/jbc.M115.687731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stennicke HR, et al. Pro-caspase-3 is a major physiologic target of caspase-8. J. Biol. Chem. 1998;273:27084–27090. doi: 10.1074/jbc.273.42.27084. [DOI] [PubMed] [Google Scholar]
- 37.Deng W, et al. Streptococcal pyrogenic exotoxin B cleaves GSDMA and triggers pyroptosis. Nature. 2022;602:496–502. doi: 10.1038/s41586-021-04384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puel A, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–68. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Billon C, Murray MH, Avdagic A, Burris TP. RORgamma regulates the NLRP3 inflammasome. J. Biol. Chem. 2019;294:10–19. doi: 10.1074/jbc.AC118.002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee GS, et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492:123–127. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao Y, et al. Transcriptional profiling identifies caspase-1 as a T cell-intrinsic regulator of Th17 differentiation. J. Exp. Med. 2020;217:e20190476. doi: 10.1084/jem.20190476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arbore G, et al. T helper 1 immunity requires complement-driven NLRP3 inflammasome activity in CD4+ T cells. Science. 2016;352:aad1210. doi: 10.1126/science.aad1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin BN, et al. T cell-intrinsic ASC critically promotes TH17-mediated experimental autoimmune encephalomyelitis. Nat. Immunol. 2016;17:583–592. doi: 10.1038/ni.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 45.Wiggins KA, et al. IL-1α cleavage by inflammatory caspases of the noncanonical inflammasome controls the senescence-associated secretory phenotype. Aging Cell. 2019;18:e12946–e12946. doi: 10.1111/acel.12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Helmstetter C, et al. Individual T helper cells have a quantitative cytokine memory. Immunity. 2015;42:108–122. doi: 10.1016/j.immuni.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xia S, et al. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature. 2021;593:607–611. doi: 10.1038/s41586-021-03478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Noster R, et al. IL-17 and GM-CSF expression are antagonistically regulated by human T helper cells. Sci. Transl. Med. 2014;6:241ra280. doi: 10.1126/scitranslmed.3008706. [DOI] [PubMed] [Google Scholar]
- 49.Matthias J, et al. Sodium chloride is an ionic checkpoint for human TH2 cells and shapes the atopic skin microenvironment. Sci. Transl. Med. 2019;11:eaau0683. doi: 10.1126/scitranslmed.aau0683. [DOI] [PubMed] [Google Scholar]
- 50.Wawrzyniak M, et al. A novel, dual cytokine-secretion assay for the purification of human Th22 cells that do not co-produce IL-17A. Allergy. 2016;71:47–57. doi: 10.1111/all.12768. [DOI] [PubMed] [Google Scholar]
- 51.Du C, Calderone RA. Phagocytosis and killing assays for Candida species. Methods Mol. Biol. 2009;499:17–26. doi: 10.1007/978-1-60327-151-6_3. [DOI] [PubMed] [Google Scholar]
- 52.Gautier L, Cope L, Bolstad BM, Irizarry RA. affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20:307–315. doi: 10.1093/bioinformatics/btg405. [DOI] [PubMed] [Google Scholar]
- 53.Ritchie ME, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andrews, S. FastQC: a quality control tool for high throughput sequence data (Online) (2010). http://www.bioinformatics.babraham.ac.uk/projects/fastqc/
- 55.Frankish A, et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 2019;47:D766–D773. doi: 10.1093/nar/gky955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Howe KL, et al. Ensembl 2021. Nucleic Acids Res. 2021;49:D884–D891. doi: 10.1093/nar/gkaa942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sherman, B.T. et al. DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res.50, W216–W221 (2022). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figs. 1–11 with legends.
Unprocessed immunoblot scans.
Supplementary Table 1 DEGs in cluster 1 versus rest clusters within TH17 cells. DEGs resulting from comparing cluster 1 versus rest clusters in one healthy donor are shown. Differential expression of IL1A in cluster 1 versus rest clusters, log(fold-change) 25.39236, P = 2.407359 × 10−3; Wilcoxon’s rank-sum test as implemented in Seurat’s function FindAllMarkers.
Coculture of monocytes with FITC-labelled C. albicans in medium control conditions. Real-time live cell in vitro imaging for 5 h. Monocytes were cultured in medium control conditions for 18 h before addition of C. albicans for another 5 h.
Coculture of monocytes with FITC-labelled C. albicans in the presence of Th17 cell supernatants. Real-time live cell in vitro imaging for 5 h. Th17 cell supernatants were harvested after 5 days of Th17 cell stimulation with anti-CD3 and anti-CD28 mAbs. Monocytes were cultured for 18 h with Th17 cell supernatants before addition of C. albicans for another 5 h.
Coculture of monocytes with FITC-labelled C. albicans in the presence of IL-1α depleted Th17 cell supernatants. Real-time live cell in vitro imaging for 5 h. Th17 cell supernatants were harvested after 5 days of Th17 cell stimulation with anti-CD3 and anti-CD28 mAbs. IL-1α was depleted by immune absorption from Th17 cell supernatants. Monocytes were cultured for 18 h with IL-1α-depleted Th17 cell supernatants before addition of C. albicans for another 5 h.
Coculture of monocytes with FITC-labelled C. albicans in the presence of recombinant IL-1α. Real-time live cell in vitro imaging for 5 h. Monocytes were cultured for 18 h with recombinant IL-1α before addition of C. albicans for another 5 h.
Data Availability Statement
Transcriptomic datasets for scRNA-seq (raw scRNA-seq fastq files, count matrix, gene and barcode files) and bulk RNA-seq (raw fastq files, raw and MRN count matrices) have been deposited in the GEO under the accession nos. GSE214519, GSE214292, GSE214475 and GSE214444. Source data are provided with this paper.
The original code used to analyze the presented data are available at GitHub (https://github.com/thisisalbert/christina_zielinski_code_repository) and Zenodo (10.5281/zenodo.7257536).