Summary
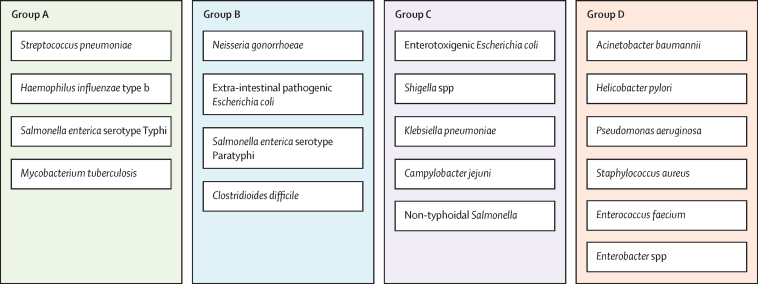
Vaccines can be highly effective tools in combating antimicrobial resistance as they reduce infections caused by antibiotic-resistant bacteria and antibiotic consumption associated with disease. This Review looks at vaccine candidates that are in development against pathogens on the 2017 WHO bacterial priority pathogen list, in addition to Clostridioides difficile and Mycobacterium tuberculosis. There were 94 active preclinical vaccine candidates and 61 active development vaccine candidates. We classified the included pathogens into the following four groups: Group A consists of pathogens for which vaccines already exist—ie, Salmonella enterica serotype Typhi, Streptococcus pneumoniae, Haemophilus influenzae type b, and M tuberculosis. Group B consists of pathogens with vaccines in advanced clinical development—ie, extra-intestinal pathogenic Escherichia coli, Salmonella enterica serotype Paratyphi A, Neisseria gonorrhoeae, and C difficile. Group C consists of pathogens with vaccines in early phases of clinical development—ie, enterotoxigenic E coli, Klebsiella pneumoniae, non-typhoidal Salmonella, Shigella spp, and Campylobacter spp. Finally, group D includes pathogens with either no candidates in clinical development or low development feasibility—ie, Pseudomonas aeruginosa, Acinetobacter baumannii, Staphylococcus aureus, Helicobacter pylori, Enterococcus faecium, and Enterobacter spp. Vaccines are already important tools in reducing antimicrobial resistance and future development will provide further opportunities to optimise the use of vaccines against resistance.
Introduction
Antimicrobial resistance (AMR) is a growing threat to global health, and was associated with 4·95 million deaths in 2019 globally, more than HIV and malaria combined.1 AMR compromises many aspects of modern medicine beyond bacterial infectious diseases; it also affects surgery, organ transplantation, and treatment for illnesses and ailments including HIV, liver and kidney disease, cancer, and physical trauma.2 Vaccines can be highly effective tools in combating AMR3, 4, 5, 6, 7, 8 and have been highlighted by WHO as such.9, 10 Vaccines work through multiple mechanisms to reduce AMR.11 Vaccines targeting bacterial pathogens reduce infections caused by antibiotic-resistant or susceptible pathogens, and contribute to the protection of unvaccinated populations if sufficient levels of immunity are maintained.12 Vaccines do this by both reducing the overall burden of infectious disease, caused by resistant or susceptible bacteria, and also by reducing antibiotic use associated with bacterial or viral infections. In addition, vaccines against viral infections—respiratory infections in particular, for example vaccines against influenza—reduce inappropriate antibiotic consumption, which is one of the key drivers of AMR.11 In this analysis, we consider only bacterial vaccines.
Although resistance has emerged for every antibiotic that has been introduced into clinical practice, resistance to bacterial vaccines is rare and tends not to render them ineffective. However, some vaccines have been shown to select for non-vaccine serotypes, causing replacement with those serotypes not covered by the vaccine over time.13 For this reason pneumococcal conjugate vaccines (PCVs) with higher and higher valences have been needed to address the increase in pneumococcal disease caused by non-PCV serotype pneumococci,14 and antibiotic resistance can continue to emerge in these serotypes.15 Despite resistance emerging in PCVs, in some cases, vaccines work to increase the viable lifetime of antibiotics. For example, PCVs cover 90% of the drug-resistant strains that cause childhood disease.16 Prevention of antibiotic-resistant strains through the use of these vaccines allows antibiotics to continue to be effective. PCVs targeting even more strains are currently under development.
Considerable advances have been made in vaccinology over the past four decades, which have been further accelerated by the response to the COVID-19 pandemic. These advances have opened up molecular targets and made it possible to vaccinate against more pathogens. Furthermore, vaccines present an opportunity to address increasingly difficult-to-treat infections caused by AMR bacteria.
Mapping out preclinical and clinical vaccine development against pathogens of critical, high, and medium concern related to drug resistance can help to understand and articulate research and development opportunities. Additionally, mapping out development clarifies how current vaccine investments are being distributed. This Review provides a mapping of the preclinical and clinical pipeline for vaccines against the pathogens highlighted by the 2017 WHO bacterial priority pathogen list (BPPL)—in addition to Clostridioides difficile and Mycobacterium tuberculosis—combined with an analysis of the feasibility of vaccine development (appendix; Table 1, Table 2).18 This list of pathogens was developed by WHO to prioritise pathogens for the research and development of new antibiotics due to emerging AMR. The inclusion of C difficile and M tuberculosis follows the approach used in previous WHO pipeline reports and recognition in the BPPL report that M tuberculosis is an established priority for WHO.18
Table 1.
Data on the preclinical and clinical development of vaccines for pathogens in three categories
| Preclinical | Phase 1 | Phase 2 | Phase 3 | ||
|---|---|---|---|---|---|
| Group A | |||||
| Streptococcus pneumoniae | 17 | 4 | 8 | 4 | |
| Haemophilus influenzae type b | 3 | 1 | 0 | 3 | |
| Salmonella enterica serotype Typhi | 8 | 1 | 1 | 3 | |
| Mycobacterium tuberculosis | 20 | 2 | 7 | 4 | |
| Group B | |||||
| Extra-intestinal pathogenic Escherichia coli | 4 | 1 | 2 | 1 | |
| Clostridioides difficile | 5 | 1 | 0 | 1 | |
| Salmonella enterica serotype Paratyphi | 4 | 1 | 1 | 1 | |
| Neisseria gonorrhoeae | 2 | 0 | 0 | 1 | |
| Group C | |||||
| Enterotoxigenic E coli | 10 | 4 | 2 | 0 | |
| Shigella spp | 10 | 6 | 2 | 0 | |
| Shigella flexneri | .. | 3 | 1 | 0 | |
| Shigella sonnei | .. | 0 | 1 | 0 | |
| S flexneri and S sonnei | .. | 3 | 0 | 0 | |
| Non-typhoidal Salmonella | 5 | 1 | 0 | 0 | |
| Klebsiella pneumoniae | 5 | 1 | 0 | 0 | |
| Campylobacter spp | 4 | 0 | 0 | 0 | |
Group A are pathogens with vaccines that are already licensed. Group B are pathogens with vaccines in late-stage clinical trials with high development feasibility. Group C are pathogens with vaccine candidates either in early clinical trials or with moderate to high feasibility of vaccine development.
Table 2.
Definition of feasibility
| Definition | |
|---|---|
| Biological feasibility | Considers progression of clinical development; existence of immunity from natural exposure; current understanding of mechanisms of immunity; and the likelihood of a vaccine protecting against most pathogenic strains |
| Product development feasibility | Considers the existence of established animal and in-vitro models to facilitate vaccine development; the ease of clinical development and setting a late-stage clinical trial; and the availability of human challenge models if these are likely to be required |
| Access and implementation feasibility | Considers the possibility of implementation within existing delivery systems, in particular childhood immunisation programmes; commercial attractiveness and whether there are likely to be high-income markets to support tiered pricing; the clarity of the licensure and policy decision pathway; and the ease of uptake and acceptability in target populations |
Each aspect was rated from very low to very high feasibility.17
Results and discussion: reviewing the pipeline
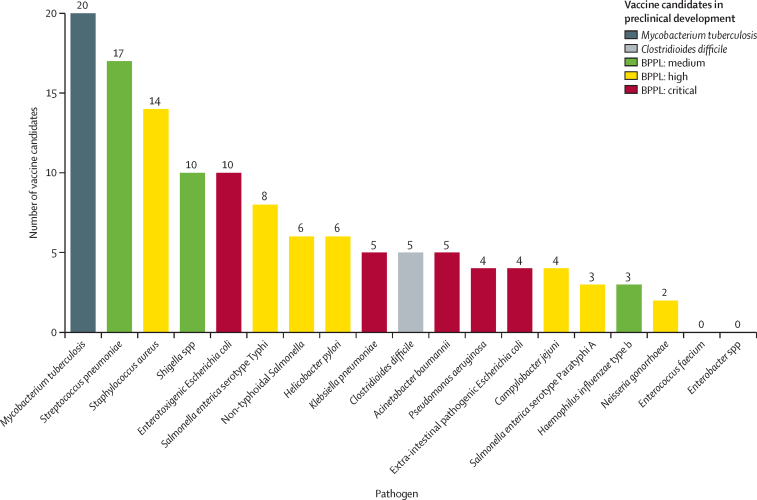
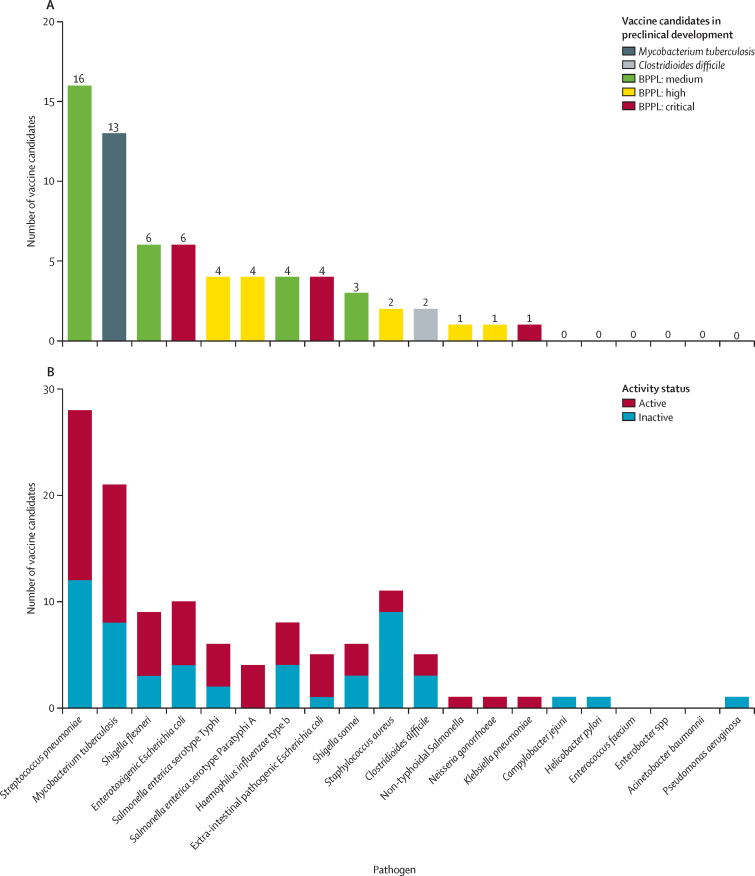
A total of 94 vaccine candidates were identified in active preclinical development (figure 1) and 61 vaccine candidates were identified in active clinical development (figure 2). Most of the vaccines that are in preclinical development target M tuberculosis (n=20), Streptococcus pneumoniae (n=17), and Staphylococcus aureus (n=14). For vaccines in the clinical development stage, most candidates target S pneumoniae (n=16), M tuberculosis (n=13), Shigella flexneri (n=6), and enterotoxigenic Escherichia coli (n=6). There are currently no vaccines under active clinical development that target Campylobacter jejuni, Helicobacter pylori, Enterococcus faecium, Enterobacter spp, Acinetobacter baumannii, or Pseudomonas aeruginosa. However, apart from E faecium and Enterobacter spp, all of these pathogens have vaccines in preclinical development.
Figure 1.
Vaccine candidates in preclinical development, categorised by pathogen and type
BPPL=bacterial priority pathogen list.
Figure 2.
Vaccine candidates in active clinical development
(A) Total number of vaccine candidates in active clinical development by pathogen. (B) Total number of candidates both in active clinical development and that have become inactive or discontinued over the past 10 years, by pathogen. Note that some vaccine candidates are double counted here as they target more than one pathogen. Pathogen type refers to status as defined by the WHO BPPL.14 BPPL=bacterial priority pathogen list.
Group A: pathogens with vaccines that are already licensed
Vaccines targeting S pneumoniae, Haemophilus influenzae type b, and Salmonella enterica serotype Typhi (S Typhi) are already licensed. Research to improve these vaccines continues and vaccine candidates in clinical development against S pneumoniae outnumber any of the other pathogens considered. Current BCG vaccines, which are licensed against M tuberculosis, continue to save the lives of many children each year. However, BCG vaccines do not provide adequate protection against pulmonary tuberculosis, a disease associated with high burden in many parts of the world.
S pneumoniae
A 23-valent pneumococcal polysaccharide vaccine has been licensed for use in children (aged ≥2 years) and adults since 1983,19 and many more vaccines have come to the market since. Two PCVs have been on the market since 2009; these are 10-valent and 13-valent vaccines recommended by WHO in childhood immunisation schedules for use in children younger than 5 years.20 These vaccines have reduced the incidence of infections caused by drug-susceptible and drug-resistant S pneumoniae. Observational studies show that in just 5 years the first PCV introduced in the USA reduced invasive pneumococcal disease caused by multidrug-resistant S pneumoniae by 84% in children younger than 2 years. Penicillin-resistant invasive pneumococcal disease was also reduced by 49% in adults older than 65 years, probably due to reduced transmission from children, as this group did not receive the vaccine.12, 17 PCV10 and PCV13 are both widely used and cover serotypes 10 and 13, respectively. Although more than 100 S pneumoniae serotypes exist, most disease-causing serotypes are covered by current vaccines. However, as vaccines reduce the incidence of vaccine serotypes, replacement serotypes, which are not covered by current vaccines, have increased in some populations.14 Under current coverage, PCVs prevent an estimated 23·8 million cases of antibiotic-treated illness among children younger than 5 years in low-income and middle-income countries (LMICs) every year.21
S pneumoniae was identified as one of the leading causes of death related to AMR infections in 2019, and it was the number one cause of death due to resistant infections in sub-Saharan Africa.1 Despite the availability of effective vaccines against S pneumoniae, worldwide coverage remains low at approximately 40% in children younger than 5 years.22 Uptake needs to be supported by global policies and interventions to improve access and affordability, particularly in low-resource settings where the burden of infectious diseases is highest. Research goals include reducing the manufacturing costs for PCVs, increasing serotype coverage and tailoring to local epidemiology, and developing new methods for protein conjugation and vaccine manufacturing.3
H influenzae type b
Effective vaccines against H influenzae type b have been available since the 1990s and WHO recommends the inclusion of H influenzae type b vaccines in infant immunisation programmes.23 Through their use in children younger than 5 years, invasive disease caused by H influenzae type b is close to being eliminated in high-income countries.3 H influenzae type b vaccines have also contributed to reducing the prevalence of some drug-resistant strains.24 Increasing the uptake of H influenzae type b vaccines globally would reduce infections caused by drug-resistant H influenzae type b.
S Typhi
More than 20 vaccines have been authorised against S Typhi.3 The WHO Strategic Advisory Group of Experts on Immunization recommends the existing WHO-approved typhoid conjugate vaccine (TCV) in favour of unconjugated Vi polysaccharide vaccine and live attenuated Ty21a vaccine due to TCV's improved immune response, longer expected duration of protection, and its suitability for use in those younger than 2 years.25 TCV has been used successfully in Pakistan to combat outbreaks of typhoid caused by drug-resistant S Typhi.26 Current research aims to incorporate multiple pathogen targets alongside S Typhi, which would make the value proposition of the vaccine even more favourable, and seeks to better understand the effect of vaccination on long-term pathogen carriage. Models suggest that the introduction of routine immunisation with TCV at age 9 months, with a catch-up campaign to age 15 years, would avert 21·2 million cases and 342 000 deaths from multidrug-resistant typhoid over the 10 years after introduction in the 73 countries eligible for Gavi, the Vaccine Alliance support.27
M tuberculosis
The BCG vaccine, active against M tuberculosis, is the most widely administered vaccine globally.28, 29 WHO continues to recommend BCG vaccination in countries and settings where the tuberculosis burden is high.30 It was developed 100 years ago and continues to effectively avert thousands of paediatric deaths every year. BCG provides long-lasting strong protection against miliary and meningeal tuberculosis, the most deadly forms of tuberculosis in children, but it is less effective for the prevention of pulmonary tuberculosis in adults. Importantly, BCG does not prevent reactivation of latent pulmonary infection—the principal source of bacillary spread in the community—and, therefore, has no activity against tuberculosis transmission.31 Given the high global burden of tuberculosis, and concerning levels of drug resistance, a new more effective tuberculosis vaccine is greatly needed. Model estimates suggest a novel vaccine could avert 115 000 deaths due to rifampicin-resistant tuberculosis between 2020 and 2035.32
At present, there are four vaccine candidates in phase 3 clinical trials targeting M tuberculosis. VPM1002 is a prophylactic recombinant BCG vaccine, currently in phase 3 trial in newborn infants to assess its efficacy, safety, and immunogenicity against M tuberculosis infection. The trial is scheduled to be completed in June, 2025 (NCT04351685). A phase 2/3 trial of VPM1002 for preventing tuberculosis recurrence in treated patients is also underway in India. GamTBvac is a prophylactic recombinant vaccine under development by the Russian Ministry of Health, that was scheduled to begin a phase 3 trial in November, 2021 (NCT04975737). MTBVAC is a live attenuated M tuberculosis candidate for which a phase 3 trial is planned to start in July, 2022, among newborn babies in sub-Saharan Africa (NCT04975178). Lastly, Immuvac is a therapeutic vaccine that uses a heat-killed Mycobacterium indicus pranii and is in a phase 3 trial in India.
There are a total of seven vaccine candidates in phase 2 clinical trials against tuberculosis, three in phase 2a and four in phase 2b. Among these, M72/AS01E is a subunit candidate, composed of an immunogenic fusion protein (M72) derived from two M tuberculosis antigens (MTB32A and MTB39A), and the adjuvant AS01E.33 A phase 2b clinical trial reported that M72/AS01E had an efficacy of nearly 54·0% in preventing active tuberculosis over 3 years in adults infected with M tuberculosis.34 A multicentre phase 3 trial is currently in preparation. H56:IC31 is a preventive recombinant subunit vaccine consisting of three antigens (ie, Ag85B, ESAT-6, and Rv2660c) currently studied in South Africa and Tanzania for prevention of recurrence (NCT03512249). DAR-901 takes a heat inactivated whole-cell approach and is prophylactic.33, 34 RUTI is a vaccine composed of M tuberculosis cell fragments and is a therapeutic vaccine candidate.
Group B: pathogens with vaccines in late-stage clinical trials with high development feasibility
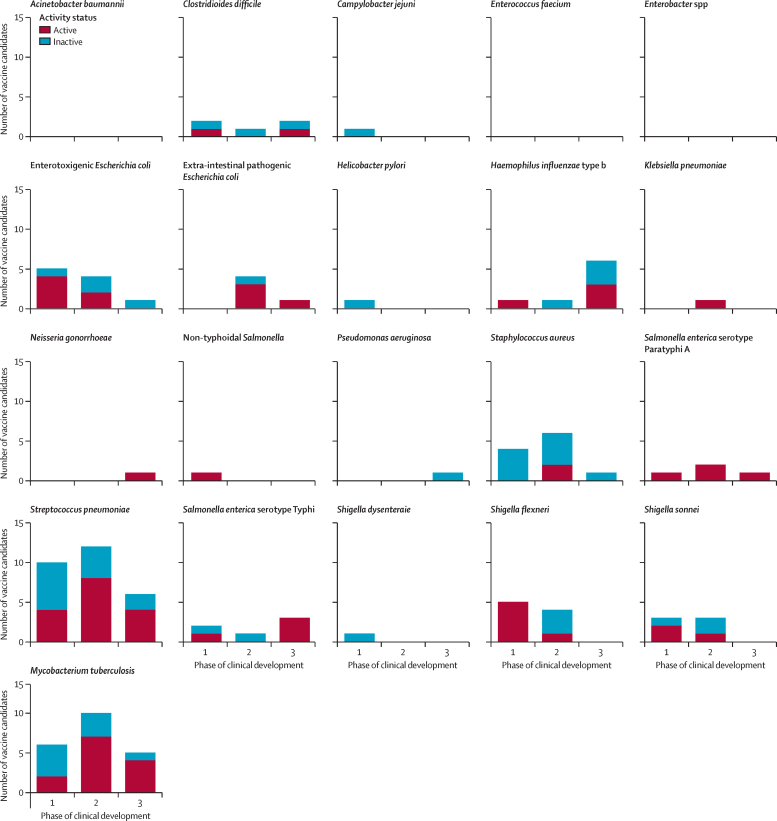
Vaccine candidates are shown by phase of clinical development in figure 3. There are currently vaccine candidates in phase 3 of development targeting: extra-intestinal pathogenic E coli, Salmonella enterica serotype Paratyphi A (S Paratyphi A), Neisseria gonorrhoeae, and C difficile. Candidates in phase 3 are the most clinically advanced, and therefore most likely to be available in the near future.
Figure 3.
Number of vaccine candidates by phase of clinical development and continued activity
Extra-intestinal pathogenic E coli
E coli is the leading cause of deaths associated with antibiotic-resistant bacterial pathogens.1 Infections caused by E coli have multiple manifestations, including both extra-intestinal pathogenic E coli and enterotoxigenic E coli. Extra-intestinal pathogenic E coli are a common cause of highly resistant hospital-acquired infections. There are multiple challenges associated with developing vaccines against hospital-acquired infections, including the relatively low incidence of infections caused by extra-intestinal pathogenic E coli in vaccine target populations (panel 1). An extra-intestinal pathogenic E coli vaccine might have different target populations depending on whether it targets invasive sepsis in infants or older adults, or urinary tract infections, in those with recurrent, complicated, or catheter-associated urinary tract infections. As E coli is a commensal pathogen, the effect on the broader microbiota and the potential consequence of disrupting it would also need to be assessed. There are four vaccine candidates in clinical development targeting extra-intestinal pathogenic E coli. The most advanced in development is ExPEC9V, a nine-valent-O-polysaccharide conjugate vaccine, currently in a phase 3 clinical trial (NCT04899336) against invasive disease, which is expected to be completed in May, 2027.
Panel 1. Challenges of developing vaccines against pathogens causing hospital-acquired infections.
Many of the WHO bacterial priority pathogens cause hospital-acquired infections, in particular Acinetobacter baumannii, Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus, extra-intestinal pathogenic Escherichia coli, Clostridioides difficile, Enterococcus faecium, and Enterobacter spp. There are multiple challenges35 for the clinical development of vaccines against these pathogens:
-
•
The relatively low incidence of infections in the vaccine target population makes efficacy trials prohibitively large in terms of trial sites and patient numbers, and expensive3
-
•
Potential target populations tend to be critically ill, with multiple comorbidities and severely compromised immune systems, making clinical endpoints hard to establish36, 37
-
•
Identification of populations at risk of hospital-acquired infections at intensive care unit admission is impracticable, due to the little time available to mount an effective immune response38
-
•
At present there is no regulatory or policy precedent for a vaccine against hospital-acquired infections
Despite these limitations, it could be possible to identify patients at high risk, such as those scheduled for elective surgery or hospital treatment, which would allow enough time for their immune systems to respond to vaccine administration. Given these challenges, regulators could explore the use of correlates of protection in phase 3 trials followed by the collection of post-licensure effectiveness data and real-world evidence. However, correlates of protection are currently absent for these pathogens. Combination vaccines might require fewer participants in clinical trials than single-target vaccines as multiple causes of disease are being targeted and incidence will be higher, which could help facilitate trials.
C difficile
Infections caused by C difficile are often difficult to manage with antibiotics, and alternative strategies are greatly needed. Clinical trial designs must take into account that the clinical endpoint is diarrhoea, which has multiple causes, particularly in older patients (aged >65 years), who are the vaccines' main target population (panel 1). Data from vaccine candidates that are currently in clinical development suggest that these agents could reduce symptomatic disease; however, C difficile could continue to be shed by the host.39 Further challenges to vaccine development against C difficile include the recruitment of patients in appropriate target populations. Many of these patients are older, severely ill, or have other comorbidities, which creates difficulties in establishing and evaluating clinical endpoints. There are currently three vaccine candidates against C difficile in clinical trials. PF-06425090 is a recombinant toxin vaccine targeting C difficile, consisting of genetically and chemically detoxified TcdA and TcdB toxins.40, 41 PF-06425090 did not meet its primary endpoint of preventing C difficile infections in a phase 3 trial (NCT03090191); however, disease severity was reduced.42 The study recruited 17 500 participants and the final analysis was done after accruing 42 cases of C difficile infection within 4 years of primary vaccination, reflecting the economic and logistical challenges of conducting studies on hospital-acquired infections.
S Paratyphi
The global mortality burden of drug-resistant S Paratyphi was estimated to be 20 224 associated deaths in 2019, with 4106 deaths directly attributed to it.1 However, the relatively low incidence in vaccine target populations would require large and long-lasting clinical trials or a controlled human infection model. A controlled human infection model has been developed but has not yet been used clinically for vaccine evaluation.43 A vaccine would most likely be co-administered in combination with TCV, improving the value proposition of both vaccines. Despite differences between S Typhi and S Paratyphi A, in particular the absence of Vi capsular polysaccharide in S Paratyphi A, the successful development of TCV suggests a vaccine against S Paratyphi A is also possible. Three vaccines are in clinical development against S Paratyphi. A phase 3 trial of the O:2, 12-TT conjugate vaccine is currently being conducted in China.
N gonorrhoeae
There are multiple challenges to vaccine development against N gonorrhoeae, including the absence of known correlates of protection, absence of immunity from natural exposure, poor understanding of immunity, and the existence of multiple pathogenic strains. Despite these challenges conserved antigenic targets have been identified. There is evidence to suggest the Bexsero vaccine (GlaxoSmithKline, Kundl, Austria), developed and licensed to prevent group B meningococcal infections, provides some protection against gonorrhoea. MenB given to children and young adults was associated with an approximately 30% reduction in gonorrhoea diagnoses.44 Two large clinical trials are underway to investigate the efficacy of Bexsero against N gonorrhoeae; however, phase 3 trial results have been delayed by the COVID-19 pandemic (NCT04415424).45 Modelling estimates suggest that gonococcal prevalence could be reduced by 40% by a vaccine with only 20% efficacy if it were given to all children aged 13 years.46 Another model in men who have sex with men (MSM) suggested that a vaccine would need a minimum efficacy of 90% and a fixed uptake of 40% to completely prevent AMR development.44 A third model in MSM estimated that even in a worst-case scenario, in which untreatable gonorrhoea infections emerge, the WHO 2016–30 target of a 90% reduction in gonorrhoea incidence would be achievable, under the condition that all MSM attending sexual health clinics receive a vaccine that offers at least 52% protection for more than 6 years.47
Group C: pathogens with vaccine candidates either in early clinical trials or with moderate to high feasibility of vaccine development
The pathogens in Group C are associated with moderate feasibility of vaccine development and include Enterotoxigenic E coli, Klebsiella pneumoniae, non-typhoidal Salmonella, Campylobacter spp, and Shigella spp. However, given the early stages of development of vaccines against these targets, no vaccine is likely to be available on the market soon.
Enterotoxigenic E coli
There are vaccine candidates against enterotoxigenic E coli and Shigella spp that are in phase 2 of clinical development. Mortality estimates are established for enterotoxigenic E coli, Campylobacter spp, and Shigella spp; however, long-term morbidity data on stunting and cognitive impairment are scarce. Better data on the economic effects of these infections are also needed to assess vaccine value and potential impact. Much of the disease burden is in LMICs, which reduces commercial attractiveness for the private sector. Three of the six vaccines in clinical trials against enterotoxigenic E coli also target Shigella spp and several candidates in preclinical development target even more combinations of pathogens. Such a multipronged approach would improve the value proposition of a vaccine. Evidence that the Dukoral cholera vaccine (Valneva, Solna, Sweden) provides 3 months' protection against some enterotoxigenic E coli strains suggests that an enterotoxigenic E coli vaccine is also possible.48, 49 Despite the diversity of enterotoxigenic E coli strains, up to 80% of those that cause disease could be covered by a vaccine targeting heat-labile toxoid and colonisation factor antigens.50 Two vaccines in phase 2 are being developed against enterotoxigenic E coli: ETVAX/dmLT, an inactivated whole cell vaccine (PACTR202010819218562);51 and CfaE+mLT, a recombinant subunit vaccine (NCT01922856).52
Shigella spp
A vaccine against Shigella spp might be used in multiple target populations, including infants (≤24 months) living in endemic regions, travellers to endemic regions, MSM, and military personnel. Although multiple strains of disease-causing Shigella spp exist, research suggests that more than 80% of these would be covered by a four-valent conjugate vaccine. More research is needed to ascertain whether conjugate vaccines will gain a sufficient immune response in the target population of those younger than 3 years; however, adjuvants could improve this response. Two vaccines targeting Shigella spp have reached phase 2 development. GlycoShig3 is a glycoconjugate subunit vaccine against S flexneri (NCT04602975; estimated study completion is Sept 30, 2023).53 WRSS2/WRSS3 is a live attenuated whole-cell vaccine targeting Shigella sonnei (NCT04242264; estimated study completion is 2023).54 There are also several vaccines in early stages of development that combine Shigella spp and enterotoxigenic E coli as targets.
Non-typhoidal Salmonella
The global burden of non-typhoidal Salmonella associated with drug-resistant infection was estimated to be 27 133 deaths in 2019.1 The development of a vaccine against non-typhoidal Salmonella, appears biologically feasible and would help to reduce the substantial burden and mortality of invasive non-typhoidal Salmonella disease.3 A large proportion of this burden occurs in Africa and is caused by the S Typhi and Salmonella enterica serotype Enteritidis.55, 56 Coverage of these serotypes could prevent most disease-causing strains. iCVD1000 is a trivalent conjugate vaccine that targets S Typhi, and non-typhoidal serotypes and Enteritidis. A phase 1 trial is scheduled for completion in September, 2022 (NCT03981952).
K pneumoniae
K pneumoniae has been identified by WHO as a critical priority resistance threat18 and one of six pathogens with the highest mortality due to antibiotic-resistant infections.1 Multiple challenges are associated with the development of vaccines against highly resistant hospital-acquired infections (panel 1). K pneumoniae is associated with a high burden of neonatal sepsis in low-resource settings and a maternal vaccine has been suggested to combat this burden. Commercial attractiveness is likely to be low as much of the burden is in LMICs; however, if there is also a market in high-income countries tiered pricing based on country income levels could make development more economically feasible. A further hurdle is the design and implementation of clinical trials involving neonates, which can be challenging to conduct. A tetravalent bioconjugated vaccine candidate, KlebV4, is being assessed with and without the AS03 adjuvant in a phase 1/2 trial (NCT04959344).
Campylobacter spp
Campylobacter spp are the leading cause of bacterial gastroenteritis in high-income countries.57 There are currently no vaccines in clinical development against C jejuni. Some vaccine candidates have been developed to the clinical stage in the past, but they have all been unsuccessful. Such a vaccine would have markets in high-income and low-income countries and potential target populations in infants and international travellers. There is a conjugate vaccine that has been developed for use in cows, which has shown Campylobacter spp to be a viable target for vaccines.58
Group D: pathogens with a small number of or no vaccine candidates in the pipeline or low vaccine development feasibility in the near future
Pathogens in group D are associated with low feasibility of vaccine development and include the priority pathogens S aureus, with one vaccine candidate in phase 2; A baumannii, P aeruginosa, and H pylori, which have no vaccine candidates in clinical development; and E faecium and Enterobacter spp, which do not have any vaccine candidates in preclinical or clinical development. There are vaccine candidates in preclinical development for all pathogens in the BPPL except for Enterobacter spp and E faecium. This exemption reflects the restricted feasibility of developing a vaccine against these two pathogens.59 Many of the pathogens of critical priority in the BPPL commonly cause difficult-to-treat hospital infections. An overview of challenges of developing vaccines against pathogens causing hospital-acquired infections is shown in panel 1.
One vaccine candidate is in phase 2 clinical trials against S aureus; it is a recombinant toxic shock syndrome toxin-1 variant vaccine. The vaccine candidate was reported to be safe and immunogenic in a phase 1 trial60 and a phase 2 trial was completed in January, 2021, the results of which are yet to be published (NCT02814708). Despite relatively high investment from industry, many of the candidates that target S aureus that have been developed, have later been unsuccessful, including nine candidates over the last 10 years that are included in this study. A four-antigen vaccine was terminated during phase 2b in 2019 due to futility (NCT02388165).4 The V710 IsdB vaccine candidate proved unsuccessful after the termination of its phase 3 trial. This cancellation came after the interim analysis reported increased mortality and adverse side-effects among participants who had taken the vaccine candidate and later developed an S aureus infection (NCT00518687). CP5-Epa and CP8-Epa vaccines also reached phase 3 of clinical trials, but are no longer under development.61
Although there are three candidates in clinical development against S aureus, this pathogen has been categorised in a low feasibility category due to extensive challenges in developing a vaccine against the pathogen in the past. There are multiple challenges to developing a successful vaccine against S aureus (panel 1). First, it is unclear which antigen targets would enable a vaccine to provide protection against S aureus infection.4 Correlates of protection are scarce, and vaccines that have shown protection in rodent models have subsequently been unsuccessful in clinical trials. Although multiple candidate monoclonal antibodies targeted against S aureus have been tested, these have also proven ineffective in clinical trials.62 Second, intermittent colonisation with S aureus occurs in approximately two-thirds of the population; however, exposure does not appear to confer natural immunity.63, 64 Finally, the diseases caused by S aureus are diverse, including bacteraemia, skin infections, pneumonia, and others, and it is not clear whether a single vaccine would be protective against multiple clinical syndromes.65
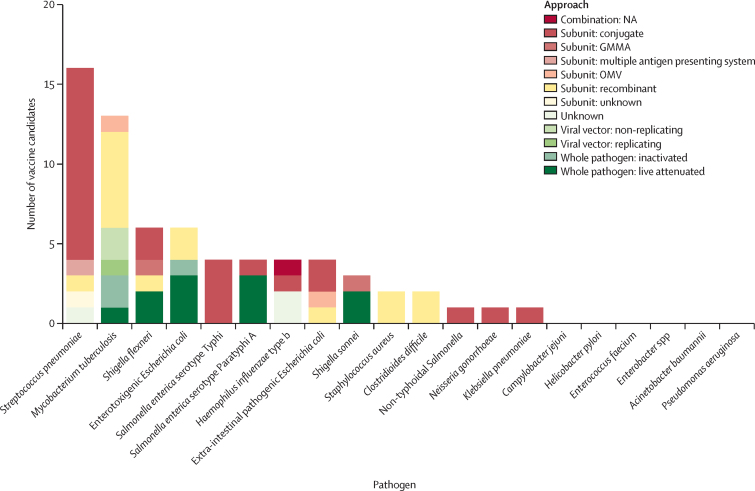
Development approaches and future trends in research and development
The vaccine candidates in clinical development use many different mechanistic approaches (figure 4). The biological approach adopted by clinical candidates was not recorded as, in many cases, this information was unavailable. However, approaches in preclinical development are likely to be even more diverse. Vaccine development during the COVID-19 pandemic carries lessons for vaccines to tackle AMR. Global recognition of COVID-19 as an urgent threat and the lifesaving potential of vaccines converged to global prioritisation, investment, and research, resulting in a reduced timeline for licensure and use. This acceleration was facilitated by an increase in available resources, and optimal approaches to both regulating and doing clinical trials in parallel. A further consequence of the COVID-19 pandemic has been advancements in vaccine technology, in particular the development of mRNA vaccines. Although only one clinical mRNA candidate targeting M tuberculosis was identified in this Review, many more candidates are likely to emerge, potentially targeting AMR pathogens. The advantages of mRNA vaccine technology include that they are less costly to manufacture and can be developed and scaled up for production more rapidly than other vaccine approaches.66, 67 The expression of the antigen occurs in the body of the vaccine recipient, avoiding complex, costly, and time-consuming steps in manufacture compared with other vaccines.68 However, research challenges to the development of mRNA vaccines against AMR pathogens include thermostability and the need for ultra-cold chain and storage. At present, multiple antigens have not been combined into a single vaccine and all currently licensed mRNA vaccines target viral pathogens.68 It is also important to highlight that mRNA technology is not going to solve other scientific challenges in vaccine development—eg, when it is unclear how the human immune response against a specific pathogen is triggered.
Figure 4.
Number of vaccine candidates in active clinical development by approach
NA=not applicable. GMMA=generalised modules for membrane antigens. OMV=outer membrane vesicles.
Another research area that would improve the value proposition of vaccines against AMR pathogens is the development of combination vaccines with multiple antigen targets. In some disease areas combination vaccines are already being developed, for example Shigella–enterotoxigenic E coli vaccine candidates and candidates targeting multiple serotypes of Salmonella. A vaccine targeting multiple key pathogens, which cause hospital-acquired infections, would allow for easier efficacy trials due to relatively increased incidence of disease in the patient population. Vaccines targeting multiple pathogens is already an area being explored for some preclinical candidates, for example the Paragon Novel Vaccine, which targets S aureus, enterotoxigenic E coli, and extra-intestinal pathogenic E coli. Another example is KapaVax, an inactivated whole-cell vaccine against K pneumoniae, P aeruginosa, and A baumannii.
Recommendations
This analysis has classified the pathogens on the BPPL, M tuberculosis, and C difficile for AMR into four distinct groups by feasibility (figure 5). Groupings were based on matching the progression of vaccine candidates in clinical and preclinical development and assessments of the feasibility of generating a vaccine. Feasibility assessments were based on analyses of biological feasibility, product development feasibility, and access and implementation feasibility. These groups and our recommendations for each are outlined in panel 2.
Figure 5.
Categorisation of priority pathogens as targets for vaccination
Group A contains pathogens with vaccines that are already licensed. Group B contains pathogens with vaccines in late-stage clinical trials with high development feasibility. Group C contains pathogens with vaccine candidates either in early clinical trials or with moderate to high feasibility of vaccine development. Group D contains pathogens with a small number or no vaccine candidates in the pipeline and low vaccine development feasibility in the near future.
Panel 2. Recommendations and summary results of vaccine feasibility for pathogens on the WHO bacterial priority pathogen list for antimicrobial resistance in addition to Mycobacterium tuberculosis and Clostridioides difficile.
Group A
Pipeline feasibility (very high)
Constitutes antimicrobial resistance (AMR) priority pathogens for which licensed vaccines already exist. Group A pathogens are associated with a very high feasibility of vaccine development and include Salmonella enterica serotype Typhi, Streptococcus pneumoniae, Haemophilus influenzae type b, and M tuberculosis.
Recommendation
Increase the coverage of authorised vaccines in line with WHO immunisation targets to maximise impact on AMR. Accelerate the development of more effective vaccines against tuberculosis.
Group B
Pipeline feasibility (high)
Constitutes AMR priority pathogens for which a vaccine candidate is in late-stage development (phase 3) and vaccines would be suitable to target AMR infections caused by these priority pathogens in the coming years. Group B pathogens are associated with a high feasibility of vaccine development and include extra-intestinal pathogenic Escherichia coli, Salmonella enterica serotype Paratyphi A, Neisseria gonorrhoeae, and C difficile.
Recommendation
Accelerate the development of vaccines for these pathogens.
Group C
Pipeline feasibility (moderate)
Constitutes AMR priority pathogens for which a vaccine candidate has either been identified in early clinical trials or been identified as a feasible vaccine target during expert review. For these pathogens, vaccines might be feasible solutions to target AMR infections. These pathogens are associated with moderate feasibility of vaccine development and include enterotoxigenic E coli, Klebsiella pneumoniae, non-typhoidal Salmonella, Campylobacter spp, and Shigella spp. Given the early stages of development, no vaccine for these pathogens will be available on the market soon.
Recommendation
Continue the development of a vaccine for these pathogens and expand knowledge of potential for vaccine use and impact and other tools to combat the AMR threat.
Group D
Pipeline feasibility (low)
Constitutes AMR priority pathogens for which no vaccine candidate has been identified in clinical development; therefore, vaccines are not a feasible solution to target AMR infections in the foreseeable future. These pathogens are associated with low feasibility of vaccine development and include the priority pathogens Acinetobacter baumannii, Pseudomonas aeruginosa, Enterobacter spp, Enterococcus faecium, Staphylococcus aureus, and Helicobacter pylori. Research and investment should explore alternative methods of control, including treatments and effective infection prevention, and should ensure access to clean water, adequate sanitation, and hygiene facilities. This focus is even more urgent as the drug development pipeline for A baumannii and P aeruginosa is also currently insufficient to adequately address the burden posed by these critical pathogens.
Recommendation
Focus on other prevention and control tools to combat the AMR threat linked to these priority pathogens. Conduct basic research to understand biological and product development barriers and ultimately facilitate vaccine development.
Conclusion
This report provides a mapping of vaccine candidates, against pathogens prioritised due to AMR. This analysis of the preclinical pipeline is a non-exhaustive list of preclinical research at the time of writing this Review. Due to the high uncertainty and turnover of programmes in this phase of drug development, the preclinical dataset is highly dynamic. In general, failure rates in vaccine development are high. The average vaccine requires a development timeline of 11 years from the preclinical phase and has a market entry probability of 6%.69 Thus, many of the vaccine candidates described in this Review are likely to be unsuccessful in clinical development. For those vaccines that make it to the market, public health driven vaccination strategies will have to be developed for the appropriate target populations. This step often proves challenging for some of the pathogens, particularly those targeting hospital-acquired infections (panel 1). Finally, if vaccines become available, they will only mitigate AMR if they are available to those who would benefit from them. Three pathogens on the BPPL already have licensed, highly effective vaccines: S pneumoniae, S Typhi, and H influenzae type b. Despite the existence of an effective vaccine, 122 000 deaths were attributed to resistant S pneumoniae globally in 2019 and, of these, 40 400 occurred in sub-Saharan Africa.1 It is essential that these tools to combat AMR, and save lives, are made available globally once developed. Access to PCV vaccines continues to be low, and the vaccine is costly for many countries, resulting in global coverage that has reached only 40% of the target population.22 Funding for vaccination campaigns, and investment for research into cheaper, more cost-effective methods of manufacture and distribution, and novel methods of delivery and administration are needed to ensure vaccines reach those who need them, in the most efficient way.
For some pathogens, the absence of dual markets across high-income and low-income countries is challenging for financing vaccine development (eg, for Shigella spp); however, the development and WHO recommendation of a vaccine against malaria in October, 2021, has shown that financing without a dual market is possible. Some ways in which financing without a dual market can be facilitated include mechanisms for pooled procurement, such as Gavi financing and country ownership in co-financing; risk-sharing mechanisms; and end-to-end planning of policy, regulatory, and financing pathways. Access to vaccines is not solely an issue of equity; populations living in areas with high levels of infectious disease provide opportunities for resistant pathogens to emerge and propagate, irrespective of country borders.70 These clinically vulnerable populations inevitably need to consume antibiotics to treat infections that might have been prevented through vaccination or access to clean water, appropriate sanitation, and hygiene facilities, further exacerbating a cycle of poverty, infectious disease, and AMR.
Search strategy and selection criteria
The method that was followed was taken from previous WHO analyses of the antimicrobial pipeline. A search of clinical trials was performed in both ClinicalTrials.gov and the International Clinical Trials Registry Platform. Search terms consisted of the pathogen name and the word “vaccine”. In addition, national databases in Russia and Japan were also searched by local experts. A review of peer-reviewed and grey literature was also done to look for candidates. PubMed was searched from 2010 to 2021, for papers in English, using the following search terms for each pathogen: ((vaccine) AND ((candidate) OR (pipeline) OR (research) OR (landscape))) AND (pathogen name) AND ((phase 1) OR (phase 2) OR (phase 3) OR (clinical)). Titles and abstracts were then screened for relevance before papers were analysed. Consulted grey literature included reports, documents and slides from WHO Product Development for Vaccines Advisory Committee meetings, key reports, the WHO tracker database (discontinued at the end of 2018), and any other grey literature that was referenced or suggested by experts. Data were also included from the WHO Global Observatory on Health Research and Development, provided by AdisInsight, and the Dynamic Dashboard from the Global Antimicrobial Resistance Research and Development Hub. Data including the vaccine candidate name, clinical trials, route of administration, approach, condition targeted, and sponsor were extracted and cleaned to remove duplicates.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
This study was funded by the Government of Germany and was done entirely independently of them. The authors alone are responsible for the views expressed in this Review and they do not necessarily represent the decisions, policy, or views of WHO.
Contributors
IF conducted data analysis and produced the first draft of the manuscript. All authors provided extensive comments on the manuscript.
Supplementary Material
References
- 1.Murray CJ, Ikuta KS, Sharara F, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Europe RAND . Wellcome Trust; London: 2021. How drug-resistant infections are undermining modern medicine—and why more research is needed now. [Google Scholar]
- 3.Gooch J, Rodriguez A, Serazin E, Schwarm M, Steene A, Cheng YY. Vaccines to tackle drug resistant infections: an evaluation of R&D opportunities. Oct 6, 2021. https://www.bcg.com/vaccines-to-tackle-drug-resistant-infections
- 4.Micoli F, Bagnoli F, Rappuoli R, Serruto D. The role of vaccines in combatting antimicrobial resistance. Nat Rev Microbiol. 2021;19:287–302. doi: 10.1038/s41579-020-00506-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipsitch M, Siber GR. How can vaccines contribute to solving the antimicrobial resistance problem? MBio. 2016;7:1–8. doi: 10.1128/mBio.00428-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bloom DE, Black S, Salisbury D, Rappuoli R. Antimicrobial resistance and the role of vaccines. Proc Natl Acad Sci USA. 2018;115:12868–12871. doi: 10.1073/pnas.1717157115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jansen KU, Knirsch C, Anderson AS. The role of vaccines in preventing bacterial antimicrobial resistance. Nat Med. 2018;24:10–19. doi: 10.1038/nm.4465. [DOI] [PubMed] [Google Scholar]
- 8.Rappuoli R, Bloom DE, Black S. Deploy vaccines to fight superbugs. Nature. 2017;552:165–167. doi: 10.1038/d41586-017-08323-0. [DOI] [PubMed] [Google Scholar]
- 9.Vekemans J, Hasso-Agopsowicz M, Kang G, et al. Leveraging vaccines to reduce antibiotic use and prevent antimicrobial resistance: a World Health Organization action framework. Clin Infect Dis. 2021;73:e1011–e1017. doi: 10.1093/cid/ciab062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO . World Health Organization; Geneva: 2015. Global action plan on antimicrobial resistance; pp. 1–28. [Google Scholar]
- 11.Atkins KE, Lafferty EI, Deeny SR, Davies NG, Robotham JV, Jit M. Use of mathematical modelling to assess the impact of vaccines on antibiotic resistance. Lancet Infect Dis. 2017;18:e204–e213. doi: 10.1016/S1473-3099(17)30478-4. [DOI] [PubMed] [Google Scholar]
- 12.Orenstein WA, Gellin BG, Beigi RH, et al. A call for greater consideration for the role of vaccines in national strategies to combat antibiotic-resistant bacteria: recommendations from the National Vaccine Advisory Committee. Public Health Rep. 2016;131:11–16. [PMC free article] [PubMed] [Google Scholar]
- 13.Lo SW, Mellor K, Cohen R, et al. Emergence of a multidrug resistant and virulent Streptococcus pneumoniae lineage mediates serotype replacement after PCV13. medRxiv. 2021 doi: 10.1101/2021.11.24.21266813. published online Nov 26. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ladhani SN, Collins S, Djennad A, et al. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–17: a prospective national observational cohort study. Lancet Infect Dis. 2018;18:441–451. doi: 10.1016/S1473-3099(18)30052-5. [DOI] [PubMed] [Google Scholar]
- 15.Lo SW, Gladstone RA, van Tonder AJ, et al. Pneumococcal lineages associated with serotype replacement and antibiotic resistance in childhood invasive pneumococcal disease in the post-PCV13 era: an international whole-genome sequencing study. Lancet Infect Dis. 2019;19:759–769. doi: 10.1016/S1473-3099(19)30297-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klugman KP, Black S. Impact of existing vaccines in reducing antibiotic resistance: primary and secondary effects. Proc Natl Acad Sci USA. 2018;115:12896–12901. doi: 10.1073/pnas.1721095115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354:1455–1463. doi: 10.1056/NEJMoa051642. [DOI] [PubMed] [Google Scholar]
- 18.WHO Global priority list of antibiotic-resistant bacteria to guide research, discovery and development of new antibiotics. 2017. https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed
- 19.Chandler T, Furmanek S, Carrico R, Balcom D, Arnold F, Ramirez J. 23-valent pneumococcal polysaccharide vaccination does not prevent community-acquired pneumonia hospitalizations due to vaccine-type Streptococcus pneumoniae. Microorganisms. 2022;10:560. doi: 10.3390/microorganisms10030560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO Summary of WHO position paper on pneumococcal conjugate vaccines in infants and children under 5 years of age, February 2019. 2019. https://cdn.who.int/media/docs/default-source/immunization/position_paper_documents/pneumococcus/who-pp-pcv-2019-summary.pdf?sfvrsn=3e4fdf81_2
- 21.Lewnard JA, Lo NC, Arinaminpathy N, Frost I, Laxminarayan R. Childhood vaccines and antibiotic use in low- and middle-income countries. Nature. 2020;581:94–99. doi: 10.1038/s41586-020-2238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO. UNICEF . World Health Organization; Geneva: 2021. Immunization.https://data.unicef.org/topic/child-health/immunization/ [Google Scholar]
- 23.WHO Haemophilus influenzae type b (Hib) vaccination position paper – July 2013. 2013. https://apps.who.int/iris/bitstream/handle/10665/242126/WER8839_413-426.PDF?sequence=1&isAllowed=y [DOI] [PubMed]
- 24.Giufrè M, Cardines R, Caporali MG, Accogli M, D'Ancona F, Cerquetti M. Ten years of Hib vaccination in Italy: prevalence of non-encapsulated Haemophilus influenzae among invasive isolates and the possible impact on antibiotic resistance. Vaccine. 2011;29:3857–3862. doi: 10.1016/j.vaccine.2011.03.059. [DOI] [PubMed] [Google Scholar]
- 25.WHO Typhoid vaccines: WHO position paper, March 2018 - recommendations. Vaccine. 2019;37:214–216. doi: 10.1016/j.vaccine.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 26.Yousafzai MT, Karim S, Qureshi S, et al. Effectiveness of typhoid conjugate vaccine against culture-confirmed Salmonella enterica serotype Typhi in an extensively drug-resistant outbreak setting of Hyderabad, Pakistan: a cohort study. Lancet Glob Health. 2021;9:e1154–e1162. doi: 10.1016/S2214-109X(21)00255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birger R, Antillón M, Bilcke J, et al. Estimating the effect of vaccination on antimicrobial-resistant typhoid fever in 73 countries supported by Gavi: a mathematical modelling study. Lancet Infect Dis. 2022;22:679–691. doi: 10.1016/S1473-3099(21)00627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dockrell HM, Smith SG. What have we learnt about BCG vaccination in the last 20 years? Front Immunol. 2017;8 doi: 10.3389/fimmu.2017.01134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO BCG vaccine. https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccines-quality/bcg
- 30.WHO BCG vaccines: WHO position paper. February 2018. https://www.who.int/publications/i/item/who-wer9308-73-96
- 31.Mangtani P, Abubakar I, Ariti C. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis. 2014;58:470–480. doi: 10.1093/cid/cit790. [DOI] [PubMed] [Google Scholar]
- 32.Fu H, Lewnard JA, Frost I, Laxminarayan R, Arinaminpathy N. Modelling the global burden of drug-resistant tuberculosis avertable by a post-exposure vaccine. Nat Commun. 2021;12:424. doi: 10.1038/s41467-020-20731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO . World Health Organization; Geneva: 2020. Vaccines and immunization: investigational vaccine candidate M72/AS01E.https://www.who.int/news-room/questions-and-answers/item/vaccines-and-immunization-investigational-vaccine-candidate-m72-as01e [Google Scholar]
- 34.Tait DR, Hatherill M, Van Der Meeren O, et al. Final analysis of a trial of M72/AS01E vaccine to prevent tuberculosis. N Engl J Med. 2019;381:2429–2439. doi: 10.1056/NEJMoa1909953. [DOI] [PubMed] [Google Scholar]
- 35.Bekeredjian-Ding I. Challenges for clinical development of vaccines for prevention of hospital-acquired bacterial infections. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.01755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pachón J, McConnell MJ. Considerations for the development of a prophylactic vaccine for Acinetobacter baumannii. Vaccine. 2014;32:2534–2536. doi: 10.1016/j.vaccine.2013.10.064. [DOI] [PubMed] [Google Scholar]
- 37.López-Siles M, Corral-Lugo A, McConnell MJ. Vaccines for multidrug resistant Gram negative bacteria: lessons from the past for guiding future success. FEMS Microbiol Rev. 2021;45 doi: 10.1093/femsre/fuaa054. [DOI] [PubMed] [Google Scholar]
- 38.Adlbrecht C, Wurm R, Depuydt P, et al. Efficacy, immunogenicity, and safety of IC43 recombinant Pseudomonas aeruginosa vaccine in mechanically ventilated intensive care patients—a randomized clinical trial. Crit Care. 2020;24:74. doi: 10.1186/s13054-020-2792-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riley TV, Lyras D, Douce GR. Status of vaccine research and development for Clostridium difficile. Vaccine. 2019;37:7300–7306. doi: 10.1016/j.vaccine.2019.02.052. [DOI] [PubMed] [Google Scholar]
- 40.Inoue M, Yonemura T, de Solom R, et al. A phase 1 randomized study assessing safety and immunogenicity of two 3-dose regimens of a Clostridium difficile vaccine in healthy older Japanese adults. Vaccine. 2019;37:2600–2607. doi: 10.1016/j.vaccine.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 41.Kitchin N, Remich SA, Peterson J, et al. A phase 2 study evaluating the safety, tolerability, and immunogenicity of two 3-dose regimens of a Clostridium difficile vaccine in healthy US adults aged 65 to 85 years. Clin Infect Dis. 2020;70:1–10. doi: 10.1093/cid/ciz153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bratulic A. Pfizer's C. difficile vaccine misses main goal of pivotal study. March 1, 2022. https://firstwordpharma.com/story/5515307
- 43.Dobinson HC, Gibani MM, Jones C, et al. Evaluation of the clinical and microbiological response to Salmonella Paratyphi A infection in the first paratyphoid human challenge model. Clin Infect Dis. 2017;64:1066–1073. doi: 10.1093/cid/cix042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heijne J, Xiridou M, Turner K, et al. The impact of vaccination on Neisseria gonorrhoeae antimicrobial resistance and prevalence in men who have sex with men: a mathematical modelling study. medRxiv. 2020 doi: 10.1101/2020.09.14.20192062. published online Sept 15. (preprint). [DOI] [Google Scholar]
- 45.Vincent LR, Jerse AE. Biological feasibility and importance of a gonorrhea vaccine for global public health. Vaccine. 2019;37:7419–7426. doi: 10.1016/j.vaccine.2018.02.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Craig AP, Gray RT, Edwards JL, et al. The potential impact of vaccination on the prevalence of gonorrhea. Vaccine. 2016;33:4520–4525. doi: 10.1016/j.vaccine.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whittles LK, White PJ, Didelot X. Assessment of the potential of vaccination to combat antibiotic resistance in gonorrhea: a modeling analysis to determine preferred product characteristics. Clin Infect Dis. 2020;71:1912–1919. doi: 10.1093/cid/ciz1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scandinavian Biopharma Promising preliminary findings from a phase 2b study of the oral vaccine ETVAX® against travelers' diarrhea in Finnish travelers to Benin, west Africa. 2020. https://scandinavianbiopharma.se/promising-preliminary-findings-from-a-phase-2b-study-of-the-oral-vaccine-etvax-against-travelers-diarrhea-in-finnish-travelers-to-benin-west-africa/
- 49.Larocque R, Harris JB. Approach to the adult with acute diarrhea in resource-rich countries. June 28, 2021. https://www.uptodate.com/contents/approach-to-the-adult-with-acute-diarrhea-in-resource-rich-settings
- 50.Wolf MK. Occurrence, distribution, and associations of O and H serogroups, colonization factor antigens, and toxins of enterotoxigenic Escherichia coli. Clin Microbiol Rev. 1997;10:569–584. doi: 10.1128/cmr.10.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qadri F, Akhtar M, Bhuiyan TR, et al. Safety and immunogenicity of the oral, inactivated, enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi children and infants: a double-blind, randomised, placebo-controlled phase 1/2 trial. Lancet Infect Dis. 2020;20:208–219. doi: 10.1016/S1473-3099(19)30571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riddle MS, Maciel M, Jr, Porter CK, et al. A first in human clinical trial assessing the safety and immunogenicity of transcutaneously delivered enterotoxigenic Escherichia coli fimbrial tip adhesin with heat-labile enterotoxin with mutation R192G. Vaccine. 2020;38:7040–7048. doi: 10.1016/j.vaccine.2020.09.025. [DOI] [PubMed] [Google Scholar]
- 53.Cohen D, Atsmon J, Artaud C, et al. Safety and immunogenicity of a synthetic carbohydrate conjugate vaccine against Shigella flexneri 2a in healthy adult volunteers: a phase 1, dose-escalating, single-blind, randomised, placebo-controlled study. Lancet Infect Dis. 2021;21:546–558. doi: 10.1016/S1473-3099(20)30488-6. [DOI] [PubMed] [Google Scholar]
- 54.Frenck RW, Jr, Baqar S, Alexander W, et al. A phase I trial to evaluate the safety and immunogenicity of WRSs2 and WRSs3; two live oral candidate vaccines against Shigella sonnei. Vaccine. 2018;36:4880–4889. doi: 10.1016/j.vaccine.2018.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agnandji ST, Lell B, Fernandes JF, et al. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med. 2012;367:2284–2295. doi: 10.1056/NEJMoa1208394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stanaway JD, Parisi A, Sarkar K, et al. The global burden of non-typhoidal salmonella invasive disease: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis. 2019;19:1312–1324. doi: 10.1016/S1473-3099(19)30418-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Galanis E. Campylobacter and bacterial gastroenteritis. CMAJ. 2007;177:570–571. doi: 10.1503/cmaj.070660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zoetis Vibrin, Campylobacter fetus bacterin. 2022. https://www2.zoetisus.com/products/beef/vibrin
- 59.WHO Bacterial vaccines in clinical and preclinical development 2021. 2022. https://www.who.int/publications/i/item/9789240052451
- 60.Schwameis M, Roppenser B, Firbas C, et al. Safety, tolerability, and immunogenicity of a recombinant toxic shock syndrome toxin (rTSST)-1 variant vaccine: a randomised, double-blind, adjuvant-controlled, dose escalation first-in-man trial. Lancet Infect Dis. 2016;16:1036–1044. doi: 10.1016/S1473-3099(16)30115-3. [DOI] [PubMed] [Google Scholar]
- 61.Shinefield H, Black S, Fattom A, et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med. 2002;346:491–496. doi: 10.1056/NEJMoa011297. [DOI] [PubMed] [Google Scholar]
- 62.Fowler VG, Jr, Proctor RA. Where does a Staphylococcus aureus vaccine stand? Clin Microbiol Infect. 2014;20(suppl 5):66–75. doi: 10.1111/1469-0691.12570. (abstr). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sakr A, Brégeon F, Mège JL, Rolain JM, Blin O. Staphylococcus aureus nasal colonization: an update on mechanisms, epidemiology, risk factors, and subsequent infections. Front Microbiol. 2018;9 doi: 10.3389/fmicb.2018.02419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wertheim HFL, Melles DC, Vos MC, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5:751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 65.Luna BM, Nielsen TB, Cheng B, et al. Vaccines targeting Staphylococcus aureus skin and bloodstream infections require different composition. PLoS One. 2019;14 doi: 10.1371/journal.pone.0217439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosa SS, Prazeres DMF, Azevedo AM, Marques MPC. mRNA vaccines manufacturing: challenges and bottlenecks. Vaccine. 2021;39:2190–2200. doi: 10.1016/j.vaccine.2021.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Munira SL, Hendriks JT, Atmosukarto II, et al. A cost analysis of producing vaccines in developing countries. Vaccine. 2019;37:1245–1251. doi: 10.1016/j.vaccine.2018.11.050. [DOI] [PubMed] [Google Scholar]
- 68.Sparrow E, Hasso-Agopsowicz M, Kaslow DC, et al. Leveraging mRNA platform technology to accelerate development of vaccines for some emerging and neglected tropical diseases through local vaccine production. Front Trop Dis. 2022 doi: 10.3389/fitd.2022.844039. published online July 4. [DOI] [Google Scholar]
- 69.Pronker ES, Weenen TC, Commandeur H, Claassen EHJHM, Osterhaus ADME. Risk in vaccine research and development quantified. PLoS One. 2013;8 doi: 10.1371/journal.pone.0057755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frost I, Laxminarayan R, McKenna N, Chai S, Joshi J. Antimicrobial resistance and primary health care. 2018. https://apps.who.int/iris/bitstream/handle/10665/326454/WHO-HIS-SDS-2018.56-eng.pdf%0Ahttps://www.who.int/docs/default-source/primary-health-care-conference/amr.pdf?sfvrsn=8817d5ba_2
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.