Abstract
Objective
To identify novel candidate genes whose expression is associated with bone mineral density (BMD) and body lean mass (LM) in children.
Methods
A tissue-specific transcriptome-wide association study (TWAS) was conducted utilizing a large-scale genome-wide association study (GWAS) dataset associated with BMD and LM and involving 10,414 participants. The measurement of BMD and LM phenotypes was made based on total-body dual-energy X-ray absorptiometry (TB-DXA) scans. TWAS was conducted by using FUSION software. Reference panels for muscle skeleton (MS), peripheral blood (NBL) and whole blood (YBL) were used for TWAS analysis. Functional enrichment and protein–protein interaction (PPI) analyses of the genes identified by TWAS were performed by using the online tool Metascape (http://metascape.org).
Results
For BMD, we identified 174 genes with P < 0.05, such as IKZF1 (P = 1.46 × 10−9) and CHKB (P = 8.31 × 10−7). For LM, we identified 208 genes with P < 0.05, such as COPS5 (P = 3.03 × 10−12) and MRPS33 (P = 5.45 × 10−10). Gene ontology (GO) enrichment analysis of the BMD-associated genes revealed 200 GO terms, such as protein catabolic process (Log P = −5.09) and steroid hormone-mediated signaling pathway (Log P = −3.13). GO enrichment analysis of the LM-associated genes detected 287 GO terms, such as the apoptotic signaling pathway (Log P = −8.08) and lipid storage (Log P = −3.55).
Conclusion
This study identified several candidate genes for BMD and LM in children, providing novel clues to the genetic mechanisms underlying the development of childhood BMD and LM.
Keywords: Transcriptome-wide association study (TWAS), Genome-wide association study (GWAS), Bone mineral density (BMD), Body lean mass (LM), Expression-trait association
Introduction
Bone mineral density (BMD) is the amount of bone mineral in bone tissue and is often measured by using dual-energy X-ray absorptiometry (DXA) [1]. It is very widely used in clinical practice to assess the health status of bone in young people to identify those with osteoporosis and to evaluate the risk of fracture. Lean mass (LM) is the composition of our body and theoretically consists of muscle, organs, and bone [2]. LM can also be measured with DXA and is often thought to be a good proxy for skeletal muscle. Both bone and skeletal muscle are important components of the musculoskeletal system and are associated with the locomotion of our body.
The mechanisms of BMD and LM remain unclear, but it is thought to be multifactorial with complex interactions between genetic susceptibility and environmental factors. The heritability of BMD was estimated to be 50–80% [3]. Studies have shown that BMD has an obvious family genetic correlation. For example, Lutz et al. reported that the BMD of daughters is strongly correlated with premenopausal mothers [4]. In addition, several genome-wide association studies have been conducted to identify the susceptibility genes of BMD [5–13]. Over 60 different loci were identified to be robustly associated with BMD at different skeletal sites, including SREBF1, TOM1L2, WNT16, and LGR4 [14]. Meanwhile, the heritability of LM was estimated to be approximately 52% [14]. Family studies have demonstrated a significant familial resemblance for lean mass, suggesting that they are under genetic control [15]. However, the genetic mechanisms underlying BMD and LM are still elusive.
In classic Genome-wide association studies (GWASs), vast association signals have been detected between genetic variants and traits or phenotypes. In most cases, the association does not represent the real causal relationship partly because the mechanistic steps between genetic variants and traits or phenotypes cannot be taken into consideration. Furthermore, several lines of evidence show that a substantial proportion of risk variants exert their influence on traits by modulating the expression levels of the target genes (for example, in the case of expression quantitative trait loci (eQTLs)) [16, 17]. In this situation, it is needed to be solved the association between gene expression and traits. However, these types of studies have been hindered by difficulties in specimen collection and the high cost of assessing genotypes, phenotypes, and gene expression levels. To address these issues, transcriptome-wide association study (TWAS) was used to identify genes whose expression is associated with complex traits based on GWAS summary data [18].
Without directly measuring the gene expression levels, TWAS approach leverages a relatively small set of reference panels with both genotype and expression data to impute the gene expression levels in large-scale GWASs. Then, the imputed gene expression levels were used to identify the expression-trait association. Through this approach, several expression-trait associations have been found for various traits or diseases [18–22]. In recent years, TWAS approach has become a useful tool to evaluate the genetics of complex traits. For example, A. Gusev, et al. identified 157 TWAS-significant genes in a schizophrenia GWAS and found that one of the genes, mapk3, showed a significant effect on neurodevelopmental phenotypes in zebrafish [19]. There are several methods can be used in the TWAS, such as FUSION, PrediXcan, UTMOST, S-MultiXcan, et al. These methods can be divided in two groups: cross-tissue TWAS and single-tissue TWAS. Cross-tissue (MultiXcan, S-MultiXcan, and UTMOST) showed improved ability to identify gene-level associations in simulated and natural data compared to single-tissue TWAS (FUSION and PrediXcan). However, cross-tissue TWAS results were not tissue-specific, and therefore the cross-tissue TWAS could not reveal tissue-specific genetic regulatory mechanisms [23].
Here, we used FUSION software because it can estimate gene-trait associations and tissue-specificity. In other words, the genes identified by FUSION software are more tissue-specificity [18]. In this study, we report a tissue-specific TWAS based on a large GWAS dataset of BMD and LM. Reference panels for different tissues/cells, including muscle skeleton (MS), peripheral blood (NBL) and whole blood (YBL) panels, were used for imputation. Several significant expression-trait association signals were detected for both BMD and LM. Moreover, an enrichment analysis was performed to explore the functional annotations.
Materials and methods
GWAS summary data
The GWAS data used in our analysis were extracted from a previously published study [14] that enrolled 10,414 participants from four pediatric cohorts, namely, the Generation R Study, the Avon Longitudinal Study of Parents and their Children (ALSPAC), the Bone Mineral Density in Childhood Study (BMDCS), and the Copenhagen Prospective Studies on Asthma in Childhood (COPSAC) cohort. The average ages of the participants from the four cohorts varied in the range of 6.21–9.94 years. The measurements of BMD and LM phenotypes were made based on total-body dual-energy X-ray absorptiometry (TB-DXA) scans. DNA samples were genotyped using the Illumina platform. SNPs with a minor allele frequency (MAF) < 0.05 were excluded. Imputation to the CEU panel of the HapMap Phase II (build 36 release 22) reference panels was performed. BMD and LM measurements were adjusted by age, sex, height, fat percent (TB-FM/weight), and study-specific covariates (genetic principal components and measurement center). The GCTA software package and linkage disequilibrium-score regression methodology were used to assess the SNP heritability.
TWAS analysis
TWAS was performed using FUSION software based on the pediatric GWAS summary data (http://gusevlab.org/projects/fusion/). The software provided an approach for identifying the association between complex traits and intermediate phenotypes (gene expression levels) without directly measuring the expression levels [18]. Given that the MS, NBL and YBL were used in previous biological studies of BMD and LM, the FUSION precomputed functional weights of gene expression of these three reference panels were used in our study [24–28]. Reference panels of different tissues were used to impute the cis genetic component expression of single nucleotide polymorphism (SNP) genotype data. In our study, the gene expression weights of the MS, NBL and YBL reference panel data, which were derived from FUSION, were used for the TWAS analysis. A P value was calculated for each analyzed gene. The significant association thresholds of MS should be P < 1.68 × 10−5 (0.05/2976), NBL should be P < 2.04 × 10−5 (0.05/2455) and YBL should be P < 1.06 × 10−5 (0.05/4701) after strict Bonferroni correction. P values between 1.68 × 10−5 (for MS, 2.04 × 10−5 for NBL, and 1.06 × 10−5 for YBL) and 0.05 were considered to be suggestive of significance.
Functional annotation and enrichment analysis
To better interpret the associated genes identified in the TWAS, the online tool Metascape was used to perform further functional enrichment analysis and protein–protein interaction (PPI) analysis (http://metascape.org) [29]. A standard accumulative hypergeometric statistical test was applied to identify ontology terms based on canonical pathway (MSigDB), hallmark gene set (MSigDB), Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway and gene ontology (GO) biological processes resources. The construction of the PPI network and associated module analysis were based on GO enrichment analysis using the plugin Molecular Complex Detection (MCODE). The MCODE algorithm was then applied to this network to identify neighborhoods where proteins are densely connected. Based on Bonferroni correction, the significance thresholds were Log P < −3.60 for BMD and Log P < −3.75 for LM. Log P values between Log P −3.60/Log P −3.75 and Log P −1.30 were considered to be suggestive of significance.
Results
Associated genes identified by the TWAS
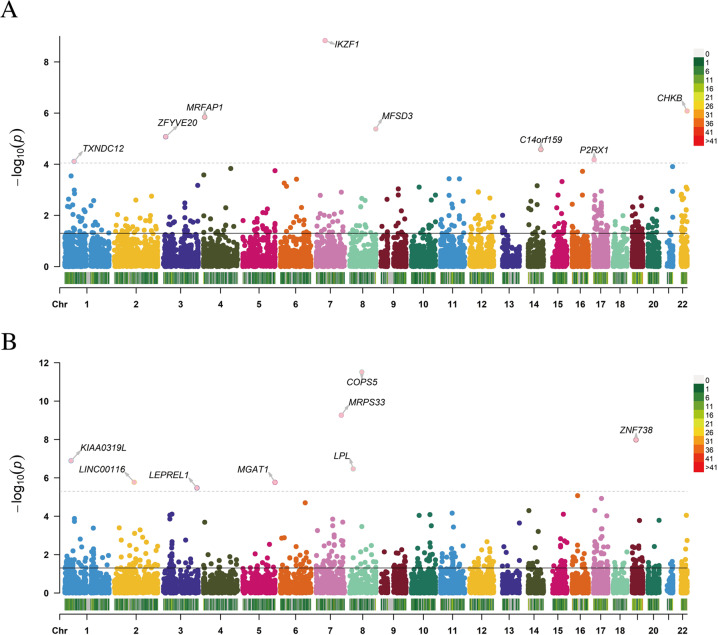
For different tissues/cells of the MS, NBL, and YBL panels, 2976, 2455, and 4701 genes were finally included in the expression-training association analysis (Table 1). For the childhood BMD phenotype, TWAS identified 120, 86, and 174 genes with P < 0.05 based on the MS, NBL, and YBL panels, such as IKZF1 (P = 1.46 × 10−9) and CHKB (P = 8.31 × 10−7). For childhood LM, we detected 145, 94, and 208 genes with P < 0.05 based on the MS, NBL, and YBL panels, such as COPS5 (P = 3.03 × 10−12) and MRPS33 (P = 5.45 × 10−10) (Fig. 1). The 10 top significant genes in the BMD and LM groups are shown in Table 2, including population, heritability of genes (HSQ), and the BEST.GWAS. ID, number of SNPs in the locus (NSNP), and TWAS P value (P TWAS).
Table 1.
The number of associated with BMD and LM identified by the TWAS
| Tissue/cell | N# | n# for BMD | n# for LM |
|---|---|---|---|
| MS | 2976 | 120 | 145 |
| NBL | 2455 | 86 | 94 |
| YBL | 4701 | 174 | 208 |
BMD bone mineral density, LM lean mass, TWAS transcriptome-wide association study
N means the number of genes finally included in the analysis of TWAS
n means the number of significantly associated genes identified in the TWAS
Fig. 1.
Manhattan plot showing TWAS-identified genes. Manhattan plot showing TWAS-identified genes and significantly expressed genes associated. with bone mineral density (A BMD) and lean mass (B LM). Each point represents a single gene, and the physical position (chromosome localization) is plotted on the x-axis, while the -log10 (P value) of the association between gene and BMD or LM is plotted on the y-axis. TWAS Transcriptome-wide association study, BMD bone mineral density, LM lean mass
Table 2.
Top genes selected by TWAS analysis (BMD and LM)
| Gene | CHR | BEST.GWAS.ID | NSNP | TWAS.Z | TWAS.P | |
|---|---|---|---|---|---|---|
| BMD | IKZF1 | 7 | rs7779747 | 534 | −6.0490 | 1.46E-09 |
| CHKB | 22 | rs761744 | 263 | −3.9353 | 8.31E-07 | |
| MRFAP1 | 4 | rs7660424 | 493 | 4.8190 | 1.44E-06 | |
| MFSD3 | 8 | rs6558318 | 186 | 4.6027 | 4.17E-06 | |
| ZFYVE20 | 3 | rs17040623 | 490 | 4.4536 | 8.44E-06 | |
| C14orf159 | 14 | rs1286341 | 450 | 4.2027 | 2.64E-05 | |
| P2RX1 | 17 | rs17822998 | 463 | 3.9892 | 6.63E-05 | |
| TXNDC12 | 1 | rs6686632 | 194 | 3.9516 | 7.76E-05 | |
| C21orf89 | 21 | rs2838846 | 522 | −3.8387 | 1.24E-04 | |
| RPS3A | 4 | rs7657668 | 264 | 3.7963 | 1.47E-04 | |
| LM | COPS5 | 8 | rs16933079 | 177 | 6.9761 | 3.03E-12 |
| MRPS33 | 7 | rs10488014 | 378 | −6.2056 | 5.45E-10 | |
| ZNF738 | 19 | rs661453 | 288 | −5.7224 | 1.05E-08 | |
| KIAA0319L | 1 | rs6668101 | 215 | 5.2800 | 1.28E-07 | |
| LPL | 8 | rs1569209 | 679 | 5.1003 | 3.39E-07 | |
| LINC00116 | 2 | rs11904760 | 72 | 4.7872 | 1.69E-06 | |
| MGAT1 | 5 | rs3733754 | 350 | −4.7852 | 1.71E-06 | |
| LEPREL1 | 3 | rs6788300 | 652 | −4.6479 | 3.35E-06 | |
| CD2BP2 | 16 | rs7196298 | 191 | −4.4548 | 8.40E-06 | |
| WNT3 | 17 | rs16941702 | 253 | −4.3818 | 1.18E-05 |
The large-scale Genome-Wide Association Study (GWAS) summary data for BMD and LM acquired from a cohort study, including 10,414 participants. The TWAS.P and TWAS.Z values were calculated by the FUSION approach (http://gusevlab.org/projects/fusion/)
TWAS Transcriptome-Wide Association Study, GWAS Genome-Wide Association Study, BMD Bone mineral density, LM Lean mass, TWAS P TWAS P value, TWAS Z TWAS Z-score, HSQ heritability of genes, NSNP number of SNPs in the locus
Functional enrichment and PPI analysis results
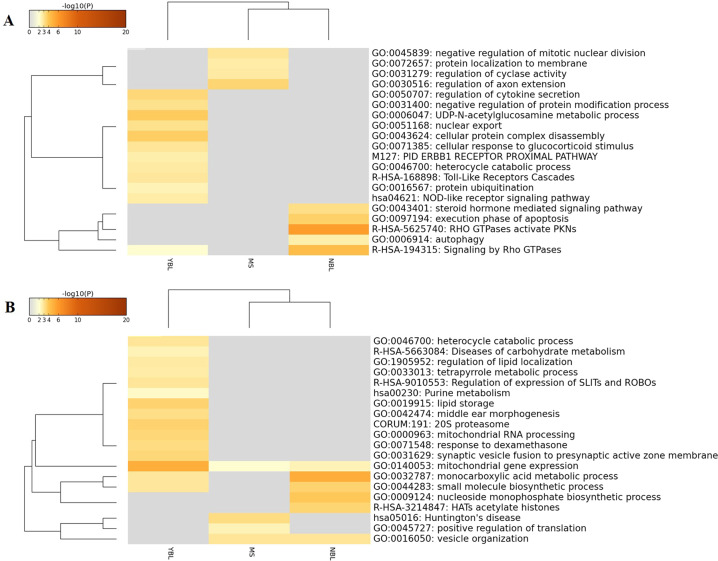
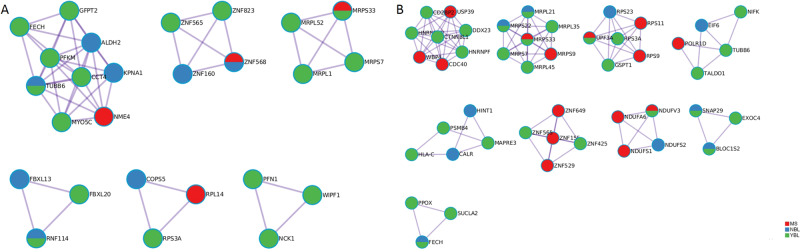
In this study, functional enrichment analysis was carried out with the following ontology sources: GO biological processes, KEGG pathways, GO molecular functions, reactome gene sets, canonical pathways, and CORUM. The heatmap of the enriched terms is shown in Fig. 2. The enrichment analysis results showed that the genes significantly associated with BMD were enriched in hormone-related categories, such as the apoptotic signaling pathway (Log P = −8.08), steroid hormone-mediated signaling pathway (Log P = −3.13), regulation of growth hormone secretion (Log P = −3.43) and cellular response to glucocorticoid stimulus (Log P = −2.95). For LM, it seems that the associated genes are more likely to be enriched in the metabolism categories of several substances, including protein catabolic process (Log P = −5.09), carbohydrate metabolism (Log P = −2.43), regulation of lipid localization (Log P = −2.80), and lipid storage (Log P = −3.55). PPI enrichment analysis was also carried out with the following databases: BioGrid7, InWeb_IM8, and OmniPath9. The MCODE networks identified for individual gene lists were pooled and are shown in Fig. 3.
Fig. 2.
The heatmap of enriched terms of BMD (A) and LM (B). The heatmap showed the enriched terms of bone mineral density (A BMD) and lean mass (B LM). BMD bone mineral density, LM lean mass
Fig. 3.
Protein-protein interaction (PPI) enrichment analysis of BMD (A) and LM (B). Protein-protein interaction (PPI) enrichment analysis was also carried out with the following databases: BioGrid7, InWeb_IM8, OmniPath9. PPI Protein-protein interaction, BMD bone mineral density, LM lean mass
Discussion
BMD and LM are complex traits that can be influenced by multiple genetic factors. Although previous studies have reported various genes associated with the two traits, limited mechanistic information was provided in these studies. Genetic variants can influence traits by regulating gene expression levels. Therefore, we conducted this tissue-specific TWAS to investigate the expression-trait associations for BMD and LM. Databases with both expression levels and genotype data for tissues/cells, namely, MS, NBL, and YBL, were used as reference panels for expression level imputation.
SREBF1 (sterol regulatory element-binding factor 1), a significant gene we identified associated with both BMD and LM, is consistent with the results reported in previous studies [14]. The active product of SREBF1, SREBP-1, regulates muscle protein synthesis by downregulating the expression of MYOD1, MYOG, and MEF2C factors, while overexpression inhibits protein synthesis and thus induces myotubular atrophy [30, 31]. In addition, decreased expression of SREBP-1 also leads to decreased NF-κB signaling, which in turn decreases osteoclast formation and bone resorption activity, while enhanced expression of SREBF, in contrast, enhances osteoblast mineralization and thus increases BMD [32]. Overall, the biological activity of SREBF is pleiotropic, with opposite effects on LM and BMD occurrence.
By using TWAS analysis, several genes, such as COPS5, were identified with LM. COP9 signaling vesicle complex 5 (COPS5) is an important member of the ubiquitin‒proteasome system that plays an important role in ubiquitin-mediated protein degradation and is also involved in the regulation of cell development [33, 34] COPS5 regulates the myosin-interacting proteins UNC-98 and UNC96 to modulate muscle protein levels [35]. Velardo et al. showed that COPS5 plays an important role in muscle development, maintenance and regeneration and is associated with the development of congenital muscular dystrophy [36]. In addition, COPS5 is necessary for osteoblast development and postnatal bone formation, and it may function by modulating the TGF and BMP signaling pathways in osteoblast progenitor cells [37]. Thus, COPS5 may affect LM by regulating muscle and bone metabolism.
For BMD, TWAS analysis also identified several genes, such as IKZF1 and CHKB. Choline kinase β (CHKB), a kinase involved in phosphatidylcholine biosynthesis, is a gene that we have identified as significantly associated with BMD. A study demonstrated that CHKB deficiency results in defective formation and function of osteoclasts and osteoblasts, leading to reduced bone mass, and is considered to be a regulator of bone homeostasis [38]. CHKB activation may influence osteoclastic bone resorption by influencing the gene expression of chloride and voltage-dependent calcium channels that are required for osteoclast activity [39, 40]. Recent studies have shown that CHKB deficiency leads to congenital myotonic dystrophy, including microcephaly and facial dysmorphism [41]. Therefore, CHKB activity and BMD are closely related, but the exact mechanism of action needs to be further investigated.
The enrichment analysis detected 200 terms associated with BMD, such as apoptotic signaling pathway (GO: 0097190, Log P = −8.08), steroid hormone mediated signaling pathway (GO: 0043401, Log P = −3.13), autophagy (GO: 0006914, Log P = −2.65), regulation of cytokine secretion (GO: 0050707, Log P = −3.43) and cellular response to glucocorticoid stimulus (GO: 0071385, Log P = −2.95). By using enrichment analysis, we found that the apoptotic signaling pathway had the most relevant association with BMD. Apoptosis plays an important role in bone growth, bone remodeling, and bone regeneration, and increased osteocyte apoptosis is often associated with rapid bone turnover [42]. According to studies, excessive use of glucocorticoids or aging can accelerate osteoblast apoptosis and result in bone loss [43, 44]. In addition, studies have demonstrated that postmenopausal women have decreased estrogen secretion, which stimulates osteoblasts to secrete cytokines such as NF-κB that activate downstream apoptotic pathways and inhibit osteoblast proliferation and differentiation, resulting in decreased bone mineral density and osteoporosis [45]. In addition, these pathways were most enriched in the hormone-related categories. Steroid hormones such as glucocorticoids were associated with decreased BMD and impaired bone microarchitecture parameters [46]. These findings seem consistent with previous knowledge of BMD.
For LM, enrichment analysis detected 287 terms that were most likely to be enriched in the metabolism categories of several substances, including protein catabolic process (GO:0030163, Log P = −5.09), diseases of carbohydrate metabolism (R-HSA-5663084, Log P = −2.43), regulation of lipid localization (GO: 1905952, Log P = −2.80), lipid storage (GO: 0019915, Log P = −3.55) and mitochondrial RNA processing (GO: 0000963, Log P = −3.41). Muscle atrophy is the most significant symptom of LM, and we found that the protein catabolic process is the most relevant pathway associated with LM. The ubiquitin‒proteasome system is the best-known cellular protein degradation system responsible for the hydrolysis of damaged proteins collected in skeletal muscle, and multiple studies have demonstrated that UPS overexpression results in skeletal muscle atrophy [47]. In addition, during famine, aging, and excessive glucocorticoid use, the glucocorticoid receptor and transcription factor forkhead box O increase the expression of MuRF1 and accelerate protein catabolism, ultimately resulting in muscle atrophy [48]. In contrast, protein supplementation can successfully prevent muscle atrophy by reversing the imbalance between protein catabolism and anabolism [49]. In addition, adequate research has demonstrated that reduced carbohydrate and lipid consumption leads to lower body weight by increasing lipid oxidation [50, 51]. Compared with BMD, LM is more likely to be regulated by biochemical metabolism.
According to the PPI network, aberrant expression of ribosomal protein (RP) is intimately linked to BMD and LM. RP refers to the proteins that comprise the ribosome and serve a key regulatory function in ribosome biosynthesis, peptide bond formation, and protein synthesis [52]. During the growth and development of bones, ribosome-mediated protein synthesis is necessary for the formation of bone matrix. According to studies, aberrant expression of some RP proteins may be linked to congenital skeletal growth abnormalities and bone marrow failure [53]. Studies on animals indicate that mice with impaired ribosome function have changed rates of matrix protein synthesis, resulting in delayed bone formation, decreased bone mass, and heightened bone fragility [54]. In addition to RPs, ZNF823, ZNF565, ZNF160, and ZNF568 are also aberrant regulatory proteins screened by the PPI network. Schnurri-3, a zinc finger protein, was demonstrated to modulate osteoclast activity to modify bone mass [55].
The TWAS analysis approach developed by Alexander G et al. was adopted in this study [18]. It can be used to identify genes whose expression is significantly associated with complex traits in individuals based on GWAS summary data without directly measuring the expression levels of these genes [18]. There are several potential advantages of using this approach. First, the expression-trait association information can provide more interpretable insights for further genetic studies, while GWASs often obtain associated loci lying in the linkage disequilibrium with multiple significant SNPs that may not be in the genes. Moreover, unlike analyses focusing on eQTL and SNP associations, TWASs can combine full cis-SNP signals, regardless of whether they are significant, to make an expression level imputation. Finally, confounding from environmental differences caused by the traits can be avoided in TWAS analyses.
There are also some limitations of this study that must be addressed. First, as the gene expression level is imputed based on the reference panels, there is a possibility that the results can be influenced by the quality and sample size of the reference data. A larger sample size and more available tissue datasets can mitigate this issue. Second, the number of imputed genes depends on the training data. Therefore, it is limited to some degree. Finally, after multiple testing and correction, the significance threshold of enrichment analysis was Log P < −3.60 for BMD and Log P < −3.75 for LM. Unfortunately, according to our results, some terms enriched in this study, such as steroid hormone-mediated signaling pathways and diseases of carbohydrate metabolism, showed suggestive associations with BMD and LM. Therefore, the results of the suggested terms should be interpreted carefully.
Conclusion
This study was a tissue-specific TWAS of the phenotypes of BMD and LM based on previous GWAS datasets. The expression levels of several genes were identified to be associated with BMD and LM. By performing gene enrichment analysis, we found that the two traits had different regulatory mechanisms. This study could provide novel insights for the further study of BMD and LM and an outline for a systematic approach for identifying functional mediators of complex disease.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 81974347 and 81802210); the Department of Science and Technology of Sichuan Province (grant number 2021YFS0122 and 2020YFS0139). Financial support had no impact on the outcomes of this study.
Data availability
The GWAS summary dataset was extracted from a previously published study (Bivariate genome-wide association analysis implicates pleiotropic effects at the SREBF1/TOM1L2 locus on bone mineral density and lean mass in children).
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Ethics approval
The source of the data was a publicly available data base and no human participants were involved, hence ethical parameters are not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Knight SM, Ring EF, Bhalla AK. Bone mineral density and osteoarthritis. Ann. Rheum. Dis. 1992;51(9):1025–1026. doi: 10.1136/ard.51.9.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.S.M. Ribeiro, J.J. Kehayias, Sarcopenia and the analysis of body composition. Adv. Nutr. (Bethesda. Md) 5(3), 260–267 (2014) [DOI] [PMC free article] [PubMed]
- 3.Ip HF, Jansen R, Abdellaoui A, Bartels M, Boomsma DI, Nivard MG. Characterizing the relation between expression QTLs and complex traits: exploring the role of tissue specificity. Behav. Genet. 2018;48(5):374–385. doi: 10.1007/s10519-018-9914-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lutz J, Tesar R. Mother-daughter pairs: spinal and femoral bone densities and dietary intakes. Am. J. Clin. Nutr. 1990;52(5):872–877. doi: 10.1093/ajcn/52.5.872. [DOI] [PubMed] [Google Scholar]
- 5.Styrkarsdottir U, Thorleifsson G, Gudjonsson SA, Sigurdsson A, Center JR, Lee SH, Nguyen TV, Kwok TCY, Lee JSW, Ho SC, Woo J, Leung PC, Kim BJ, Rafnar T, Kiemeney LA, Ingvarsson T, Koh JM, Tang NLS, Eisman JA, Christiansen C, Sigurdsson G, Thorsteinsdottir U, Stefansson K. Sequence variants in the PTCH1 gene associate with spine bone mineral density and osteoporotic fractures. Nat. Commun. 2016;7:10129. doi: 10.1038/ncomms10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng HF, Forgetta V, Hsu YH, Estrada K, Rosello-Diez A, Leo PJ, Dahia CL, Park-Min KH, Tobias JH, Kooperberg C, Kleinman A, Styrkarsdottir U, Liu CT, Uggla C, Evans DS, Nielson CM, Walter K, Pettersson-Kymmer U, McCarthy S, Eriksson J, Kwan T, Jhamai M, Trajanoska K, Memari Y, Min J, Huang J, Danecek P, Wilmot B, Li R, Chou WC, Mokry LE, Moayyeri A, Claussnitzer M, Cheng CH, Cheung W, Medina-Gómez C, Ge B, Chen SH, Choi K, Oei L, Fraser J, Kraaij R, Hibbs MA, Gregson CL, Paquette D, Hofman A, Wibom C, Tranah GJ, Marshall M, Gardiner BB, Cremin K, Auer P, Hsu L, Ring S, Tung JY, Thorleifsson G, Enneman AW, van Schoor NM, de Groot LC, van der Velde N, Melin B, Kemp JP, Christiansen C, Sayers A, Zhou Y, Calderari S, van Rooij J, Carlson C, Peters U, Berlivet S, Dostie J, Uitterlinden AG, Williams SR, Farber C, Grinberg D, LaCroix AZ, Haessler J, Chasman DI, Giulianini F, Rose LM, Ridker PM, Eisman JA, Nguyen TV, Center JR, Nogues X, Garcia-Giralt N, Launer LL, Gudnason V, Mellström D, Vandenput L, Amin N, van Duijn CM, Karlsson MK, Ljunggren Ö, Svensson O, Hallmans G, Rousseau F, Giroux S, Bussière J, Arp PP, Koromani F, Prince RL, Lewis JR, Langdahl BL, Hermann AP, Jensen JE, Kaptoge S, Khaw KT, Reeve J, Formosa MM, Xuereb-Anastasi A, Åkesson K, McGuigan FE, Garg G, Olmos JM, Zarrabeitia MT, Riancho JA, Ralston SH, Alonso N, Jiang X, Goltzman D, Pastinen T, Grundberg E, Gauguier D, Orwoll ES, Karasik D, Davey-Smith G, Smith AV, Siggeirsdottir K, Harris TB, Zillikens MC, van Meurs JB, Thorsteinsdottir U, Maurano MT, Timpson NJ, Soranzo N, Durbin R, Wilson SG, Ntzani EE, Brown MA, Stefansson K, Hinds DA, Spector T, Cupples LA, Ohlsson C, Greenwood CM, Jackson RD, Rowe DW, Loomis CA, Evans DM, Ackert-Bicknell CL, Joyner AL, Duncan EL, Kiel DP, Rivadeneira F, Richards JB. Whole-genome sequencing identifies EN1 as a determinant of bone density and fracture. Nature. 2015;526(7571):112–117. doi: 10.1038/nature14878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivadeneira F, Styrkársdottir U, Estrada K, Halldórsson BV, Hsu YH, Richards JB, Zillikens MC, Kavvoura FK, Amin N, Aulchenko YS, Cupples LA, Deloukas P, Demissie S, Grundberg E, Hofman A, Kong A, Karasik D, van Meurs JB, Oostra B, Pastinen T, Pols HA, Sigurdsson G, Soranzo N, Thorleifsson G, Thorsteinsdottir U, Williams FM, Wilson SG, Zhou Y, Ralston SH, van Duijn CM, Spector T, Kiel DP, Stefansson K, Ioannidis JP, Uitterlinden AG. Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat. Genet. 2009;41(11):1199–1206. doi: 10.1038/ng.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richards JB, Rivadeneira F, Inouye M, Pastinen TM, Soranzo N, Wilson SG, Andrew T, Falchi M, Gwilliam R, Ahmadi KR, Valdes AM, Arp P, Whittaker P, Verlaan DJ, Jhamai M, Kumanduri V, Moorhouse M, van Meurs JB, Hofman A, Pols HA, Hart D, Zhai G, Kato BS, Mullin BH, Zhang F, Deloukas P, Uitterlinden AG, Spector TD. Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet. 2008;371(9623):1505–1512. doi: 10.1016/S0140-6736(08)60599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Estrada K, Styrkarsdottir U, Evangelou E, Hsu YH, Duncan EL, Ntzani EE, Oei L, Albagha OM, Amin N, Kemp JP, Koller DL, Li G, Liu CT, Minster RL, Moayyeri A, Vandenput L, Willner D, Xiao SM, Yerges-Armstrong LM, Zheng HF, Alonso N, Eriksson J, Kammerer CM, Kaptoge SK, Leo PJ, Thorleifsson G, Wilson SG, Wilson JF, Aalto V, Alen M, Aragaki AK, Aspelund T, Center JR, Dailiana Z, Duggan DJ, Garcia M, Garcia-Giralt N, Giroux S, Hallmans G, Hocking LJ, Husted LB, Jameson KA, Khusainova R, Kim GS, Kooperberg C, Koromila T, Kruk M, Laaksonen M, Lacroix AZ, Lee SH, Leung PC, Lewis JR, Masi L, Mencej-Bedrac S, Nguyen TV, Nogues X, Patel MS, Prezelj J, Rose LM, Scollen S, Siggeirsdottir K, Smith AV, Svensson O, Trompet S, Trummer O, van Schoor NM, Woo J, Zhu K, Balcells S, Brandi ML, Buckley BM, Cheng S, Christiansen C, Cooper C, Dedoussis G, Ford I, Frost M, Goltzman D, González-Macías J, Kähönen M, Karlsson M, Khusnutdinova E, Koh JM, Kollia P, Langdahl BL, Leslie WD, Lips P, Ljunggren Ö, Lorenc RS, Marc J, Mellström D, Obermayer-Pietsch B, Olmos JM, Pettersson-Kymmer U, Reid DM, Riancho JA, Ridker PM, Rousseau F, Slagboom PE, Tang NL, Urreizti R, Van Hul W, Viikari J, Zarrabeitia MT, Aulchenko YS, Castano-Betancourt M, Grundberg E, Herrera L, Ingvarsson T, Johannsdottir H, Kwan T, Li R, Luben R, Medina-Gómez C, Palsson ST, Reppe S, Rotter JI, Sigurdsson G, van Meurs JB, Verlaan D, Williams FM, Wood AR, Zhou Y, Gautvik KM, Pastinen T, Raychaudhuri S, Cauley JA, Chasman DI, Clark GR, Cummings SR, Danoy P, Dennison EM, Eastell R, Eisman JA, Gudnason V, Hofman A, Jackson RD, Jones G, Jukema JW, Khaw KT, Lehtimäki T, Liu Y, Lorentzon M, McCloskey E, Mitchell BD, Nandakumar K, Nicholson GC, Oostra BA, Peacock M, Pols HA, Prince RL, Raitakari O, Reid IR, Robbins J, Sambrook PN, Sham PC, Shuldiner AR, Tylavsky FA, van Duijn CM,Wareham NJ, Cupples LA, Econs MJ, Evans DM, Harris TB, Kung AW, Psaty BM, Reeve J, Spector TD, Streeten EA, Zillikens MC, Thorsteinsdottir U, Ohlsson C, Karasik D, Richards JB, Brown MA, Stefansson K, Uitterlinden AG, Ralston SH, Ioannidis JP, Kiel DP, Rivadeneira F. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat. Genet. 2012;44(5):491–501. doi: 10.1038/ng.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koller DL, Zheng HF, Karasik D, Yerges-Armstrong L, Liu CT, McGuigan F, Kemp JP, Giroux S, Lai D, Edenberg HJ, Peacock M, Czerwinski SA, Choh AC, McMahon G, St Pourcain B, Timpson NJ, Lawlor DA, Evans DM, Towne B, Blangero J, Carless MA, Kammerer C, Goltzman D, Kovacs CS, Prior JC, Spector TD, Rousseau F, Tobias JH, Akesson K, Econs MJ, Mitchell BD, Richards JB, Kiel DP, Foroud T. Meta-analysis of genome-wide studies identifies WNT16 and ESR1 SNPs associated with bone mineral density in premenopausal women. J. Bone Miner. Res.: Off. J. Am. Soc. Bone Miner. Res. 2013;28(3):547–558. doi: 10.1002/jbmr.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Styrkarsdottir U, Thorleifsson G, Sulem P, Gudbjartsson DF, Sigurdsson A, Jonasdottir A, Jonasdottir A, Oddsson A, Helgason A, Magnusson OT, Walters GB, Frigge ML, Helgadottir HT, Johannsdottir H, Bergsteinsdottir K, Ogmundsdottir MH, Center JR, Nguyen TV, Eisman JA, Christiansen C, Steingrimsson E, Jonasson JG, Tryggvadottir L, Eyjolfsson GI, Theodors A, Jonsson T, Ingvarsson T, Olafsson I, Rafnar T, Kong A, Sigurdsson G, Masson G, Thorsteinsdottir U, Stefansson K. Nonsense mutation in the LGR4 gene is associated with several human diseases and other traits. Nature. 2013;497(7450):517–520. doi: 10.1038/nature12124. [DOI] [PubMed] [Google Scholar]
- 12.Styrkarsdottir U, Thorleifsson G, Eiriksdottir B, Gudjonsson SA, Ingvarsson T, Center JR, Nguyen TV, Eisman JA, Christiansen C, Thorsteinsdottir U, Sigurdsson G, Stefansson K. Two rare mutations in the COL1A2 gene associate with low bone mineral density and fractures in Iceland. J. Bone Miner. Res.: Off. J. Am. Soc. Bone Miner. Res. 2016;31(1):173–179. doi: 10.1002/jbmr.2604. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Choi HJ, Estrada K, Leo PJ, Li J, Pei YF, Zhang Y, Lin Y, Shen H, Liu YZ, Liu Y, Zhao Y, Zhang JG, Tian Q, Wang YP, Han Y, Ran S, Hai R, Zhu XZ, Wu S, Yan H, Liu X, Yang TL, Guo Y, Zhang F, Guo YF, Chen Y, Chen X, Tan L, Zhang L, Deng FY, Deng H, Rivadeneira F, Duncan EL, Lee JY, Han BG, Cho NH, Nicholson GC, McCloskey E, Eastell R, Prince RL, Eisman JA, Jones G, Reid IR, Sambrook PN, Dennison EM, Danoy P, Yerges-Armstrong LM, Streeten EA, Hu T, Xiang S, Papasian CJ, Brown MA, Shin CS, Uitterlinden AG, Deng HW. Multistage genome-wide association meta-analyses identified two new loci for bone mineral density. Hum. Mol. Genet. 2014;23(7):1923–1933. doi: 10.1093/hmg/ddt575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medina-Gomez C, Kemp JP, Dimou NL, Kreiner E, Chesi A, Zemel BS, Bønnelykke K, Boer CG, Ahluwalia TS, Bisgaard H, Evangelou E, Heppe DHM, Bonewald LF, Gorski JP, Ghanbari M, Demissie S, Duque G, Maurano MT, Kiel DP, Hsu YH, B CJVDE, Ackert-Bicknell C, Reppe S, Gautvik KM, Raastad T, Karasik D, van de Peppel J, Jaddoe VWV, Uitterlinden AG, Tobias JH, Grant SFA, Bagos PG, Evans DM, Rivadeneira F. Bivariate genome-wide association meta-analysis of pediatric musculoskeletal traits reveals pleiotropic effects at the SREBF1/TOM1L2 locus. Nat. Commun. 2017;8(1):121. doi: 10.1038/s41467-017-00108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arden NK, Spector TD. Genetic influences on muscle strength, lean body mass, and bone mineral density: a twin study. J. Bone Miner. Res.: Off. J. Am. Soc. Bone Miner. Res. 1997;12(12):2076–2081. doi: 10.1359/jbmr.1997.12.12.2076. [DOI] [PubMed] [Google Scholar]
- 16.Mancuso N, Shi H, Goddard P, Kichaev G, Gusev A, Pasaniuc B. Integrating gene expression with summary association statistics to identify genes associated with 30 complex traits. Am. J. Hum. Genet. 2017;100(3):473. doi: 10.1016/j.ajhg.2017.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicolae DL, Gamazon E, Zhang W, Duan S, Dolan ME, Cox NJ. Trait-associated SNPs are more likely to be eQTLs: annotation to enhance discovery from GWAS. Plos Genet. 2010;6(4):e1000888. doi: 10.1371/journal.pgen.1000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.A. Gusev, A. Ko, Integrative approaches for large-scale transcriptome-wide association studies. 48(3), 245–252 (2016) [DOI] [PMC free article] [PubMed]
- 19.Gusev A, Mancuso N, Won H, Kousi M, Finucane HK, Reshef Y, Song L, Safi A, Mccarroll S, Neale BM. Transcriptome-wide association study of schizophrenia and chromatin activity yields mechanistic disease insights. Nat. Genet. 2018;50(4):538–548. doi: 10.1038/s41588-018-0092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.S. Thériault, N. Gaudreault, M. Lamontagne, M. Rosa, M. C. Boulanger, D. Messika-Zeitoun, M. A. Clavel, R. Capoulade, F. Dagenais, P. Pibarot, A transcriptome-wide association study identifies PALMD as a susceptibility gene for calcific aortic valve stenosis. Nat. Commun. 9(1), (2018) [DOI] [PMC free article] [PubMed]
- 21.Jiang T, Wang Y, Zhu M, Wang Y, Huang M, Jin G, Guo X, Sha J, Dai J, Hu Z. Transcriptome-wide association study revealed two novel genes associated with nonobstructive azoospermia in a Chinese population. Fertil. Steril. 2017;108(6):1056–1062. doi: 10.1016/j.fertnstert.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 22.Lin H, Lunetta KL, Zhao Q, Rong J, Benjamin EJ, Mendelson MM, Joehanes R, Levy D, Larson MG, Murabito JM. Transcriptome-wide association study of inflammatory biologic age. Aging. 2017;9(11):2288–2301. doi: 10.18632/aging.101321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barbeira AN, Pividori M, Zheng J, Wheeler HE, Nicolae DL, Im HK. Integrating predicted transcriptome from multiple tissues improves association detection. PLoS Genet. 2019;15(1):e1007889. doi: 10.1371/journal.pgen.1007889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernandez CJ, Moeller AH. The microbiome: A heritable contributor to bone morphology? Semin. Cell Developmental Biol. 2022;123:82–87. doi: 10.1016/j.semcdb.2021.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan M. L., L. R. Chen, H. M. Tsao, K. H. Chen, Iron deficiency anemia as a risk factor for osteoporosis in Taiwan: a nationwide population-based study. Nutrients 9(6), (2017) [DOI] [PMC free article] [PubMed]
- 26.Valderrábano RJ, Lui LY, Lee J, Cummings SR, Orwoll ES, Hoffman AR, Wu JY. Bone density loss is associated with blood cell counts. J. Bone Miner. Res.: Off. J. Am. Soc. Bone Miner. Res. 2017;32(2):212–220. doi: 10.1002/jbmr.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Y. Ingenbleek, Plasma transthyretin as a biomarker of sarcopenia in elderly subjects. Nutrients. 11(4), (2019) [DOI] [PMC free article] [PubMed]
- 28.Kehoe K, Noels H, Theelen W, De Hert E, Xu S, Verrijken A, Arnould T, Fransen E, Hermans N, Lambeir AM, Venge P, Van Gaal L, De Meester I. Prolyl carboxypeptidase activity in the circulation and its correlation with body weight and adipose tissue in lean and obese subjects. PloS One. 2018;13(5):e0197603. doi: 10.1371/journal.pone.0197603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, Benner C, Chanda SK. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019;10(1):1523. doi: 10.1038/s41467-019-09234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dessalle K, Euthine V, Chanon S, Delarichaudy J, Fujii I, Rome S, Vidal H, Nemoz G, Simon C, Lefai E. SREBP-1 transcription factors regulate skeletal muscle cell size by controlling protein synthesis through myogenic regulatory factors. PloS One. 2012;7(11):e50878. doi: 10.1371/journal.pone.0050878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lecomte V, Meugnier E, Euthine V, Durand C, Freyssenet D, Nemoz G, Rome S, Vidal H, Lefai E. A new role for sterol regulatory element binding protein 1 transcription factors in the regulation of muscle mass and muscle cell differentiation. Mol. Cell. Biol. 2010;30(5):1182–1198. doi: 10.1128/MCB.00690-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.C. Shochat, Z. Wang, C. Mo, S. Nelson, R. Donaka, J. Huang, D. Karasik, M. Brotto, Deletion of SREBF1, a functional bone-muscle pleiotropic gene, alters bone density and lipid signaling in zebrafish. Endocrinology. 162(1), (2021) [DOI] [PMC free article] [PubMed]
- 33.Zhang XC, Chen J, Su CH, Yang HY, Lee MH. Roles for CSN5 in control of p53/MDM2 activities. J. Cell. Biochem. 2008;103(4):1219–1230. doi: 10.1002/jcb.21504. [DOI] [PubMed] [Google Scholar]
- 34.Liu G, Claret FX, Zhou F, Pan Y. Jab1/COPS5 as a novel biomarker for diagnosis, prognosis, therapy prediction and therapeutic tools for human cancer. Front. Pharmacol. 2018;9:135. doi: 10.3389/fphar.2018.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller RK, Qadota H, Stark TJ, Mercer KB, Wortham TS, Anyanful A, Benian GM. CSN-5, a component of the COP9 signalosome complex, regulates the levels of UNC-96 and UNC-98, two components of M-lines in Caenorhabditis elegans muscle. Mol. Biol. Cell. 2009;20(15):3608–3616. doi: 10.1091/mbc.E09-03-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sacks D, Baxter B, Campbell BCV, Carpenter JS, Cognard C, Dippel D, Eesa M, Fischer U, Hausegger K, Hirsch JA, Shazam Hussain M, Jansen O, Jayaraman MV, Khalessi AA, Kluck BW, Lavine S, Meyers PM, Ramee S, Rüfenacht DA, Schirmer CM, Vorwerk D. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int. J. Stroke.: Off. J. Int. Stroke. Soc. 2018;13(6):612–632. doi: 10.1177/1747493018778713. [DOI] [PubMed] [Google Scholar]
- 37.Samsa WE, Mamidi MK, Hausman BS, Bashur LA, Greenfield EM, Zhou G. The master developmental regulator Jab1/Cops5/Csn5 is essential for proper bone growth and survival in mice. Bone. 2021;143:115733. doi: 10.1016/j.bone.2020.115733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kular J, Tickner JC, Pavlos NJ, Viola HM, Abel T, Lim BS, Yang X, Chen H, Cook R, Hool LC, Zheng MH, Xu J. Choline kinase β mutant mice exhibit reduced phosphocholine, elevated osteoclast activity, and low bone mass. J. Biol. Chem. 2015;290(3):1729–1742. doi: 10.1074/jbc.M114.567966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okamoto F, Kajiya H, Toh K, Uchida S, Yoshikawa M, Sasaki S, Kido MA, Tanaka T, Okabe K. Intracellular ClC-3 chloride channels promote bone resorption in vitro through organelle acidification in mouse osteoclasts. Am. J. Physiol. Cell Physiol. 2008;294(3):C693–701. doi: 10.1152/ajpcell.00251.2007. [DOI] [PubMed] [Google Scholar]
- 40.Kornak U, Kasper D, Bösl MR, Kaiser E, Schweizer M, Schulz A, Friedrich W, Delling G, Jentsch TJ. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell. 2001;104(2):205–215. doi: 10.1016/s0092-8674(01)00206-9. [DOI] [PubMed] [Google Scholar]
- 41.Haliloglu G, Talim B, Sel CG, Topaloglu H. Clinical characteristics of megaconial congenital muscular dystrophy due to choline kinase beta gene defects in a series of 15 patients. J. Inherit. Metab. Dis. 2015;38(6):1099–1108. doi: 10.1007/s10545-015-9856-2. [DOI] [PubMed] [Google Scholar]
- 42.Hock JM, Krishnan V, Onyia JE, Bidwell JP, Milas J, Stanislaus D. Osteoblast apoptosis and bone turnover. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2001;16(6):975–984. doi: 10.1359/jbmr.2001.16.6.975. [DOI] [PubMed] [Google Scholar]
- 43.Moriishi T, Maruyama Z, Fukuyama R, Ito M, Miyazaki T, Kitaura H, Ohnishi H, Furuichi T, Kawai Y, Masuyama R, Komori H, Takada K, Kawaguchi H, Komori T. Overexpression of Bcl2 in osteoblasts inhibits osteoblast differentiation and induces osteocyte apoptosis. PloS One. 2011;6(11):e27487. doi: 10.1371/journal.pone.0027487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weinstein RS. Glucocorticoid-induced osteonecrosis. Endocrine. 2012;41(2):183–190. doi: 10.1007/s12020-011-9580-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jimi E, Aoki K, Saito H, D’Acquisto F, May MJ, Nakamura I, Sudo T, Kojima T, Okamoto F, Fukushima H, Okabe K, Ohya K, Ghosh S. Selective inhibition of NF-kappa B blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat. Med. 2004;10(6):617–624. doi: 10.1038/nm1054. [DOI] [PubMed] [Google Scholar]
- 46.Adhikary S, Choudhary D, Ahmad N, Karvande A, Kumar A, Banala VT, Mishra PR, Trivedi R. Dietary flavonoid kaempferol inhibits glucocorticoid-induced bone loss by promoting osteoblast survival. Nutr. (Burbank, Los Angeles Cty., Calif.) 2018;53:64–76. doi: 10.1016/j.nut.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 47.Khalil R. Ubiquitin-proteasome pathway and muscle atrophy. Adv. Exp. Med. Biol. 2018;1088:235–248. doi: 10.1007/978-981-13-1435-3_10. [DOI] [PubMed] [Google Scholar]
- 48.S.M. Ebert, A. Al-Zougbi, S.C. Bodine, C.M. Adams, Skeletal muscle atrophy: discovery of mechanisms and potential therapies. Physiology (Bethesda. Md) 34(4), 232–239 (2019). [DOI] [PMC free article] [PubMed]
- 49.Dardevet D, Rémond D, Peyron MA, Papet I, Savary-Auzeloux I, Mosoni L. Muscle wasting and resistance of muscle anabolism: the “anabolic threshold concept” for adapted nutritional strategies during sarcopenia. TheScientificWorldJournal. 2012;2012:269531. doi: 10.1100/2012/269531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adam-Perrot A, Clifton P, Brouns F. Low-carbohydrate diets: nutritional and physiological aspects. Obes. Rev. 2006;7(1):49–58. doi: 10.1111/j.1467-789X.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- 51.Bradley U, Spence M, Courtney CH, McKinley MC, Ennis CN, McCance DR, McEneny J, Bell PM, Young IS, Hunter SJ. Low-fat versus low-carbohydrate weight reduction diets: effects on weight loss, insulin resistance, and cardiovascular risk: a randomized control trial. Diabetes. 2009;58(12):2741–2748. doi: 10.2337/db09-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dresios J, Panopoulos P, Frantziou CP, Synetos D. Yeast ribosomal protein deletion mutants possess altered peptidyltransferase activity and different sensitivity to cycloheximide. Biochemistry. 2001;40(27):8101–8108. doi: 10.1021/bi0025722. [DOI] [PubMed] [Google Scholar]
- 53.Liu JM, Ellis SR. Ribosomes and marrow failure: coincidental association or molecular paradigm? Blood. 2006;107(12):4583–4588. doi: 10.1182/blood-2005-12-4831. [DOI] [PubMed] [Google Scholar]
- 54.Oristian DS, Sloofman LG, Zhou X, Wang L, Farach-Carson MC, Kirn-Safran CB. Ribosomal protein L29/HIP deficiency delays osteogenesis and increases fragility of adult bone in mice. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2009;27(1):28–35. doi: 10.1002/jor.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones DC, Wein MN, Oukka M, Hofstaetter JG, Glimcher MJ, Glimcher LH. Regulation of adult bone mass by the zinc finger adapter protein Schnurri-3. Science. 2006;312(5777):1223–1227. doi: 10.1126/science.1126313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The GWAS summary dataset was extracted from a previously published study (Bivariate genome-wide association analysis implicates pleiotropic effects at the SREBF1/TOM1L2 locus on bone mineral density and lean mass in children).