Abstract
Objectives:
To assess the utility of neutrophil/lymphocyte ratio (NLR) and platelet/lymphocyte ratio (PLR) in predicting radiographic sacroiliitis and active disease in axial spondyloarthritis (axSpA) and to explore the association of tumor necrosis factor inhibitors (TNFi) use with these laboratory values, compared to traditional inflammatory markers.
Methods:
Observational data from the Program to Understand the Longterm outcomes in SpondyloARthritis registry were analyzed. Receiver Operating Characteristics curves were generated to calculate laboratory cut-off values; these values were used in multivariable logistic regression models to identify radiographic sacroiliitis and active disease. Logistic regression was also used to determine likelihood of elevated laboratory values after initiation of TNFi.
Results:
The majority of study participants (n=354) were Caucasian, male, and HLAB27 positive. NLR (OR=1.459, p=0.034), PLR (OR=4.842, p<0.001), erythrocyte sedimentation rate (ESR; OR=4.397, p<0.001), and C-reactive protein (CRP; OR=2.911, p=0.001) were independent predictors of radiographic sacroiliitis. Models including PLR with traditional biomarkers performed better than those with traditional biomarkers alone. NLR (OR=6.931, p=0.002) and CRP (OR=2.678, p=0.004) predicted of disease activity but the model including both NLR and CRP performed better than CRP alone. TNFi use reduced the odds of elevated NLR (OR=0.172, p<0.001), PLR (OR=0.073, p<0.001), ESR (OR=0.319, p<0.001), and CRP (OR=0.407, p<0.001), but models including NLR or PLR and traditional biomarkers performed best.
Conclusions:
These findings demonstrate an association of NLR and PLR with sacroiliitis and disease activity; NLR and PLR are responsive to TNFi treatment. The NLR and PLR add useful clinical information to established biomarkers and may assist in disease management.
Keywords: Axial Spondyloarthritis, Biomarker, Sacroiliitis
Introduction:
Axial spondyloarthritis (axSpA) is a chronic, inflammatory joint disorder involving the axial skeleton, classically the sacroiliac (SI) joints and spine. In its more advanced form, ankylosing spondylitis (AS), erosions of the SI joints and spine may lead to irreversible fusion. AxSpA, especially if untreated, diminishes quality of life in the form of functional impairment, depression (1), and economic hardships with elevated healthcare costs, work limitations, and lost productivity (2).
Though no cure exists for axSpA, treatments now include non-steroidal anti-inflammatories and an expanding armamentarium of nonbiologic and biologic disease modifying anti-rheumatic drugs. Based on limited observation and interventional data, the use of tumor necrosis factor inhibitors (TNFi) can improve symptoms and may retard disease progression (3,4). There is also evidence that treatment in early disease stages is of greatest benefit (3) and improve long-term outcomes (5).
Unfortunately, clinicians face substantial challenges in determining how to appropriately employ current treatments due to the inability to precisely classify patients’ disease activity. Current practice guidelines include the use of patient-reported outcomes (PROs) and a conditional recommendation (based on a very low level of evidence) to utilize a limited set of laboratory biomarkers: the C-reactive Protein (CRP) and the erythrocyte sedimentation rate (ESR)(6). While these are the most commonly used biomarkers, they have poor sensitivity and specificity and poor correlations with self-reported or physician assessed disease activity (7,8).
The most common employed PRO is the Bath Ankylosing Spondylitis Activity Index (BASDAI). Limitations of the BASDAI include patient subjectivity, failure to capture the influence of extra-articular manifestations, and failure to identify those patients likely to respond to TNFi (9). Despite its limitations, the BASDAI is used in clinical practice and is the gold standard for measuring disease activity in clinical trials due to its ease of administration.
Imaging based approaches suffer from similar limitations. Plain film radiographs, for example, only demonstrate structural features, are difficult to interpret, are inconsistent between readers, and most importantly, can require up to 10 years after the appearance of signs and symptoms before revealing abnormalities (10,11). In fact, current guidelines advise against the use of routine radiographs for monitoring disease progression (6). Magnetic Resonance Imaging (MRI) can detect subtle inflammatory changes of axSpA, though cost, accessibility, and expertise in interpretation limit its utility.
Thus, current laboratory and imaging biomarkers do not comprehensively represent the disease process in axSpA. Fortunately, prior epidemiological studies have identified that the neutrophil lymphocyte ratio (NLR) and platelet lymphocyte ratio (PLR) are useful tools in the diagnosis, prognosis, and assessment of disease activity in several rheumatologic illnesses including Sjogren’s syndrome, systemic lupus erythematosus, and rheumatoid arthritis (12–15).
These lab parameters are inexpensive and easily available from assays of complete blood counts (CBC) with differentials, which are routinely performed to monitor drug toxicity in axSpA patients. Some preliminary studies have also suggested that the NLR and PLR may have some utility as a biomarker of axSpA in smaller Eurasian populations (16–19). Unfortunately, these findings have not been adequately validated in a U.S. population nor have prior studies explored the utility of these newer biomarkers in predicting sacroiliitis on radiographs. There are also no data linking NLR or PLR with PROs nor any investigation demonstrating that they vary in response to initiation of pharmacologic therapies. Finally, prior studies have also not assessed the utility of these newer biomarkers compared to existing biomarkers, such as ESR and CRP.
This study evaluated the usefulness of the NLR and PLR as biomarkers in subjects with axSpA using a large prospective registry of U.S. veterans. The aims of the study were to determine: whether NLR and PLR are associated with existing disease activity biomarkers; whether NLR and PLR can predict active disease status (as defined by BASDAI); whether NLR and PLR drawn during the 10 year window prior to an x-ray can predict the presence of radiographic sacroiliitis; and whether TNFi use is associated with an elevated NLR and PLR. We hypothesized that NLR and PLR were associated with existing biomarkers, sacroiliitis, and disease activity. We further hypothesized that the association with disease activity and sacroiliitis would exist even after taking into account CRP concentration and ESR level. Lastly, we anticipated that TNFi use would be associated with lower odds for an elevated NLR and PLR.
Methods:
2.1. Study setting
This secondary analysis relied on data collected by the US Department of Veterans Affairs (VA) Program to Understand the Longterm outcomes in SpondyloARthritis (PULSAR) registry, included data from eleven participating VA Medical Centers. PULSAR is an ongoing, prospective, longitudinal clinical registry and biologic repository initiated in 2007 for the study of spondyloarthritis and related conditions.
2.2. Data Sources and Variables
The PULSAR registry records clinical data collected during routine care by the treating rheumatologist at enrollment and longitudinally. At enrollment, patients provide investigators with the following data: self-reported gender and race, date of birth, years of education, alcohol use, and smoking status. Date of diagnosis and the clinical characteristics of the respective spondyloarthritis are also recorded. Patients complete the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) as a disease activity measurement at enrollment and at follow up visits.
Laboratory data obtained during the course of clinical management were extracted via the VA Corporate Data Warehouse (CDW) using the LabChem Domain and accessed through the secure VA Informatics and Computing Infrastructure (VINCI) programming environment. The LabChemSID variable was used to identify specific laboratory values corresponding to date and absolute neutrophil count, lymphocyte count, platelet count, ESR and CRP. NLR and PLR were calculated based on these laboratory values; NLR is the proportion of the absolute neutrophil count to the lymphocyte count and PLR is the proportion of the platelet count to the lymphocyte count. The observation date was defined as the date of the laboratory blood draw. Other data extracted from the CDW included detailed pharmacy information, including prescription start and end dates. TNFi medication courses were determined according to the approach previously described (20).
Radiographic images were obtained from the Computerized Patient Record System (CPRS) and Compensation and Pension Record Interchange (CAPRI). Plain film sacroiliac joints were scored by trained readers (RS, EK, EM, EA, KM) in accordance with the modified New York (mNY) Criteria (21). Patients were designated as having radiographic sacroiliitis if they met the mNY criteria – a score of 2 or more bilaterally or a score of 3 or more unilaterally. Inter-reader and intra-reader reliability was calculated using the kappa statistic.
Control variables used in the multiple variable models were as follows: gender, race, age, disease duration, HLAB27 status, comorbidities, tobacco use, alcohol use, education level, marital status, working status and physician global assessment.
2.3. Study population
Study participants included subjects enrolled in the PULSAR registry. Subjects were at least 18 years of age and receiving care at a Veterans Affairs Medical Center. The recruitment of patients with HLAB27 associated conditions occurred in an otherwise unselected fashion from the population of subjects attending the rheumatology clinic at each location. This study was restricted to patients with diagnosis of AxSpA; patients met either the mNY Criteria (1984) or the Assessment of Spondyloarthritis international Society (ASAS) classification criteria (2009) for AS and AxSpA, respectively (22,23), or carried a diagnosis of AS or AxSpA by a medical provider.
Laboratory values drawn within a 10-year interval preceding the acquisition date of x-ray were included in analysis to predict the presence of radiographic sacroiliitis; for this analysis, laboratory values outside of this window were excluded as were patients without available x-rays. For analysis to predict disease activity, only those observations with BASDAI scores and laboratory values acquired on the same day were included; observations with BASDAI and laboratory values acquired on different days were excluded. Lastly, to determine likelihood of elevated laboratory values after TNFi initiation, analysis included only the initial course of TNFi (i.e., TNFi-experienced/exposed patients were excluded).
2.4. Statistical analysis
Demographic characteristics were described using continuous and categorical variables. Continuous variables that conformed to a normal distribution were expressed as the mean ± standard deviation (SD) or median and interquartile range (IQR) if the data were parametric or non-parametric, respectively. Univariate linear regression was used to describe the relationship between NLR, PLR and existing biomarkers (ESR, CRP).
Logistic Receiver Operating Characteristics (ROC) curves were generated to describe the relationship between NLR, PLR, ESR, CRP and two categorical variables: sacroiliitis (as defined by mNY criteria) and active disease (as defined by BASDAI ≥ 4). Area under the curve (AUC) and Youden’s index was calculated to determine cut-off values for NLR, PLR, ESR, and CRP. These cut-off values were then employed to calculate sensitivity, and specificity for each laboratory value and to define elevated laboratory values in subsequent analyses. Control variables were entered into a multiple variable logistic regression model to determine the likelihood of the outcome variables (active disease and sacroiliitis). The model was simplified using a backward stepwise procedure until the model contained only those variables with p < 0.05. As the variables of interest, the laboratory values (NLR, PLR, ESR, CRP) were forced into the simplified multiple variable model. In addition, NLR and PLR were assessed while controlling for CRP or ESR in order to determine whether NLR and PLR associate with active disease or sacroiliitis after accounting for CRP and ESR. Model selection utilized both the Akaike Information Criterion (AIC) and Bayesian Information Criteria (BIC).
Using an approach similar to that described above, multiple variable logistic regression models were constructed to determine the likelihood of elevated laboratory values associated with TNFi use. For these analyses, we tested for an association between current TNFi use and elevated NLR, PLR, ESR, or CRP. Cut-off values for these laboratory values were based on the ROC curves for active disease described above.
Because it was unclear where concomitant peripheral arthritis may have influenced the performance of NLR and PLR, we performed a post-hoc sensitivity analysis excluding those subjects with peripheral arthritis.
For each analysis, the threshold for significance was established at a p <0.05 and confidence intervals set at 95%. All statistical analyses were performed using StataMP v17 (StataCorp, College Station, TX, USA).
2.5. Ethics
Each participating site received Institutional Review Board approval prior to initiation of the study. Patients consented and completed agreements to disclose health information at the time of enrollment. An independent Scientific Ethics Advisory Committee approved this sub-study. No vulnerable populations are enrolled in PULSAR and this study is in accordance with the Declaration of Helsinki (24).
Results:
3.1. Cohort Characteristics
The PULSAR database consists of over 1,350 veterans enrolled at eleven sites of which 354 veterans had a diagnosis of axSpA or AS and were included in our study (Table 1). Most subjects identified as male (92.4%) and Caucasian (74.9%), reflecting the underlying demographics of the VA. HLAB27 positivity was seen in 71.8%. There were 2,733 patient observations and the mean BASDAI across all appointments was 5.35. Most patients had history of TNF inhibitor use (68.9%).
Table 1.
Demographic and Clinical Characteristics of Study Group
| Variables | n/mean | % or Standard Deviation (SD) | |
|---|---|---|---|
|
| |||
| Number of patients with axSpA/AS | 354 | - | |
|
| |||
| Number of observations | 2733 | - | |
|
| |||
| Mean age at enrollment | 55.11 | 12.726 (SD) | |
|
| |||
| Mean disease duration at enrollment | 17.36 | 14.905 (SD) | |
|
| |||
| Self-identified gender | Male Female Unknown/did not disclose |
327 21 6 |
92.4% 5.9% 1.7% |
|
| |||
| Race | Caucasian African American Hispanic American Indian/Pacific Islander Asian Other Unknown/did not disclose |
257 45 30 5 5 1 11 |
74.9% 13.1% 8.8% 1.5% 1.5% 0.3% 3.1% |
|
| |||
| HLAB27 status | Positive Negative Unknown |
254 43 57 |
71.8% 12.1% 16.1% |
|
| |||
| Cigarette smoking status | Current Former Never Unknown |
91 135 96 32 |
27.1% 38.1% 25.7% 9.0% |
|
| |||
| Alcohol use | Current Former Never Unknown |
135 54 40 125 |
38.1% 15.3% 11.3% 35.3% |
|
| |||
| Mean BASDAI | 5.35 (mean) | 1.879 (SD) | |
|
| |||
| TNFi use | All Adalimumab Etanercept Certolizumab Golimumab Infliximab |
244 145 99 15 30 55 |
68.9% 41.0% 28.0% 4.2% 8.5% 15.5% |
|
| |||
| Number of observations | Absolute neutrophils Platelets Lymphocytes ESR CRP NLR PLR |
10,191 8,363 10,296 5,558 4,990 10,189 6,997 |
- - - - - - - |
|
| |||
| Mean laboratory values prior to TNFi use | NLR PLR ESR CRP |
2.93 134.33 22.74 1.74 |
3.029(SD) 97.18(SD) 22.36(SD) 2.81(SD) |
axSpA, axial spondyloarthritis; AS, ankylosing spondylitis, BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; TNFi, tumor necrosis factor inhibitor; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; NLR, neutrophil/lymphocyte ratio; PLR, platelet/lymphocyte ratio
3.2. Correlation between NLR, PLR and ESR, CRP
Linear regression showed a strong association of NLR with ESR (r = 7.203, p < 0.001) and CRP (r = 0.751, p < 0.001). PLR also strongly associated with ESR (r = 0.022, p < 0.001) and CRP (r = 0.211, p < 0.001) (Supplementary table 1).
3.3. Value of NLR, PLR, ESR, and CRP in predicting the presence of sacroiliitis, as defined by mNY criteria
Our inter-reader correlation for scoring radiographs was moderate (К = 0.421) and intra-reader correlation was also moderate (K = 0.571). ROC curves were plotted to illustrate the ability of NLR, PLR, ESR, and CRP to detect sacroiliitis on radiographs (Figure 1). Using the optimal cut-offs, the sensitivity of each laboratory measure ranged from 84% (CRP) to 91% (ESR), while the specificity ranged from 75% (NLR) to 89% (PLR). The Area Under the Curve (AUC) was 0.851 for NLR, and 0.940 for PLR, compared with 0.934 for ESR and 0.874 for CRP.
Figure 1.
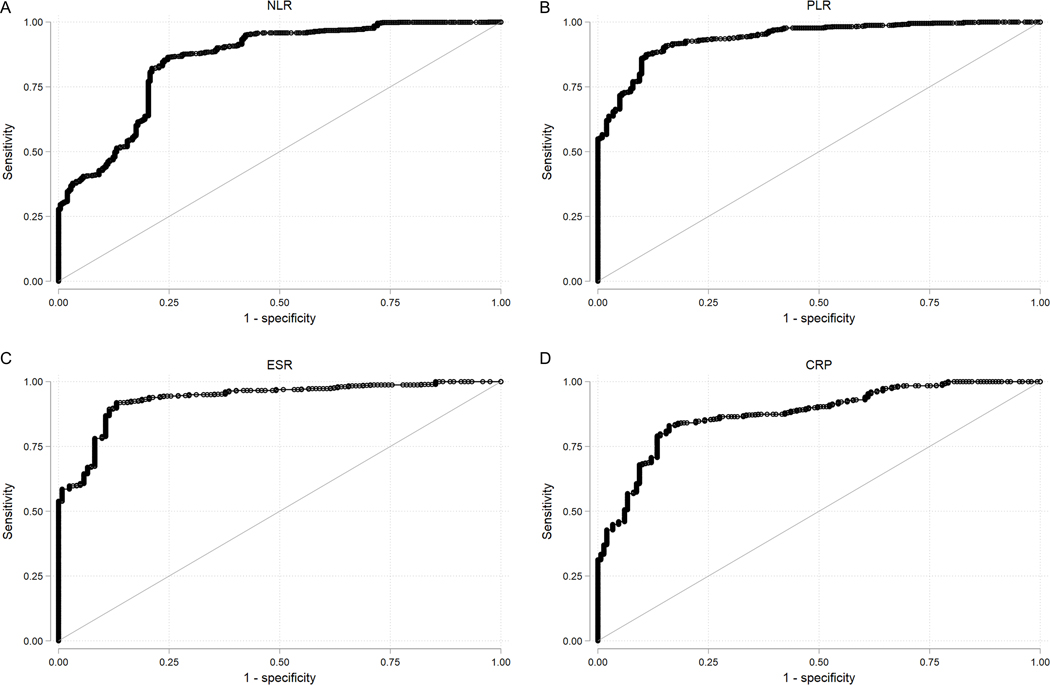
ROC Curve with the outcome of sacroiliitis, defined according to the mNY criteria
NLR cut-off value = 2.280, sensitivity = 0.864, specificity = 0.753, AUC = 0.851. PLR cut-off value = 115.833, sensitivity 0.874, specificity 0.891, AUC = 0.940. ESR cut-off value = 15.000, sensitivity = 0.907, specificity = 0.869, AUC = 0.934. CRP cut-off value 1.490, sensitivity 0.840, specificity 0.819, AUC = 0.874.
ROC, Receiver Operating Characteristic; mNY, modified New York; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
Univariate logistic regression showed statistically significant but weak relationship between PLR (OR = 1.016, p < 0.001), ESR (OR = 1.059, p < 0.001), CRP (OR = 1.613, p = 0.001) and presence of sacroiliitis on x-ray imaging; NLR showed only a trend towards association with sacroiliitis (OR 1.112, p = 0.056) (Supplementary table 2).
Multivariate logistic regression analyses controlling for gender, age, duration of disease, tobacco use, education, marital status, and working status demonstrated somewhat stronger relationships (Table 2). An NLR cut-off of 2.280 (OR = 1.459, p = 0.034), PLR cut-off of 115.833 (OR = 4.842, p < 0.001), ESR cut-off of 15.0 mm/HR (OR = 4.397, p < 0.001), and CRP cut-off of 1.49 mg/dL (OR = 2.911, p = 0.001) were independent predictors of sacroiliitis.
Table 2.
Multivariate logistic regression assessing for association of various laboratory values and sacroiliitis, defined according to the mNY criteria
| Laboratory variables | Number of observations | Odds ratio (95% CI) | p-value | AIC | BIC |
|---|---|---|---|---|---|
| NLR | 1451 | 1.459 (1.028–2.069) | 0.034 | 955.419 | 1002.940 |
| PLR | 990 | 4.842 (2.029–11.549) | <0.001 | 526.977 | 571.057 |
| ESR | 669 | 4.397 (2.128–9.085) | <0.001 | 365.383 | 405.935 |
| CRP | 904 | 2.911 (1.518–5.584) | 0.001 | 566.035 | 609.296 |
| PLR (+ ESR) | 560 | 8.200 (1.352–49.731) | 0.022 | 272.468 | 315.748 |
| PLR (+ CRP) | 549 | 18.361 (3.230–104.379) | 0.001 | 286.151 | 329.323 |
All models controlled for gender, age, duration of disease, tobacco use, education, marital status, working status. The final models (+ ESR and + CRP) tested for associations of PLR and sacroiliitis after inclusion either ESR or CRP in the model.
NLR was not significant (P>0.05) for any model that included ESR and CRP (data not shown).
mNY, modified New York; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; CI, confidence interval; AIC, Akaike Information Criteria; BIC, Bayesian Information Criteria; mm/Hr, millimeters per hour; mg/dL, milligrams per deciliter
PLR remained a significant predictor of sacroiliitis when controlling for CRP (OR = 18.361, p = 0.001), or ESR (OR = 8.200, p = 0.022) in addition to the previously mentioned control variables. The models including PLR and traditional biomarkers of disease activity (ESR and CRP) performed better than those with traditional biomarkers alone, according to the AIC and BIC.
3.4. Value of NLR, PLR, ESR, and CRP in predicting Patient Reported Outcome (BASDAI)
Additional ROC curves were plotted to illustrate the ability of NLR, PLR, ESR, and CRP to predict active disease (BASDAI ≥ 4) (Figure 2) on the same day of the laboratory draw. Using the optimal cut-offs, the sensitivity of each laboratory measure ranged from 36% (PLR) to 76% (ESR), while the specificity ranged from 52% (ESR) to 87% (PLR). The AUC was 0.678 for NLR and 0.648 for PLR, compared with 0.673 for ESR and 0.681 for CRP.
Figure 2.
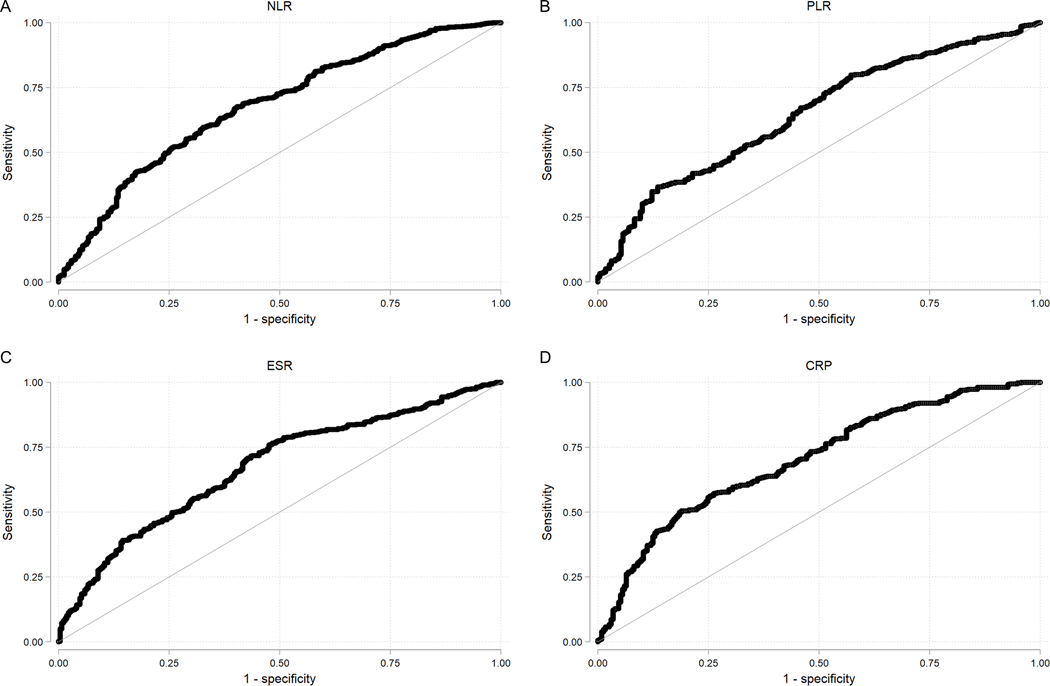
ROC Curves with the outcome of active disease, defined by BASDAI ≥ 4
NLR cut-off value = 3.669, sensitivity = 0.672, specificity = 0.599, AUC = 0.678. PLR cut-off value = 242.466, sensitivity 0.363, specificity 0.865, AUC = 0.648. ESR cut-off value = 22.000, sensitivity = 0.756, specificity = 0.524, AUC = 0.673. CRP cut-off value 1.980, sensitivity 0.523, specificity 0.780, AUC = 0.701.
ROC, Receiver Operating Characteristic; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
Univariate logistic regression showed statistically significant but weak relationships between NLR (OR = 1.197, p = 0.037), ESR (OR = 1.013, p = 0.021), CRP (1.267, p = 0.004) and active disease; no statistically significant OR was found between PLR and active disease (OR = 1.000, p = 0.945) (Supplementary table 3).
Multivariate logistic regression analysis controlling for age, duration of disease, duration of TNF inhibitor use, smoking pack year history, and working status again demonstrated stronger relationships (Table 3). An NLR cut-off of 3.669 (OR = 6.931, p = 0.002) and CRP cut-off of 1.980 (OR = 2.678, p = 0.004) were independent predictors of active disease; ESR was not an independent predictor of active disease in the multivariate model. (OR = 1.333, p = 0.148). NLR remained a significant predictor of active disease (OR 12.182, p = 0.015) when controlling for CRP in addition to the previously mentioned control variables. The model including NLR and CRP performed better than the model with CRP alone, according to the AIC and BIC.
Table 3.
Multivariate logistic regression assessing for association of various laboratory values and active disease, defined by BASDAI ≥ 4
| Laboratory variables (cut-off values) | Number of observations | Odds ratio (95% CI) | p-value | AIC | BIC |
|---|---|---|---|---|---|
| NLR (3.669) | 1026 | 6.931 (2.094–22.946) | 0.002 | 1180.231 | 1219.699 |
| PLR (242.466) | Did not perform multivariate logistic regression, univariate regression showed no statistical significance | ||||
| ESR (22.000 mm/Hr) | 858 | 1.333 (0.903–1.966) | 0.148 | not performed | not performed |
| CRP (1.980 mg/dL) | 758 | 2.745 (1.479–5.096) | 0.001 | 869.018 | 906.063 |
| NLR (+ CRP) | 635 | 12.182 (1.611–92.109) | 0.015 | 703.121 | 743.204 |
Controlled for age, duration of disease, duration on TNFi, pack years smoking, working status.
The final model (+ CRP) tested for associations of NLR and BASDAI > 4 after included CRP in the model.
BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; CI, confidence interval; AIC, Akaike Information Criteria; BIC, Bayesian Information Criteria; mm/Hr, millimeters per hour; mg/dL, milligrams per deciliter
3.5. Likelihood of change in NLR, PLR, ESR, and CRP and after initial TNFi use
Using the optimal cut-offs determined in the previous section (to predict active disease), multivariate logistic regression controlling for race, age, disease duration, Charlson Comorbidity index (which controls for the presence of 20 common health conditions), alcohol, and marital status was performed to determine the likelihood of change above this cut-off value after initial TNFi use (Table 4). TNFi use reduced the odds of an NLR above the cut-off by 83% (OR = 0.172, p < 0.001), PLR above the cut-off by 93% (OR = 0.073, p < 0.001), ESR above the cut-off by 70% (OR = 0.319, p < 0.001), and CRP above the cut-off by 60% (0.407, p < 0.001). The models including NLR or PLR combined with traditional biomarkers of disease activity (ESR and CRP) performed better than those with traditional biomarkers alone, according to the AIC and BIC.
Table 4.
Logistic regression assessing for association of TNFi use and elevated laboratory values
| Laboratory variables (cut-off values) | Number of observations | Odds ratio (95% CI) | p-value | AIC | BIC |
|---|---|---|---|---|---|
| NLR (3.669) | 779 | 0.172 (0.100–0.295) | <0.001 | 465.386 | 502.650 |
| PLR (242.466) | 727 | 0.073 (0.024–0.219) | <0.001 | 168.163 | 204.874 |
| ESR (22.000 mm/Hr) | 724 | 0.319 (0.221–0.463) | <0.001 | 803.004 | 839.682 |
| CRP (1.980 mg/dL) | 688 | 0.407 (0.268–0.618) | <0.001 | 636.908 | 673.178 |
| NLR (+ ESR) | 672 | 0.159 (0.085–0.299) | <0.001 | 373.007 | 413.599 |
| NLR (+ CRP) | 602 | 0.197 (0.107–0.359) | <0.001 | 373.807 | 413.409 |
| PLR (+ ESR) | 263 | 0.059 (0.015–0.237) | <0.001 | 128.216 | 156.793 |
| PLR (+ CRP) | 146 | 0.084 (0.024–0.296) | <0.001 | 120.333 | 144.202 |
Controlled for race, age, disease duration, Charlson Comorbidity index, alcohol use, marital status.
The final models (+ ESR and + CRP) tested for associations of PLR and sacroiliitis after included either ESR or CRP in the model.
TNFi, tumor necrosis factor inhibitor; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; CI, confidence interval; AIC, Akaike Information Criteria; BIC, Bayesian Information Criteria; mm/Hr, millimeters per hour; mg/dL, milligrams per deciliter
3.6. Sensitivity analysis
A series of sensitivity analyses were performed excluding subjects with peripheral arthritis. These had no appreciable effect on the results of the associations of patient reported outcomes and TNFi use [Supplement table 6 and 7]. For the study assessing for an association with sacroiliitis, there was a modest reduction in the odds ratio (18.361 vs 14.639) and the p-value showed only a trend towards significance [Supplement table 5].
Discussion:
Despite significant advances in the treatment of axSpA, clinicians continue to face challenges in making a timely diagnosis, predicting the risk of sacroiliitis, classifying disease activity, and assessing response to therapies; these challenges are compounded in the setting of a normal CRP, that can occur in up to 40–50% of patients (8). There is need for inexpensive, readily available, simple-to-implement biomarkers that can advance and optimize diagnosis and treatment in axSpA.
Our study addresses an area of immense importance and practical utility by building on previous reports evaluating the NLR and PLR as biomarkers of inflammation by applying them to a registry of US Veterans with axSpA. Our results showed the value of these biomarkers in predicting the odds of sacroiliitis and predicting the odds of active disease as defined by the PRO BASDAI. We also demonstrate the likelihood of elevated biomarkers after starting initial TNFi. Most importantly, our results suggest that the addition of the NLR or PLR to models of existing biomarkers may provide better models rather than duplicative results. Further investigations are necessary to corroborate our findings before they should be routinely employed in clinical care.
We demonstrated that NLR, PLR, ESR, and CRP values above a cut-off value and drawn within a ten-year window prior to obtaining an x-ray were independent predictors of an x-ray finding of sacroiliitis as defined by the mNY criteria. Elevated CRP levels have been long thought to correlate with radiographic progression of sacroiliitis (25,26) though much less is known about NLR, PLR and ESR. Our findings were similar to results reported by Wang et al., in which patients with radiographic axSpA had higher NLR than those with non-radiographic axSpA (16). In our study, the model containing CRP alone outperformed the model with NLR alone; however, both of these models were inferior to the models for PLR and ESR, which each increased the odds of finding sacroiliitis on x-ray by more than four-fold. The best model included PLR and controlled for ESR. If our findings are confirmed, these laboratory markers may eventually be useful in large studies as a proxy for the risk of developing sacroiliitis when x-rays are unavailable.
Furthermore, we showed that NLR and CRP values above a cut-off value increased the odds of having active disease by nearly seven- and three-fold, respectively. This result was consistent with findings from a study of Iraqi patients (18) but were in contrast with two studies from Turkey in which NLR reported no correlation with BASDAI (19,27). Importantly, all three prior studies were substantially less powered and had racially different study populations compared with our cohort. We also found that the addition of the NLR cut-off value to a model controlled for CRP increased the OR while resulting in a lower AIC and BIC, suggesting a better model to predict active disease. Interestingly, neither PLR nor ESR had statistical significance in our multivariate model and were not predictors of active disease.
Lastly, we showed that initiation of TNFi (in those patients never on TNFi) decreased the odds of NLR, PLR, ESR, and CRP above cut-off values by several fold. We confirmed this finding with linear regression showing inverse relationship between laboratory values and initial TNFi use. Other studies have shown lower mean values in those on TNFi compared with those patients not on TNF (19) and ours is the first to show this relationship in a multivariate regression. It is unclear from our results whether the relationship of TNFi initiation and changes in NLR and PLR represent direct effects of these medication or result from changes in disease activity. However, our findings do demonstrate that the PLR containing model outperformed models with NLR, ESR, or CRP. Models with the addition of NLR or PLR and controlled for ESR or CRP were the best fit and consistent with our hypothesis. The best models contained PLR. This suggests a potentially important role of PLR (and NLR) in assessing TNFi response in patients with axSpA, a finding that has been shared in several prior studies including a small study of 35 axSpA patients by Qian et al (28) where elevated platelet counts were shown to predict poor response to TNFis. NLR and PLR reduction was also associated with decreased disease activity in psoriasis and associated with biologic drug treatment (29).
The association of NLR and PLR with all outcomes were largely insensitive to presence of peripheral arthritis. It is unclear whether this reflects the fact that the presence peripheral arthritis may not impact the NLR or PLR in this patient population (i.e. observed association were due only to axial disease) or whether there was insufficient peripheral arthritis in our patient population to identify associations.
There were limitations to this study. Ours was a secondary analysis of prospectively collected data, which is exploratory in nature, rather than a randomized study design. The findings of this study may not be generalizable to other populations, particularly women (veterans and non-veterans alike), younger patients, racial minorities, and to those patients on non-TNFi medications. Furthermore, the CBC with differential is a product in circulation that does not necessarily reflect activity within the diseased joint or entheseal tissue. The NLR and PLR (like the ESR and CRP) are not specific to axSpA only and may be elevated in other inflammatory conditions. Furthermore, our study did not control for body mass index (BMI); an individual’s BMI may influence CRP. Though it might function as a potential confounder, it is unclear whether there is an association between BMI and the NLR or PLR. Preliminary studies suggest a positive linear correlation between absolute counts of neutrophils, platelets, and lymphocytes and CRP, but less is known of the effects of BMI on cellular ratios, such as NLR and PLR (30–32). Lastly, there are inherent limitations of administrative data, including misclassification.
Recognizing these limitations, there are substantial strengths to this study. To our knowledge, this is the largest investigation showing the value of the NLR and PLR in axSpA. It is the first within an U.S. population, and the only study that employs multiple variable analysis and predictive modeling. Data were collected prospectively and the cohort is clinically well-characterized. It also assesses NLR and PLR in multiple clinical domains. The inter- and intra-reader agreement in our study was moderate and consistent with the existing literature (33,34).
There are pathophysiologic findings that may bolster the argument that these biomarkers can serve as proxies for disease activity in axSpA. The importance of the innate immune response has been increasingly recognized in the pathogenesis of axSpA (35). The importance of neutrophils in axSpA has been demonstrated in the SKG mouse model by Rosenzweig et. al. in which neutrophils are shown to play a role in shaping Th17 mediated immunity and promotion of disease-causing T-cells in axSpA (36). A recently published study by Stavre et. al. 2022 also examining SKG mice proposes a role for neutrophils in early enthesitis (37), which is one of the cardinal manifestations of spondyloarthritis. Platelet activation has also been suggested as a sign of axSpA exacerbation (38). Furthermore, new bone formation in axSpA is believed to be controlled by Wnt/B-catenin signaling, of which Dkk-1 is an inhibitor; platelets represent a major source of Dkk-1 in humans and are proposed to be downregulated due to dysfunctional platelets in patients with axSpA (39). Additionally, lymphopenia has been accepted as a marker for systemic inflammation in many diseases.. While the relevance of the NLR and PLR to specific biologic mechanisms has yet to be fully articulated, based on emerging evidence, there remains biological plausibility for the role of NLR and PLR as biomarkers in axSpA. These biomarkers may be evaluated in future studies as potential risk factors for an axSpA diagnosis.
The NLR and PLR are globally available and inexpensive laboratory parameters that strongly correlate with inflammation. Their components (neutrophils, lymphocytes, and platelets) are stable and not typically influenced by hydration status or blood specimen handling. Thus, they have the potential to serve as useful inflammatory biomarkers in axSpA, especially in under-resourced regions and in the 40–50% of patients with normal ESR or CRP in the presence of clinical activity (8).
Further randomized studies are needed to fully understand the role of neutrophils, platelets, and lymphocytes in the pathogenesis of axSpA, including the development of sacroiliitis. It is also unclear whether non-TNFi therapies cause alterations in the NLR or PLR. Nevertheless, our study demonstrates that NLR and PLR adds important and useful clinical information to the established biomarkers of disease activity in axSpA. Given the substantial difficulty in assessing active disease and in risk-stratifying individuals with axSpA, our findings hold out the promise that an economical and important tool may be readily available for our management of the disease.
Supplementary Material
Funding Sources:
This VA PULSAR registry is supported in part by the Rocky Mountain Regional Veterans Affairs Medical Center. Dr. Sen is supported by a VA OAA GME Enhancement Award, NIH 5T32AR007534-35, and Spondyloarthritis Research and Treatment Network Pilot Project Grant. Dr. Caplan is supported by the U.S. Department of Veterans Affairs, an NIH All of Us Study sub-award (Denver Research Institute), a Spondyloarthritis Research and Treatment Network sub-award, and a Shear Family Foundation grant (CU Foundation). Dr. Napier is supported by VA Career Development Award (CDA2) IK2 BX004523, Spondyloarthritis Research and Treatment Network Junior Investigator grant, and grants from the Arthritis National Research Foundation and Spondyloarthritis Association of America. Dr. Kerr receives research funding for research outside of the submitted work or serves as a consultant for Aurinia, Novartis, Bristol Myers Squibb, Pfizer, and Horizon. Dr. Walsh receives research funding for research outside of the submitted work or serves as a consultant for AbbVie, Merck, Pfizer, UCB, Eli Lilly, Novartis, and Amgen. Dr. Raychaudhuri receives research funding for research outside of the submitted work or serves as a consultant for Johnson & Johnson, Abbvie, Sun Pharma, UCB, Novartis, and Pfizer. All other authors have no relevant conflicts of interest to disclose. The sponsors were not involved in the design or conduct of the study; collection, management, analysis and interpretation of data; preparation, review, or approval of the manuscript; nor the decision to submit the manuscript for publication. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Footnotes
Authorship:
(a) substantial contributions to the conception and design (RS, LC); or the acquisition (all authors except RN and AF), analysis (RS, LC), or interpretation of the data (all authors),
(b) the drafting of the article or critical revision for important intellectual content (all authors),
(c) final approval of the version to be published (all authors),
(d) agreement to be accountable for all aspects of the work (all authors)
Assurances: This study was approved by the Colorado Multiple Institutional Review Board under protocol 10–0212 and the PULSAR Scientific and Ethics Advisory Committee review. All analyses were performed by RS and LC. All the authors had access to the data.
Conflict of Interest: All authors acknowledge that they have no financial nor personal relationships with other people or organizations that could inappropriately influence the content of this article.
References:
- 1.Zhao S, Thong D, Miller N, Duffield SJ, Hughes DM, Chadwick L, et al. The prevalence of depression in axial spondyloarthritis and its association with disease activity: A systematic review and meta-analysis. Arthritis Res Ther. 2018. Jul 11;20(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh Jessica A., Song Xue, Kim Gilwan, Park Y. Healthcare Utilization and Direct Costs in Patients with Ankylosing Spondylitis Using a Large US Administrative Claims Database. Rheumatol Ther. 2018. Aug 18;5(2):463–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haroon N, Inman RD, Learch TJ, Weisman MH, Lee M, Rahbar MH, et al. The impact of tumor necrosis factor α inhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum. 2013. Oct;65(10):2645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wanders A, Van Der Heijde D, Landewé R, Béhier JM, Calin A, Olivieri I, et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: A randomized clinical trial. Arthritis Rheum. 2005;52(6):1756–65. [DOI] [PubMed] [Google Scholar]
- 5.Maas F, Arends S, Brouwer E, Essers I, van der Veer E, Efde M, et al. Reduction in Spinal Radiographic Progression in Ankylosing Spondylitis Patients Receiving Prolonged Treatment With Tumor Necrosis Factor Inhibitors. Arthritis Care Res. 2017. Jul 1;69(7):1011–9. [DOI] [PubMed] [Google Scholar]
- 6.Ward MM, Deodhar A, Gensler LS, Dubreuil M, Yu D, Khan MA, et al. 2019. Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis. Arthritis Rheumatol. 2019 Oct 1;71(10):1599–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spoorenberg Anneke, van der Heijde, D, Dougados, M, de Vlam K, Mielants H, van der Tempel, H, van der Linden S. Relative value of erythrocyte sedimentation rate and C-reactive protein in assessment of disease activity in ankylosing spondylitis. J Rheumatol. 1999;26(4):980–4. [PubMed] [Google Scholar]
- 8.Ruof J, Stucki G. Validity aspects of erythrocyte sedimentation rate and C-reactive protein in ankylosing spondylitis: a literature review. J Rheumatol. 1999;26(4):966–70. [PubMed] [Google Scholar]
- 9.Vastesaeger N, Cruyssen B Vander, Mulero J, Gratacós Masmitjá J, Zarco P, Almodovar R, et al. ASDAS high disease activity versus BASDAI elevation in patients with ankylosing spondylitis as selection criterion for anti-TNF therapy. Reumatol Clin. 2014;10(4):204–9. [DOI] [PubMed] [Google Scholar]
- 10.Mau W, Zeidler H, Mau R, Majewski A, Freyschmidt J, Stangel W DH. Clinical features and prognosis of patients with possible ankylosing spondylitis. Results of a 10-year followup. J Rheumatol. 1988;15(7):1109–14. [PubMed] [Google Scholar]
- 11.Feldtkeller E, Khan MA, Van Der Heijde D, Van Der Linden S, Braun J. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int. 2003;23(2):61–6. [DOI] [PubMed] [Google Scholar]
- 12.Hu Z-D, Sun Y, Guo J, Huang Y-L, Qin B-D, Gao Q, et al. Red blood cell distribution width and neutrophil/lymphocyte ratio are positively correlated with disease activity in primary Sjögren’s syndrome. Clin Biochem. 2014. Dec;47(18):287–90. [DOI] [PubMed] [Google Scholar]
- 13.Qin B, Ma N, Tang Q, Wei T, Yang M, Fu H, et al. Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) were useful markers in assessment of inflammatory response and disease activity in SLE patients. Mod Rheumatol. 2016. May 3;26(3):372–6. [DOI] [PubMed] [Google Scholar]
- 14.Li M, Xie L. Correlation between NLR, PLR, and LMR and Disease Activity, Efficacy Assessment in Rheumatoid Arthritis. Evidence-based Complement Altern Med. 2021;2021(4433141):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Chen Q, Chen DY, Xu XZ, Liu YY, Yin TT, Li D. Platelet/lymphocyte, lymphocyte/monocyte, and neutrophil/lymphocyte ratios as biomarkers in patients with rheumatoid arthritis and rheumatoid arthritis-associated interstitial lung disease. Med Sci Monit. 2019. Aug 29;25:6474–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Su J, Yuan Y, Jin X, Shen B, Lu G. The role of lymphocyte-monocyte ratio on axial spondyloarthritis diagnosis and sacroiliitis staging. BMC Musculoskelet Disord. 2021. Dec 1;22(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu S, Ma Y, Wu M, Zhang X, Yang J, Deng J, et al. Neutrophil lymphocyte ratio in patients with ankylosing spondylitis: A systematic review and meta-analysis. Mod Rheumatol. 2020. Jan 2;30(1):141–8. [DOI] [PubMed] [Google Scholar]
- 18.Al-Osami MH, Awadh NI, Khalid KB, Awadh AI. Neutrophil/lymphocyte and platelet/lymphocyte ratios as potential markers of disease activity in patients with Ankylosing spondylitis: A case-control study. Adv Rheumatol. 2020;60(1):1–9. [DOI] [PubMed] [Google Scholar]
- 19.Gökmen F, Akbal A, Reşorlu H, Gökmen E, Güven M, Aras AB, et al. Neutrophil-Lymphocyte Ratio Connected to Treatment Options and Inflammation Markers of Ankylosing Spondylitis. J Clin Lab Anal. 2015;29(4):294–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bekele DI, Cheng E, Reimold A, Geier C, Ganuthula K, Walsh JA, et al. Tumor necrosis factor inhibitor (TNFi) persistence and reasons for discontinuation in a predominantly male cohort with axial spondyloarthritis. Rheumatol Int. 2021. Nov 1; [DOI] [PubMed] [Google Scholar]
- 21.Van Der Linden Hans Valkenburg SA, Cats A. Official Journal of the American Rheumatism Association Section of the Arthritis Foundation EVALUATION OF DIAGNOSTIC CRITERIA FOR ANKYLOSING SPONDYLITIS A Proposal for Modification of the New York Criteria. [DOI] [PubMed] [Google Scholar]
- 22.Van Der Linden Hans Valkenburg SA, Cats A. EVALUATION OF DIAGNOSTIC CRITERIA FOR ANKYLOSING SPONDYLITIS. A proposal for modification of the New York criteria. Arthritis Rheum [Internet]. 1984. Apr 27 [cited 2022 Feb 7];27(4):361–8. Available from: Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria - PubMed (nih.gov) [DOI] [PubMed] [Google Scholar]
- 23.Rudwaleit M, Van Der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): Validation and final selection. Ann Rheum Dis. 2009;68(6):777–83. [DOI] [PubMed] [Google Scholar]
- 24.WMA DECLARATION OF HELSINKI – ETHICAL PRINCIPLES FOR MEDICAL RESEARCH INVOLVING HUMAN SUBJECTS [Internet]. 2013. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ [PubMed] [Google Scholar]
- 25.Reveille JD. Biomarkers for diagnosis, monitoring of progression, and treatment responses in ankylosing spondylitis and axial spondyloarthritis. Clin Rheumatol. 2015. Jun 28;34(6):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.López-Medina C, Ramiro S, Van Der Heijde D, Sieper J, Dougados M, Molto A. Characteristics and burden of disease in patients with radiographic and non-radiographic axial Spondyloarthritis: A comparison by systematic literature review and meta-analysis. RMD Open. 2019. Nov 1;5(2):e001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mercan R, Bitik B, Tufan A, Bozbulut UB, Atas N, Ozturk MA, et al. The Association Between Neutrophil/Lymphocyte Ratio and Disease Activity in Rheumatoid Arthritis and Ankylosing Spondylitis. J Clin Lab Anal. 2016. Sep 1;30(5):597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qian H, Chen R, Wang B, Yuan X, Chen S, Liu Y, et al. Associations of Platelet Count with Inflammation and Response to Anti-TNF-α Therapy in Patients with Ankylosing Spondylitis. Front Pharmacol. 2020. Nov 6;11(559593):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Najar Nobari N, Shahidi Dadras M, Nasiri S, Abdollahimajd F, Gheisari M. Neutrophil/platelet to lymphocyte ratio in monitoring of response to TNF-α inhibitors in psoriatic patients. Dermatol Ther. 2020. Jul 1;33(4):e13457. [DOI] [PubMed] [Google Scholar]
- 30.Furuncuoǧlu Y, Tulgar S, Dogan AN, Cakar S, Tulgar YK, Cakiroglu B. How obesity affects the neutrophil/lymphocyte and platelet/lymphocyte ratio, systemic immune-inflammatory index and platelet indices: A retrospective study. Eur Rev Med Pharmacol Sci. 2016;20(7):1300–6. [PubMed] [Google Scholar]
- 31.Samocha-Bonet D, Justo D, Rogowski O, Saar N, Abu-Abeid S, Shenkerman G, et al. Platelet counts and platelet activation markers in obese subjects. Mediators Inflamm. 2008;2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goudswaard LJ, Corbin LJ, Burley KL, Mumford A, Akbari P, Soranzo N, et al. Higher body mass index raises immature platelet count: evidence from Mendelian randomization analyses. medRxiv [Internet]. 2021;2021.05.19.21257443. Available from: https://www.medrxiv.org/content/10.1101/2021.05.19.21257443v1%0A https://www.medrxiv.org/content/10.1101/2021.05.19.21257443v1.abstract [Google Scholar]
- 33.Omar A, Sari I, Bedaiwi M, Salonen D, Haroon N, Inman RD. Analysis of dedicated sacroiliac views to improve reliability of conventional pelvic radiographs. Rheumatol (United Kingdom). 2017;56(10):1740–5. [DOI] [PubMed] [Google Scholar]
- 34.Braga MV, de Oliveira SC, Vasconcelos AHC, Lopes JR, de Macedo Filho CL, Ramos LMA, et al. Prevalence of sacroiliitis and acute and structural changes on MRI in patients with psoriatic arthritis. Sci Rep [Internet]. 2020;10(1):1–8. Available from: 10.1038/s41598-020-68456-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mauro D, Thomas R, Guggino G, Lories R, Brown MA, Ciccia F. Ankylosing spondylitis: an autoimmune or autoinflammatory disease? Nat Rev Rheumatol. 2021. Jul 10;17(7):387–404. [DOI] [PubMed] [Google Scholar]
- 36.Rosenzweig H, Vance E, Sen R, Caplan L, Napier R. A Role for Neutrophils in Disease Onset and Severity of Spondyloarthritis [abstract]. Arthritis Rheumatol. 2021;73(Supplement 10). [Google Scholar]
- 37.Stavre Z, Bridgewood C, Zhou Q, Maeda Y, Huang T ting, Karman J, et al. A role for neutrophils in early enthesitis in spondyloarthritis. Arthritis Res Ther [Internet]. 2022;24(1):1–11. Available from: 10.1186/s13075-021-02693-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang F, Yan CG, Xiang HY, Xing T, Wang NS. The significance of platelet activation in ankylosing spondylitis. Clin Rheumatol. 2008;27(6):767–9. [DOI] [PubMed] [Google Scholar]
- 39.Czepiel M, Stec M, Korkosz M, Guła Z, Błyszczuk P, Baran J, et al. Down-Regulation of Dkk-1 in Platelets of Patients With Axial Spondyloarthritis. Arthritis Rheumatol. 2021;73(10):1831–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


