Abstract
Objective:
To evaluate how stress related to the coronavirus disease 2019 (COVID-19) pandemic has affected women’s menstrual cycles. We hypothesized that women with high levels of COVID-related stress would have more menstrual changes compared to those with lower stress.
Methods:
Using a cross-sectional study design, we recruited a representative sample of US adult women of reproductive age (18–45 years) using non-hormonal birth control to participate in an online REDCap survey. COVID stress was assessed with the COVID-19 Perceived Stress Scale (PSS-10-C) and dichotomized as low stress (scores <25) and high stress (scores ≥ 25). Self-reported menstrual outcomes were defined as changes in cycle length, duration, flow, and increased frequency of spotting between cycles. We used chi-square (χ2) and Fisher’s exact tests to compare differences in outcome between the two stress groups and logistic regression models for effect estimates.
Results:
A total of 354 women of reproductive age across the US completed both menstrual and COVID-19 stress components of our survey. Over half of these women reported at least one change in their menstrual cycle since the start of the pandemic (n=191) and 10.5% reported high COVID-related stress (n=37). Compared to those with low COVID-19 stress, a greater proportion of women with high COVID-19 stress reported changes in cycle length (shorter or longer; p=0.008), changes in period duration (shorter or longer; p<0.0001), heavier menstrual flow (p=0.035) and increased frequency of spotting between cycles (p=0.006) compared to pre-pandemic times. After adjusting for age, smoking history, obesity, education and mental health history, high COVID-19 stress was associated with increased odds of changes in: menstrual cycle length (adj OR: 2.32; 95% CI: 1.12, 4.85), duration (adj OR: 2.38; 95% CI: 1.14, 4.98), and spotting (adj OR: 2.32; 95% CI: 1.03, 5.22). Our data also demonstrated a nonsignificant trend of heavier menstrual flow among women with high COVID-related stress (adj OR: 1.61; 95% CI: 0.77, 3.34).
Conclusions:
High COVID-19-related stress is associated with significant changes in menstrual cycle length, alterations in period duration and increased intermenstrual spotting as compared to prior to the pandemic. Given that menstrual health is frequently an indicator of women’s overall well-being, clinicians, researchers, and public health officials must consider the association between COVID-19 stress and menstrual disturbances.
Précis:
Coronavirus disease 2019 (COVID-19) stress is associated with changes in menstrual cycle parameters, and thus, clinicians should consider this association when assessing a patient’s menstrual health and providing education.
Introduction:
In March of 2020, the World Health Organization characterized the coronavirus disease (COVID-19) as a global pandemic.1 At the time of the study, May 2021, over 3.5 million deaths worldwide had been attributed to COVID-19, with over 500,000 deaths recorded in the United States (US) alone.2 The Centers for Disease Control and Prevention now cites just over 1,000,000 US deaths from COVID as of August 2022.3 The harrowing loss of life due to this global pandemic along with the ensuing public health interventions and indirect economic effects have resulted in unprecedented societal disruption, which have led to a spike in emotional distress and psychiatric symptoms.4 In the US, women have shouldered more childcare duties during the pandemic5 and find COVID-induced changes to daily activities, along with the potential risk of a COVID-19 infection significantly more stressful than men.6
High stress has been associated with aberrant menstrual changes in women.6–10 Disruptions in women’s menstrual cycles, such as amenorrhea, can not only be detrimental to reproductive goals,11they have been associated with undesired mental health,12 respiratory,13 and cardiovascular outcomes.14 Although reports suggest greater effects of COVID-related stress on women than men during the pandemic,15,16 little research has been conducted on the relationship between COVID-19 related stress and women’s menstrual cycles, an important indicator of overall wellbeing.
In this study, we evaluate how stress related to the COVID-19 pandemic has affected women’s menstrual cycle length, duration, flow, and frequency of spotting between cycles. We hypothesized that women with high levels of COVID-related stress will report changes in all four menstrual parameters (cycle length, duration, flow, and spotting) compared to pre-pandemic times.
Methods:
Because it was important to reach a geographically and racially diverse population of women across the US, we used a cross-sectional study design to recruit a sample of US adult women between the ages of 18–45 years using Dynata, a survey sampling company, which maintains a demographically diverse web panel of survey takers across the US.17–19 Dynata’s panel members are randomly routed to available surveys based on eligibility criteria of open surveys, and receive participation rewards based on Dynata’s incentive system.17–19 Our recruitment plan involved the use of “soft” quotas, aligned with the US census data to ensure geographic, racial, and ethnic diversity in our sample. In research, “soft quotas” can either mean an absolute minimum that researchers expect to be exceeded, or a quota for which near enough is good enough.20 For this study, using ‘soft’ quotas in our recruitment and sampling scheme allowed us to monitor the geographic, racial, and ethnicity distributions of the study population and modify/target the distribution of subsequent invitations to participate to grossly reflect the US census data.
The title of the survey that was distributed to Dynata’s panelists was “Women’s Covid-related stress, menstrual health and wellbeing,” accompanied by a summary detailing that the survey was intended to investigate the effect of Covid-related stress on women’s menstrual health and overall well-being. Participants completed the anonymous, web-based survey using REDCap (Research Electronic Data Capture, Vanderbilt University). Survey finishers received participation rewards per Dynata’s incentive system.17–19
The study’s inclusion criteria included: i) self-identifies as a woman ii) self-reported age between 18–45 years iii) resides in a US state/territory. Women over 45 years were excluded to avoid hormonal irregularities associated with the menopausal transition.21–23 To capture naturally cycling women, we excluded women who: were menopausal or post-menopausal prior to the pandemic, had undergone a hysterectomy, currently pregnant, were less than 3 months post-partum, currently receiving exogenous glucocorticoids, had received infertility treatments prior to the pandemic, or were currently taking hormonal birth control.
All survey questions were reviewed for relevance and context by the research team and pre-tested with a sub-sample of women within the target population for face and content validity. Informed consent was obtained from all research participants. The study was approved by the University of Rochester Institutional Review Board (STUDY00005980).
Menstrual parameters were self-reported based on the following questions:
Menstrual cycle length:
“Since the COVID-19 pandemic began in March 2020 has the length of your menstrual cycle changed? (the time from day 1 of one cycle until day 1 of the next cycle)”. Response options (randomized): No change/Shorter/Longer/I have not had my period since the pandemic began in March 2020
Cycle Duration:
“Since the COVID-19 pandemic began in March 2020, has the duration of your periods changed? (days of flow per period)” Response options (randomized): No change/Shorter/Longer/I have not had my period since the pandemic began in March 2020
Cycle Flow:
“Since the COVID-19 pandemic began in March 2020, has your menstrual flow changed? (amount of bleeding)” Response options (randomized): No change/Lighter/Heavier/I have not had my period since the pandemic began in March 2020
Spotting:
“Since the COVID-19 pandemic began in March 2020, have you begun having spotting between periods?” Response options (randomized): Yes/No/ I have not had my period since the pandemic began in March 2020
Participants who selected “no change/no” across all four parameters (length and duration and flow and spotting) were categorized as “no change” and all others were grouped as “change.” The two groups were then compared in bivariate and multivariable regression analyses.
Participants’ COVID-related stress was assessed with the COVID-19 Perceived Stress Scale (PSS-10-C).24 The PSS-10-C has 10 items, which are ranked on a 5-point Likert scale of “0-Never” to “4-Always.” Scores range from 0–40, with higher scores indicative of greater stress. We defined low COVID-19 stress as PSS-10-C scores <25 and high COVID-19 stress as scores ≥25, in accordance with the literature.24
Covariates included age, race, ethnicity, educational attainment, marital status, number of living children (under 18), and smoking status. Participants were also asked about their pre-pandemic menstrual functioning, assessed by how many periods they experienced per year prior to the pandemic, along with comorbidities diagnosed prior to and during the pandemic. Comorbidities included reproductive/gynecological (endometriosis, leiomyomas or myomas, polycystic ovarian syndrome (PCOS), and uterine polyps), thyroid disease, obesity, sexually transmitted infections (STI), and mental health history (anorexia, anxiety, depression and other mood disorders). We also asked about COVID-19 vaccination status, as preliminary reports suggest an association between psychological stress and vaccination hesitancy.25
We used descriptive statistics (proportions, means, medians, ranges and standard deviations) to describe the study sample. Pearson’s chi-square and t-tests were used to compare survey responses in bivariate analyses, to identify important covariates. We used logistic regression models to estimate crude and adjusted effect sizes. In “minimally adjusted” models, we only adjusted for predictors (variables that were associated with menstrual change only) and confounders (variables that were associated with both COVID stress and menstrual change). In “fully adjusted” models, we adjusted for all variables that were statistically significant in bivariate analyses if they had also been adjusted for in previously published menstruation studies. We used the standard p <0.05 cut-off to determine statistical significance in all our analyses. Analyses were completed using Stata (StataCorp LLC, College Station, TX).
Results:
The survey launched on May 4th 2021, and ended on May 7th 2021. A total of 1,037 survey takers (Figure 1) met the first set of inclusion criteria and consented to participate in the study and 948 (91%) were deemed “completes,” meaning that they proceeded through all questions to the final survey page. The remaining 89 did not make it to the final survey page and were considered “incompletes.” Despite being considered a “complete,” participants may have had missing data and not answered all relevant questions. Thus, we indicate the final sample sizes for each of the analyses in the Tables as appropriate. (See Appendix 1, http://links.lww.com/xxx, for comparison of differences between “completes” and “incompletes”) The mean age of the completes was 32.62 years (+/− 7.06 SD) and the mean number of children <18 for the sample was 1.1 (+/− 1.31 SD). Three hundred and seventy-four naturally-cycling women met the second set of gynecologic and reproductive inclusion criteria for the menstrual analysis group (i.e. not menopausal or post-menopausal prior to the pandemic, had not undergone a hysterectomy, not currently pregnant or less than 3 months post-partum, not currently receiving exogenous glucocorticoids, had not received infertility treatments prior to the pandemic, not currently taking hormonal birth control) and were asked the menstrual assessment questions (Figure 1). (See Appendix 1, http://links.lww.com/xxx, for comparison of the excluded non-natural cyclers (n=569) to the included natural cyclers (n=374) on key covariates).
Figure 1.
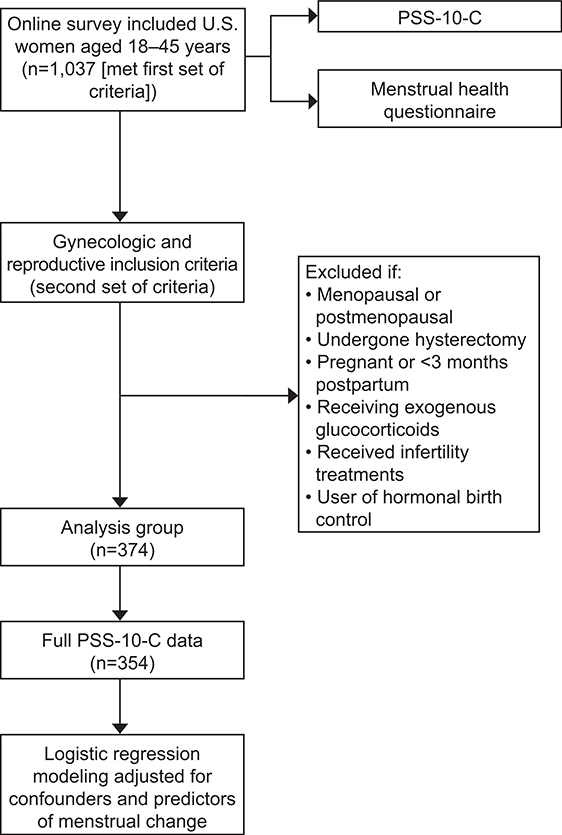
Schematic of study methods. PSS-10-C, coronavirus 2019 perceived stress scale.
Our recruitment strategy, modeled by the US census data, resulted in a diverse representation of survey participants across each US state and geographic region (Table 1). With regards to race, compared to the census data, there was an over-representation of Asian, American Indian/Alaskan Native and those who identify as an unlisted race, and an under-representation of those of Hispanic heritage. The educational attainment was high compared to the census data, with about a quarter (23.9%) of all participants having at least a Master’s degree. However, among the women who met the second set of gynecologic and reproductive inclusion criteria (Table 1), only 15.3% had a Master’s level of education which was close to the census rate of 13.0%, and there was an over-representation of women with high school equivalent education or less (44.0%) compared to census data (38.0%). Less than 5% had been divorced/separated (Table 1). However, because we only surveyed US women between the ages of 18–45, differences in the study data compared to the larger census population should be interpreted with caution as they are likely due to the age-restricted nature of the study’s inclusion criteria.
Table 1.
Demographic characteristics of participants
| Characteristic | Total Study Sample meeting 1st set of eligibility criteria n=1037 |
Participants meeting 2nd set of eligibility criteria n=374 |
Census Data |
|---|---|---|---|
| Race 47 | |||
| Missing | n=141 | n=1 | |
| Non-Missing | n=896 | n=373 | |
| Asian | 86 (9.6%) | 33 (8.9%) | 5.9% |
| American Indian/Alaska Native | 17 (1.9%) | 9 (2.4%) | 1.3% |
| Black | 76 (8.5%) | 40 (10.7%) | 13.4% |
| Native Hawaiian | 2 (0.2%) | 2 (0.5%) | 0.2% |
| White | 680 (75.9%) | 270 (72.4%) | 76.3% |
| None of the above | 35 (3.9%) | 19 (5.1%) | 2.8% |
| Ethnicity | |||
| Missing | n=154 | n=5 | |
| Non-Missing | n=883 | n=369 | |
| Hispanic | 122 (13.8%) | 51 (13.8%) | 18.5% |
| Non-Hispanic | 761 (86.2%) | 318 (86.2%) | 60.1% |
| Educational attainment 48 | |||
| Missing | n=140 | n=1 | |
| Non-Missing | n=897 | n=373 | |
| High School or less | 310 (34.6%) | 164 (44.0%) | 38.0% |
| Technical Training/Associates/Bachelor’s | 373 (41.6%) | 152 (40.7%) | 50.0% |
| Master’s Degree or Higher | 214 (23.9%) | 57 (15.3%) | 13.0% |
| Relationship status 49 | |||
| Missing | n=141 | n=0 | |
| Non-Missing | n=896 | n=374 | |
| Single/in a relationship but not married | 435 (48.6%) | 217 (58.0%) | 30.7% |
| Married | 421 (47.0%) | 140 (37.4%) | 46.3% |
| Divorced/Separated/Other | 40 (4.5%) | 17 (4.6%) | 23.0% |
| Geographic region* 47 | |||
| Missing | n=214 | n=30 | |
| Non-Missing | n=823 | n=344 | |
| Northeast | 107 (13.0%) | 49 (14.3%) | 19.2% |
| Southeast | 164 (19.9%) | 79 (23.0%) | 25.6% |
| Midwest | 122 (14.8%) | 46 (13.4%) | 20.6% |
| Southwest | 159 (19.3%) | 51 (14.8%) | 12.8% |
| West | 271 (32.9%) | 119 (34.6%) | 20.8% |
Northeast: Maine, Massachusetts, Rhode Island, Connecticut, New Hampshire, Vermont, New York, Pennsylvania, New Jersey, Delaware, Maryland, Washington DC
Southeast: West Virginia, Virginia, Kentucky, Tennessee, North Carolina, South Carolina, Georgia, Alabama, Mississippi, Arkansas, Louisiana, Florida
Midwest: Ohio, Indiana, Michigan, Illinois, Missouri, Wisconsin, Minnesota, Iowa, Kansas, Nebraska, South Dakota, North Dakota
Southwest: Texas, Oklahoma, New Mexico, Arizona
West: Colorado, Wyoming, Montana, Idaho, Washington, Oregon, Utah, Nevada, California, Alaska, Hawaii
Of the 1037 participants who met the 1st set of eligibility criteria, 838 completed the PSS-10-C scale, and were dichotomized as high (score ≥25 on PSS-10-C, n=93) or low (score <25 on PSS-10-C scale, n=745) COVID-stress. As shown in Table 2, women with high COVID-19 stress were significantly younger than those with low stress (p= 0.003), and more likely to identify as long-term tobacco users or endorse recent smoking cessations since the pandemic (p=0.034). Additionally, there was a greater prevalence of obesity (p=0.006) and mental health history (p<0.0001) among the high stress group compared to the low stress group. When considering only the women who met our gynecologic and reproductive inclusion criteria, 354 had complete COVID-stress data for bivariate analyses, with 89.5% meeting the cut-off for low COVID-stress (n=317), and 10.5% categorized as high COVID stress (n=37, Table 2). Women in the high COVID-stress group were still significantly younger than those in the low stress group (p=0.048) and had a greater prevalence of mental health history (p=0.001). Mental health was also associated with menstrual change (p<0.001, see Appendix 1, http://links.lww.com/xxx), as was education (p=0.012, see Appendix 1, http://links.lww.com/xxx). Thus age, smoking status, obesity status, mental health status, and education were included as covariates in our multivariable models, mimicking what has been done in other menstruation literature.10,26–28
Table 2.
Characteristics of Survey Participants across COVID-19 Stress Levels
| Participants meeting 1st set of eligibility criteria with COVID-stress data (n=838) | |||
|---|---|---|---|
| Characteristic | Low COVID-19 Stress (n=745) | High COVID-19 Stress (n=93) | p value |
| Age ╪ | |||
| Mean | 32.7 ± 6.84 | 30.3 ± 7.39 | 0.003 |
| Median | 33 | 30 | |
| Range | 18–45 | 18–45 | |
| Race | |||
| Black | 63 (8.5%) | 10 (10.8%) | 0.769 |
| White | 557 (75.5%) | 69 (74.2%) | 0.769 |
| None of the above | 118 (16.0%) | 14 (15.1%) | |
| Missing | 7 (0.9%) | 0 (0.0%) | |
| Ethnicity | |||
| Hispanic | 102 (14.0%) | 10 (11.0%) | 0.434 |
| Non-Hispanic | 628 (86.0%) | 81 (89.0%) | |
| Missing | 15 (2.0%) | 2 (2.2%) | |
| Number of children | |||
| Mean | 1.13 ± 1.32 | 1.1 ± 1.32 | 0.631 |
| Median | 1 | 1 | 0.631 |
| Range | 0–11 | 0–8 | |
| Current marital status | |||
| Single | 211 (28.5%) | 24 (25.8%) | 0.596 |
| In relationship, but not married | 149 (20.1%) | 24 (25.8%) | 0.596 |
| Married | 346 (46.8%) | 42 (45.2%) | |
| Divorced/Separated/Widowed/Other | 34 (4.6%) | 3 (3.2%) | |
| Missing | 5 (0.7%) | 0 (0.0%) | |
| Partner gender (only asked of those who indicated that they had a partner) | |||
| Male partner | 362 (88.7%) | 55 (91.7%) | 0.495 |
| Female partner | 46 (11.3%) | 5 (8.3%) | |
| Missing/not asked | 337 (45.2%) | 33 (35.5%) | |
| Educational attainment ¥¥ ╪ | |||
| Less than or equal to High School | 259 (35.0%) | 32 (34.4%) | 0.804 |
| Technical/ Associate’s/ Bachelor’s Degree | 311 (42.0%) | 42 (45.2%) | 0.804 |
| Master’s or other Advanced Degrees | 170 (23.0%) | 19 (20.4%) | |
| Missing | 5 (0.7%) | 0 (0.0%) | |
| Smoking Status ╪ | |||
| Long term tobacco user, even before pandemic | 235 (31.8%) | 40 (43.0%) | 0.034 |
| Recently started smoking since the pandemic | 59 (8.0%) | 5 (5.4%) | |
| Recently stopped smoking | 69 (9.3%) | 13 (14.0%) | 0.034 |
| Has never used tobacco | 377 (51.0%) | 35 (37.6%) | |
| Missing | 5 (0.7%) | 0 (0.0%) | |
| Pre-pandemic menstrual functioning (Only asked of those not using non-hormonal birth control) | |||
| Amenorrhea (0–3 periods/ year) | 27 (8.6%) | 0 (0.0%) | 0.211 |
| Oligomenorrhea (4–7 periods/ year) | 29 (9.3%) | 2 (5.4%) | 0.211 |
| Normal cycle (8–14 periods/ year) | 238 (76.0%) | 22 (89.2%) | |
| Polymenorrhea (15 or more periods/ year) | 19 (6.1%) | 2 (5.4%) | |
| Missing/not asked | 432 (58.0%) | 67 (72.0%) | |
| COVID-19 vaccination status | |||
| Vaccinated | 344 (46.6%) | 50 (53.8%) | 0.189 |
| Not Vaccinated | 395 (53.5%) | 43 (46.2%) | |
| Missing | 6 (0.8) | 0 (0.0%) | |
| Reproductive/gynecologic comorbidity | |||
| Yes | 151 (20.4%) | 19 (20.4%) | 0.986 |
| No | 591 (79.6%) | 74 (79.6%) | |
| Missing | 3 (0.4%) | 0 (0.0%) | |
| Thyroid comorbidity | |||
| Yes | 52 (7.0%) | 8 (8.6%) | 0.575 |
| No | 690 (93.0%) | 85 (91.4%) | |
| Missing | 3 (0.4%) | 0 (0.0%) | |
| Obesity Status ╪ | |||
| Obese | 91 (12.3%) | 21 (22.6%) | 0.006 |
| Not obese | 651 (87.7%) | 72 (77.4%) | |
| Missing | 3 (0.4%) | 0 (0.0%) | |
| Mental health comorbidity ¥¥ ╪ ** | |||
| Yes | 356 (47.4%) | 69 (74.2%) | <0.0001 |
| No | 386 (52.6%) | 24 (25.8%) | |
| Missing | 3 (0.4%) | 93 (0.0%) | |
| Sexually Transmitted Infection | |||
| Yes | 46 (6.2%) | 6 (6.5%) | 0.757 |
| No | 699 (93.8%) | 87 (93.5%) | |
| Missing | 0 (0.0%) | 0 (0.0%) | |
| Participants meeting 2nd set of eligibility criteria with COVID-stress data (=354) | |||
| Characteristic | Low COVID-19 Stress(n=317) | High COVID-19 Stress(n=37) | p value |
| Age ╪ | |||
| Mean | 32.5 ± 7.2 | 30.4 ± 8.3 | 0.048 |
| Median | 33 | 30 | |
| Range | 0–11 | 0–4 | |
| Race | |||
| Black | 35 (11.1%) | 4 (10.8%) | 0.981 |
| White | 226 (71.5%) | 27 (73.0%) | 0.981 |
| None of the above | 55 (17.4%) | 6 (16.2%) | |
| Missing | 1 (0.3%) | 0 (0.0%) | |
| Ethnicity | |||
| Hispanic | 43 (13.7%) | 5 (13.9%) | 0.980 |
| Non-Hispanic | 270 (86.3%) | 31 (86.1%) | |
| Missing | 4 (1.3%) | 1 (2.7%) | |
| Number of children | |||
| Mean | 0.95 ± 1.3 | 0.79 ± 1.1 | 0.429 |
| Median | 1 | 0 | |
| Range | 0–11 | 0–4 | |
| Current relationship status | |||
| Single | 108 (34.1%) | 14 (37.8%) | 0.595 |
| In relationship, but not married | 75 (23.7%) | 9 (24.3%) | |
| Married | 119 (37.5%) | 14 (37.8%) | |
| Divorced/Separated/Widowed/ Other | 15 (4.7%) | 0 (0.0%) | |
| Missing | 0 (0.0%) | 37 (0.0%) | |
| Partner gender (only asked of those who indicated that they had a partner) | |||
| Male partner | 146 (94.8%) | 20 (100%) | 0.297 |
| Female partner | 8 (5.19%) | 0 (0.0%) | |
| Missing/Not asked | 163 (51.4%) | 17 (46.0%) | |
| Educational attainment | |||
| Less than or equal to High School | 143 (45.3%) | 13 (35.1%) | 0.269 |
| Technical/ Associate’s/ Bachelor’s Degree | 127 (40.2%) | 20 (54.1%) | |
| Master’s or other Advanced Degrees | 46 (14.6%) | 4 (10.8%) | |
| Missing | 1 (0.3%) | 0 (0.0%) | |
| Smoking Status | |||
| Long term tobacco user, even before pandemic | 90 (28.4%) | 12 (32.4%) | 0.119 |
| Recently started smoking since the pandemic | 11 (3.5%) | 1 (2.7%) | |
| Recently stopped smoking | 25 (7.9%) | 7 (18.9%) | |
| Has never used tobacco | 191 (60.3%) | 17 (46.0%) | |
| Missing | 0 (0.0%) | 0 (0.0%) | |
| Pre-pandemic menstrual functioning (Only asked of those not using non-hormonal birth control) | |||
| Amenorrhea (0–3 periods/ year) | 27 (8.7%) | 0 (0.0%) | 0.219 |
| Oligomenorrhea (4–7 periods/ year) | 28 (9.0%) | 2 (5.4%) | |
| Normal cycle (8–14 periods/ year) | 238 (76.3%) | 33 (89.2%) | |
| Polymenorrhea (15 or more periods/ year) | 19 (6.1%) | 2 (5.4%) | |
| Missing/Not asked | 5 (1.6%) | 0 (0.0%) | |
| COVID-19 vaccination status | |||
| Vaccinated | 113 (35.9%) | 14 (37.8%) | 0.814 |
| Not Vaccinated | 202 (64.1%) | 23 (62.2%) | |
| Missing | 2 (0.6%) | 0 (0.0%) | |
| Reproductive/gynecologic comorbidity | |||
| Yes | 41 (12.9%) | 5 (13.5%) | 1.00 |
| No | 276 (87.1%) | 32 (86.5%) | |
| Missing | 0 (0.0%) | 0 (0.0%) | |
| Thyroid comorbidity | |||
| Yes | 14 (4.4%) | 3 (8.1%) | 0.403 |
| No | 303 (95.6%) | 34 (91.9%) | |
| Missing | 0 (0.0%) | 0 (0.0%) | |
| Obesity Status | |||
| Obese | 31 (9.8%) | 7 (18.9%) | 0.089 |
| Not obese | 286 (90.2%) | 30 (81.1%) | |
| Missing | 0 (0.0%) | 0 (0.0%) | |
| Mental health comorbidity ¥¥ ╪ ** | |||
| Yes | 128 (40.4%) | 26 (70.3%) | 0.001 |
| No | 189 (59.6%) | 11 (29.7%) | |
| Missing | 0 (0.0%) | 0 (0.0%) | |
| Sexually Transmitted Infection | |||
| Yes | 8 (2.5%) | 1 (2.7%) | 1.00 |
| No | 309 (97.5%) | 36 (97.3%) | |
| Missing | 0 (0.0%) | 0 (0.0%) | |
Low COVID-19 Stress, score <25 on PSS-10-C scale; High COVID-19 Stress, score ≥25 on PSS-10-C.
Data are n (%) unless otherwise specified.
Reproductive/ gynecologic diagnoses included endometriosis, fibroids/myomas, polycystic ovarian syndrome (PCOS), and uterine polyps
Mental health diagnoses included anorexia, anxiety, depression and other mood disorders
included in minimally adjusted model
included in fully adjusted model
Of the women who met the 2nd set of eligibility criteria, 180 reported no changes in their menstrual function (categorized as the “no change” group) and 191 reported at least one change in their period length, duration, flow or spotting (“change” group). Twenty-three women of the 191 (12%) reported a change in all four menstrual parameters (see Appendix 1, http://links.lww.com/xxx).
As shown in Table 3, a greater proportion of women in the high-stress group experienced menstrual cycle changes, as hypothesized. High COVID-19 stress was significantly associated with both shorter and longer period lengths (p=0.008), both shorter and longer period durations (p<0.0001), heavier menstrual flow (p=0.035), and increased spotting between cycles (p=0.006).
Table 3.
Assessing the association between perceived COVID-19 stress and menstrual changes (n=354)
| Low COVID-19 Stress (n=317) | High COVID-19 Stress (n=37) | p value | |
|---|---|---|---|
|
| |||
| Menstrual Cycle Length (n = 353) | 0.008 | ||
| No change | 224 (70.9) | 18 (48.6) | |
| Shorter | 37 (11.7) | 7 (18.9) | |
| Longer | 38 (12.0) | 11 (29.7) | |
| No period since pandemic began | 17 (5.4) | 1 (2.7) | |
| Missing | 1 (0.27) | 0 (0%) | |
| Period Duration (n=354) | <0.0001 | ||
| No change | 225 (71.0) | 18 (48.7) | |
| Shorter | 41 (12.9) | 7 (18.9) | |
| Longer | 33 (10.4) | 12 (32.4) | |
| No period since pandemic began | 18 (5.7) | 0 (0.0) | |
| Missing | 0 (0.0) | 0 (0%) | |
| Menstrual Flow (n=354) | 0.035 | ||
| No change | 207 (65.3) | 19 (51.4) | |
| Lighter | 37 (11.7) | 4 (10.8) | |
| Heavier | 59 (18.6) | 14 (37.8) | |
| No period since pandemic began | 14 (4.4) | 0 (0.0) | |
| Missing | 0 (0.0) | 0 (0.0) | |
| Spotting Between Cycles (n=353) | 0.006 | ||
| No spotting | 255 (80.7) | 25 (67.6) | |
| Spotting between cycles | 43 (13.6) | 12 (32.4) | |
| No period since pandemic began | 18 (5.7) | 0 (0.0) | |
| Missing | 1 (0.27) | 0 (0.0) | |
Low COVID-19 Stress, score <25 on PSS-10-C scale; High COVID-19 Stress, score ≥25 on PSS-10-C. Data are n (%) unless otherwise specified.
Compared to those in the low stress group, a significantly greater proportion of women with high COVID-related stress reported shorter (11.7% vs 18.9% respectively) and longer menstrual cycle lengths (12.0% vs 29.7% respectively) compared to pre-pandemic times. In minimally adjusted models, high COVID-19 stress was associated with twice the odds of changes in menstrual cycle length compared to low COVID stress (adj. OR 2.15; 95% CI: 1.05, 4.39; p=0.035, Table 4). The odds were even greater in fully adjusted models (adj. OR 2.32; 95% CI: 1.12, 4.85; Table 4).
Table 4.
Odds of change in menstrual cycle parameters associated with high COVID-19 stress (vs Low) ¥¥ (n=354)
| Outcome | Crude | Minimally adjusted model* | Fully adjusted model** | |||
|---|---|---|---|---|---|---|
|
| ||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
|
| ||||||
| Menstrual cycle length change (vs “no change”) | 2.57 | 1.29–5.11 | 2.15 | 1.05–4.49 | 2.32 | 1.12–4.85 |
| Period duration change (vs “no change”) | 2.58 | 1.30–5.14 | 2.08 | 1.02–4.25 | 2.38 | 1.14–4.98 |
| Menstrual flow change (vs “no change”) | 1.78 | 0.90–3.54 | 1.47 | 0.72–2.99 | 1.61 | 0.77–3.34 |
| Spotting between cycles (vs “no change”) | 2.85 | 1.33–6.09 | 2.48 | 1.12–5.49 | 2.32 | 1.03–5.22 |
Low COVID-19 Stress, score <25 on PSS-10-C scale; High COVID-19 Stress, score ≥25 on PSS-10-C.
Change refers to any response other than “No change” across each parameter.
model adjusted for education and mental health
model adjusted for education, mental health, age, smoking status and obesity status
With regards to menstrual period duration, a significantly greater proportion of women with high COVID-19 stress reported that they experienced shorter periods (12.9% vs 18.9%) and longer periods (10.4% vs 32.4%) since the start of the pandemic. Minimally adjusted models showed that high COVID-19 stress was associated with 108% greater odds of changes in period duration during the pandemic, compared to low COVID-19 stress (adj. OR 2.08; 95% CI: 1.02, 4.25; p= 0.04, Table 4). Again, the effect estimates increased in fully adjusted models (adj. OR 2.38; 95% CI: 1.14, 4.98; Table 4).
Furthermore, a greater percentage of women with high COVID-related stress endorsed experiencing a heavier menstrual flow during the pandemic (37.8%) as compared to those with low stress (18.6%, p= 0.035, Table 3). In regression analyses however, the effect estimates were not statistically significant, though they were in the hypothesized direction (Table 4).
Similarly, 32.4% of high stress women reported spotting between their menstrual cycles during the pandemic, while about half that proportion of low stress women endorsed this symptom (13.6%, p= 0.006, Table 3). In regression analyses, high COVID-19 stress was associated with over twice the odds of spotting compared to low COVID-19 stress in both minimally (adj. OR 2.48; 95% CI: 1.12, 5.49) and fully adjusted (adj. OR 2.32; 95% CI: 1.03, 5.22; Table 4) models.
Discussion:
We surveyed a geographically representative and racially diverse sample of US women of reproductive age to evaluate how stress related to the COVID-19 pandemic has affected their menstrual cycles. Over half of the participants who met the study’s gynecologic and reproductive criteria and had complete COVID-stress data reported at least one change in their period length, duration, flow, or spotting (n=191), and an alarming 12% of these women reported changes in all four menstrual parameters. We found that high COVID-19 stress is significantly associated with both shorter and longer period length (p<0.008), both shorter and longer period durations (p<0.0001), heavier menstrual flow (p=0.035), and increased spotting between cycles (p=0.006). Multivariable analyses showed that high COVID-19 stress was associated with at least twice the odds of menstrual perturbations in crude, minimally, and fully adjusted regression estimates for period length, duration and spotting. Although the association between COVID-19 stress and heavier menstrual flow was not statistically significant in our findings, the clinical relevance of the correlation between the two variables cannot be neglected as menorrhagia has been associated with anemia29 and the economic costs of menstruation, “the tampon tax,” can be burdensome to individuals.30 Given the economic ramifications that the pandemic has had on populations worldwide, for women who experience abnormal bleeding, changes in cycle length or duration, and intermenstrual spotting, the burden of the additional “tampon tax” could be mentally burdensome and financially prohibitive.
Our findings align with early indications of COVID-related menstrual disruptions in the emerging literature. Initial reports from a study on Australian Olympic trainees show that 19.6% and 24.7% of hormonal contraceptive users and natural cyclers, respectively, have reported a change to their menstrual cycles since the onset of the pandemic, a marked increase from the percentage of natural cyclers who reported changes prior to the pandemic.11 Emerging research from the National Institutes of Health (NIH) and Centers for Disease Control and Prevention (CDC) also indicate that females with long-COVID-19 (lingering COVID-19 symptoms that can up to months after the initial infection) endorse a range of menstrual changes, including cycle irregularities, abnormal clotting, and severe premenstrual syndromes.11, 31
Stress pathways are known to interact with and modulate the menstrual cycle, which is regulated by the hypothalamus-pituitary-ovarian (HPO) axis through hormonally mediated feedback loops.32,33 Epidemiological studies have long pointed to an association between stressful events and menstrual perturbations. Menstrual cycle irregularities have been documented in women experiencing war7 and psychological stress in the workplace.10,34 Additionally, high incidence of amenorrhea was reported in active-duty females and army nurses in the British and American camps during World War II9 and documented in women who were enslaved during the era of the Trans-Atlantic slave trade.35–37 To our knowledge, the role of COVID-related stress in relation to menstrual cycle changes has yet to be fully elucidated. In contrast to our findings, Nguyen et al. did not find an association between COVID-related stress and menstrual changes.38 A potential contributor to this discrepancy could be the fact that the authors of this paper utilized a two question Likert style assessment to query COVID-related stress rather than a validated questionnaire such as the PSS-10-C to more accurately assess stress associated with the pandemic. Unlike Nguyen et al., two other studies have pointed to an association between COVID-related stress and menstrual changes. A recent study of female healthcare workers in Turkey concluded that reported COVID-19 stress was a significant predictor of menstrual irregularities, although the type of menstrual disturbances were not specified.39 Similarly, Ozimek et al. observed that women with high perceived stress during the pandemic were more likely to experience a longer duration of menses and heavier bleeding during menses compared to those with moderate stress.40 However, unlike ours, the study sample was not reflective of US census in multiple demographic factors including race, socioeconomic status and geographic distribution. Our findings demonstrate an association between high COVID-related stress and menstrual changes on a granular level and within a more diverse group of women across various educational, racial and ethnic, and regional backgrounds in the US. Because we report menstruation parameters as discrete categories of cycle length, period duration, menstrual flow, and spotting changes, the findings can be pinpointed to specific menstrual parameters, such as cycle length, which is known to be clinically relevant to future health risks.14 Additionally, our sampling scheme allowed us to sample US women exposed to varying degrees of COVID-19 infection rates, restrictions, mandates, and policies, and to understand what aspects of their menstrual cycle have been affected by COVID-related stressors.
Our study has some limitations, however. The first is the potential for recall bias in participant survey responses, where participants may have over- or under- reported the observed changes in their menstrual cycle throughout the pandemic. However, the validity of self-reports of women’s reproductive history, compared to the gold standard of medical records has been reported in the range of 92.9%–100%41 implying that the probability of biased reporting in this study was low. Future studies should validate participants’ self-reports with objective assessments of menstrual functioning, such as hormonal biomarkers, which could be combined with prospective menstrual logs to provide researchers long-term assessments of menstrual disruptions second to COVID-related stress. The online nature of the survey could have resulted in a sampling/selection bias favoring individuals with internet access and ample time for research participation, a potential explanation for why the education level of the sample is higher than national averages. However, a comparison of survey completers and non-completers showed very few differences between the two, and even highlighted more participation by Black citizens than would be expected. Additionally, the research was limited to participants who self-identify as women, thus excluding gender minorities who may not identify as women, but do menstruate. Future studies should be more inclusive of not only racially diverse participants but also sexual and gender minority groups as emerging reports have highlighted the disproportionate effects of the pandemic on these individuals’ mental health and well-being.42, 43
In this study, we found that high COVID-19 stress is associated with increased risk of changes in multiple menstrual cycle parameters. Menstrual outcomes provide insight into numerous aspects of women’s overall wellbeing, including cardiovascular,14 endocrinologic,44 reproductive,45 and menopausal health outcomes.46 Given the importance of the menstrual cycle as an indicator of women’s overall well-being, reproductive health care professionals should be attuned to COVID-related stress levels as a potential factor affecting their patients’ menstrual health.
Supplementary Material
Acknowledgements:
Funding support was provided for Dr. Martina Anto-Ocrah by NIH grant #5T32NS007338–30. At the time of press, Dr. Martina Anto-Ocrah (MAO) was funded by NIH NINDS K01 Award#7K01NS121199–02.
Financial Disclosure
Each author has confirmed compliance with the journal’s requirements for authorship.
Footnotes
The authors did not report any potential conflicts of interest.
PEER REVIEW HISTORY
Received July 17, 2022. Received in revised form September 5, 2022. Accepted September 15, 2022. Peer reviews and author correspondence are available at http://links.lww.com/xxx.
References:
- 1.World Health Organization. Archived: WHO Timeline – COVID-19. Accessed July 8, 2021 https://www.who.int/news/item/27-04-2020-who-timeline---COVID-19
- 2.World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Accessed July 8, 2021.https://covid19.who.int/
- 3.Centers for Disease Control and Prevention. COVID-19 Forecasts: Death. Accessed September 4, 2022. https://www.cdc.gov/coronavirus/2019-ncov/science/forecasting/forecasting-us.html
- 4.Kalin NH. COVID-19 and Stress-Related Disorders. American Journal of Psychiatry 2021;178(6):471–474. doi: 10.1176/appi.ajp.2021.21040371 [DOI] [PubMed] [Google Scholar]
- 5.Zamarro G, & Prados MJ. Gender differences in couples’ division of childcare, work and mental health during COVID-19. Review of Economics of the Household 2021; 19(1): 11–40. 10.1007/s11150-020-09534-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park CL, Russell BS, Fendrich M, Finkelstein-Fox L, Hutchison M, Becker J. Americans’ COVID-19 Stress, Coping, and Adherence to CDC Guidelines. Journal of General Internal Medicine 2020;35(8):2296–2303. doi: 10.1007/s11606-020-05898-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hannoun AB, Nassar AH, Usta IM, Zreik TG, Abu M, Antoine A. Effect of War on the Menstrual Cycle. Obstetrics & Gynecology 2007; 109 (4): 929–932. doi: 10.1097/01.AOG.0000257170.83920.de [DOI] [PubMed] [Google Scholar]
- 8.Petit NF. Dysfunctional uterine bleeding in active-duty women: scope of the problem and management issues. Womens Health Issues 1996; 6, 358–61. [PubMed] [Google Scholar]
- 9.Whitacre FE, Barrera B, Briones TN, Suaco BS, and Paz A. War Amenorrhea. The Journal of American Medical Association 1944; 124(7): 399–403. 10.1001/jama.1944.02850070001001 [DOI] [Google Scholar]
- 10.Fenster L, Waller K, Chen J, Hubbard AE, Windham GC, Elkin E, et al. Psychological stress in the workplace and menstrual function. American Journal of Epidemiology 1999; 149(2): 127–13 Today. Long COVID and periods: The unspoken impact on female well-being. Accessed July 7, 2021. https://www.medicalnewstoday.com/articles/long-covid-and-periods-the-unspoken-impact-on-female-well-being [DOI] [PubMed] [Google Scholar]
- 11.Medical News Today. Long COVID and periods: The unspoken impact on female well-being. Accessed July 7, 2021. https://www.medicalnewstoday.com/articles/long-covid-and-periods-the-unspoken-impact-on-female-well-being
- 12.Fourestié V, Lignières B, Roudot-Thoraval F, Fulli-Lemaire I, Nahoul K, Cremniter D, et al. Suicide attempts in hypo-oestrogenic phases of the menstrual cycle. The Lancet 1986; 328: 1357–1360. 10.1097/00006842-200001000-00008 [DOI] [PubMed] [Google Scholar]
- 13.Skobeloff EM, Spivey WH, Silverman R, Eskin BA, Harchelroad F, Alessi TV. The effect of the menstrual cycle on asthma presentations in the emergency department. Arch Intern Med 1996; 156:1837–40. 10.1001/archinte.156.16.1837 [DOI] [PubMed] [Google Scholar]
- 14.Solomon CG, Hu FB, Dunaif A, Rich-Edwards JE, Stampfer MJ, Willett WC, et al. Menstrual Cycle Irregularity and Risk for Future Cardiovascular Disease. The Journal of Clinical Endocrinology & Metabolism 2002;87(5):2013–2017. doi: 10.1210/jcem.87.5.8471 [DOI] [PubMed] [Google Scholar]
- 15.McNamara A, Harris R, Minahan C. Menstrual Cycle Change During COVID-19. Sharing some early results. British Journal of Sports Medicine 2020. https://blogs.bmj.com/bjsm/2020/11/20/menstrual-cycle-change-during-COVID-19/. [Google Scholar]
- 16.Yuksel B, Ozgor F. Effect of the COVID-19 pandemic on female sexual behavior. International Journal of Gynecology & Obstetrics 2020;150(1):98–102. doi: 10.1002/ijgo.13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dynata. Accessed May 15, 2021. https://www.dynata.com/.
- 18.Vordenberg SE, Zikmund-Fisher BJ. Characteristics of older adults predict concern about stopping medications. J Am Pharm Assoc 2020;60(6):773–780. 10.1016/j.japh.2020.01.019 [DOI] [PubMed] [Google Scholar]
- 19.Vordenberg SE, Zikmund-Fisher BJ. Older adults’ strategies for obtaining medication refills in hypothetical scenarios in the face of COVID-19 risk. J Am Pharm Assoc 2020;60(6):915–922.e914. 10.1016/j.japh.2020.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bock T What are Survey Quotas? Accessed 6/8/2022, https://www.displayr.com/what-are-survey-quotas/
- 21.Ripley DL, Harrison-Felix C, Sendroy-Terrill M, Cusick CP, Dannels-McClure A, Morey C. The impact of female reproductive function on outcomes after traumatic brain injury. Arch Phys Med Rehabil 2008;89(6):1090–1096. 10.1016/j.apmr.2007.10.038 [DOI] [PubMed] [Google Scholar]
- 22.Anto-Ocrah M, Cafferky, V., Lewis, V. Pregnancy after concussion: A clarion call for attention?. Journal of Head Trauma Rehabilitation 2021; 01;37(4):E268–E279. 10.1097/HTR.0000000000000723 [DOI] [PubMed] [Google Scholar]
- 23.Anto-Ocrah M, Bazarian J, Lewis V, Jones CM, Jusko TA, Van Wijngaarden E. Risk of female sexual dysfunction following concussion in women of reproductive age. Brain Inj 2019;33(11):1449–1459. 10.1080/02699052.2019.1644377 [DOI] [PubMed] [Google Scholar]
- 24.Campo-Arias A, Pedrozo-Cortés MJ, Pedrozo-Pupo JC. Pandemic-Related Perceived Stress Scale of COVID-19: An exploration of online psychometric performance [Escala de estrés percibido relacionado con la pandemia de COVID-19: una exploración del desempeño psicométrico en línea]. Revista Colombiana de Psiquiatría (English ed.) 2020; 49(4): 229–230. 10.1016/j.rcpeng.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Y, Zhang R, Zhou Z, Fan J, Liang J, Cai L, et al. Parental psychological distress and attitudes towards COVID-19 vaccination: A cross-sectional survey in Shenzhen, China. Journal of Affective Disorders 2021; 292: 552–558. 10.1016/j.jad.2021.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harlow SD, Matanoski GM. The association between weight, physical activity, and stress and variation in the length of the menstrual cycle. Am J Epidemiol 1991; 133(1):38–49. doi: 10.1093/oxfordjournals.aje.a115800 [DOI] [PubMed] [Google Scholar]
- 27.Hahn KA, Wise LA, Riis AH, Mikkelsen EM, Rothman KJ, Banholzer K, Hatch EE. Correlates of menstrual cycle characteristics among nulliparous Danish women. Clinical Epidemiology 2013; 5(1): 311–319. 10.2147/CLEP.S46712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shapley M, Jordan K, & Croft PR. Increased vaginal bleeding and psychological distress: a longitudinal study of their relationship in the community. BJOG: An International Journal of Obstetrics & Gynaecology 2003; 110(6): 548–554. 10.1046/j.1471-0528.2003.02458.x [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Bleeding Disorders in Women- Heavy Menstrual Bleeding. Accessed on August 15, 2021. https://www.cdc.gov/ncbddd/blooddisorders/women/menorrhagia.html.
- 30.Global Citizen. The Tampon Tax: Everything You Need to Know. Accessed on August 15, 2021. https://www.globalcitizen.org/en/content/tampon-tax-explained-definition-facts-statistics/#:~:text=The%20tampon%20tax%20is%20a%20charge%20on%20menstrual,and%20erectile%20dysfunction%20pills%20%E2%80%94%20are%20typically%20tax-exempt.
- 31.Vox. The many strange long-term symptoms of COVID-19, explained. Accessed on July 7, 2021. https://www.vox.com/22166236/long-term-side-effects-COVID-19-symptoms-heart-fatigue
- 32.Fritz MA, Speroff L. Clinical gynecologic endocrinology and infertility. Eighth edition. Wolters Kluwer Health/Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 33.Chrousos GP, Torpy DJ, & Gold PW. Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: Clinical implications. Annals of Internal Medicine 1998; 129(3): 229–240. 10.7326/0003-4819-129-3-199808010-00012 [DOI] [PubMed] [Google Scholar]
- 34.László KD, Gyorffy Z, Adám S, Csoboth C, Kopp MS. Work-related stress factors and menstrual pain: a nation-wide representative survey. Journal of Psychosomatic Obstetrics and Gynaecology 2008; 29(2): 133–138. 10.1080/01674820701804423 [DOI] [PubMed] [Google Scholar]
- 35.Morgan K Slave Women and Reproduction in Jamaica, c.1776–1834. History (London) 2006; 91(302): 231–253. 10.1111/j.1468-229X.2006.00365.x [DOI] [Google Scholar]
- 36.Klein HS, Engerman SL. Fertility differentials between slaves in the United States and the British West Indies: a note on lactation practices and their possible implications. The William and Mary quarterly 1978; 35(2): 357–374. 10.2307/1921839 [DOI] [PubMed] [Google Scholar]
- 37.Lantz H, Hendrix L. Black fertility and the black family in nineteenth century: a reexamination of the past. Journal of Family History 1978; 3(3): 251–261. 10.1177/036319907800300303 [DOI] [PubMed] [Google Scholar]
- 38.Nguyen BT, Pang RD, Nelson AL, Pearson JT, Benhar Noccioli E, Reissner HR, et al. Detecting variations in ovulation and menstruation during the COVID-19 pandemic, using real-world mobile app data. PLoS ONE 2021; 16(10): e0258314. 10.1371/journal.pone.0258314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takmaz T, Gundogmus I, Okten SB, Gunduz A. The impact of COVID-19–588related mentalhealth issues on menstrual cycle characteristics of female589healthcare providers.The Journal of Obstetrics and Gynaecology Research 2021;59047(9), 3241–3249. 10.1111/jog.14900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozimek N, Velez K, Anvari H, Butler L, Goldman KN, Woitowich NC. Impact of Stress on Menstrual Cyclicity During the Coronavirus Disease 2019 Pandemic: A Survey Study. Journal of Women’s Health 2022; 31(1), 84–90. 10.1089/jwh.2021.0158 [DOI] [PubMed] [Google Scholar]
- 41.Hassan MA, Killick SR. Ultrasound diagnosis of polycystic ovaries in women who have no symptoms of polycystic ovary syndrome is not associated with subfecundity or subfertility. Fertil Steril 2003; 80(4): 966–75. DOI: 10.1016/s0015-0282(03)01010-0 [DOI] [PubMed] [Google Scholar]
- 42.Kamal K, Li JJ, Hahm HC, Liu CH. Psychiatric impacts of the COVID-19 global pandemic on U.S. sexual and gender minority young adults. Psychiatry Research 2021; 299: 113855–113855. 10.1016/j.psychres.2021.113855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phillips G, Felt D, Ruprecht MM, Wang X, Xu J, Pérez-Bill E, et al. Addressing the Disproportionate Impacts of the COVID-19 Pandemic on Sexual and Gender Minority Populations in the United States: Actions Toward Equity. LGBT Health 2020; 7(6): 279–282. 10.1089/lgbt.2020.0187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Solomon CG. Long or Highly Irregular Menstrual Cycles as a Marker for Risk of Type 2 Diabetes Mellitus. JAMA 2001; 286(19): 2421. 10.1001/jama.286.19.2421 [DOI] [PubMed] [Google Scholar]
- 45.Crawford NM, Pritchard DA, Herring AH, Steiner AZ. Prospective evaluation of luteal phase length and natural fertility. Fertil Steril 2017; 107: 749–755. DOI: 10.1016/j.fertnstert.2016.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Landgren BM, Collins A, Csemiczky G, Burger HG, Baksheev L, Robertson DM. Menopause Transition: Annual Changes in Serum Hormonal Patterns over the Menstrual Cycle in Women during a Nine-Year Period Prior to Menopause. The Journal of Clinical Endocrinology & Metabolism 2004;89(6):2763–2769. doi: 10.1210/jc.2003-030824 [DOI] [PubMed] [Google Scholar]
- 47.United States Census. Accessed April 15, 2021. https://www.census.gov/quickfacts/fact/table/US/PST045219
- 48.United States Census Bureau. Educational Attainment in the United States: 2020. Accessed July 7, 2021. https://www.census.gov/data/tables/2020/demo/educational-attainment/cps-detailed-tables.html
- 49.United States Census Bureau. Marital Status in the United States. Accessed July 9, 2021. https://www.census.gov/library/visualizations/interactive/marital-status-in-united-states.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


