Abstract
Objective:
We aimed to evaluate whether delivering early in the coronavirus disease 2019 (COVID-19) pandemic was associated with increased risk of maternal death or serious morbidity from common obstetric complications compared with a historical control period.
Methods:
This was a multicenter retrospective cohort study with manual medical record abstraction performed by centrally trained and certified research personnel at 17 U.S. hospitals. Individuals who gave birth on randomly selected dates in 2019 (before the pandemic) and 2020 (during the pandemic) were compared. Hospital, healthcare system, and community SARS-CoV-2 risk mitigation strategies in response to the early COVID-19 pandemic are described. The primary outcome was a composite of maternal death or serious morbidity from common obstetric complications including hypertensive disorders of pregnancy (eclampsia, end organ dysfunction, or need for acute antihypertensive therapy), postpartum hemorrhage (operative intervention or receipt of 4 units or more blood products), and infections other than SARS-CoV-2 (sepsis, pelvic abscess, prolonged IV antibiotics, bacteremia, deep surgical site infection). The major secondary outcome was cesarean birth.
Results:
Overall 12,133 patients giving birth during and 9,709 before the pandemic were included. Hospital, healthcare system and community SARS-CoV-2 mitigation strategies were employed at all sites for a proportion of 2020 with a peak in modifications from March to June 2020. Of those delivering during the pandemic, 3% had a positive SARS-CoV-2 test during pregnancy through 42 days postpartum. Giving birth during the pandemic was not associated with a change in the frequency of the primary composite outcome (9.3 vs 8.9%, aRR 1.02, 95% CI 0.93–1.11), or cesarean birth (32.4 vs 31.3%, aRR 1.02, 95% CI 0.97–1.07). No maternal deaths were observed.
Conclusion:
Despite substantial hospital, healthcare and community modifications, giving birth during the early COVID-19 pandemic was not associated with higher rates of serious maternal morbidity from common obstetric complications.
Clinical Trial Registration:
Precis:
Despite healthcare and societal modifications, giving birth during the early COVID-19 pandemic was not associated with increased risk of serious maternal morbidity from common obstetric complications.
INTRODUCTION
Early in the coronavirus disease 2019 (COVID-19) pandemic, major shifts occurred in healthcare delivery including reallocation of staff to other areas of the hospital, decrease in trainees in the hospital, increased use of telehealth, and changes in capacity to conduct elective surgeries and procedures. Similarly, there were societal changes to decrease viral transmission such as school closures and lockdowns. Studying the effects of these changes is critical as we prepare to navigate the ongoing COVID-19 pandemic and future pandemics.
Studies in non-pregnant individuals have observed increased all-cause mortality during the pandemic in the United States.1 Similarly, in obstetrics, data from the National Center for Health Services demonstrate an increase in maternal mortality from 754 deaths in 2019 to 861 deaths in 2020.2 The proportion of these deaths that are directly attributable to COVID-19 remains unknown. While some deaths will undoubtedly be linked directly to the virus, other deaths may be due to indirect effects of the pandemic such as medical access disruption or maternal stressors.3 While those with SARS-CoV-2 infection in pregnancy are at increased risk of adverse pregnancy outcomes4–6, especially in the setting of greater COVID-19 severity4–5, the effect of the pandemic on outcomes of obstetric patients overall remains uncertain.
Serious maternal morbidity is closely linked to maternal mortality and is often along the pathway to maternal death. Therefore, we sought to evaluate whether birthing individuals during the early COVID-19 pandemic in the U.S. were at increased risk of maternal death or serious morbidity from common obstetric complications during the pandemic compared with a historic control group at the same hospitals in the year prior to the pandemic. In addition, we described the specific hospital, healthcare system, or community-level changes that occurred over the early pandemic at these hospital sites.
METHODS
This was a retrospective cohort study of pregnant individuals with a singleton or twin gestation who gave birth from March through December in the years 2019 and 2020 at one of 17 U.S. hospitals participating in the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units (MFMU) Network Gestational Research Assessments for COVID-19 (GRAVID) study. Prior publications include patients with SARS-CoV-2 and the randomly-selected delivery controls from 2020 that are included in this analysis.4–5 GRAVID was performed under waiver of consent with IRB approval at all participating institutions. The study protocol was registered on clinicaltrials.gov (NCT04519502 prior to the initiation of data abstraction.
The exposure was giving birth during the early COVID-19 pandemic. Patients who gave birth on randomly selected dates in 2020 were included as deliveries during the pandemic. Six weekdays and two weekend days per month were sampled from March through May 2020 when we anticipated the largest surge of COVID-19, and three weekdays and one weekend day per month were randomly selected from June through December 2020. The historical control group was composed of patients who gave birth at the same hospitals before the pandemic (deliveries during 2019); three weekdays and one weekend day per month were randomly selected from March through December 2019. All of the MFMU sites used the same randomly selected delivery dates. Random selection was based on a uniform distribution in which each weekday and weekend day would have equal probability of random selection. Both weekdays and weekend days were sampled intentionally as staffing and delivery volumes were anticipated to differ. Data were abstracted from the medical record by centrally trained and certified research staff and outcomes assessed through 42 days postpartum.
The primary outcome was a composite of death from any cause or serious maternal morbidity related to common obstetric complications: hypertensive disorders of pregnancy, postpartum hemorrhage, or infection other than SARS-CoV-2. Serious morbidity was defined by the NICHD MFMU Steering Committee a priori as clinically significant endpoints for morbidity. Serious morbidity related to hypertensive disorders of pregnancy included eclampsia, hemolysis elevated liver enzymes, and low platelets (HELLP) syndrome, pulmonary edema on chest x-ray, severe hypertension (>160/110 mmHg) with acute administration of antihypertensive therapy, hepatic rupture, impaired liver function (>2 times the upper limit of normal), renal insufficiency (Cr ≥ 1.2 mg/dL), thrombocytopenia (platelets <100,000/μL), or placental abruption. Serious morbidity related to postpartum hemorrhage included transfusion of four or more units of packed red blood cells, surgical or radiological interventions to control bleeding and related complications. Serious morbidity related to infection included sepsis (infection with end organ dysfunction), bacteremia, endometritis requiring intravenous antibiotic therapy for > 24 hours, deep incisional surgical site infection, or pelvic abscess.
The major secondary outcome was cesarean birth. Other maternal secondary outcomes included severe maternal morbidity defined as recommended by the American College of Obstetricians and Gynecologists (ACOG) and Society for Maternal-Fetal Medicine (SMFM) as ICU admission or transfusion of four or more units of blood.7 Rates of ICU admission, length of ICU stay, and length of hospital stay were also evaluated. Neonatal secondary outcomes included perinatal death, neonatal ICU admission, and length of neonatal ICU stay. Among those who delivered at or beyond 20 weeks’ gestation, a perinatal preterm and term adverse composite outcome were also evaluated. The preterm composite included fetal or neonatal death, severe bronchopulmonary dysplasia, grade III or IV intraventricular hemorrhage, Bell Stage 2A or greater necrotizing enterocolitis, periventricular leukomalacia, stage III or IV retinopathy of prematurity, or neonatal sepsis with positive blood cultures. The term composite included fetal or neonatal death, respiratory support within first 72 hours (beyond support for transition in the delivery room), Apgar score less than or equal to 3 at 5 minutes, hypoxic ischemic encephalopathy, seizure, infection (sepsis or pneumonia), birth trauma, meconium aspiration syndrome, intracranial or subgaleal hemorrhage, or hypotension requiring vasopressor support. Neonatal outcomes were collected during the delivery hospitalization.
Date of implementation and discontinuation of modifications to hospitals, healthcare systems and community risk mitigation strategies for SARS-CoV-2 during 2020 were recorded at the individual site level. Specific modifications that were recorded are included in Appendix 2, available online at http://links.lww.com/xxx.
Data from the MFMU Network’s Assessment of Perinatal Excellence (APEX) cohort study were used to provide estimates on outcome rates for the sample size calculation.13 The rate of serious maternal morbidity in APEX was 5.1%. With an estimated sample size of 10,600 deliveries for 2019 and 13,800 deliveries for 2020, the study had more than 90% power to show a 30% increase in the rate of the primary composite maternal morbidity end point, assuming the rate was at least 3% in calendar year 2019 with a 2-sided alpha of 0.05. A 30% relative increase in the rate of the composite maternal morbidity end point was thought to be a clinically meaningful difference and was selected by the MFMU Steering Committee prior to study initiation.
For the primary objective, patients who gave birth during the pandemic were compared with patients who gave birth before the pandemic. Descriptive summary statistics were calculated for baseline characteristics and for modifications to hospitals, healthcare systems, and community risk mitigation strategies for SARS-CoV-2 during 2020.
For the main analysis, patients who delivered during the pandemic were compared to those delivered before the pandemic using the Wilcoxon rank sum test for continuous variables and Chi-square or Fisher’s exact test for categorical variables, as appropriate. Multivariable modeling was not performed for outcomes with low frequencies. Covariates for modeling included MFMU site and factors based on clinical relevance including maternal age, body mass index (BMI) at first prenatal visit or (if that was not available) pre-pregnancy weight reported in the medical record, and major medical comorbidity including any of the following: asthma of any severity or chronic obstructive pulmonary disease, chronic hypertension, or pregestational diabetes. Models for the primary outcome also included obstetric history categorized as no prior deliveries > 20 weeks’ gestation, prior delivery with a hypertensive disorder or preterm birth, or prior delivery without hypertensive disorder or preterm birth. The model for cesarean birth included history of cesarean birth (categorized as no prior pregnancy 20 weeks of gestation or longer, history of only vaginal births, or any prior cesarean birth) in addition to the baseline demographics variables previously described.
To account for the random sampling of individuals, weighted analyses were performed. For maternal outcomes, Poisson regression models were used to estimate relative risks and 95% confidence intervals (CIs). To account for patients with twin gestations, models based on a generalized estimating equations framework with exchangeable correlation structure were used to estimate relative risks for neonatal outcomes. For skewed continuous variables, medians were presented for descriptive purposes and the natural log-transformed value was used in the regression model to estimate differences in the means of the log-transformed values.
A planned sensitivity analysis was performed in which those with documented SARS-CoV-2 infection (positive nucleic acid or antigen test in the outpatient or inpatient setting) at any time during pregnancy through 42 days postpartum were excluded from the analysis. An additional sensitivity analysis was performed in which missing BMI values were imputed based on a generalized linear model. The imputation modeled the natural-log scale BMI with linear, quadratic, and cubic natural-log scale BMI at delivery calculated from the most recent pregnancy weight before delivery. For patients with BMI at delivery and without prenatal (or prepregnancy) BMI available, imputed BMI values were the back-transformed predicted values based on the model.
Subgroup analyses were conducted by race and ethnicity, parity, and insurance status to determine whether the association or lack thereof prevailed throughout particular subgroups of patients. Race and ethnicity was evaluated given the known association between maternal race and morbidity.8 Likelihood ratio tests were used to evaluate interactions between the exposure and a pre-specified subgroup. For each subgroup, stratified analyses were only conducted for outcomes if there was evidence of significant effect modification.
Nominal two-sided p-values are reported. P value less than 0.05 was considered statistically significant. No adjustment was made for multiple comparisons. Statistical analyses were performed using SAS statistical software version 9.4.
RESULTS
During the pandemic in 2020, 12,133 patients gave birth to 12,407 neonates on randomly selected delivery dates. Of those individuals, 3.1% (n=381/12133, 95% CI 2.8–3.5%) had a documented SARS-CoV-2 infection during pregnancy through 42 days postpartum (Figure 1). The proportion of patients with SARS-CoV-2 from March through December 2020 varied consistent with surges in infection rates across the MFMU (Figure 2). Before the pandemic in 2019, 9,709 patients gave birth to 9,938 neonates on randomly selected delivery dates. Demographic characteristics are in Table 1.
Figure 1.
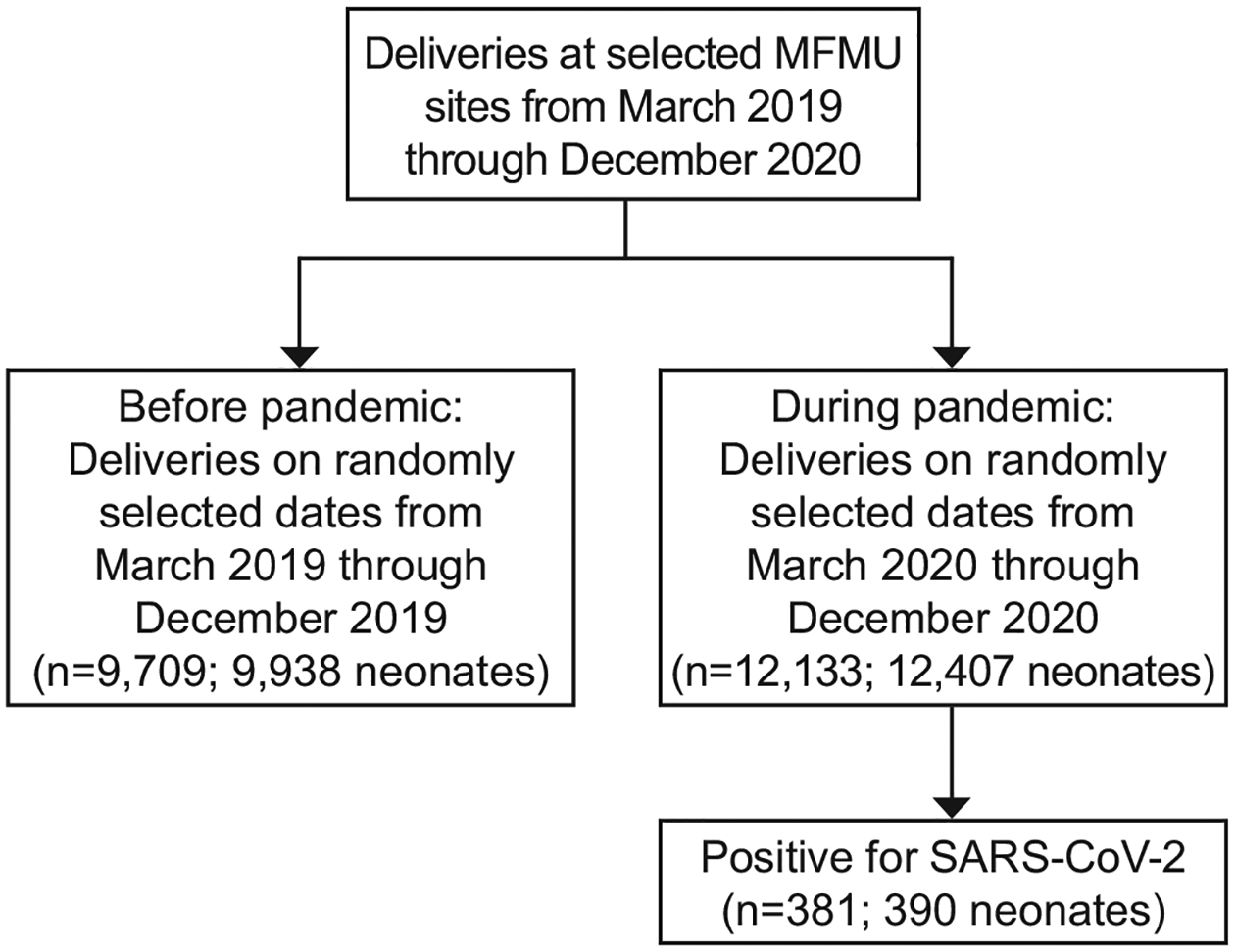
Study population. MFMU, Maternal-Fetal Medicine Units Network; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Figure 2.
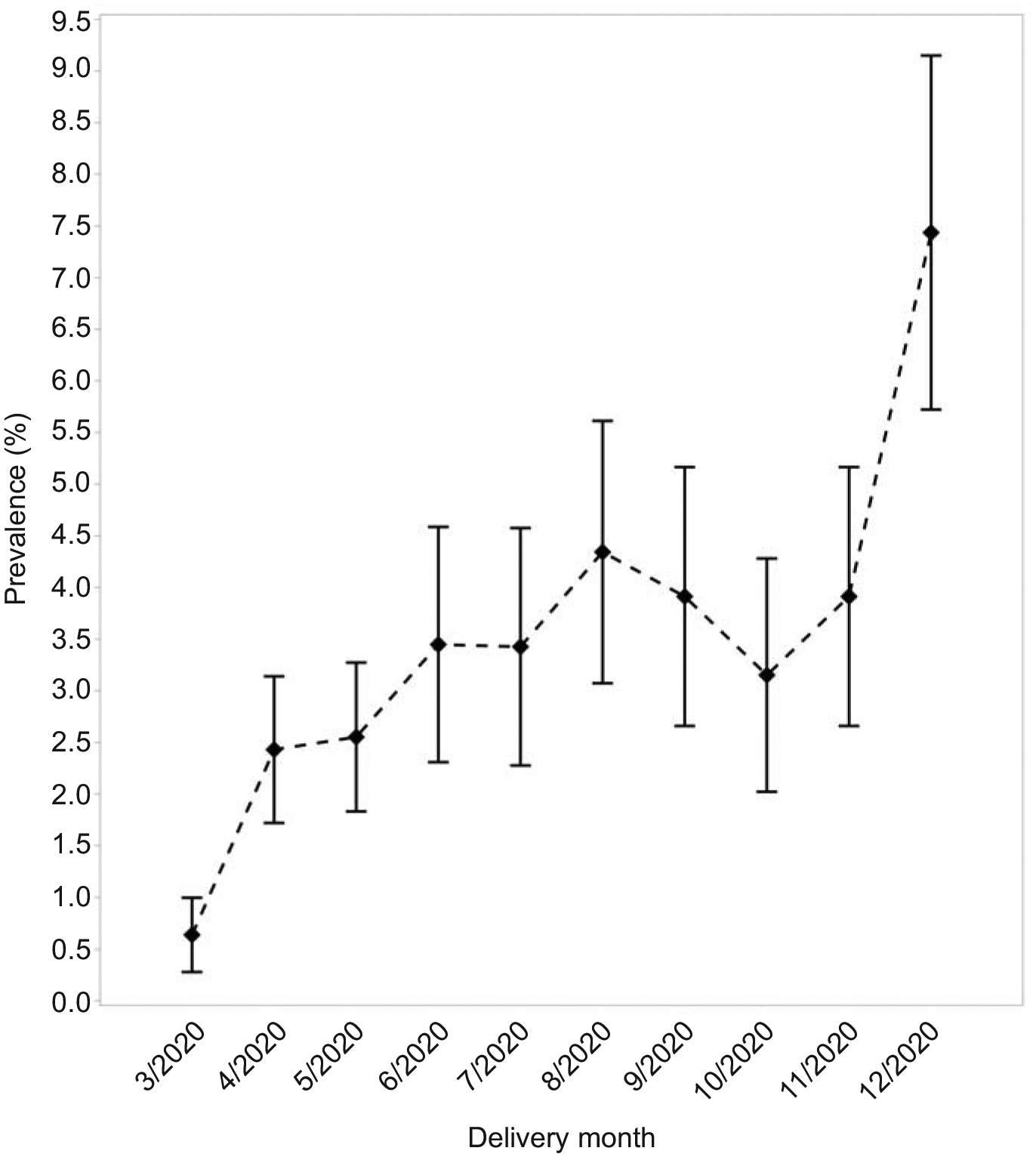
Graphical depiction of the proportion of patients positive for severe acute respiratory syndrome coronavirus 2, across the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network over the study period on a monthly basis. Squares indicates percent, bars indicate 95% CI.
Table 1.
Demographics and Baseline Characteristics
| During Pandemic (n=12133) | Before Pandemic (n=9709) | p-value | |
|---|---|---|---|
| Age (years) | 29.9 +/− 5.84 [n=12130] | 29.7 +/− 5.85 | 0.02 |
| Body mass index (kg/m^2) | 26.6 (23.0 – 32.0) [n=11008] | 26.6 (22.9 – 32.1) [n=8604] | 0.37 |
| Race and ethnicity | n=11550 | n=9193 | 0.07 |
| American Indian / Alaskan Native | 43 (0.4) | 20 (0.2) | |
| Asian | 584 (5.1) | 523 (5.7) | |
| Hispanic | 2822 (24.4) | 2228 (24.2) | |
| Native Hawaiian / Pacific Islander | 29 (0.3) | 35 (0.4) | |
| Non-Hispanic Black | 2624 (22.7) | 2077 (22.6) | |
| Non-Hispanic White | 5387 (46.6) | 4252 (46.3) | |
| More than one race | 61 (0.5) | 58 (0.6) | |
| No prior pregnancy 20 weeks or longer | 4913/12120 (40.5) | 3868/9703 (39.9) | 0.31 |
| Previous preterm birth (20 to less than 37 weeks) | 1130/12120 (9.3) | 924/9703 (9.5) | 0.62 |
| Previous cesarean birth | 2232/12120 (18.4) | 1777/9703 (18.3) | 0.85 |
| Previous hypertensive disorder of pregnancy | 971/12120 (8.0) | 707/9703 (7.3) | 0.05 |
| Private insurance | 6501/12054 (53.9) | 5202/9610 (54.1) | 0.77 |
| Smoked during this pregnancy | 866/12133 (7.1) | 748/9709 (7.7) | 0.11 |
| Any substance use during this pregnancy | 992 (8.2) | 855 (8.8) | 0.10 |
| Immunocompromising condition | 169 (1.4) | 132 (1.4) | 0.83 |
| Asthma or chronic obstructive pulmonary disease | 1614 (13.3) | 1206 (12.4) | 0.05 |
| Pregestational diabetes | 278 (2.3) | 237 (2.4) | 0.47 |
| Thrombophilia | 81 (0.7) | 86 (0.9) | 0.07 |
| Chronic hypertension | 677 (5.6) | 498 (5.1) | 0.14 |
| Chronic cardiovascular disease | 179 (1.5) | 108 (1.1) | 0.02 |
| Chronic renal disease | 60 (0.5) | 43 (0.4) | 0.58 |
| Chronic liver disease | 111 (0.9) | 86 (0.9) | 0.82 |
| Thyroid disease | 808 (6.7) | 652 (6.7) | 0.87 |
| Neurocognitive disorder | 1584 (13.1) | 1084 (11.2) | <.001 |
| Neuromuscular disorder | 54 (0.4) | 40 (0.4) | 0.71 |
| Seizure disorder | 151 (1.2) | 134 (1.4) | 0.38 |
| Inflammatory bowel disease | 131 (1.1) | 103 (1.1) | 0.89 |
| Any co-morbidity (asthma/COPD, pregestational diabetes, chronic hypertension) | 2312 (19.1) | 1721 (17.7) | 0.01 |
Data are mean +/− standard deviation, median (Q1 - Q3), or n (%) unless otherwise specified.
During the pandemic in 2020, there were modifications to hospitals, healthcare systems and community-level mitigation strategies for SARS-CoV-2, which peaked from March through June 2020 (Figure 3).
Figure 3.
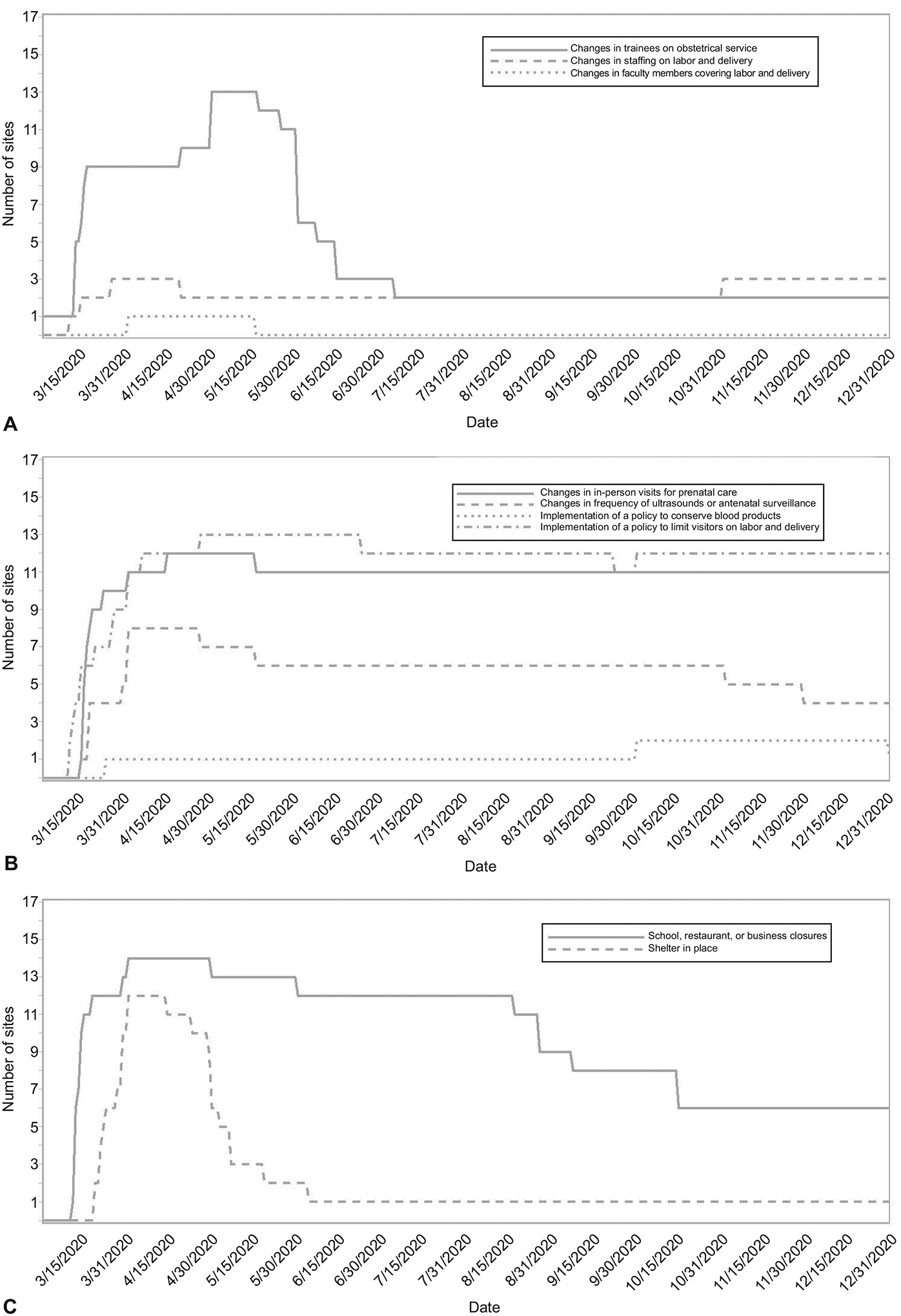
Descriptive data for modifications to hospital, health care system, and community-level mitigation strategies for severe acute respiratory syndrome coronavirus 2 during the pandemic in 2020. A. Hospital-level modifications including changes to the compliment of trainees on the obstetric service (medical students, residents, or fellows), changes in staffing on Labor and Delivery, changes in faculty members on Labor and Delivery (eg, coverage by individuals who do not normally do obstetrics as part of their practices). B. Health care systems modifications including changes in frequency of in-person visits or use of telehealth, changes in frequency of ultrasonograms or antenatal surveillance, implementation of a policy to conserve blood products, and implementation of a policy to limit the number of visitors on Labor and Delivery. C. Community-level modifications including school, restaurant, or business closures, and shelter-in-place orders.
The incidence of the primary composite outcome was 9.3% (95% CI 8.8–9.8) during the pandemic and 8.9% (95% CI 8.3–9.5) before the pandemic. Giving birth during the pandemic was not associated with the primary composite of maternal death or serious morbidity (9.3% vs 8.9%, aRR 1.02, 95% CI 0.93–1.11) (Figure 4, Table 2). There were no maternal deaths in either group which precluded comparison for this component of the primary outcome. In sensitivity analyses that either excluded those with a positive SARS-CoV-2 test or imputed BMI, the results were unchanged (Appendixes 3 and 4, available online at http://links.lww.com/xxx).
Figure 4.
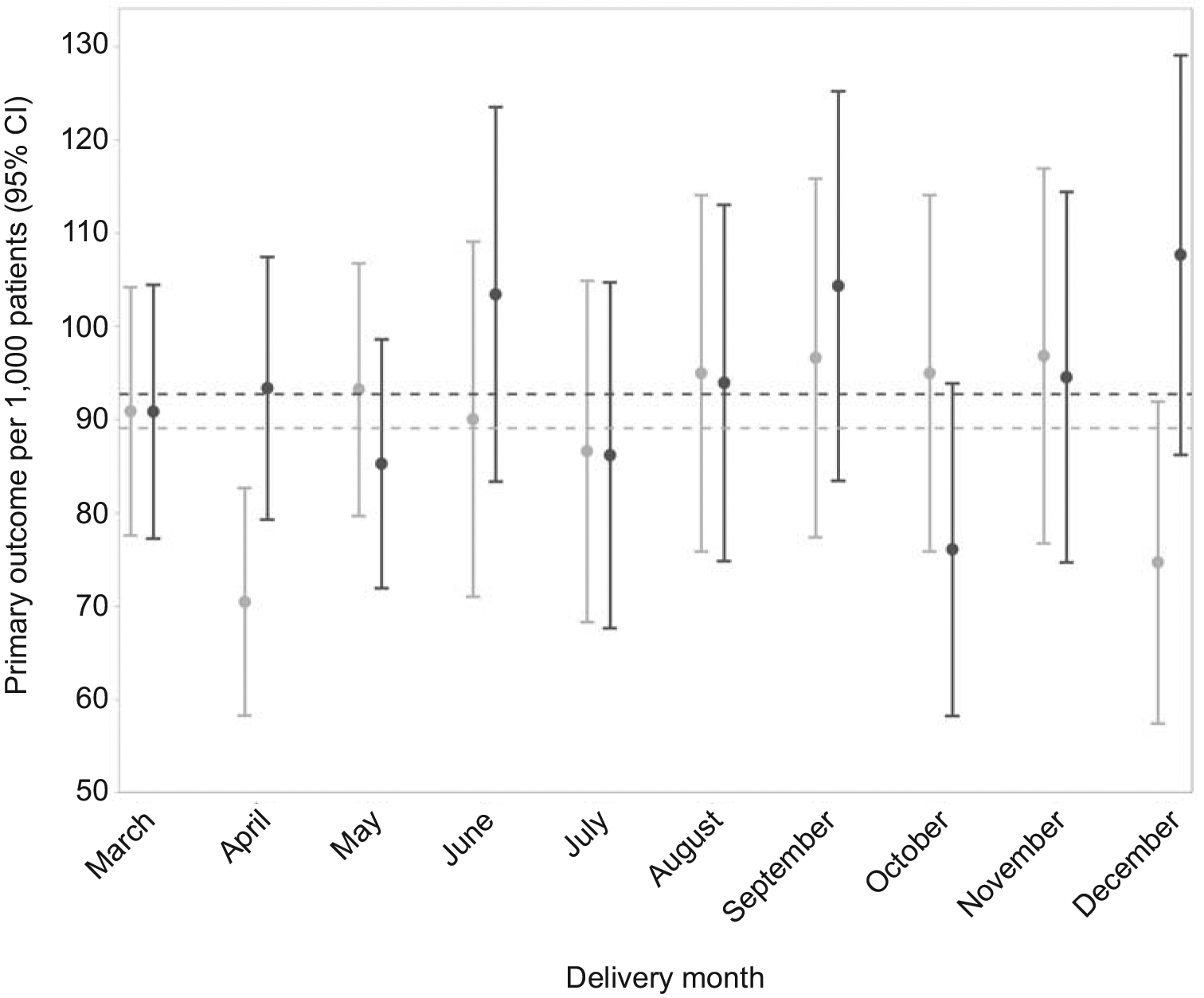
Prevalence of primary composite outcome of maternal death or serious morbidity from common obstetric complications with 95% CIs by month. Before pandemic is represented in light grey and during pandemic is represented in dark grey. Dashed lines denote the overall prevalence of the primary outcome for March through December of each calendar year.
Table 2.
Primary and Secondary Maternal and Neonatal Outcomes
| During Pandemic (n=12133) | Before Pandemic (n=9709) | RR (95% CI) | aRR (95% CI) | |
|---|---|---|---|---|
| Primary Composite of Maternal Death or Serious Morbidity from Common Obstetric Complications | 1125 (9.3) | 865 (8.9) | 1.05 (0.97 – 1.14) | 1.02 (0.93 – 1.11) |
| Death | 0 | 0 | ||
| Hypertensive disorders of pregnancy | 797 (6.6) | 641 (6.6) | 1.00 (0.91 – 1.11) | 0.95 (0.86 – 1.05) |
| Postpartum hemorrhage | 292 (2.4) | 215 (2.2) | 1.10 (0.93 – 1.29) | 1.12 (0.94 – 1.33) |
| Infection | 109 (0.9) | 83 (0.9) | 1.02 (0.78 – 1.33) | 1.04 (0.78 – 1.38) |
| Secondary Maternal Outcomes | ||||
| Cesarean birth | 3930 (32.4) | 3041 (31.3) | 1.04 (0.99 – 1.08) | 1.02 (0.97 – 1.07) |
| ACOG and SMFM definition severe morbidity | 174 (1.4) | 188 (1.9) | 0.71 (0.59 – 0.87) | 0.69 (0.56 – 0.84) |
| ICU admission | 150 (1.2) | 169 (1.7) | 0.68 (0.55 – 0.83) | 0.67 (0.53 – 0.83) |
| Number of ICU days | 2.0 (1.0 – 3.0) | 2.0 (1.0 – 3.0) | −0.1 (−0.2 – 0.1)* | −0.15 (−0.30 – 0.00)* |
| Venous thromboembolism (DVT/PE) | 9 (0.1) | 13 (0.1) | 0.58 (0.26 – 1.32) | |
| Superficial or deep incisional surgical site infection | 30 (0.8) | 18 (0.6) | 1.36 (0.79 – 2.36) | 1.25 (0.70 – 2.22) |
| Number of in-patient hospitalization days | 2.0 (2.0 – 3.0) | 3.0 (2.0 – 3.0) | −0.1 (−0.1 – −0.1)* | −0.09 (−0.11 – −0.08)* |
| Length of stay (days) | 2.0 (2.0 – 3.0) | 3.0 (2.0 – 3.0) | −0.1 (−0.1 – −0.1)* | −0.09 (−0.11 – −0.08)* |
| Neonatal Outcomes | During Pandemic (n=12407) | Before Pandemic (n=9938) | RR (95% CI) | aRR (95% CI) |
| Stillbirth at 20 weeks’ gestation or later | 91 (0.7) | 62 (0.6) | 1.12 (0.84 – 1.51) | 1.05 (0.76 – 1.46) |
| Neonatal death | 70 (0.6) | 69 (0.7) | 0.84 (0.61 – 1.14) | 0.88 (0.62 – 1.25) |
| Live births or stillbirth 20 weeks or later | 12339 | 9895 | ||
| Perinatal preterm composite | 257 (2.1) | 211 (2.1) | 0.98 (0.83 – 1.16) | 1.05 (0.87 – 1.27) |
| Perinatal term composite | 727 (5.9) | 618 (6.2) | 0.94 (0.85 – 1.04) | 0.95 (0.85 – 1.05) |
| Live births | 12248 | 9833 | ||
| NICU admission | 2183 (17.8) | 1901 (19.3) | 0.93 (0.88 – 0.98) | 0.92 (0.87 – 0.98) |
| Number of days in NICU | 7 (3 – 21) | 7 (3 – 20) | 0.01 (−0.07 – 0.09)* | 0.04 (−0.04 – 0.12)* |
RR, relative risk; aRR, adjusted relative risk; CI, confidence interval.
Data are n (%) or median (IQR), unless otherwise specified.
difference in means of the natural-log transform.
Model for primary composite of maternal death or serious morbidity adjusted for MFMU site, maternal age, body mass index, any co-morbidity (asthma/COPD, pregestational diabetes, chronic hypertension), and obstetric history (no prior pregnancy, prior pregnancy without PTB/preeclampsia, or prior pregnancy with PTB/preeclampsia).
Model for cesarean birth adjusted for MFMU site, maternal age, body mass index, any co-morbidity (asthma/COPD, pregestational diabetes, chronic hypertension), and prior delivery route (no prior pregnancy, vaginal only, or cesarean).
All other models adjusted for MFMU site, maternal age, body mass index, and any co-morbidity (asthma/COPD, pregestational diabetes, chronic hypertension).
Giving birth during the pandemic was not associated with cesarean birth (32.4% vs 31.3%, aRR 1.02, 95% CI 0.97–1.07). ACOG and SMFM-defined severe morbidity7 which includes ICU admission (1.4% vs 1.9%, aRR 0.69, 95% CI 0.56–0.84) as well as ICU admission alone (1.2% vs 1.7%, aRR 0.67, 95% CI 0.53–0.83) were less frequent during the pandemic than before the pandemic. Among individuals admitted to the ICU, there was no difference in number of ICU days (median: 2 vs 2 days, adjusted mean difference of log-transform (95% CI): −0.15 (−0.30 – 0.0)). Overall length of hospital stay was shorter during the pandemic than before the pandemic (median: 2 vs 3 days, adjusted mean difference of log-transform (95% CI): −0.09 days (−0.11 – −0.08)).
Neonatal outcomes did not differ for those who gave birth during the pandemic compared with those who gave birth prior to the pandemic (Table 2). In sensitivity analyses that either excluded those with a positive SARS-CoV-2 test or imputed BMI, the results were unchanged (Appendixes 3 and 4, http://links.lww.com/xxx).
There was no significant interaction between race and ethnicity or insurance status and giving birth during the pandemic for the primary outcome. Parity had a significant interaction with giving birth during the pandemic for the composite primary outcome (no prior pregnancy 20 weeks or longer: aRR 0.84, 95% 0.64–1.09; prior pregnancy 20 weeks or longer: aRR 0.51, 95% CI 0.36–0.71) meaning that parous individuals were significantly less likely to experience the composite outcome than nulliparous individuals during versus before the pandemic. Similarly, there was a significant interaction between parity and giving birth during the pandemic for ICU admission (no prior pregnancy 20 weeks or longer: aRR 0.80, 95% 0.60–1.06; prior pregnancy 20 weeks or longer: aRR 0.50, 95% CI 0.35–0.72) meaning that parous individuals were significantly less likely to experience an ICU admission than nulliparous individuals during versus before the pandemic (Appendixes 5–7, available online at http://links.lww.com/xxx).
DISCUSSION
In a multicenter U.S. cohort, we found no association between giving birth during the early COVID-19 pandemic and a composite outcome of maternal death or serious morbidity from common obstetric complications overall when compared with historic controls.
In a prior publication from the NICHD MFMU GRAVID study, SARS-CoV-2 infection in pregnancy was associated with our primary composite outcome of death or serious morbidity.5 It is reassuring to find that in the population overall, similar effects were not observed. The current study included patients with a positive SARS-CoV-2 test who gave birth on randomly selected days in 2020; these individuals accounted for approximately 3% of the cohort who gave birth during the pandemic. Results did not differ when these patients were excluded in sensitivity analyses.
We hypothesized that giving birth during the early COVID-19 pandemic would be associated with serious morbidity from common obstetric complications due to changes in healthcare delivery, delays in presentation to care, and delays in timely intervention in the hospital. Other studies have demonstrated increased rates and severity of diabetic ketoacidosis in children.9,10 Within obstetrics and gynecology, the COVID-19 pandemic has been associated with increased rates of rupture of ectopic pregnancy and lower rates of obstetric and gynecologic emergency department visits early in the pandemic.11,12 It could be that our observed lack of association with serious morbidity and mortality in the obstetric population speaks to the necessity of continuing to operate labor and delivery with relatively normal function even during a pandemic.
The observed lower prevalence of ICU admission in the obstetric population early in the COVID-19 pandemic may reflect decreased ICU bed availability to those with serious illness other than COVID-19. However, we do not have detailed data regarding hospital bed shortages at the individual sites. The association between giving birth during the COVID-19 pandemic and shorter hospital stays has been observed in other studies.14 We followed patients through 42 days postpartum so any increased risk for mortality or serious morbidity associated with these shorter hospital stays would have been identified.
Notably, there were no maternal deaths in our cohort on the randomly selected delivery dates through 42 days postpartum. Given that maternal death is a rare outcome, we did not have a sufficient sample size to examine this component of the composite individually. Larger studies are required to examine maternal mortality at the population level, as there are initial concerning findings for an increase in maternal mortality during the pandemic.2, 15 Our study also does not examine pregnancy-related deaths through 1 year postpartum as recommended by the Centers for Disease Control and Prevention16, as there are cases in which pregnancy initiates a chain of events resulting in death later than our examined window of 42 days postpartum.
Strengths of this study include manual medical record abstraction to evaluate serious morbidity beyond what can be ascertained from billing or diagnostic codes, representation from multiple hospital sites increases generalizability, and the ability to evaluate rare but serious obstetric complications that are clinically meaningful. We were also able to describe the care modifications that occurred during the pandemic across the sites. Finally, we performed sensitivity analyses and demonstrated that our results were robust even when excluding those affected directly by SARS-CoV-2.
The study has several limitations. First, these data were collected early in the pandemic (March-Dec 2020) and we could not evaluate whether there were differences in outcomes for obstetrical patients during subsequent surges of infection in the U.S. However, this was the phase of the most drastic healthcare and community-level modifications across the country and no difference was detected. Second, while the sample size was sufficient for our primary outcome, differences in rare but important outcomes such as maternal death could not be evaluated. Third, most of the participating hospital sites were academic medical centers which limits generalizability. Similarly, most of the MFMU Centers are in the Northeast, and these hospitals may have experienced more disruption to care early in the pandemic when little was known about SARS-CoV-2 transmission. However, we hypothesize this would have increased the likelihood of finding an increase in maternal morbidity, and we did not find one. Finally, the baseline rate of morbidity was higher than anticipated. The study was initially powered to detect a relative 30% increase from a baseline rate of 3%. With the higher baseline rate of 8.9%, with an alpha of 0.05 and our available sample size, we had 90% power to detect a relative increase of 15% (to 10.2%) or relative risk of 1.15.
In summary, no association was found between giving birth during the pandemic and serious maternal morbidity from common obstetric complications. These data are reassuring in that, even during a healthcare crisis, outcomes for individuals giving birth remained similar to those prior to the early COVID-19 pandemic. Nonetheless, investigation of maternal morbidity and mortality during the COVID-19 pandemic in settings outside of the U.S., or in regions of the country that are underrepresented in this cohort including more rural settings, remains critical as we learn from our initial response to COVID-19 globally.
Supplementary Material
Funding:
This work is funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (UG1 HD087230, UG1 HD027869, UG1 HD027915, UG1 HD034208, UG1 HD040500, UG1 HD040485, UG1 HD053097, UG1 HD040544, UG1 HD040545, UG1 HD040560, UG1 HD040512, UG1 HD087192, U10 HD036801) and the National Center for Advancing Translational Sciences (UL1TR001873). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial Disclosure:
Torri D. Metz reports personal fees from Pfizer for her role as a medical consultant for a SARS-CoV-2 vaccination in pregnancy study, grants from Pfizer for role as a site PI for SARS-CoV-2 vaccination in pregnancy study, grants from Pfizer for role as a site PI for RSV vaccination in pregnancy study, and grants from Gestvision for role as a site PI for a preeclampsia study outside the submitted work. Brenna L. Hughes reports personal fees from Merck for her role on a Medical Advisory Board outside of the submitted work. Tracy A. Manuck reports money was paid to her institution from the NIH (NICHD and NIEHS) and the State of North Carolina (PFAST Network Grant). She also received Cefalo Bowes grant funding (local UNC obgyn grant funding) where she was a mentor to fellow physicians. Hyagriv N. Simhan reports that he is an LLC Co-founder of Naima Health and personal fees from UptoDate outside of the submitted work. Cynthia Gyamfi-Bannerman reports receiving payment from Medela and Hologic. Alan T.N. Tita reports grants from CDC and from Pfizer for a COVID-19 in pregnancy trial outside of the submitted work. The other authors did not report any potential conflicts of interest.
Authors’ Data Sharing Statement
Will individual participant data be available (including data dictionaries)? Yes
What data in particular will be shared? De-identified study data will be shared through NICHD Data and Specimen Hub (DASH) following reasonable request within 1 year of study publication.
What other documents will be available? None
When will data be available (start and end dates)? Within 1 year of manuscript publication
By what access criteria will data be shared (including with whom, for what types of analyses, and by what mechanism)? Access to data will follow requirements in place through DASH.
References:
- 1.Woolf SH, Chapman DA, Sabo RT, Zimmerman EB. Excess deaths from COVID-19 and other causes in the US, March 1, 2020 to January 2, 2021. JAMA 2021; 325(17): 1786–9. doi: 10.1001/jama.2021.5199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoyert DL. Maternal mortality rates in the United States, 2020. NCHS Health E-Stats. 2022. DOI: 10.15620/cdc:113967. [DOI] [Google Scholar]
- 3.Metz TD, Collier C, Hollier LM. Maternal mortality from coronavirus disease 2019 (COVID-19) in the United States. Obstet Gynecol 2020; 136(2):313–6. doi: 10.1097/AOG.0000000000004024 [DOI] [PubMed] [Google Scholar]
- 4.Metz TD, Clifton RC, Hughes BL, et al. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID-19). Obstet Gynecol 2021; 137(4):571–80. doi: 10.1097/AOG.0000000000004339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metz TD, Clifton RG, Hughes BL, et al. Association of SARS-CoV-2 Infection with Serious Maternal Morbidity and Mortality from Obstetric Complications. JAMA 2022; 327(8):748–59. doi: 10.1001/jama.2022.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID Multinational Cohort Study. JAMA Pediatr 2021;175:817–826. doi: 10.1001/jamapediatrics.2021.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Severe maternal morbidity: screening and review. Obstetric Care Consensus No. 5. American College of Obstetricians and Gynecologists. Obstet Gynecol 2016; 128: e54–60. doi: 10.1016/j.ajog.2016.07.050 [DOI] [PubMed] [Google Scholar]
- 8.Petersen EE, Davis NL, Goodman D, et al. Racial/Ethnic Disparities in Pregnancy-Related Deaths – United States, 2007–16. MMWR Morb Mortal Weekly Rep 2019;68(35):762–5. doi: 10.15585/mmwr.mm6835a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dzygalo K, Nowaczyk J, Szwilling A, Kowalska A. Increased frequency of severe diabetic ketoacidosis at type I diabetes onset among children during COVID-19 pandemic lockdown: an observational cohort study. Pediatr Endocrinol Diabetes Metab 2020; 26(4):167–75. doi: 10.5114/pedm.2020.101003 [DOI] [PubMed] [Google Scholar]
- 10.McGlacken-Byrne SM, Drew SEV, Turner K, Peters C, Amin R. The SARS-CoV-2 pandemic is associated with increased severity of presentation of childhood onset type 1 diabetes mellitus: A multi-centre study of the first COVID-19 wave. Diabet Med 2021; 38(9): e14640. doi: 10.1111/dme.14640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toma H, Bank TC, Hoffman MK. Care for women with ectopic pregnancies during the coronavirus disease 2019 (COVID-19) pandemic. Obstet Gynecol 2021; 137(6):1041–2. Doi: 10.1097/AOG.0000000000004392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abel MK, Alavi M, Tierney C, Weintraub M, Ritterman M, Avins A, Zaritsky E. Coronavirus disease 2019 (COVID 19) and the incidence of obstetric and gynecologic emergency department visits in an integrated healthcare system. Obstet Gynecol 2021; 137(4):581–3. doi: 10.1097/AOG.0000000000004331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grobman WA, Bailit JL, Rice MM, et al. Can differences in obstetric outcomes be explained by differences in the care provided? The MFMU Network APEX study. Am J Obstet Gynecol 2014; 211(2):147.e1–147.e16. doi: 10.1016/j.ajog.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Handley S, Gallagher K, Lindgren E, et al. Postpartum length of stay and hospital readmission before and during the coronavirus disease 2019 (COVID-19) pandemic. Obstet Gynecol 2022; 139(3):381–90. doi: 10.1097/AOG.0000000000004687. [DOI] [PubMed] [Google Scholar]
- 15.Molina RL, Tsai TC, Dai D, et al. Comparison of pregnancy and birth outcomes before vs during the COVID-19 pandemic. JAMA Netw Open 2022; 5(8):e2226531. doi: 10.1001/jamanetworkopen.2022.26531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention, Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion. Preventing pregnancy-related deaths. https://www.cdc.gov/reproductivehealth/maternal-mortality/preventing-pregnancy-related-deaths.html. Accessed August 20, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Will individual participant data be available (including data dictionaries)? Yes
What data in particular will be shared? De-identified study data will be shared through NICHD Data and Specimen Hub (DASH) following reasonable request within 1 year of study publication.
What other documents will be available? None
When will data be available (start and end dates)? Within 1 year of manuscript publication
By what access criteria will data be shared (including with whom, for what types of analyses, and by what mechanism)? Access to data will follow requirements in place through DASH.


