Abstract
Objectives
Although long COVID-19 is widely recognized in adults, less information is available about this condition in children, especially in developing countries. Here, we studied the long-term symptoms of SARS-CoV-2 infection beyond 3 months and the associated risk factors in a pediatric population.
Methods
This observational study included 639 Argentinian children and adolescents with previously confirmed COVID-19 from June 2020-June 2021 and 577 children without previous COVID-19. Parents completed a survey about symptoms that their child had for >3 months after the diagnosis of SARS-CoV-2 infection.
Results
At least one persistent symptom was observed more frequently in children with previous COVID-19 than in the non-COVID-19 group (34% vs 13%, P <0.0001). SARS-CoV-2 infection increased the risk of headache, dizziness, loss of taste, dyspnea, cough, fatigue, muscle pain, and loss of weight by three- to seven-fold. The loss of smell was only reported in infected children. After controlling for the other variables, older age, symptomatic COVID-19, and comorbidities were independent predictors of long-term symptoms.
Conclusions
One-third of children experienced persistent symptoms after COVID-19. Older age, symptomatic infection, and comorbidities were shown to be risk factors for long COVID-19. Pediatric long COVID-19 is a new condition that requires further investigation.
Keywords: Child, SARS-CoV-2, Long COVID-19, Comorbidity
INTRODUCTION
Almost 600 million people have been infected with SARS-CoV-2 since March 2020 worldwide. Pediatric COVID-19 accounts for 2-7% of the total confirmed cases [1,2]. Based on the prevalence reported thus far, there could be 100 million people now living with persistent symptoms after COVID-19 [3], [4], [5].
During the 1st year of the pandemic, the focus was primarily aimed at the acute phase of infection with SARS-CoV-2. Then, there was growing recognition that the COVID-19 pandemic has left a significant proportion of the population experiencing symptoms in the long-term. The survivors of the SARS epidemic of 2003 and the Middle East respiratory syndrome coronavirus outbreak of 2012 have already demonstrated a constellation of persistent symptoms, reinforcing the concerns for the significant sequelae of COVID-19 [6,7].
Most of our current insights into long COVID-19 come from adults who have recovered from symptomatic SARS-CoV-2 infection [8], [9], [10], [11], [12], [13]. However, long-term consequences can occur after SARS-CoV-2 infection in children, even in previously asymptomatic patients [14]. Long COVID-19 is a heterogeneous syndrome for which a precise definition remains lacking. The underlying mechanisms remain to be clearly established [12,15]. Post-COVID-19 condition or long COVID-19 occurs in children and young people with a history of SARS-CoV-2 infection, with one or more persistent physical symptoms for a minimum duration of 12 weeks after acute infection that cannot be explained by an alternative diagnosis. The symptoms impact everyday functioning and may fluctuate or relapse over time [16]. The CLoCk study suggests that, in the United Kingdom alone, tens of thousands of children and young people might have long COVID-19 [17]. A recent meta-analysis [18] including 21 studies of long COVID-19 in children reported a prevalence of long COVID-19 in 25% of the study population. Most of these studies were carried out in high-income countries (18 of 21 were from Europe), and only one study was performed in Latin America, which included 53 Brazilian children [19].
This study aimed to determine the long-term symptoms of SARS-CoV-2 infection beyond 3 months and the associated risk factors in 639 children and adolescents (COVID-19 group) in Argentina. The non-COVID-19 group included 577 children without a history of SARS-CoV-2 infection. We also focused on the differences between subgroups defined by age, sex, presence of underlying diseases, presence of symptoms, and severity during acute COVID-19.
METHODS
Study population
Children and adolescents with or without a history of SARS-CoV-2 infection admitted to the Hospital General de Niños Pedro de Elizalde, Hospital Universitario Austral, Hospital Dr. Salvador Mazza, Hospital Pediátrico Juan Pablo II, and Policlínico Regional Juan Domingo Perón between June 2020 and June 2021 were invited to participate. Two pediatric cohorts were enrolled in this observational study. The first cohort (“COVID-19”) consisted of 664 children with polymerase chain reaction-confirmed SARS-CoV-2 infection, aged between 1 and 17 years. A total of 639 children were finally included in the analysis. They were initially admitted to the previously mentioned hospitals during the course of acute infection and enrolled 6 months later. The severity of acute COVID-19 was assessed according to the World Health Organization (WHO) criteria. Asymptomatic patients were those who remained without symptoms throughout the infection. Mild patients showed only nonspecific symptoms (fever, fatigue, headache, myalgia, cough, and diarrhea) with no evidence of pneumonia or hypoxia. Moderate patients presented with nonsevere pneumonia (cough or fast breathing and/or chest indrawing). Severe children had severe pneumonia (difficulty breathing and respiratory distress) and/or lethargy and convulsions. Children with multisystem inflammatory syndrome in children (MIS-C) diagnosis and those with incomplete data were excluded. The second cohort (“non-COVID-19”) consisted of 751 children without a history of COVID-19 disease who were age- and sex-matched and enrolled in the same participant hospitals. A total of 577 children were finally included in the analysis. Children with the following criteria were included in the non-COVID-19 group: (i) children who tested negative for SARS-CoV-2, presented with acute symptoms, and/or were in close contact with a COVID-19 case; (ii) nontested children reporting neither symptoms nor history of school or household close contact assisted for well-child visits in some of the participating hospitals. Lockdown measures were implemented in Argentina from March 2020 until December 2020, including school closure. All children from both cohorts were unvaccinated because pediatric COVID-19 vaccines were not approved in our country at the time of the survey. Parental perception of changes in children's emotional, behavioral, and health status in the 6 months after diagnosis in the COVID-19 group or in the 6 months before the survey in the non-COVID-19 group was assessed between January and June 2021. The children's evaluations were carried out on a single occasion.
Questionnaire
The questionnaire was adapted from the WHO case report form for post-COVID-19 conditions for adults [20]. Participating parents completed online surveys. The questionnaire consisted of several questions with mandatory responses (Supplementary Table 1). The first part of the survey included demographic data, date of COVID-19 diagnostic confirmation, acute COVID-19 symptoms and severity, and comorbidities. The last part of the survey asked for persistent or newly developed symptoms that lasted for more than 3 months after COVID-19 diagnosis and were not explained by other reasons. Considering that the young age of patients could affect the parental perception of some symptoms, such as anxiety, mood swings, depression, headache, dizziness, loss of smell, loss of taste, forgetfulness, and concentration difficulties, only children aged ≥5 years were screened for these symptoms. Parents or legal guardians of children gave full written informed consent to participate in the study. This study was conducted in accordance with the Declaration of Helsinki and the institutional review board approved the study.
Statistical analysis
Descriptive statistics were calculated for baseline characteristics. Continuous variables are summarized as medians (with interquartile ranges), and categorical variables are summarized as frequencies (with percentages). Two groups were compared using Fisher's exact test. The data were further analyzed by fitting logistic regression models for persistent symptoms that were statistically significant in the univariate analysis comparing patients with COVID-19 and non-COVID-19 after adjusting for age, sex, presence of comorbidities, and presence of symptoms during the acute phase of COVID-19. The results are reported as unadjusted odds ratios (ORs) and adjusted ORs (aORs) with 95% confidence intervals. An upset plot was used to present the coexistence of persistent symptom categories. To identify children at higher risk of long COVID-19, a logistic regression was conducted considering age, sex, categories of disease severity, presence of symptoms at acute COVID-19, and different comorbidities as predictor variables. A P-value <0.05 was considered statistically significant. The statistical analyses were performed with GraphPad Prism v.8 (GraphPad Software) and R (version R-3.6.1) using the packages ggplot2 and UpSetR.
RESULTS
Characteristics of the study cohorts
A total of 1415 children and adolescents with and without previous COVID-19 agreed to participate in this study. The flowchart of enrollment is shown in Supplementary Figure 1. A total of 639 children with COVID-19 were finally included in the analysis. The median age and interquartile range of the COVID-19 cohort was 7 years (3-12), and 47% (n = 298) were girls. All had polymerase chain reaction-confirmed SARS-CoV-2 infection. A total of 80% were tested because they had COVID-19 symptoms (n = 512), whereas 20% were tested because they were identified having been in close contact with someone who had COVID-19 (n = 127). A total of 71% of children (n = 454) had no comorbidities, with respiratory disease (mainly asthma) being the most prevalent underlying problem (13%, n = 80). The cohort included patients with asymptomatic (20%, n = 127), mild (70%, n = 447), moderate (8%, n = 49), and severe (2%, n = 16) disease. Eight of the 639 previously infected children required pediatric intensive care unit admission during acute COVID-19.
The non-COVID-19 group included 577 children. The median age and interquartile range was 8 years (5-12), and 48% (n = 277) were girls. A total of 86% percent of the patients in the non-COVID-19 cohort (n = 495) had no comorbidities, with respiratory disease (again, mainly asthma) being the most prevalent underlying condition (8%, n = 45). The characteristics of both cohorts are summarized in Table 1 .
Table 1.
Characteristics of the children with or without COVID-19 included in the study.
| COVID-19 (N = 639) | Non-COVID-19 (N = 577) | |
|---|---|---|
| Age (median, interquartile range) | 7 (3-12) | 8 (5-12) |
| Female, n (%) | 298 (47) | 277 (48) |
| Comorbidities, n (%) | ||
| None | 454 (71) | 495 (86) |
| Prematurity | 34 (5) | 18 (3) |
| Respiratory disease | 80 (13) | 45 (8) |
| Heart disease | 20 (3) | 9 (2) |
| Renal disease | 12 (2) | 2 (0.3) |
| Neurological disease | 42 (7) | 10 (2) |
| Obesity | 23 (4) | 8 (1) |
| Undernutrition | 10 (2) | 0 |
| Cancer | 13 (2) | 1 (0.2) |
| Diabetes | 9 (1) | 2 (0.3) |
| Genetic disorder | 8 (1) | 1 (0.2) |
| Immunodeficiency | 10 (2) | 3 (0.5) |
| Othera | 4 (0.6) | 4 (0.7) |
| Acute COVID-19 severity, n (%)b | ||
| Asymptomatic | 127 (20) | - |
| Mild | 447 (70) | - |
| Moderate | 49 (8) | - |
| Severe | 16 (2) | - |
| Pneumonia, n (%) | 32 (5) | 0 |
| O2 requirement, n (%) | 50 (8) | 0 |
| Pediatric intensive care unit admission, n (%) | 8 (1) | 0 |
| Persistence of COVID-19 symptoms, n (%) | ||
| None or symptoms <3 months | 420 (66) | 503 (87) |
| Symptoms >3 months | 219 (34) | 74 (13) |
Other comorbidities included autoimmunity and tuberculosis.
Disease severity of COVID-19 was assessed according to the criteria from World Health Organization.
Evaluation of persistent symptoms after COVID-19
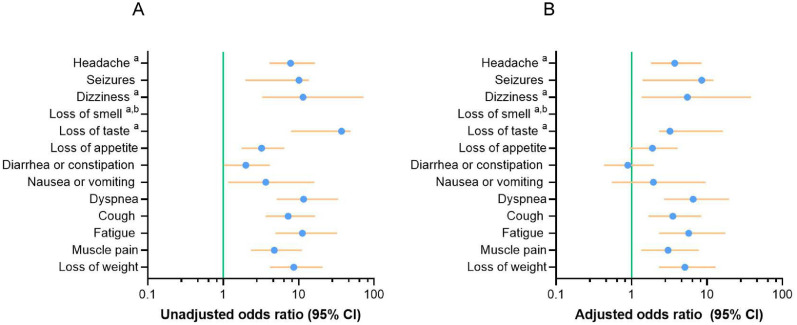
A higher proportion of the parents of children with previous COVID-19 (34%, n = 219) reported that their children remained unwell and had at least one persistent symptom for more than 3 months, unlike the perception of parents of the non-COVID-19 cohort (13%, n = 74, P <0.0001; Table 1). Symptoms were grouped into different categories, such as mental, neurological, sensory, cognition, gastrointestinal, respiratory, cardiologic, and others, as described elsewhere [21]. Anxiety, mood swings, depression, headache, dizziness, loss of smell, loss of taste, forgetfulness, and concentration difficulties were screened only in children aged ≥5 years (COVID-19, n = 388 and non-COVID-19, n = 477). A total of 13 of the 19 symptoms analyzed were significantly more prevalent in the COVID-19 group (n = 639) than in the non-COVID-19 group (n = 577, Table 2 ). Neurological symptoms were more common in the COVID-19 group than in the non-COVID-19 group: headache (59/388 vs 10/477, P <0.001), seizures (11/639 vs 1/577, P <0.007), and dizziness (19/388 vs 2/477, P <0.001). The differences in sensory problems between the COVID-19 and non-COVID-19 groups involved loss of taste (30/388 vs 1/477, P <0.001) and loss of appetite (41/639 vs 12/577, P <0.001). Persistent loss of smell was only reported in the COVID-19 group (33/388 vs 0/477, P <0.001). The differences in gastrointestinal conditions between the COVID-19 and non-COVID-19 groups involved diarrhea or constipation (26/639 vs 12/577, P <0.049) and nausea or vomiting (12/639 vs 3/577, P <0.038). The differences in respiratory conditions between the COVID-19 and non-COVID-19 groups included dyspnea (26/639 vs 5/577, P <0.001) and cough (71/639 vs 8/577, P <0.001). Finally, the differences in fatigue (56/639 vs 5/577, P <0.001), muscle pain (49/639 vs 8/577, P <0.001), and loss of weight (51/639 vs 7/577, P <0.001) between the COVID-19 and the non-COVID-19 groups were also found. In short, we found that 13 of the 19 symptoms analyzed were significantly more prevalent in the COVID-19 group, including neurological, sensorial, and respiratory symptoms, as well as fatigue, muscle pain, and weight loss (Table 2). To further analyze the relationship between persistent symptoms and different variables, such as age, sex, comorbidities, and symptomatic COVID-19, we conducted an adjusted logistic regression model for the 13 persistent symptoms that were significantly different between COVID-19 and non-COVID-19 patients. This analysis included group, age, sex, presence of any comorbidity, and presence of symptoms at the acute phase of infection as predictor variables. We observed that eight persistent symptoms were independently associated with previous COVID-19. The differences in neurological symptoms included headache (aOR 3.74 [1.81-8.46], P <0.001) and dizziness (aOR 5.50 [1.36-38.25], P <0.037). The differences in sensory problems included loss of taste (aOR 3.23 [2.31-16.24], P <0.019). Logistic regression analysis regarding loss of smell was not possible because it was only reported among children with COVID-19. The differences in respiratory symptoms included dyspnea (aOR 6.53 [2.70-19.60], P <0.001) and cough (aOR 3.52 [1.67-8.42], P <0.002). The differences in fatigue (aOR 5.72 [2.32-17.46], P <0.001), muscle pain (aOR 3.05 [1.34-7.78], P <0.012) and loss of weight (aOR 5.09 [2.31-12.96], P <0.001) were also found as shown in Figure 1 and Supplementary Table 2.
Table 2.
Persistent symptoms lasting at least 3 months reported in children with or without previous SARS-CoV-2 infection.
| COVID-19 (N = 639) | Non-COVID-19 (N = 577) | P-valueb | |
|---|---|---|---|
| Mental health | |||
| Anxietya | 34 (9) | 54 (12) | 0.142 |
| Mood swingsa | 29 (7) | 50 (11) | 0.076 |
| Depressiona | 26 (7) | 32 (7) | 0.999 |
| Neurological | |||
| Headachea | 59 (15) | 10 (2) | <0.001 |
| Seizures | 11 (2) | 1 (0.2) | 0.007 |
| Dizzinessa | 19 (5) | 2 (0.4) | <0.001 |
| Sensory problems | |||
| Loss of smella | 33 (9) | 0 | <0.001 |
| Loss of tastea | 30 (8) | 1 (0.2) | <0.001 |
| Loss of appetite | 41 (6) | 12 (2) | <0.001 |
| Cognition | |||
| Forgetfulnessa | 17 (4) | 20 (4) | 0.999 |
| Difficulty concentrationa | 29 (7) | 38 (9) | 0.612 |
| Gastrointestinal | |||
| Diarrhea or constipation | 26 (4) | 12 (2) | 0.049 |
| Nausea or vomiting | 12 (2) | 3 (0.5) | 0.038 |
| Respiratory | |||
| Dyspnea | 26 (4) | 5 (1) | <0.001 |
| Cough | 71 (11) | 8 (1) | <0.001 |
| Cardiologic | |||
| Palpitations | 18 (3) | 13 (2) | 0.588 |
| Fatigue | 56 (9) | 5 (1) | <0.001 |
| Muscle pain | 49 (8) | 8 (1) | <0.001 |
| Loss of weight | 51 (8) | 7 (1) | <0.001 |
Anxiety, mood swings, depression, headache, dizziness, loss of smell, loss of taste, forgetfulness, and difficulty concentrating were requested only in children ≥5 years (cases, n = 388 and controls, n = 447).
Fisher's exact test. N (%) are shown.
Figure 1.
Forest plots showing the unadjusted and adjusted ORs of symptoms lasting at least 3 months. Unadjusted (a) and adjusted ORs (b) for cohort, age, sex, presence of any comorbidity, and presence of symptoms among COVID-19 and non-COVID-19 (n = 1216) is shown.
Abbreviation: OR, odds ratio.
Cohorts were coded as follows: 0 = non-COVID-19, 1 = COVID-19. The dots indicate the OR and the horizontal lines indicate the lower and upper limits of the 95% confidence interval.
aHeadache, dizziness, loss of smell, and loss of taste were requested only in children ≥5 years (n = 835).
bLogistic regression analysis was not possible for loss of smell, which was only reported among COVID-19 children.
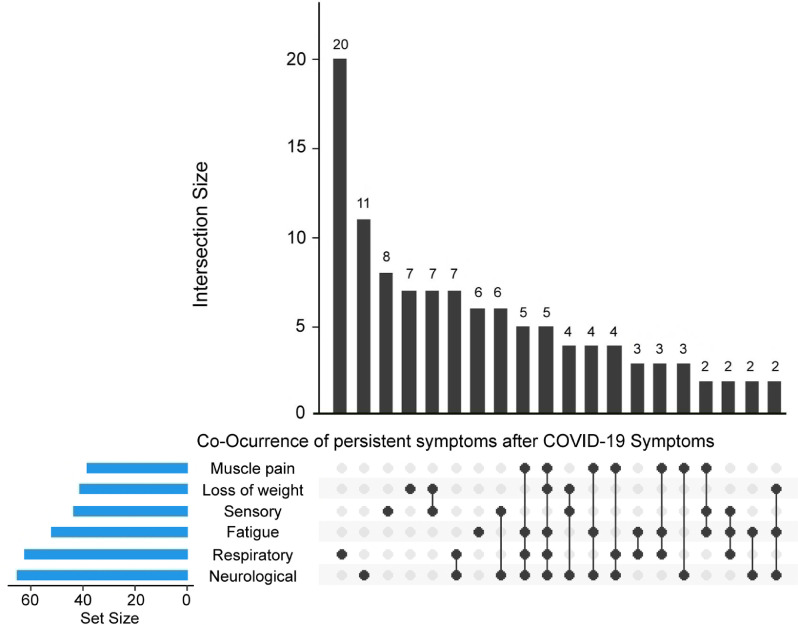
Subsequently, we studied the coexistence of different categories of persistent symptoms in children aged ≥5 years with COVID-19, who were able to report all the studied symptoms. A total of 61% (n = 83) of these children reported persistent symptoms from more than one category. The most commonly co-occurring categories were loss of weight and sensory symptoms in 5% (n = 7) of children, whereas respiratory and neurological symptoms were present in 4% (n = 6) of children. A total of 35% (n = 48) of children had persistent symptoms from three or more different categories. The coexistence of persistent symptom categories is presented in Figure 2 .
Figure 2.
Upset plot showing the coexistence of symptoms categories lasting at least 3 months in children with previous COVID-19 ≥5 years. Each bar (intersection size) shows the number of children who reported some particular category symptom or combination of categories (neurological, respiratory, sensory, fatigue, muscle pain, and loss of weight). Underneath it is a graphical table showing what those combinations are. The black dots and lines show the combination of symptoms that make up each cluster or subset of symptoms. The smaller bar chart (set size) to the left of the graphical table shows the overall size of each category. The top 20 intersections are shown.
Identification of children at higher risk of persistent symptoms after COVID-19
To identify potential risk factors for developing persistent symptoms in the COVID-19 group, we performed a logistic regression analysis focused on age, sex, categories of disease severity; presence of symptoms in the course of acute COVID-19; respiratory, heart, renal, or neurologic disease; prematurity; cancer; diabetes; immunodeficiency; obesity; undernutrition and genetic disorders as predictor variables. This logistic regression model showed that older age (B = 0.102, P <0.001); presence of symptoms in the course of acute infection (B = 1.591, P <0.013); and underlying diseases, such as respiratory (B = 0.847, P <0.005) or renal diseases (B = 1.366, P <0.043) and diabetes (B = 2.034, P <0.031), were significantly associated with the persistence of symptoms lasting at least 3 months after COVID-19. Disease severity was not a significant predictor variable of persistent symptoms. In fact, we did not find differences between asymptomatic vs mild (P = 0.663), vs moderate (P = 0.930), or vs severe disease (P = 0.859), as shown in Table 3 .
Table 3.
Predictor variables for developing persistent symptoms lasting at least 3 months in children with COVID-19.
| B coefficient | Standard error | z-value | P-value | |
|---|---|---|---|---|
| Intercept | -2.157 | 0.322 | -6.702 | <0.001 |
| Age (years) | 0.102 | 0.019 | 5.468 | <0.001 |
| Gender (female/male) | -0.351 | 0.196 | -1.797 | 0.072 |
| Severitya | ||||
| Mild (no/yes) | -0.262 | 0.601 | -0.436 | 0.663 |
| Moderate (no/yes) | -0.060 | 0.682 | -0.088 | 0.930 |
| Severe (no/yes) | 0.145 | 0.812 | 0.178 | 0.858 |
| Presence of symptoms (no/yes) | 1.591 | 0.642 | 2.475 | 0.013 |
| Comorbidities (no/yes) | ||||
| Prematurity | 0.305 | 0.415 | 0.734 | 0.463 |
| Respiratory disease | 0.847 | 0.300 | 2.826 | 0.005 |
| Heart disease | 0.858 | 0.547 | 1.568 | 0.117 |
| Renal disease | 1.366 | 0.675 | 2.025 | 0.043 |
| Neurological disease | 0.313 | 0.396 | 0.790 | 0.430 |
| Obesity | 0.420 | 0.493 | 0.853 | 0.394 |
| Undernutrition | -2.298 | 1.198 | -1.918 | 0.055 |
| Cancer | -0.117 | 0.880 | -0.189 | 0.850 |
| Diabetes | 2.034 | 0.945 | 2.152 | 0.031 |
| Genetic disorder | 0.769 | 0.894 | 0.869 | 0.390 |
| Immunodeficiency | 0.349 | 0.874 | 0.399 | 0.690 |
Logistic regression test using different predictor variables in children with COVID-19. Data were coded as follows: age (as numeric variable, grand mean-centered), sex (0 = female, 1 = male), mild (0 = no, 1 = yes), moderate (0 = no, 1 = yes), severe (0 = no, 1 = yes), presence of symptoms at acute COVID-19 (0 = no, 1 = yes), presence of prematurity (0 = no, 1 = yes), respiratory disease (0 = no, 1 = yes), heart disease (0 = no, 1 = yes), renal disease (0 = no, 1 = yes), neurological disease (0 = no, 1 = yes), obesity (0 = no, 1 = yes), undernutrition (0 = no, 1 = yes), cancer (0 = no, 1 = yes), diabetes (0 = no, 1 = yes), genetic disorder (0 = no, 1 = yes), immunodeficiency (0 = no, 1 = yes). Estimated coefficients (B) and their standard errors, z-value and P-value are listed. a Asymptomatic category was used as reference variable.
DISCUSSION
Although long COVID-19 is widely recognized in adults [8,9,12,13], its relevance in children remains to be established. Early reports from Buosenso (Italy) [14] and Say (Australia) [22] suggested that children with asymptomatic or mild COVID-19 could develop persisting symptoms; although, these children were followed up for a relatively short time after diagnosis. Ludvigsson et al. (Sweden) [2] published a systematic review indicating that children display long COVID-19 symptoms similar to those reported in adults. Conversely, Denina et al. (Italy) [23], described a small group of hospitalized children with COVID-19 displaying no long‐term sequelae. In addition, six [17,19,[24], [25], [26], [27]] of eight previous studies on long COVID-19 in children and adolescents that included a control group performed in Brazil, Denmark, and the United Kingdom found a higher frequency and persistence of symptoms in previously SARS-CoV-2 infected children than in the control group, whereas another two studies from Germany and Switzerland reported no differences between groups [28,29]. A recent review by Lopez-Leon et al. [18] showed that the prevalence of long COVID-19 in children was 25%, revealing important differences between the studies. The most prevalent clinical manifestations reported were mood symptoms, fatigue, and sleeping disorders. Compared with uninfected children, SARS-CoV-2-infected children had a higher risk of persistent dyspnea, anosmia, ageusia, and fever. Conversely, Buonsenso et al. [30], analyzed long COVID-19 in adults and children living in the same household in Italy. With a median follow-up post-SARS-CoV-2 acute infection of 77 days, the authors reported that 67% of adults and 32% of children showed at least one persistent symptom, concluding that children can experience long COVID-19, although less frequently than cohabitant adults. Notably, most of the previous studies focused on pediatric long COVID-19 were carried out in high-income countries (18 of 21 were from Europe), but only one study, which included 53 symptomatic Brazilian children, was performed in Latin America [19]. This prospective study found that 23% of children had at least one symptom lasting >12 weeks. The most frequently reported symptoms at the longitudinal follow-up visit were headache (19%), tiredness (9%), dyspnea (8%), and concentration difficulty (4%).
In this study, we found that ∼30% of the children from our cohort of children with COVID-19 had persistent symptoms for more than 3 months after their initial illness, with headache, cough, and fatigue being the most common symptoms in comparison with children in the non-COVID-19 cohort. These observations are in agreement with those described by other reports in children and adults [5,13,[25], [26], [27],31]. Our study showed that a previous SARS-CoV-2 infection increased the risk of nine persistent symptoms lasting more than 3 months by three- to seven-fold, specifically, neurological (headache and dizziness) and sensory conditions (loss of smell and loss of taste), respiratory symptoms (dyspnea and cough), fatigue, muscle pain, and loss of weight. Sensory symptoms affecting smell were reported exclusively in children with COVID-19. Moreover, we found that more than 50% of the children with long COVID-19 showed two or more categories of persistent symptoms at the time of enrollment. In line with our results, Zavala et al. [27] also identified nine prevalent symptoms in children with long COVID-19 that clustered into three categories: mental health, neurologic, and sensory conditions. Similarly, Osmanov et al. [21] showed that almost one in ten children had multiple symptoms, with two or more categories of persistent symptoms present at the time of the follow-up. Moreover, a recent retrospective study highlighted that chronic anosmia after COVID-19 can significantly affect children's eating habits and everyday activities [32]. Finally, although no substantial differences were identified between results reported in low- and high-income countries, the obtained results do provide a sense of the scale of the problem. The long-term impact of this pandemic will especially affect the quality of life of children from low-income countries, children from minorities, and children living with disabilities who will require an additional support.
We analyzed not only physical symptoms but also emotional or behavioral changes. No differences were observed between the COVID-19 and non-COVID-19 groups for any mental health symptom. The symptoms included anxiety, mood swings, and depression. Approximately one in ten parents noticed changes in the mental health of their children in both cohorts, which could be attributed, at least in part, to the prolonged period of isolation imposed in our country. The lockdown measures were implemented in Argentina from March 2020 until December 2020.
Older age and male sex are well-established risk factors for severe COVID-19 outcomes in adults. Various pre-existing conditions have also been associated with an increased risk of severe disease [11,33]. Looking at risk factors for long COVID-19 in children, we observed that the risk of developing persistent symptoms increases with age. Our multivariate logistic regression analysis also showed that both the presence of symptoms at the acute phase of the infection and comorbidities, such as respiratory or renal diseases and diabetes, were also predictor variables significantly associated with a higher risk of developing long-term symptoms. Our results are in line with a previous report showing that being older than 6 years and presenting with allergic diseases are risk factors for long COVID-19 [21]. The mechanistic basis for long COVID-19 in children and adults remain to be elucidated, but the presence of minuscule clots, viral reservoirs, or dysregulated immune responses have been proposed as possible contributors [15,34]. Moreover, our results suggest that a subgroup of children who experienced SARS-CoV-2 infection should be specifically monitored after diagnosis. In agreement with Buosenso et al. and Erol et al. [14,35], we also found that the severity of acute infection was not associated with a higher risk of long-term COVID-19.
Most long COVID-19 studies have focused on clinical manifestations, some of which are difficult to standardize. Therefore, research needs to be expanded to characterize the underlying mechanisms responsible for this condition. In this regard, evidence of lung perfusion defects and ongoing inflammation [36], as well as evidence of orbitofrontal cortex hypometabolism associated with persistent neuropsychiatric symptoms after SARS-CoV-2 infection, have been reported [37]. Likewise, the presence of perinuclear antineutrophil cytoplasmic antibodies and abnormal D-dimer levels have been associated with long COVID-19 [38,39].
Our study has several limitations. Different symptoms appear to be related to pediatric long COVID-19 [40]. We included symptoms listed in the validated case report form for adult post-COVID-19 conditions from the WHO. However, the list of symptoms we used was not complete. Moreover, all enrolled children were interviewed just once, and the retrospective recall up to 6 months might have caused recall bias. As expected, parents showed imprecision in answering some questions, such as those related to changes in sensory symptoms in very young children and those related to some neurological symptoms. In addition, we did not perform serological studies in the children included in the non-COVID-19 group; therefore, we cannot rule out that some have experienced asymptomatic SARS-CoV-2 infection. Although our adjusted logistic regression model included the presence of comorbidities as a predictor variable, it did not evaluate the influence of each particular comorbidity. The incidence of certain comorbidities, such as immunodeficiencies, cancer, and obesity, was markedly higher in the COVID-19 cohort, whereas the incidence of other comorbidities showed more modest differences. This issue, together with the complexity of the epidemiological scenario, could have introduced some undetected bias that may not have been controlled in our model. Finally, all the participants in our cohorts were recruited and followed up before the emergence of the Omicron variant, which is widely circulating today. The frequency and severity of long COVID-19 associated with Omicron infection remain to be analyzed.
Tens of millions of children have been infected with SARS-CoV-2. Even considering the low incidence of long COVID-19 in children, millions of children experience and will experience its consequences. This situation should be considered, particularly in developing countries with strong disparities in access to effective health care. A clear definition and accurate data on the long-term effects associated with COVID-19 in children and adolescents are urgently needed to ensure an adequate diagnosis, treatment, and follow-up for a multiplicity of clinical manifestations associated with this new disease.
Declaration of competing interest
The authors have no competing interest to declare.
Acknowledgments
Funding
This work was supported by grants from the National Agency for Promotion of Science and Technology, Argentina (PMO BID PICTO 2021-0007 and PMO BID PICT 2018-02548 to L.A.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethical approval statement
This study was conducted in accordance with the Declaration of Helsinki. The institutional review board approved the study (Hospital General de Niños Pedro de Elizalde #1226/20, Hospital Universitario Austral #P21-064 and IATIMET Universidad de Buenos Aires #1.0/150621). This study followed the strengthening the reporting of observational studies in epidemiology guidelines for cohort studies.
Acknowledgments
The authors express their sincerest thanks to all the participating children and their families.
Author contributions
Conception and design: V.S., S.R., F.F., J.G., L.A.; Enrollment of subjects and acquisition of data: S.R., M.B., L.H., M.U., M.M.P., C.CH., C.E., L.S.A., M.L., L.D.M., C.D., S.H.A., L.M., L.S., M.G., C.R., I.S.; Analysis and interpretation of data: V.S., J.M.G.P., M.A.P., N.L., R.M., J.G., L.A.; Drafting the article for important intellectual content: V.S., F.F., J.G., L.A.; All authors contributed, verified the underlying data, and approved the submitted version of the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2023.01.031.
Appendix. Supplementary materials
References
- 1.Idele P, Anthony D, You D, Luo C, Mofenson L. The evolving picture of SARS-CoV-2 and COVID-19 in children: critical knowledge gaps. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2020-003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ceban F, Ling S, Lui LMW, Lee Y, Gill H, Teopiz KM, et al. Fatigue and cognitive impairment in post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav Immun. 2022;101:93–135. doi: 10.1016/j.bbi.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cutler DM. The costs of long COVID. JAMA Health Forum. 2022;3 doi: 10.1001/jamahealthforum.2022.1809. [DOI] [PubMed] [Google Scholar]
- 5.Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: A meta-analysis and systematic review. J Infect Dis. 2022;226:1593–1607. doi: 10.1093/infdis/jiac136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed H, Patel K, Greenwood DC, Halpin S, Lewthwaite P, Salawu A, et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J Rehabil Med. 2020;52:jrm00063. doi: 10.2340/16501977-2694. [DOI] [PubMed] [Google Scholar]
- 7.Hui DS, Joynt GM, Wong KT, Gomersall CD, Li TS, Antonio G, et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60:401–409. doi: 10.1136/thx.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blomberg B, Mohn KG, Brokstad KA, Zhou F, Linchausen DW, Hansen BA, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021;27:1607–1613. doi: 10.1038/s41591-021-01433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Oliveira JF, de Ávila RE, de Oliveira NR, da Cunha Severino Sampaio N, Botelho M, Gonçalves FA, et al. Persistent symptoms, quality of life, and risk factors in long COVID: a cross-sectional study of hospitalized patients in Brazil. Int J Infect Dis. 2022;122:1044–1051. doi: 10.1016/j.ijid.2022.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacob L, Koyanagi A, Smith L, Tanislav C, Konrad M, van der Beck S, et al. Prevalence of, and factors associated with, long-term COVID-19 sick leave in working-age patients followed in general practices in Germany. Int J Infect Dis. 2021;109:203–208. doi: 10.1016/j.ijid.2021.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seeßle J, Waterboer T, Hippchen T, Simon J, Kirchner M, Lim A, et al. Persistent symptoms in adult patients 1 year after coronavirus disease 2019 (COVID-19): A prospective cohort study. Clin Infect Dis. 2022;74:1191–1198. doi: 10.1093/cid/ciab611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buonsenso D, Munblit D, De Rose C, Sinatti D, Ricchiuto A, Carfi A, et al. Preliminary evidence on long COVID in children. Acta Paediatr. 2021;110:2208–2211. doi: 10.1111/apa.15870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Couzin-Frankel J. Clues to long COVID. Science. 2022;376:1261–1265. doi: 10.1126/science.add4297. [DOI] [PubMed] [Google Scholar]
- 16.Stephenson T, Allin B, Nugawela MD, Rojas N, Dalrymple E, Pinto Pereira S, et al. Long COVID (post-COVID-19 condition) in children: a modified Delphi process. Arch Dis Child. 2022;107:674–680. doi: 10.1136/archdischild-2021-323624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stephenson T, Pinto Pereira SM, Shafran R, de Stavola BL, Rojas N, McOwat K, et al. Physical and mental health 3 months after SARS-CoV-2 infection (long COVID) among adolescents in England (CLoCk): a national matched cohort study. Lancet Child Adolesc Health. 2022;6:230–239. doi: 10.1016/S2352-4642(22)00022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez-Leon S, Wegman-Ostrosky T, Ayuzo del Valle NC, Perelman C, Sepulveda R, Rebolledo PA, et al. Long-COVID in children and adolescents: a systematic review and meta-analyses. Sci Rep. 2022;12:9950. doi: 10.1038/s41598-022-13495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fink TT, Marques HHS, Gualano B, Lindoso L, Bain V, Astley C, et al. Persistent symptoms and decreased health-related quality of life after symptomatic pediatric COVID-19: a prospective study in a Latin American tertiary hospital. Clinics (Sao Paulo) 2021;76:e3511. doi: 10.6061/clinics/2021/e3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wise J. Long covid: WHO calls on countries to offer patients more rehabilitation. BMJ. 2021;372:n405. doi: 10.1136/bmj.n405. [DOI] [PubMed] [Google Scholar]
- 21.Osmanov IM, Spiridonova E, Bobkova P, Gamirova A, Shikhaleva A, Andreeva M, et al. Risk factors for post-COVID-19 condition in previously hospitalised children using the ISARIC Global follow-up protocol: a prospective cohort study. Eur Respir J. 2022;59 doi: 10.1183/13993003.01341-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Say D, Crawford N, McNab S, Wurzel D, Steer A, Tosif S. Post-acute COVID-19 outcomes in children with mild and asymptomatic disease. Lancet Child Adolesc Health. 2021;5:e22–e23. doi: 10.1016/S2352-4642(21)00124-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denina M, Pruccoli G, Scolfaro C, Mignone F, Zoppo M, Giraudo I, et al. Sequelae of COVID-19 in hospitalized children: a 4-months follow-up. Pediatr Infect Dis J. 2020;39:e458–e459. doi: 10.1097/INF.0000000000002937. [DOI] [PubMed] [Google Scholar]
- 24.Kikkenborg Berg S, Dam Nielsen S, Nygaard U, Bundgaard H, Palm P, Rotvig C, et al. Long COVID symptoms in SARS-CoV-2-positive adolescents and matched controls (LongCOVIDKidsDK): a national, cross-sectional study. Lancet Child Adolesc Health. 2022;6:240–248. doi: 10.1016/S2352-4642(22)00004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller F, Nguyen DV, Navaratnam AM, Shrotri M, Kovar J, Hayward AC, et al. Prevalence and characteristics of persistent symptoms in children during the COVID-19 pandemic: evidence from a household cohort study in England and Wales. Pediatr Infect Dis J. 2022;41:979–984. doi: 10.1097/INF.0000000000003715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molteni E, Sudre CH, Canas LS, Bhopal SS, Hughes RC, Antonelli M, et al. Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2. Lancet Child Adolesc Health. 2021;5:708–718. doi: 10.1016/S2352-4642(21)00198-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zavala M, Ireland G, Amin-Chowdhury Z, Ramsay ME, Ladhani SN. Acute and persistent symptoms in children with polymerase chain reaction (PCR)–confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection compared with test-negative children in England: active, prospective, national surveillance. Clin Infect Dis. 2022;75:e191–e200. doi: 10.1093/cid/ciab991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blankenburg J, Wekenborg MK, Reichert J, Kirsten C, Kahre E, Haag L, et al. Comparison of mental health outcomes in seropositive and seronegative adolescents during the COVID19 pandemic. Sci Rep. 2022;12:2246. doi: 10.1038/s41598-022-06166-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radtke T, Ulyte A, Puhan MA, Kriemler S. Long-term symptoms after SARS-CoV-2 infection in children and adolescents. JAMA. 2021;326:869–871. doi: 10.1001/jama.2021.11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buonsenso D, Munblit D, Pazukhina E, Ricchiuto A, Sinatti D, Zona M, et al. Post-COVID condition in adults and children living in the same household in Italy: a prospective cohort study using the ISARIC global follow-up protocol. Front Pediatr. 2022;10 doi: 10.3389/fped.2022.834875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Logue JK, Franko NM, McCulloch DJ, McDonald D, Magedson A, Wolf CR, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buonsenso D, Martino L, Morello R, De Rose C, Valentini P. Chronic olfactory dysfunction in children with long COVID: a retrospective study. Children (Basel) 2022;9:3–10. doi: 10.3390/children9081251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinstock LB, Brook JB, Walters AS, Goris A, Afrin LB, Molderings GJ. Mast cell activation symptoms are prevalent in Long-COVID. Int J Infect Dis. 2021;112:217–226. doi: 10.1016/j.ijid.2021.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erol N, Alpinar A, Erol C, Sari E, Alkan K. Intriguing new faces of Covid-19: persisting clinical symptoms and cardiac effects in children. Cardiol Young. 2022;32:1085–1091. doi: 10.1017/S1047951121003693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buonsenso D, Di Giuda D, Sigfrid L, Pizzuto DA, Di Sante G, De Rose C, et al. Evidence of lung perfusion defects and ongoing inflammation in an adolescent with post-acute sequelae of SARS-CoV-2 infection. Lancet Child Adolesc Health. 2021;5:677–680. doi: 10.1016/S2352-4642(21)00196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cocciolillo F, Di Giuda D, Morello R, De Rose C, Valentini P, Buonsenso D. Orbito-frontal cortex hypometabolism in children with post-COVID condition (long COVID): a preliminary experience. Pediatr Infect Dis J. 2022;41:663–665. doi: 10.1097/INF.0000000000003578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buonsenso D. A call to invest in immunological determinants of pediatric post-COVID conditions: insights from a post-COVID pANCA+ diffuse alveolar hemorrhage in an adolescent. Pediatr Pulmonol. 2022;57:2861–2862. doi: 10.1002/ppul.26097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Gennaro L, Valentini P, Sorrentino S, Ferretti MA, De Candia E, Basso M, et al. Extended coagulation profile of children with Long Covid: a prospective study. Sci Rep. 2022;12:18392. doi: 10.1038/s41598-022-23168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimmermann P, Pittet LF, Curtis N. How common is long COVID in children and adolescents? Pediatr Infect Dis J. 2021;40:e482–e487. doi: 10.1097/INF.0000000000003328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.