Abstract
Background
This Italian multicentric retrospective study aimed to investigate the possible changes in outcomes of patients undergoing surgery for gastrointestinal cancers during the COVID-19 pandemic.
Method
Our primary endpoint was to determine whether the pandemic scenario increased the rate of patients with colorectal, gastroesophageal, and pancreatic cancers resected at an advanced stage in 2020 compared to 2019. Considering different cancer staging systems, we divided tumors into early stages and advanced stages, using pathological outcomes. Furthermore, to assess the impact of the COVID-19 pandemic on surgical outcomes, perioperative data of both 2020 and 2019 were also examined.
Results
Overall, a total of 8250 patients, 4370 (53%) and 3880 (47%) were surgically treated during 2019 and 2020 respectively, in 62 Italian surgical Units. In 2020, the rate of patients treated with an advanced pathological stage was not different compared to 2019 (P = 0.25). Nevertheless, the analysis of quarters revealed that in the second half of 2020 the rate of advanced cancer resected, tented to be higher compared with the same months of 2019 (P = 0.05). During the pandemic year ‘Charlson Comorbidity Index score of cancer patients (5.38 ± 2.08 vs 5.28 ± 2.22, P = 0.036), neoadjuvant treatments (23.9% vs. 19.5%, P < 0.001), rate of urgent diagnosis (24.2% vs 20.3%, P < 0.001), colorectal cancer urgent resection (9.4% vs. 7.37, P < 0.001), and the rate of positive nodes on the total nodes resected per surgery increased significantly (7 vs 9% - 2.02 ± 4.21 vs 2.39 ± 5.23, P < 0.001).
Conclusions
Although the SARS-CoV-2 pandemic did not influence the pathological stage of colorectal, gastroesophageal, and pancreatic cancers at the time of surgery, our study revealed that the pandemic scenario negatively impacted on several perioperative and post-operative outcomes.
Keywords: COVID-19, Advanced cancer, Gastrointestinal cancers, Colorectal cancer, Pancreatic cancer, Gastroesophageal cancer
1. Introduction
On March the 9th, 2020, the first national lockdown was imposed across Italy by the Prime Minister to flatten the curve of the COVID-19 pandemic and reduce its potential impact on the population. Health resources were mostly dedicated to the care of COVID-19 infected patients.
These measures dramatically impacted cancer screening programs, routine diagnostic examinations, elective oncological treatments, and emergency surgery [[1], [2], [3], [4]]. Preoperative delay of oncological surgery increased [5], and a significant increase in the number of avoidable cancer-related deaths is expected in the near future [1,6].
Italy was one of the first and most affected Countries by the pandemic among Western Countries, and the actual extent of the disruption in the provision of Italian surgical services for oncological patients has only been partially investigated [4,7,8].
This multicentric national study aimed to investigate the possible changes in outcomes of patients undergoing surgery for colorectal, gastroesophageal, and pancreatic cancers in the year before the pandemic outbreak and the year after.
2. Methods
Data were collected from a national multicentric retrospective cohort study including adult patients that underwent surgery for colorectal, gastroesophageal, and pancreatic cancers from January 2019 to December 2020 in 62 Italian surgical divisions. This research was approved by the ethics committee of the coordinating center (reference number 18886) and registered at ClinicalTrial.gov (NCT04686747). As of the anonymized nature of patient data and retrospective design of the study, the informed consent was waived.
Local principal investigators were responsible for the acquisition of ethical approval to participate in the study.
All Italian surgical divisions treating colorectal, gastroesophageal, and pancreatic cancers were eligible for participation. Sixty-two Italian surgical divisions were included in this study. Data were collected from a disease-specific database sent to participating hospitals.
All adult patients surgically treated for localized, locally advanced, or metastatic cancers with curative or palliative intent during the study period were included in the study. Exclusion criteria were patients under 18 years of age or with multiple tumors, patients not surgically treated and hospitals that were not able to provide data for both 2019 and 2020.
Demographic data (Body Mass Index – BMI, age, sex, American Society of Anesthesiologists classification score – ASA, Charlson Comorbidity Index - CCI score) preoperative outcomes (rate of urgent diagnosis secondary to symptomatic cancers, rate of neoadjuvant treatments–NCTs, interval from diagnosis to operation in days), perioperative outcomes (i.e., rate of urgent colorectal cancer resection, rate of unresectable cancer, rate of minimally invasive procedure, length of hospital stay, rate of major postoperative complications) were compared between the two years. Furthermore, in order to collect homogeneous data, early oncological outcomes (i.e., rate of adjuvant therapy, number of lymph nodes retrieved, rate of positive nodes, rate of patients with positive nodes) were evaluated only in patients treated with curative intents. The primary endpoint was to demonstrate whether the COVID-19 pandemic increased the rate of colorectal, gastroesophageal, and pancreatic cancers surgically treated at an advanced stage in 2020. Considering different cancer staging systems (colorectal, gastroesophageal, and pancreatic cancers), we divided tumors into early stages (non-nodal, non-metastatic patients, R0 resection) and advanced stages (nodal, metastatic patients, and R1-R2 resection) according to the AJCC Cancer Staging Manual, 8th edition [9]. The secondary endpoint was to study the impact of the COVID-19 pandemic on perioperative and postoperative outcomes.
Urgent diagnosis was defined as diagnosis performed after an admission to the emergency department secondary to symptomatic cancer (e.g. bleeding, bowel perforation, symptoms of acute intestinal obstruction). The interval from diagnosis to surgery was calculated as the interval between the day of diagnosis (i.e., the date of endoscopy, CT scan, and histopathologic diagnosis) and the day of surgery, patients undergoing NCT were excluded. Cancer resectability was specified for each cancer: for example carcinosis peritonei, or not resectable M1 or T4 neoplasm were criteria for colorectal cancers resectability. Further details are provided in the study protocol (Appendix S2).
To better investigate the pandemic's influence on gastrointestinal cancer surgery, we divided the two years in quarters:
-
●
Q1: from January 1 to March 31
-
●
Q2: from April 1 to June 30
-
●
Q3: from July 1to September 30
-
●
Q4: from October 1 to December 31.
Than we compared the trend of surgical activity, both elective and emergent surgery, the rate of advanced resected cancers, as well as the rate of palliative and emergent surgical procedures performed during the quarters of the two years.
This paper was drafted according to the STrengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist [10].
2.1. Statistical analysis
The quantitative variables included in the study are presented as mean ± standard deviation at the general level and divided by year. Qualitative (categorical) variables are represented as percentages and absolute values, both at a general level and divided by year. Quantitative variables of interest were compared using two-tailed Student's t-test (in case of heteroskedasticity of variances). A comparison between qualitative variables of interest will be carried out to evaluate the association among them through an extension of the chi-square test, suitable for multi-centric studies (the Cochran – Mantel – Haenszel test). This test was used also for distribution comparison among quarters between 2019 and 2020. A p-value ≤0.05 was considered statistically significant.
Missing data were excluded listwise, which could have affected some numerical discrepancies in the calculation of frequencies. Since the majority of outcomes were provided for about 75–100% of patients, after data aggregation and before the analysis, patients with more than 25% of missing data of a specific outcome were excluded from the analysis of the single outcome. Moreover, we reported the rate of patients with available data for outcomes that was statistically significant.
3. Results
Overall, 62 Italian surgical divisions sent complete data for both years for a total of 8250 patients, 4370 (53%) and 3880 (47%) operated during 2019 and 2020, respectively. Of these, 730 (9%) were patients that underwent surgery for gastroesophageal cancer, 1816 (22%) for pancreatic and 5704 (69%) for colorectal cancer (Fig. 1 - Table 1 ). More than 50% of centers (37 out of 62) were of North Italy.
Fig. 1.
Geographical distribution of participating centers. Blue: coordinating center.
Table 1.
Population study divided by years. CR: Colorectal cancer patients; Pan: pancreatic cancer patients; GE: gastroesophageal cancer patients.
| 2019 | 2020 | Tot. | % | |
|---|---|---|---|---|
| Cancer Type | ||||
|
3058 | 2646 | 5704 | 69 |
|
924 | 892 | 1816 | 22 |
|
388 | 342 | 730 | 9 |
| Tot. | 4370 | 3880 | 8250 | |
3.1. Primary endpoint
The rate of patients operated at an advanced stage during the pandemic year was 51% compared to 49% in the year before. Although in 2020, the rate of patients with an advanced stage tended to be higher, this difference was not statistically significant (P = 0.25, 90% of patients with available data). To better investigate this outcome we analyzed the sub group of colorectal cancer patients, as they made the majority of patients included in this study. Although during 2020 the rate of advanced stage colorectal cancer raised, this difference was not statistically significant (46% vs 44%, P = 0.23, 93% of patients with available data).
3.2. Secondary endpoints
3.2.1. Demographic data (Table 2)
Table 2.
Demographic data. BMI: body Mass Index; CCI: Charlson Comorbidity Index; ASA: American Society of Anesthesiologists. *Mean ± standard deviation.
| 2019 | 2020 | P | Rate of patients with available data, % | |
|---|---|---|---|---|
| Age* | 69.26 (12.13) | 69.1 (12.17) | 0.55 | 100 |
| BMI* | 25.18 (4.32) | 25.21 (4.22) | 0.11 | 83 |
| CCI* | 5.28 (2.22) | 5.38 (2.08) | 0.04 | 91 |
| Sex | 0.98 | 78 | ||
|
1497 (43.5) | 1301 (43.6) | ||
|
1942 (56.5) | 1686 (56.4) | ||
| ASA | 0.74 | 93 | ||
|
2247 (55.3) | 1986 (54.9) | ||
|
1820 (44.7) | 1633 (45.1) |
The analysis of demographic data showed no differences in terms of mean age, sex, mean BMI, and preoperative ASA score between the two groups. Nevertheless, patients with diagnosis of cancer in 2020 had a higher Charlson Comorbidity Index - (CCI) score (5.38 ± 2.08 vs 5.28 ± 2.22, P = 0.04–91% of patients with available data).
3.2.2. Pre-operative outcomes
During the pandemic year, 24.2% of patients had a cancer diagnosis consequent to an urgent presentation compared to that of 20.3% in 2019 (P < 0.001–100% patients with available data).
In 2020, the number of patients that underwent NCT increased (24.1% vs. 19.5%), and this difference was statistically significant (P < 0.001–91% of patients with available data). To better understand this outcome we analyzed the colorectal cancer sub group, the most frequent tumor in the study. The rate of NCT for rectal cancer increased in the 2020 compared to the 2019 (389 vs 365 patients, P < 0.001–87% of patients with available data).
The mean time from diagnosis to surgery increased significantly during the pandemic year, being 56.8 days in 2019 and 64.2 days in 2020 (P < 0.001–90% of patients with available data).
3.2.3. Perioperative outcomes
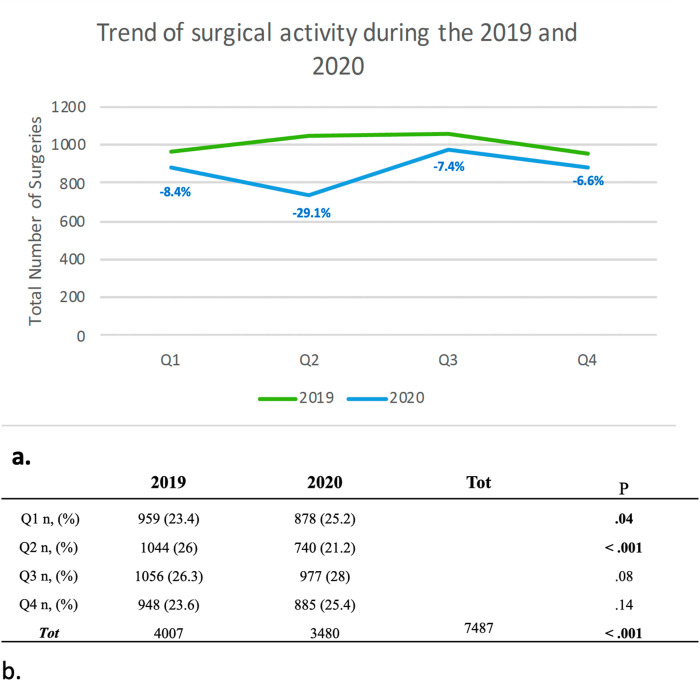
Overall, in 2020, rather than in 2019, a reduction in surgical activity of 5.2% was registered and this difference was statistically significant (P < 0.001–91% of patients with available data). Quarters analysis showed that surgical volume decreased significantly during Q1 of 2020 (−8%, P = 0.04), until the beginning of Q2 in 2020 which showed the most important reduction compared to that in the same months of 2019 (−29%, P < 0.001). (Fig. 2 ).
Fig. 2.
a. Line graph showing the trend of surgical activity during the 2019 and 2020; b. Trend of surgical activity during the 2019 and 2020 analyzed on 7487 patients (91%) with available data. Q1-4: quarters.
During the Q3 and Q4 of 2020, an increased rate of advanced cancers resected was noted, compared with Q3 and Q4 of 2019, and this difference tended to be statistically significant (50% and 49% vs 48% and 45% - P = 0.05). Conversely, palliative and urgent surgical procedures increased during the last two quarters of 2020 compared with the same months of 2019, without a statistical significance (P = 0.6 and P = 0.7, respectively).
In 2020 there was a significant increase in urgent colorectal cancer resection (9.3 vs. 7.4%, P = 0.005–100% of patients with available data). The analysis of data on cancer resectability showed that 5.9% of patients operated in 2019 were unresectable, compared to 6.4% of patients resected in 2020 and this difference was not statistically significant (P = 0.28). During both years, there were no differences in terms of minimally invasive surgery (MIS) (63.4% vs. 62.1%, P = 0.23). Interestingly, conversion rate during 2020 was lower than that in 2019 (7.2 vs 9.2%), and this difference was statistically significant (P = 0.01–94% of patients with available data). To better assess these findings, we analyzed the subgroup of patients with colorectal cancer since it was the most frequent included in the study. The analysis of data showed that the rate of MIS for colorectal cancer resections decreased significantly in 2020 (78.4 vs 75.6%, P = 0.009–99% of patients with available data). Nevertheless, conversion rate was significantly lower during the pandemic year than in 2019 ( 6.9 vs 8.7 %, P = 0.03–93% of patients with available data).
The mean LOS was significantly shorter in 2020 (12.1 ± 11.4 vs 11.6 ± 11 days, P = 0.04–99% of patients with available data). The analysis of major postoperative complications (grade III-V, according to the Clavien–Dindo classification system [11]) demonstrated no difference between the two years (13.7 vs 12.8%, P = 0.20). Thirty-day readmission rate occurred more frequently in patients operated in 2020 (5 vs. 4.4%), without a significant difference (P = 0.16). Similarly, the 30-day mortality rate was higher in 2020 than in 2019, but without a significant difference (1.9 vs 2.5, P = 0.09).
3.2.4. Early oncological outcomes (Table 3)
Table 3.
Perioperative outcomes. NCTs: Neoadjuvant treatments. *Mean ± standard deviation.
| 2019 | 2020 | P | Rate of patients with available data, % | |
|---|---|---|---|---|
| Preoperative | ||||
| Urgent diagnosis n, (%) | 857(20.3) | 900 (24.2) | < .001 | 97 |
| NCTs n, (%) | 778 (19.5) | 843 (24.1) | < .001 | 91 |
| Interval of time diagnosis - surgery (days) * | 56.8 (8.48) | 64.2 (53.03) | < .001 | 90 |
| Perioperative | ||||
| Urgent CRC resection n, (%) | 224 (7.3) | 248 (9.3) | .005 | 100 |
| Unresectable n, (%) | 244 (5.9) | 244 (6.4) | .28 | 95 |
| Palliative procedure n, (%) | 249 (5.7) | 256 (6.6) | .11 | 99 |
| MI procedure n, (%) | 2746 (63.4) | 2399 (62.1) | .23 | 99 |
| Conversion rate n, (%) | 234 (9.2) | 2399 (7.2) | .01 | 94 |
| MI CRC resection n, (%) | 2379 (78.4) | 1986 (75.6) | .009 | 99 |
| Conversion rate of CRC resection n, (%) | 191 (8.7) | 130 (6.9) | .03 | 93 |
| Postoperative outcomes | ||||
| LOS days * | 12.1 (11.4) | 11.6 (11) | .04 | 99 |
| Postoperative Complications III-V n, (%) | 583 (13.7) | 487 (12.8) | .20 | 97 |
| 30 days re- admission n, (%) | 180 (4.4) | 189 (5) | .16 | 95 |
| 30 days mortality n, (%) | 81 (1.9) | 93 (2.5) | .09 | 96 |
| Short-term oncological outcomes | ||||
| Adjuvant treatment n,(%) | 1200 (35.9) | 1084 (36.2) | .80 | 77 |
| Number of Lymph nodes Retrieved* | 25.4 (15.6) | 24.6 (15) | .03 | 89 |
| Lymph nodes + % - n (*) | 7 – 1.96(4.18) | 9 – 2.22 (4.63) | < .001 | 92 |
| Patients with Lymph nodes + n, (%) | 1603 (41.6) | 1587(43.) | .23 | 92 |
| Resection margin involvement (R1-R2) n, (%) | 302 (7.9) | 253 (7.4) | .4 | 91 |
| Lymphovascular and perineural invasion n, (%) | 1929 (62.1) | 1727 (63.6) | .25 | 75 |
These outcomes were evaluated only in patients treated with a curative intent during the two years. The rate of adjuvant treatments of patients with positive lymph nodes, or with R1/R2 resected cancers and of tumors with lymphovascular and perineural invasion were not statistically different between the two years. The mean number of retrieved lymph nodes was higher in 2019 compared to 2020 (25.4 vs 24.6 nodes, P < 0.05–89% of patients with available data). Conversely, the rate of positive nodes on the total nodes resected per surgery was significantly higher in 2020 compared to that in 2019 (9% vs 7% - 2.22 ± 4.63 vs 1.96 ± 4.18, P < 0.001–92% of patients with available data).
4. Discussion
The results of the COVID-AGICT study revealed that in 2020, the pandemic conditions did not increase the rate of colorectal, gastroesophageal, and pancreatic cancers diagnosed and operated at an advanced stage. However, the analysis of quarters revealed that in the second half of 2020 the rate of advanced cancer resected, tented to be higher compared with the same months of 2019. Nevertheless, our study demonstrated that patients who were diagnosed with cancer in 2020 had more comorbidities. Furthermore, increased preoperative delay and decreased surgical activity were observed during the first half of 2020. Consequently, the diagnosis of cancers after urgent admission and the rate of NCTs, as well as the rate of urgent colorectal resection increased. Despite this, a reduction in the conversion rate to open surgery and mean LOS was found in 2020. Finally, during the pandemic year, the rate of positive lymph nodes increased significantly without a significant impact on other pathological outcomes. The SARS-CoV-2 pandemic had catastrophic consequences not only for patients infected by COVID-19. Screening systems were damaged and the waiting time to elective surgery increased [5].
Although several studies have shown that short-term oncological outcomes were not compromised by the COVID-19 pandemic, the disruption of standard cancer care will have negative consequences on long-term oncological outcomes [6,12,13].
Pathological findings from our study showed no difference in stages in 2020 compared to 2019, whereas an increased number of positive nodes was found in 2020. Several studies have found no statistically significant difference in the pathological stage between the pandemic period and the previous year [[14], [15], [16]]. A recently published multicentric retrospective study analyzed the impact of the pandemic on patients with colorectal cancer treated in 2019 and 2020 in 20 hospitals of northern Italy. The authors found that patients treated between March and December 2020 had an increased risk of advanced disease in terms of associated symptoms, cancer location, clinical T4 stage and number of liver metastases, compared with those in the same period in 2019 [16].
Nodal status is an important prognostic factor [18,19]. Patients treated with a curative intent during 2020 were found to have an increased rate of positive lymph nodes. The increased rate of urgent surgery, that usually involves patients with a poor preoperative workup, and the delayed time from diagnosis to surgery shown in our study, could partially explain this result [20,21]. As other pathological outcomes evaluated in COVID-AGICT were not compromised, the increased of lymph nodes positivity could represent the initial impact on pathological outcomes for patients resected during the COVID-19 pandemic period.
Preoperative delay was the most important concern of the surgical community for oncological patients during the pandemic period [[6], [22], [34]]. We demonstrated that the interval from diagnosis to surgery increased during the entire 2020 from 76.7 days in 2019 to 93.9 days in the pandemic year (P < 0.001). The COVIDSurg collaborative group recently published an international prospective cohort study to assess the nonoperation rate (defined as the proportion of patients who did not undergo planned surgery) of patients who were candidates for curative surgery during the COVID-19 pandemic. The authors included 20006 patients from 61 countries and 15 cancer types and demonstrated that one in seven patients who were residing in regions with full lockdowns experienced longer preoperative delays and did not undergo planned surgery [5].
The reduction in surgical activity caused by the outbreak secondary to the COVID-19 pandemic was another important finding of our study. In the first two quarters of 2020, during the first pandemic wave, the surgical activity decreased up to 29%. Recently, an Italian multicentric study investigated the influence of COVID-19 on the diagnostic and therapeutic pathways of surgical pancreatic diseases across 10 Italian referral centers. The authors included 1423 patients: 638, and 785 patients in 2020 and 2019, respectively. This study demonstrated an 18.7% reduction in the surgical activity in the first six months of 2020 (P < 0.0001), with the most significant impact during the lockdown period (phase 1: −33.9%; P < 0.0001) and the partial easing of restrictions (phase 2: −23.9%; P = 0.01) compared to that in the same weeks of 2019 [23].
Although the primary endpoint did not show a worsening of stage in 2020, other points of our investigation could show indirectly that as the pandemic spread, the rate of advanced cancers at the diagnosis increased. First, patients with cancers diagnosed during the pandemic year had a higher CCI score (5.38 ± 2.08 vs 5.28 ± 2.22, P = 0.036). A recent multicentric study published by the Covid ICE International Collaborative Group compared the outcomes of patients admitted to 45 international emergency surgical units during the months of March and April 2020 (COVID-19 pandemic outbreak) and the same months in 2019 (pre-Covid-19). The authors showed that during the COVID period the rate of patients with ASA>1 patients with frailty score >2 increased from 46% to 53% during the COVID-19 pandemic outbreak [24]. This difference could be a consequence of a higher rate of symptomatic cancers diagnosed in 2020, and patients with more comorbidities were more likely to attend the hospital than the healthier ones.
Indeed, as showed by others studies [16,25,26], during the pandemic year the rate of cancer diagnosis following an emergency admission (24.2% vs. 20.3%, P < 0 0.001) and of urgent colorectal cancer resection (9.4% vs. 7.37, P < 0.001) increased significantly. These findings could reflect that during 2020, the analyzed tumors were more symptomatic, requiring an emergent admission, and could be an indirect sign of a worsened stage.
The COVID-AGICT study showed that during 2020, more neoadjuvant treatments were delivered, increasing from 19.5% to 23.9% (P < 0.001). Morris et al. demonstrated a decrease in the number of operations occurring in parallel with an increase of 44% in the use of radiotherapy, predominantly in the form of short-course radiotherapy for rectal cancer in England, between April and August 2020 [26]. Furthermore, Quero et al. showed that patients with pancreatic cancer in 2020 underwent neoadjuvant treatment more frequently than those in 2019 (29.9 vs. 23.7%, P = 0.009) [23]. The increase in neoadjuvant treatments in 2020 could reflect an indirect sign of advanced disease during this period. At the same time NCTs could also be implemented for early-stage cancers to avoid disease progression caused by increased preoperative delay. Than NCT may be considered as a “bridge to surgery strategy”, used during the 2020 to minimize the COVID-19 effect on disease progression.
In 2020, particularly during the early phase, the feasibility and safety of minimally invasive surgery were debated and discouraged to minimize the risk of inhalation of the surgical plume [26,28,29]. To date, there is no clear evidence suggesting the transmission of viral particles through surgical smoke [30,31]. We demonstrated that during 2020 in Italy, there was no reduction in MIS and that the conversion rate to open approach was significantly reduced. To better understand these findings, we analyzed the colorectal cancer subgroup, the most frequent tumors included in the study, and cancers that are more suitable for MIS. Indeed, the analysis showed that the rate of MIS for colorectal cancer resection decreased significantly in 2020 (78.4 vs 75.5%, P < 0.001). At the same time, conversion rate to open approach reduced significantly in 2020 (9.5 vs 7.41%, P = 0.03). The reduction in MIS for colorectal cancer patients could reflect the consequences of restrictions suggested by several guidelines and the significant increase in emergency resection in 2020. The lower conversion rate of the entire cohort of patients and that of the colorectal cancer patient group could be a consequence of better patient selection for MIS in 2020 [24].
The COVID-AGICT study showed that during 2020, the LOS was shorter than that in 2019 (12.1 vs 11.6 days, P = 0.037). An Italian retrospective study by Spinelli et al. investigated the impact of a pandemic scenario on the quality of a long-established enhanced recovery protocol colorectal surgery program. The study revealed that in 2020, the LOS was globally shorter than that in the same period in 2019 (4.3 vs 6.2 d) [14]. These findings could reflect the change in the mindset that the pandemic was induced not only by clinicians, but also by patient behaviors. In our case, we were anxious to release our patients as soon as the discharge criteria were met, to reduce the in-hospital risk of coronavirus infection and, at the same time, to furnish more resources possible for oncological and emergency patients. During the COVID period, patients’ awareness increased regarding the risks of COVID-19 infection secondary to prolonged contact with health care workers [32] and a higher presence of family caregivers, as the lockdown forced people to stay home, resulting in a higher motivation for patients to leave the hospital as soon as possible.
The findings of this study should be interpreted with caution because of its limitations that should be acknowledged. First, it was retrospective in nature. Second, the COVID-AGICT study did not considered the patients who did not undergo surgery during the 2020 for the same cancers (i.e. for advanced malignancy, severe comorbidities). This may influenced the understanding on any epidemiology modification during this period: at the same time our study investigated a specific population of oncological patients. Third, some data were not detailed and could have influenced the final outcomes. Fourth, the COVID-AGICT study included five types of gastrointestinal cancers: perioperative and postoperative outcomes could be influenced by the intrinsic biological difference of these cancers. It could jeopardize the strength of COVID AGICT study findings and their generatability. For example neoadjuvant therapy, that has different effects and indications for these five cancer, as well as for the rate of postoperative complications that are influenced by the type of the surgical procedure. Fifth, it must be considered that the time period of our study may be too short to see any difference in pathological outcomes, and others studies analyzing also the year 2021 could show interesting results. Finally, 69% of patients included in our cohort had a colorectal cancer, which could have influenced the final outcomes.
Although the included centers and the number of patients (more than 60 centers and 8000 patients) might not represents the real influence of pandemic on the Italian gastro-intestinal surgery, to the best of our knowledge, the COVID-AGICT study is the first study to assess the impact of the pandemic on the diagnosis and surgical treatment of gastroesophageal, pancreatic, and colorectal cancers across Italy, analyzing several preoperative and postoperative outcomes in detail during the first waves of COVID-19.
5. Conclusion
Although the pandemic scenario did not influence the stage of gastroesophageal, pancreatic, and colorectal cancers, the COVID-AGICT study revealed that during 2020, the CCI score of cancer patients, neoadjuvant treatments, rate of urgent diagnosis, colorectal cancer urgency resection, and number of positive lymph nodes increased, significantly.
To date we are in a different era with vaccines. Nevertheless, new SARS-CoV-2 variants could lead in the future to new pandemic scenario [33]. Our findings should serve as a lesson to guide specific assessment of the clinical impact of the pandemic on gastrointestinal cancer patients in the future. In the future, specific pathways for diagnosis and treatment of GI cancers should be preserved to offer the same standard of treatment also in these difficult moments [2,5].
Conflict of interest disclosures
The authors have no conflicts of interest or financial ties to disclose related to the research presented.
Sources of funding for research
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The study was preregistered without an analysis plan
This paper reports the results of NCT04686747 preregistered studies, which can be accessed at https://clinicaltrials.gov/ct2/show/NCT04686747.
Whether the paper has been presented in part elsewhere
Not applicable.
The COVID – AGICT
study group is an Italian collaboration, hosted centrally from the Misericordia Hospital, Grosseto. In appendix 1 is provided a complete list of the group.
Data access, responsibility, and analysis
Giuliani Giuseppe had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Data will be made available upon reasonable request.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.suronc.2023.101907.
Contributor Information
COVID-AGICT Collaborative group:
Lorenzo De Franco, Roberto Benigni, Angela Tribuzi, Ubaldo Marra, Michele Di Marino, Chiara Cova, Beatrice Bianchi, Sara Nobile, Luigi Zorcolo, Giorgio Lisi, Fabrizio Allisiardi, Michele Grieco, Carolina Righetti, Marco Frisini, Alberto Brolese, Michele Grassia, Andrea Lucchi, Giulia Bagaglini, Giuseppe S. Sica, Michele Manara, Luca Turati, Lorenzo Macone, Roberta Carminati, Pierpaolo Mariani, Gianluca Rizzo, Claudio Coco, Francesca Pennetti Pennella, Fabio Rondelli, Lucia Romano, Antonio Giuliani, Raffaele Palaia, Andrea Belli, Vittorio Albino, Maddalena Leongito, Giulia David, Pasquale Misitano, Silvia Pasulo, Gian Luca Baiocchi, Roberta La Mendola, Mohamnad Abu Hilal, Ludovica Baldari, Elisa Cassinotti, Luigi Boni, Gabriella Teresa Capolupo, Marco Caricato, Enrico Pinotti, Mauro Montuori, Cristina Bombardini, Gabriele Anania, Rigers Dibra, Gennaro Martines, Leonardo Solaini, Giorgio Ercolani, Renato Oliva, Maria Vittoria Carati, Gian Luca Grazi, Giacomo Ghio, Francesco Marchegiani, Salvatore Pucciarelli, Filippo La Torre, Immacolata Iannone, Dimitri Krizzuk, Francesco Sammartino, Giorgia Catalano, Paolo Strignano, Renato Romagnoli, Domenico Piccione, Bruno Nardo, Rossella Reddavid, Maurizio Degiuli, Martino Gerosa, Dario Maggioni, Michele Zuolo, Marco Rigamonti, Omar Ghazouani, Raffaele Galleano, Andrea Percivale, Luca Tirloni, Luca Moraldi, Nicolò Fabbri, Carlo Vittorio Feo, Samuele Colombo, Salomone Di Saverio, Giuseppe Barbato, Francesco Coratti, Andrea Sagnotta, Stefano Mancini, Nicola Cillara, Antonello Deserra, Alessandro Cannavera, and Giampaolo Formisano
Appendix 1. COVID-AGICT Collaborative Group
Giuseppe Giuliani MD1, Francesco Guerra MD1, Simona Messinese MD 2, Francesco Santelli PhD 3, Lucia Salvischiani 1, Sofia Esposito MD 13, Luca Ferraro72, Alessandro Esposito MD 4, Matteo De Pastena MD 4, Daniela Rega MD 5, Paolo Delrio MD 5, Carlotta La Raja MD 6,7, Antonino Spinelli MD 6,7, Simonetta Massaron MD 8, Paola De Nardi MD 8, Emanuele Federico Kauffmann MD 9, Ugo Boggi MD 9, Simona Deidda MD 10, Angelo Restivo MD 10, Alessandra Marano MD 11, Felice Borghi MD 12, Micaela Piccoli MD 13, Norma Depalma MD 14, Stefano D'Ugo14, Marcello Spampinato MD 14, Federico Cozzani MD 15, Paolo Del Rio MD 15, Rosa Marcellinaro MD 16, Massimo Carlini MD 16, Raffaele De Rosa MD 17, Stefano Scabini MD 17, Fabio Maiello18, Roberto Polastri MD 18, Giulia Turri MD 19, Corrado Pedrazzani MD 19, Monica Zese MD 20, Dario Parini MD 20, Andrea Casaril MD 21, Gianluigi Moretto MD 21, Antonio De Leo MD 22, Marco Catarci MD 22, Renza Trapani MD 23, Sandro Zonta MD 23, Patrizia Marsanic MD 24, Andrea Muratore MD 24, Gregorio Di Franco MD 25, Luca Morelli MD 25, Alessandro Coppola MD 26, Damiano Caputo MD 27,28, Jacopo Andreuccetti MD 29, Giusto Pignata MD 29, Laura Mastrangelo MD 30, Elio Jovine MD 30, Michele Mazzola MD 31, Giovanni Ferrari MD 31, Lorenzo Mariani MD 32, Graziano Ceccarelli MD 32, Rocco Giuseppe MD 33, Stefano Bolzon MD 33, Mariateresa Grasso MD 34, Silvio Testa MD 34, Paola Germani MD 35, Nicolò de Manzini MD 35, Serena Langella MD 36, Alessandro Ferrero MD 36, Diego Coletta58, Lorenzo De Franco 1, Roberto Benigni 1, Angela Tribuzi 1, Ubaldo Marra 1, Michele Di Marino1, Chiara Cova 4, Beatrice Bianchi 4, Sara Nobile 4, Luigi Zorcolo MD10, Giorgio Lisi 16, Fabrizio Allisiardi 16, Michele Grieco 16, Carolina Righetti MD 18, Marco Frisini 37, Alberto Brolese 37, Michele Grassia 38, Andrea Lucchi 38, Giulia Bagaglini 39, Giuseppe S Sica 39, Michele Manara 40, Luca Turati 40, Lorenzo Macone41, Roberta Carminati 41, Pierpaolo Mariani 41, Gianluca Rizzo 73, Claudio Coco 42, Francesca Pennetti Pennella 43, Fabio Rondelli 43, Lucia Romano 44, Antonio Giuliani 44, Raffaele Palaia 45, Andrea Belli 45, Vittorio Albino 46, Maddalena Leongito 46, Giulia David47, Pasquale Misitano 47, Silvia Pasulo 48, Gian Luca Baiocchi 48, Roberta La Mendola 49, Mohamnad Abu Hilal 49, Ludovica Baldari 50, Elisa Cassinotti 50, Luigi Boni 50, Gabriella Teresa Capolupo51, Marco Caricato 51, Enrico Pinotti 52, Mauro Montuori 52, Cristina Bombardini 53, Gabriele Anania 53, Rigers Dibra 54, Gennaro Martines 54, Leonardo Solaini 55, Giorgio Ercolani 55, Renato Oliva 56, Maria Vittoria Carati 56, Gian Luca Grazi 56, Giacomo Ghio 57, Francesco Marchegiani 57, Salvatore Pucciarelli 57, Filippo La Torre 58, Immacolata Iannone 59, Dimitri Krizzuk 60, Francesco Sammartino 60, Giorgia Catalano 61, Paolo Strignano 61, Renato Romagnoli 61, Domenico Piccione 62, Bruno Nardo 62, Rossella Reddavid 63, Maurizio Degiuli 63, Martino Gerosa 64, Dario Maggioni 64, Michele Zuolo 65, Marco Rigamonti 65, Omar Ghazouani 66, Raffaele Galleano 66, Andrea Percivale 66, Luca Tirloni 67, Luca Moraldi 67, Nicolò Fabbri 68, Carlo Vittorio Feo 68, Samuele Colombo 69, Salomone Di Saverio 69, Giuseppe Barbato 70, Francesco Coratti 70, Andrea Sagnotta 71, Stefano Mancini 71, Nicola Cillara74, Antonello Deserra74, Alessandro Cannavera74, Giampaolo Formisano 72, Paolo Pietro Bianchi72, Carmelo Bengala 2, Andrea Coratti MD 1.
Affiliations:
-
1.
Department of General and Emergency Surgery, Misericordia Hospital, Azienda Usl Toscana Sud Est. School of robotic surgery. Grosseto, Italy.
-
2.
Medical Oncology Unit, Misericordia Hospital, Grosseto, Italy.
-
3.
Department of Economics, Business, Mathematics and Statistics (DEAMS), University of Trieste, Trieste, Italy.
-
4.
Department of General and Pancreatic Surgery, The Pancreas Institute, University of Verona Hospital Trust, Piazzale L.A. Scuro, 10, 37134, Verona, Italy.
-
5.
Colorectal Surgical Oncology, Abdominal Oncology Department, Fondazione Giovanni Pascale IRCCS, Naples, Italy.
-
6.
Department of Biomedical Sciences, Humanitas University, Via Rita Levi Montalcini 4, Pieve Emanuele, 20072 Milan, Italy.
-
7.
IRCCS Humanitas Research Hospital, Division of Colon and Rectal Surgery, Via Manzoni 56, Rozzano, 20089 Milan, Italy.
-
8.
Division of Gastrointestinal Surgery, San Raffaele Hospital, Milan, Italy.
-
9.
Division of General and Transplant Surgery, University of Pisa, Pisa, Italy.
-
10.
Department of Surgery, Colorectal Surgery Center, University of Cagliari, Cagliari, Italy.
-
11.
General and Specialist Surgery Department, Emergency General Surgery Unit, A.O.U. Città della Salute e della Scienza di Torino, Turin, Italy.
-
12.
Oncological Surgery, Candiolo Cancer Institute-FPO-IRCCS, Candiolo, 10060 Torino, Italy.
-
13.
Department of General, Emergency Surgery and New Technologies, Baggiovara General Hospital, AOU Modena, Italy.
-
14.
Department of General Surgery, "Vito Fazzi" Hospital, Piazza Muratore 1–73100, Lecce, Italy.
-
15.
General Surgery Unit, Parma University Hospital, Parma, Italy.
-
16.
Department of General Surgery, S. Eugenio Hospital, Piazzale dell'Umanesimo, 10, 00144, Rome, Italy.
-
17.
Surgical Oncology Surgery, IRCCS Policlinico San Martino, Genoa, Italy.
-
18.
Department of Surgery - General Surgery Unit, Hospital of Biella, Biella, Italy.
-
19.
Department of Surgical Sciences, Dentistry, Gynecology and Pediatrics, Unit of General and Hepatobiliary Surgery, University and Hospital Trust of Verona, 37134, Verona, Italy.
-
20.
Department of General and Urgent surgery. Santa Maria della Misericordia Hospital, Rovigo, Italy.
-
21.
Department of Surgery, "Pederzoli" Hospital, Peschiera del Garda, Verona, Italy.
-
22.
General Surgery Unit, Sandro Pertini Hospital, ASL Roma 2, Via dei Monti Tiburtini, 385, 00157, Rome, Italy.
-
23.
Department of General Surgery, Ospedale San Biagio, ASL VCO, Domodossola, Italy.
-
24.
Surgical Department, E. Agnelli Hospital, 10064 Pinerolo, Italy.
-
25.
General Surgery Unit, Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, Via Paradisa 2, 56125, Pisa, Italy.
-
26.
Dipartimento di chirurgia, Sapienza Università di Roma, Rome, Italy.
-
27.
Research Unit of Generale Surgery, Department of Medicine and Surgery, University Campus Bio-Medico di Roma, Via Alvaro del Portillo 200,00128 Rome, Italy.
-
28.
Operative Research Unit of General Surgery, Fondazione Policlinico Universitario Campus Bio-Medico, Via Alvaro del Portillo, 200 - 00128 Roma, Italy.
-
29.
Second General Surgery, ASST Spedali Civili di Brescia, Brescia, Italy.
-
30.
Division of General and Emergency Surgery, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
-
31.
Division of Minimally-Invasive Surgical Oncology, ASST Grande Ospedale Metropolitano Niguarda, Piazza Ospedale Maggiore, 3 20162 Milan, Italy.
-
32.
General Surgery, San Giovanni Battista Hospital, USL Umbria 2, Foligno, Italy.
-
33.
Hepatobiliary, Pancreatic and General Surgery Unit, Department of Surgery, Azienda Unità Sanitaria Locale Area Vasta Romagna, Santa Maria delle Croci - Ravenna Hospital, Ravenna, Italy.
-
34.
S.C. Chirurgia Generale, Ospedale S.Andrea, Vercelli, Italy.
-
35.
Surgical Clinic Unit, University Hospital of Trieste, Trieste, Italy.
-
36.
Department of General and Oncological Surgery, Mauriziano Hospital, Largo Turati 62, 10128, Turin, Italy.
-
37.
APSS, Department of General Surgery & HPB Unit, Largo Medaglie d'oro 9, 38122, Trento, Italy.
-
38.
Division of Surgery, Ospedale "Ceccarini", AUSL Romagna, Riccione, Italy.
-
39.
University of Rome Tor Vergata, Department of Surgery, Rome, Italy.
-
40.
Surgical Oncology Unit - Treviglio Hospital ASST Bergamo Ovest.
-
41.
ASST Bergamo Est, P.O. Pesenti Fenaroli, General Surgery Unit, Alzano Lombardo, Bergamo, Italy.
-
42.
U.O.C. Chirurgia Generale 2 - Fondazione Policlinico Universitario Agostino Gemelli IRCCS; Università Cattolica del Sacro Cuore - Rome, Italy U.O.C. Radioterapia Oncologica - Fondazione Policlinico Universitario Agostino Gemelli IRCCS; Università Cattolica del Sacro Cuore - Rome, Italy.
-
43.
Department of Surgical Specialties, SC General Surgery and Surgical Specialties, St Maria Hospital, Terni, Italy.
-
44.
Department of Biotechnological and Applied Clinical Sciences, San Salvatore Hospital, Università degli studi dell’Aquila, L'Aquila, Italy.
-
45.
Department of Abdominal Oncology, Division of Gastro-esophageal and pancreatic Surgical Oncology, Istituto Nazionale Tumori, Fondazione G. Pascale, IRCCS, Naples 80131, Italy.
-
46.
Department of Abdominal Oncology, Division of Gastro-esophageal Surgical Oncology, Istituto Nazionale Tumori, Fondazione G. Pascale, IRCCS, Naples 80131, Italy.
-
47.
Chirurgia Generale - Sant'Anna Como (san fermo della battaglia) ASST-lariana.
-
48.
Surgical Clinic, Department of Experimental and Clinical Sciences, University of Brescia, 25123 Brescia, Italy
-
49.
Hepato-Bilio-Pancreatic Minimally Invasive Surgery, Poliambulanza Foundation Hospital, Brescia, Italy.
-
50.
Department of Surgery, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico di Milano, University of Milan, Milan, Italy.
-
51.
Colorectal Surgery Unit, Fondazione Policlinico Campus Bio-Medico, Università Campus Bio-Medico di Roma, Rome, Italy.
-
52.
Department of Surgery, Ponte San Pietro Hospital, Bergamo, Italy.
-
53.
Department of Surgery, Section of Chirurgia 1, Sant'Anna University Hospital of Ferrara, Cona, Italy.
-
54.
Deparment of Emergency and Organ Transplantation, University "Aldo Moro" of Bari, Piazza G Cesare, 11, 70124, Bari, Italy.
-
55.
Department of Medical and Surgical Sciences, University of Bologna, Morgagni-Pierantoni Hospital, Forlì, Italy.
-
56.
IRCCS Regina Elena National Cancer Institute - Rome, Italy.
-
57.
Department of Surgical Oncology and Gastroenterology Sciences, First Surgical Clinic, University of Padua, Padua (PD), Italy.
-
58.
Department of Surgical Sciences, Policlinico Umberto I University Hospital, Sapienza University of Rome, Rome, Italy.
-
59.
Department of Surgery, “Pietro Valdoni”, “Sapienza” University of Rome, Italy.
-
60.
Unit of general surgery - Aurelia Hospital- Rome, Italy.
-
61.
General Surgery 2U - Liver Transplant Unit, A.O.U. Città della Salute e della Scienza di Torino, University of Turin, Turin, Italy.
-
62.
General and Oncological Surgery Unit, Department of Surgery, Annunziata Hospital, Cosenza, Italy.
-
63.
Division of Surgical Oncology and Digestive Surgery, Department of Oncology, San Luigi University Hospital, University of Turin, Turin, Italy.
-
64.
Laparoscopic and Oncological General Surgery Department, ASST Monza, Desio Hospital, Via Mazzini 1, Desio, Italy.
-
65.
Department of General Surgery, Cles Hospital, Cles, Italy.
-
66.
Department of Surgery - Santa Corona Hospital - Pietra Ligure – Italy.
-
67.
Division of Oncologic Surgery and Robotics, Department of Oncology, Careggi University Hospital, Florence, Italy.
-
68.
Department of Surgery, Section of General Surgery, Ospedale del Delta, Azienda USL of Ferrara, University of Ferrara, Ferrara 44023, Italy.
-
69.
Department of General Surgery, Ospedale Civile "Madonna del Soccorso", San Benedetto del Tronto, AP, Italy.
-
70.
Digestive Surgery Unit, Department of Clinical and Experimental Medicine, Careggi University Hospital, Florence, Italy.
-
71.
Department of General Surgery and Surgical Oncology, San Filippo Neri Hospital, Rome 00135, Italy.
-
72.
Division of General and Robotic Surgery, Dipartimento di Scienze della Salute, Università di Milano, ASST Santi Paolo e Carlo, 20142 Milano, Italy.
-
73.
UOC Chirurgia dell’Apparato Digerente e del Colon-Retto Ospedale Fatebenefratelli Isola Tiberina - Gemelli Isola Rome, Italy.
-
74.
UOC Chirurgia Generale PO Santissima Trinità ASL Cagliari - Cagliar, Italy.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Maringe C., Spicer J., Morris M., et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVIDSurg Collaborative: Global guidance for surgical care during the COVID-19 pandemic. Br. J. Surg. 10.1002/bjs.11646 [epub ahead of print on April 15, 2020]. [DOI] [PMC free article] [PubMed]
- 3.Saini K.S., de Las Heras B., de Castro J., et al. Effect of the COVID-19 pandemic on cancer treatment and research. Lancet Haematol. 2020;7:e432–e435. doi: 10.1016/S2352-3026(20)30123-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patriti A., Eugeni E., Guerra F. What happened to surgical emergencies in the era of COVID-19 outbreak? Considerations of surgeons working in an Italian COVID-19 red zone. Updates Surg. 2020 Jun;72(2):309–310. doi: 10.1007/s13304-020-00779-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.COVIDSurg Collaborative Effect of COVID-19 pandemic lockdowns on planned cancer surgery for 15 tumour types in 61 countries: an international, prospective, cohort study. Lancet Oncol. 2021 Nov;22(11):1507–1517. doi: 10.1016/S1470-2045(21)00493-9. Epub 2021 Oct 5. PMID: 34624250; PMCID: PMC8492020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.London J.W., Fazio-Eynullayeva E., Palchuk M.B., et al. Effects of the COVID-19 pandemic on cancer-related patient encounters. JCO Clin. Cancer Inform. 2020;4:657–665. doi: 10.1200/CCI.20.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torzilli G., Viganò L., Galvanin J., Castoro C., Quagliuolo V., Spinelli A., Zerbi A., Donadon M., Montorsi M., COVID-SURGE-ITA group A snapshot of elective oncological surgery in Italy during COVID-19 emergency: pearls, pitfalls, and perspectives. Ann. Surg. 2020 Aug;272(2):e112–e117. doi: 10.1097/SLA.0000000000004081. PMID: 32675512; PMCID: PMC7373476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Saverio S., Pata F., Gallo G., Carrano F., Scorza A., Sileri P., Smart N., Spinelli A., Pellino G. Coronavirus pandemic and colorectal surgery: practical advice based on the Italian experience. Colorectal Dis. 2020 Jun;22(6):625–634. doi: 10.1111/codi.15056. Epub 2020 Jun 1. PMID: 32233064. [DOI] [PubMed] [Google Scholar]
- 9.Amin M.B., Edge S., Greene F., Byrd D.R., Brookland R.K., Washington M.K., Gershenwald J.E., Compton C.C., Hess K.R., et al., editors. AJCC Cancer Staging Manual. eighth ed. Springer International Publishing: American Joint Commission on Cancer; 2017. [cited 2016 Dec 28] [Google Scholar]
- 10.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P., STROBE initiative The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J. Clin. Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Dindo D., Demartines N., Clavien P. Classification of surgical complications. Ann. Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richards M., Anderson M., Carter P., et al. The impact of the COVID-19 pandemic on cancer care. Nat. Can. (Que.) 2020;1:565–567. doi: 10.1038/s43018-020-0074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton W. Cancer diagnostic delay in the COVID-19 era: what happens next? Lancet Oncol. 2020 Aug;21(8):1000–1002. doi: 10.1016/S1470-2045(20)30391-0. Epub 2020 Jul 20. PMID: 32702312; PMCID: PMC7834491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spinelli A., Carvello M., Carrano F.M., Pasini F., Foppa C., Taffurelli G., Ugolini G., Montroni I. Reduced duration of stay after elective colorectal surgery during the peak phase of COVID-19 pandemic: a positive effect of infection risk awareness? Surgery. 2021 Aug;170(2):558–562. doi: 10.1016/j.surg.2020.12.017. Epub 2020 Dec 23. PMID: 33714617; PMCID: PMC7757347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arneiro A.J., Ramos M.F.K.P., Pereira M.A., Dias A.R., Zilberstein B., Ribeiro Junior U., Nahas S.C. Impact of COVID-19 pandemic on the surgical treatment of gastric cancer. Clinics. 2021 Nov 26;76 doi: 10.6061/clinics/2021/e3508. PMID: 34852144; PMCID: PMC8595635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rottoli M., Pellino G., Spinelli A., Flacco M.E., Manzoli L., Morino M., Pucciarelli S., Jovine E., Abu Hilal M., Rosati R., Ferrero A., Pietrabissa A., Guaglio M., de Manzini N., Pilati P., Cassinotti E., Pignata G., Goletti O., Opocher E., Danelli P., Sampietro G., Olmi S., Portolani N., Poggioli G., COVID-CRC Collaborative Group Impact of COVID-19 on the oncological outcomes of colorectal cancer surgery in northern Italy in 2019 and 2020: multicentre comparative cohort study. BJS Open. 2022 Jan 6;6(1):zrab139. doi: 10.1093/bjsopen/zrab139. PMID: 35143629; PMCID: PMC8830755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Homma Y., Hamano T., Otsuki Y., Shimizu S., Kobayashi Y. Total number of lymph node metastases is a more significant risk factor for poor prognosis than positive lateral lymph node metastasis. Surg. Today. 2015 Feb;45(2):168–174. doi: 10.1007/s00595-014-0913-5. Epub 2014 May 15. PMID: 24831659. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki O., Sekishita Y., Shiono T., Ono K., Fujimori M., Kondo S. Number of lymph node metastases is better predictor of prognosis than level of lymph node metastasis in patients with node-positive colon cancer. J. Am. Coll. Surg. 2006 May;202(5):732–736. doi: 10.1016/j.jamcollsurg.2006.02.007. PMID: 16648012. [DOI] [PubMed] [Google Scholar]
- 20.Patriti A., Baiocchi G.L., Catena F., Marini P., Catarci M. FACS on behalf of the Associazione Chirurghi Ospedalieri Italiani (ACOI). Emergency general surgery in Italy during the COVID-19 outbreak: first survey from the real life. World J. Emerg. Surg. 2020 May 24;15(1):36. doi: 10.1186/s13017-020-00314-3. PMID: 32448333; PMCID: PMC7245630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reichert M., Sartelli M., Weigand M.A., Hecker M., Oppelt P.U., Noll J., Askevold I.H., Liese J., Padberg W., Coccolini F., Catena F., Hecker A. WSES COVID-19 emergency surgery survey collaboration group. Two years later: is the SARS-CoV-2 pandemic still having an impact on emergency surgery? An international cross-sectional survey among WSES members. World J. Emerg. Surg. 2022 Jun 16;17(1):34. doi: 10.1186/s13017-022-00424-0. Erratum in: World J Emerg Surg. 2022 Jul 8;17(1):39. PMID: 35710386; PMCID: PMC9202986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vose J.M. Delay in cancer screening and diagnosis during the COVID-19 pandemic: what is the cost? Oncology (Williston Park) 2020 Sep 15;34(9):343. doi: 10.46883/ONC.2020.3409.0343. PMID: 32965661. [DOI] [PubMed] [Google Scholar]
- 23.Quero G., Pecorelli N., Paiella S., Fiorillo C., Petrone M.C., Rosa F., Capretti G., Laterza V., Kauffmann E., Nobile S., Butturini G., Ferrari G., Coratti A., Casadei R., Mazzaferro V., Boggi U., Zerbi A., Salvia R., Falconi M., Alfieri S. Quantitative assessment of the impact of COVID-19 pandemic on pancreatic surgery: an Italian multicenter analysis of 1423 cases from 10 tertiary referral centers. Updates Surg. 2022 Feb;74(1):255–266. doi: 10.1007/s13304-021-01171-8. Epub 2021 Nov 24. PMID: 34817837; PMCID: PMC8611384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tebala G.D., Milani M.S., Bignell M., Bond-Smith G., Lewis C., Cirocchi R., Di Saverio S., Catena F., Scatizzi M., Marini P., CovidICE-International Collaborative Emergency surgery admissions and the COVID-19 pandemic: did the first wave really change our practice? Results of an ACOI/WSES international retrospective cohort audit on 6263 patients. World J. Emerg. Surg. 2022 Jan 28;17(1):8. doi: 10.1186/s13017-022-00407-1. PMID: 35090519; PMCID: PMC8795350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shinkwin M., Silva L., Vogel I., Reeves N., Cornish J., Horwood J., Davies M.M., Torkington J., Ansell J. COVID-19 and the emergency presentation of colorectal cancer. Colorectal Dis. 2021 Aug;23(8):2014–2019. doi: 10.1111/codi.15662. Epub 2021 Apr 24. PMID: 33793063; PMCID: PMC8250723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris E.J.A., Goldacre R., Spata E., Mafham M., Finan P.J., Shelton J., Richards M., Spencer K., Emberson J., Hollings S., Curnow P., Gair D., Sebag-Montefiore D., Cunningham C., Rutter M.D., Nicholson B.D., Rashbass J., Landray M., Collins R., Casadei B., Baigent C. Impact of the COVID-19 pandemic on the detection and management of colorectal cancer in England: a population-based study. Lancet Gastroenterol. Hepatol. 2021 Mar;6(3):199–208. doi: 10.1016/S2468-1253(21)00005-4. Epub 2021 Jan 15. PMID: 33453763; PMCID: PMC7808901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santoro G.A., Grossi U., Murad-Regadas S., Nunoo-Mensah J.W., Mellgren A., Di Tanna G.L., Gallo G., Tsang C., Wexner S.D., DECOR-19 Collaborative Group DElayed COloRectal cancer care during COVID-19 Pandemic (DECOR-19): global perspective from an international survey. Surgery. 2021 Apr;169(4):796–807. doi: 10.1016/j.surg.2020.11.008. Epub 2020 Nov 17. PMID: 33353731; PMCID: PMC7670903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allaix M.E., Lo Secco G., Velluti F., De Paolis P., Arolfo S., Morino M. Colorectal surgery during the COVID-19 outbreak: do we need to change? Updates Surg. 2021 Feb;73(1):173–177. doi: 10.1007/s13304-020-00947-8. Epub 2021 Jan 2. PMID: 33387170; PMCID: PMC7778389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mintz Y., Arezzo A., Boni L., Baldari L., Cassinotti E., Brodie R., Uranues S., Zheng M., Fingerhut A. The risk of COVID-19 transmission by laparoscopic smoke may be lower than for laparotomy: a narrative review. Surg. Endosc. 2020 Aug;34(8):3298–3305. doi: 10.1007/s00464-020-07652-y. Epub 2020 May 26. PMID: 32458289; PMCID: PMC7250491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kameyama H., Otani T., Yamazaki T., Iwaya A., Uehara H., Harada R., Hirai M., Komatsu M., Kubota A., Katada T., Kobayashi K., Sato D., Yokoyama N., Kuwabara S., Tanaka Y., Sawakami K. Comparison of surgical smoke between open surgery and laparoscopic surgery for colorectal disease in the COVID-19 era. Surg. Endosc. 2022 Feb;36(2):1243–1250. doi: 10.1007/s00464-021-08394-1. Epub 2021 Feb 22. PMID: 33616729; PMCID: PMC7899056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin C., Montesinos I., Dauby N., et al. Dynamic of SARS-CoV-2 RT-PCR positivity and seroprevalence among high-risk health care workers and hospital staff. J. Hosp. Infect. 2020;106:102e106. doi: 10.1016/j.jhin.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fontanet A., Autran B., Lina B., Kieny M.P., Karim S.S.A., Sridhar D. SARS-CoV-2 variants and ending the COVID-19 pandemic. Lancet. 2021;397:952–954. doi: 10.1016/S0140-6736(21)00370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caricato M, Baiocchi GL, Crafa F, Scabini S, Brisinda G, Clementi M, Sica G, Delrio P, Longo G, Anania G, de Manzini N, Amodio P, Lucchi A, Baldazzi G, Garulli G, Patriti A, Pirozzi F, Pavanello M, Carrara A, Campagnacci R, Liverani A, Muratore A, Siquini W, De Luca R, Mancini S, Borghi F, Di Cosmo M, Persiani R, Pedrazzani C, Scaramuzzi M, Scatizzi M, Vettoretto N, Totis M, Gennai A, Marini P, Basti M, Viola M, Ruffo G, Catarci M. Italian Colorectal Anastomotic Leakage (iCral) study group. Colorectal surgery in Italy during the Covid19 outbreak: a survey from the iCral study group. Updates Surg. 2020 Jun;72(2):249–257. doi: 10.1007/s13304-020-00760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.