Abstract
Transmembrane proteins comprise ~30% of the mammalian proteome, mediating metabolism, signaling, transport, and many other functions required for cellular life. The microenvironment of integral membrane proteins (IMPs) is intrinsically different from cytoplasmic ones, with IMPs solvated by a compositionally and biophysically complex lipid matrix. These solvating lipids affect protein structure and function in a variety of ways, from stereospecific, high-affinity protein-lipid interactions to modulation by bulk membrane properties. Specific examples of functional modulation of IMPs by their solvating membranes have been reported for various transporters, channels, and signal receptors; however, generalizable mechanistic principles governing IMP regulation by lipid environments are neither widely appreciated nor completely understood. Here, we review recent insights into the inter-relationships between complex mammalian lipidomes, the membrane physicochemical properties resulting from such lipid collectives, and the regulation of IMPs by either or both. The recent proliferation of high-resolution methods to study such lipid-protein interactions has led to generalizable insights, which are synthesized into a general framework termed the “functional paralipidome” for understanding the mutual regulation between membrane proteins and their surrounding lipid microenvironments.
INTRODUCTION
Functional interactions between proteins and other biomolecules are fundamental to molecular cell biology. Constituting ~30% of the mammalian proteome 1 and 60% of all drug targets 2, integral membrane proteins are solvated by complex mixtures of lipids that influence their structures, dynamics, and functions. However, in comparison to protein-protein and protein-nucleic acid interactions, the interactions between membrane proteins and their specific lipid nano-environments are not nearly as well characterized. For many IMP drug targets, the protein-lipid interface provides an important site through which pharmaceuticals enter the protein before accessing their binding sites, which are themselves often relatively hydrophobic 3. Despite the obvious fundamental and biomedical impacts, knowledge gaps regarding lipid-mediated IMP regulation persist because both membrane proteins and their associated lipids are ill-suited to classical analytical and conceptual paradigms of molecular cell biology. Practically, both IMPs and lipids are hydrophobic and poorly soluble, and co-assemble into large structures (i.e. membranes) that are problematic for many common structural and biochemical approaches. Conceptually, lipids can serve simultaneously as solvents, substrates, and regulatory co-factors for membrane protein activity (Fig 1) and these roles are often entangled and overlapping.
Figure 1. Inter-relationships between membrane lipidomes, protein structure/function, and collective membrane physical properties.
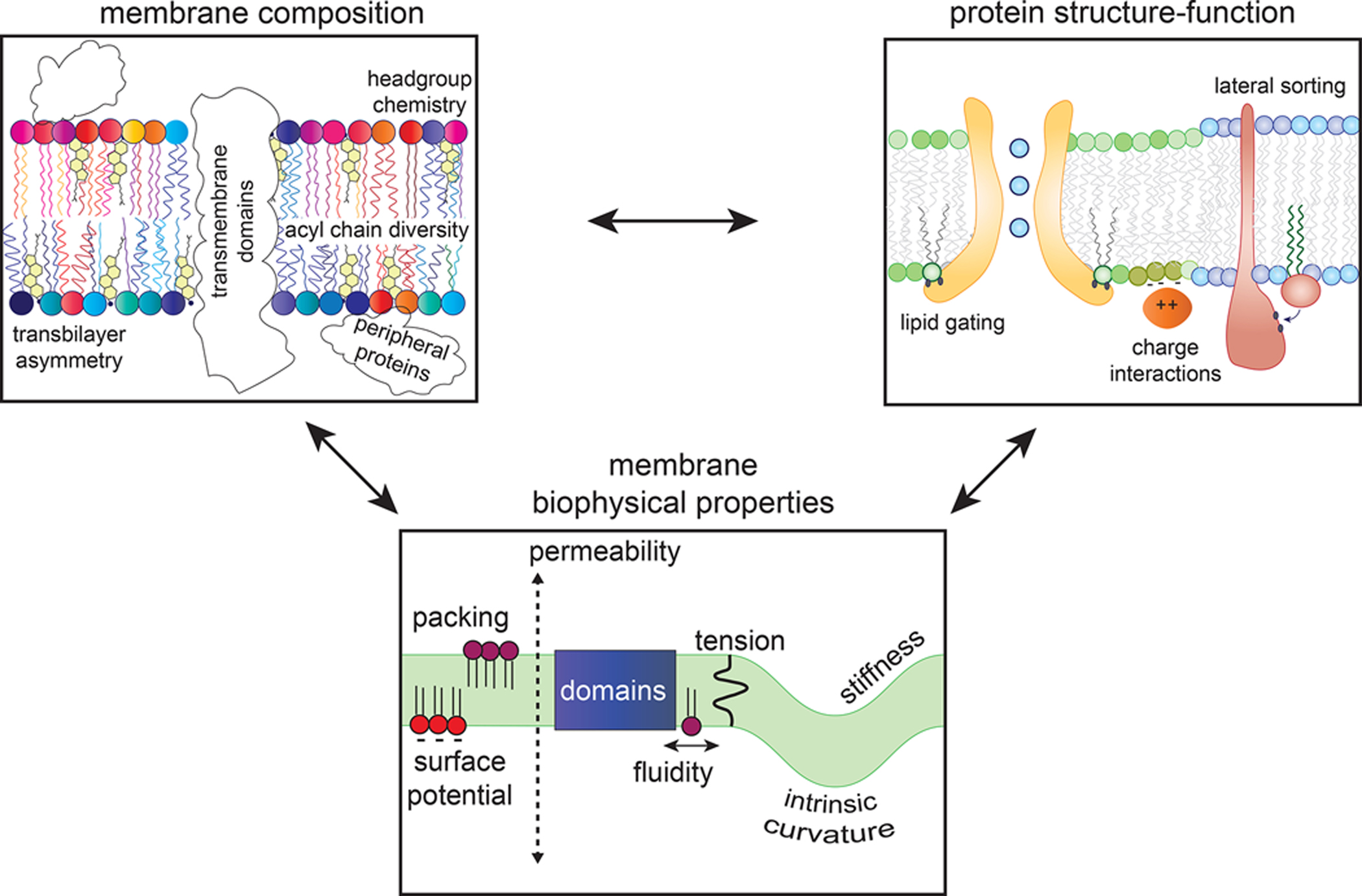
Individual lipid molecules can serve as specific cofactors for MPs, but lipids also collectively comprise the complex, dynamic solvent that determines the functional behavior of MPs. In turn, IMPs produce and transduce signals that can regulate membrane lipidomes, which themselves determine the biophysical properties of a given membrane.
The scope for protein regulation by membranes and their constituent lipids has been broadened by recent discoveries that reveal cellular lipidomes to be much more complex, diverse, and variable than is typically appreciated 4. Mammalian cells produce hundreds of distinct lipid species, and the specific lipid complement can vary dramatically depending on cell type 5,6, metabolic state 7, disease state 8, and external inputs (e.g. from the diet) 6,9. In some cases, a single specific lipid species appears to be required for regulating protein function, as was reported for the interaction between the transmembrane domain of the trafficking protein p24 and a sphingomyelin lipid containing an 18-carbon acyl chain (C18-SM) 10. Such remarkable specificity combined with the complexity of mammalian lipidomes suggests the potential for a layer of regulation of IMPs by lipids that has been underappreciated.
Here, we discuss illustrative recent examples of protein regulation by interactions with their solvating membranes and their constituent lipids. We describe distinct roles of individual lipid molecules as specific protein cofactors versus collective properties like membrane thickness or packing. We focus specifically on transmembrane rather than peripheral or lipidated proteins, whose lipid interactions constitute an important, but separate, area of membrane biology 11,12 (for readability, integral membrane proteins will be referred to hereafter as IMPs). Another broad area that will not be covered in this review are the various enzymes involved in production, degradation, and regulation of the lipidome whose functionality inherently involves lipid interactions. Finally, while there have been extensive demonstrations of functionally relevant binding between membrane proteins and lipids (many expertly reviewed previously 13–18) and protein-lipid interfaces as drug targets 3, our intent is not to document an exhaustive list of such examples. We instead present an experimentally informed and theoretically reinforced framework to rationalize the influence of lipids and bulk membrane properties on protein function.
IMP REGULATION BY MEMBRANE BIOPHYSICS
The combination of a cell’s lipidome and its collection of IMPs produces a bilayer membrane with remarkable properties. On molecular scales, biomembranes are fluids whose lipids and proteins mix and interact via diffusion in two dimensions. At intermediate scales we can speak of collective properties such as membrane thickness and lipid tilt. At still larger length scales, the same materials behave as thin-yet-robust elastic sheets that can be stretched, bent, and shaped into the variety of morphologies required for cellular functions 19. Across the length scales, the membrane acts on membrane-embedded proteins to regulate their interactions, conformations, localizations, and functions 20 (Fig 2).
Figure 2. How collective membrane properties can affect protein organization and function.
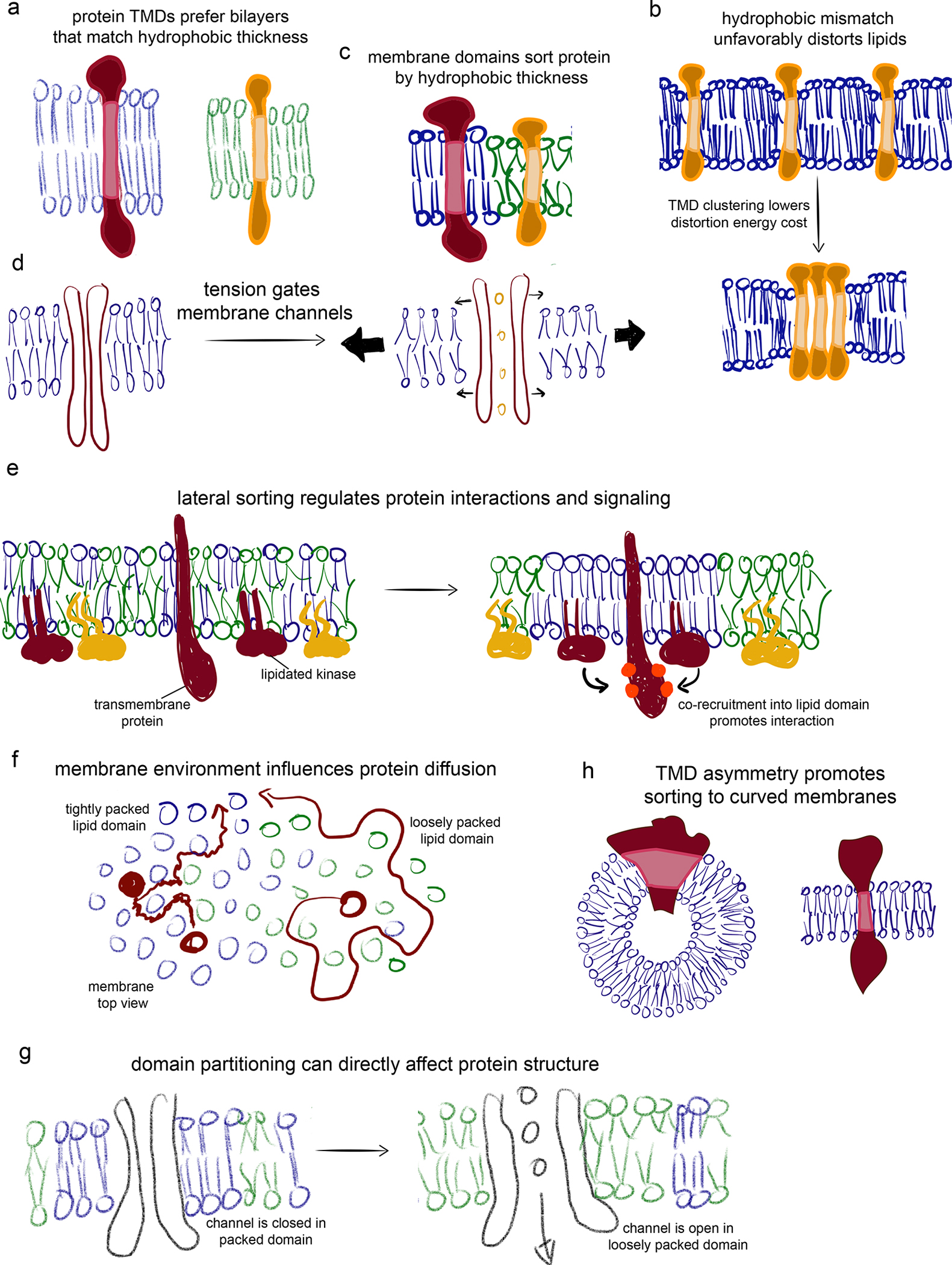
(a) Membranes comprised of lipids with long acyl chains and/or cholesterol have thicker hydrophobic cores and thus prefer IMPs with longer hydrophobic transmembrane domains (vice versa for thinner membranes). (b) In membranes containing domains of different thicknesses, proteins can be sorted by their TMD length. (c) Hydrophobic mismatches create disturbances in optimal lipid configurations. These can be minimized by clustering of misfit TMDs. (d) Mechanical tension applied to a membrane decreases lipid packing, thins the membrane, and disorders lipid acyl chains. These effects can be transduced by transmembrane channels to sense touch and pressure. (e) IMPs sorting via preferences for lipid domains can facilitate interactions with other domain residents or restrict collisions with domain-excluded components. (f) Domains can affect IMP dynamics, with more ordered and tightly packed domains slowing protein diffusion. (g) The distinct compositions and physical properties of various membrane environments can directly regulate protein structure and activity. (h) TMD shape can promote sorting to membrane subdomains of different curvature.
Thickness mismatch can drive lateral and subcellular protein sorting
A fundamental structural feature of membranes is their thickness, which is largely determined by the length and order of lipids’ hydrophobic chains. These chains can vary from 12 carbons up to 24 carbons in mammalian cells, suggesting a range of possible membrane thicknesses from ~3–4.5 nm 21. While there have been few direct measurements of biomembrane thickness, X-ray scattering of purified membranes indicates that plasma membranes are >10% thicker than internal membranes 22 (Fig 3). More recently, direct images of membrane thickness variations in biomimetic and bioderived membranes 21,23 have been obtained by cryoEM, with similar images of cryopreserved cells suggesting feasibility of biomembrane thickness measurements in situ 24.
Figure 3. Physical and compositional variations in subcellular membranes.
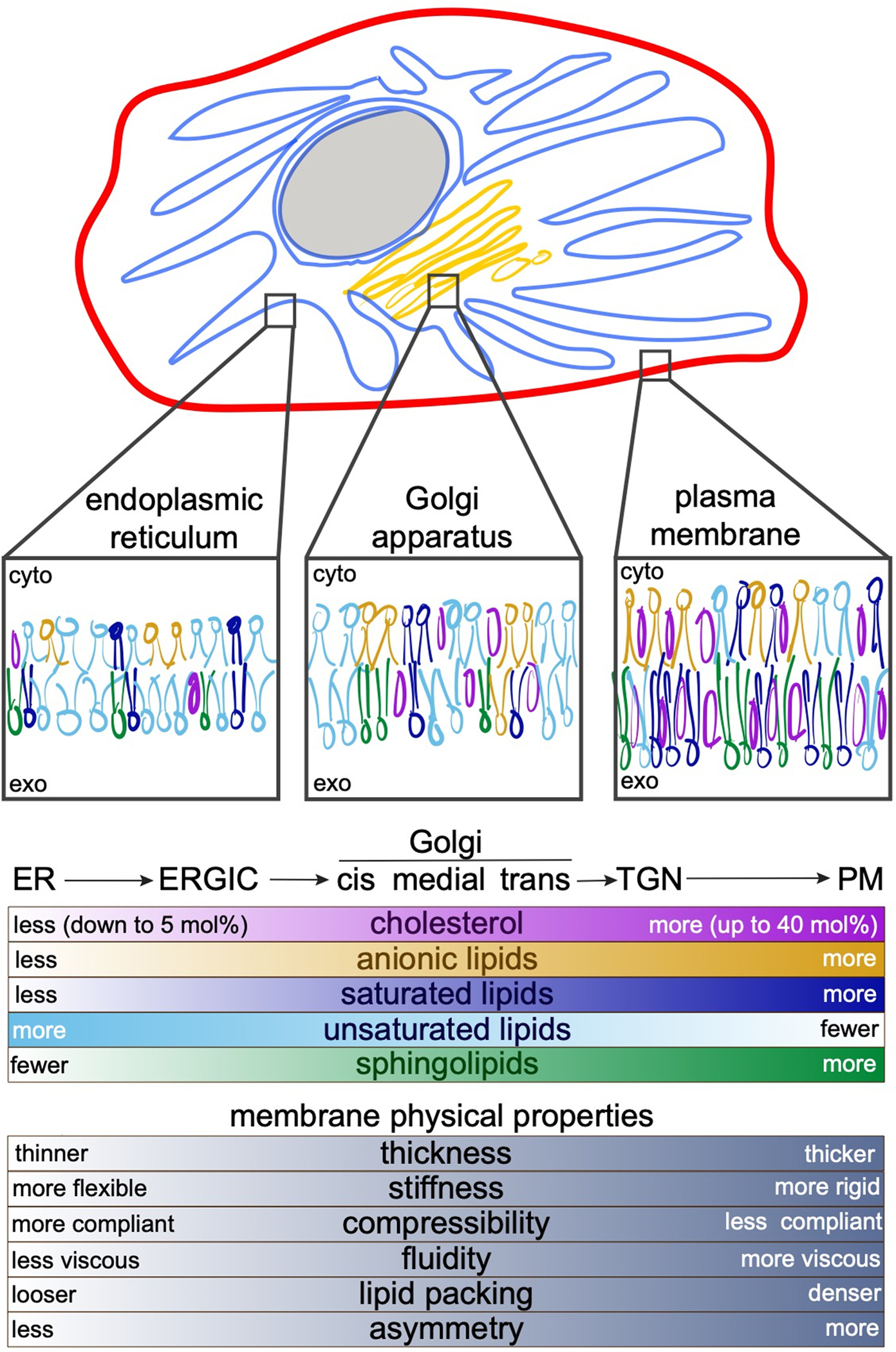
Membranes of various subcellular organelles can differ dramatically in their compositions and resulting physical properties. The secretory pathway is experienced by most MPs, because they are synthesized in the ER, modified and sorted in the Golgi, and function at the PM. In their journey through these membranes, IMPs experience increasing cholesterol, sphingolipids, and saturated lipid concentrations. The membranes likely become more asymmetric with respect to the lipid compositions of the two bilayer leaflets, with the outer leaflet become more tightly packed and ordered, while the inner leaflet adopts greater negative charge due to increasing concentrations of anionic lipids. As they progress through the secretory pathway, membrane also become less fluid, more rigid, and thicker. In polarized cells, specialized regions of the PM like the apical PM of epithelial cells 22,175,176 may be especially rich in tightly packing lipids and therefore thicker, less permeable, and more viscous. These features can be used to sort proteins between organelles and regulate their function within them. For example, TMDs of IMPs in the PM are longer and more asymmetric (i.e. bulkier near the exoplasmic leaflet, thinner near the cytoplasmic) compared to those of the ER and Golgi 30,163.
Variations in membrane thickness become relevant for proteins when the lengths of their hydrophobic membrane spanning regions (their transmembrane domains, TMDs) do not match the hydrophobic thickness of the surrounding membranes (Fig 2a)25. Such ‘hydrophobic mismatch’ is energetically unfavorable because lipids must deform (e.g. compress) to avoid exposure of hydrophobic regions to water (Fig 2b). At the extremes, sufficient compression of the membranes allow lipids to scramble from one leaflet to the other 26. In a membrane of uniform thickness, misfit IMPs may cluster to minimize membrane distortion 27 (Fig 2c). In membranes with coexisting domains of different thicknesses, proteins are laterally sorted to their least-mismatched domain (Fig 2c). It should be emphasized that these descriptions treat the membrane as a homogenous continuum, with elastic costs imposed by bending or stretching. However, at the molecular level relevant for protein interactions, membranes are composed of discrete lipid species with complex biological lipidomes comprised of many different species capable of conforming to various shapes and sizes of proteins. Thus, in biological contexts, elastic considerations must be considered alongside more local effects (e.g. sorting of short lipids near short proteins)28,29.
The energetics of hydrophobic mismatch are likely responsible for the conserved variation in TMD length between subcellular organelles, with TMDs of the endoplasmic reticulum and Golgi being shorter than those of proteins that reside in the plasma membrane (PM) 30 (Fig 3). These variations correlate closely with the presumed cholesterol gradients between those membranes, suggesting that cholesterol concentrations are important determinants of organellar membrane properties (Fig 3). The striking correlation between TMD length and hydrophobic thickness of organellar membranes suggests that proteins and lipids co-segregate based on their biophysical properties, and that such segregation may be a prerequisite to proper subcellular trafficking 31. Yeast use an analogous mechanism for sorting membrane proteins between the mother cell and the daughter bud. Here, a thick ER domain at the cleavage furrow appears to act as a filter, preferentially allowing long-TMD proteins to pass through 32. A recent report suggested that analogous domains in the mammalian ER may be organized by interactions between a multipass protein sigma-1 receptor (S1R) and cholesterol 33, and that such domains may mediate intra-organelle contacts 33,34.
Just as hydrophobic mistmatch applies stress to bilayer lipids (Fig 2b), that stress is also propagated to the proteins. In some cases, this stress can change their conformation and function (Fig 2f)25. This effect has been most clearly demonstrated for pumps and transporters, including the Na+/K+ ATPase 35, a bacterial aquaporin, and the sarcoplasmic ER Ca2+ ATPase (SERCA) 36, and certain GPCRs 37, all of which show clear variation in activity as a function of membrane thickness, with maximal activity in optimally matching membranes (for in-depth discussion of hydrophobic mismatch, see 13,38). More recently, the oligomeric assembly of a bacterial antiporter was shown to be driven by hydrophobic mismatch between the membrane and one protein surface that becomes buried upon homodimerization, lowering the overall free energy of the system 28. Doping membranes with short-chain lipids that solvate this special surface reduces dimerization, demonstrating the critical importance of lipid context in IMP functional assembly.
The notion that bilayer thickness regulates protein function suggests that this thickness must be sensed and regulated by cells. A prototype membrane thickness sensor is the bacterial “molecular caliper” called DesK 39, which changes conformation as a function of membrane thickness to activate a B. subtilis two-component signaling system. Intriguingly, the originally proposed function of this protein is for sensing temperature, with the rationale that colder temperatures lead to thicker membranes as lipids pack more tightly together and their acyl chains elongate.
A related but distinct principle is protein sorting by membrane curvature. An array of peripheral proteins, many containing banana-shaped features called BAR domains, are known to preferentially bind highly curved membranes and also to induce such curvature when applied to flat membranes 40. Such curvature selectivity is often accomplished by amphipathic helices that insert into one leaflet of the bilayer 41. However, some transmembrane proteins also appear to sense curvature 42, preferentially enriching in highly curved membrane tubes (Fig 2h). Other IMPs generate membrane curvature by repulsive interactions of their extramembrane domains 43,44. Finally, post-translational modifications near the transmembrane domain can affect proteins’ curvature preference, which may be used to sort proteins in the secretory pathway 45.
Protein sensors of membrane fluidity and lipid packing
The fluidity of biomembranes is fundamental to their function, as it allows proteins and lipids to explore the cell surface and find interaction partners. The viscosity of this fluid largely determines the lateral diffusion of IMPs and lipids, and therefore the frequency of the encounters that underlie signaling and other cellular processes. An elegant recent demonstration of this principle linked cellular respiration rates to the fluidity of the inner mitochondrial membrane via the intramembrane diffusion of quinones, key intermediates of the electron transport chain 46.
The intrinsic capacity for cells to autonomously maintain their membrane fluidity has been recognized since the 1970s 47. This behavior was first identified in bacteria using fluorescent probes that measure membrane viscosity via their rotational motion, reported by the time-dependent loss of fluorescence polarization. These measurements revealed a remarkable homeostasis mechanism, wherein membrane viscosity remained unchanged despite changes in cells’ growth temperature via regulation of the lipidome (increased unsaturation as growth temperature is lowered). This adaptation, termed homeoviscous adaptation 47, is in striking contrast to synthetic membranes, which (unable to modify their composition) become more viscous at lower temperature as hydrocarbon chains order, packing lipids closer together. Similar regulation of membrane fluidity in response to temperature variation has been reported across the tree of life 48, from yeast to plants 49, fish 50 and worms 51. Recently, an analogous lipidomic and biophysical homeostasis has been described across various mammalian cell types whose membrane properties were challenged by lipid inputs from the diet 9.
Homeoviscous adaptation requires a mechanism for sensing membrane fluidity, which must then be linked to the machinery that regulates membrane composition. Viscosity is inherently a dynamical (rather than structural) property; thus a direct sensor of viscosity would have to integrate the dynamics of a IMP over time. For example, a change in viscosity might change the rate of conformational dynamics of a MP, resulting in accumulation or depletion of a downstream signal. A mechanism like this was recently proposed for a yeast transmembrane protein called Mga2 52, a critical regulator of unsaturated lipid production (a good start for controlling viscosity). However, the dynamic explanation for Mga2’s function has come under recent scrutiny 53,54 — it appears instead that dimerization of the TMD domain of Mga2 responds to subtle changes in lipid packing in the hydrocarbon core.
These findings raise questions regarding what, exactly, cells are aiming to maintain and what they can sense to do so. While membrane viscosity (as measured by probes embedded in the hydrophobic bilayer core) is clearly maintained in various settings, there remains the possibility that viscosity is an epiphenomenon that changes together with other membrane properties. For example, Ire1, an important regulator of the unfolded protein response (UPR), appears to be activated by changes in membrane compressibility, i.e. how much energy it takes to stretch or compress a bilayer. The TMD of Ire1 contains an amphipathic helix that “squeezes” the ER membrane. When the ER membrane becomes too saturated, it becomes more difficult to compress, causing the Ire1 TMD to oligomerize and creating a signal to produce the stress response 55.
Force from membranes regulates proteins
In addition to a plethora of biochemical inputs, cells must transduce mechanical stimuli such as membrane stretching/compression, changing elastic moduli, and localized stresses. For many of these functions, the membrane provides the medium that transmits mechanical information to protein sensors (Fig 2d), such as the molecular mechanosensors of the MscL and MscS families 56. Functioning as cellular osmoregulators, these channels open in response to tension applied to their surrounding membrane, as in the case of hypo-osmotic shock. The structural mechanisms of such lipid-mediated opening have recently been described, with lipids playing roles in both conducting mechanical force to the protein and direct occlusion of the water permeation path 57. A similar role has been ascribed to mechanosensitive mammalian channels known as Piezo1 and Piezo2 58, that transduce touch and strain (e.g. due to arterial pressure). The mechanism of Piezo gating appears to involve their deformation of the surrounding membrane to form a dimple around the protein 59. When the membrane is stretched, the dimple is straightened, and the channel opens. Other eukaryotic mechanosensors function via analogous mechanisms, suggesting that cellular mechanical stimuli may be generically sensed via membrane tension 60. Under special circumstances, this mechanism may also run in the opposite direction, with conformational changes in transmembrane proteins producing tension in membranes. This is the case for the electromotive protein Prestin 61,62, which produces the remarkable cellular contraction required for auditory signal amplification in mammalian outer hair cells 63.
Lipid phases in protein organization
Lipids self-organize into membranes but can also spontaneously demix into coexisting domains via liquid-liquid phase separation. This self-organizing capacity is functionalized by cells for lateral membrane organization into functional microdomains known as lipid rafts 64. After a cycle of excitement, confusion, and controversy, the raft concept has emerged as an important paradigm in membrane biology 65. Building upon a mountain of biophysical insights in synthetic model membranes 66–69, a critical confirmation of the raft concept was the observation 70,71 and biophysical characterization of lipid liquid-liquid phase separation in isolated mammalian plasma membranes 72–75 and vacuoles of living yeast cells 76,77. The nature, compositions, functions, and controversies surrounding membrane rafts have been extensively reviewed 64,78 and will not be further discussed here.
Membrane domains function largely by sorting proteins laterally via their preference for distinct lipid environments (Fig 2c). The structural bases for these preferences have been characterized by quantitative measurements of protein and lipid partitioning in isolated PM vesicles (often called Giant Plasma Membrane Vesicles 79). For single-pass proteins, the TMD is the major determinant of raft microdomain affinity. Long 80, thin 81 TMDs, supported by post-translational palmitoylation 82, tend to prefer ordered membrane domains. The principles governing lipid anchored protein partitioning are similarly well understood, with proteins anchored by sterols and saturated acyl chains preferring ordered domains while short, branched, or unsaturated lipid anchors drive affinity for disordered domains 83. The determinants for multi-pass transmembrane proteins are less clear, though recent observations suggest that most are excluded from the ordered phase of GPMVs 84. Together, these features allow the assembly of membrane proteins and lipids into signaling complexes 85 (Fig 2e), enable transmembrane protein trafficking 31,80, and support assembly of complex multimolecular machines like viruses 86,87.
Lipid microdomains may also affect protein function beyond simply reorganizing their lateral organization. First, it is likely that the large differences in lipid composition between various microdomains may influence protein conformation, as detailed below (Fig 2g). In some cases, it has been shown directly that cholesterol-induced lipid ordering can meaningfully modulate a protein’s conformation landscape, as for rhodopsin 88, other GPCRs 89, and potassium channels 90. Another possibility is that membrane domains may regulate protein-protein interaction dwell times by decreasing their diffusivity (Fig 2f).
The most notable protein-lipid interaction implicated in microdomain formation exists within a subtype of ordered membrane microdomain called caveolae, which are small invaginations of the PM dependent on the protein caveolin and its interaction with cholesterol 91. Historically, caveolae were often conflated with rafts because they share many biophysical, compositional, and functional qualities 92. Although ordered lipid domains are clearly possible without caveolar proteins, caveolae likely represent a functionally important, stabilized, immobilized assembly of ordered lipids 93. These stabilized caveolar domains dramatically enrich cholesterol compared to the surrounding PM 94 and their formation absolutely requires high levels of cholesterol 95, possibly mediated by putative cholesterol-binding sequences present in the unusual membrane-embedded domain of caveolin-1 96. Interestingly, caveolae have been shown to disassemble in response to membrane stretch 97, resulting in release of caveolar-sequestered lipids, which significantly change the surrounding lipid content 93. This coupling suggests a functional interplay between lipid-mediated lateral organization and mechanical stresses on the membrane.
INTERACTIONS OF MEMBRANE PROTEINS WITH LIPIDS
In addition to being potential binding partners and functional cofactors, lipids are also the solvating medium for membrane proteins. While many peripheral proteins are recruited to membranes by tight binding to specific lipid headgroups 11,12, defining interactions between IMPs and lipids is conceptually more difficult. Several models for such interactions have been proposed, from specificity for a single lipid species 10, to a stably-associated, selective lipid ring 98, to non-selective transmembrane domain solvation 99. These apparently distinct interaction modes, however, differ only in degree, not quality. This continuum of possible interactions presents a challenge to the interpretation of experimental data, since different experimental (and simulation) techniques are typically restricted to a relatively narrow dynamic range, and therefore only reveal a correspondingly narrow window of protein-lipid interactions. Moreover, the apparent affinity of a particular lipid-protein interaction will depend on the specific mixture of lipids in which the protein finds itself. We return to this problem below in the section on “Interpreting Protein-Lipid Interaction”, after discussing early evidence that IMPs recruit particular lipid environments, and then structural evidence for specific interactions.
Early evidence for annular lipids
The first direct evidence for lipid protein interactions was obtained by EPR spectroscopy (expertly reviewed in 98). Experiments in the early 1970s using spin-labeled lipids observed a spectrally distinct fraction of lipids around bovine cytochrome oxidase, interpreted as a layer of bound lipids around the protein’s perimeter 100 (Fig 5a). Later analysis of this ‘bound’ fraction using conventional binding isotherms suggested a single type of “binding site” 101. Modeling of EPR lineshapes then permitted determination of lipid exchange rates between the protein surface and the bulk, finding them to be about 10−7 sec−1 (or a lifetime at the protein surface of 100 nsec) 102.
Figure 5. Possible modes of lipid interactions with transmembrane proteins.
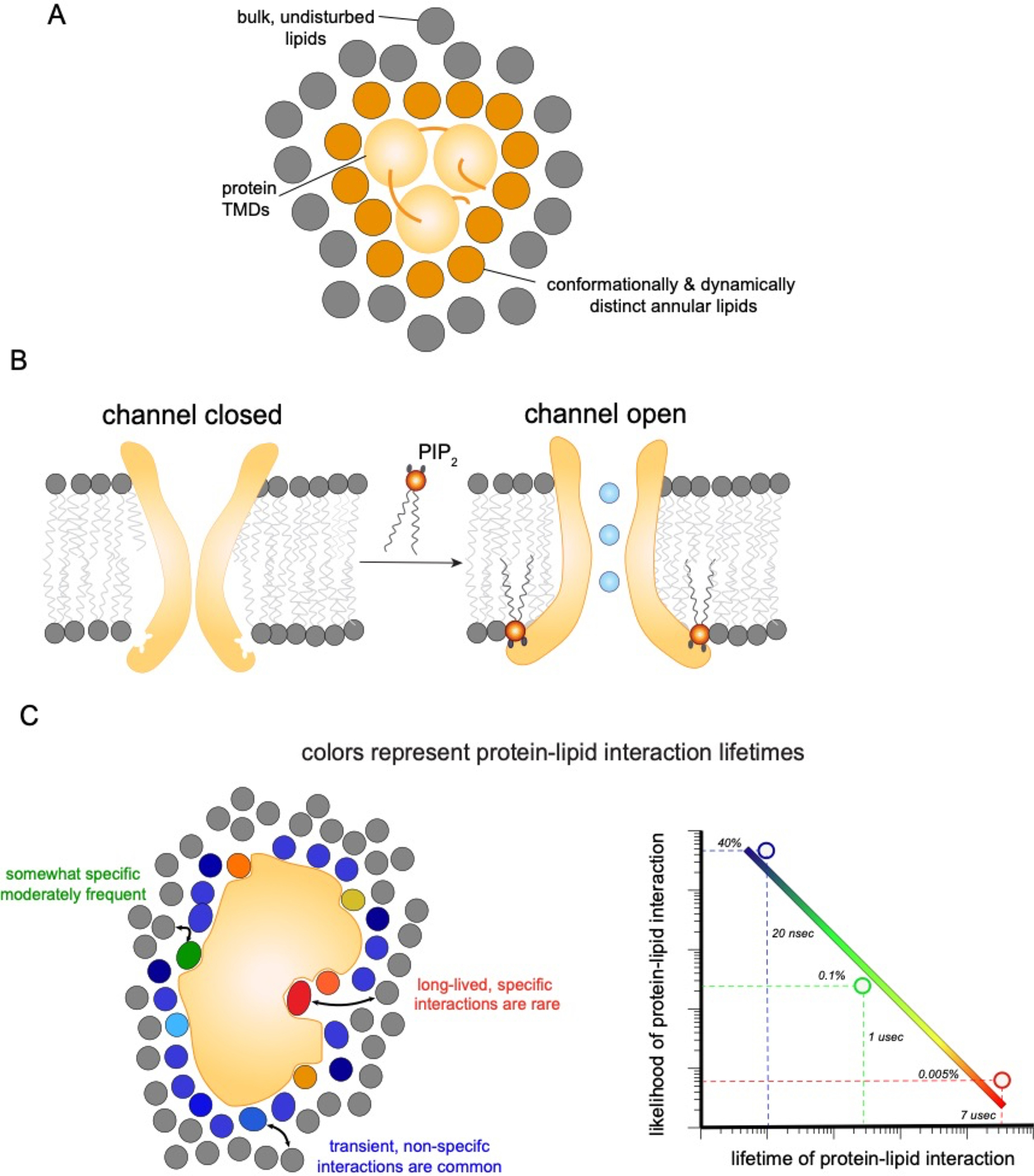
(a) A shell of annular lipids stably associates with transmembrane protein regions. The exchange rate of these annular lipids with the bulk is slow, such that these lipids can be conceptualized as an extended part of the protein. (b) Charged and large lipids like PIP2 (orange), can be tightly and specifically bound by protein domains to introduce large scale conformational changes. In these cases, lipids act as allosteric ligands for protein function. (c) Interactions between IMPs and individual lipid molecules (colored) are in constant competition with those from bulk lipids (gray). The affinity for a particular lipid species determines how likely it is to occupy a particular site, relative to its bulk concentration. The lifetime of those interactions is directly related to affinity. Simulations suggest that protein-lipid interactions span a range of lifetimes from relatively high affinity interactions (which are rare) to very common short-lived interactions (nanosecond range), which indicate rapid lipid exchange not influenced by protein binding. These observations imply that there is no single characteristic scale for protein-lipid interactions, but rather that specific, semi-selective, and bulk solvent-like effects could potentially be simultaneously relevant.
These experiments indicated that lipids in the immediate environment (first solvation shell) of a protein are different from lipids in the bulk – dynamically restricted and slower to exchange. This set of observations was later synthesized into the concept of a “lipid annulus,” with properties and composition distinct from the bulk membrane (Fig 5a). However, since the EPR signal is averaged over the entire protein surface, it affords no insight into localization of lipid-protein interactions within the annulus, which must be obtained by other techniques. For example, selectivity of anionic lipid interactions with a potassium channel was reported using brominated lipids to quench Trp residues on lipid-facing domains of KcsA 103.
Ligand-like, specific protein-lipid binding
What about even more localized, more specific lipid interactions? Structurally detailed information on lipid-protein binding is sometimes obtained in high resolution structures, at the cost of removing the protein from its native environment. Early advances in structural methods for membrane proteins led to several well-resolved transmembrane protein structures, with some of these revealing electron densities consistent with lipid molecules 104–106. A lipid surviving the process of solubilization, purification, and crystallization suggested a significantly tighter binding than the few kT obtained for lipid-protein interactions from EPR measurements, raising doubts about the presence of a single population of “annular lipids,” and leading to the concept of specific lipid binding sites. (A comprehensive list of structural data is beyond the scope of this review, and would anyway make for tedious reading.) The curious reader is directed to several excellent recent reviews 16,107.
While specific lipid structures are often poorly resolved, suggesting that the lipids do not adopt a specific conformation or that a variety of different lipids may be bound, occasionally specific lipids are clearly identifiable and are then found to act as essential regulators of protein activity. A famous example is direct binding and activation of the inward rectifying potassium channel Kir2.2 by the phosphorylated lipid PIP2 (phosphatidyl inositol 4,5 bisphosphate) 108 (Fig 5b). This mechanism may be used to dynamically regulate Kir channels, as PIP2 can be rapidly produced, consumed, or sequestered by various cytoplasmic proteins 109. A non-exclusive alternative is that PIP2-binding signals arrival of the potassium channels on the PM (where PIP2 is enriched, Fig 3), preventing ion leakage during biosynthesis or trafficking. An intriguing wrinkle is that Kir channels also interact with another lipid characteristic of the PM, cholesterol 110. However, while PIP2 activates the channel, cholesterol appears to suppress the open state 111. This modulation of Kir activity has prompted the intriguing hypothesis that translocation to cholesterol-rich lipid rafts may be a mechanism for functional regulation of these channels 90,111. Other, weaker lipid association sites on Kir2.2 have also been reported, with a complex interplay and competition between them 112.
The example of Kir channels is instructive, as their regulation by PIP2 and cholesterol appears to be characteristic of many cell-surface ion channels 113, transporters 114, and receptors 107. Quite why these particular lipids are so over-represented in membrane protein structures is somewhat mysterious. The case for PIP2 may be clearer: it has a large, highly charged, stereospecific headgroup that can be coordinated by positively charged binding pockets. PIP2 composition and localization are tightly regulated by enzymatic control, while its confinement to the inner leaflet of the PM may make PIP2 a useful coincidence detector for cell surface localization. Because of these features, it is easy to imagine how and why specific PIP2 binding has evolved. Similar arguments could be made for other relatively inabundant, highly localized anionic lipids, including other phosphatidylinositides, cardiolipin 115, and phosphatidic acid 116.
Cholesterol, on the other hand, has none of these features, being deeply membrane embedded, almost entirely hydrophobic, and present in many cellular membranes. Perhaps its most outstanding characteristic is its high concentration in the PM (30–40%), with the many biophysical sequalae associated with cholesterol-rich membranes (higher lipid packing, ordered domains, increased thickness – see Fig 3). However, cholesterol’s structure and dynamics are entirely distinct from other membrane lipids, containing five rigidly coupled rings rather than long, floppy tails. This relative rigidity means that cholesterol pays a smaller entropic price for being bound to protein surfaces. These features perhaps contribute to the many examples of cholesterol binding to, and occasionally functionally regulating, membrane proteins, including multipass receptors 117–124, channels 125,126, transmembrane oligomers 124,127, and even single-pass transmembrane domains 128. Interestingly, despite the ubiquity of reported cholesterol interaction sites, their structural determinants remain ambiguous. While “Cholesterol Recognition/interaction Amino acid Consensus (CRAC)” sites have been widely reported, the predictiveness and structural basis of this motif has been questioned 129.
In addition to the many reports of cholesterol and PIP2 binding are more rare cases of other lipids interacting with and regulating membrane proteins. A notable category are glycosylated sphingolipids, which are often ligands for soluble protein binding (e.g. for bacterial toxins 130,131 or viruses 132), but also can interact in cis (i.e. on same membrane) with membrane proteins 133,134.
INTERPRETING PROTEIN-LIPID INTERACTION
Despite the tremendous technological advances across various structural and biophysical techniques, there remain conceptual shortcomings in our understanding of the mutual regulation between membrane proteins and their associated lipids. These ambiguities remain because of challenges in interpreting measurements of protein-lipid interactions, and because of limitations in molecular simulation techniques.
For example, densities consistent with lipids have often been identified in crystal and cryoEM structures of membrane proteins and such observations are often taken as bona fide evidence of protein-lipid binding. However, it should be noted that lipid structures are rarely well-resolved, leaving doubt to their specific identity. In some cases, the poor resolution may be due to lipids’ inherent flexibility. Another possibility is that the lipid component may be intrinsically heterogenous, with a variety of potential lipids filling a given hydrophobic cavity within a protein. Similarly, an amphiphilic detergent molecule may fill the niche that would have been occupied by lipids in situ. These possibilities imply that while some lipid is an essential component of a membrane protein’s structure, there may be very weak selectivity for any particular lipid type. Such sites would be more akin to solvating water molecules for soluble proteins rather than specific ligands.
On the other hand, evidence for more selective interactions has come from some of the most elegant lipid-IMP binding experiments to date, which used native mass spectrometry to directly reveal protein-lipid coupling and the structural and functional consequences thereof 135 (Fig 4d). These experiments generally show that structurally diverse lipids bind with similar affinities 136,137, though with some important exceptions as in the bacterial transporter LeuT, where phosphatidylglycerol appears to be essential for dimerization 138.
Figure 4. Methods to study MP-lipid interactions.
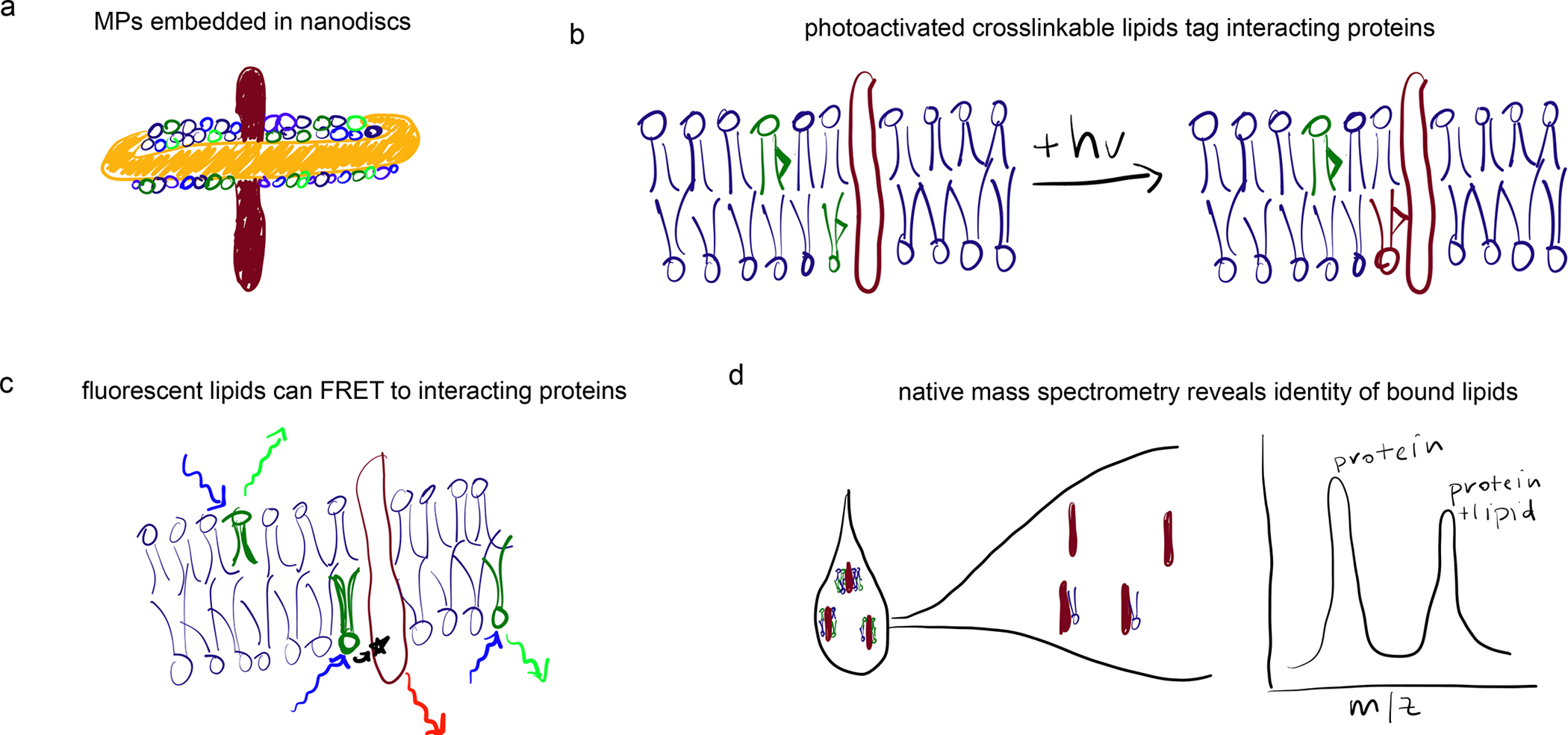
(a) Nanodiscs supported via amphiphilic protein or polymer scaffolds provide a native-like membrane environment for MPs. (b) Photoactivatable crosslinks can be introduced into lipid acyl chains, facilitating identification of protein-lipid interactions in situ. (c) FRET between fluorescently labeled lipids and proteins can report on interaction potentials. (d) Mass spectrometry of undigested proteins extracted from cells can reveal exact molecular identities and stoichiometries of bound lipids.
Lipids bearing photoactivated crosslinking groups are a powerful technology for assaying protein-lipid interactions (Box 1, Fig 4b). However, interpretations of such experiments must account for the likelihood of labeling without specific interactions. This is because reactive lipids present in the membrane at any reasonable abundance will have access to most IMPs. Consider a moderately-sized membrane protein, such as a GPCR with a diameter ~3 nm, which would be surrounded at all times by ~40 bilayer lipids 139 (e.g. Fig 5c). Assuming no selectivity for any set of lipids, a cross-linker or other labeled lipid present at 1 mol% would be present in the first interacting shell of ~40% of the protein ensemble. In principle, it is possible that more specific interactions could be captured above such a high background of non-specific labeling, but it is an inescapable conclusion that highly abundant lipids (e.g. cholesterol in the PM) are encountering most membrane proteins most of the time. Specificity in such experiments is often demonstrated through identification of the putative lipid-binding site, whose mutation then reduces labeling. While such observations support specificity, it is essential to verify that mutations do not dramatically affect the lipid-facing interface of the protein (e.g. by disrupting TMD oligomers) or change a protein’s subcellular localization. In the latter case, the different lipid compositions of various subcellular membranes (Fig 3) would be expected to affect the likelihood of labeling. Similar issues may affect FRET-based experiments (Fig 4c).
Box 1: Structural methods to study interactions between lipids and integral membrane proteins.
Structural biology: X-ray crystallography and, more recently, cryogenic electron microscopy (cryo-EM) have been extensively applied to membrane proteins, with thousands of structures already solved 177 and more being rapidly produced. In almost all cases, native lipids are replaced by detergents during the purification procedure. However, supplementing proteins crystals or crystallization solutions with lipids can sometimes reveal lipid binding sites, and, more importantly, demonstrate structural and functional effects of bound lipids, as is the case for PIP2’s gating of the inward rectifying potassium channel Kir2.2 108.
Electron crystallography: A specialized application of crystallography for analysis of lipid-protein interactions relies on electron diffraction to achieve atomic-level resolution of structure of both protein and lipids for specialized membrane proteins that can form two-dimensional crystals embedded within a lipid matrix. For example, the seven-pass channel bacteriorhodopsin was imaged within its native membrane environment 178 to reveal intimate integration of lipids within the transmembrane domain bundle and between tightly packed oligomers 179. Notably, despite the unprecedented resolution, the specific structures of these lipids could not be identified, likely because they are not specific. That is, they are both conformationally flexible (i.e. disordered) and compositionally flexible (different lipid species can be accommodated at the same location). The apotheosis of this technique was a <2 A resolution structure of the water channel aquaporin 0 (AQP0) 99, complete with surrounding lipids and water molecules in the pore. Here again, no stereospecific lipid interactions were observed.
Nanodiscs: an important addition to structural biology of protein-lipid interactions has been the development of nanodiscs 149 (Fig 4a). The intrinsic bilayer-stabilizing properties of apolipoproteins enabled the design of a family of membrane scaffolding proteins (MSP). When mixed with lipids and membrane proteins of interest, these MSPs facilitate formation of ~10 nm discs in which the protein target is solvated by a semi-native lipid bilayer. Combined with cryoEM, nanodiscs have propelled an explosion of structural insights into membrane protein structure 149. More recently, synthetic polymer scaffolds have proven capable of producing similar protein/lipid nanodiscs 150. Importantly, these can extract proteins directly from cellular membranes, obviating the need for detergents and suggesting the potential for isolating the native paralipidomes of membrane proteins 146.
Native mass spectrometry (MS): the development of “soft ionization” methods like Electron Spray Ionization (ESI) has facilitated mass spectrometry of intact complex biomolecules. ESI-MS was an enabling technology for shotgun lipidomics, allowing identification and quantification of hundreds of lipid species without the need for chromatographic separation or fragmentation 180. More recently, similar methodology was applied to membrane proteins 181, revealing that intact folded proteins could be stripped of their detergent and analyzed for oligomerization, lipid binding, and most importantly, the structural and functional consequences of lipid-protein interactions 135 (Fig 4d).
Given the difficulties in directly identifying lipid-MP interactions, why not look for titratable effects of a given lipid on protein structure or function? For soluble small molecules, such dose-dependence and saturability are usually accepted as bona fide evidence of specific biomolecular interactions. Unfortunately, such experiments can be quite difficult to perform rigorously for MPs, owing to the difficulty of achieving sufficient dilution over a wide range of concentrations. This barrier has been recently overcome by elegant experiments on the lipid dependence of the dimerization of a chloride transporter, showing that lipid dependence of IMP function is not well described by a simple binding isotherm, as would be predicted by the classic binding and linkage model 28. Instead, at low concentration of a short chain lipid (which would serve as a putative “ligand”), the dependence of the dimerization equilibrium depends logarithmically on concentration, indicating a preferential solvation effect, first described in the context of soluble protein folding 140. This effect is conceptually very different from either a ligand-like activity or regulation by bulk membrane properties.
Moreover, changing membrane composition during a titration experiment also changes collective membrane properties, which can influence protein structure and function independently from specific interactions, as discussed above (Fig 1). Cholesterol is especially problematic, since increasing membrane cholesterol will also increase membrane thickness and has a strong effect on the chemical potential of other lipids, especially sphingomyelin. As an example, consider an IMP with a binding site for cholesterol in two different membranes: (1) a polyunsaturated phospholipid with 30 mol% cholesterol versus (2) a saturated sphingomyelin with the same fraction of cholesterol. Because cholesterol interacts preferentially with sphingolipids and saturated lipids, its chemical activity is much lower in the second case and therefore the occupancy of the cholesterol binding site will be lower compared to the unsaturated/cholesterol mixture. While similar effects may complicate binding equilibria for soluble proteins, they are much more significant for lipid-protein interactions, where the solvent is crowded and is almost never dilute 141. Such collective effects are often complex, dose-dependent, and saturable, complicating simple interpretations of titration experiments.
Driven partly by these challenges, simulations have become an important complement to experimental measurements of lipid binding (recently reviewed in 142), revealing a broad spectrum of lipid-protein interactions 122(Fig 5c). Imagining lipid-protein interactions to be either tightly bound and ligand-like or transient and solvent-like has been the dominant paradigm since the early EPR measurements described above, guiding experimental design and interpretation of data. However, evidence from computer simulations suggests that such binary classification is misguided. The distribution of first solvation shell lifetimes for lipids around a GPCR is very broad, decaying as a power law in both atomistic 139 and coarse-grained 122 simulations (Fig 5c), covering the entire dynamic range of the simulation — longer than 30 μsec. Similarly broad lifetime distributions have been reported for rhodopsin 143. These observations suggest that lipid-protein interaction strengths are very heterogeneous, and therefore describing lipid-protein affinities only on the average is likely to yield misleading conclusions.
THE FUNCTIONAL PARALIPIDOME MODEL
While details of specific lipid-protein interactions, and/or solvent-like effects, and/or effects of material properties (like membrane thickness) can sometimes explain functional regulation of IMPs, the functional consequences of lipids on proteins can be conceptualized without reliance on a specific mechanism, as recently described 144. The basic concept is illustrated in Figure 6a–b, which shows an IMP that has two different conformations (A and B), each of which selects a different local lipid environment due to the distinct lipid interaction interfaces of each conformation.
Figure 6. The functional paralipidome.
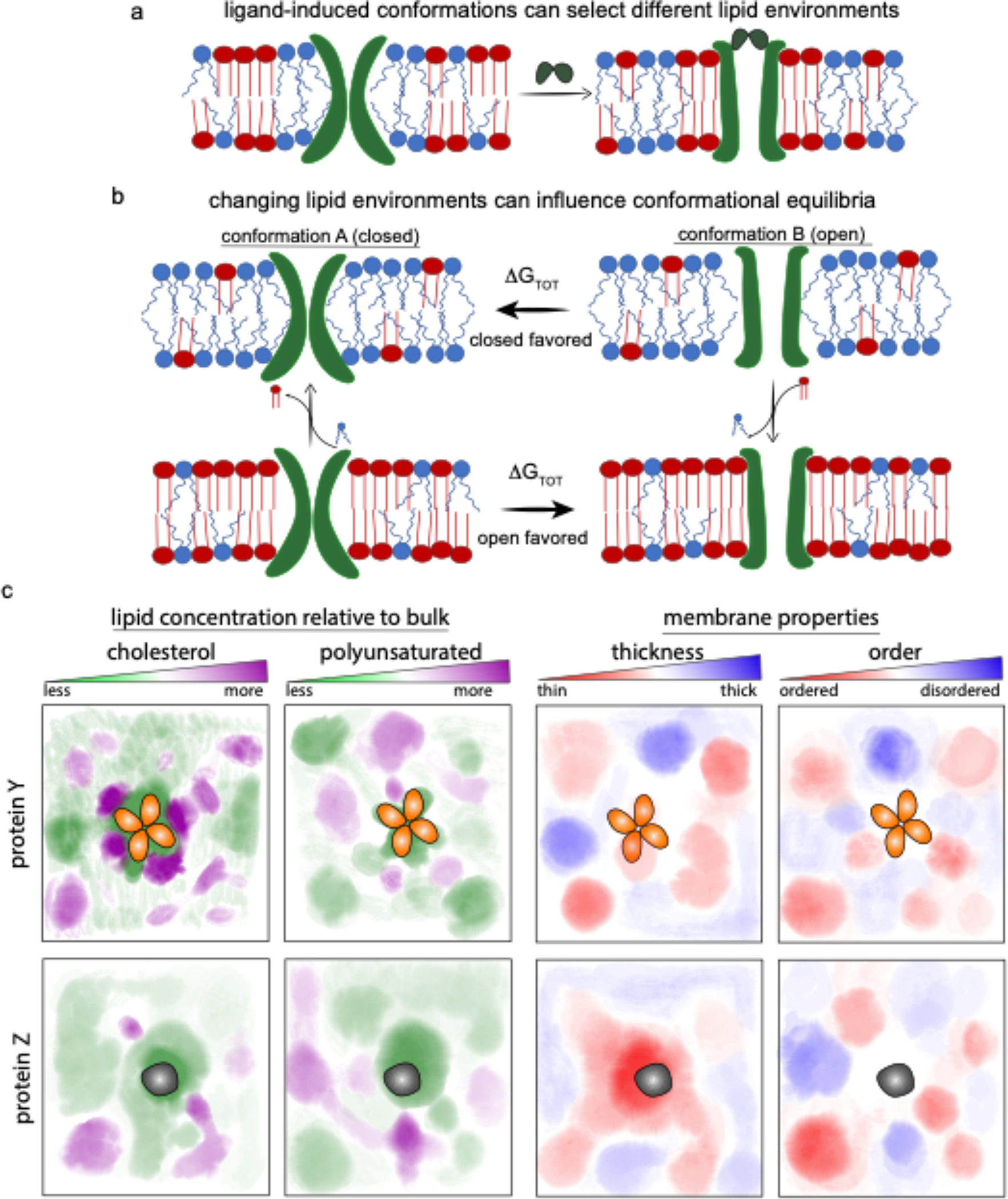
(a) IMPs can prefer distinct lipid environments in different conformations. (b) Conversely, the conformational equilibrium of a protein is determined in part by the lipid environment. If the closed state of an ion channel (A) recruits unsaturated lipids (blue) into its paralipidome (top left), the open state will be favored in membranes rich unsaturated lipids (i.e. those in which the chemical activity of unsaturated lipids is higher; top panels). (bottom panels) Membranes rich in saturated lipids, which preferentially solvate or bind the open conformation (B), will tend to favor the open state. Importantly, these effects depend not on absolute compositions, but rather on chemical activities. For example, inclusion of other lipids (e.g. cholesterol) into the bulk may influence the chemical activity of saturated lipids and thereby change the conformational equilibria. (c) various IMPs have unique preferences of their local membrane nano-environment (i.e. membrane fingerprints), selecting both paralipidomes and consequent biophysical parameters based on subtleties of their conformation and dynamics.
The function of this protein depends on the fraction of the conformational ensemble in conformation A versus conformation B — for example, “B” might be the open state of an ion channel while “A” is the closed state. The relative population of the two states depends on the free energy difference between states A and B, which includes contributions from the membrane. The total free energy difference ΔGtot between states A and B is a sum of different types of interaction
where ΔGP−P is the contribution from interactions within the protein which differ in A and B, ΔGP−L are changes in lipid-protein interactions in A versus B, and ΔGL−L are the contributions from the bulk lipid environment.
The effect of the local lipid environment, or “paralipidome,” is determined by ΔGP−L. The model is agnostic regarding the origins of ΔGP−L, which can encompass specific lipid binding favored in one conformation over the other, or may represent the accumulation of many weaker, “solvent-like” interactions 143, or changes in protein shape and lipid environment that minimize thickness mismatches or other stresses 29,145. Since the conformational equilibrium of an IMP (and therefore its function) may depend on all such interactions, the paralipidome model offers a way to rationalize their effects without recourse to (often undetermined) particulars (Fig 6b–c).
The model is relatively simple, requiring only that (a) IMPs recruit a local lipid environment (paralipidome) that is distinct in composition from the bulk, and (b) different conformational states recruit distinct paralipidomes (Fig 6a–c). There is extensive evidence for both aspects. Proteins’ recruitment of selective paralipidomes has been experimentally documented 146,147, but is perhaps most clearly evident in simulations. For example, in a compositionally complex model of a mammalian plasma membrane, ten different membrane proteins each recruited a unique “fingerprint” of membrane nano-environments, defined by subtle-yet-clear enrichment of specific lipid subtypes. These enrichments were spatially defined, with hotspots for various headgroups, lipid saturations, and sterols 122,148 (Fig 6c). Experimentally, nanodiscs have become an important tool for interrogating and controlling the local lipid environment (Fig 4a). Originally constructed from modified apolipoproteins and widely used for structural biology 149, protein-scaffolded nanodiscs required detergent solubilization of target proteins, which limits their utility for exploring native paralipidomes. More recently, synthetic polymers have replaced proteins as nanodisc-stabilizing scaffolds, with the advantage that these do not require pre-solubilization of IMPs 150. This approach has already been used to identify enrichment of certain lipid species in the paralipidomes of proteins 146. Remarkably, some proteins appear to modulate the physical properties of their surrounding lipids, as was elegantly shown for leaflet-selective lipid ordering by gap junction channels composed of connexin proteins 151. It is important to emphasize here that neither protein- nor polymer-scaffolded nanodiscs extract the “native” paralipidome of a IMP 152, nor necessarily recapitulate the native properties of a membrane 153. How much these issues will limit their usefulness for studies of paralipidomes remains to be seen.
There is also substantial evidence from simulations for recruitment of different lipid environments depending on conformation. Coarse-grained simulations of the A2A adenosine receptor revealed lipid-dependent partitioning of the receptor between regions of distinct composition 154. All atom simulations of the same protein showed state-dependent recruitment of distinct lipid environments 144; similar results were obtained for rhodopsin 143. Although direct detection of lipid environments is challenging experimentally (as discussed above), coupling of receptor state and lipid composition has been observed many times, from classic experiments on gramicidin A 29,155,156 to more recent measurements of cholesterol-dependent GPCR activation 157–159.
CONCLUSIONS AND PERSPECTIVES
Returning to the central question of this review: how do membrane proteins interact with lipids? Do lipids comprise a stable annulus, traveling with a protein like a greasy tutu (Fig 5a)? Or do lipids bind selectively like soluble ligands, allosterically modulating protein function (Fig 5b)? Or is there no specificity at all, just a random sea of lipid solvent for transmembrane domains? All three scenarios are likely relevant in various contexts. On the one hand, lipid dynamics and conformations are clearly affected by proximity to a protein — that is, lipids near a protein are different than those far from it. On the other hand, few of these lipids are bound in any thermodynamically meaningful sense, in that they are rapidly replaced by other, often different, lipids from the bulk. Some lipids (cholesterol, PIP2) have potential for relatively high-affinity interactions, while most others interact with similar affinity and are therefore largely interchangeable (from a protein’s perspective). Functionally, strongly binding lipids can act as ligands or co-factors, driving conformation and oligomerization, while other proteins maybe be entirely agnostic about their solvating lipids. We submit that the combination of these factors drives the formation of a local paralipidome with a unique set of compositional and biophysical features (Figs 5c & 6c). Paralipidome features can, in turn, be modulated by external factors to affect structural and functional changes in IMPs. These changes could be physical (e.g. membrane tension) or biochemical, including enzymatic conversion of lipid species or metabolic changes to bulk lipidomes (Fig 6a–b).
This hypothesis makes an urgent call for methods to detail protein paralipidomes. While computational measurements are providing deep insights, these must be supported by experimental approaches. Native-MS 147 and nanodiscs will be critically important approaches moving forward, though an emphasis on avoiding detergent solubilization of IMPs is needed. In cellular systems, super-resolution spectroscopy 160 and single-particle tracking 161 can be used to quantitatively evaluate lipid-protein interactions in situ. High resolution structures will continue to be important, perhaps soon obtained in situ. A major advance in this realm could be identification of multiple structures within a single preparation and modulation of this conformational landscape by lipid components, as recently suggested for the temperature-sensing channel TRPV3 162.
In what cellular contexts might changes in local lipid complements (i.e. paralipidomes) be relevant for regulating protein function? Perhaps the simplest argument can be made for sub-cellular localization. Each organellar membrane supports unique bilayer characteristics, often with major divergences between them (see Fig 3). For example, eukaryote PMs are very rich in sterols, with mammalian PMs containing up to 40 mol% 163. This high concentration stands in contrast to most intracellular organelles 164. It is plausible that lipid-determined conformational equilibria may restrict protein activity to only particular membranes within a cell. A protein’s paralipidome might also be regulated by recruiting it to a distinct lipid subdomain via reversible post-translational modifications. For example, palmitoylation (i.e. addition of a long, saturated acyl chain) is an essential driver of a protein’s affinity for ordered microdomains, is rapidly reversible 165,166, and is present on hundreds of PM proteins 167. Ordered phases in synthetic systems can be enriched by up to 10-fold in saturated lipids and 3–5-fold in cholesterol 168,169 with qualitatively similar enrichments reported in isolated PMs 75,170,171. Finally, large-scale metabolic activity may, in special cases, drive sufficient lipidomic changes to affect protein structures, e.g. the enzymatic conversion of sphingomyelin to ceramide during apoptosis or incorporation of exogenous fatty acids into phospholipids 9.
Perhaps the most significant feature of biomembranes omitted from the discussions above is their compositional and biophysical asymmetry 172. Most well studied has been the mammalian plasma membrane, where the cytoplasmic leaflet is rich in unsaturated, charged, loosely packed, amino-headgroup lipids opposite an external-facing leaflet that is uncharged, more saturated, and tightly packed 163. Similar asymmetries are likely present in other organelles, e.g. endosomes 163 and the ER 173. An under-appreciated and low-energy way of rapidly and dramatically changing proteins’ lipid environments is by releasing this lipid asymmetry via scramblase channels 174, which likely induces major changes in the composition of both leaflets 172.
Box 2: Methods to study lipids - integral membrane protein interaction dynamics.
Electron paramagnetic resonance (EPR) spectroscopy: classical studies that established the concept of distinct lipid properties in the annulus surrounding a transmembrane protein relied on lipids labeled with a stable free-radical (e.g. nitroxide). The dynamics of such “spin probes” report on the fluidity and polarity (i.e. hydration) environment of the membrane nano-environment 52,182. A subset of spin-labeled lipids is slowed by the presence of transmembrane proteins 98, providing evidence for protein-lipid interactions.
Molecular dynamics simulations: structural techniques can provide high-resolution information about protein conformation and lipid binding sites, but the picture is usually static, obscuring the eternal, jiggling dance of biomolecules at biological temperatures. Insights into the dynamic behavior of these systems can be provided by computational simulations (reviewed in 16). With increasing computational power, large and complex systems like membrane-embedded proteins can now be simulated over relevant time-scales to measure protein-lipid on/off-rates 139, binding energetics 142, and preferential solvation by specific lipid subtypes 148.
Fluorescence microscopy and spectroscopy: complementary to structural and computational methods, protein-membrane interactions can be investigated in situ using fluorescent lipids. Interactions can be identified via microscopic co-localization or fluorescent lipid detection after protein-pulldown 183. Higher resolution and quantitation is achieved via spectroscopic methods, e.g. fluorescence resonance energy transfer (FRET) between a fluorescent lipid and protein 10 (Fig 4c). Similarly, single-molecule techniques can reveal changes in conformation or assembly induced by membrane environments 184. An important consideration is that fluorescent tags are often large on the scale of a lipid and can therefore change essential aspects of lipid structure and interactions. To address this limitation, structurally and functionally similar analogs of both phospholipids 183 and sterols 185 have been developed and characterized.
Photo-activatable lipids: lipids can be engineered to contain small moieties that allow photo-conversion between a stable chemical bond (e.g. diazirine) and a highly reactive one (e.g. carbene free radical). Upon UV-activation these lipids rapidly react with their neighboring molecules, including other lipids and transmembrane proteins (Fig 4b). Inclusion of fluorescence or radioactivity into such photo-activated lipids allows detection of protein-lipid crosslinks by standard biochemical methods (e.g. electrophoresis or chromatography) 15,186. Several of the above moieties can be combined to make multi-functional lipid analogs, containing photo-labile caging, cross-linking, and fluorescent groups for powerful, versatile, and temporally resolved analysis of protein-lipid dynamics 187,188.
Electrophysiology: one of the few methods capable of directly testing membrane protein function in different lipid environments takes advantage of the fact that many such proteins conduct ions across a membrane, and therefore their activity can be assayed by electrical signals. Practically, this is often accomplished via a patch clamp, wherein a patch of membrane forms the seal between two electrically isolated compartments and flow of ions through individual channels or transporters is detected as current. These membrane patches are usually derived from cells, whose lipid composition can be manipulated 111,189. Alternatively, similar techniques can be applies to patches of reconstituted liposomes 190, or even whole giant liposomes 191, whose lipid composition can be precisely controlled.
ACKNOWLEDGEMENTS
Major acknowledgement goes to Dr Kandice Levental for insightful feedback and for generating the beautiful and informative figures for the manuscript. We also acknowledge the members of the Levental and Lyman labs for critical reading and enlightening discussions that have polished the ideas in the manuscript. This work was supported by NIH/National Institute of General Medical Sciences (GM134949, GM124072, GM120351), the Volkswagen Foundation (grant 93091), and the Human Frontiers Science Program (RGP0059/2019).
Glossary
- Unfolded protein response
a conserved cellular response to various stresses to the protein folding and secretory systems, can also be induced by lipid perturbations
- Shotgun lipidomics
a mass spectrometric technique for identifying and quantifying the lipid components of a complex sample (e.g. cell membrane) without prior chromatographic separation
- PIP2
Phosphatidyl inositol (4,5) bisphosphate, a highly charged and tightly regulated lipid type that can associate tightly with IMPs through both electrostatic and stereospecific interactions
- Nanodisc
an experimental construct containing an IMP, lipids, and a scaffold that solubilizes them. The scaffold can be a protein (MSP) or synthetic polymer (SMA)
- Native mass spectrometry
a mass spectrometric technique capable of measuring molecular weights of large macromolecules (i.e. proteins and their complexes) without fragmentation
- Paralipidome
the preferred lipid nanoenvironments, and their resulting membrane properties, that solvate transmembrane proteins
Footnotes
-
Jensen, M. O. & Mouritsen, O. G. Lipids do influence protein function - the hydrophobic matching hypothesis revisited. Biochimica Et Biophysica Acta-Biomembranes 1666, 205–226, doi:10.1016/j.bbamem.2004.06.009 (2004).
- A detailed discussion of experimental observations documenting how proteins perturb their lipid environments and, vice versa, how membrane properties like hydrophobic mismatch and curvature stress affect protein function
-
Budin, I. et al. Viscous control of cellular respiration by membrane lipid composition. Science, doi:10.1126/science.aat7925 (2018).
- Clever combination of genetics, biophysics, and mathematical model to explain how membrane viscosity regulates cell growth via diffusion of electron carriers in the respiratory chain
-
Chadda, R., N. Bernhardt, E. G. Kelley, S. C. Teixeira, K. Griffith, A. Gil-Ley, T. N. Ozturk, L. E. Hughes, A. Forsythe, V. Krishnamani, J. D. Faraldo-Gomez and J. L. Robertson (2021). “Membrane transporter dimerization driven by differential lipid solvation energetics of dissociated and associated states.” Elife 10.
- Unique biochemical approaches are combined with simulations to show that IMP oligomerization is mediated by the energetics of TMD solvation by lipids, rather than direct lipid binding
-
Halbleib, K. et al. Activation of the unfolded protein response by lipid bilayer stress. Molecular cell 67, 673–684 e678, doi:10.1016/j.molcel.2017.06.012 (2017).
- Demonstration that proteins can sense membrane properties by compessing the bilayer to signal aberrant lipid compositions during cell stress
-
Gonen, T., Y. Cheng, P. Sliz, Y. Hiroaki, Y. Fujiyoshi, S. C. Harrison and T. Walz (2005). Lipid-protein interactions in double-layered two-dimensional AQP0 crystals. Nature 438(7068): 633–638.
- Ground-breaking study using electron diffraction from two-dimension crystals of a membrane protein, AQP0, providing direct evidence for lipid-protein interaction and the water conduction mechanism
-
Levental, K. R. et al. omega-3 polyunsaturated fatty acids direct differentiation of the membrane phenotype in mesenchymal stem cells to potentiate osteogenesis. Science advances 3, eaao1193, doi:10.1126/sciadv.aao1193 (2017).
- Demonstrates that membrane lipidomes can be comprehensively remodeled by exogenous, dietary fatty acids and that these effects can modulate stem cell differentiation
-
Laganowsky, A., E. Reading, T. M. Allison, M. B. Ulmschneider, M. T. Degiacomi, A. J. Baldwin and C. V. Robinson (2014). “Membrane proteins bind lipids selectively to modulate their structure and function.” Nature 510(7503): 172–175.
- Native mass spectrometry used to demonstrate that lipids can remain bound to proteins through the extraction and ionization process and be identified directly by their molecular mass
-
Leonard, A. N. and E. Lyman (2021). “Activation of G-protein-coupled receptors is thermodynamically linked to lipid solvation.” Biophys J 120(9): 1777–1787.
- Conceptual underpinning of the functional paralipidome model, demonstrating via computational and theoretical analysis how lipid nanoenvironments could influence protein conformational equilibria
-
Lorent, J. H. et al. Plasma membranes are asymmetric in lipid unsaturation, packing and protein shape. Nat Chem Biol 16, 644–652, doi:10.1038/s41589-020-0529-6 (2020).
- Experiments and computational modeling detail the lipidomic and biophysical asymmetry of a mammalian plasma membrane, while bioinformatics shows that membrane asymmetry is reflected in asymmetry of protein TMDs, which is conserved througout Eukaryota
-
Flores, J. A., B. G. Haddad, K. A. Dolan, J. B. Myers, C. C. Yoshioka, J. Copperman, D. M. Zuckerman and S. L. Reichow (2020). “Connexin-46/50 in a dynamic lipid environment resolved by CryoEM at 1.9 A.” Nat Commun 11(1): 4331.
- Ultra-high resolution structures of native connexin channels, which span two bilayers, reveal novel details of IMP-membrane interactions, including remarkably ordering of outer leaflet lipids
-
Shurer, C. R., J. C. Kuo, L. M. Roberts, J. G. Gandhi, M. J. Colville, T. A. Enoki, H. Pan, J. Su, J. M. Noble, M. J. Hollander, J. P. O’Donnell, R. Yin, K. Pedram, L. Mockl, L. F. Kourkoutis, W. E. Moerner, C. R. Bertozzi, G. W. Feigenson, H. L. Reesink and M. J. Paszek (2019). “Physical Principles of Membrane Shape Regulation by the Glycocalyx.” Cell 177(7): 1757–1770 e1721.
- Protein engineering and scanning electrom microscopy reveal remarkable morphological transformations of living cell membranes induced by the glycocalyx
-
Contreras, F. X., A. M. Ernst, P. Haberkant, P. Bjorkholm, E. Lindahl, B. Gonen, C. Tischer, A. Elofsson, G. von Heijne, C. Thiele, R. Pepperkok, F. Wieland and B. Brugger (2012). “Molecular recognition of a single sphingolipid species by a protein’s transmembrane domain.” Nature 481(7382): 525–529.
- Demonstration of the remarkable specificity of binding between a single-pass transmembrane domain and a particular sub-species of sphingomyelin
-
van ‘t Klooster, J. S. et al. Periprotein lipidomes of Saccharomyces cerevisiae provide a flexible environment for conformational changes of membrane proteins. eLife 9, doi:10.7554/eLife.57003 (2020).
- Synthetic copolymer nanodiscs were used to extract microcompartments of the yeast PM defined by two different proteins, identifying distinct compositions of the ‘periprotein lipidomes’ compared to each other and the rest of the PM
References
- 1.Krogh A, Larsson B, von Heijne G & Sonnhammer EL Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. Journal of molecular biology 305, 567–580 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Overington JP, Al-Lazikani B & Hopkins AL How many drug targets are there? Nature reviews. Drug discovery 5, 993–996 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Payandeh J & Volgraf M Ligand binding at the protein-lipid interface: strategic considerations for drug design. Nature reviews. Drug discovery 20, 710–722 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Harayama T & Riezman H Understanding the diversity of membrane lipid composition. Nat Rev Mol Cell Biol 19, 281–296 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Symons JL et al. Lipidomic atlas of mammalian cell membranes reveals hierarchical variation induced by culture conditions, subcellular membranes, and cell lineages. Soft Matter (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levental KR et al. omega-3 polyunsaturated fatty acids direct differentiation of the membrane phenotype in mesenchymal stem cells to potentiate osteogenesis. Science advances 3, eaao1193 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han X Lipidomics for studying metabolism. Nat Rev Endocrinol 12, 668–679 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Eiriksson FF et al. Lipidomic study of cell lines reveals differences between breast cancer subtypes. PloS one 15, e0231289 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levental KR et al. Lipidomic and biophysical homeostasis of mammalian membranes counteracts dietary lipid perturbations to maintain cellular fitness. Nature communications 11, 1339 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Contreras FX et al. Molecular recognition of a single sphingolipid species by a protein’s transmembrane domain. Nature 481, 525–529 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Lemmon MA Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol 9, 99–111 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Moravcevic K, Oxley CL & Lemmon MA Conditional peripheral membrane proteins: facing up to limited specificity. Structure 20, 15–27 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee AG How lipids affect the activities of integral membrane proteins. Biochim Biophys Acta 1666, 62–87 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Barrera NP, Zhou M & Robinson CV The role of lipids in defining membrane protein interactions: insights from mass spectrometry. Trends in cell biology 23, 1–8 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Contreras FX, Ernst AM, Wieland F & Brugger B Specificity of intramembrane protein-lipid interactions. Cold Spring Harbor perspectives in biology 3 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corradi V et al. Emerging Diversity in Lipid-Protein Interactions. Chem Rev 119, 5775–5848 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sych T, Levental KR & Sezgin E Lipid-Protein Interactions in Plasma Membrane Organization and Function. Annual review of biophysics 51, 135–156 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ernst M & Robertson JL The Role of the Membrane in Transporter Folding and Activity. Journal of molecular biology 433, 167103 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simunovic M, Prevost C, Callan-Jones A & Bassereau P Physical basis of some membrane shaping mechanisms. Philos Trans A Math Phys Eng Sci 374 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips R, Ursell T, Wiggins P & Sens P Emerging roles for lipids in shaping membrane-protein function. Nature 459, 379–385 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heberle FA et al. Direct label-free imaging of nanodomains in biomimetic and biological membranes by cryogenic electron microscopy. Proc Natl Acad Sci U S A 117, 19943–19952 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitra K, Ubarretxena-Belandia I, Taguchi T, Warren G & Engelman DM Modulation of the bilayer thickness of exocytic pathway membranes by membrane proteins rather than cholesterol. Proc Natl Acad Sci U S A 101, 4083–4088 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cornell CE, Mileant A, Thakkar N, Lee KK & Keller SL Direct imaging of liquid domains in membranes by cryo-electron tomography. Proc Natl Acad Sci U S A 117, 19713–19719 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer TD, Dash PK, Liu J & Waxham MN Morphology of mitochondria in spatially restricted axons revealed by cryo-electron tomography. PLoS Biol 16, e2006169 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersen OS & Koeppe RE, 2nd. Bilayer thickness and membrane protein function: an energetic perspective. Annu Rev Biophys Biomol Struct 36, 107–130 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Falzone ME et al. TMEM16 scramblases thin the membrane to enable lipid scrambling. Nature communications 13, 2604 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaiser H-J et al. Lateral sorting in model membranes by cholesterol-mediated hydrophobic matching. Proceedings of the National Academy of Sciences of the United States of America 108, 16628–16633 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chadda R et al. Membrane transporter dimerization driven by differential lipid solvation energetics of dissociated and associated states. eLife 10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beaven AH et al. Gramicidin A Channel Formation Induces Local Lipid Redistribution I: Experiment and Simulation. Biophys J 112, 1185–1197 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharpe HJ, Stevens TJ & Munro S A comprehensive comparison of transmembrane domains reveals organelle-specific properties. Cell 142, 158–169 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diaz-Rohrer B, Levental KR & Levental I Rafting through traffic: Membrane domains in cellular logistics. Biochim. Biophys. Acta-Biomembr. 1838, 3003–3013 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Prasad R, Sliwa-Gonzalez A & Barral Y Mapping bilayer thickness in the ER membrane. Science advances 6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhemkov V et al. The role of sigma 1 receptor in organization of endoplasmic reticulum signaling microdomains. eLife 10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King C, Sengupta P, Seo AY & Lippincott-Schwartz J ER membranes exhibit phase behavior at sites of organelle contact. Proc Natl Acad Sci U S A 117, 7225–7235 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cornelius F Modulation of Na, K-ATPase and Na-ATPase activity by phospholipids and cholesterol. I. Steady-state kinetics. Biochemistry 40, 8842–8851 (2001). [DOI] [PubMed] [Google Scholar]
- 36.Lee AG Lipid-protein interactions in biological membranes: a structural perspective. Biochim Biophys Acta 1612, 1–40 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Botelho AV, Huber T, Sakmar TP & Brown MF Curvature and hydrophobic forces drive oligomerization and modulate activity of rhodopsin in membranes. Biophys J 91, 4464–4477 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jensen MO & Mouritsen OG Lipids do influence protein function - the hydrophobic matching hypothesis revisited. Biochimica Et Biophysica Acta-Biomembranes 1666, 205–226 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Cybulski LE et al. Activation of the bacterial thermosensor DesK involves a serine zipper dimerization motif that is modulated by bilayer thickness. Proc Natl Acad Sci U S A 112, 6353–6358 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simunovic M, Evergren E, Callan-Jones A & Bassereau P Curving Cells Inside and Out: Roles of BAR Domain Proteins in Membrane Shaping and Its Cellular Implications. Annu Rev Cell Dev Biol 35, 111–129 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Cui H, Lyman E & Voth GA Mechanism of membrane curvature sensing by amphipathic helix containing proteins. Biophys J 100, 1271–1279 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aimon S et al. Membrane shape modulates transmembrane protein distribution. Dev Cell 28, 212–218 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shurer CR et al. Physical Principles of Membrane Shape Regulation by the Glycocalyx. Cell 177, 1757–1770 e1721 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stachowiak JC, Hayden CC & Sasaki DY Steric confinement of proteins on lipid membranes can drive curvature and tubulation. Proc Natl Acad Sci U S A 107, 7781–7786 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ernst AM et al. S-Palmitoylation Sorts Membrane Cargo for Anterograde Transport in the Golgi. Dev Cell 47, 479–493 e477 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Budin I et al. Viscous control of cellular respiration by membrane lipid composition. Science (2018). [DOI] [PubMed] [Google Scholar]
- 47.Sinensky M Homeoviscous adaptation--a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A 71, 522–525 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ernst R, Ejsing CS & Antonny B Homeoviscous adaptation and the regulation of membrane lipids. Journal of molecular biology 428, 4776–4791 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Martiniere A et al. Homeostasis of plasma membrane viscosity in fluctuating temperatures. New Phytol 192, 328–337 (2011). [DOI] [PubMed] [Google Scholar]
- 50.Behan-Martin MK, Jones GR, Bowler K & Cossins AR A near perfect temperature adaptation of bilayer order in vertebrate brain membranes. Biochim Biophys Acta 1151, 216–222 (1993). [DOI] [PubMed] [Google Scholar]
- 51.Ruiz M et al. Membrane fluidity is regulated by the C. elegans transmembrane protein FLD-1 and its human homologs TLCD1/2. eLife 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Covino R et al. A eukaryotic sensor for membrane lipid saturation. Molecular cell 63, 49–59 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Ballweg S et al. Regulation of lipid saturation without sensing membrane fluidity. Nature communications 11, 756 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Radanovic T, Reinhard J, Ballweg S, Pesek K & Ernst R An Emerging Group of Membrane Property Sensors Controls the Physical State of Organellar Membranes to Maintain Their Identity. BioEssays : news and reviews in molecular, cellular and developmental biology, e1700250 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Halbleib K et al. Activation of the unfolded protein response by lipid bilayer stress. Molecular cell 67, 673–684 e678 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Cox CD, Bavi N & Martinac B Bacterial Mechanosensors. Annu Rev Physiol 80, 71–93 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Reddy B, Bavi N, Lu A, Park Y & Perozo E Molecular basis of force-from-lipids gating in the mechanosensitive channel MscS. eLife 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coste B et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin YC et al. Force-induced conformational changes in PIEZO1. Nature 573, 230–234 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brohawn SG, Su Z & MacKinnon R Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels. Proc Natl Acad Sci U S A 111, 3614–3619 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bavi N et al. The conformational cycle of prestin underlies outer-hair cell electromotility. Nature 600, 553–558 (2021). [DOI] [PubMed] [Google Scholar]
- 62.Ge J et al. Molecular mechanism of prestin electromotive signal amplification. Cell (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dallos P et al. Prestin-based outer hair cell motility is necessary for mammalian cochlear amplification. Neuron 58, 333–339 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sezgin E, Levental I, Mayor S & Eggeling C The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nat Rev Mol Cell Biol 18, 361–374 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lingwood D & Simons K Lipid rafts as a membrane-organizing principle. Science 327, 46–50 (2010). [DOI] [PubMed] [Google Scholar]
- 66.Veatch SL & Keller SL Organization in lipid membranes containing cholesterol. Phys Rev Lett 89, 268101 (2002). [DOI] [PubMed] [Google Scholar]
- 67.Veatch SL & Keller SL Seeing spots: complex phase behavior in simple membranes. Biochim Biophys Acta 1746, 172–185 (2005). [DOI] [PubMed] [Google Scholar]
- 68.Sodt AJ, Sandar ML, Gawrisch K, Pastor RW & Lyman E The Molecular Structure of the Liquid-Ordered Phase of Lipid Bilayers. Journal of the American Chemical Society 136, 725–732 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sodt AJ, Pastor RW & Lyman E Hexagonal Substructure and Hydrogen Bonding in Liquid-Ordered Phases Containing Palmitoyl Sphingomyelin. Biophys J 109, 948–955 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baumgart T et al. Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc Natl Acad Sci U S A 104, 3165–3170 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lingwood D, Ries J, Schwille P & Simons K Plasma membranes are poised for activation of raft phase coalescence at physiological temperature. Proc Natl Acad Sci U S A 105, 10005–10010 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Levental KR & Levental I Giant plasma membrane vesicles: models for understanding membrane organization. Current topics in membranes 75, 25–57 (2015). [DOI] [PubMed] [Google Scholar]
- 73.Veatch SL et al. Critical fluctuations in plasma membrane vesicles. ACS Chem Biol 3, 287–293 (2008). [DOI] [PubMed] [Google Scholar]
- 74.Levental I et al. Cholesterol-dependent phase separation in cell-derived giant plasma-membrane vesicles. Biochem J 424, 163–167 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Levental KR et al. Polyunsaturated lipids regulate membrane domain stability by tuning membrane order. Biophys J 110(8), 1800–1810 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Toulmay A & Prinz WA Direct imaging reveals stable, micrometer-scale lipid domains that segregate proteins in live cells. J Cell Biol 202, 35–44 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rayermann SP, Rayermann GE, Cornell CE, Merz AJ & Keller SL Hallmarks of reversible separation of living, unperturbed cell membranes into two liquid phases. Biophys J 113, 2425–2432 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Levental I, Levental KR & Heberle FA Lipid rafts: controversies resolved, mysteries remain. Trends in cell biology 30, 341–353 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sezgin E et al. Elucidating membrane structure and protein behavior using giant plasma membrane vesicles. Nat Protoc 7, 1042–1051 (2012). [DOI] [PubMed] [Google Scholar]
- 80.Diaz-Rohrer BB, Levental KR, Simons K & Levental I Membrane raft association is a determinant of plasma membrane localization. Proc Natl Acad Sci U S A 111, 8500–8505 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lorent JH et al. Structural determinants and functional consequences of protein affinity for membrane rafts. Nature communications 8, 1219 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Levental I, Lingwood D, Grzybek M, Coskun U & Simons K Palmitoylation regulates raft affinity for the majority of integral raft proteins. Proc Natl Acad Sci U S A 107, 22050–22054 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Levental I, Grzybek M & Simons K Greasing their way: lipid modifications determine protein association with membrane rafts. Biochemistry 49, 6305–6316 (2010). [DOI] [PubMed] [Google Scholar]
- 84.Castello-Serrano I, Lorent JH, Ippolito R, Levental KR & Levental I Myelin-Associated MAL and PLP Are unusual among multipass transmembrane proteins in preferring ordered membrane domains. J Phys Chem B 124, 5930–5939 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stone MB, Shelby SA, Nunez MF, Wisser K & Veatch SL Protein sorting by lipid phase-like domains supports emergent signaling function in B lymphocyte plasma membranes. eLife 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sengupta P et al. A lipid-based partitioning mechanism for selective incorporation of proteins into membranes of HIV particles. Nat Cell Biol 21, 452–461 (2019). [DOI] [PubMed] [Google Scholar]
- 87.Ono A & Freed EO Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proceedings of the National Academy of Sciences of the United States of America 98, 13925–13930 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mitchell DC, Straume M, Miller JL & Litman BJ Modulation of metarhodopsin formation by cholesterol-induced ordering of bilayer lipids. Biochemistry 29, 9143–9149 (1990). [DOI] [PubMed] [Google Scholar]
- 89.Huang SK et al. Allosteric modulation of the adenosine A2A receptor by cholesterol. bioRxiv, 2021.2009.2013.460151 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tikku S et al. Relationship between Kir2.1/Kir2.3 activity and their distributions between cholesterol-rich and cholesterol-poor membrane domains. American journal of physiology. Cell physiology 293, C440–450 (2007). [DOI] [PubMed] [Google Scholar]
- 91.Murata M et al. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci U S A 92, 10339–10343 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Parton RG & Simons K The multiple faces of caveolae. Nat Rev Mol Cell Biol 8, 185–194 (2007). [DOI] [PubMed] [Google Scholar]
- 93.Parton RG, Kozlov MM & Ariotti N Caveolae and lipid sorting: Shaping the cellular response to stress. J Cell Biol 219 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ortegren U et al. Lipids and glycosphingolipids in caveolae and surrounding plasma membrane of primary rat adipocytes. European journal of biochemistry / FEBS 271, 2028–2036 (2004). [DOI] [PubMed] [Google Scholar]
- 95.Rothberg KG et al. Caveolin, a protein component of caveolae membrane coats. Cell 68, 673–682 (1992). [DOI] [PubMed] [Google Scholar]
- 96.Epand RM, Sayer BG & Epand RF Caveolin scaffolding region and cholesterol-rich domains in membranes. Journal of molecular biology 345, 339–350 (2005). [DOI] [PubMed] [Google Scholar]
- 97.Sinha B et al. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 144, 402–413 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marsh D Protein modulation of lipids, and vice-versa, in membranes. Biochim Biophys Acta 1778, 1545–1575 (2008). [DOI] [PubMed] [Google Scholar]
- 99.Gonen T et al. Lipid-protein interactions in double-layered two-dimensional AQP0 crystals. Nature 438, 633–638 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jost PC, Griffith OH, Capaldi RA & Vanderkooi G Evidence for boundary lipid in membranes. Proc Natl Acad Sci U S A 70, 480–484 (1973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brotherus JR et al. Lipid--protein multiple binding equilibria in membranes. Biochemistry 20, 5261–5267 (1981). [DOI] [PubMed] [Google Scholar]
- 102.Horvath LI, Brophy PJ & Marsh D Exchange rates at the lipid-protein interface of myelin proteolipid protein studied by spin-label electron spin resonance. Biochemistry 27, 46–52 (1988). [DOI] [PubMed] [Google Scholar]
- 103.Marius P, Alvis SJ, East JM & Lee AG The interfacial lipid binding site on the potassium channel KcsA is specific for anionic phospholipids. Biophys J 89, 4081–4089 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McAuley KE et al. Structural details of an interaction between cardiolipin and an integral membrane protein. Proc Natl Acad Sci U S A 96, 14706–14711 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Belrhali H et al. Protein, lipid and water organization in bacteriorhodopsin crystals: a molecular view of the purple membrane at 1.9 A resolution. Structure 7, 909–917 (1999). [DOI] [PubMed] [Google Scholar]
- 106.Zhou Y, Morais-Cabral JH, Kaufman A & MacKinnon R Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature 414, 43–48 (2001). [DOI] [PubMed] [Google Scholar]
- 107.Duncan AL, Song W & Sansom MSP Lipid-Dependent Regulation of Ion Channels and G Protein-Coupled Receptors: Insights from Structures and Simulations. Annu Rev Pharmacol Toxicol 60, 31–50 (2020). [DOI] [PubMed] [Google Scholar]
- 108.Hansen SB, Tao X & MacKinnon R Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2. Nature 477, 495–498 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McLaughlin S, Wang J, Gambhir A & Murray D PIP(2) and proteins: interactions, organization, and information flow. Annu Rev Biophys Biomol Struct 31, 151–175 (2002). [DOI] [PubMed] [Google Scholar]
- 110.Rosenhouse-Dantsker A, Noskov S, Durdagi S, Logothetis DE & Levitan I Identification of novel cholesterol-binding regions in Kir2 channels. J Biol Chem 288, 31154–31164 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Romanenko VG et al. Cholesterol sensitivity and lipid raft targeting of Kir2.1 channels. Biophys J 87, 3850–3861 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Duncan AL, Corey RA & Sansom MSP Defining how multiple lipid species interact with inward rectifier potassium (Kir2) channels. Proc Natl Acad Sci U S A 117, 7803–7813 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zaydman MA et al. Kv7.1 ion channels require a lipid to couple voltage sensing to pore opening. Proc Natl Acad Sci U S A 110, 13180–13185 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hilgemann DW & Ball R Regulation of cardiac Na+, Ca2+ exchange and KATP potassium channels by PIP2. Science 273, 956–959 (1996). [DOI] [PubMed] [Google Scholar]
- 115.Hedger G et al. Lipid-Loving ANTs: Molecular Simulations of Cardiolipin Interactions and the Organization of the Adenine Nucleotide Translocase in Model Mitochondrial Membranes. Biochemistry 55, 6238–6249 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hite RK, Butterwick JA & MacKinnon R Phosphatidic acid modulation of Kv channel voltage sensor function. eLife 3 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zocher M, Zhang C, Rasmussen SG, Kobilka BK & Muller DJ Cholesterol increases kinetic, energetic, and mechanical stability of the human beta2-adrenergic receptor. Proc Natl Acad Sci U S A 109, E3463–3472 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cherezov V et al. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science 318, 1258–1265 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu W et al. Structural basis for allosteric regulation of GPCRs by sodium ions. Science 337, 232–236 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Guixa-Gonzalez R et al. Membrane cholesterol access into a G-protein-coupled receptor. Nature communications 8, 14505 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Manna M et al. Mechanism of allosteric regulation of beta2-adrenergic receptor by cholesterol. eLife 5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rouviere E, Arnarez C, Yang L & Lyman E Identification of Two New Cholesterol Interaction Sites on the A2A Adenosine Receptor. Biophys J 113, 2415–2424 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee JY & Lyman E Predictions for cholesterol interaction sites on the A2A adenosine receptor. J Am Chem Soc 134, 16512–16515 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nelson LD, Johnson AE & London E How interaction of perfringolysin O with membranes is controlled by sterol structure, lipid structure, and physiological low pH: insights into the origin of perfringolysin O-lipid raft interaction. J Biol Chem 283, 4632–4642 (2008). [DOI] [PubMed] [Google Scholar]
- 125.D’Avanzo N, Hyrc K, Enkvetchakul D, Covey DF & Nichols CG Enantioselective protein-sterol interactions mediate regulation of both prokaryotic and eukaryotic inward rectifier K+ channels by cholesterol. PloS one 6, e19393 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gutorov R et al. Modulation of Transient Receptor Potential C Channel Activity by Cholesterol. Front Pharmacol 10, 1487 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Elkins MR et al. Cholesterol-binding site of the influenza M2 protein in lipid bilayers from solid-state NMR. Proc Natl Acad Sci U S A 114, 12946–12951 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Barrett PJ et al. The amyloid precursor protein has a flexible transmembrane domain and binds cholesterol. Science 336, 1168–1171 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Marlow B, Kuenze G, Li B, Sanders CR & Meiler J Structural determinants of cholesterol recognition in helical integral membrane proteins. Biophys J 120, 1592–1604 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Romer W et al. Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature 450, 670–675 (2007). [DOI] [PubMed] [Google Scholar]
- 131.Flores A et al. Gangliosides interact with synaptotagmin to form the high-affinity receptor complex for botulinum neurotoxin B. Proc Natl Acad Sci U S A 116, 18098–18108 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ewers H et al. GM1 structure determines SV40-induced membrane invagination and infection. Nat Cell Biol 12, 11–18; sup pp 11–12 (2010). [DOI] [PubMed] [Google Scholar]
- 133.Coskun U, Grzybek M, Drechsel D & Simons K Regulation of human EGF receptor by lipids. Proc Natl Acad Sci U S A (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Song W, Yen HY, Robinson CV & Sansom MSP State-dependent Lipid Interactions with the A2a Receptor Revealed by MD Simulations Using In Vivo-Mimetic Membranes. Structure 27, 392–403 e393 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Laganowsky A et al. Membrane proteins bind lipids selectively to modulate their structure and function. Nature 510, 172–175 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cong X et al. Determining Membrane Protein-Lipid Binding Thermodynamics Using Native Mass Spectrometry. J Am Chem Soc 138, 4346–4349 (2016). [DOI] [PubMed] [Google Scholar]
- 137.Patrick JW et al. Allostery revealed within lipid binding events to membrane proteins. Proc Natl Acad Sci U S A 115, 2976–2981 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gupta K et al. The role of interfacial lipids in stabilizing membrane protein oligomers. Nature 541, 421–424 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang L & Lyman E Local Enrichment of Unsaturated Chains around the A2A Adenosine Receptor. Biochemistry 58, 4096–4105 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tanford C Extension of the theory of linked functions to incorporate the effects of protein hydration. Journal of molecular biology 39, 539–544 (1969). [DOI] [PubMed] [Google Scholar]
- 141.Salari R, Joseph T, Lohia R, Henin J & Brannigan G A Streamlined, General Approach for Computing Ligand Binding Free Energies and Its Application to GPCR-Bound Cholesterol. J Chem Theory Comput 14, 6560–6573 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Corey RA, Stansfeld PJ & Sansom MSP The energetics of protein-lipid interactions as viewed by molecular simulations. Biochemical Society transactions 48, 25–37 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Salas-Estrada LA, Leioatts N, Romo TD & Grossfield A Lipids Alter Rhodopsin Function via Ligand-like and Solvent-like Interactions. Biophys J 114, 355–367 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Leonard AN & Lyman E Activation of G-protein-coupled receptors is thermodynamically linked to lipid solvation. Biophys J 120, 1777–1787 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Huber T, Botelho AV, Beyer K & Brown MF Membrane model for the G-protein-coupled receptor rhodopsin: hydrophobic interface and dynamical structure. Biophys J 86, 2078–2100 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.van ‘t Klooster JS et al. Periprotein lipidomes of Saccharomyces cerevisiae provide a flexible environment for conformational changes of membrane proteins. eLife 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gupta K et al. Identifying key membrane protein lipid interactions using mass spectrometry. Nat Protoc 13, 1106–1120 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Corradi V et al. Lipid-Protein Interactions Are Unique Fingerprints for Membrane Proteins. ACS Cent Sci 4, 709–717 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Denisov IG & Sligar SG Nanodiscs for structural and functional studies of membrane proteins. Nature structural & molecular biology 23, 481–486 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dorr JM et al. Detergent-free isolation, characterization, and functional reconstitution of a tetrameric K+ channel: the power of native nanodiscs. Proc Natl Acad Sci U S A 111, 18607–18612 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Flores JA et al. Connexin-46/50 in a dynamic lipid environment resolved by CryoEM at 1.9 A. Nature communications 11, 4331 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cuevas Arenas R et al. Fast Collisional Lipid Transfer Among Polymer-Bounded Nanodiscs. Sci Rep 7, 45875 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Stepien P et al. Complexity of seemingly simple lipid nanodiscs. Biochim Biophys Acta Biomembr 1862, 183420 (2020). [DOI] [PubMed] [Google Scholar]
- 154.Javanainen M et al. Reduced level of docosahexaenoic acid shifts GPCR neuroreceptors to less ordered membrane regions. PLoS computational biology 15, e1007033 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sodt AJ, Beaven AH, Andersen OS, Im W & Pastor RW Gramicidin A Channel Formation Induces Local Lipid Redistribution II: A 3D Continuum Elastic Model. Biophys J 112, 1198–1213 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lundbaek JA, Collingwood SA, Ingolfsson HI, Kapoor R & Andersen OS Lipid bilayer regulation of membrane protein function: gramicidin channels as molecular force probes. J R Soc Interface 7, 373–395 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.McGraw C, Yang L, Levental I, Lyman E & Robinson AS Membrane cholesterol depletion reduces downstream signaling activity of the adenosine A2A receptor. Biochim Biophys Acta Biomembr 1861, 760–767 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gutierrez MG, Mansfield KS & Malmstadt N The Functional Activity of the Human Serotonin 5-HT1A Receptor Is Controlled by Lipid Bilayer Composition. Biophys J 110, 2486–2495 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kumar GA et al. A molecular sensor for cholesterol in the human serotonin1A receptor. Science advances 7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Barbotin A et al. z-STED Imaging and Spectroscopy to Investigate Nanoscale Membrane Structure and Dynamics. Biophys J 118, 2448–2457 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Komura N et al. Raft-based interactions of gangliosides with a GPI-anchored receptor. Nat Chem Biol 12, 402–410 (2016). [DOI] [PubMed] [Google Scholar]
- 162.Nadezhdin KD et al. Structural mechanism of heat-induced opening of a temperature-sensitive TRP channel. Nature structural & molecular biology 28, 564–572 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lorent JH et al. Plasma membranes are asymmetric in lipid unsaturation, packing and protein shape. Nat Chem Biol 16, 644–652 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.van Meer G, Voelker DR & Feigenson GW Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 9, 112–124 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Akimzhanov AM & Boehning D Rapid and transient palmitoylation of the tyrosine kinase Lck mediates Fas signaling. Proc Natl Acad Sci U S A 112, 11876–11880 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Martin BR, Wang C, Adibekian A, Tully SE & Cravatt BF Global profiling of dynamic protein palmitoylation. Nat Methods 9, 84–89 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Martin BR & Cravatt BF Large-scale profiling of protein palmitoylation in mammalian cells. Nat Methods 6, 135–138 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Veatch SL, Gawrisch K & Keller SL Closed-loop miscibility gap and quantitative tie-lines in ternary membranes containing diphytanoyl PC. Biophys J 90, 4428–4436 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Feigenson GW & Buboltz JT Ternary phase diagram of dipalmitoyl-PC/dilauroyl-PC/cholesterol: nanoscopic domain formation driven by cholesterol. Biophys J 80, 2775–2788 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sezgin E et al. Partitioning, diffusion, and ligand binding of raft lipid analogs in model and cellular plasma membranes. Biochim Biophys Acta 1818, 1777–1784 (2012). [DOI] [PubMed] [Google Scholar]
- 171.Kaiser HJ et al. Order of lipid phases in model and plasma membranes. Proc Natl Acad Sci U S A 106, 16645–16650 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Doktorova M, Symons JL & Levental I Structural and functional consequences of reversible lipid asymmetry in living membranes. Nat Chem Biol 16, 1321–1330 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tsuji T et al. Predominant localization of phosphatidylserine at the cytoplasmic leaflet of the ER, and its TMEM16K-dependent redistribution. Proc Natl Acad Sci U S A 116, 13368–13373 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Malvezzi M et al. Ca2+-dependent phospholipid scrambling by a reconstituted TMEM16 ion channel. Nature communications 4, 2367 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gerl MJ et al. Quantitative analysis of the lipidomes of the influenza virus envelope and MDCK cell apical membrane. J Cell Biol 196, 213–221 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sampaio JL et al. Membrane lipidome of an epithelial cell line. Proc Natl Acad Sci U S A 108, 1903–1907 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Newport TD, Sansom MSP & Stansfeld PJ The MemProtMD database: a resource for membrane-embedded protein structures and their lipid interactions. Nucleic acids research 47, D390–D397 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kimura Y et al. Surface of bacteriorhodopsin revealed by high-resolution electron crystallography. Nature 389, 206–211 (1997). [DOI] [PubMed] [Google Scholar]
- 179.Reichow SL & Gonen T Lipid-protein interactions probed by electron crystallography. Curr Opin Struct Biol 19, 560–565 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Han X, Yang K & Gross RW Multi-dimensional mass spectrometry-based shotgun lipidomics and novel strategies for lipidomic analyses. Mass Spectrometry Reviews 31, 134–178 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Laganowsky A, Reading E, Hopper JT & Robinson CV Mass spectrometry of intact membrane protein complexes. Nat Protoc 8, 639–651 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Franck JM, Chandrasekaran S, Dzikovski B, Dunnam CR & Freed JH Focus: Two-dimensional electron-electron double resonance and molecular motions: The challenge of higher frequencies. J Chem Phys 142, 212302 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kuerschner L et al. Polyene-lipids: a new tool to image lipids. Nat Methods 2, 39–45 (2005). [DOI] [PubMed] [Google Scholar]
- 184.Ward AE, Ye Y, Schuster JA, Wei S & Barrera FN Single-molecule fluorescence vistas of how lipids regulate membrane proteins. Biochemical Society transactions 49, 1685–1694 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.McIntosh AL et al. Fluorescence techniques using dehydroergosterol to study cholesterol trafficking. Lipids 43, 1185–1208 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Thiele C, Hannah MJ, Fahrenholz F & Huttner WB Cholesterol binds to synaptophysin and is required for biogenesis of synaptic vesicles. Nat Cell Biol 2, 42–49 (2000). [DOI] [PubMed] [Google Scholar]
- 187.Hoglinger D et al. Trifunctional lipid probes for comprehensive studies of single lipid species in living cells. Proc Natl Acad Sci U S A 114, 1566–1571 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Schuhmacher M et al. Live-cell lipid biochemistry reveals a role of diacylglycerol side-chain composition for cellular lipid dynamics and protein affinities. Proc Natl Acad Sci U S A 117, 7729–7738 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Romero LO et al. Dietary fatty acids fine-tune Piezo1 mechanical response. Nature communications 10, 1200 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chakrapani S, Cordero-Morales JF & Perozo E A quantitative description of KcsA gating I: macroscopic currents. J Gen Physiol 130, 465–478 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Garten M et al. Whole-GUV patch-clamping. Proc Natl Acad Sci U S A 114, 328–333 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]


