The benefit of dietary fiber in promoting human health has been long recognized and is largely based on the lower prevalence of Western diseases such as diabetes, heart disease, and colorectal cancer among populations that consume higher amounts of dietary fiber.1–4 While not well understood, the protective effects of fiber had been previously attributed to faster transit time and stool size, which may facilitate removal of toxic metabolic products.5 However, recent studies show that microbial fermentation likely underlies its benefits of dietary fiber.6, 7 Gut microbiota harbor a diverse set of enzymes such as glycoside hydrolases and polysaccharide lyases, which help break down specific linkages in complex carbohydrates derived from host glycans or dietary fiber into simple sugars. The enzymes profile of these communities will likely determine the specific carbohydrates that can be fermented by an individual’s gut microbiota. The resulting fermentation end-products, such as short chain fatty acids8 (SCFA), regulate important aspects of host physiology including metabolism, cell turnover, and the immune system. Hence, one would expect to see beneficial effects with fiber supplementation. However, human interventional studies show significant inter-individual variability in responses to fiber as well as differences based on fiber type.9 In the current issue of Gastroenterology, Armstrong et al.10 systematically address the complexity that underlies differences in response to fiber by investigating the effects of different β-fructan fibers on barrier function and inflammation using a combination of human specimens, ex vivo culture of colonic biopsies, and cell culture models.
One of the challenges in the field is that carbohydrates that differ in chemical composition and size resulting in varying potential to undergo microbial fermentation along with non-fermentable components such as lignin are all categorized as fiber. Armstrong et al.10 found that certain β-fructans such as fructo-oligosaccharide (FOS) and inulin, but not barley, maltodextrin or starch triggered a pro-inflammatory response in THP-1-derived macrophage cell lines and primary peripheral blood mononuclear cells from healthy donors as evidenced by increased release of IL-1β. This suggests that different carbohydrates classified as fiber can evoke different biologic responses. However, Armstrong et al.10 found the pro-inflammatory effect was not only dependent on the fiber type, but also on immune status of an individual and the fermentative capacity of their gut microbiota (Figure 1A).
Figure 1.
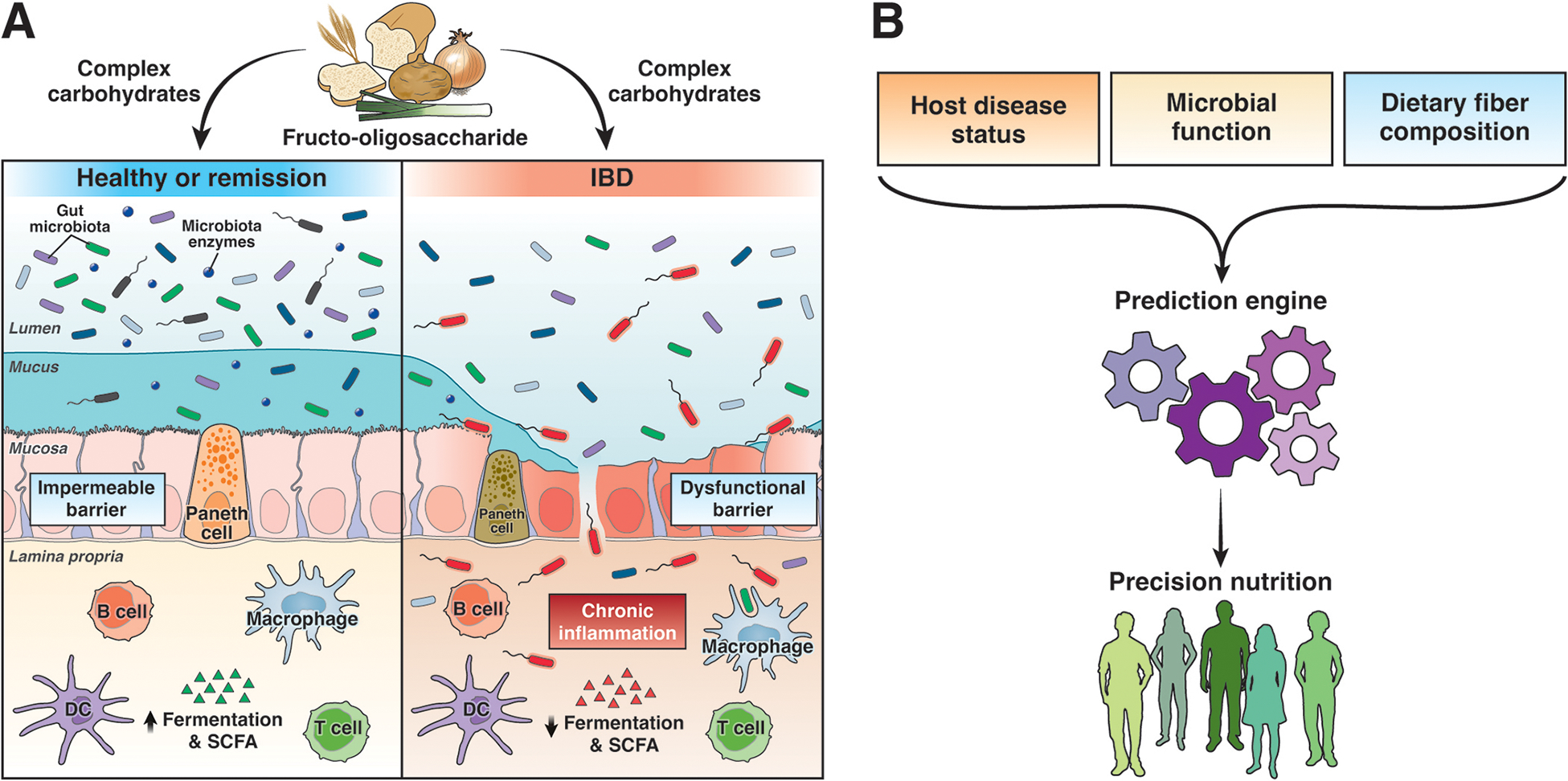
(A) Outline of factors described in the study and addition potential determinants of the effect of differet fibers on host function. (B) Precision nutrition approach will require itegrating the different host, microbial, and diet features. SCFA, short chain fatty acid.
The authors cultured colonic biopsies from pediatric patients with Crohn’s disease and ulcerative colitis (UC) with both active and quiescent disease and from non-inflammatory bowel disease (IBD) controls. They found higher levels of CD45+ cells in biopsies from IBD patients. FOS significantly increased IL-1β secretion in colonic biopsies from patients with active IBD and to a lesser extent from those with quiescent disease, but reduced IL-1β secretion in biopsies from non-IBD controls. The pro-inflammatory effect of FOS was mediated via the NLRP3 and TLR2 pathway. Thus, FOS can differentially affect immune responses based on the underlying immune cell population in the gut. Interestingly, the authors also found that avoidance of FOS among pediatric IBD patients correlated with pro-inflammatory response to FOS, suggesting that FOS consumption during health may reduce the severity of inflammation subsequently. This observation raises additional questions about the mechanisms underlying the effect of distinct fibers on the gut-immune system in different states of health and disease.
Armstrong et al.10 assessed the effect of microbial fermentative capacity on the inflammatory response by exposing THP-1 cells to supernatants from colonic washes cultured in the presence or absence of FOS. They found the fermentative capacity of the gut microbiota as evidenced by levels of FOS and SCFA negatively correlated with the inflammatory response evoked by FOS. The dampened immune response was dependent on both a reduction in FOS as well as an increase in SCFA, suggesting potential complementary mechanisms underlying this effect. To complement their observations with findings in human subjects, authors used samples from a clinical trial of adult UC patients treated with β-fructans and found that symptom flares during β-fructan supplementation correlated with increased inflammatory cytokines in intestinal biopsy lysates.
The findings of Armstrong et al. raise as many questions as they answer. In this study authors deconstructed the complexity of food by investigating individual carbohydrates with varying complexity. Authors found select carbohydrates can affect immune function but as these molecules were purified from chicory roots, the potential role of microbial contaminants that may co-purify with β-fructans cannot be ruled out. Moving forward, it will be important to build back this complexity by combining different carbohydrates to better understand the impact of complex foods as well as modifications that occur with food preparation and cooking. Interventional studies11 have shown that rapid shifts in the gut microbiota and associated changes in microbial metabolism occur with short-term dietary changes and that food-derived microbes can be detected in the distal gut. Single fiber effects do not occur in isolation. Indeed, others have reported the benefits of combining fiber consumption with reduced protein intake for reducing colitis severity in animal models.7
While the study focuses on mucosa-associated microbiota, it is potentially important to also assess the luminal microbiota to determine overall fermentative capacity in different segments of the gut (Figure 1A). Further, the study uses metagenomics to assess the functional capacity of the microbiome, but it is difficult to predict the phenotypic ability of bacteria to utilize specific carbohydrates based on metagenomic sequences alone, given that bacteria do not express all their genes in a given environment. In addition to promoting the growth of the specific microbes that utilize them, dietary carbohydrates can also promote other microbes that depend on the end-products of primary fermenters (cross-feeding). Hence, it is not surprising that while functional differences were observed in the microbiota between FOS responders and non-responders, these differences do not directly explain the differential capacity for FOS fermentation. Thus, it will be important to complement sequencing data with biochemical characterization to determine specific enzymatic activity in a microbial community which in turn provides a therapeutic target (Figure 1A). The acquisition of enzymes capable of digesting algae by the microbiome in Japanese individuals12 highlights one potential pathway for introducing missing enzymatic capabilities into a microbial community.
The findings of Armstrong et al. are compelling and have implications beyond IBD. Another recent study found specific foods (gluten, wheat, soy, milk) may evoke pain through local immune responses with mast cell activation in patients with irritable bowel syndrome.13 It is plausible based that differential fermentative capacity of small intestinal microbiomes plays a role in determining food-evoked pain. The current study highlights the complexity of factors involved in an individual’s response to fiber, such as the carbohydrate chemical structure, the enzyme repertoire of the gut microbiota, and host immune status. While there are likely additional determinants, these observations help explain the inter-individual differences in response to fiber supplementation and underscore the need for precision nutrition approaches rather than the one-size-fits-all fiber supplementation strategy in disease states (Figure 1B). A broader approach of fiber supplementation may still be relevant in health, especially among populations with overall low fiber consumption.
Funding:
AS is supported by NIDDK K23DK122015, R03DK132446. PCK is supported by NIH R01DK114007.
Footnotes
Disclosures: AS serves on Ardelyx Scientific Communications Advisory Board for irritable bowel syndrome with constipation. PCK is an ad hoc consultant for Pendulum Therapeutics, IP Group Inc., and Intrinsic Medicine. PCK holds the patent US20170042860A1 “Methods and materials for using Ruminococcus gnavus or Clostridium sporogenes to treat gastrointestinal disorders” for use of tryptamine producing bacteria to treat gastrointestinal disorders. Mayo Clinic and PCK have a financial interest related to use of tryptamine-producing bacteria.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Makki K, Deehan EC, Walter J, et al. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018;23:705–715. [DOI] [PubMed] [Google Scholar]
- 2.Sonnenburg JL, Sonnenburg ED. Vulnerability of the industrialized microbiota. Science 2019;366. [DOI] [PubMed] [Google Scholar]
- 3.Sonnenburg ED, Sonnenburg JL. The ancestral and industrialized gut microbiota and implications for human health. Nat Rev Microbiol 2019;17:383–390. [DOI] [PubMed] [Google Scholar]
- 4.Burkitt DP, Walker AR, Painter NS. Effect of dietary fibre on stools and the transit-times, and its role in the causation of disease. Lancet 1972;2:1408–12. [DOI] [PubMed] [Google Scholar]
- 5.Trowell H, Burkitt D. Physiological role of dietary fiber: a ten-year review. Bol Asoc Med P R 1986;78:541–4. [PubMed] [Google Scholar]
- 6.Desai MS, Seekatz AM, Koropatkin NM, et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016;167:1339–1353 e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llewellyn SR, Britton GJ, Contijoch EJ, et al. Interactions Between Diet and the Intestinal Microbiota Alter Intestinal Permeability and Colitis Severity in Mice. Gastroenterology 2018;154:1037–1046 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee M, Chang EB. Inflammatory Bowel Diseases (IBD) and the Microbiome-Searching the Crime Scene for Clues. Gastroenterology 2021;160:524–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters V, Dijkstra G, Campmans-Kuijpers MJE. Are all dietary fibers equal for patients with inflammatory bowel disease? A systematic review of randomized controlled trials. Nutr Rev 2022;80:1179–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armstrong HK, Bording-Jorgensen M, Santer DM, et al. Unfermented beta-fructan fibers fuel inflammation in select inflammatory bowel disease patients. Gastroenterology 2022. [DOI] [PubMed] [Google Scholar]
- 11.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014;505:559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hehemann JH, Correc G, Barbeyron T, et al. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature 2010;464:908–12. [DOI] [PubMed] [Google Scholar]
- 13.Aguilera-Lizarraga J, Florens MV, Viola MF, et al. Local immune response to food antigens drives meal-induced abdominal pain. Nature 2021;590:151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroeder BO, Birchenough GMH, Stahlman M, et al. Bifidobacteria or Fiber Protects against Diet-Induced Microbiota-Mediated Colonic Mucus Deterioration. Cell Host Microbe 2018;23:27–40 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]


