Abstract
Objective:
Gabapentin can treat neuropathic pain syndromes and has increasingly been prescribed to treat nociplastic pain. Some patients with knee osteoarthritis (OA) suffer from both nociceptive and nociplastic pain. We examined the cost-effectiveness of adding gabapentin to knee OA care.
Method:
We used the Osteoarthritis Policy Model, a validated Monte Carlo simulation of knee OA, to examine the value of gabapentin in treating knee OA by comparing three strategies: 1) usual care, gabapentin sparing (UC-GS); 2) targeted gabapentin (TG), which provides gabapentin plus usual care for those who screen positive for nociplastic pain on the modified PainDETECT questionnaire (mPD-Q) and usual care only for those who screen negative; and 3) universal gabapentin plus usual care (UG). Outcomes included cumulative quality-adjusted life years (QALYs), lifetime direct medical costs, and incremental cost-effectiveness ratios (ICERs), discounted at 3% annually. We derived model inputs from published literature and national databases and varied key input parameters in sensitivity analyses.
Results:
UC-GS dominated both gabapentin-containing strategies, as it led to lower costs and more QALYs. TG resulted in a cost increase of $689 and a cumulative QALY reduction of 0.012 QALYs. UG resulted in a further $1,868 cost increase and 0.036 QALY decrease. The results were robust to plausible changes in input parameters. The lowest TG strategy ICER of $53,000/QALY was reported when mPD-Q specificity was increased to 100% and AE rate was reduced to 0%.
Conclusion:
Incorporating gabapentin into care for patients with knee OA does not appear to offer good value.
Keywords: Gabapentin, Cost-Efficacy, Knee Osteoarthritis, Pain
Introduction
Symptomatic knee osteoarthritis (OA) is a chronic, costly, and disabling condition that affects over 14 million Americans1, 2. Pain is the central concern of patients with OA as it limits function, reduces quality of life, and drives individuals to seek treatment3. With no disease-modifying treatments available, pharmacologic OA-pain management agents include acetaminophen, non-steroidal anti-inflammatory drugs (NSAIDs), injections of corticosteroids or hyaluronate products, and/or opioids4. These medications offer only modest efficacy and are not tailored to specific pain mechanisms5.
Knee OA pain has long been considered nociceptive, arising from abnormal loading of the damaged joint6. However, roughly one in three patients with knee OA report neuropathic-like pain symptoms – such as allodynia and hyperalgesia –suggesting that increased sensitization and additional pain mechanisms are present5, 7–11. Mechanistically, neuropathic pain follows from nervous system lesions – which have not been observed in knee OA12. The International Association for the Study of Pain (IASP) defines nociplastic pain as altered nociception without clear evidence of tissue damage or disease of the somatosensory system causing the pain12, 13. Consequently, we grouped various non-nociceptive components of some patients’ pain profiles under the umbrella term: nociplastic pain14. Patients with nociplastic pain may experience less relief from OA treatments, including NSAIDs, corticosteroid injections and total knee replacement7, 12, 15–17.
Gabapentinoids are centrally-acting, brain-stabilizing drugs with analgesic efficacy in various neuropathic and nociplastic pain conditions18, 19. Therefore, gabapentinoids may present an opportunity to better treat those with knee OA and nociplastic pain. However, gabapentinoids carry risks of adverse events (AEs), including a 5% chance of drug abuse/misuse and 3% chance of overdose19–22. Screening for nociplastic pain could help identify patients most likely to benefit while sparing others of gabapentinoids’ serious AEs8, 9, 11, 23. Our analysis focuses on gabapentin, which has shown no difference in safety nor efficacy from pregabalin, the other commonly prescribed gabapentinoid24.
Use of gabapentinoids has skyrocketed. In the US, the rate of ambulatory visits involving gabapentinoids rose from ~1% in 2003 to ~3.5% in 201625. In the UK, prescription rates for patients with knee OA has increased over 17-fold since 200026. Yet, to our knowledge, there are no analyses that weigh the risks of AEs with the pain benefits from gabapentin for knee OA. In this analysis, we examine the clinical and economic impact of gabapentin in addition to usual care for patients with knee OA.
Materials and Methods
Analytic Overview
The Osteoarthritis Policy Model (OAPol) is a validated microsimulation model of the natural history and management of knee OA27. We used OAPol to examine if adding gabapentin to usual care (UC) for patients with knee OA and inadequate pain relief from NSAIDs could be cost-effective.
The key outcomes were cumulative quality-adjusted life years (QALYs), lifetime medical costs, and incremental cost-effectiveness ratios (ICERs). We calculated ICERs as the difference in lifetime direct medical costs divided by the difference in QALYs between two strategies. Each year lived is quality-adjusted by a qualify of life (QoL) utility value (1 for perfect health), stratified by BMI, knee pain, age, number of comorbidities, and treatment-related complications. All costs are reported in 2020 USD. Analyses were conducted from a healthcare sector perspective, which includes direct medical costs paid by all payers, including public, private, and individuals28. Costs and QALYs were discounted 3% annually28. Traditionally, cost-effectiveness analyses are anchored in willingness-to-pay (WTP) thresholds, with $50,000 and $100,000/QALY serving as well-established benchmarks29. Here, we report ICERs alone, allowing stakeholders to determine cost-effectiveness. Any strategy or combination of strategies that increased cost and decreased QALYs was labeled “dominated.” To address uncertainty in our inputs, we drew 1000 sets of values for nociplastic pain prevalence, mPD-Q sensitivity and specificity, gabapentin efficacy, gabapentin discontinuation rate, AE rates and QoL decrements associated with AEs from plausible probability distributions specific to each parameter (TA Table 8). We constructed a cost-effectiveness acceptability curve to depict the proportion of model runs where each strategy was preferred at a specific WTP threshold. A strategy was preferred if it produced the greatest number of QALYs while maintaining an ICER below the given WTP threshold. Whenever possible, this cost-effectiveness analysis adhered to the 2022 Consolidated Health Economic Evaluation Reporting Standards statement. We also report results from a benefit-harm analysis, a method for quantitative assessment of the overall value of a treatment based on consideration of its benefits and side-effects. This framework provides an objective and transparent mechanism for combining the various outcomes of treatment into a single ‘net benefit’ metric that can inform clinical decision-making.30
The OAPol Model
OAPol uses prespecified distributions of demographic and clinical characteristics (e.g., age and BMI), comorbidities (e.g., cardiovascular disease and diabetes), and structural and symptomatic severity of knee OA to simulate different patient populations. Using Monte Carlo simulation, in each annual model cycle, subjects may transition to different health states, such as more severe knee OA, according to probabilities determined by their current health state. Subjects accrue QALYs and costs each cycle based on their clinical and/or demographic characteristics. Simulated subjects are followed until death, accounting for all potential benefits and drawbacks of treatment over their remaining lifespan. The average cumulative QALYs and costs for the entire simulated population are used in calculating ICERs.
Knee OA symptom severity is defined by pain ratings on the 100-point Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scale, and ratings are split into five pain groups (<1, 1–15, 16–40, 41–70, and 71–100). Additional details about OAPol are published elsewhere and available in the Technical Appendix (TA)27, 31.
Treatments can reduce patient pain in the model. Initial pain reductions are drawn from probability distributions, derived from treatment-specific published literature and secondary analysis of relevant cohort data, to account for variation in pain relief experienced by different individuals. Thus, some individuals receive little-to-no pain relief, while others experience extensive relief from the same treatment. Each cycle following an initial pain reduction, transition probabilities determine whether a subject’s pain relief is sustained. If pain relief is not sustained, a “regimen failure” occurs and the subject is evaluated for the next regimen (Figure 1). Voluntary discontinuation and/or adverse events can also lead to regimen discontinuation. Regimen costs include medication costs as well as dispensation, procedures, physician visits, and AE-associated costs.
Figure 1.
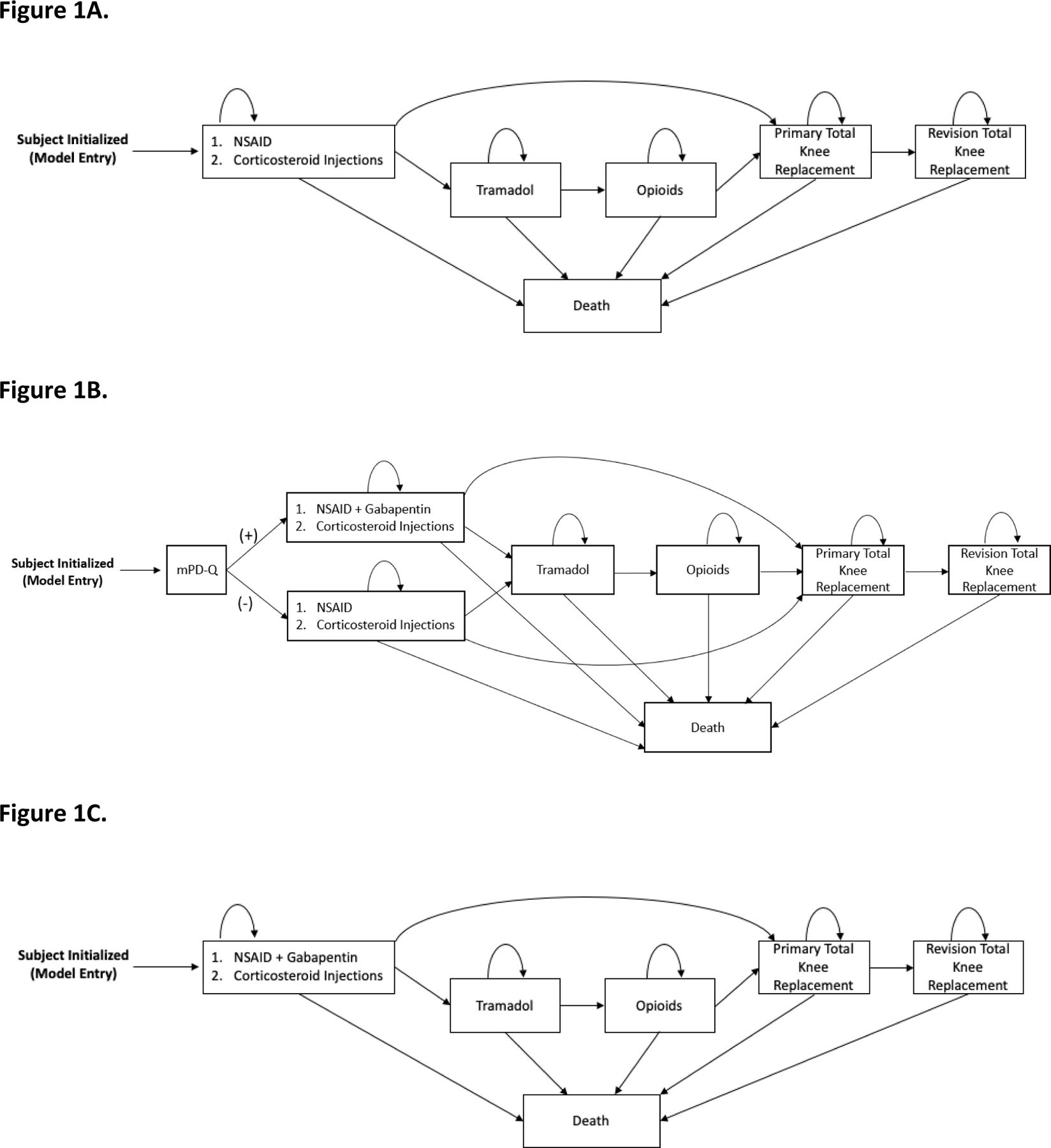
Panel A. The usual care, gabapentin sparing (UC-GS) treatment sequence for knee OA in the OAPol model. Subjects are initialized based on specific cohort characteristics and progress through the regimens outlined, including an NSAID plus corticosteroid injections, tramadol, opioids (for some subjects, others skip the tramadol and opioid regimens), total knee replacement, and revision total knee replacement. Subjects remain on each treatment until it is no longer effective. Death can occur at any point in the sequence.
Panel B. The targeted gabapentin (TG) treatment sequence for knee OA in the OAPol model. Subjects are initialized based on specific cohort characteristics and are screened for nociplastic pain using the modified PainDETECT questionnaire (mPD-Q) at initialization. If they screen positive for nociplastic pain, they receive gabapentin in addition to an NSAID plus corticosteroid injections before moving on to the rest of the UC treatment sequence of tramadol, opioids (for some subjects, others skip the tramadol and opioid regimens), total knee replacement, and revision total knee replacement. If they screen negative for nociplastic pain, they proceed normally through the UC sequence. Subjects remain on each treatment until it is no longer effective. Death can occur at any point in the sequence.
Panel C. The universal gabapentin treatment sequence for knee OA in the OAPol model. Subjects are initialized based on specific cohort characteristics and receive gabapentin in addition to an NSAID plus corticosteroid injections before moving on to the rest of the UC treatment sequence of tramadol, opioids (for some subjects, others skip the tramadol and opioid regimens), total knee replacement, and revision total knee replacement. Subjects remain on each treatment until it is no longer effective. Death can occur at any point in the sequence.
Cohort Characteristics
We modeled a population with demographic and clinical characteristics from the Glucosamine/chondroitin Arthritis Intervention Trial (GAIT) cohort32. We chose these data as they represent a population of patients with knee OA in whom NSAIDs did not lead to satisfactory pain relief. The baseline characteristics of our cohort included a starting age of 58.6 years (SD: 10.3), BMI of 31.7 kg/m2 (SD: 7.1) and average WOMAC pain of 47.2 (SD: 14.7).
Our cohort was divided into two sub-cohorts with distinct pain phenotypes: those with nociceptive pain only and those with nociceptive and nociplastic pain. Based on estimates of nociplastic pain prevalence in knee OA, the nociceptive plus nociplastic cohort prevalence was set to 30%10. Within this group, we assumed that 50% of a subject’s pain was nociceptive and the other 50% nociplastic. In the nociceptive only cohort, all pain was nociceptive (TA Figure 1). We assigned a baseline pain score of 41 to the nociceptive only cohort and 61 to the nociceptive plus nociplastic cohort. With the nociceptive plus nociplastic cohort prevalence at 30% (and thus the nociceptive-only at 70%), this ensured that the overall (combined knee OA) cohort had an average starting pain of 47, equivalent to the GAIT cohort32. These pain values are consistent with studies demonstrating that individuals with nociplastic and nociceptive pain have greater overall pain levels compared to those with nociceptive pain only5, 16.
Prevalence and incidence of comorbidities were derived from the 2017–2018 National Health and Nutrition Examination Survey (NHANES) and were stratified by age, race, and sex33. Background medical costs were derived from the Medicare Current Beneficiary Survey (MCBS)34, and we used a risk-adjustment model developed by Pope et al. to stratify costs by age and comorbidities35. Annual QoL values were derived from The Osteoarthritis Initiative (OAI) and were stratified by age, BMI, pain severity and comorbidities36.
Treatment Strategies
We examined three treatment strategies (Figure 1):
Usual care, gabapentin sparing (UC-GS) – participants are treated with a sequence of a second NSAID plus corticosteroid injections, tramadol, opioids (oxycodone), TKR, and revision TKR.
Targeted gabapentin (TG) – participants are screened for nociplastic pain using the modified PainDETECT Questionnaire (mPD-Q)8 and receive gabapentin if positive for nociplastic pain, in addition to a second NSAID; if negative, participants receive only NSAIDs. All receive the same UC treatment thereafter.
Universal gabapentin (UG) – all participants receive gabapentin in addition to a second NSAID before moving on to the rest of the UC sequence.
QALYs and lifetime medical cost estimates for each of the three strategies were calculated by combining weighted averages of the outputs from the following four OAPol runs, based on nociplastic prevalence as well as the sensitivity and specificity of the mPD-Q:
Nociceptive only cohort – treated with UC
Nociceptive only cohort – UC and gabapentin
Nociceptive plus nociplastic cohort – UC
Nociceptive plus nociplastic cohort – UC and gabapentin
For example, the base case UC-GS strategy is a weighted average of run 1 (70%) and run 3 (30%). The UG strategy is a weighted average of run 2 (70%) and 4 (30%). The weights for the TG strategy depend on the sensitivity and specificity of the mPD-Q.
We assumed that NSAIDs only treat nociceptive pain and gabapentin only treats nociplastic pain. Thus, in the base case, the nociceptive only cohort received pain relief strictly from NSAIDs, while the nociceptive plus nociplastic cohort received pain relief from both NSAIDs and gabapentin (TA). Gabapentin offered pain relief to only the nociceptive plus nociplastic cohort, but costs and risks of AEs were incurred by both cohorts.
Efficacy, utilization rates and AE profiles of the UC regimens have been previously published (TA)27, 31. Informed by MCBS34, we set 5% of subjects to receive opioids. Total knee replacement efficacy for subjects’ pain was decreased for subjects receiving opioids (TA)31.
Impact of Nociplastic Pain on UC Regimen Efficacies
Studies investigating corticosteroid injections and total knee replacements report worse outcomes in persons with nociplastic pain7, 16, 17. Patients with nociceptive pain only and those with nociceptive and nociplastic pain receive similar absolute reductions in pain due to the given intervention – the worse outcomes reported reflect a variation in initial pain. Those with nociplastic pain have a higher baseline pain, resulting in higher outcome pain, even though the raw benefit received is the same. The equivalent absolute pain decrement represents nociceptive pain relief. Thus, we assume that corticosteroid injections, TKR, and revision TKR strictly treat nociceptive pain (TA). For the tramadol and opioid regimens, we assume equivalent efficacy for nociceptive and nociplastic pain.
Nociplastic Pain Screening Tool
We used the modified 9-item PainDETECT Questionnaire (mPD-Q) to screen for nociplastic pain as it can be completed and scored quickly in clinical settings8, 11. PainDETECT is a validated, self-administered questionnaire that rates seven pathological pain qualities for a total score ranging from −1–38 to aid in identifying persons with a neuropathic pain component8, 37. Scores between 13 and 18 suggest a possible neuropathic component, while scores of 19 and higher are indicative of neuropathic pain11. The mPD-Q was modified for patients with knee OA by adjusting question phrasing to specifically address knee pain as opposed to overall pain8; the modified questionnaire was validated against quantitative sensory testing (QST) and reports a sensitivity of 58% and specificity of 71% when a score of 12 is used as the cut-off point for “neuropathic-like” pain11. Given that neuropathy is not recognized to occur in knee OA, we adopted the current IASP terminology of “nociplastic pain” to characterize the neuropathic-like pain symptoms captured with the same mPD-Q cutoff14.
Gabapentin Characteristics
Efficacy
We derived gabapentin’s pain efficacy (40.6% reduction of nociplastic pain) from a Cochrane systematic review18 including 7 studies and 1439 participants that compared gabapentin to placebo in treating painful diabetic neuropathy (TA). We assumed the sustainability of this pain benefit was like that reported in NSAID trials38. With no data available for gabapentin’s efficacy in treating knee OA nociplastic pain specifically, we used diabetic neuropathy as a proxy. To address uncertainty in using a proxy, we performed extensive sensitivity analyses for this parameter.
Adverse Events (AEs)
We derived AEs that resulted in immediate discontinuation of gabapentin (drug abuse/misuse, overdose, suicidal behavior, and death) and those that did not warrant discontinuation (dizziness, somnolence, peripheral edema, nausea) from published literature19–22 (TA). Only AEs that occurred at rates significantly different from placebo were included. Ten percent of the cohort experienced AEs resulting in immediate discontinuation, while 35% experienced other AEs19–22. Overall, 16% of users discontinued gabapentin by choice with 7% due to AEs that did not necessitate discontinuation21.
Cost
We derived the annual cost of a 600 mg dose taken 3x/day of generic gabapentin from Red Book Online39. We used the average wholesale acquisition cost for 100-unit packages, which was $320/year. In addition to the pharmaceutical cost, we included a monthly dispensing fee40, bringing the yearly price of gabapentin to $463.
Sensitivity Analyses
We varied input parameters in one-way, two-way, and scenario-based deterministic sensitivity analyses (DSAs) to understand the breadth of possible outcomes. Ranges of values for these analyses were informed by base-case results. We also conducted a probabilistic sensitivity analysis (PSA) to understand the likelihood of possible outcomes when parameter values were simultaneously selected from plausible probability distributions.
One-Way Sensitivity Analyses
We varied the following parameters: initial pain distribution of nociplastic vs. nociceptive pain in the nociceptive plus nociplastic cohort (50% nociplastic to 95% nociplastic); screening test sensitivity (58–100%) and specificity (71–100%); nociplastic prevalence (20–40%)10; gabapentin nociplastic pain efficacy (20–60% reduction); gabapentin nociceptive pain efficacy (4–20% reduction); and, adverse events (AEs necessitating discontinuation varied from 10% to 0%, AEs not necessitating discontinuation varied from 17.5% to 0%). These ranges are summarized in TA Table 7.
Two-Way Sensitivity Analyses
We conducted additional sensitivity analyses by simultaneously varying two parameters using the same ranges as in the one-way analyses (TA Table 7).
Scenario-Based Deterministic Sensitivity Analyses
To approximate an idealized version of more reliable screening tools like QST, we considered a “perfect” test where both specificity and sensitivity of the mPD-Q were set to 100%. Additionally, we considered an equal pain case where the initial WOMAC pain score for both the nociceptive only and nociceptive plus nociplastic cohorts were set to 47 (SD: 14.9) (TA Figure 2).
Results
Base Case
The UC-GS strategy led to 11.44 QALYs (95% credible interval (CI): 11.42–11.46) and a cumulative lifetime medical cost estimate of $221,301 (95% CI: $221,035-$221,544). The TG and UG strategies led to 11.43 QALYs (95% CI: 11.42–11.46) and 11.41 QALYs (95% CI: 11.39–11.45), and lifetime medical costs of $221,990 (95% CI: $221,484-$222,264) and $223,000 (95% CI: $222,319-$223,248), respectively. Thus, the UC-GS strategy dominated both the TG and UG strategies as both gabapentin-containing strategies increased costs and decreased QALYs. All strategies resulted in comparable opioid and TKR utilization rates (Table 2). Non-quality-adjusted life expectancies were 23.86 (95% CI: 23.85–23.87), 23.85 (95% CI: 23.85–23.87) and 23.84 (95% CI: 23.83–23.88) years for the UC-GS, TG and UG strategies respectively.
Table 2.
Cost-effectiveness analysis results for adding gabapentin to the usual care knee OA treatment sequence in three scenarios.
| Base Case | ||||||
|---|---|---|---|---|---|---|
| Strategy † | QALY | Cost | ICER | % Using Opioids | % Receiving TKR | TKR Age |
| UC-GS | 11.44 | $221,301 | -- | 5.9% | 53.9% | 64.8 |
| TG | 11.43 | $221,990 | Dominated# | 5.9% | 54.0% | 64.8 |
| UG | 11.41 | $223,169 | Dominated# | 5.6% | 54.3% | 64.7 |
| Perfect Screening Test * | ||||||
| Strategy † | QALY | Cost | ICER | % Using Opioids | % Receiving TKR | TKR Age |
| UC-GS | 11.44 | $221,301 | -- | 5.9% | 53.9% | 64.8 |
| TG | 11.44 | $221,810 | Dominated# | 5.9% | 53.9% | 64.8 |
| UG | 11.41 | $223,169 | Dominated# | 5.6% | 54.3% | 64.7 |
| Equal Pain + | ||||||
| Strategy † | QALY | Cost | ICER | % Using Opioids | % Receiving TKR | TKR Age |
| UC-GS | 11.45 | $221,428 | -- | 5.8% | 54.5% | 64.7 |
| TG | 11.44 | $222,151 | Dominated# | 5.7% | 54.6% | 64.7 |
| UG | 11.42 | $223,352 | Dominated# | 5.4% | 55.0% | 64.6 |
Perfect screening test refers to the scenario where both mPD-Q sensitivity and specificity were set to 100%
Equal pain refers to the scenario where both the nociceptive only and nociceptive plus nociplastic cohorts initialized with an equivalent WOMAC pain score of 47
UC-GS is the usual care, gabapentin sparing strategy; TG is the targeted gabapentin strategy; UG is the universal gabapentin strategy
Any strategy or combination of strategies that increased cost and decreased QALY was labeled, “Dominated.”
Sensitivity Analyses
One-way Deterministic Sensitivity Analyses
Across all one-way analyses, QALYs ranged from 11.38–11.47, 11.38–11.46 and 11.36–11.45 for UC-GS, TG, and UG, respectively. Lifetime costs remained at $221,301, $221,990, and $223,169 for UC-GS, TG, and UG, respectively. The UC-GS strategy dominated the other strategies in nearly all sensitivity analyses (TA Table 9). When the AE rate leading to immediate withdrawal was decreased from 10% to 0%, the TG strategy had an ICER of $161,000/QALY, the most favorable ICER observed in these analyses.
Two-way Deterministic Sensitivity Analyses
The results were not sensitive to simultaneous change in nociplastic pain prevalence and mPD-Q accuracy, nor gabapentin efficacy and mPD-Q accuracy. Across all ranges considered, the UC-GS strategy dominated. When the nociceptive plus nociplastic cohort’s pain distribution was varied alongside mPD-Q sensitivity, the UC-GS strategy dominated across all ranges. However, when the pain distribution was set to 95% nociplastic and 5% nociceptive, and mPD-Q specificity was set to 100%, the TG strategy had an ICER of $119,000/QALY. The UG strategy remained dominated. For these analyses, QALYs ranged from 11.38–11.44 and 11.38–11.45 for UC-GS and TG, respectively. Lifetime costs ranged from $221,185-$221,811 and $221,460-$222,707 for UC-GS and TG, respectively (TA Table 10).
Results from the two-way sensitivity analyses are displayed in Figure 2 and TA Table 10. In every scenario and range considered, either the UC-GS strategy dominated both gabapentin strategies, or the TG strategy had a lower ICER than the UG strategy. Consequently, we show only the TG ICERs. With mPD-Q sensitivity at 100% and AE rate at 0%, TG had an ICER of $110,000/QALY (Figure 2A). With mPD-Q specificity at 100% and AE rate at 1%, 0.5%, and 0%, the TG ICERs were $79,000, $58,000, and $53,000/QALY, respectively (Figure 2B). When gabapentin efficacy and AE rate (type that results in immediate discontinuation) were varied simultaneously, the lowest TG ICER of $108,000/QALY resulted when gabapentin’s nociplastic pain reduction was increased from 40.6% (base case) to 60%, and its AE rate was decreased from 10% (base case) to 0%.
Figure 2.
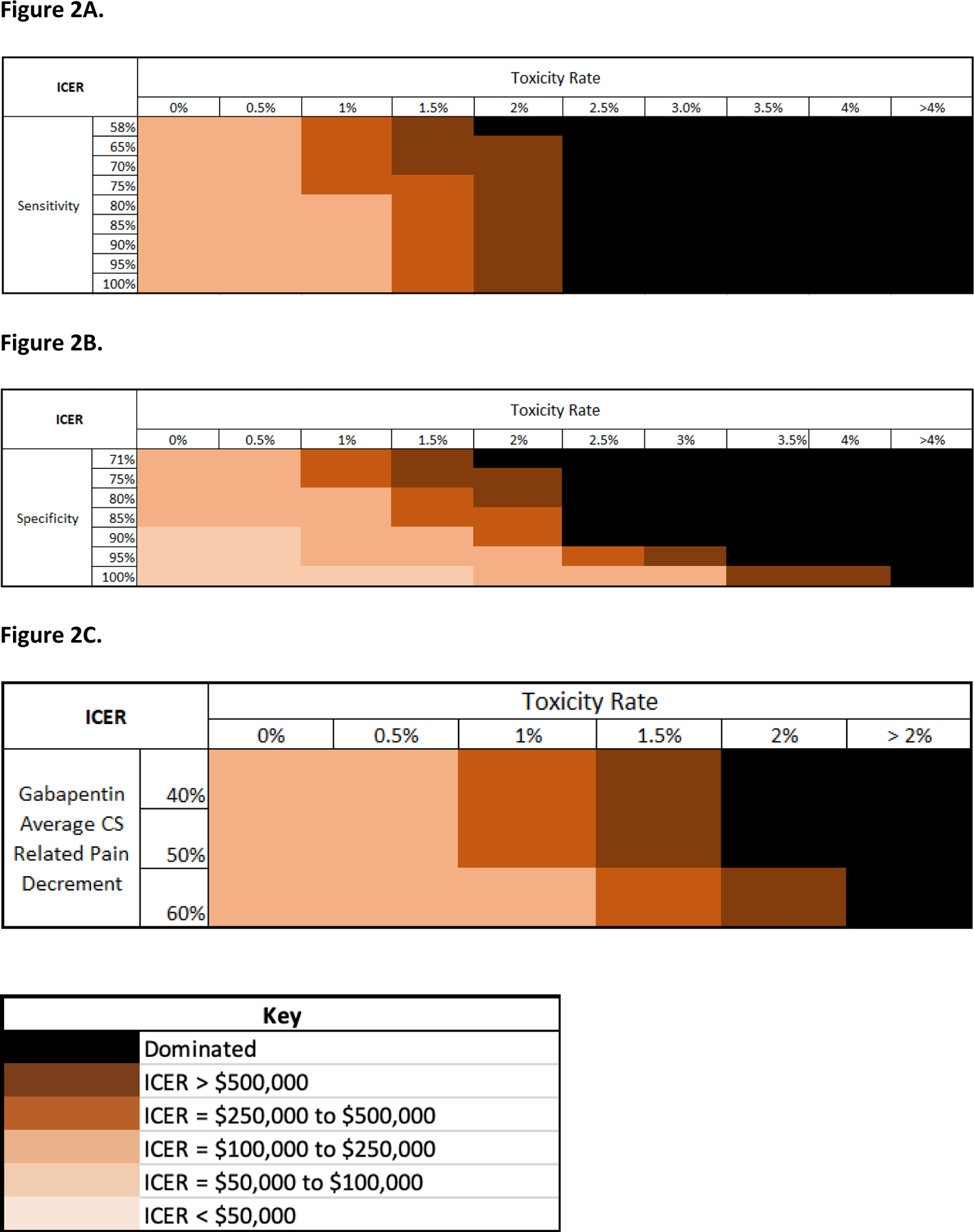
Panel A. Bivariate heat map of the modified PainDETECT questionnaire (mPD-Q) sensitivity and adverse event (AE) rate for the targeted gabapentin (TG) strategy. The AE rate refers to the likelihood of experiencing one of the three AEs (drug abuse/misuse, overdose, suicidal behavior, and deaths) that lead to immediate discontinuation of gabapentin. Incremental cost-effectiveness ratios (ICERs) for the TG strategy were calculated for increasing mPD-Q sensitivity values and decreasing AE rates. The AE rate was decreased from the base case value of 10% to 0% and the mPD-Q sensitivity was increased from the base case value of 58% to 100%. All other parameters were held at base case values. ICERs were categorized into buckets and assigned an orange color as specified in the key.
Panel B. Bivariate heat map of the modified PainDETECT questionnaire (mPD-Q) specificity and adverse event rate for the targeted gabapentin (TG) strategy. The AE rate refers to the likelihood of experiencing one of the three AEs (drug abuse/misuse, overdose, suicidal behavior, and deaths) that lead to immediate discontinuation of gabapentin. Incremental cost-effectiveness ratios (ICERs) for the TG strategy were calculated for increasing mPD-Q specificity values and decreasing AE rates. The AE rate was decreased from the base case value of 10% to 0% and the mPD-Q specificity was increased from the base case value of 71% to 100%. All other parameters were held at base case values. ICERs were categorized into buckets and assigned an orange color as specified in the key.
Panel C. Bivariate heat map of gabapentin efficacy and adverse event rate for the targeted gabapentin strategy. The AE rate refers to the likelihood of experiencing one of the three AEs (drug abuse/misuse, overdose, suicidal behavior, and deaths) that lead to immediate discontinuation of gabapentin. Incremental cost-effectiveness ratios (ICERs) for the TG strategy were calculated for increasing gabapentin efficacy values and decreasing AE rates. The AE rate was decreased from the base case value of 10% to 0% and gabapentin efficacy was increased from the base case value of 40.6% to 60% nociplastic pain reduction.
Scenario-based Sensitivity Analyses
In both the “perfect” test and equal pain scenarios, the UC-GS strategy dominated the TG and UG strategies (Table 2).
Probabilistic Sensitivity Analyses
The distribution of model outcomes in the cost-effectiveness plane resulting from the PSA is illustrated in Figure 3. Using these data, 95% credible intervals were constructed for lifetime medical costs and QALYs gained for each strategy (TA Table 11). Figure 4 depicts the results of the PSA in a cost-effectiveness acceptability curve. This shows the proportion of times each strategy was the cost-effective option at willingness-to-pay thresholds of $0 to $200,000/QALY. At a WTP threshold of $50,000/QALY, UC-GS was the cost-effective strategy in 100% of cases. At the $200,000/QALY WTP threshold, the TG strategy was cost-effective in 5.7% of cases and, at the $100,000/QALY WTP threshold, the TG strategy was cost-effective in 0.8% of cases. UG was not cost-effective at any WTP threshold considered.
Figure 3.
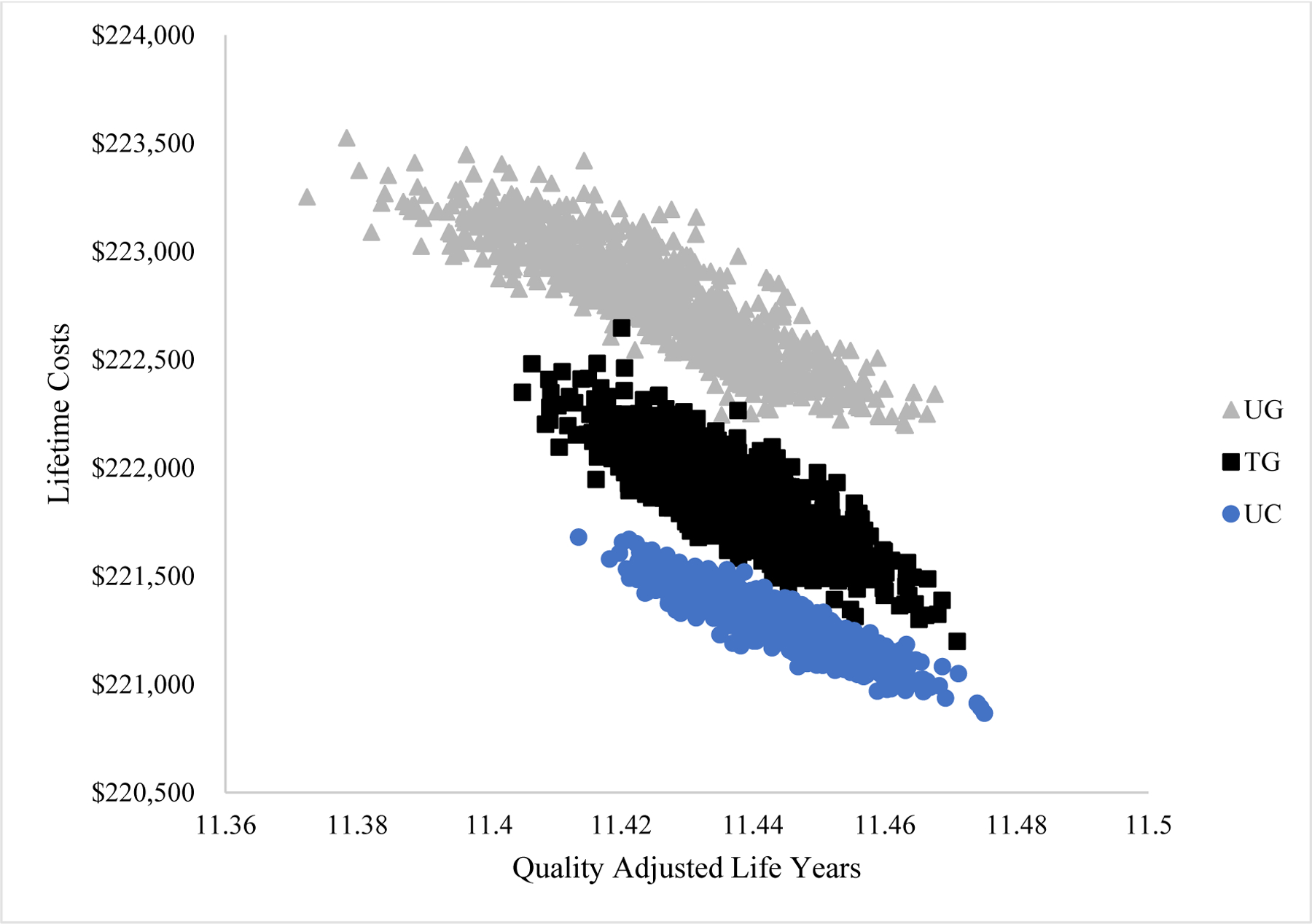
The cost-effectiveness points distribution corresponding to the probabilistic sensitivity analyses that were conducted varying gabapentin efficacy, nociplastic prevalence, discontinuation rates, mPD-Q sensitivity and specificity, AE rates and AE QoL multipliers. One thousand sets of probabilistically chosen inputs were drawn from plausible distributions. Each point in the plane marks the average lifetime medical cost and average cumulative quality adjusted life years for a single strategy from one of the 1,000 model runs in the probabilistic sensitivity analysis. Lifetime medical costs are reported in 2020 USD and treatment efficacy is reported in quality adjusted life years. The three treatment strategies include usual care (UC), universal gabapentin (UG), and targeted gabapentin (TG).
Figure 4.

Probabilistic sensitivity analyses were conducted varying gabapentin efficacy, nociplastic prevalence, discontinuation rates, mPD-Q sensitivity and specificity, AE rates and AE QoL multipliers. 1,000 iterations of probabilistically chosen inputs were drawn. Incremental cost-effectiveness ratios (ICERs) were calculated for each iteration comparing the usual care, gabapentin sparing (green line), targeted gabapentin (orange line), and universal gabapentin (purple line) treatment strategies. The ICERs for these strategies were compared at a range of willingness-to-pay (WTP) thresholds, and that which produced the greatest number of quality-adjusted life years while remaining below the WTP threshold was termed the preferred strategy. The probability of being the preferred strategy is plotted against WTP thresholds from $0 to $200,000/QALY.
Discussion
We used a widely-published, validated computer simulation model to evaluate the cost-effectiveness of adding gabapentin to usual care in treating knee OA. Results from our analysis suggest that adding gabapentin to usual care for patients with knee OA leads to greater costs without additional benefits measured by cumulative QALYs. The UC-GS strategy dominated both TG and UG as it led to lower lifetime medical costs and more QALYs. Sensitivity analyses showed that AE rates were the driving factors of gabapentin’s value. The TG strategy led to ICERs below $175,000/QALY only when the likelihood of experiencing an AE that resulted in immediate discontinuation dropped from 10% (base case) to 0.5%.
The finding that gabapentin does not provide good value is driven primarily by high gabapentin AE rates and secondarily by modest gabapentin efficacy. The TG strategy should help ensure that only those who would benefit from gabapentin would also take on the AE risks. However, our results show that this is not enough to increase gabapentin’s value. Even in the “perfect” test scenario, UC-GS dominated. Furthermore, gabapentin use did not delay TKR treatment and the age of TKR was consistent across strategies (65 years). AEs associated with gabapentin treatment led to higher discontinuation rates such that simulated individuals spent comparable amounts of time on the NSAIDs plus gabapentin and the NSAIDs only treatment regimens, despite gabapentin’s additional pain efficacy. In the one-way sensitivity analysis that varied AE rates, the TG strategy had an ICER of $161,000/QALY when the likelihood of experiencing any of the AEs that necessitated immediate discontinuation was set to 0%. The ICER only dropped to $103,000/QALY when the mPD-Q specificity was simultaneously increased to 85%. With the specificity at 100%, the screening strategy ICER reduced to $53,000/QALY. All other analyses led to TG ICERs above the $100,000/QALY WTP threshold. These analyses show that gabapentin-related AEs had a decisive impact on our results. Unless there is some combination of a more accurate nociplastic screening tool, larger pain reduction, and no AEs, the gabapentin-based strategies do not offer sufficient value.
To the best of our knowledge, this is the first study to examine the cost-effectiveness of gabapentin for treating knee OA pain. For a drug with such high prescription rates25, 26, the literature on its cost-effectiveness remains surprisingly sparse. A 2019 knee OA randomized controlled trial41 reported greater WOMAC pain reduction in the gabapentin group than the acetaminophen group; however, they did not separate the overall cohort by pain status and did not perform a cost-effectiveness analysis (CEA). Gabapentin’s pain benefits must be contextualized by their significant AE profile, which includes overdose and suicidal behavior.
CEAs have shown several pharmacologic treatments to be cost-effective for knee OA including hyaluronate and platelet-rich plasma injections.42 However these non-centrally acting treatments may not be as effective among patients with nociplastic knee OA pain.12 CEAs conducted for both gabapentin and pregabalin in treating neuropathy showed that the value of one over the other relies on just 6–9 pain-free days43, 44. When considered over a lifetime, these differences are negligible and confirm our decision to use gabapentin as a proxy for the gabapentinoids family. O’Connor et al.45 and Bellows et al. 46 found that both duloxetine and desipramine dominated pregabalin and gabapentin in treating painful diabetic neuropathy. In Bellows et al.’s PSA results, gabapentin was the most cost-effective treatment just 14% of the time at a WTP threshold of $50,000/QALY46. Similarly, our results show that incorporating gabapentin into knee OA care is not cost-effective compared to usual care. Furthermore, duloxetine was found to be cost-effective compared to usual care in treating knee OA47.
Our results should be viewed considering several limitations. First, we limited our analyses to OA treatments covered by major payers in the US. Due to a dearth of published trials evaluating gabapentin use in pain-stratified knee OA cohorts, studies used to derive gabapentin efficacy only included patients with diabetic neuropathy. While nociplastic pain involves similar symptoms, assuming gabapentin would be equally efficacious in treating nociplastic and neuropathic pain is perhaps an overestimate. We address this limitation with sensitivity analyses that vary gabapentin efficacy. To model the distinct impact of gabapentin versus NSAIDs on pain, we assumed that total pain could be split into two discrete categories. We are unaware of measures that accurately quantify nociplastic vs. nociceptive pain. Sensitivity analyses varying the relative proportions of nociceptive and nociplastic pain showed that our base case conclusions were robust across all scenarios considered. We acknowledge evidence that suggests some usual care treatments for OA, such as injections and TKRs, may affect nociplastic pain48, and that opioid treatments may not affect those with and without nociplastic pain equally49. However, these assumptions affect all strategies similarly and likely do not confound our comparative results. Moreover, we assume that gabapentin adverse event occurrence is independent of all patient characteristics. While we do perform sensitivity analyses concerning the AE rates for gabapentin, we did not perform a specific subgroup analysis of those experiencing serious gabapentin AEs because we are not aware of any prognostic tools to determine if one individual is more likely to experience a severe AE. Finally, we assumed that gabapentin is only efficacious for nociplastic pain but found this to not affect results in sensitivity analyses.
Nociplastic pain is prevalent in the knee OA community; identifying individuals with non-nociceptive pain components could offer opportunities for personalized treatment strategies5, 9, 10. Those affected by nociplastic pain are at a disadvantage as they are likely to receive less benefit from NSAIDs, corticosteroid injections, and total knee replacements. Gabapentin prescription rates over the past decade have increased in hopes of providing more targeted pain relief for those with nociplastic pain25, 26. However, our analysis suggests that gabapentin is not cost-effective in treating patients with knee OA, even if we could accurately identify those with nociplastic pain 100% of the time, due to its serious AE profile. Further research is needed to determine what interventions could offer value to this subset of patients with knee OA.
Supplementary Material
Table 1.
Key model inputs (costs in 2020 USD).
|
Overall Cohort Characteristics
| |||
|---|---|---|---|
| Parameter | Mean (SD) | Source | |
| Age (years) | 58.6 (10.3) | Derived from Clegg et al.32 | |
| Female | 64.1% | ||
| Race/Ethnicity | |||
| White non-Hispanic | 80.8% | ||
| White Hispanic | 4.0% | ||
| Black non-Hispanic | 14.4% | ||
| Black Hispanic | 0.7% | ||
| BMI | 31.7 (7.0) | ||
| Kellgren Lawrence (KL) grade | |||
| 2 | 55.3% | ||
| 3 | 44.7% | ||
| Starting WOMAC knee pain | 47 (14.7) | ||
|
Nociceptive Only Cohort Characteristics | |||
| Parameter | Mean (SD) | Source | |
|
| |||
| Prevalence | 70% | Derived from Zoli et al.10, Wiffen et al.18 | |
|
| |||
| Starting WOMAC knee pain | 41 (14.9) | Derived from Clegg et al.32 | |
|
Nociceptive + Nociplastic Cohort Characteristics | |||
| Parameter | Mean (SD) | Source | |
|
| |||
| Prevalence | 30% | Derived from Zolio et al.10, Wiffen et al.18 | |
|
| |||
| Starting WOMAC knee pain | 61 (14.9) | Derived from Clegg et al.32 | |
|
| |||
| Percent nociceptive pain | 50% | Assumptiona | |
| Percent nociplastic pain | 50% | ||
|
Gabapentin Intervention Parameters | |||
| Parameter | Mean (SD) | Source | |
|
| |||
| Annual cost | $463 | Derived from Red Book39, CCPA40 (TA) | |
|
| |||
| Percent reduction in nociceptive WOMAC pain | 0% | Assumptionb | |
|
| |||
| Percent reduction in nociplastic WOMAC pain | 40.6% | Derived from Wiffen et al.18 | |
|
| |||
| Annual probability treatment stops providing pain relief* | 24% - 75% | Derived from Scott et al.38 | |
|
| |||
| Discontinuation rate** | 16% | FDA21 | |
|
| |||
| Adverse Events | Probability † | Source | |
|
| |||
| Dizziness | 28% | FDA21 | |
| Somnolence | 21% | ||
| Peripheral edema | 8% | ||
| Nausea | 13% | ||
| Suicidal behavior & death | 0.9% | Molero et al.20 | |
| Overdose | 3.5% | ||
|
| |||
| Drug abuse/misuse | 5.7% | Evoy et al.22 | |
|
Screening Test Parameters | |||
| Parameter | Value | Source | |
|
| |||
| Sensitivity | 58% | Hochman et al.11 | |
| Specificity | 71% | ||
|
| |||
| Cost | $0 | Assumptionc | |
|
Other Intervention Costs | |||
| Intervention | Cost (year 1) | Cost (years 2+) | Source |
|
| |||
| NSAIDS/PT/Braces | $903 | $722 | Derived from Losina et al.1 (TA) |
|
| |||
| Corticosteroid injections | $574 | $574 | Derived from Losina et al.1 (TA) |
|
| |||
| Tramadol | $580 | $716 | Derived from Huizinga et al.31 (TA) |
|
| |||
| Opioids (oxycodone) | $1,076 | $1,328 | Derived from Huizinga et al.31 (TA) |
|
| |||
| Primary TKR | $20,120 | $119 | Derived from Stanley et al.50 (TA) |
| Revision TKR | $28,111 | $119 | |
Assumption = 50% of the cohort’s pain is nociceptive, 50% is nociplastic
Assumption = gabapentin is only efficacious in treating nociplastic pain
Assumption = the mPD-Q questionnaire costs $0
The range spans all pain groups
This value does not include the 100% discontinuation rate of the three more severe adverse events
Rates of adverse events are halved in years 2+
This range refers to pain decrements for pain group 3 to pain group 5. To be in pain group 3, a person must have a WOMAC score of at least 15
These values were diminished if a person had previously taken opioids
Support:
National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) grants K24 AR070892, RO1 AR074290, P30 AR07257
Role of the funding source
This work is supported in part by the NIH/NIAMS grants K24 AR070892, RO1 AR074290, P30 AR07257, K01 AR075879. The funder (NIH/NIAMS) has not seen or participated in this analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
Outside of NIH/NIAMS funding, all authors have not received any other financial support for this manuscript. Outside of this work, Dr. Tuhina Neogi, Dr. Jamie E. Collins, Dr. Jeffrey N. Katz and Dr. Elena Losina currently receive support for research grants from the NIH (Neogi, Collins, Losina), Biosplice (Katz, Losina) and Pfizer (Losina). Dr. Tuhina Neogi, Dr. Jamie E. Collins and Dr. Elena Losina also received consultancy payments from Pfizer (Neogi, Losina), Lilly (Neogi), Regeneron (Neogi), Novartis (Neogi) and Boston Imaging Core Labs (Collins). Gordon P. Bensen, Alec C. Rogers, Valia P. Leifer, Aleksandra M. Kostic, Dr. Robert R. Edwards, Dr. A. David Paltiel and Dr. David J. Hunter have no disclosures.
References
- 1.Losina E, Paltiel AD, Weinstein AM, Yelin E, Hunter DJ, Chen SP, et al. Lifetime medical costs of knee osteoarthritis management in the United States: impact of extending indications for total knee arthroplasty. Arthritis Care Res (Hoboken) 2015; 67: 203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deshpande BR, Katz JN, Solomon DH, Yelin EH, Hunter DJ, Messier SP, et al. Number of Persons With Symptomatic Knee Osteoarthritis in the US: Impact of Race and Ethnicity, Age, Sex, and Obesity. Arthritis Care Res (Hoboken) 2016; 68: 1743–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neogi T The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage 2013; 21: 1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res (Hoboken) 2020; 72: 149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011; 152: S2–s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu K, Robbins SR, McDougall JJ. Osteoarthritis: the genesis of pain. Rheumatology 2018; 57: iv43–iv50. [DOI] [PubMed] [Google Scholar]
- 7.Kurien T, Arendt-Nielsen L, Petersen KK, Graven-Nielsen T, Scammell BE. Preoperative Neuropathic Pain-like Symptoms and Central Pain Mechanisms in Knee Osteoarthritis Predicts Poor Outcome 6 Months After Total Knee Replacement Surgery. J Pain 2018; 19: 1329–1341. [DOI] [PubMed] [Google Scholar]
- 8.Hochman JR, Gagliese L, Davis AM, Hawker GA. Neuropathic pain symptoms in a community knee OA cohort. Osteoarthritis Cartilage 2011; 19: 647–654. [DOI] [PubMed] [Google Scholar]
- 9.López-Ruiz M, Losilla JM, Monfort J, Portell M, Gutiérrez T, Poca V, et al. Central sensitization in knee osteoarthritis and fibromyalgia: Beyond depression and anxiety. PLoS One 2019; 14: e0225836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zolio L, Lim KY, McKenzie JE, Yan MK, Estee M, Hussain SM, et al. Systematic review and meta-analysis of the prevalence of neuropathic-like pain and/or pain sensitization in people with knee and hip osteoarthritis. Osteoarthritis Cartilage 2021. [DOI] [PubMed]
- 11.Hochman JR, Davis AM, Elkayam J, Gagliese L, Hawker GA. Neuropathic pain symptoms on the modified painDETECT correlate with signs of central sensitization in knee osteoarthritis. Osteoarthritis Cartilage 2013; 21: 1236–1242. [DOI] [PubMed] [Google Scholar]
- 12.Fitzcharles MA, Cohen SP, Clauw DJ, Littlejohn G, Usui C, Häuser W. Nociplastic pain: towards an understanding of prevalent pain conditions. Lancet 2021; 397: 2098–2110. [DOI] [PubMed] [Google Scholar]
- 13.IASP Terminology. vol. 2021: International Association for the Study of Pain 2017. [Google Scholar]
- 14.Bailly F, Cantagrel A, Bertin P, Perrot S, Thomas T, Lansaman T, et al. Part of pain labelled neuropathic in rheumatic disease might be rather nociplastic. RMD Open 2020; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore RA, Chi CC, Wiffen PJ, Derry S, Rice AS. Oral nonsteroidal anti-inflammatory drugs for neuropathic pain. Cochrane Database Syst Rev 2015; 2015: Cd010902. [DOI] [PMC free article] [PubMed]
- 16.Dave AJ, Selzer F, Losina E, Usiskin I, Collins JE, Lee YC, et al. The association of pre-operative body pain diagram scores with pain outcomes following total knee arthroplasty. Osteoarthritis Cartilage 2017; 25: 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jørgensen TS, Graven-Nielsen T, Ellegaard K, Danneskiold-Samsøe B, Bliddal H, Henriksen M. Intra-Articular Analgesia and Steroid Reduce Pain Sensitivity in Knee OA Patients: An Interventional Cohort Study. Pain Res Treat 2014; 2014: 710490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiffen PJ, Derry S, Bell RF, Rice AS, Tölle TR, Phillips T, et al. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev 2017; 6: Cd007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaheen A, Alam SM, Ahmad A, Khan M. Clinical efficacy and tolerability of Gabapentinoids with current prescription patterns in patients with Neuropathic pain. Pak J Med Sci 2019; 35: 1505–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molero Y, Larsson H, D’Onofrio BM, Sharp DJ, Fazel S. Associations between gabapentinoids and suicidal behaviour, unintentional overdoses, injuries, road traffic incidents, and violent crime: population based cohort study in Sweden. Bmj 2019; 365: l2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Full Prescribing Information: Neurontin (gabapentin) vol. 4168942: US Food and Drug Administration 2017. [Google Scholar]
- 22.Evoy KE, Covvey JR, Peckham AM, Ochs L, Hultgren KE. Reports of gabapentin and pregabalin abuse, misuse, dependence, or overdose: An analysis of the Food And Drug Administration Adverse Events Reporting System (FAERS). Res Social Adm Pharm 2019; 15: 953–958. [DOI] [PubMed] [Google Scholar]
- 23.Fingleton C, Smart K, Moloney N, Fullen BM, Doody C. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage 2015; 23: 1043–1056. [DOI] [PubMed] [Google Scholar]
- 24.Davari M, Amani B, Amani B, Khanijahani A, Akbarzadeh A, Shabestan R. Pregabalin and gabapentin in neuropathic pain management after spinal cord injury: a systematic review and meta-analysis. Korean J Pain 2020; 33: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou L, Bhattacharjee S, Kwoh CK, Tighe PJ, Malone DC, Slack M, et al. Trends, Patient and Prescriber Characteristics in Gabapentinoid Use in a Sample of United States Ambulatory Care Visits from 2003 to 2016. Journal of Clinical Medicine 2020; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Appleyard T, Ashworth J, Bedson J, Yu D, Peat G. Trends in gabapentinoid prescribing in patients with osteoarthritis: a United Kingdom national cohort study in primary care. Osteoarthritis Cartilage 2019; 27: 1437–1444. [DOI] [PubMed] [Google Scholar]
- 27.Losina E, Walensky RP, Reichmann WM, Holt HL, Gerlovin H, Solomon DH, et al. Impact of obesity and knee osteoarthritis on morbidity and mortality in older Americans. Ann Intern Med 2011; 154: 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. Jama 2016; 316: 1093–1103. [DOI] [PubMed] [Google Scholar]
- 29.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 2014; 371: 796–797. [DOI] [PubMed] [Google Scholar]
- 30.Felli JC, Noel RA, Cavazzoni PA. A multiattribute model for evaluating the benefit-risk profiles of treatment alternatives. Med Decis Making 2009; 29: 104–115. [DOI] [PubMed] [Google Scholar]
- 31.Huizinga JL, Stanley EE, Sullivan JK, Song S, Hunter DJ, Paltiel AD, et al. Societal Cost of Opioid Use in Symptomatic Knee Osteoarthritis Patients in the United States. Arthritis Care Res (Hoboken) 2021. [DOI] [PMC free article] [PubMed]
- 32.Clegg DO, Reda DJ, Harris CL, Klein MA, O’Dell JR, Hooper MM, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med 2006; 354: 795–808. [DOI] [PubMed] [Google Scholar]
- 33.National Health and Nutrition Examination Survey (NHANES). Online: Centers for Disease Control and Prevention 2017–2018.
- 34.Medicare Current Beneficiaries Survey (MCBS). Centers for Medicare and Medicaid Services 2014–2016.
- 35.Pope GKJ, Ingber M, Freeman S, Sekar R, Newhart C. Evaluation of the CMS-HCC Risk Adjustment Model RTI International 2011.
- 36.Osteoarthritis Initiative. University of California, San Fransisco: 2013. [Google Scholar]
- 37.Sumitani H, Matsubayashi Y, Tsuchida R, Oshima Y, Takeshita K, Yamada Y. Validation of Pain Severity Assessment using the PainDETECT Questionnaire. International Journal of Anesthesiology & Pain Medicine 2017; 03. [Google Scholar]
- 38.Scott DL, Berry H, Capell H, Coppock J, Daymond T, Doyle DV, et al. The long-term effects of non-steroidal anti-inflammatory drugs in osteoarthritis of the knee: a randomized placebo-controlled trial. Rheumatology (Oxford) 2000; 39: 1095–1101. [DOI] [PubMed] [Google Scholar]
- 39.Red Book Online. vol. 2020: Truven Health Analytics Inc
- 40.The Cost of Dispensing Study: an independent comparative analysis of U.S. prescription dispensing costs. Coalition for Community Pharmacy Action: National Community Pharmacists Association 2015.
- 41.Enteshari-Moghaddam A, Azami A, Isazadehfar K, Mohebbi H, Habibzadeh A, Jahanpanah P. Efficacy of duloxetine and gabapentin in pain reduction in patients with knee osteoarthritis. Clin Rheumatol 2019; 38: 2873–2880. [DOI] [PubMed] [Google Scholar]
- 42.Samuelson EM, Ebel JA, Reynolds SB, Arnold RM, Brown DE. The Cost-Effectiveness of Platelet-Rich Plasma Compared With Hyaluronic Acid Injections for the Treatment of Knee Osteoarthritis. Arthroscopy 2020; 36: 3072–3078. [DOI] [PubMed] [Google Scholar]
- 43.Tarride JE, Gordon A, Vera-Llonch M, Dukes E, Rousseau C. Cost-effectiveness of pregabalin for the management of neuropathic pain associated with diabetic peripheral neuropathy and postherpetic neuralgia: a Canadian perspective. Clin Ther 2006; 28: 1922–1934. [DOI] [PubMed] [Google Scholar]
- 44.Athanasakis K, Petrakis I, Karampli E, Vitsou E, Lyras L, Kyriopoulos J. Pregabalin versus gabapentin in the management of peripheral neuropathic pain associated with post-herpetic neuralgia and diabetic neuropathy: a cost effectiveness analysis for the Greek healthcare setting. BMC Neurology 2013; 13: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Connor AB, Noyes K, Holloway RG. A cost-utility comparison of four first-line medications in painful diabetic neuropathy. Pharmacoeconomics 2008; 26: 1045–1064. [DOI] [PubMed] [Google Scholar]
- 46.Bellows BK, Nelson RE, Oderda GM, LaFleur J. Long-term cost-effectiveness of initiating treatment for painful diabetic neuropathy with pregabalin, duloxetine, gabapentin, or desipramine. Pain 2016; 157: 203–213. [DOI] [PubMed] [Google Scholar]
- 47.Sullivan JK, Huizinga J, Edwards RR, Hunter DJ, Neogi T, Yelin E, et al. Cost-effectiveness of duloxetine for knee OA subjects: the role of pain severity. Osteoarthritis Cartilage 2021; 29: 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arant KR, Katz JN, Neogi T. Quantitative sensory testing: identifying pain characteristics in patients with osteoarthritis. Osteoarthritis and Cartilage 2021. [DOI] [PMC free article] [PubMed]
- 49.Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician 2011; 14: 145–161. [PubMed] [Google Scholar]
- 50.Stanley EE, Trentadue TP, Smith KC, Sullivan JK, Thornhill TS, Lange J, et al. Cost-effectiveness of dental antibiotic prophylaxis in total knee arthroplasty recipients with type II diabetes mellitus. Osteoarthritis and Cartilage Open 2020; 2: 100084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


