Abstract
Developing chemical tools to detect and influence biological processes is a cornerstone of chemical biology. Here we combine two tools which rely on orthogonality– perfluorocarbons and multiplexed shortwave infrared fluorescence imaging– to track the biodistribution of nanoemulsions in real time in living mice. Drawing inspiration from fluorous and shortwave infrared (SWIR) fluorophore development, we prepared two SWIR-emissive, fluorous-soluble chromenylium polymethine dyes. These are the most red-shifted fluorous fluorophores– “fluorofluorophores”– to date. After characterizing the dyes, their utility was demonstrated by tracking perfluorocarbon nanoemulsion biodistribution in vivo. Using an excitation-multiplexed approach to visualize two variables simultaneously, we gained insight into the importance of size and surfactant identity on biodistribution.
Keywords: perfluorocarbon emulsion, fluorescence imaging, shortwave infrared, excitation-based multiplexed imaging, biodistribution
Graphical Abstract
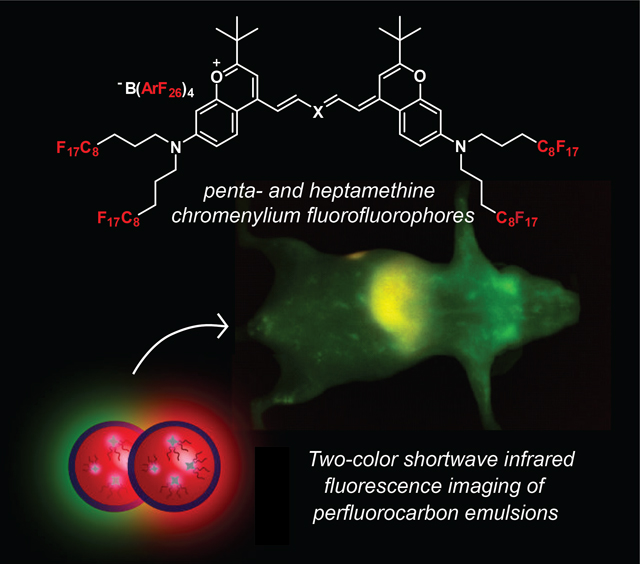
Fluorofluorophores (fluoro references to fluorine and fluorescence) are chemical tools to study perfluorocarbons. To understand the biodistribution of perfluorocarbons in animals, fluorofluorophores that emit past 1000 nm are necessary. Here we describe the synthesis, characterization, and application of shortwave infrared (SWIR)-emissive fluorofluorophores towards understanding how to control the pharmacokinetics of perfluorocarbon nanoemulsions in mammals.
Introduction
The complexity of living systems has prompted the design of chemical probes and bioorthogonal delivery vehicles which have minimal interactions with the plethora of functionality present in biomolecules. Initially, the concept of bioorthogonality was introduced to describe covalent reactions that can occur in the presence of biomolecules and cells.[1] However, as efforts to bring bioorthogonal chemistries into mammals have increased, its definition has evolved to encompass non-covalent chemistries[2] and nanosystems.[3] One such functionality that falls into the expanded definition of bioorthogonal and has also seen clinical success is the perfluorocarbon.[4],[5]
Perfluorocarbons (PFCs) are compounds with a large percentage (often >60 wt%) of nonpolarizable sp3 C–F bonds, which phase separate from aqueous and organic solutions to form a “fluorous” phase (Figure 1A).[6],[7] PFCs are not metabolized by mammals and, due to their low boiling points, can be readily cleared by exhalation.[8] In addition, their rigid structure and lack of van der Waals interactions provides a high free volume that leads to impressive gas solubilities.[9] These properties have resulted in a variety of uses of PFCs in mammals including artificial blood,[10] liquid ventilation,[11] enhanced photodynamic therapy,[12] and anti-fouling medical device coatings.[13],[14] The unique properties of PFCs have also found use in materials,[15] cells,[16] and model organisms.[17],[18]
Figure 1.
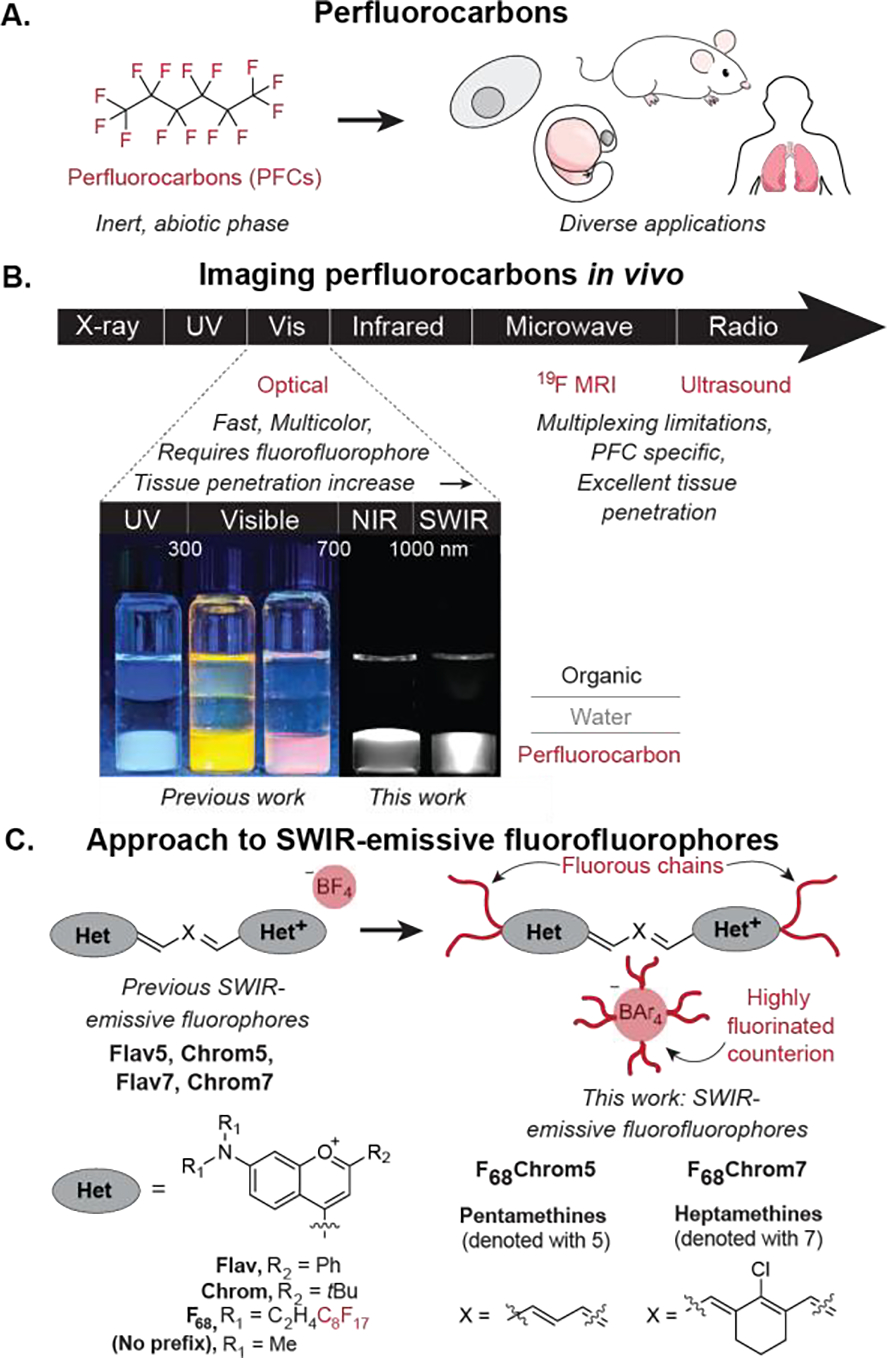
Perfluorocarbons and methods to visualize them. A) Perfluorocarbons and their broad utility. B) In vivo imaging methods for perfluorocarbons and their benefits and limitations. C) Transformation of benzopyrylium polymethine dyes into fluorofluorophores.
The tracking of PFC materials in vivo has traditionally relied on 19F magnetic resonance imaging (MRI), taking advantage of the ½ spin of fluorine,[19] or ultrasound imaging, due to the low boiling points of perfluorocarbons (Figure 1B).[20] However, these imaging modalities have limitations in sensitivity, speed, resolution and/or multiplexing.[21],[22] An alternative approach to visualizing PFCs is to label them with a “fluorofluorophore,” a fluorous-soluble fluorophore.[23] This strategy decouples the identity of the fluorous solvent from the imaging method. Previously, we reported on a palette of fluorofluorophores spanning the visible, far-red, and near infrared (NIR) regions, which have enabled the fluorescent tracking of PFCs in cells and zebrafish (Figure S1).[24] We now extend these fluorophores past 800 nm and report two fluorophores which enable real time imaging of PFCs in mice using shortwave infrared detection on an InGaAs camera. These fluorophores can be used in concert with each other, facilitating direct comparison of two PFC materials in the same animal through a paired imaging approach.[25],[26],[27] Paired imaging increases statistical significance of results while halving the overall number of animals required.[28]
The shortwave infrared region of the electromagnetic spectrum (SWIR, 1000–2000 nm) allows for high resolution, non-invasive optical imaging in animals.[29],[30] This region is superior to the visible and near-infrared regions for in vivo imaging due to decreased light scattering and minimal background autofluorescence.[31] We previously reported SWIR-emissive flavylium and chromenylium polymethine fluorophores and applied these dyes in an excitation-matched imaging configuration to enable real-time multicolor imaging.[32],[33] While displaying excellent brightness for the SWIR region, the flavylium and chromenylium fluorophores are considerably hydrophobic, which bring solubility and aggregation challenges. Here, we combine the strategies of fluorous tagging and counterion exchange (Figure 1C) to produce chromenylium pentamethine and heptamethine dyes with good solubility and brightness in PFC solvents. We use these two fluorophores to simultaneously track different populations of PFC nanoemulsions in mice in the first SWIR paired imaging experiments.
PFC nanoemulsions are droplets of fluorous solvent stabilized by a surfactant. These nanoemulsions were initially developed and FDA-approved for oxygen delivery and have since been used as a diagnostic as well as for an expanded scope of oxygen delivery applications.[34] Additional efforts harnessing the orthogonal nature of the PFCs for drug or biomolecule delivery are underway by our group and others.[35],[36],[37] Our previous work has probed the effect of the surface chemistry of PFC nanoemulsions in cell culture using visible fluorofluorophores.[38] However, we had been unable to perform analogous studies in mice due to the lack of fluorofluorophores available for imaging through tissue. With the development of SWIR fluorofluorophores, we are able to visualize multiple PFC nanoemulsions in animals non-invasively, in real-time, and allow for rapid assaying of the biodistribution of these unique soft, bioorthogonal nanomaterials. Indeed, using two-color paired imaging experiments, we demonstrate using minimal mice that varying the size of the PFC nanoemulsions leads to selective accumulation in the spleen within 1 hour, while modification of the surfactant identity results in more subtle biodistribution differences.
Results and Discussion
To generate SWIR-fluorescent fluorous soluble dyes, we combined our previous efforts towards fluorofluorophore development with SWIR flavylium and chromenylium fluorophores. We had previously prepared UV and visible fluorofluorophores (Figure S1) from fluorinated aminophenol 1 (Scheme 1). Fortunately, SWIR-emissive Flav7, Chrom7, and Chrom5 (Figure 1C) are each prepared in three steps from 3-dimethylaminophenol. Thus, we envisioned 1 to be a suitable building block for fluorous variants of flavylium and chromenylium dyes. We first applied this synthetic pathway to the synthesis of F68Flav7; however, F68Flav7 did not display adequate solubility in fluorous solvents even after exchanging the counterion[39] and we directed our attention to the more soluble chromenylium scaffold.
Scheme 1.
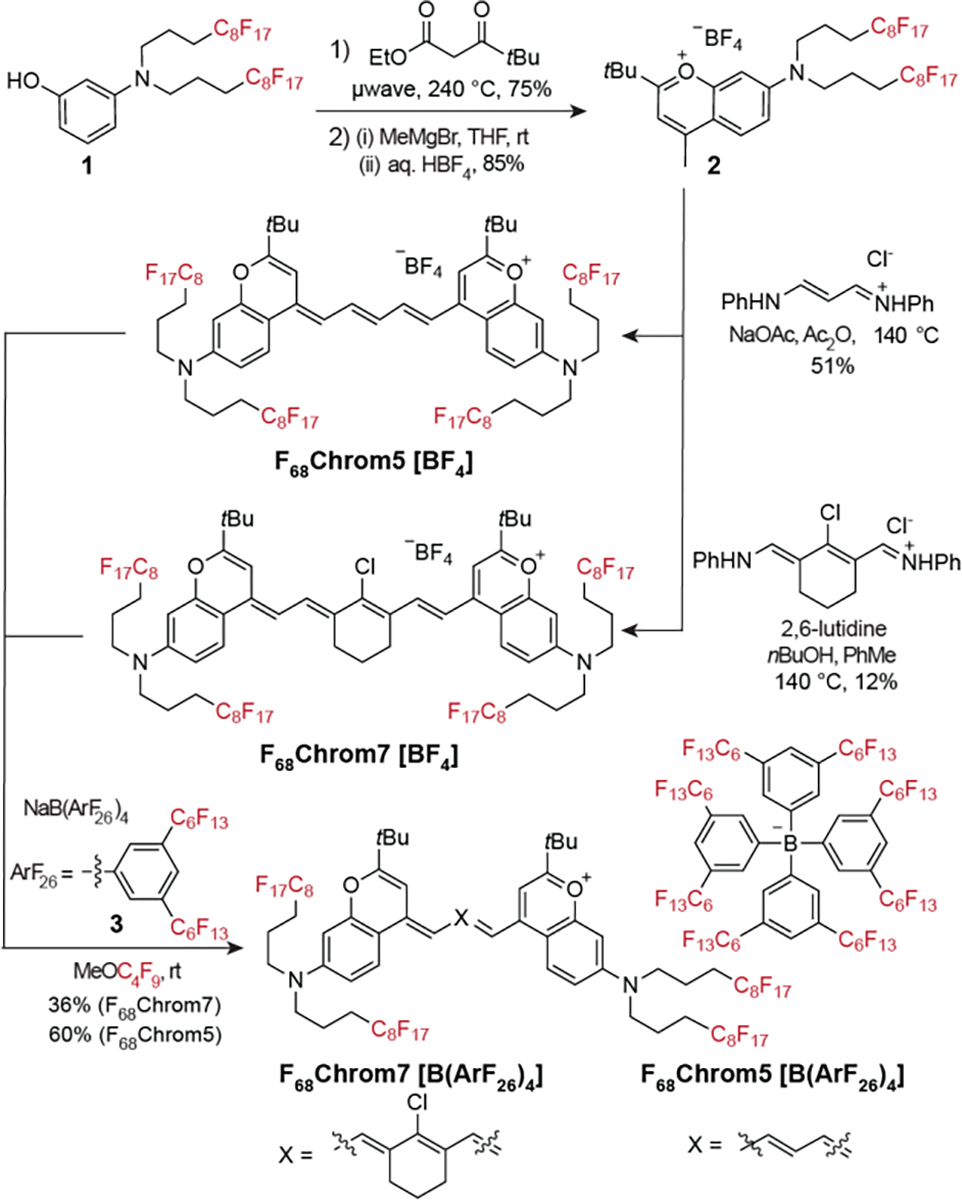
Synthetic scheme for F68Chrom5[BF4] and F68Chrom7[BF4]. Subsequent counterion exchange with NaB(ArF26)4 yields F68Chrom5[B(ArF26)4] and F68Chrom7[B(ArF26)4].
To prepare fluorous-tagged chromenylium dyes, fluorinated aminophenol 1 underwent Mentzer pyrone synthesis with ethyl pivaloylacetate followed by methyl Grignard addition to yield chromenylium 2 with a BF4 counteranion. Heterocycle 2 was converted to pentamethine and heptamethine fluorophores by condensation of 0.5 equivalent of N-(3-(phenylamino)allylidene) aniline or 1 equivalent of N-[(3-(anilinomethylene)-2-chloro-1-cyclohexen-1-yl)methylene]aniline to generate F68Chrom5[BF4] and F68Chrom7[BF4], respectively. The “F68” corresponds to the number of fluorine atoms on the chromophore scaffold, while the 5 and 7 correspond to the length of the polymethine chain. The counterion is indicated in square brackets. With F68Chrom5 and F68Chrom7 in hand, we explored the solubility and photophysical properties of these SWIR-emissive fluorofluorophores.
F68Chrom5[BF4] and F68Chrom7[BF4] proved to be relatively insoluble in both organic and fluorous solvents, only displaying adequate solubility in solvents known to solubilize semifluorinated compounds such as acetone and hexafluoroisopropanol. These results suggested that more fluorous content was necessary to achieve a true fluorofluorophore. We more than doubled the fluorine content by exchanging the BF4 counteranion with highly fluorinated aryl borate 3, denoted here as B(ArF26)4 and previously reported by the Gladysz and Buhlmann groups.[40],[41] Gratifyingly, the counterion exchanged dyes F68Chrom5[B(ArF26)4] and F68Chrom7[B(ArF26)4] were well-solubilized in PFC solvents such as perfluorooctyl bromide (PFOB) and perfluoromethyl cyclohexane (PFMC). Solubility limit studies were performed in PFOB and PFMC by preparing saturated solutions of the dyes, removing an aliquot, and determining the concentration using absorbance measurements and Beer’s law (Figure 2A, Table S2). These studies were especially enlightening for F68Chrom5, in which counterion B(ArF26)4 enables ~7.4-fold higher solubility in perfluoromethylcyclohexane (PFMC) and ~5.4-fold higher solubility in PFOB. The B(ArF26)4 counterion does not improve solubility limits for F68Chrom7 as drastically as its pentamethine counterpart.
Figure 2.
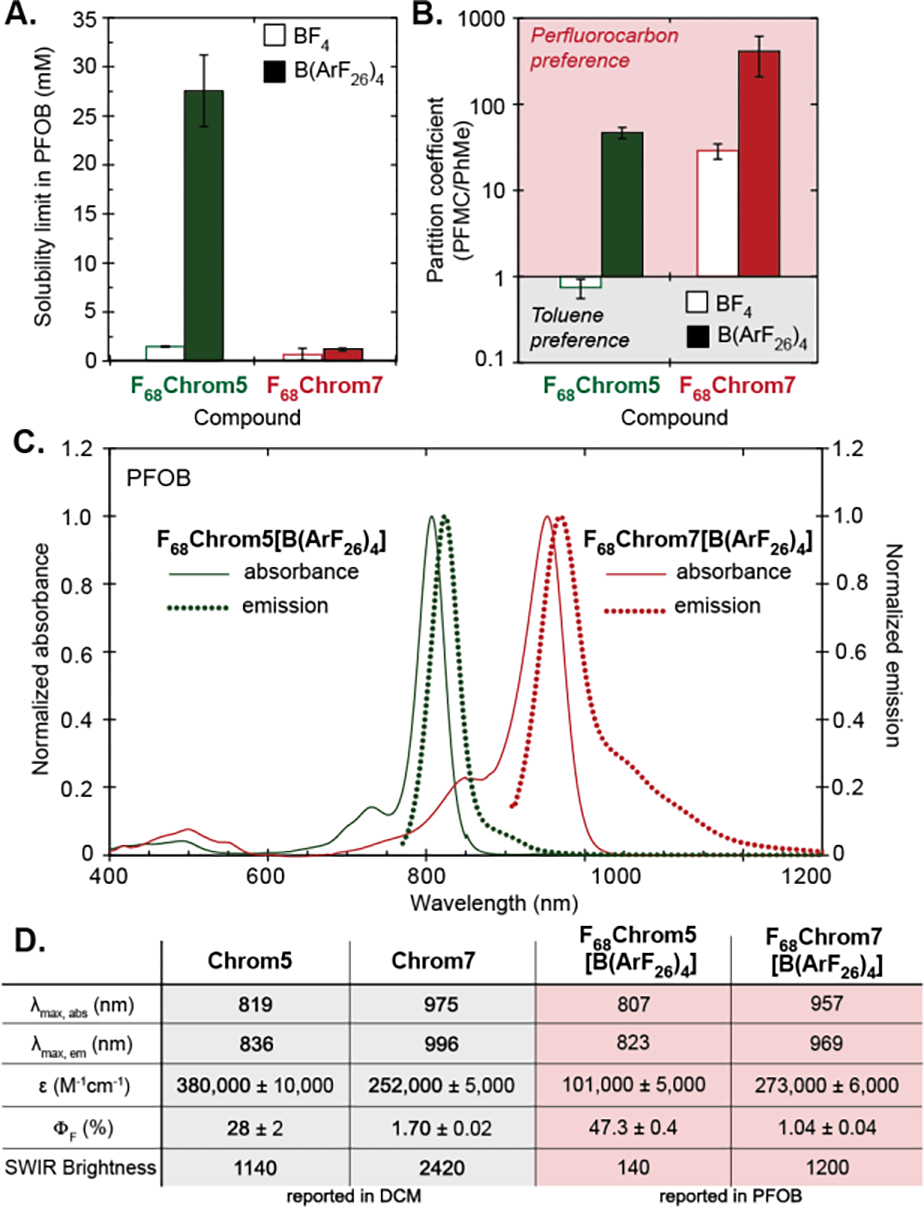
Photophysical characterizations of F68Chrom5 and F68Chrom7. A) Solubility limit (mM) measured by saturating a perfluorooctylbromide (PFOB) solution. B) Fluorous partition coefficient measured in a perfluoromethylcyclohexane:toluene system at 3 mM. C) Normalized absorption and emission spectra taken in PFOB at 2 μM. D) Table of photophysical properties comparing F68Chrom (reported in PFOB) dyes with their organic congeners (reported in dichloromethane). See Table S1 for errors.
Another valuable parameter to characterize fluorous solubility is the partition coefficient between PFMC and toluene, deemed logF.[6] Quantification of the logF for the F68Chrom dyes also shows a significant change between the BF4 and B(ArF26)4 F68Chrom5 dyes where the preference for toluene is switched to preference for the fluorous solvent in a ~65-fold increase (Figure 2B). A less drastic but still significant change was observed for F68Chrom7, displaying a ~14-fold increase in preference for fluorous solvents upon counterion exchange from BF4 to B(ArF26)4. These data suggest that F68Chrom5[B(ArF26)4] and F68Chrom7[B(ArF26)4] are well-retained in PFC even in the presence of hydrophobic species, an essential quality for using fluorofluorophores in vivo.
We evaluated the photophysical properties of F68Chrom5[B(ArF26)4] and F68Chrom7[B(ArF26)4] in PFOB, making comparisons to nonfluorinated variants Chrom7 and Chrom5 measured in dichloromethane. The absorbance spectra of both fluorofluorophores are narrow with a small vibronic shoulder indicative of a delocalized cyanine state.[42] The emission is similarly narrow with a long tail, consistent with polymethine fluorophores.[43],[44] Note that for F68Chrom5[B(ArF26)4] the tail extends above 1000 nm, facilitating imaging with a InGaAs camera. Both fluorous variants displayed a slight blue-shift in λmax from the parent fluorophores, with λmax,abs = 957 nm for F68Chrom7[B(ArF26)4] and λmax,abs = 807 nm for F68Chrom5[B(ArF26)4] (Figure 2C). The absorption coefficients (ε) for F68Chrom7[B(ArF26)4] and F68Chrom5[B(ArF26)4] were lower than observed for Chrom7 and Chrom5 (Figure 2D), consistent with the effects of PFC solvent on ε observed for other fluorofluorophores.[24] The quantum yield (ΦF) values for the fluorofluorophores did display deviation from the organic congeners; however, these values remained good to excellent for their respective regions of the electromagnetic spectrum. The ΦF = 1.04% for F68Chrom7[B(ArF26)4] was lower than that of Chrom7 (ΦF = 1.7%) while the ΦF of F68Chrom5[B(ArF26)4] at 47.3% was significantly higher than the ΦF of Chrom5 (ΦF = 28%). The most important metric for imaging experiments is the brightness where overall brightness = εmax × ΦF and, relevant to our excitation-based multiplexing experiments, SWIR brightness = εmax × αΦF, where α = the percentage of emission above 1000 nm. When considering overall brightness, the F68Chrom5[B(ArF26)4] is the superior dye; however, for SWIR imaging experiments the more red-shifted yet less emissive F68Chrom7[B(ArF26)4] displays superior brightness. Importantly, both fluorofluorophores have sufficient brightness and photostability (Figure S2) for use in real-time whole animal imaging experiments, and display monomeric spectral shapes in fluorous solvent.
With fluorofluorophores applicable for use in mice obtained, we demonstrated their in vivo utility by tracking perfluorocarbon nanoemulsions. PFC nanoemulsions have a long history of use in vivo, yet only one example images them optically in whole animals (structure of fluorophore not disclosed).[45] For our initial studies, we used Pluronic F-68 as a surfactant[46] and PFOB for the perfluorocarbon[47] as these components are both FDA-approved. We were able to prepare NIR and SWIR emissive nanoemulsions by predissolving F68Chrom5 or F68Chrom7 in PFOB and emulsifying with PBS containing 2.7 wt% Pluronic F-68 (Figure 3A, S3). This generated unimodal 200 nm-sized emulsions. Using a continuous partition between an aqueous PFC nanoemulsion solution and 1-octanol, we observed <1% loss of fluorophore to the 1-octanol for dyes F68Chrom5[B(ArF26)4] and F68Chrom7[B(ArF26)4]. Notably, for the pentamethine dye the counterion exchange proved important as 30% leaching was observed when F68Chrom5[BF4] was incorporated in the nanoemulsions (Figure S4). These data are consistent with the solubility and logF data.
Figure 3.
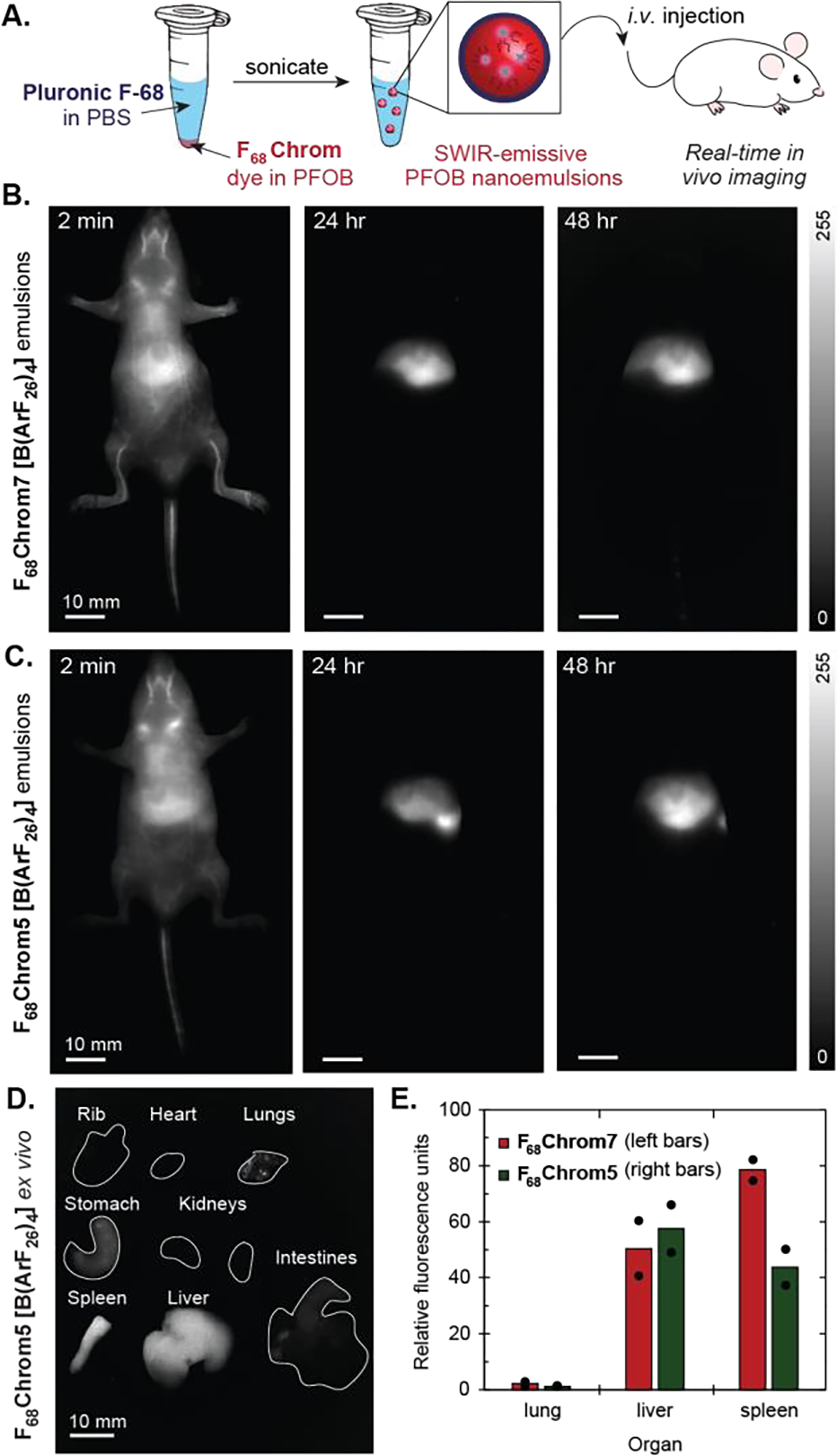
Single color imaging of perfluorocarbon nanoemulsions in nude mice. A) Schematic of PFOB nanoemulsion preparation and subsequent injection in vivo. B) Time course of F68Chrom7-labelled emulsions (200 nm in diameter) over 48 hours (ex. 974 nm, 100 mW/cm2, 1100 nm LP, 5–10 ms ET). C) Time course of F68Chrom5-labelled emulsions (200 nm in diameter) over 48 hours (ex. 785 nm, 50 mW/cm2, 1100 nm LP, 5–7 ms ET). D) Ex vivo fluorescence image of F68Chrom7. Ex. 974 nm, 100 mW/cm2, 1100 nm LP, 5 ms ET. E) Quantification of ex vivo images (see Figure S6 for bar graph with all organs). Signal was normalized to the lowest intensity organ. Dots overlaid on bar graph are individual mice. Red (left bars) indicate PFC nanoemulsions containing F68Chrom7, green bars (right bars) indicate PFC nanoemulsions containing F68Chrom5 B-D) Scale bar = 10 mm.
We first evaluated how emulsions containing F68Chrom5[B(ArF26)4] or F68Chrom7[B(ArF26)4] behaved in mice by performing single color imaging experiments (Figure 3B,C). All in vivo experiments were performed with the B(ArF26)4 counterion and for simplicity the counterion will not be defined during the imaging discussions. We injected 100 μL of emulsions labeled with either F68Chrom5 or F68Chrom7 and tracked the movement of the emulsions through the vasculature, heart, and liver almost immediately. Both fluorophores enabled video rate imaging at 100 frames per second (fps). There were no significant changes in biodistribution between 1 and 2 days (Figure 3B,C). After 48 hours, an ex vivo analysis was performed in which the heart, lungs, sternum, stomach, kidneys, intestines, liver, and spleen were excised and their fluorescence intensity quantified (Figure 3D,E, S5). As is typical for nanomaterials, we found significant accumulation in the liver and spleen. Importantly, we confirmed that the identity of the dye does not impact the overall trends in biodistribution, which provided support that the dye was not leaching from the PFC and labelling biomolecules outside of the emulsions.
Next, we confirmed that the PFC nanoemulsions containing F68Chrom5 and F68Chrom7 could be orthogonally excited and employed in an excitation multiplexed imaging experiment. Excitation at 785 nm (flux set to 50 mW/cm2) and 974 nm (flux set to 100 mW/cm2) were chosen for F68Chrom5 and F68Chrom7 nanoemulsions, respectively. Power densities of irradiation were guided by the International Commission on Non-Ionizing Radiation Protection. Experiments in capillaries to decrease crosstalk between channels yielded concentrations of 0.012 mM F68Chrom7 and 0.048 mM F68Chrom5 as optimal (~1% crosstalk, Figure S5).
Spurred by these findings, we looked to vary parameters of the perfluorocarbon emulsions and compare two different emulsions in the same mouse. These direct two-color comparison experiments are referred to as paired imaging. The first variable we tested was the size of the nanoemulsions. It is well-established from the use of PFC nanoemulsions for oxygen delivery that the size of the nanoemulsions affects their serum half-lives, with smaller nanoemulsions displaying longer lifetimes in the bloodstream.[48] By varying the amount of Pluronic F-68 surfactant from 0.6–16 wt%, we were able to prepare nanoemulsions that ranged from 100–300 nm in size (Figure 4A). We loaded the smaller nanoemulsions (100, 150 nm) with F68Chrom5 and the larger nanoemulsions (200, 300 nm) with F68Chrom7. Two paired imaging experiments were performed comparing the 100 and 200 nm emulsions as well as the 150 and 300 nm emulsions. These experiments involved injecting a mix of the PFC nanoemulsions intravenously and immediately imaging with an excitation multiplexed in vivo imaging setup (Figure 4B). We observed a striking size-dependent localization with 100 nm emulsions displaying diffuse labeling throughout the animal and the larger nanoemulsions showing liver and spleen localization (Figure 4C,D, S6, S7). Indeed, the early literature on PFC emulsions corroborates this result, as it has been proposed that the reticuloendothelial macrophages are responsible for depositing perfluorocarbons into the liver and spleen.[49],[50] For the case of the 300 nm emulsions, these nanomaterials displayed 5-fold preferential labeling for the spleen vs. the liver, providing a potential delivery vehicle for spleen-targeted immunotherapies.[51] A similar size dependence for spleen localization has been observed for PEG-coated polystyrene (240 nm)[52] and cyanoacrylate nanoparticles (220 nm).[53],[54]
Figure 4.
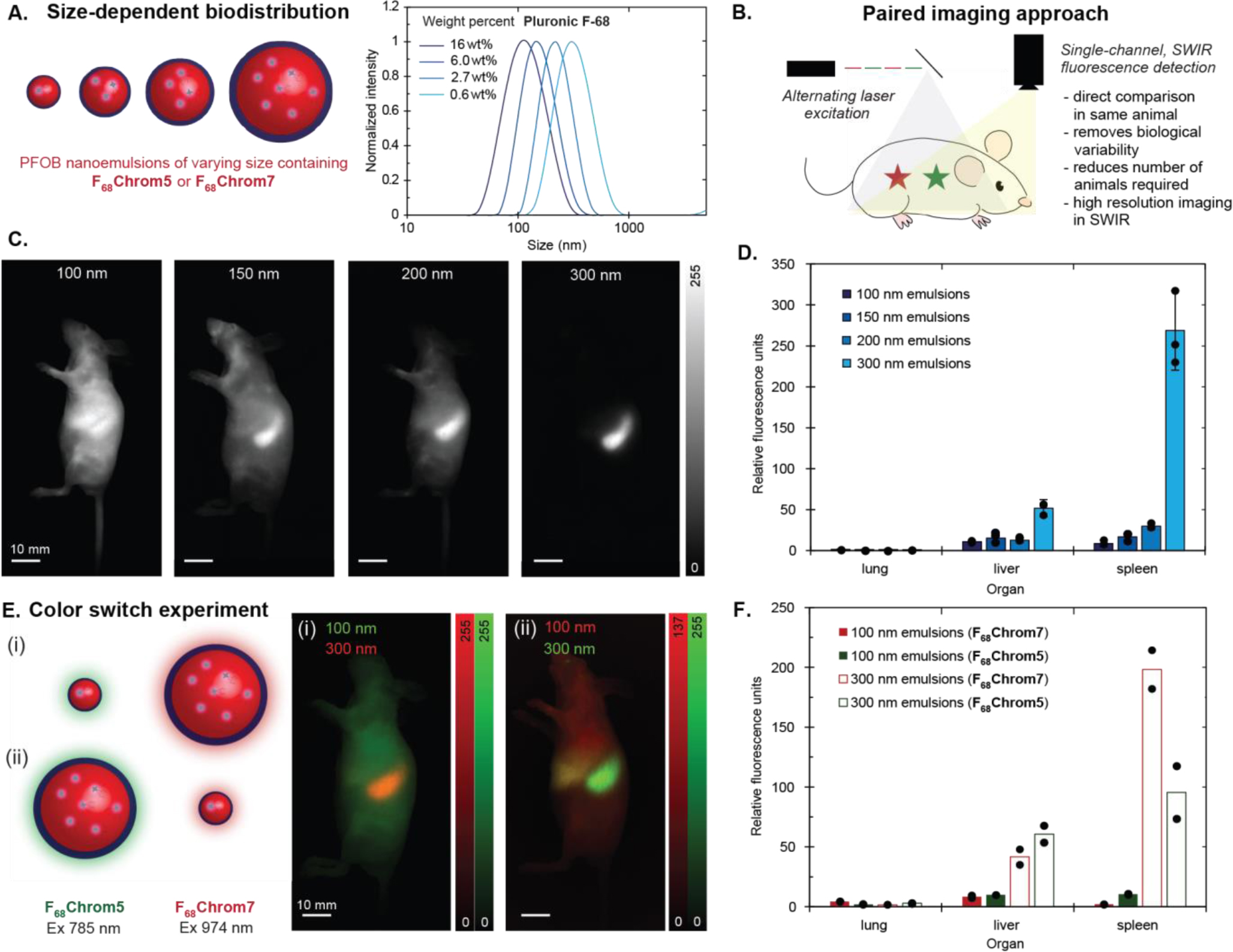
Paired in vivo experiments with Pluronic F-68–stabilized emulsions. A) Schematic and dynamic light scattering analysis of emulsions. Sizes ranging from 100 to 300 nm were achieved by varying the weight percent of surfactant from 0.6 to 16 wt%. B) Schematic illustrating excitation-multiplexed imaging of an animal labelled with two fluorophores with different absorbance. Triggered orthogonal excitation and subsequent deconvolution allows for color assignment. C) Single color sagittal images of nude mice with varying emulsion sizes, taken at 48 hours post-injection (F68Chrom7 ex. 974 nm, 100 mW/cm2; F68Chrom5 ex. 785 nm, 50 mW/cm2; 1100 nm LP, 4–6 ms ET). 100 μL of F68Chrom5-labelled 100 nm emulsions and 100 μL of F68Chrom7-labelled 200 nm emulsions were i.v. injected into one animal. 100 μL of F68Chrom5-labelled 150 nm emulsions and F68Chrom7-labelled 300 nm emulsions were i.v. injected into another animal. See Figure S7 and S8 for replicates. Scale bars = 10 mm. D) Ex vivo images were captured after the 48 hour post-injection. The mean fluorescence intensity from each organ was quantified in ImageJ and normalized to the lowest intensity organ value. The quantification of ex vivo images is displayed in the bar graph. See Figure S7 and S8 for bar graph with all organs. Dots overlaid on bar graph are replicates. Error bars are the standard deviation of the mean, where n = 3. E) Color switch experiment in which 100 or 300 nm emulsions were labelled with either F68Chrom5 or F68Chrom7. (i) Experimental schematic and two color sagittal image of F68Chrom5-labelled 100 nm emulsions + F68Chrom7-labelled 300 nm emulsions, taken at 48 hours post-injection. 100 μL of each emulsion suspension was injected and imaged with the same acquisition settings as in 4C. (ii) Experimental schematic and two color sagittal image of F68Chrom5-labelled 300 nm emulsions + F68Chrom7-labelled 100 nm emulsions, taken at 48 hours post-injection. 100 μL of each emulsion suspension was injected and imaged with the same acquisition settings as in 4C. Scale bars = 10 mm. F) Ex vivo images were captured after the 48 hour post-injection images. The mean fluorescence intensity from each organ was quantified in ImageJ, normalized to the lowest intensity organ value, and plotted. See Figure S9 for bar graph with all organs. Dots overlaid on bar graph are replicates. 100 nm emulsions in solid bars; 300 nm emulsion in unfilled bars. Red bars represent F68Chrom7. Green bars represent F68Chrom5. Red bars are plotted to the right of the green bars.
To validate the results from the paired imaging studies, we performed a color switch experiment (Figure 4E). 100 nm emulsions were prepared containing F68Chrom5 and 300 nm emulsions were prepared with F68Chrom7. These emulsions were mixed, i.v. injected, and the animals were immediately imaged. After 48 hours, the mice were sacrificed and their organs were analyzed ex vivo. Simultaneously, we performed an analogous experiment with 100 nm emulsions labeled with F68Chrom7 and 300 nm emulsions labeled with F68Chrom5. We compared the non-invasive imaging results (Figure 4E) as well as ex vivo fluorescence quantification (Figure 4F) between the two experiments. We found that overall trends in biodistribution were not impacted by the identity of the dye sequestered in the emulsions (Figure S9, S10).
Finally, we were interested in the effect of the surfactant on the localization of the PFC nanoemulsions. The Pluronic F-68 surfactant was the original surfactant used for PFC nanoemulsions in humans;[55] yet, it is not readily customized to allow for targeted or smart nanocarriers. We have explored poly(2-oxazoline) (POx)-based amphiphiles as an alternative to Pluronic F-68 due to its synthetic modularity and control (Figure 5A).[56],[57] We have developed amphiphile 4 as a suitable replacement for Pluronic F-68 and evaluated 4 and its derivatives extensively in vitro and in cellulo. However, until now we lacked the fluorofluorophores to properly evaluate POx-stabilized nanoemulsions in mice. With F68Chrom5 and F68Chrom7, we were now able to perform direct comparisons of these custom nanoemulsions in vivo, focusing on formulations which showed differences in cellulo (Figure 5B).
Figure 5.
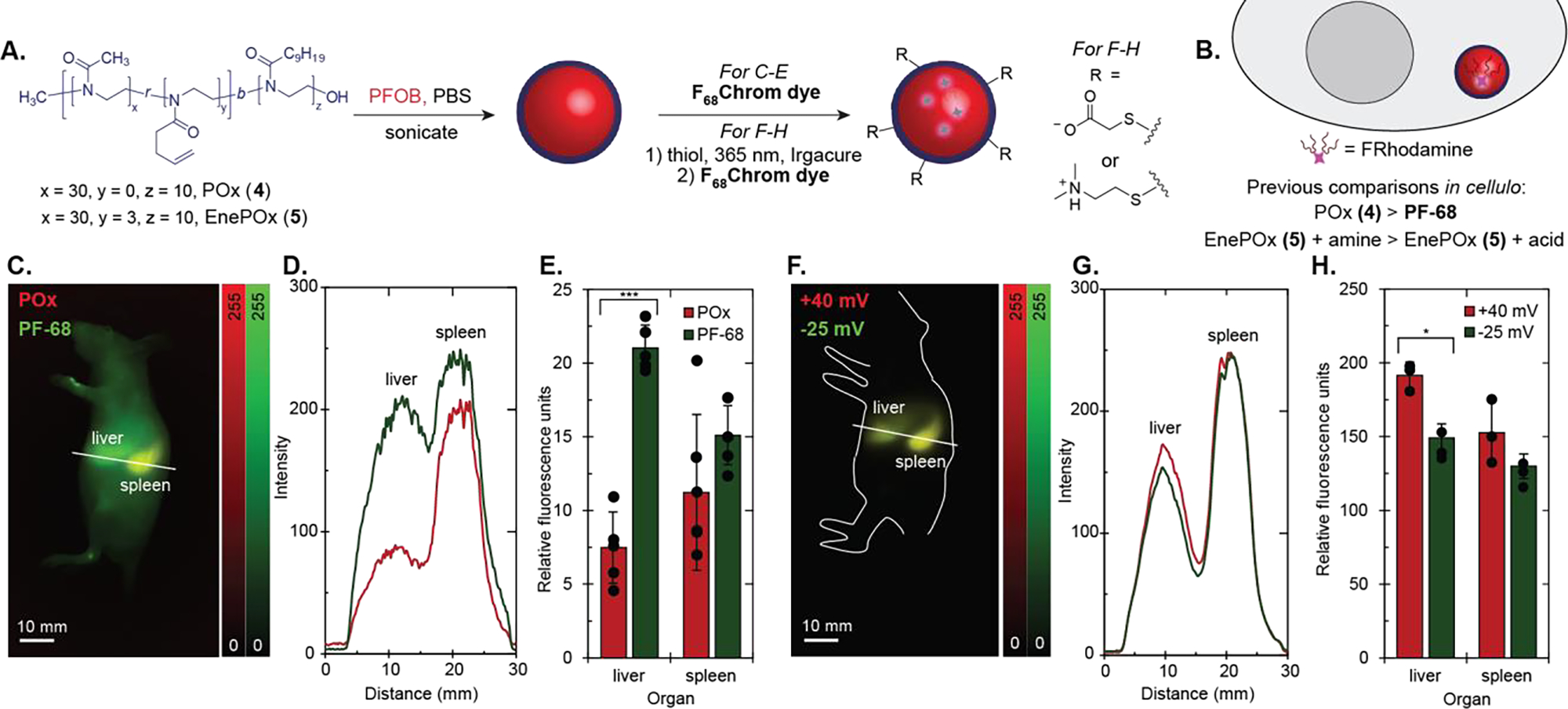
Imaging PFC nanoemulsions stabilized by poly(oxazoline) amphiphiles in vivo. A) Schematic of PFOB emulsions stabilized by POx (4) or EnePOx (5) at 2.7 wt% and subsequent functionalization of emulsions by thiol-ene chemistry. B) Summary of results from previous work in cellulo using this methodology. C) Two color sagittal image of an animal injected with Pluronic F-68 (PF-68)-stabilized emulsions and POx-stabilized emulsions, taken at 48 hours post-injection. F68Chrom7 ex. 974 nm, 100 mW/cm2; F68Chrom5 ex. 785 nm, 50 mW/cm2; 1100 nm LP, 5–7 ms ET). 100 μL of F68Chrom5-labelled PF-68 emulsions and 100 μL of F68Chrom7-labelled POx emulsions were i.v. injected into one animal. Scale bars = 10 mm. D) Cross-section intensity profile of the line drawn in 5C, where the green line represents the profile for PF-68 emulsions and the red line represents the profile for POx emulsions. E) Ex vivo images were captured after the 48 hour post-injection images. The mean fluorescence intensity from each organ was quantified using ImageJ, normalized to the lowest intensity organ value, and plotted. See Figure S11 for bar graph with all organs. Dots overlaid on bar graph are replicates. Error bars are the standard deviation of the mean, where n = 5. Red bars on the left indicate POx emulsions and green bars on the right indicate Pluronic F-68 emulsions. F) Two color sagittal image of an animal injected with EnePOx-stabilized emulsions, taken at 48 hours post-injection. 100 μL of F68Chrom5-labelled negatively charged (−25 mV) emulsions and 100 μL of F68Chrom7-labelled positively charged (+40 mV) emulsions were i.v. injected into one animal. Images were taken with the same settings as in 5C. Scale bars = 10 mm. G) Cross-section intensity profile of the line drawn in 5F, where the green line represents the profile for negatively charged emulsions and the red line represents the profile for positively charged emulsions. H) Ex vivo images were captured after the 48 hour post-injection images. The mean fluorescence intensity from each organ was quantified using the ImageJ measurement function, normalized to the lowest intensity organ value, and plotted. See Figure S13 for bar graph with all organs. Signal was normalized to the lowest intensity organ. Dots overlaid on bar graph are replicates. Red bars on the left indicate positively charged emulsions and green bars on the right indicate negatively charged emulsions. Error bars are the standard deviation of the mean, where n = 3. * = p < 0.05, *** = p < 0.001
First, we validated that POx-stabilized nanoemulsions were stable in vivo and behaved similarly to the previously FDA-approved Pluronic F-68 nanoemulsions. We compared PFOB-nanoemulsions stabilized by 4 or Pluronic F-68 containing F68Chrom5 or F68Chrom7 in five replicates of a paired imaging experiment (Figure 5C–D, S10). We imaged the mice non-invasively over 48 hours and then performed an ex vivo organ analysis. The only significant difference observed was that 4-stabilized nanoemulsions displayed lower liver uptake than Pluronic F-68-stabilized nanoemulsions. Decreased liver uptake is an encouraging result for implementing POx-stabilized nanoemulsions for targeted delivery applications.
The success of the POx-stabilized perfluorocarbon nanoemulsions in vivo sets the stage for advancing these delivery vehicles through defined surface modification. Previously, we had developed a method to decouple the effects of nanoemulsion size and charge in cellulo by implementing a post-emulsification modification procedure using thiol-ene chemistry (Figure 5A). This method required alkene-containing amphiphile 5 in place of 4. Our in cellulo work using a rhodamine fluorofluorophore suggested that the surface charge of the nanoemulsions was a significant factor in cellular uptake, particularly for non-macrophage cells. [38]
By using the SWIR-emissive fluorofluorophores, we were able to apply the same methodology used for the cellular microscopy experiments to in vivo fluorescence imaging. Briefly, we prepared 250 nm emulsions from 5 and treated half with mercaptoacetic acid and the other half with 2-dimethylaminoethanethiol. The emulsions were irradiated with 365 nm light for 1 hour at 4 °C and purified via centrifugation and resuspension. The acid-functionalized emulsions displayed a zeta potential of −25 mV, and the tertiary amine-functionalized emulsions displayed a zeta potential of +40 mV (Figure S12). These emulsions were then loaded with F68Chrom5 or F68Chrom7 and injected into animals. The emulsions labelled the liver and spleen predominantly, with an unchanging biodistribution profile from 1 hour after injection to 48 hours (Figure 5F–H). Cross-sections from the paired imaging experiment indicate complete colocalization (Figure 5G, S12). Upon ex vivo analysis, we did observe a significant difference (p = 0.03) in preference for liver but the overall change was modest.
Taken together, the new fluorofluorophores F68Chrom5 and F68Chrom7 allowed for direct comparisons of PFC nanoemulsions in vivo. These experiments demonstrated that POx-stabilized nanoemulsions are promising alternatives for Pluronic F-68, displaying lower liver uptake but otherwise similar biodistribution. It appears that the surface charge of the nanoemulsions does not play a large role in mice. This could be due to 1) protein corona effects, which are well-precedented to have a large impact on biodistribution of foreign particles in animals,[58] or 2) the majority of cellular uptake in vivo is from macrophages,[59],[60],[61],[62] which displayed less charge-dependent uptake in our in cellulo studies.[38] Future studies evaluating these hypotheses are underway.
Conclusion
We have prepared two chromenylium fluorofluorophores, F68Chrom5 and F68Chrom7, for imaging in the shortwave infrared region of the electromagnetic spectrum. F68Chrom5 and F68Chrom7 were prepared by combining the building block previously employed for the synthesis of visible fluorofluorophores with chromenylium polymethine dye synthesis. Counterion exchange from tetrafluoroborate to the highly fluorinated B(ArF26)4 anion enhanced the fluorous solubility, retention, and photophysical properties. These low energy fluorofluorophores are ideal for imaging through tissue as demonstrated by two-color shortwave infrared imaging in mice with PFC nanoemulsions containing F68Chrom5 or F68Chrom7. Paired experiments allowed for two nanoemulsions to be compared in the same animal, reducing biological variability and animal count. Notable results from the paired PFC nanoemulsion experiments include 1) 300 nm emulsions display significant localization in the spleen over the liver and 2) altering the surfactant from the traditional poly(ethylene glycol) to poly(methyl-2-oxazoline) results in decreased liver accumulation. We expect the F68Chrom5 and F68Chrom7 fluorofluorophores will continue to advance our knowledge of PFC nanomaterials in vivo as well as be applied to emerging applications of PFCs such as in vivo mechanical force measurements[18] and droplet-based sensors.[63]
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health (1R01EB027172 to E.M.S., 5R01GM135380 to E.M.S); Chan Zuckerberg Initiative (2020-225707 to E.M.S.), Tobacco Related Disease Research Program (T32DT4847 to E.Y.L), UCLA (Dissertation Year Fellowship to I.L.; Cota Robles Fellowship to J.G.), and Welch Foundation (A-1656 to J.A.G.) NMR spectrometers are supported by the National Science Foundation under equipment grant no. CHE-1048804. We would also like to thank the Radu group at UCLA Department of Radiology for helpful discussion and members of the Sletten Group for critical reading of the manuscript.
Footnotes
Supporting information for this article is given via a link at the end of the document
References
- [1].Prescher JA, Bertozzi CR, Nat. Chem. Biol. 2005, 1, 13–21. [DOI] [PubMed] [Google Scholar]
- [2].Yoder N, Yuksel D, Dafik L, Kumar K, Curr. Opin. Chem. Biol. 2006, 10, 576–583. [DOI] [PubMed] [Google Scholar]
- [3].Zhang X, Liu Y, Gopalakrishnan S, Castellanos-Garcia L, Li G, Malassiné M, Uddin I, Huang R, Luther DC, Vachet RW, Rotello VM, ACS Nano 2020, 14, 4767–4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Miller MA, Sletten EM, ChemBioChem 2020, 21, 3451–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chirizzi C, Morasso C, Caldarone AA, Tommasini M, Corsi F, Chaabane L, Vanna R, Bombelli FB, Metrangolo P, J. Am. Chem. Soc. 2021, 143, 12253–12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gladysz John. A., Curran DP, Horváth István T., Eds., Handbook of Fluorous Chemistry, John Wiley & Sons, 2004. [Google Scholar]
- [7].Horváth IT, Rábai J, Science 1994, 266, 72–75. [DOI] [PubMed] [Google Scholar]
- [8].Lowe KC, Comp. Biochem. Physiol. Part A Physiol. 1987, 87, 825–838. [DOI] [PubMed] [Google Scholar]
- [9].Pierre M, Riess JG, Krafft MP, Riess JG, Adv. Colloid Interface Sci. 2021, 184, 102407. [DOI] [PubMed] [Google Scholar]
- [10].Riess JG, Artif. Cells, Blood Substitutes, Biotechnol. 2005, 33, 47–63. [DOI] [PubMed] [Google Scholar]
- [11].Sarkar S, Paswan A, Prakas S, Anesth Essays Res 2014, 8, 277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cheng Y, Cheng H, Jiang C, Qiu X, Wang K, Huan W, Yuan A, Wu J, Hu Y, Nat. Commun. 2015, 6, 8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Krishnan S, Weinman CJ, Ober CK, J. Mater. Chem. 2008, 18, 3405–3413. [Google Scholar]
- [14].Lv J, Cheng Y, Chem. Soc. Rev. 2021, 50, 5435–5467. [DOI] [PubMed] [Google Scholar]
- [15].Zarzar LD, Sresht V, Sletten EM, Kalow JA, Blankschtein D, Swager TM, Nature 2015, 518, 520–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fabiilli ML, Lee JA, Kripfgans OD, Carson PL, Fowlkes JB, Pharm. Res. 2010, 27, 2753–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Campàs O, Mammoto T, Hasso S, Sperling RA, O’Connell D, Bischof AG, Maas R, Weitz DA, Mahadevan L, Ingber DE, Nat. Methods 2014, 11, 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mongera A, Rowghanian P, Gustafson HJ, Shelton E, Kealhofer DA, Carn EK, Serwane F, Lucio AA, Giammona J, Campàs O, Nature 2018, 561, 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tirotta I, Dichiarante V, Pigliacelli C, Cavallo G, Terraneo G, Bombelli FB, Metrangolo P, Resnati G, Chem. Rev. 2015, 115, 1106–1129. [DOI] [PubMed] [Google Scholar]
- [20].Cosco D, Fattal E, Fresta M, Tsapis N, J. Fluor. Chem. 2015, 171, 18–26. [Google Scholar]
- [21].Janasik D, Krawczyk T, Chem. – A Eur. J. 2022, 28, e202102556. [DOI] [PubMed] [Google Scholar]
- [22].Pirovano G, Roberts S, Kossatz S, Reiner T, J. Nucl. Med. 2020, 61, 1419–1427. [DOI] [PubMed] [Google Scholar]
- [23].Sletten EM, Swager TM, J. Am. Chem. Soc. 2014, 136, 13574–13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lim I, Vian A, van de Wouw HL, Day RA, Gomez C, Liu Y, Rheingold AL, Campàs O, Sletten EM, J. Am. Chem. Soc. 2020, 142, 16072–16081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pressman D, Day ED, Blau M, Cancer Res. 1957, 17, 845–50. [PubMed] [Google Scholar]
- [26].Tichauer KM, Samkoe KS, Sexton KJ, Hextrum SK, Yang HH, Klubben WS, Gunn JR, Hasan T, Pogue BW, Mol. Imaging Biol. 2012, 14, 584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li C, Xu X, McMahon N, Alhaj Ibrahim O, Sattar HA, Tichauer KM, Contrast Media Mol. Imaging 2019, 2019, 7561862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schreiber CL, Zhai C, Dempsey JM, McGarraugh HH, Matthews BP, Christmann CR, Smith BD, Bioconjug. Chem. 2020, 31, 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hong G, Antaris AL, Dai H, Nat. Biomed. Eng. 2017, 1, 10. [Google Scholar]
- [30].Kenry, Duan Y, Liu B, Adv. Mater. 2018, 30, 1802394. [DOI] [PubMed] [Google Scholar]
- [31].Ding F, Zhan Y, Lu X, Sun Y, Chem. Sci. 2018, 9, 4370–4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cosco ED, Spearman AL, Ramakrishnan S, Lingg JGP, Saccomano M, Pengshung M, Arús BA, Wong KCY, Glasl S, Ntziachristos V, Warmer M, McLaughlin RR, Bruns OT, Sletten EM, Nat. Chem. 2020, 12, 1123–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cosco ED, Arús BA, Spearman AL, Atallah TL, Lim I, Leland OS, Caram JR, Bischof TS, Bruns OT, Sletten EM, J. Am. Chem. Soc. 2021, 143, 6836–6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhang C, Yan K, Fu C, Peng H, Hawker CJ, Whittaker AK, Chem. Rev. 2022, 122, 167–208. [DOI] [PubMed] [Google Scholar]
- [35].Sloand JN, Nguyen TT, Zinck SA, Cook EC, Zimudzi TJ, Showalter SA, Glick AB, Simon JC, Medina SH, ACS Nano 2020, 14, 4061–4073. [DOI] [PubMed] [Google Scholar]
- [36].Herneisey M, Salcedo PF, Domenech T, Bagia C, George SS, Tunney R, Velankar S, Hitchens TK, Janjic JM, ACS Med. Chem. Lett. 2020, 11, 2032–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Nichols JM, Crelli CV, Liu L, Pham HV, Janjic JM, Shepherd AJ, J. Neuroinflammation 2021, 18, 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Estabrook DA, Ennis AF, Day RA, Sletten EM, Chem. Sci. 2019, 10, 3994–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cosco ED, Polymethine Fluorophores for in Vivo Shortwave Infrared Imaging, University of California, Los Angeles, 2020. [Google Scholar]
- [40].Ghosh SK, Ojeda AS, Guerrero-Leal J, Bhuvanesh N, Gladysz JA, Inorg. Chem. 2013, 52, 9369–9378. [DOI] [PubMed] [Google Scholar]
- [41].Boswell PG, Bühlmann P, J. Am. Chem. Soc. 2005, 127, 8958–8959. [DOI] [PubMed] [Google Scholar]
- [42].Pascal S, Haefele A, Monnereau C, Charaf-Eddin A, Jacquemin D, Le Guennic B, Andraud C, Maury O, J. Phys. Chem. A 2014, 118, 4038–4047. [DOI] [PubMed] [Google Scholar]
- [43].Zhu S, Tian R, Antaris AL, Chen X, Dai H, Adv. Mater. 2019, 31, 1900321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhu S, Yung BC, Chandra S, Niu G, Antaris AL, Chen X, Theranostics 2018, 8, 4141–4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Balducci A, Wen Y, Zhang Y, Helfer BM, Hitchens TK, Meng WS, Wesa AK, Janjic JM, Oncoimmunology 2013, 2, e23034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pitto-Barry A, Barry NPE, Polym. Chem. 2014, 5, 3291–3297. [Google Scholar]
- [47].Keipert PE, Artif. Cells, Blood Substitutes, Biotechnol. 1995, 23, 381–394. [DOI] [PubMed] [Google Scholar]
- [48].Riess JG, Le Blanc M, Pure Appl. Chem. 2013, 54, 2383–2406. [Google Scholar]
- [49].Tsuda Y, Yamanouchi K, Yokoyama K, Suyama T, Watanabe M, Ohyanagi H, Saitoh Y, Biomater. Artif. Cells Artif. Organs 1988, 16, 473–483. [DOI] [PubMed] [Google Scholar]
- [50].Flaim SF, Artif. Cells, Blood Substitutes, Biotechnol. 1994, 22, 1043–1054. [DOI] [PubMed] [Google Scholar]
- [51].Zhai Y, He X, Li Y, Han R, Ma Y, Gao P, Qian Z, Gu Y, Li S, Sci. Adv. 2021, 7, eabi6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Moghimi SM, Hedeman H, Muir IS, Illum L, Davis SS, Biochim. Biophys. Acta - Gen. Subj. 1993, 1157, 233–240. [DOI] [PubMed] [Google Scholar]
- [53].Fang C, Shi B, Pei Y-Y, Hong M-H, Wu J, Chen H-Z, Eur. J. Pharm. Sci. 2006, 27, 27–36. [DOI] [PubMed] [Google Scholar]
- [54].Yoo J-W, Chambers E, Mitragotri S, Curr. Pharm. Des. 2010, 16, 2298–2307. [DOI] [PubMed] [Google Scholar]
- [55].Mitsuno T, Ohyanagi H, Naito R, Ann. Surg. 1982, 195, 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hoogenboom R, Angew. Chemie - Int. Ed. 2009, 48, 7978–7994. [DOI] [PubMed] [Google Scholar]
- [57].Day RA, Estabrook DA, Wu C, Chapman JO, Togle AJ, Sletten EM, ACS Appl. Mater. Interfaces 2020, 12, 38887–38898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Cai R, Chen C, Adv. Mater. 2019, 31, 1805740. [DOI] [PubMed] [Google Scholar]
- [59].Wu L, Liu F, Liu S, Xu X, Liu Z, Sun X, Int. J. Nanomedicine 2020, 15, 7377–7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Jägers J, Wrobeln A, Ferenz KB, Pflugers Arch. Eur. J. Physiol. 2021, 473, 139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Holman R, Lorton O, Guillemin PC, Desgranges S, Contino-Pépin C, Salomir R, Front. Chem. 2022, 9, 810029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Schmieder AH, Caruthers SD, Keupp J, Wickline SA, Lanza GM, Engineering 2015, 1, 475–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Joseph JM, Gigliobianco MR, Firouzabadi BM, Censi R, Di Martino P, Pharmaceutics 2022, 14, 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


