Abstract
The ability of animal cells to sense, adhere to and remodel their local extracellular matrix (ECM) is central to control of cell shape, mechanical responsiveness, motility and signalling and hence to development, tissue formation, wound healing and the immune response. Cell-ECM interactions occur at a variety of specialised, multi-protein adhesion complexes that serve to physically link the ECM, predominantly via clustered transmembrane receptors of the integrin family, to the cytoskeleton and the intracellular signalling apparatus. Here we review how the interplay of mechanical forces, biochemical signalling, and molecular self-organisation determines the composition, organisation, mechanosensitivity and dynamics of adhesions. Progress in the identification of core common interactome modules and characterisation of rearrangements in response to force, together with advanced imaging approaches, has improved understanding of adhesion maturation and turnover and the relationships between adhesion structures and functions. Perturbations of adhesion contribute to a broad range of disease and to age-related dysfunction, thus an improved understanding may facilitate therapeutic intervention in these conditions.
Introduction
Cell-extracellular matrix (ECM) interactions play integral roles in developmental morphogenesis and physiological functions. In metazoans, the ECM serves as an active biomaterial scaffold, spanning multiple orders of magnitude of length scale1. Cells in vivo interact extensively and dynamically with the ECM, actively contributing to both ECM biogenesis and remodelling, while themselves being profoundly influenced by the ECM physicochemical properties. The sensitivity of cells to cues presented by diverse ECM scaffolds underlies a broad array of cell-type specific responses ranging from motility to metabolism and survival2–5. The cell-ECM interaction is intrinsically mechanical; cells generate forces which drive coordinated shape changes during cell locomotion and which remodel and mechanically alter the ECM. Cells also mechanochemically interrogate their ECM microenvironment, with specific ECM adhesions and local ECM deformations registered and transduced into biochemical signals that propagate downstream along various complex pathways. The multifaceted involvement of cell-ECM adhesion-dependent mechanobiology in many aspects of cellular physiology is becoming well recognised4,6,7. Proper mechanosensitivity and mechanoresponsiveness of cell-ECM interactions are essential for normal physiology and their dysfunctions can be pivotal in a range of pathological conditions, including aging, inflammation, and cancer4,6–8.
Since its identification in the 1960–70s, a combination of biochemical, biophysical and cell culture models have provided extensive information on the structures and interactions of the molecules involved in cell-ECM adhesion, their organisation into ordered multi-protein complexes and their interface with signalling and cytoskeletal networks9,10. This, together with genetic analyses in model organisms, has helped build a detailed picture of the roles and mechanisms of action of cell adhesions and the central roles that the integrin family of transmembrane heterodimeric adhesion receptors play11. Integrins directly engage ECM proteins and connect to the cytoskeletal scaffold via various intermediary adaptor proteins, providing access points for combinatorial regulation and control. Several types of micron-scale multi-protein complexes that mediate cell-ECM adhesion are recognised [Box 1], typically linking ECM-bound integrins to the actin cytoskeleton or intermediate filaments via distinct configurations of myriad adaptor and signalling proteins. Importantly, these connections between adhesion complexes and the actomyosin contractility apparatus are dynamic and mechanosensitive, actively controlling cell shape and the force generation that propels adherent cell migration. Advanced biochemical and biophysical tools [Box 2] are revealing the structural and mechanical organisation of core cell-ECM adhesion complexes and how dynamic association of regulatory and signalling components connect cell-ECM adhesion to virtually all other cellular signalling circuits12. The vast literature precludes a comprehensive review of all aspects of cell-ECM adhesion and we refer readers to recent detailed reviews on integrin mechanosensing, ECM, cell migration and integrins in disease2,3,6–9,11,13. Here we focus on the dynamic multi-protein assemblies through which integrins mediate cell-ECM interactions and interface with the cytoskeleton. We seek to provide a current view of the molecular composition and organisation of integrin-mediated adhesions and how their dynamics is influenced by mechanical and signalling activities. We do not review adhesions in 3D matrix, as these have recently been reviewed elsewhere14, instead we largely limit our discussion to the abundant data from high-resolution experimental systems where cells adhere to defined planar artificial substrates, as this facilitates detailed reductionist biochemical, biophysical and imaging approaches.
Box 1 |. Integrin-based adhesions.
There is considerable heterogeneity in IACs and, while strict definitions have not been uniformly established, IACs have been classified based on size, composition, lifetime, cellular distribution and function9,189,206,207. In some cases, differences represent steps along a maturation process (depicted in the cartoon of a top view of a polarised migrating fibroblast) while others are more specialised, or cell-type specific structures.
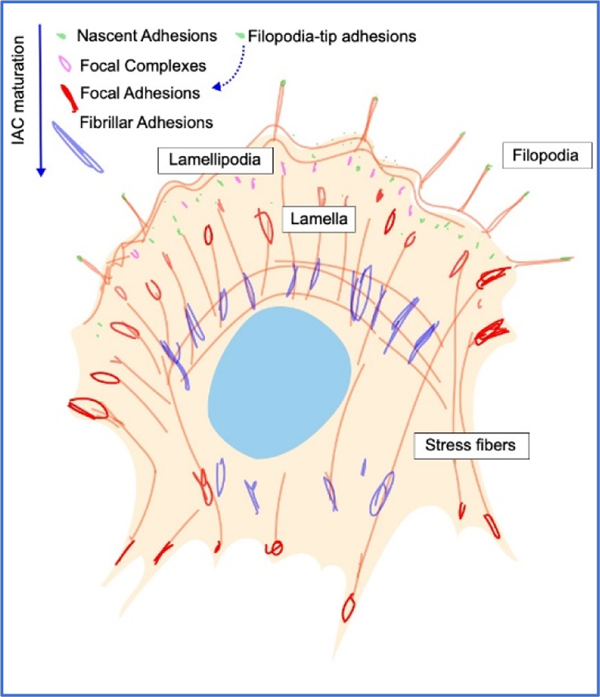
Nascent Adhesions are the first adhesion structures identifiable during lamellipodial extension, forming at the cell edge, containing ~50 integrins and having a diameter of <0.5 μm9,62. In addition to integrin, these structures are enriched for kindlin, talin, FAK, paxillin and α-actinin. Nascent adhesions are transient, either disassembling or maturing into larger focal complexes.
Filopodia-tip adhesions are small IACs composed of a unique subset of adhesome proteins, enriched in myosin X and distinct from other adhesions208,209. Upon stabilisation, filopodia-tip adhesions can give rise to nascent adhesions and mature into focal adhesions208.
Focal complexes are small (<1 μm) dot-like adhesions formed at the transition zone between the lamellum and lamellipodium. These structures contain core IAC components, link to the actin cytoskeleton and strengthen in response to forces.
Focal adhesions (FA), the most extensively studied and best characterised adhesions, form as focal complexes mature in response to increased forces applied from actomyosin contractility or through externally applied forces9,189,207. Force-mediated recruitment of additional IAC proteins promotes a stronger association between integrin and actin and allows assembly of ordered elongated structures structure with sizes of ~ 2–5 μm.
Fibrillar adhesions can arise from further maturation of FA in some cell types. These long thin or beaded structures are centrally located, vary in length (~1–10 μm) and are characterised by enrichment of the ECM protein fibronectin, its receptor, α5β1 integrin, and the cytoplasmic actin-binding protein tensin. Integrins in fibrillar adhesions mediate fibronectin fibrillogenesis9,207.
Invadosomes (invadopodia and podosomes) are small (~2 μm diameter) cylindrical structures containing typical IAC proteins but are also rich in actin regulators gelsolin, Arp2/3, (N-) WASP, adaptor proteins Tks4 and Tks5. They are generally termed invadopodia in cancer cells and podosomes in normal cells, such as macrophages, osteoclasts and endothelial cells207. Unlike FA, they have been associated with reduced cellular tension and are organised with a central actin core surrounded by actin regulators and signalling proteins. Forces generated by polymerisation of the actin core drive protrusion from the cell body and are associated with local matrix metalloprotease activation.
Reticular adhesions (clathrin plaques, flat clathrin lattices) are recently characterised, atypical αvβ5-containing adhesions, probably formed via frustrated endocytosis, particularly under low force conditions172,188,189. They lack many of classical IAC components, including talin and a link to the actin cytoskeleton, but are enriched in endocytic components, including clathrin and endocytic adaptors Numb and Dab2 and have important roles in cell migration, signalling and coordination of mitosis45.
Hemidesmosomes are specialised epithelial adhesions linking basement membrane laminins to the intermediate filament cytoskeleton via α6β4 integrins206. They lack canonical IAC proteins and instead are enriched for transmembrane proteins bullous pemphigoid antigen 180 and the tetraspanin CD151 and cytoplasmic plakin proteins (plectin and BP230) that link to intermediate filament cytoskeleton and can also crosslink actin and intermediate filaments. Hemidesmosomes can form adjacent to FA and mechanical and signalling crosstalk between hemidesmosomes and FA influences stable adhesion and migration207.
Box 2 |. Toolbox for investigating IAC mechanobiology.
Over the past 20 years, a wide variety of tools have been used for both the application and readout of mechanical force at IACs210. The tools highlighted here, in conjunction with genetic, pharmacological, cell biological, and biomaterials perturbations have transformed our understanding of IAC mechanobiology.
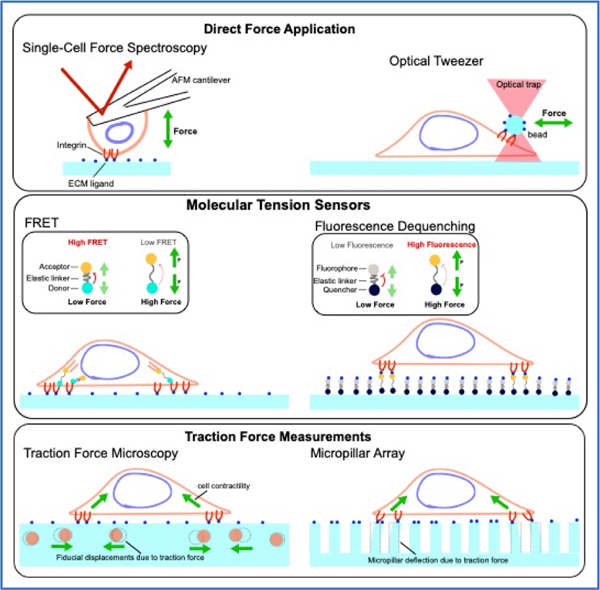
Direct Force Application. Optical/magnetic tweezers can deliver targeted force to cells using optical/magnetic beads coated with the appropriate adhesion receptor ligand. Alternatively, a family of Atomic Force Microscopy (AFM) techniques, can use functionalised tips of cantilevers to probe cells. Cells can also be positioned on cantilevers or micropipettes to probe the mechanical responses of the adhesive contact, such as in Single Cell Force Spectroscopy108. More global acute force application to IACs are commonly applied by stretching elastic substrates109,211.
Super-resolution Microscopy (SRM). Imaging cellular structures beyond the diffraction limit often involves a trade-off between imaging speed, range, spatial resolution, live-cell compatibility, data throughput, and method complexity. For probing nanoscale organisation in fixed specimens, STED (Stimulated Emission Depletion) microscopy and single-molecule localisation microscopy can achieve ~20 nm resolution in 2D (x,y) but higher z resolution requires more specialised interferometric systems such as iPALM (interferometric PhotoActivated Localisation Microscopy)57. For live-cell imaging, Structured Illumination Microscopy offers a versatile approach but with the spatial resolution generally limited to the 100–200 nm range.
Single-molecule tracking (SMT). Motion of integrin and other IAC proteins in live cells can be imaged using TIRF (Total Internal Reflection Fluorescence) microscopy or a similar illumination configuration, followed by tracking and statistical analysis of motion characteristics60,103,212,213. SMT trajectory can be categorised as random (Brownian) diffusion, sub-diffusive confined diffusion, immobilised, and active transport, which report on local microenvironment, binding interactions, and ensemble heterogeneity212. A key technical limitation of SMT has been the short (typically a few seconds) observation lifetime due to photobleaching, but recent photostability enhancement have enabled ultra-long trajectory analysis of integrins104.
Fluorescence Correlation Spectroscopy (FCS). FCS and a related method, Fluorescence Cross-Correlation Spectroscopy, is based on statistical analysis of the fluorescence fluctuations, which can be used to infer stoichiometry, diffusion coefficients, and protein-protein interactions. Due to its reliance on fluctuations, FCS is primarily suitable for probing IAC proteins in small diffusible complexes62.
Fluorescence Recovery/Loss After Photobleaching/Photoactivation (FRAP/FLAP). Spatiotemporal dynamics in IACs in the seconds and microns regime can be probed by FRAP or FLAP which monitor the exchange of fluorescently-tagged proteins between IAC-resident and cytoplasmic pools. The exchange lifetimes of various IAC proteins have been shown to exhibit different correlation with contractility and substrate rigidity159.
Traction force microscopy (TFM) and micropillar array. Traction force exerted by cells can deform pliable substrates, and thus force vectors can be inferred. Microfabricated elastomeric structures such as micropillar array can probe force read-outs at discrete points. In contrast, TFM made use of continuum substrate with randomly distributed fiducial marks but require mathematical reconstruction of force vectors210. Development of SRM-enhanced TFM has enabled higher-resolution force measurements to be performed214.
Molecular tension sensors. Biopolymers such as DNA or proteins with calibrated force-extension relationship can be used as a molecular tension gauge akin to a spring in a mechanical balance. Commonly used modes of optical read-outs include Forster Resonance Energy Transfer (FRET) and dequenching or binding-induced fluorescence215–218. Extracellular tension sensors incorporating ligand, such as RGD, can measure forces exerted through integrins. This class of sensors can utilise optimal synthetic fluorophores and permit single-molecule sensitivity217. Intracellular tension sensors based on fluorescent proteins FRET-pairs have the advantage of being genetically encoded but the typical photostability limitations of fluorescent proteins. Active development continues to add to a large library of IAC protein tension sensors (see 219 for technical discussion).
Molecular composition of adhesions
Dynamic cell adhesion to ECM substrates is largely mediated by micron-scale clusters of integrins with associated signalling, scaffolding and cytoskeletal proteins. These integrin adhesion complexes (IACs) can be classified based on their size, composition, organisation, lifetime, localisation and function (Box 1). As depicted in Fig 1a and b, and discussed in more detail in later sections, adhesions form when nanoclusters of activated integrins engage the ECM and associate with cytoplasmic adaptor proteins, such as talin, linking integrins to the actin cytoskeleton. Rho-mediated activation of myosin contractility drives adhesion maturation through talin-mediated engagement of the actin filaments undergoing retrograde flow leading to mechanical activation of talin, recruitment of additional adaptor and signalling proteins, and stabilisation of active integrin. This maturation and remodelling of IACs is strongly influenced by factors such as cell type, contractility and the composition and rigidity of the ECM substrate. New adhesion formation, adhesion maturation and subsequent adhesion turnover, driven by disassembly in response to loss of tension or the internalisation of integrins through endocytosis, enables the cell to sense and respond to changes in the local extracellular mechanical environment and permits intracellular signals to control adhesion dynamics that enable cell migration and influence ECM organisation. A detailed mechanistic understanding of IAC assembly, dynamics and function requires identification of the proteins present in these multi-protein assemblies and elucidation of their key interaction networks. Here we briefly summarise current information on the integrin family and discuss recent advances through mass spectrometry-based identification of integrin-associated proteins.
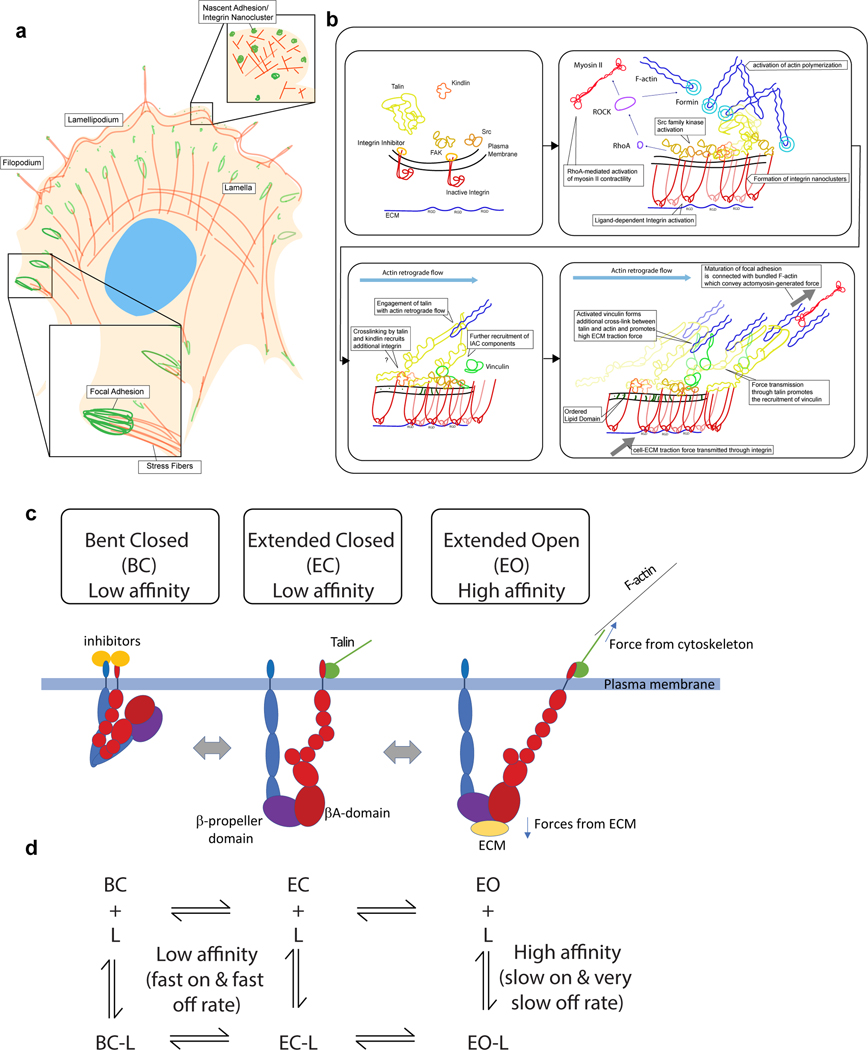
Figure 1 |. Integrin conformational dynamics at integrin-mediated adhesions.
a | Cartoon of the top view of a spread polarised cell with IACs and actin structures indicated. b | Depiction of key steps in the formation and maturation of IACs from initial nascent adhesions to mature FA. c | General schematic depicting the three major conformations of integrin – bent closed (BC), extended closed (EC) and extended open (EO) – along with key interacting proteins and sites of force application. α-subunit is shown in blue and β-subunit in red. Inhibitors (such as filamin199,200, ICAP-1201, or SHARPIN202) binding to α or β cytoplasmic tails are depicted as yellow circles while activating proteins such as talin and kindlin that directly or indirectly link to the cytoskeleton are shown in green. d | Reaction scheme showing the kinetics of integrin and ligand binding taking into account integrin conformational change – based on results in 31.
Integrin adhesion receptors.
By engaging ECM components with their extracellular domains and cytoskeletal and signalling proteins via their cytoplasmic tails, integrins provide a transmembrane link between the ECM and the cytoskeleton, transmitting chemical and mechanical signals into and out of the cell11. Integrins are conserved throughout metazoans11, and loss of function experiments in a range of model organisms (including mice, zebrafish, Drosophila, C. elegans and Xenopus) establishes essential roles for integrins in cell adhesion and tissue integrity15–19. Functional integrin receptors are obligate heterodimers of an α-subunit and β-subunit that are mostly unrelated to one another in sequence but are both type I transmembrane proteins with large multidomain extracellular portions, a single-pass transmembrane region and a generally short cytoplasmic tail11 (Fig 1c). The number of different α- and β-subunits per species appears to increase with organismal complexity, with mammals having 18 α-subunits and 8 β-subunits that can combine into 24 distinct αβ heterodimers11. Each heterodimer exhibits distinct, but sometimes overlapping, specificity for ECM ligands, such as collagens, laminins, fibronectin and vitronectin, allowing the precise repertoire of integrins expressed on the cell surface to determine the cellular response to complex ECMs.
Integrins are well understood at the structural and functional level, and have been reviewed in detail elsewhere9–11,20. In the early 2000s, crystallography first revealed the domain architecture of integrin extracellular domains and with electron microscopy (EM) provided insights into conformational regulation of integrins (reviewed in10,21,22) (Fig. 1c). In most integrins, interactions between the β-propeller in the α-subunit and the βA domain in the β-subunit form the ligand binding “head” of the heterodimer, this head is connected to the transmembrane domains and the cytoplasmic tails via α- and β-subunit “legs”. The structures of the extracellular portions of many integrin heterodimers have now been resolved by x-ray crystallography and a notable feature is that the ligand-binding headpiece folds back against the α and β subunit legs resulting in a bent structure (Fig. 1c). While the extent of bending may vary between different heterodimers, bent structures can be seen in cryo-EM images of purified integrins23–26 and most recently bent αIIbβ3 integrins have been visualised in cryo-electron tomograms of membrane protrusions in platelets27. It is now generally accepted that in the bent conformation integrins exhibit low affinity for extracellular ligands and the legs, trans-membrane domains and cytoplasmic tails of the α- and β-subunits are close to one another11,21,22. Additional EM, cryo-EM, super-resolution microscopy (SRM) imaging, biophysical studies, mutational analyses and x-ray crystallography of smaller portions of integrin, support the idea that integrins adopt at least two additional conformations; an extended-closed conformation that also has low affinity for ligand, and a high-affinity extended-open conformation where the head piece is open coupling conformational change in the ligand-binding head with separation of the subunit legs, transmembrane and cytoplasmic regions11,21–26,28 (Fig. 1c). Integrin affinity for ECM, and hence integrin-mediated adhesions, are thus influenced by the dynamic equilibrium between the bent closed, extended closed and extended open integrin conformations29–32 (Fig. 1d). These equilibria are altered by the binding of proteins to the short cytoplasmic tails of integrins and through mechanical forces applied to integrins via actomyosin-mediated contractility, extracellular fluid flow and ECM tension6,11,20. Thus, the low-affinity bent state is often supported by the association of inhibitory proteins with the α- or β-subunit cytoplasmic tails, stabilising the association of α and β tails and inhibiting the binding of activating proteins, while binding of the FERM domain at the N-terminus of the cytoskeletal adaptor protein talin (Box 3) to the β-subunit cytoplasmic tail destabilises the association between the α- and β-subunits, favouring high-affinity extended integrin conformations33–35. As talin also contains binding sites for F-actin and other IAC proteins, including vinculin33 (Box 3), it can link active integrins to the actin cytoskeleton allowing force transmission, and help pattern and organise protein networks at IACs (Fig. 1c). This multi-step process, by which intracellular signals increase integrin affinity for ligands is termed activation, or inside-out signalling, and kindlin (Box 3) binding to integrin β tails also contributes, although the molecular mechanisms are less clearly defined20,33. Notably, using well-characterised conformation-specific Fabs to stabilize α4β1 and α5β1 integrins into ensembles of defined conformational states, recent detailed analysis of integrin-binding kinetics31 has revealed that low-affinity integrin conformations (both the bent closed and extended closed) bind ligand more rapidly than high-affinity extended open conformations but that the extended open states have much lower off-rates (Fig. 1d). The increased on-rate, together with a higher fraction of low-affinity integrins on the cell surface leads the authors to suggest that integrin binding will occur in the low affinity form, prior to inside-out activation, but that ligand binding and activation would rapidly lead to conversion to the high-affinity extended open form that permits coupling to the actin cytoskeleton supporting cell adhesion and the recruitment of additional intracellular binding partners that mechanically reinforce the link and allow subsequent downstream signal transduction.
Box 3 |. Key IAC proteins.
The core integrin adhesome contains many proteins. Integrins are discussed in detail in the main text, but here we highlight a small subset of other adhesome proteins with well-established roles in IACs.
Talins (talin 1 and 2) are large (~270 kDa) proteins that contains an N-terminal head, composed of an atypical FERM domain (with four subdomains F0, F1, F2 and F3) that binds to the integrin β-subunit cytoplasmic tail, destabilising the association between the α- and β-subunits, favouring integrin extension33–35. The head also contains binding sites for phosphoinositide lipids, Rap1 GTPases and F-actin. Talin head connects to the rod via a flexible linker containing a calpain cleavage site. The rod is composed of 13 helical bundles and contains additional binding sites for integrin, vinculin (at least 11), actin (at least 2), KANK and other IAC proteins. Homodimerisation occurs via the C-terminus. Talin binding partners regulate its transitions between a compact, globular auto-inhibited form where the head binds helical bundles from the rod, and an extended active form that can link activate integrins to the actin cytoskeleton, provide a molecular scaffold for IAC assembly, and transmit and respond to mechanical force.
Vinculin is a conformationally autoinhibited ~120 kDa protein with multiple binding sites for other IAC proteins. Its N-terminal domain binds talin, α-actinin or its own C-terminal domain during autoinhibition, while C-terminal domains can bind F-actin, paxillin and PIP2 phosphoinositides94. By binding talin and F-actin, vinculin reinforces the talin-mediated integrin-actin connection.
Kindlins are a family of 3 (kindlin-1, −2 and −3) atypical FERM domain-containing proteins20,33,220. The kindlin FERM is most closely related to that of talin but, uniquely, contains a PH domain inserted in the F2 subdomain. Kindlin binding to integrin β cytoplasmic tails contributes to integrin activation and links integrins to other IAC components, notably the IPP complex and paxillin. Unlike talin or vinculin, most evidence suggests kindlins are not regulated by autoinhibition but oligomerisation may regulate kindlin functions220,221. Loss-of-function kindlin-1 and kindlin-3 mutations cause Kindler syndrome20,33,222,223 and leukocyte adhesion deficiency type-III20,33,222,223 respectively.
IPP complex - the IPP complex is an essential trimeric protein complex made up of the pseudokinase integrin-linked kinase (ILK) and its obligate partners PINCH1 or PINCH2 and α, β or γ parvin. The N-terminal ankyrin-repeat domain of ILK engages the first of PINCH’s five LIM domain while the C-terminal ILK pseudokinase domain binds the second CH domain of parvin11. The IPP complex can scaffold many adaptor and signalling proteins including kindlin (via ILK), paxillin (via parvin) and Rsu1 (via PINCH) and apparently directly links to actin (via both PINCH and parvin).
KANKs are a family of 4 related proteins (KANK1–4) recently implicated in controlling IAC functions. KANKs contain an N-terminal talin-binding KN motif, a central coiled-coil domain that mediates oligomerisation and a C-terminal ankyrin-repeat domain. KANKs localise to the periphery of mature FAs and modulate IACs by binding talin, altering its activation state and its link to F-actin, by modulating Rho activity and, through interactions with the cortical microtubule stabilising complex (CMSC), by recruiting microtubules to IACs influencing IAC turnover43,53–55,156,172,178.
Paxillin, and the related Hic-5 and leupaxin, are key IAC adaptor proteins51,224. Paxillin is an essential 68 kDa, tyrosine-phosphorylated protein composed of an N-terminal portion containing multiple LD motifs followed by 4 tandem LIM domains at the C-terminus. Paxillin binding proteins include parvin, kindlin, vinculin, talin, FAK and Cas.
Focal adhesion kinase (FAK), contains an N-terminal FERM domain, a central tyrosine kinase domain and a C-terminal focal adhesion-targeting domain that binds talin and paxillin among other proteins225. FAK phosphorylation of IAC substrates, such as paxillin, generates additional protein interaction sites regulating FA growth and dynamics.
The integrin adhesome.
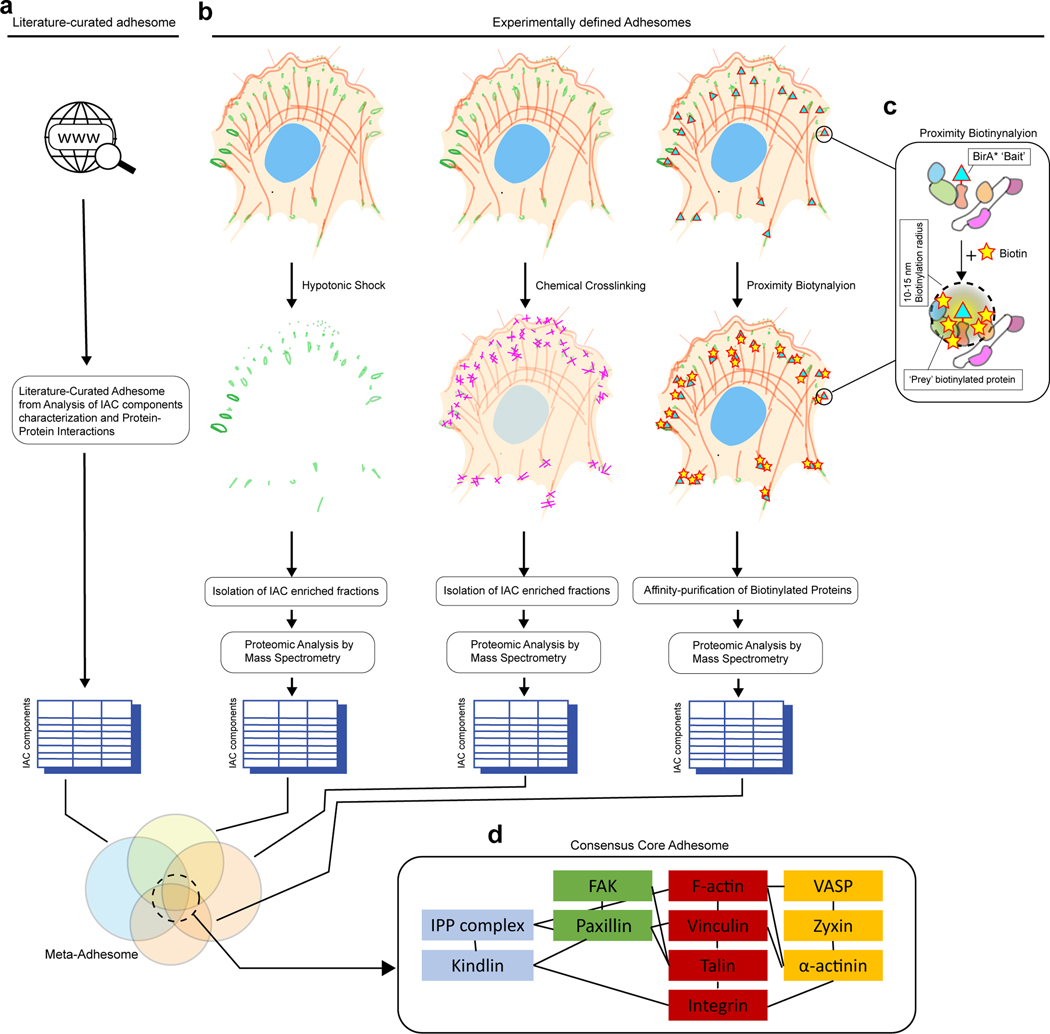
The proteins associated with IACs (extracellular, membrane bound and cytoplasmic) are collectively termed the integrin adhesome. IACs were originally characterised using microscopy and many constituents, including talin, vinculin and integrin, were identified by immunofluorescence, through expression of fluorescently-tagged proteins, biochemical interactions and yeast 2-hybrid screens36. Building on this, the adhesome was initially predicted from sequence similarities with known IAC-associated proteins, the presence of specific predicted domains, and extensive literature analysis37,38 (Fig. 2a). However, mass spectrometry proteomics of purified IACs has now expanded the number of integrin adhesome proteins to several thousands36,39,40(Fig. 2b). Profiling the adhesome of different cells, or on different matrices, has allowed comparison of cell-type or integrin specific factors and has facilitated investigation of phosphorylation of adhesome components41–44. Different adhesions can also be compared with this method. Indeed, despite the requirement for purification of protein complexes from cell populations, which may result in mixing different types of adhesion, proteomic analyses identified a new class of β5 integrin-enriched adhesion, the reticular adhesion45 (Box 1). Detailed bioinformatic analysis of adhesomes from various cell types, under differing mechanical and signalling conditions, has revealed a consensus adhesome of only around 60 proteins12,40,46 (Supplementary Table 1). Comparison of these components with the literature curated list, suggests that they are enriched in core structural IAC proteins, perhaps because these are most easily purified in the complex. The complexity of the adhesome prevents us from providing detailed descriptions of each component but four canonical interrelated signalling/cytoskeletal modules have been identified in the consensus adhesome12,40,46 (Fig 2d).
Figure 2 |. Cataloging the Molecular components of IACs.
a | Literature-curated adhesome is assembled from the analysis of published studies describing IAC locations and protein-protein interactions. b | Experimentally-defined adhesomes are obtained from mass spectrometry-based proteomic analysis using various enrichment strategies, such as hypotonic shock, chemical crosslinking, or proximity biotinylation. Enriched IAC-containing fractions are then subjected to tryptic digestion and mass spectrometric analysis. c | Proximity biotinylation enables inference of protein interactions networks. Bait moiety such as BirA*, a promiscuous biotin ligase from Escherichia coli, can be fused to a ‘bait’ protein that localize to IACs. Incubation with biotin results in BirA*-catalyzed biotinylization of nearby ‘prey’ protein within 10–15 nm radius. Biotinylated ‘prey’ proteins can then be affinity-purified and subjected to mass spectrometric analysis. d | Core consensus adhesome. Meta-analysis of literature-curated adhesome and experimentally-defined adhesomes, together with proximity biotinylation data, have largely converged on a set of core consensus adhesome components. Protein-protein interactions are depicted by black lines. Color-coding indicate distinct modular sub-networks.
While proteomic identification of adhesome proteins complements more focused functional studies on individual components, it does not by itself provide protein-protein interaction data. Therefore, several groups initiated BioID proximity-dependent labelling approaches, allowing mass spectrometric identification of proteins within 15–25 nm of specific bait proteins – IAC proteins fused to a biotin ligase47–50 (Fig. 2c). This approach, which has been applied to both focal adhesions (FA) and hemidesmosomes (Box 1), avoids the requirement to purify IACs for analysis, removing the need for reversible chemical crosslinking and presumably facilitating identification of weaker, more transient interactions beyond the core structural components47–52. Furthermore, separately labelling several different IAC proteins allows multiplexing analysis facilitating generation of proximity maps that are more likely to reveal molecular interactions and help map protein localisations and interactions in sub-regions of adhesions. Initial studies focused on a few IAC bait proteins, notably paxillin, kindlin-2 and talin-1, but multiplexed data from 16 IAC baits spanning all 4 canonical adhesome modules (Fig 2d) have now been reported50. Hierarchical clustering of bait proteins based on their interactions led to 5 clusters of baits that aligns relatively well with the canonical modules defined by proteomic analysis of purified IACs. Thus, in vivo proximity labelling generally supports models derived from proteomics of purified IACs (Supplementary Table 1), however additional factors not captured in the purified adhesomes were observed. Notably, KANK2 was identified as paxillin- and kindlin-proximal in initial studies47 and this was strongly supported in later larger studies where KANK2, and other members of the cortical microtubule stabilising complex (CMSC), were robustly identified with many IAC bait proteins50. Focused studies have confirmed the importance of KANKs (Box 3) in IACs43,53–55. The integration of advanced imaging studies (discussed below) with new proximity interaction data on IAC components, should contribute to a clearer picture of the molecular organisation of adhesions during assembly, maturation and turnover (Fig 1a,b).
Molecular organisation of adhesions
Advances in our understanding of the structural organisation of cell-ECM adhesions owe much to improved biophysical and bioimaging methods. Resolving individual IAC structures revealed the presence of nanoscale compartments, termed nanoclusters here, which may comprise the structural and functional modules of IACs.
Nanocluster subunits of adhesions.
Although the presence of sub-diffraction-limited IAC structures such as nascent adhesions [Box 1] have long been appreciated56, the development of SRM in mid-2000s57, enabled investigation of the nanoscale internal organisation of IACs (Box 2). Various SRM techniques all revealed extensive structural granularity in FA, typically characterised by ‘nanoclusters’, nodules of high local IAC protein density, embedded within lower density regions58–61. As discussed below, nanocluster organisation appears to originate with initial adhesion formation, triggered by integrin activation (Fig. 1b). Using conformation-specific monoclonal antibodies, active and inactive integrin α5β1 are found to segregate into distinct nanoclusters (Fig. 3b), suggestive of cooperativity within nanoclusters and implying that nanoclusters could be functionally regulated as distinct substructures61.
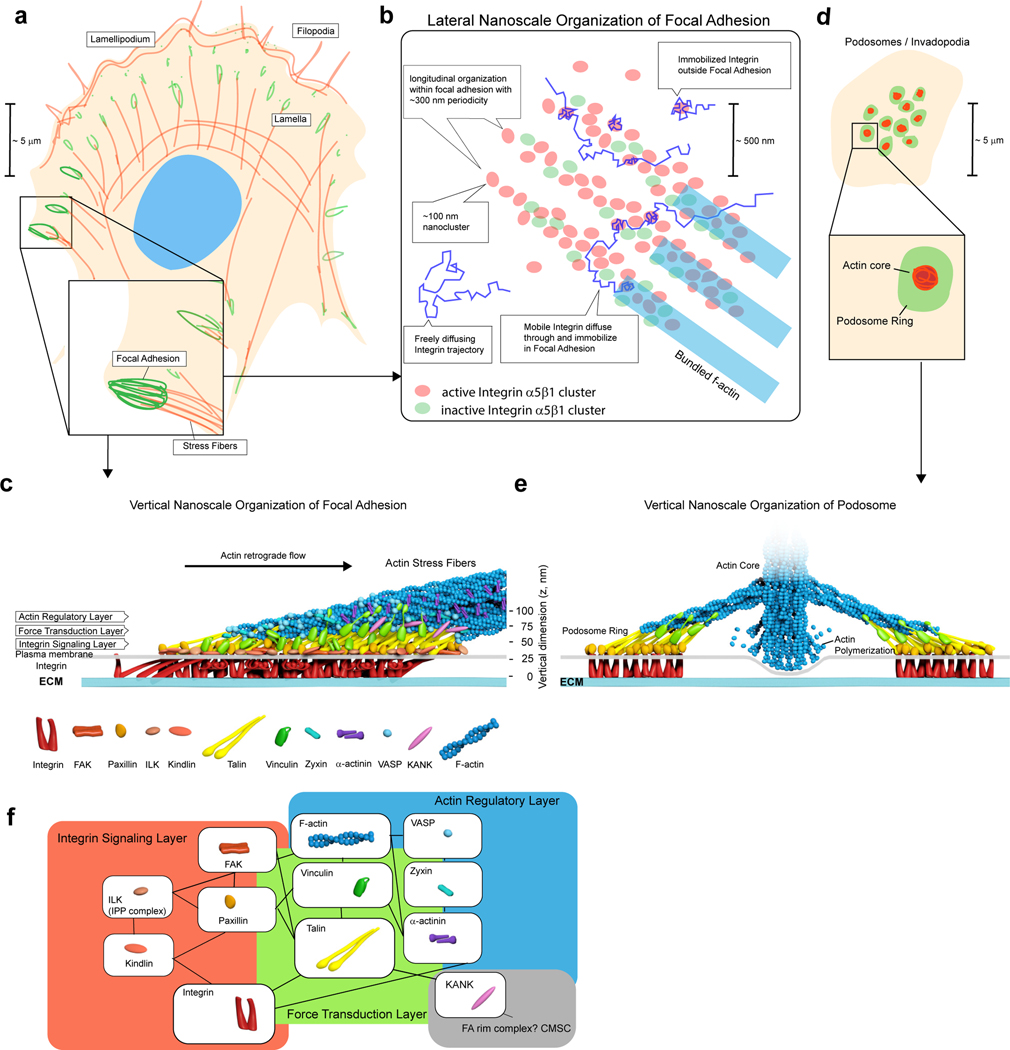
Figure 3 |. Molecular organisation of IACs.
a - b | Lateral composite nanocluster organisation. In mature FAs, extensive granularity and occasional alignments of nanoclusters into linear chains along actin templates can be observed in certain cell types203,204. For integrin α5β1, active and inactive integrin segregate into distinct nanoclusters61 suggesting that nanoclusters could function as discrete units. Single-molecule analysis of integrin motion provides a consistent picture whereby integrin can diffuse freely through IACs. Integrin is immobilised in distinct loci which are comparatively enriched in IACs. c | Multilaminar architecture of FA. Schematic diagram depicting vertical organisation of various IAC components that have been analysed by SRM studies59,87,89,205. Integrins are depicted in clusters of inactive (bent-closed), active (extended-open), as well as in tilted orientation as inferred from fluorescence anisotropy measurements137. d | Podosomes or invadopodia are arrays of typically centrally located IAC structures, consisting of actin-rich core that protrude against and degrade ECM, surrounded by ring-like plaques containing IAC components. e | Vertical organisation of podosome ring featuring polarised orientation of talin, and similar organisation of paxillin and vinculin as in FA. f | Comparison of SRM-based spatial organization with sub-networks of IAC components identified by proteomic analysis (Fig. 2d). A putative IAC compartment anchored by KANK proteins that may potentially interface with microtubules is also depicted.
Multiple factors serve as gatekeepers for the maturation of IAC from the initial nanocluster or nascent adhesion stage (Fig. 1b). The molecular chronology of initial IAC organization in the protruding lamellipodia of migrating cells62 has been probed by Fluorescence Correlation Spectroscopy (Box 2), which identified kindlin-2 as the earliest IAC component recruited, followed closely by talin in a myosin II-dependent fashion. Transient α5β1 and α-actinin-containing complexes was also identified as precursors to the formation of nascent adhesion. The integration of advanced imaging with ECM nanopatterning offers opportunities to systematically dissect the influence of ligand geometry, mobility and stoichiometry, and of mechanical force. The nanoscale geometric requirement for IAC formation was first probed by micelle-based nanoparticle patterning, which revealed that a variety of cell types adhered and robustly formed IAC on substrates with ligand spacing in the ~60 nm range more effectively than on ~110 nm ligand spacing63,64. This was followed by studies combining supported lipid bilayers presenting mobile RGD ligands with nano-patterned mobility barriers or ligand-decorated pedestals65, which enabled the contribution of ligand mobility and ligand immobilization to be distinguished. In fibroblasts, the assembly of IAC nanoclusters involves the initial clustering of mobile RGD ligands, actin polymizeration, and the early recruitment of FAK, paxillin, and talin (Fig. 1b, Box 3), in conjunction with Src-dependent cell spreading65,66. On a uniform supported bilayer, these initial IAC nanoclusters are mobile but unable to promote further adhesion maturation, presumably due to the inability of the supported bilayer to support high force. In contrast, on nanopatterned supported lipid bilayers, the rigid mobility barrier serves to immobilize the IAC nanoclusters allowing force transmission, thus enabling IAC maturation, contractility, and further cell spreading. Nanocluster maturation also appears to orchestrate a local remodelling of the plasma membrane, as characterised by fluorescence anisotropy of GPI-anchored proteins67. Initial integrin nanoclusters are associated with disordered lipid domains, but significant lipid order, sometimes called membrane rafts68, is observed upon integrin immobilisation, activation of actomyosin contractility via FAK/Src/ROCK signalling, and the recruitment of activated vinculin (Fig. 1b)67. Importantly, using SRM-based molecule counting, the molecular stoichiometry of IAC nanoclusters formed on either supported bilayer or ECM-coated substrate were determined to be largely comparable, containing ~50 molecules of integrin αvβ3 within ~100 nm puncta69. Since lateral interaction of integrins with membrane proteins such as syndecan70, tetraspanin71,72, and growth factor receptors73 have long been known to influence integrin-dependent signaling, often in association with specialized lipid domains such as membrane rafts74 or tetraspanin microdomains75, revisiting and redefining their structure and dynamics with the latest imaging and molecular manipulation tools will likely become a fruitful avenue for future investigation.
Since major components of ECM are high molecular-weight fibrous glycoproteins, ECM ligand presentation in vivo is expected to be hetergeneous at the nanoscale. The configuration of ECM fibres of various geometry has recently been recapitulated by patterning RGD-ligands with <30 nm spatial features, comparable to the binding footprint of single integrin molecules76. This experimental platform revealed that robust IAC nanocluster formation requires 2-dimensional ligand presentation, either as parallel or crossing lines, over the range of >40 nm, as opposed to linear single fiber (1-D). Additionally, it was also shown that while conformational activation of integrin is necessary for nanocluster nucleation, conformationally-activated, ligand-binding defective, integrin αvβ3 mutants are recruited into preformed clusters, suggesting capture of exposed integrin β cytoplasmic tails by adaptor proteins such as talin76. Indeed, it has long been known that inactive integrins or even isolated integrin β tails fused to unrelated transmembrane and extracellular domains can be recruited to adhesions and that this depends on interactions of the β tail with adaptors77. Among such adaptors, talin, with an elongated dimension of >60–80 nm and the potential to dimerize, is proposed to determine to dimension of the nanocluster and sensitivity to ligand presentation geometry as discussed further below78.
Molecular architecture of adhesions.
Our current understanding of the 3D molecular architecture of IACs is based primarily on studies of FA or podosomes. Immuno-Electron Microscopy79, Atomic Force Microscopy80, and Cryo-Electron Tomography81 have defined the thin (<200 nm) sub-diffractive vertical (z) dimensionality of FA and identified a limited number of IAC protein complexes. However, systematic 3D-mapping of protein organisation in IACs is significantly accelerated by SRM methods such as iPALM57,59,82,83(Box 2). These studies showed that IAC proteins exhibit characteristic vertical (z-axis) density distribution, indicative of organisation into partially overlapping layers relative to the plasma membrane (Fig. 3c)59. Relative to the fibronectin-coated substrate surface (z = 0 nm), the integrin cytoplasmic tail was found to locate at z~30 nm, consistent with the extended open conformation28,59. The ~20 nm band centering around the integrin cytoplasmic tails has been termed the integrin signalling layer (ISL), within which the peak density of ILK, FAK, kindlin-2, and paxillin are located59,84–88. The intermediate layer, immediately adjacent to the ISL, is termed Force Transduction Layer (FTL) primarily due to the presence of key mechanotransducer proteins such as talin and vinculin. Above the FTL, the actin regulatory layer (ARL) corresponds to the interfacial zone where stress fibers are coupled to FAs, with high densities of F-actin and actin binding proteins such as VASP and α-actinin. To date, 3D nanoscale protein mapping has been reported for IACs from cell lines of epithelial, endothelial, and fibroblast origins as well as pluripotent stem cells59,82,84,85,87,89. Additionally, in podosomes, talin, vinculin, and paxillin localise to comparable positions as in FA (Fig. 3d–e)90. In general, the multilayer hierarchy of IAC organisation is broadly similar in all these studies, albeit with notable variations in the absolute z-position of the layers (likely due to varying ECM thickness in different experimental settings84) and, as discussed further below, in FTL proteins such as vinculin. Importantly, SRM-based protein mapping results are in remarkable agreement with the BioID proximity-dependent labelling results described above47,50 (Fig. 3f), suggesting that the multilayer stratification captures a broad structural framework underlying IAC molecular architecture.
What controls the molecular architecture of IACs? Thus far, talin is the most notable determinant of structural FA organisation. SRM imaging of talin with fluorophores at the N- or C-terminus, showed it to adopt a highly polarised orientation (Fig. 3c, 4b) with the integrin-binding N-terminus in ISL and actin-binding C-terminus in ARL, separated by 20–30 nm along the z-axis perpendicular to the plasma membrane58,59. When endogenous talin is substituted by engineered talin of altered lengths (by deletion or insertion of central rod domains), the N-terminus remains anchored in the ISL but the z-positions of the C-terminus and ARL proteins such as VASP are modulated accordingly85. Geometric analysis of molecular extension from the N- and C- terminal positions suggests that talin could be stretched to ~100 nm or greater, presumably due to partial unfolding of mechanosensitive helical bundles that harbour cryptic vinculin binding sites85,91. Consistent with this, the position of the talin-C terminus appears to depend on vinculin in both FA and podosomes58,90. Thus, in mature IACs, talin is molecularly oriented to span from ISL through FTL and connect with the F-actin cytoskeleton in ARL85,92. Therefore, talin serves as the IAC ‘backbone’, forming the core force-transmission molecular chain that effectively templates the binding of other IAC components, most notably vinculin (Fig. 3c, 4b–d). Furthermore, since the talin dimerisation domain and an F-actin binding site are located at the very C-terminus, N-terminal integrin-binding domains of talin could conceivably capture integrins within a radius comparable to talin molecular length. This has been proposed to provide the physical basis for the minimal ligand spacing and integrin nanocluster organisation discussed earlier76. Notably, functional studies on talin mutants in Drosophila have confirmed that talin is essential for virtually all integrin functions, but suggested that talin may be organised differently in adhesions of different tissues93. Higher load-bearing structures in muscles were found to be organised like classical mammalian FA but in adhesions in the wing and epidermal cells talin could function using secondary integrin-binding sites and assemble parallel to the membrane. The importance of vinculin-talin interactions also varied with tissue and adhesion type. The extent to which this variability extends to mammalian adhesions has not been extensively investigated.
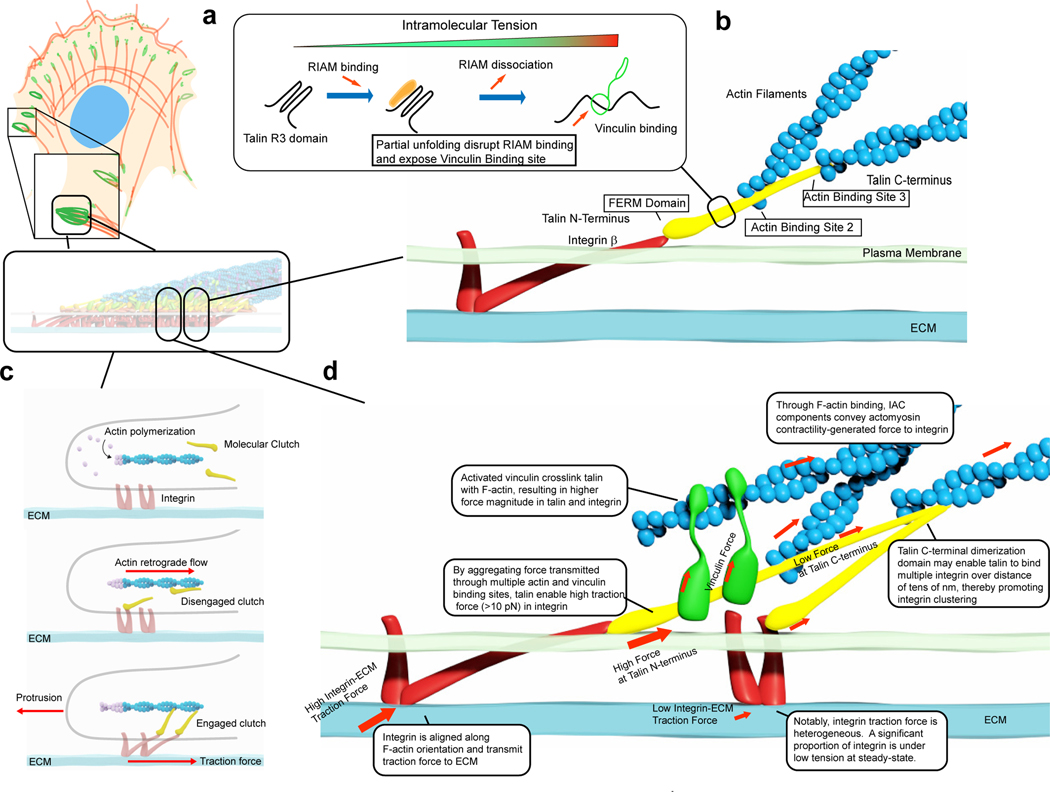
Figure 4 |. Molecular-scale mechanoregulation of IACs.
a | Mechanotransduction by force-dependent conformational changes. Several IAC proteins such as talin, vinculin, RIAM, α-actinin, KANK1 and filamin A contain domains that can be partially unfolded under piconewton physiological forces91,127–130. For example, talin R3 domain is a 4-bundle of α-helix that contains a binding site for RIAM. RIAM can bind talin under low intramolecular tension but upon the increase in intramolecular tension, R3 undergoes partial unfolding that displaces RIAM and in turn exposes a binding site to vinculin128. b | Integrin-Talin-Actin as the structural and mechanical ‘backbone’ of the IACs. In mature focal adhesions, talin adopt a vertically polarized orientation with N-terminal FERM domain engaging integrin b cytoplasmic tail. Actin binding sites 2 and 3 provide direct linkage to the F-actin cytoskeleton while indirect cross-linking is mediated by vinculin. c | Molecular clutch model of IAC mechanotransduction. Clutch molecules are capable of binding to both integrin and actin filaments. If molecular clutch is disengaged (middle panel), rearward force generated by actin polymerization in lamellipodia is primarily channeled to actin retrograde flow. Upon molecular clutch engagement (bottom panel), actin retrograde flow is mechanically coupled into traction force and support leading edge protrusion. d | Molecular-scale force transmission in IACs. Schematic diagram of force transmission in integrin-talinvinculin-actin complexes. Single-integrin force vector is primarily co-aligned with the F-actin orientation, potentially implicating a significant degree of tilting and stretching of the integrin ectodomain136,137. Integrin force is thought to be transmitted via talin, which may serve as a force aggregator as reflected by the intramolecular force gradient due to multiple vinculin and actin binding sites144,145. Load-bearing by integrin and talin are variable with high-load bearing molecules (>10 pN) comprising a subpopulation, dependent on vinculin139,145. Thus at steady-state only a subset of integrin-talin-vinculin-actin complexes in IACs are fully tensioned139.
In addition to talin, the FA organisation of kindlin-2 and vinculin have been explored by SRM. Kindlin-2 localisation to ISL appears primarily dependent on its phospholipid-binding Pleckstrin Homology (PH) domain88. On the other hand, multiple factors influence vinculin redistribution between ISL, FTL, and ARL. Vinculin is a conformationally autoinhibited protein with multiple binding sites for IAC proteins (Box 3)94. Binding to phospho-paxillin (Y31 and Y118) promotes ISL localisation of autoinhibited vinculin, while binding to talin favours FTL localisation58. During IAC maturation, vinculin localisation can progressively elevate to ARL58. Curiously, whereas in fibroblast and endothelial IACs, vinculin is oriented with an elevated C-terminus58,85, in pluripotent stem cells unknown molecular mechanisms invert vinculin orientation84,87. Altogether, these observations hint that certain proteins such as talin may provide a ‘chassis’-like structural framework upon which regulatable ‘moving parts’ components such as vinculin can modulate the structural, mechanical, and signalling functions of IACs95. However, current views of IAC compositional and structural organisation are static snapshots, inevitably biased toward stable and abundant structural motifs, and may overlook important dynamic aspects of IAC function.
Linking Adhesion Structure, Dynamics, and Trafficking to Mechanosensitivity
Mechanical force has emerged as a central unifying element influencing IAC dynamics96–99. It has been well established that cellular forces generated by actin polymerization and myosin II contractility are propagated to integrin via key adaptor IAC proteins (Fig. 4d)98–100. Force transmitted through integrin to ECM is commonly described as traction force (Fig. 4c), which drives cell shape changes, cell migration, rigidity sensing, and ECM remodelling. Mechanosensitivity of IAC is highly complex and encompasses numerous aspects of IAC structure, function, and regulations, spanning from variations in IAC lifetime associated with adhesion formation, maturation, remodelling and turnover, as well as changes in the movement and interactions of individual IAC molecules in response to recruitment of new binding partners, post-translational modifications, alterations in membrane environment and mechanical deformations99. Here, building from detailed single molecule studies to targeted vesicular traffic of adhesion components, we focus our discussion on how the interplay of molecular dynamics, conformational changes, transient force-regulated protein interactions, and intracellular transport collaborate to influence IAC mechanosensitivity.
Single-molecule dynamics within adhesions.
One measure of adhesion dynamics involves the motion of individual molecules within adhesions as studied by Single-Molecule Tracking (SMT) (Box 2). As expected, this reveals the enrichment of immobilised integrins inside and diffusive integrins outside IACs. Perhaps more surprisingly, a significant fraction of diffusive integrins can also be found inside IACs, and immobilised integrins also exist outside of IACs. Integrin single-molecule dynamics also appears to be sub-type specific60, and sensitive to local cellular activity such as lamellipodial protrusion101 or membrane curvature induced by the glycocalyx102. Longer time-scale SMT revealed that integrins repeatedly entered and exited FA and underwent transient ECM- and cytoskeleton-mediated arrest both inside and outside FA, but were arrested ~80% of the time within adhesions compared to only ~50% of the time outside adhesions. While integrins display primarily 2D diffusion characteristics, cytoplasmic IAC components can exhibit either 2D or 3D diffusion. For example, 2D diffusion of kindlin-2 and Rac1 can be attributed to their respective plasma membrane-binding sites88,103. In contrast, talin appears to become instantaneously immobilized in FA without prior inplane diffusion, indicative of its entry by 3D diffusion from a different focal plane60. Altogether, the ability of IAC proteins to freely percolate into IACs are consistent with the composite nanocluster organisation of IACs (Fig. 3a–b)60,104,105. These observations also underscored how, at the single molecule level, the dynamics of an individual IAC component is distinct from the micron-scale structural dynamics of the IAC.
Temporal responses of adhesions to applied forces.
Acute application of external force is instrumental for probing how IAC dynamics are influenced by mechanical stimuli. It is well documented that over long timescales of minutes-hours, cells respond to mechanical stretching or shear flow by concerted modulation of IAC assembly and disassembly and global cytoskeletal reorganisation to drive cell shape changes, reorientation, and activation of myriad transcription factors106,107. Force response of IAC on shorter timescale can be probed by Single-Cell Force Spectroscopy (SCFS) [Box 2], which showed that cell-ECM adhesion can be strengthened rapidly with sub-second response time108. The functions of a subset of IAC components such as integrin, talin, kindlin, FAK, and Src as well as Arp2/3 and mDia1 formin have been implicated in this process. Because this timescale is faster than visible IAC maturation [Box 1], such rapid responses potentially depends on some forms of pre-existing IAC structures, although whether these structures correspond to immobilised integrin outside of IACs observed by single-molecule analysis (Fig. 3b) remains to be established60.
The development of a substrate stretching platform compatible with SRM has revealed how acute force application elicits complex responses in cells that involve both simple elastic deformation and amplified displacement109. Differential motion observed for the N- and C-termini of talin are consistent with talin stretching, in agreement with the current model of IAC organization (Fig. 3c, 4b). Interestingly, the magnitude of actin cytoskeleton displacement is larger than the magnitude of the substrate deformation. Such overcompensated actin motion has been shown to be myosin II-dependent but the underlying mechanisms remain otherwise unclear. Responses to acute force application likely involve mechanically activated ion channels, which regulate cellular entry of various ions such as calcium, magnesium, potassium, and protons, all potent regulators of numerous signalling pathways110. Of these, the effects of calcium ions are best characterized111; for example, calpain-mediated proteolysis is known to regulate IAC disassembly112,113 via direct cleavage of core IAC proteins such as talin, FAK, or paxillin113–116. Beyond calcium, intracellular pH, which is regulated downstream of integrin-ECM engagement by proton antiporters such as the sodium hydrogen exchanger (NHE-1)117–120, is also a major regulator of cell volume, cytoskeletal dynamics, and biological activity of core IAC proteins such as talin121. The multifaceted involvement of mechanically activated ion channels in cell migration has been reviewed recently122, and their interactions with IAC mechanotransduction are now receiving increasing attention, with particular interest in the Piezo family of ion channels which are considered to be primarily dependent on mechanical cues for their activation123.
Force-dependent protein conformational changes contribute to IAC mechanosensitivity.
In response to mechanical force, biomolecules may undergo conformational changes, while non-covalent intermolecular contacts may dissociate. These molecular events, along with mechanosensitive ion channels124, comprise the elemental steps in molecular mechanotransduction125. Direct force input by optical or magnetic tweezers (Box 2) can perturb purified protein complexes with sub-molecular precision, and has been instrumental in characterising mechanical bond dynamics of integrin and IAC proteins (i.e. catch and slip bond behaviors126). Additionally, a growing list of IAC proteins have been shown to contain mechanically sensitive domains that can be partially unfolded under physiological forces to disrupt binding sites, expose additional binding sites, or otherwise modulate biochemical activity91,127–130 (Fig 4a). Of these, cell biological observations have largely corroborated the mechanosensitive recruitment of vinculin to talin in IACs52,131–133, though force-independent steps in talin-vinculin interactions may be relevant under certain contexts134,135.
Molecular-scale force transmission in adhesions.
In IACs of living cells, mechanical force can be probed using various molecular tension gauges (Box 2), in some cases at the single-molecule level. These measurements confirmed that the direction of mechanical force supported by individual integrin is largely co-axial with IAC and F-actin alignment136, consistent with the molecular alignment of integrins determined by fluorescence anisotropy137,138. Integrin load-bearing is spatially heterogeneous and dependent on vinculin, with high-load integrins (>10 pN) enriched in pheripheral FA, while the majority of integrins sustain a minimal loading of 1–3 pN139,140. Intramolecular tension measurements in integrin αLβ2, talin, and vinculin have established their general dependence on actomyosin contractility, while unveiling the underlying molecular complexity that arises from the differential mechanobiological contribution of distinct protein moieties. For example, both NPXF motifs (binding sites for talin and kindlin-2) in the integrin β2 are required for force transmission through integrin141. Intramolecular force in talin is sensitive to substrate rigitidy, dependent on the vinculin cross-bridge with actin, and closely correlated with filament density as observed by correlative light-electron microscopy142. Talin engagement with F-actin is mediated by either central or C-terminal actin binding sites (ABS2 and ABS3, respectively), which seemingly show different affinities for stress fibers and lamellipodial F-actin networks143,144. Furthermore, the magnitude of talin intramolecular tension is clearly non-uniform, with lower tensions near the C-terminus, and higher tensions near the N-terminus145 (Fig. 4d). This complexity may be a consequence of the multiplicity of binding sites for vinculin (at least 11) and actin (at least 2) in talin, and has been proposed to play higher-order mechanosignalling roles, through combinatorial engagement of different sites that could give rise to a wide variety of signalling outcomes146. Beyond cell culture, a Drosophila melanogaster strain with endogenous talin completely replaced by a FRET talin tension sensor shows that as pupae develop, the magnitude of talin tension at the myotendinous junction unexpectedly and progressively decreases147, possibly due to ongoing talin recruitment to myotendinous junctions during development, decreasing the magnitude of mechanical force per individual talin molecule.
Internally generated cellular forces arising from contractility, membrane tension, and actin polymerisation also drive various types of large-scale intracellular motion such as actin retrograde flow148. The observation that IAC proteins exhibit retrograde flow with variable degrees of coupling to actin flow is consistent with the ‘molecular clutch’ model of IAC mechanotransduction148–150 (Fig. 4c, for review see ref.151). For example, the retrograde flow vectors of talin and vinculin are moderately coupled to actin retrograde flow, while integrin, FAK, and paxillin exhibit minimal motion coupling, largely consistent with the current nanoscale structural model described above (Fig. 3c)59,148. How IAC component dynamics are converted into traction stress are, however, complex. IAC-mediated traction increases proportionally to actin retrograde flow in the slow flow regime but then decreases at flow speeds beyond ~10 nm/s152. Furthermore, IAC traction undergoes intra-IAC fluctuations153,154 and has dual actin retrograde flow-dependent and -independent components143 which may arise from differential coupling of IACs to distinct F-actin networks of lamellipodia (dendritic meshworks) and stress fibers (bundled F-actin), respectively155. Complexity is further compounded by recent studies that converge on talin as the entry point for microtubule-dependent mechanoregulatory signals via KANK proteins54,55,156.
Dynamics of protein organisation in adhesions.
IAC proteins undergo dynamic exchange with the cytoplasmic pool over time scales of minutes157–161. Largely consistent with the spatial organisation determined by proximity labeling50 and SRM59, correlative analysis of FCCS and FRAP dynamics [Box 2] identified multiple pre-complexes that can be concomitantly incorporated into or dissociated from IACs, such as the IPP complex, FAK/paxillin/Cas and Vinculin/Paxillin [Box 3]. Pre-complex of vinculin with a specific binding site on talin is also shown to favor rapid IAC maturation135. Importantly, consistent with their structural and mechanical roles, increasing substrate rigidity promotes long IAC residence time in a subset of IAC components such as talin, vinculin, and tensin159.
Interestingly, the dense aggregation, dynamic exchange and the multivalent organisation of many IAC components have led to conjecture that liquid-liquid phase separation (LLPS) could contribute to IAC dynamics and functions162. For example, LLPS of GIT1/βPIX protein complex has been reported to contribute to its signalling function in IACs163 while LLPS of p130Cas and FAK was linked to promotion of the formation of kindlin-containing molecular aggregates analogous to nascent adhesion integrin nanoclusters discussed above164. Additionally, the ability of LIMD1 to form condensates was found to be mechanosensitive and to contribute to IAC maturation and durotaxis165. LIMD1-dependent LLPS involves LIM domains and intrinsically disordered regions, common motifs in IAC components166,167. The molecular mechanisms underlying biomolecular LLPS and their potential roles in biological processes, including cell adhesion, remain active topics of research and debate162,168–170. However, while it is possible that the LLPS paradigm may be more broadly applicable to IAC functions and dynamics, it is important to note that most characterised LLPS phenomena involve micron-scale liquid-like structures, whereas the length scale of IAC structural granularity and anisotropic compartmentalisation is at the nanoscale. This involves much fewer molecules (~10s-100s) and these length scale and stoichiometry differences may require further theoretical and experimental elaboration.
Vesicular traffic in adhesion dynamics.
IACs are highly dynamic in terms of movement of individual molecules, protein responsiveness to mechanical forces and coupling of integrin adhesion to the actin cytoskeleton. Superimposed on these molecular dynamics, IAC formation, maturation and turnover is linked to the exocytic delivery of integrins to, and endocytic removal of integrins from, adhesions. Integrins, as the major transmembrane proteins in IACs, are the primary point of regulation by vesicular trafficking but alteration in integrin levels in turn influences the integrin associated protein networks present in IACs. As vesicular trafficking of integrins has been extensively reviewed elsewhere8,171,172, we limit discussion to a few recent findings linked to IAC organisation and to mechanical regulation of integrin traffic.
Expression of β1 integrins with a pH-sensitive fluorescent protein inserted into a loop within the extracellular hybrid domain has allowed direct visualisation of integrin exocytosis and revealed that integrin exocytosis occurs preferentially in proximilty to FA173. While the mechanisms specifically driving targeted integrin exocytosis, and the functional significance of localised integrin delivery, are yet to be established, increasing evidence suggests that many cargos are delivered close to FA in processes regulated by Rab and Rho small GTPases, contractility and microtubules174–177. KANK proteins (Box 3) provide mechanosensitive mechanisms for recruitment of microtubules to adhesions43,53,54,172,178 and conventional microscopy shows KANK distributed at the edges of FA. SRM studies revealed that KANK assembles in vertical walls at the outer rim of cornerstone FA, the large adhesions found at the periphery of colonies of pluripotent stem cells87, where it is presumably well placed to link to microtubules. Microtubule targeting apparently promotes FA disassembly in several ways179–182; including, influencing local Rho signalling to myosin contractility55, delivering proteases that locally degrade the ECM177 and triggering integrin endocytosis176,181,182. How the local exocytosis of integrin heterodimers, that might be expected to promote FA growth, is spatially and temporally coordinated with exocytosis of proteases that trigger FA disassembly and integrin endocytosis, and whether KANK proteins play roles in this, has not yet been determined.
Integrin endocytosis, which can proceed through a variety of pathways, has been extensively reviewed elsewhere171,172,183. Internalised integrins are recycled from endosomes back to the cell surface via several different routes with many of the Rab GTPases regulating these processes now known and specific integrin-associated adaptors enabling selective integrin endocytosis recently identified184–187. In this regard, it is noteworthy that recently characterised αvβ5 integrin-containing atypical adhesions, variously termed reticular adhesions, clathrin plaques and flat clathrin lattices (Box 1) probably form via frustrated endocytosis, particularly under low force conditions172,188,189. These structures seem to play roles in crosstalk between adhesion signalling and growth factor signalling and, because integrin-ECM interactions may mechanically prevent internalisation of these latices, they are likely to enhance and prolong growth factor signalling at the cell periphery190. Although αvβ5 appears to be preferentially localised to reticular adhesions, β1 integrins in tubular clathrin/AP-2 lattices support 3D cell migration in collagen fibers191. In addition, the mechanical properties of the ECM and the extent of cellular contractility seem to influence integrin endocytosis through the force-regulated recruitment of endocytic adaptors to IACs. For example, Dab2, an adaptor involved in clathrin-mediated endocytosis, is selectively recruited to β3 integrin clusters in low force environments, preventing recruitment of other cannonical IAC components and facilitating integrin internalisation187
Open questions in mechanoregulation of adhesions.
The process by which cells generate mechanosensitive responses ultimately arise from the complex networks of molecular-scale transactions which integrate mechanical information with biochemical signals, and thus adhesion mechanoregulation is an intrinsically multi-scale phenomenon. Understanding such information processing at the molecular level requires a better definition of the activity, stoichiometry, connectivity, and structural organization of IAC components, not only within the adhesions but also in their interrelated biological context. Case in point, the functions of many proteins within the current empirical list of 60 core IAC components (Supplementary Table 1) are still not well understood, especially in terms of adhesion mechanosensitivity40. In addition to cell biological characterisation, the bottom-up approach of biochemical reconstitution that could integrate adhesive components with active contractility systems192–194 will be highly instructive, while conversely, large-scale genetic screening would be important for discovery of less abundant but essential components. Notably there also remains much to explore in terms of the feedbacks between IAC mechanoregulation and regulatory mechanisms at the levels of transcription, translation, and proteostasis. As noted earlier, how mechanically regulated ion channels influence IACs, and vice versa, is another important question. Furthermore, given that thus far the majority of IAC proteins have been studied under ectopic expression conditions, the use of genome editing techniques to enable the analysis of IAC proteins under endogenous gene regulatory control and at endogenous levels of expression will be an important next step.
Imaging-based structural analysis of adhesions by SRM has been expedient but it should be highlighted that the inherent limitations of fluorescent imaging, whereby at most a few molecular species are observed at a time, inevitably mask the richly diverse molecular context surrounding any cellular structures. A more complete picture can be anticipated to emerge with recent advances in volumetric electron microscopy techniques81,195 and their correlation with fluorescence microscopy83,195,196. Integration of the wealth of quantitative findings on structure, dynamics, intracellular transport and mechanics in IACs into a comprehensive theoretical framework remains a work in progress. The relationship between IAC properties and mechanical inputs/outputs tends to be multiphasic and cannot be captured by simple deterministic models. Presently, coarse-grained models based on the ‘molecular clutch’ treatment139,149,151 can account for IAC mechanosensitivity to substrate rigidity, integrin sub-types, and ligand-spacing at the bulk population level197,198. However, a significant gap remains between the ‘understandable’ models which inevitably involve too much abstraction of molecular-scale intricacies and the atomistic molecular dynamics simulations which are still limited in time-scale, size-scale, and length-scale. Theoretical development bridging these multi-scale structure and dynamics will be crucial for further progress in understanding IAC structure-function.
Several lines of evidence, including detailed single-molecule analysis of mechanical loads, point to the tendency of cells to overcompensate for initial mechanical stimuli. For example, mature IACs in cells, and in Drosophila myotendinous junctions, appear to contain an excess of integrins or force-bearing proteins such as talin, such that a significant proportion of these molecules barely convey mechanical loads139,140,145,147. This bias toward over-constructing mechanical structures may have adaptation advantages especially when considering that mechanical inputs that cells experience can be nearly instantaneous, far faster than the response time of biochemical feedbacks. Thus, pre-assembled mechanical structures may enable cells to counteract these mechanical and structural challenges adequately, preventing irreparable mechanically-induced cell damage. IAC over-maturation thus may serve as a feed forward control whereby cells anticipate and preadapt for further mechanical insults. In other words, overcompensated IAC assembly may provide an ‘insurance’ mechanism against mechanical disruption of tissue cells. In conjunction with this, active pathways that attenuate or disassemble IACs (such as integrin inactivation, degradation, trafficking, and turnover9,53,177) can be expected to play key roles in maintaining proper homeostatic balance. However, more work is needed to clarify this aspect of IAC regulation, especially in complex in vivo settings, and in how pathological conditions may arise from their dysregulations [Box 4].
Box 4 |. Dysregulation of integrin adhesions.
Given their diverse roles in cell behaviour, it is unsurprising that perturbations in IAC composition, assembly or function are linked to a broad range of pathologies including developmental disorders, cardiovascular disease, inflammation, fibrosis and cancer6,8, and that integrins are potential therapeutic targets in these areas13.
Diseases linked to mutations in IAC components.
Mutations in at least 52 adhesome genes are reported to cause specific genetic diseases222. In the case of integrins, loss-of-function mutations in either subunit of the platelet integrin αIIbβ3, which normally binds fibrinogen to support platelet aggregation, causes the bleeding disorder Glanzmann thrombasthenia226. Notably, β3 also pairs with αv subunits and β3 mutations are also associated with cardiovascular disorders and increased cancer risk222. Mutations in the α7 integrin disrupts ECM-attachment of muscle cells, resulting in muscular dystrophy222, suggesting that integrin-mediated attachments operate in parallel with the dystrophin-associated glycoprotein complex, with disruption of either leading to cycles of mechanical failures causing muscular dystrophies. Indeed, viral delivery of the α7 gene prolongs survival in mouse models of Duchenne muscular dystrophy227. Loss of the hemidesmosomal (Box 1) integrin α6β4 results in defective keratinocyte adhesion to basement membrane laminins, leading to the skin blistering disease junctional epidermolysis bullosa222,228. Notably, keratinocytes also adhere to the basement membrane via FA-localised α3β1 integrins and loss-of-function α3 mutations in humans and mice results in skin fragility as well as lung and kidney defects222,228. Finally, loss-of-function mutations in β2 integrins impairs leukocyte adhesion to the endothelium, preventing extravasation from the bloodstream, causing the rare primary immunodeficiency, leukocyte adhesion deficiency (LAD) type I223. LAD can also arise due to mutations in cystic fibrosis transmembrane conductance regulator (CFTR) that, for unknown reasons, impair monocyte integrin activation (LAD-IV)223,229, while LAD-III, which combines features of both LAD-I and Glanzmann thrombasthenia, occurs due to defective activation of haematopoietic β1, β2 and β3 integrins, arising from loss of the integrin-activating protein kindlin-320,33,222,223. Similarly, loss of the epithelial-restricted kindlin-1 causes Kindler syndrome, a disorder characterised by skin blistering, poikiloderma and photosensitivity caused by defective keratinocyte adhesion to the epidermal basement membrane20,33,222. In addition to impairing cell adhesion, it is conceivable that IAC protein mutations may drive disease by altering force transmission, cytoskeletal linkage and signalling. For example, mutations in filamins, vinculin, metavinculin, α-actinins and ZASP are linked to cardiomyopathies, myopathies, skeletal and connective tissue disorders and kidney disease222,230. The central role of adhesion in cell behaviour, makes it likely that disease-associated IAC protein mutations will continue to be uncovered.
IACs in Fibrosis.
Pathological fibrosis arises due to dysregulation of normal tissue repair processes that locally deposit fibronectin and collagens, increase tissue stiffness, and stimulate cell contractility during wound healing and tissue remodelling, and has been implicated in 45% of deaths in the industrialised world231,232. Various stimuli, but particularly chronic inflammation, drive fibrosis and this involves communication between multiple cell types231,232. The major pro-fibrogenic cytokine, transforming growth factor-β (TGFβ) is stored as an ECM-bound non-covalent complex of the latency associated peptide (LAP) and the proteolytically processed TGFβ cytokine, and regulated, site-specific integrin αvβ1, αvβ3, αvβ5, αvβ6 or αvβ8 binding to the LAP triggers either mechanical or force-independent TGFβ release24,231–233 permitting binding and activation of TGFβ receptors. Small molecule αv integrin inhibitors are therefore potential novel oral therapeutics for fibrotic disorders13,231,232. IAC signalling in response to binding the stiff fibrotic matrix also leads to alterations in cell morphology, contractility, migration and proliferation that contribute to fibrotic disease7,231,232.
Adhesion remodelling in aging.
Connections from integrin-based cell adhesion to cellular senescence have been inferred in multiple pathological contexts, including cardiovascular diseases and fibrosis234–237, but the underlying molecular mechanisms are still emerging. Senescence is associated with morphological changes characteristic of cells adhering to stiff ECM (e.g. increased spreading area, reduced cell motility, increased FAK and paxillin phosphorylation, and high Rac1 and cdc42 activity)238. Signalling from multiple integrins has been implicated in pro-senescent responses. Integrin β3 expression is increased in senescent fibroblasts, activating the TGF-β pathway driving senescent phenotypes239. Binding of heparan sulphate proteoglycan and CCN1 by integrin α6β1 induces p53 and Rac1-Nox1 signalling leading to ROS-dependent activation of the pRb DNA damage response pathway that leads to fibroblast senescence235. Endothelial cells senescence increases traction forces, generating prominent actin stress fibres and FA, impairing remodelling and reorientation in response to shear flow240,241. In one recent study, age-associated loss of the FA-localised βPIX/GIT complex correlates with induced cellular senescence, via increased calpain cleavage of amphiphysin1. This results in reduced clathrin-mediated integrin endocytosis disrupting integrin signalling and ROS production. GIT1/2 competes with calpain cleavage of paxillin counteracting this effect242. Interestingly, βPIX is enriched in nascent adhesions and negatively regulates FA maturation243. Thus, mechanotransduction pathways associated with FA maturation could be coupled to senescence, however, the extent to which the senescent-associated hyperadhesive state contributes to senescence progression, rather than occurring as a consequence of aging-associated dysfunction, has not been fully characterised.
Conclusions and perspectives
It is now broadly appreciated that the dynamic, mechanosensitive behaviour of IAC components is essential in sensing the mechanobiological status of cells and in coordinating a host of cellular processes with the local mechanical environment. Many advances have relied on detailed biochemical, biophysical and proteomic studies in well-developed cell culture models, such as fibroblasts spreading on rigid model ECMs, but the task of leveraging what we have learned at the molecular and cellular scales into in vivo functional and pathological insights remain very much a work in progress. A common thread emerging from various molecular-scale interrogations is the tendency of mechanosensitive IAC assemblies to overshoot the input mechanical stimuli. In this light, aspects of physiological dysfunctions associated with IAC dysregulation, such as in fibrosis, aging, or cancer metastasis, could perhaps be seen as arising when the pro-migratory, pro-contractility mechanosensitive apparatus is out of control. How cells tune mechanosensitive circuitry to calibrate their proper mechanobiological responses in vivo is an important question that needs greater definition. In physiological settings, IACs are present and essential but their manifestations are highly dependent on cell type and tissue contexts, and often highly divergent from IACs in cell culture. We consider it likely that the structural organisation and behaviour of IAC modular nanoclusters is maintained and functionally relevant in tissue cells, but bringing nanoscale experimental precision [Box 2] to either the in vivo context or to otherwise sufficiently complex experimental model systems remains technically challenging. Nonetheless, continued studies probing the similarities and differences between adhesion organization in 2D and 3D matrix are essential. We also note that, to date, detailed investigations in IACs have focused overwhelmingly on a subset of the 24 known mammalian integrins, predominantly α5β1, αvβ3, αIIbβ3 and a few leukocyte integrins. Given that virtually all in vivo ECMs are heterogeneous mixtures, that most cells express multiple integrin subtypes, and the crosstalk between different integrins and between integrins and non-integrin co-receptors73, it is virtually guaranteed that many salient aspects of in vivo IACs are yet to be discovered. Technologically sophisticated but relatively low throughput techniques have been instrumental for dissecting IAC structure, functions, and dynamics thus far, which perhaps in part limit the range of model systems studied. Future efforts to streamline, multiplex, and democratise advanced experimental techniques will be key to tackling the complexity of IAC functions at tissue and organismal levels.
Supplementary Material
Supplementary Table 1
Comparison of consensus adhesome from meta-analysis of enriched IAC proteomes, proximity-dependent adhesome, and super-resolution microscopy-based analysis.
The consensus adhesome list of 60 IAC components40 are sorted based on their physical organization as determined by super-resolution microscopy59,85,87–89, proximity-dependent adhesome bait clustering50, and the putative signaling axes as determined from the meta-adhesome and proximity-dependent adhesome40,50. B1–5: Bait cluster from proximity-dependent adhesome analysis. ISL: Integrin Signaling Layer; FTL: Force Transduction Layer; ARL: Actin Regulatory Layer.
Acknowledgements
PK acknowledges funding support from the Mechanobiology Institute intramural fund, Singapore Ministry of Education Academic Research Fund Tier 2 (MOE2019-T2-2-014) and Tier 3 (MOE-T3-2020-0001). DAC acknowledges funding support from the National Institutes of Health (R01GM134148 and R01GM138411).
Glossary
- FERM domain
The band 4.1, ezrin, radixin and moesin domain is found in various cytoskeletal-associated proteins, and typically contains three subdomains (F1, F2 and F3) arranged in a cloverleaf formation
- Fragment antigen-binding (Fab)
A subregion of antibody molecule that contain the antigen recognition sites, which can be proteolytically cleaved and isolated from whole antibody molecule for monovalent labeling of specific epitopes
- mass spectrometry proteomics
Analytical techniques allowing the identification of proteins in complex samples on the basis of the mass to charge ratios of charged fragments
- cortical microtubule stabilising complex
Micrometer-sized, patch-like, multi-protein complexs localised in proximity to FA and containing LL5β, liprins and ELKS adaptor proteins. Responsible for tethering microtubules at the cell cortex and linking them to FA via KANK
- conformation-specific monoclonal antibodies
Antibody that recognise the specific conformation or target proteins and thus can serve as reporters for protein activation state or to selectively stabilise certain conformational states. E.g. antibodies that recognise activated integrin α5β1
- micelle
Particle suspension usually formed by segregation of hydrophobic moiety in lipid-like molecule away from aqueous phase. The dimensions of micelle particles can be precisely determined by the molecular structure of the lipid, thus close-packing of micelle particles can be applied to achieve patterning at the nanoscale
- supported lipid bilayer
An experimental platform using fluid biological membranes formed on glass substrates to facilitate imaging-based study of membrane-dependent processes
- RGD ligands
Proteins or peptides containing the tripeptide arginine, glycine, aspartate motif that serves as an integrin binding site in ECM proteins such as fibronectin and vitronectin
- fluorescence anisotropy
A measurement of the fluorescence emission intensity differences along different polarisation axes which can be used to estimate molecular orientation
- Glycosylphosphatidylinositol (GPI)-anchored proteins
Peripheral membrane proteins that are anchored to the extracellular leaflet of the plasma membrane via posttranslational modification that conjugate the protein C-terminus with polysaccharide linkage to GPI membrane lipid
- membrane rafts
Relatively ordered lateral domains (10–200 nm) in the plasma membranes that are enriched in cholesterol and sphingolipids
- Syndecan
A family of homodimeric transmembrane proteoglycans that serve as receptors to ECM and growth factors via their extracellular glycosaminoglycan chains, and which contain small cytoplasmic domains with interaction sites for Src and Fyn tyrosine kinases and the PDZ (Post-synaptic-density-95/disc large tumour suppressor/zonula occludens-1) protein-protein interaction domain
- Tetraspanin
A family of transmembrane proteins containing four transmembrane helices that can be organized into tetraspanin-enriched microdomains (TEMs) within the plasma membranes and which form direct protein-protein interactions with a wide range of proteins including multiple integrins
- immuno-Electron Microscopy
An electron microscopy technique for localising proteins in ultrastructure, in which specific biomolecules are identified using antibodies conjugated with electron dense nanoparticles
- cryptic vinculin binding sites
Amphipathic α-helix with high binding affinity to vinculin head domain that are sterically sequestered by folded protein tertiary structure, but which can be exposed upon mechanically induced protein conformational changes
- pleckstrin Homology (PH) domain
Protein domains with a binding site for phosphatidylinositol glycolipid which confer the targeting ability to various cellular membrane compartments
- conformationally autoinhibited protein
Proteins whose active site can bind to a different domain of the same protein. Thus, conformational changes to alleviate active site blockage is required to activate its biological activity
- glycocalyx
Carbohydrate-rich biopolymer matrix associated with the exterior plasma membrane that plays important roles in cellular protection, cell-cell signalling, adhesion, and recognition
- mechanically activated ion channels
Mechanically activated ion channels are transmembrane protein pores whose opening for specific ion conductance directly depends upon mechanical force, and which include Calcium channels such as Piezo family, members of the Transient Receptor Potential (TRP) superfamily such as TRPV4, and Potassium channels such as TREK1–2 and TRAAK. Additionally, certain ligand-gated or voltage-dependent ion channels such as Kv1.1, Nav1.5, and Hv1 have been shown to modulated by mechanical force
- calpain
Calcium-dependent proteases that cleave specific sites in a number of IAC components such as talin, FAK, and paxillin
- proton antiporter
Integral membrane proteins that regulate of intracellular pH by coupling the export of proton with the import of cations into cells. Examples include sodium-hydrogen antiporter-1 (NHE-1)
- catch and slip bond
Intermolecular interface whose bond strength (typically measured as bond lifetime) varies as a function of force. With higher forces slip bonds become weaker (i.e. shorter lifetime) and catch bonds become stronger (i.e. longer lifetime)
- arp2/3
A heteroheptameric protein complex that promote the formation of branched actin networks through filamentous actin nucleation and branching activities
- formin
A family of proteins that promote processive actin polymerisation leading to the linear elongation of filamentous actin; examples include diaphanous formins (mDia1–3)
- molecular clutch
A physical model describing mechanical force transmission between cell surface receptors through the flexible adaptor (or clutch) molecules that dynamically connect to actin or other cytoskeletal networks
- liquid-liquid phase separation (LLPS)
A dynamically reversible de-mixing process in which spatially distinct phases of liquids are formed with different composition and physicochemical properties
- p130Cas
130-kDa Crk-Associted Substrate (Cas) protein is an important scaffold protein encoded by BCAR1 (Breast Cancer AntiEstrogen Resistance 1) gene. p130Cas contains numerous phosphorylation sites for Src-family kinases and serine/threonine kinases, a proline rich region, Src Homology 3 (SH3) domain, and binding sites to numerous IAC components such as FAK, paxillin and kindlin
- durotaxis
Directional cell migration along a gradient of ECM rigidity, typically toward regions of higher stiffness
- lim domain
Protein domains found in a family of IAC proteins such as zyxin, paxillin, and LIMD1, that are capable of selectively binding to actin filaments under mechanical tension, named after LIN-11, ISL1 and MEC-3 proteins
- intrinsically disordered region (IDR)
Polypeptide segments that lack ordered conformation. IDR play diverse and important roles in protein functions including serving as flexible linkers between protein domains, forming ligand recognition sites, and participating in liquid-liquid phase separation
- pH-sensitive Fluorescent protein
Variants of fluorescent proteins engineered to be sensitive to pH – useful in assessing vesicular traffic as fluorescence is quenched at the low pH of the vesicle lumen and restored at neutral pH following vesicular fusion
- small GTPases
A large superfamily of small (20–25 kDa) signalling proteins that serve as key signalling hubs for a broad range of cellular processes by interacting with many partner proteins in a GTP regulated manner. Key small GTPases that influence IAC and cellular mechanobiology include Rho family proteins such as Rac1, RhoA, and Cdc42
- frustrated endocytosis
A modification of the classical clathrin-mediated endocytosis where a mechanical obstruction prevents endocytic structures from forming an invagination, resulting in long-lived, clathrin-coated, plasma membrane structures
- myotendinous junction
Contact zone between muscles and tendon consisting of highly involuted muscle cell plasma membrane and serving as the main force transmission interface
- feed forward
In feed forward control, a signal is transmitted to other part of the system to prepare for predicted future inputs, in contrast to feedback in which a signal arise in response to past inputs
- fibrinogen
Blood clotting factors which are cleaved by thrombin to form fibrin that form blood clot
- cystic fibrosis transmembrane conductance regulator (CFTR)
Transmembrane glycoprotein functioning as a chloride channel. Mutations in the CFTR gene cause cystic fibrosis and leukocyte adhesion deficiency type IV
- poikiloderma
Skin condition characterised by patches or hyper- or hypo-pigmentation associated with atrophy
- latency associated peptide (LAP)
LAP, the heavily glycosylated disulphide-linked dimer of the pro-peptide of the TGFβ cytokine, binds TGFβ preventing its interaction with its receptors
- senescence-associated secretory phenotypes
The secretion of a broad array of signalling molecules by senescent cells, including pro-inflammtory interleukins IL-1 and IL-6, chemokines, and extracellular proteases
- Senescence
Proliferative arrest of cells during aging or in response to various stimuli such as genome instability or DNA damage. Contributes to developmental morphogenesis, suppresses tumour formation, and limits fibrosis but in aging cells reduces cellular functions and regenerative capacities
- βPIX
A protein encoded by ARHGEF7 gene, also known as, p21-activated protein kinase exchange factor β, which can interact with GIT dimers and activate Rac1 and Cdc42 through its GTP exchange factor activitiy
- GIT
GIT1 and GIT2 are related ARF GTPase Activating proteins which form stable complex with βPIX and play important roles in regulating small GTPases signalling
- Reactive Oxygen species (ROS)
Byproducts of oxidative respiration by mitochondria such as hydrogen peroxide play important roles in normal cellular processes including cell signalling and migration. Excess ROS production gives rise to oxidative stress and damages cellular structure
- Src-family kinase
A family of multi-domain signalling and adaptor proteins containing a tyrosine kinase domain, Src Homology domain 2 (SH2) which binds phosphorylated tyrosine, and Src Homology domain 3 (SH3) which binds proline-rich peptide motif
- phosphoinositides
Membrane phospholipids with phosphorylated inositol headgroups, e.g., PIP2 (phosphatidyl inositol (4,5) bisphosphate) and PIP3 (phosphatidyl inositol (3,4,5) trisphosphate), recognised by different protein domains and important in signalling, vesicular trafficking, and cellular compartmentalisation
- dystrophin-associated glycoprotein complex
Multi-protein complex found in a variety of tissues that supports cell adhesion to laminin and linkage to the cytoskeleton. α-dystroglycan binds laminin and associates with the transmembrane protein β-dystroglycan which engages the intracellular actin-binding adaptor protein dystrophin. Mutations in the complex are associated with muscular dystrophies and cardiomyopathies
- basement membrane
Sheet of ECM rich in laminins and type IV collagen to which epithelial and endothelial layers adhere. The basement membrane (sometimes termed the basal lamina) forms a barrier between tissues and provides a structural support and important signalling cues to epithelial and endothelial cells
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Humphrey JD, Dufresne ER & Schwartz MA Mechanotransduction and extracellular matrix homeostasis. Nature reviews. Molecular cell biology 15, 802–812, doi: 10.1038/nrm3896 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.SenGupta S, Parent CA & Bear JE The principles of directed cell migration. Nature reviews. Molecular cell biology 22, 529–547, doi: 10.1038/s41580-021-00366-6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romani P, Valcarcel-Jimenez L, Frezza C.& Dupont S.Crosstalk between mechanotransduction and metabolism. Nature reviews. Molecular cell biology 22, 22–38, doi: 10.1038/s41580-020-00306-w (2021). [DOI] [PubMed] [Google Scholar]
- 4.Cooper J.& Giancotti FG Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 35, 347–367, doi: 10.1016/j.ccell.2019.01.007 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnans C, Chou J.& Werb Z.Remodelling the extracellular matrix in development and disease. Nature reviews Molecular cell biology 15, 786–801 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kechagia JZ, Ivaska J.& Roca-Cusachs P.Integrins as biomechanical sensors of the microenvironment. Nature reviews. Molecular cell biology 20, 457–473, doi: 10.1038/s41580-019-0134-2 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Hayward MK, Muncie JM & Weaver VM Tissue mechanics in stem cell fate, development, and cancer. Dev Cell 56, 1833–1847, doi: 10.1016/j.devcel.2021.05.011 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamidi H.& Ivaska J.Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer 18, 533–548, doi: 10.1038/s41568-018-0038-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chastney MR, Conway JRW & Ivaska J.Integrin adhesion complexes. Current biology : CB 31, R536–R542, doi: 10.1016/j.cub.2021.01.038 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Bachmann M, Kukkurainen S, Hytonen VP & Wehrle-Haller B.Cell Adhesion by Integrins. Physiol Rev 99, 1655–1699, doi: 10.1152/physrev.00036.2018 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Kadry YA & Calderwood DA Chapter 22: Structural and signaling functions of integrins. Biochim Biophys Acta Biomembr 1862, 183206, doi: 10.1016/j.bbamem.2020.183206 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humphries JD, Chastney MR, Askari JA & Humphries MJ Signal transduction via integrin adhesion complexes. Curr Opin Cell Biol 56, 14–21, doi: 10.1016/j.ceb.2018.08.004 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Slack RJ, Macdonald SJF, Roper JA, Jenkins RG & Hatley RJD Emerging therapeutic opportunities for integrin inhibitors. Nat Rev Drug Discov, doi: 10.1038/s41573-021-00284-4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamada KM, Doyle AD & Lu J.Cell-3D matrix interactions: recent advances and opportunities. Trends Cell Biol, doi: 10.1016/j.tcb.2022.03.002 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouvard D.et al. Functional consequences of integrin gene mutations in mice. Circulation research 89, 211–223 (2001). [DOI] [PubMed] [Google Scholar]
- 16.Julich D, Geisler R.& Holley SA Integrinalpha5 and delta/notch signaling have complementary spatiotemporal requirements during zebrafish somitogenesis. Dev.Cell 8, 575–586 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Williams BD & Waterston RH Genes critical for muscle development and function in Caenorhabditis elegans identified through lethal mutations. J.Cell Biol 124, 475–490 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeSimone DW, Dzamba B.& Davidson LA Using Xenopus embryos to investigate integrin function. Methods Enzymol 426, 403–414, doi: 10.1016/S0076-6879(07)26017-3 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Maartens AP & Brown NH Anchors and signals: the diverse roles of integrins in development. Curr Top Dev Biol 112, 233–272, doi: 10.1016/bs.ctdb.2014.11.020 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Sun Z, Costell M.& Fassler R.Integrin activation by talin, kindlin and mechanical forces. Nat Cell Biol 21, 25–31, doi: 10.1038/s41556-018-0234-9 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Luo BH, Carman CV & Springer TA Structural Basis of Integrin Regulation and Signaling. Annu.Rev.Immunol (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell ID & Humphries MJ Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol 3, doi: 10.1101/cshperspect.a004994 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cormier A.et al. Cryo-EM structure of the alphavbeta8 integrin reveals a mechanism for stabilizing integrin extension. Nat Struct Mol Biol 25, 698–704, doi: 10.1038/s41594-018-0093-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell MG et al. Cryo-EM Reveals Integrin-Mediated TGF-beta Activation without Release from Latent TGF-beta. Cell 180, 490–501 e416, doi: 10.1016/j.cell.2019.12.030 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schumacher S.et al. Structural insights into integrin alpha5beta1 opening by fibronectin ligand. Sci Adv 7, doi: 10.1126/sciadv.abe9716 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nesic D.et al. Electron microscopy shows that binding of monoclonal antibody PT25–2 primes integrin alphaIIbbeta3 for ligand binding. Blood Adv 5, 1781–1790, doi: 10.1182/bloodadvances.2020004166 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorrentino S.et al. Structural analysis of receptors and actin polarity in platelet protrusions. Proc Natl Acad Sci U S A 118, doi: 10.1073/pnas.2105004118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore TI, Aaron J, Chew TL & Springer TA Measuring Integrin Conformational Change on the Cell Surface with Super-Resolution Microscopy. Cell Rep 22, 1903–1912, doi: 10.1016/j.celrep.2018.01.062 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J.et al. Conformational equilibria and intrinsic affinities define integrin activation. EMBO J 36, 629–645, doi: 10.15252/embj.201695803 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J.& Springer TA Integrin extension enables ultrasensitive regulation by cytoskeletal force. Proc Natl Acad Sci U S A 114, 4685–4690, doi: 10.1073/pnas.1704171114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, Yan J.& Springer TA Low-affinity integrin states have faster ligand-binding kinetics than the high-affinity state. Elife 10, doi: 10.7554/eLife.73359 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mould AP et al. Evidence that monoclonal antibodies directed against the integrin beta subunit plexin/semaphorin/integrin domain stimulate function by inducing receptor extension. J.Biol.Chem 280, 4238–4246 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calderwood DA, Campbell ID & Critchley DR Talins and kindlins: partners in integrin-mediated adhesion. Nature reviews. Molecular cell biology 14, 503–517, doi: 10.1038/nrm3624 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tadokoro S.et al. Talin binding to integrin b tails: a final common step in integrin activation. Science 302, 103–106 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Lagarrigue F, Kim C.& Ginsberg MH The Rap1-RIAM-talin axis of integrin activation and blood cell function. Blood 128, 479–487, doi: 10.1182/blood-2015-12-638700 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geiger T.& Zaidel-Bar R.Opening the floodgates: proteomics and the integrin adhesome. Curr Opin Cell Biol 24, 562–568, doi: 10.1016/j.ceb.2012.05.004 (2012). [DOI] [PubMed] [Google Scholar]
- 37.Whittaker CA et al. The echinoderm adhesome. Dev Biol 300, 252–266, doi: 10.1016/j.ydbio.2006.07.044 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R.& Geiger B.Functional atlas of the integrin adhesome. Nat Cell Biol. 9, 858–867 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Humphries JD et al. Proteomic analysis of integrin-associated complexes identifies RCC2 as a dual regulator of Rac1 and Arf6. Sci.Signal 2, ra51, doi:2/87/ra51 [pii]; 10.1126/scisignal.2000396 [doi] (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horton ER et al. Definition of a consensus integrin adhesome and its dynamics during adhesion complex assembly and disassembly. Nat Cell Biol 17, 1577–1587, doi: 10.1038/ncb3257 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atkinson SJ et al. The beta3-integrin endothelial adhesome regulates microtubule-dependent cell migration. EMBO Rep 19, doi: 10.15252/embr.201744578 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maddala R.& Rao PV Global phosphotyrosinylated protein profile of cell-matrix adhesion complexes of trabecular meshwork cells. Am J Physiol Cell Physiol 319, C288–C299, doi: 10.1152/ajpcell.00537.2019 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paradzik M.et al. KANK2 Links alphaVbeta5 Focal Adhesions to Microtubules and Regulates Sensitivity to Microtubule Poisons and Cell Migration. Front Cell Dev Biol 8, 125, doi: 10.3389/fcell.2020.00125 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Randles MJ et al. Basement membrane ligands initiate distinct signalling networks to direct cell shape. Matrix Biol 90, 61–78, doi: 10.1016/j.matbio.2020.02.005 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lock JG et al. Reticular adhesions are a distinct class of cell-matrix adhesions that mediate attachment during mitosis. Nat Cell Biol 20, 1290–1302, doi: 10.1038/s41556-018-0220-2 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Horton ER et al. The integrin adhesome network at a glance. J Cell Sci 129, 4159–4163, doi: 10.1242/jcs.192054 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong JM et al. Proximity biotinylation provides insight into the molecular composition of focal adhesions at the nanometer scale. Sci Signal 9, rs4, doi: 10.1126/scisignal.aaf3572 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Myllymaki SM et al. Assembly of the beta4-Integrin Interactome Based on Proximal Biotinylation in the Presence and Absence of Heterodimerization. Mol Cell Proteomics 18, 277–293, doi: 10.1074/mcp.RA118.001095 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Te Molder L, Hoekman L, Kreft M, Bleijerveld O.& Sonnenberg A.Comparative interactomics analysis reveals potential regulators of alpha6beta4 distribution in keratinocytes. Biol Open 9, doi: 10.1242/bio.054155 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chastney MR et al. Topological features of integrin adhesion complexes revealed by multiplexed proximity biotinylation. J Cell Biol 219, doi: 10.1083/jcb.202003038 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mekhdjian AH et al. Integrin-mediated traction force enhances paxillin molecular associations and adhesion dynamics that increase the invasiveness of tumor cells into a three-dimensional extracellular matrix. Mol Biol Cell 28, 1467–1488, doi: 10.1091/mbc.E16-09-0654 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rahikainen R, Ohman T, Turkki P, Varjosalo M.& Hytonen VP Talin-mediated force transmission and talin rod domain unfolding independently regulate adhesion signaling. J Cell Sci 132, doi: 10.1242/jcs.226514 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Bouchet BP et al. Talin-KANK1 interaction controls the recruitment of cortical microtubule stabilizing complexes to focal adhesions. Elife 5, doi: 10.7554/eLife.18124 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun Z.et al. Kank2 activates talin, reduces force transduction across integrins and induces central adhesion formation. Nat Cell Biol 18, 941–953, doi: 10.1038/ncb3402 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rafiq NBM et al. A mechano-signalling network linking microtubules, myosin IIA filaments and integrin-based adhesions. Nat Mater 18, 638–649, doi: 10.1038/s41563-019-0371-y (2019). [DOI] [PubMed] [Google Scholar]
- 56.Choi CK et al. Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nature cell biology 10, 1039–1050, doi: 10.1038/ncb1763 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bertocchi C, Goh WI, Zhang Z.& Kanchanawong P.Nanoscale imaging by superresolution fluorescence microscopy and its emerging applications in biomedical research. Crit Rev Biomed Eng 41, 281–308 (2013). [DOI] [PubMed] [Google Scholar]
- 58.Case LB et al. Molecular mechanism of vinculin activation and nanoscale spatial organization in focal adhesions. Nature cell biology, doi: 10.1038/ncb3180 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kanchanawong P.et al. Nanoscale architecture of integrin-based cell adhesions. Nature 468, 580–584 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rossier O.et al. Integrins β1 and β3 exhibit distinct dynamic nanoscale organizations inside focal adhesions. Nature cell biology 14, 1057–1067 (2012). [DOI] [PubMed] [Google Scholar]
- 61.Spiess M.et al. Active and inactive β1 integrins segregate into distinct nanoclusters in focal adhesions. 217, 1929–1940 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bachir AI et al. Integrin-associated complexes form hierarchically with variable stoichiometry in nascent adhesions. Curr Biol 24, 1845–1853, doi: 10.1016/j.cub.2014.07.011 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cavalcanti-Adam EA et al. Cell spreading and focal adhesion dynamics are regulated by spacing of integrin ligands. Biophysical journal 92, 2964–2974 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arnold M.et al. Activation of integrin function by nanopatterned adhesive interfaces. ChemPhysChem 5, 383–388 (2004). [DOI] [PubMed] [Google Scholar]
- 65.Yu C. h., Law JBK, Suryana M, Low HY & Sheetz MP Early integrin binding to Arg-Gly-Asp peptide activates actin polymerization and contractile movement that stimulates outward translocation. Proceedings of the National Academy of Sciences 108, 20585–20590 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iskratsch T.et al. FHOD1 is needed for directed forces and adhesion maturation during cell spreading and migration. 27, 545–559 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kalappurakkal JM et al. Acto-myosin driven functional nanoclusters of GPI-anchored proteins are generated by integrin receptor signaling. bioRxiv doi: 10.1101/232223 Accepted in Cell.(2019), doi:doi: 10.1101/232223 Accepted in Cell.(2019) (2017). [DOI] [Google Scholar]
- 68.Sezgin E, Levental I, Mayor S.& Eggeling C.The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nature Reviews Molecular Cell Biology 18, 361 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Changede R, Xu X, Margadant F.& Sheetz MP Nascent integrin adhesions form on all matrix rigidities after integrin activation. Developmental Cell 35, 614–621 (2015). [DOI] [PubMed] [Google Scholar]
- 70.Morgan MR, Humphries MJ & Bass MD Synergistic control of cell adhesion by integrins and syndecans. Nature reviews 8, 957–969, doi: 10.1038/nrm2289 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Powner D, Kopp PM, Monkley SJ, Critchley DR & Berditchevski F.Tetraspanin CD9 in cell migration. Biochem Soc Trans 39, 563–567, doi: 10.1042/BST0390563 (2011). [DOI] [PubMed] [Google Scholar]
- 72.Yáñez-Mó M, Barreiro O, Gordon-Alonso M, Sala-Valdés M.& Sánchez-Madrid F. J. T. i. c. b. Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. 19, 434–446 (2009). [DOI] [PubMed] [Google Scholar]
- 73.Ivaska J, Heino J. J. A. r. o. c.& biology d.Cooperation between integrins and growth factor receptors in signaling and endocytosis. 27, 291–320 (2011). [DOI] [PubMed] [Google Scholar]
- 74.del Pozo MA et al. Integrins regulate Rac targeting by internalization of membrane domains. 303, 839–842 (2004). [DOI] [PubMed] [Google Scholar]
- 75.Hemler M. E. J. N. r. M. c. b. Tetraspanin functions and associated microdomains. 6, 801–811 (2005). [DOI] [PubMed] [Google Scholar]
- 76.Changede R, Cai H, Wind SJ & Sheetz MPJN m. Integrin nanoclusters can bridge thin matrix fibres to form cell–matrix adhesions. 18, 1366–1375 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.LaFlamme SE, Thomas LA, Yamada SS & Yamada KM Single subunit chimeric integrins as mimics and inhibitors of endogenous integrin functions in receptor localization, cell spreading and migration, and matrix assembly. J.Cell Biol 126, 1287–1298 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Critchley DR Biochemical and structural properties of the integrin-associated cytoskeletal protein talin. Annu Rev Biophys 38, 235–254 (2009). [DOI] [PubMed] [Google Scholar]
- 79.Chen W-T & Singer S.Immunoelectron microscopic studies of the sites of cell-substratum and cell-cell contacts in cultured fibroblasts. The Journal of cell biology 95, 205–222 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Franz CM & Muller DJ Analyzing focal adhesion structure by atomic force microscopy. Journal of cell science 118, 5315–5323 (2005). [DOI] [PubMed] [Google Scholar]
- 81.Patla I.et al. Dissecting the molecular architecture of integrin adhesion sites by cryo-electron tomography. Nature cell biology 12, 909–915, doi: 10.1038/ncb2095 (2010). [DOI] [PubMed] [Google Scholar]
- 82.Paszek MJ et al. Scanning angle interference microscopy reveals cell dynamics at the nanoscale. Nature Methods 9, 825–827, doi: 10.1038/nmeth.2077 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shtengel G.et al. Imaging cellular ultrastructure by PALM, iPALM, and correlative iPALM-EM. Methods Cell Biol 123, 273–294, doi: 10.1016/B978-0-12-420138-5.00015-X (2014). [DOI] [PubMed] [Google Scholar]
- 84.Xia S, Yim EK & Kanchanawong P.Molecular organization of integrin-based adhesion complexes in mouse Embryonic Stem Cells. ACS Biomaterials Science & Engineering DOI: 10.1021/acsbiomaterials.8b01124 (2019). [DOI] [PubMed] [Google Scholar]
- 85.Liu J.et al. Talin determines the nanoscale architecture of focal adhesions. Proceedings of the National Academy of Sciences 112, E4864–E4873 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kanchanawong P.& Waterman CM Localization-based super-resolution imaging of cellular structures. Methods Mol Biol 1046, 59–84, doi: 10.1007/978-1-62703-538-5_4 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stubb A.et al. Superresolution architecture of cornerstone focal adhesions in human pluripotent stem cells. Nat Commun 10, 4756, doi: 10.1038/s41467-019-12611-w (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Orré T.et al. Molecular motion and tridimensional nanoscale localization of kindlin control integrin activation in focal adhesions. Nat Commun 12, 1–17 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xia S, Yim EKF & Kanchanawong P.Molecular Organization of Integrin-Based Adhesion Complexes in Mouse Embryonic Stem Cells. ACS Biomaterials Science & Engineering 5, 3828–3842, doi: 10.1021/acsbiomaterials.8b01124 (2019). [DOI] [PubMed] [Google Scholar]
- 90.Bouissou A.et al. Podosome force generation machinery: a local balance between protrusion at the core and traction at the ring. 11, 4028–4040 (2017). [DOI] [PubMed] [Google Scholar]
- 91.del Rio A.et al. Stretching single talin rod molecules activates vinculin binding. Science (New York, N.Y 323, 638–641 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barnett SF & Kanchanawong P.Visualizing the ‘backbone’of focal adhesions. Emerging Topics in Life Sciences 2, 677–680 (2018). [DOI] [PubMed] [Google Scholar]
- 93.Klapholz B.et al. Alternative mechanisms for talin to mediate integrin function. Current biology : CB 25, 847–857, doi: 10.1016/j.cub.2015.01.043 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peng X, Nelson ES, Maiers JL & DeMali KA New insights into vinculin function and regulation. Int Rev Cell Mol Biol 287, 191–231, doi: 10.1016/B978-0-12-386043-9.00005-0 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xia S.& Kanchanawong P.in Semin Cell Dev Biol. (Academic Press; ). [DOI] [PubMed] [Google Scholar]
- 96.Vogel V.& Sheetz M.Local force and geometry sensing regulate cell functions. Nature Reviews Molecular Cell Biology 7, 265–275 (2006). [DOI] [PubMed] [Google Scholar]
- 97.Iskratsch T, Wolfenson H.& Sheetz MP Appreciating force and shape [mdash] the rise of mechanotransduction in cell biology. Nature Reviews Molecular Cell Biology 15, 825–833 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hoffman BD, Grashoff C.& Schwartz MA Dynamic molecular processes mediate cellular mechanotransduction. Nature 475, 316–323, doi: 10.1038/nature10316 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gardel ML, Schneider IC, Aratyn-Schaus Y.& Waterman CM Mechanical integration of actin and adhesion dynamics in cell migration. Annu Rev Cell Dev Biol 26, 315–333, doi: 10.1146/annurev.cellbio.011209.122036 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Parsons JT, Horwitz AR & Schwartz MA Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nature reviews. Molecular cell biology 11, 633–643, doi: 10.1038/nrm2957 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jaqaman K, Galbraith JA, Davidson MW & Galbraith CG Changes in single-molecule integrin dynamics linked to local cellular behavior. Molecular biology of the cell 27, 1561–1569 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Paszek MJ et al. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature 511, 319–325, doi: 10.1038/nature13535 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shibata AC et al. Rac1 recruitment to the archipelago structure of the focal adhesion through the fluid membrane as revealed by single‐molecule analysis. Cytoskeleton (Hoboken) 70, 161–177 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tsunoyama TA et al. Super-long single-molecule tracking reveals dynamic-anchorage-induced integrin function. Nat Chem Biol 14, 497 (2018). [DOI] [PubMed] [Google Scholar]
- 105.Kanchanawong P.et al. Analysis of Single Integrin Behavior in Living Cells. Biophysical Journal 98, 558a (2010). [Google Scholar]
- 106.Friedrich O.et al. Stretch in focus: 2D inplane cell stretch systems for studies of cardiac mechano-signaling. 7, 55 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kamble H, Barton MJ, Jun M, Park S.& Nguyen N.-T. J. L. o. a. C. Cell stretching devices as research tools: engineering and biological considerations. 16, 3193–3203 (2016). [DOI] [PubMed] [Google Scholar]
- 108.Strohmeyer N, Bharadwaj M, Costell M, Fässler R.& Müller DJ Fibronectin-bound α5β1 integrins sense load and signal to reinforce adhesion in less than a second. Nat Mater 16, 1262 (2017). [DOI] [PubMed] [Google Scholar]
- 109.Massou S.et al. Cell stretching is amplified by active actin remodelling to deform and recruit proteins in mechanosensitive structures. Nature cell biology 22, 1011–1023 (2020). [DOI] [PubMed] [Google Scholar]
- 110.Murthy SE, Dubin AE & Patapoutian A. J. N.r. M. c. b. Piezos thrive under pressure: mechanically activated ion channels in health and disease. 18, 771–783 (2017). [DOI] [PubMed] [Google Scholar]
- 111.Schwartz MA, Brown EJ & Fazeli BA 50-kda integrin-associated protein is required for integrin-regulated calcium entry in endothelial cells. J.Biol.Chem 268, 19931–19934 (1993). [PubMed] [Google Scholar]
- 112.Carragher N.et al. Calpain 2 and Src dependence distinguishes mesenchymal and amoeboid modes of tumour cell invasion: a link to integrin function. Oncogene 25, 5726–5740 (2006). [DOI] [PubMed] [Google Scholar]
- 113.Franco SJ et al. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nature cell biology 6, 977–983 (2004). [DOI] [PubMed] [Google Scholar]
- 114.Cortesio CL, Boateng LR, Piazza TM, Bennin DA & Huttenlocher A.Calpain-mediated proteolysis of paxillin negatively regulates focal adhesion dynamics and cell migration. Journal of Biological Chemistry 286, 9998–10006 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chan KT, Bennin DA & Huttenlocher A.Regulation of adhesion dynamics by calpain-mediated proteolysis of focal adhesion kinase (FAK). Journal of Biological Chemistry 285, 11418–11426 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saxena M, Changede R, Hone J, Wolfenson H.& Sheetz MP Force-induced calpain cleavage of talin is critical for growth, adhesion development, and rigidity sensing. Nano letters 17, 7242–7251 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schwartz MA, Both G.& Lechene C. J. P. o. t. N. A. o. S. Effect of cell spreading on cytoplasmic pH in normal and transformed fibroblasts. 86, 4525–4529 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schwartz MA, Lechene C.& Ingber DE Insoluble fibronectin activates the Na/H antiporter by clustering and immobilizing integrin alpha 5 beta 1, independent of cell shape. Proceedings of the National Academy of Sciences 88, 7849–7853 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Webb BA, Chimenti M, Jacobson MP & Barber DLJNRC Dysregulated pH: a perfect storm for cancer progression. 11, 671–677 (2011). [DOI] [PubMed] [Google Scholar]
- 120.Pedersen S.& Counillon L.The SLC9A-C mammalian Na+/H+ exchanger family: molecules, mechanisms, and physiology. Physiol Rev 99, 2015–2113 (2019). [DOI] [PubMed] [Google Scholar]
- 121.Srivastava J.et al. Structural model and functional significance of pH-dependent talin-actin binding for focal adhesion remodeling. Proc Natl Acad Sci U S A 105, 14436–14441, doi: 10.1073/pnas.0805163105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Coutino BC, Mayor RJC & Development. Mechanosensitive ion channels in cell migration. 166, 203683 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kefauver J, Ward A.& Patapoutian AJN Discoveries in structure and physiology of mechanically activated ion channels. 587, 567–576 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jin P, Jan LY & Jan Y.-N. J. A. r. o. n. Mechanosensitive ion channels: structural features relevant to mechanotransduction mechanisms. 43, 207–229 (2020). [DOI] [PubMed] [Google Scholar]
- 125.Wolfenson H, Yang B.& Sheetz M. P. J. A. r. o. p. Steps in mechanotransduction pathways that control cell morphology. 81, 585–605 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kong F, García AJ, Mould AP, Humphries MJ & Zhu C.Demonstration of catch bonds between an integrin and its ligand. The Journal of cell biology 185, 1275 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yao M.et al. The mechanical response of talin. Nat Commun 7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vigouroux C, Henriot V.& Le Clainche C.Talin dissociates from RIAM and associates to vinculin sequentially in response to the actomyosin force. Nat Commun 11, 1–11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Le S.et al. Mechanotransmission and mechanosensing of human alpha-actinin 1. Cell Rep 21, 2714–2723 (2017). [DOI] [PubMed] [Google Scholar]
- 130.Rognoni L, Stigler J, Pelz B, Ylänne J.& Rief M.Dynamic force sensing of filamin revealed in single-molecule experiments. Proceedings of the National Academy of Sciences 109, 19679–19684 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Atherton P.et al. Vinculin controls talin engagement with the actomyosin machinery. Nat Commun 6, 10038 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Margadant F.et al. Mechanotransduction in vivo by repeated talin stretch-relaxation events depends upon vinculin. PLoS biology 9, e1001223, doi: 10.1371/journal.pbio.1001223 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hu X.et al. Cooperative Vinculin Binding to Talin Mapped by Time Resolved Super Resolution Microscopy. Nano letters (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Atherton P.et al. Relief of talin autoinhibition triggers a force-independent association with vinculin. Journal of Cell Biology 219 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Han SJ et al. Pre-complexation of talin and vinculin without tension is required for efficient nascent adhesion maturation. Elife 10, e66151 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brockman JM et al. Mapping the 3D orientation of piconewton integrin traction forces. Nature methods 15, 115–118 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Swaminathan V.et al. Actin retrograde flow actively aligns and orients ligand-engaged integrins in focal adhesions. Proceedings of the National Academy of Sciences 114, 10648–10653 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nordenfelt P.et al. Direction of actin flow dictates integrin LFA-1 orientation during leukocyte migration. Nat Commun 8, 1–16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tan SJ et al. Regulation and dynamics of force transmission at individual cell-matrix adhesion bonds. Science advances 6, eaax0317 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chang AC et al. Single Molecule Force Measurements in Living Cells Reveal a Minimally Tensioned Integrin State. ACS Nano (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nordenfelt P, Elliott HL & Springer TA Coordinated integrin activation by actin-dependent force during T-cell migration. Nat Commun 7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kumar A.et al. Local tension on talin in focal adhesions correlates with F-actin alignment at the nanometer scale. Biophysical journal 115, 1569–1579 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Driscoll TP, Ahn SJ, Huang B, Kumar A.& Schwartz MA Actin flow-dependent and-independent force transmission through integrins. Proceedings of the National Academy of Sciences 117, 32413–32422 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kumar A.et al. Talin tension sensor reveals novel features of focal adhesion force transmission and mechanosensitivity. The Journal of cell biology 213, 371–383 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ringer P.et al. Multiplexing molecular tension sensors reveals piconewton force gradient across talin-1. Nature Methods 14, 1090 (2017). [DOI] [PubMed] [Google Scholar]
- 146.Goult BT, Brown NH & Schwartz MA Talin in mechanotransduction and mechanomemory at a glance. Journal of cell science 134, jcs258749 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lemke SB, Weidemann T, Cost A-L, Grashoff C.& Schnorrer F.A small proportion of Talin molecules transmit forces at developing muscle attachments in vivo. PLoS biology 17, e3000057 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hu K, Ji L, Applegate KT, Danuser G.& Waterman-Storer CM Differential transmission of actin motion within focal adhesions. Science (New York, N.Y 315, 111–115 (2007). [DOI] [PubMed] [Google Scholar]
- 149.Chan CE & Odde DJ Traction dynamics of filopodia on compliant substrates. Science (New York, N.Y 322, 1687–1691 (2008). [DOI] [PubMed] [Google Scholar]
- 150.Brown CM et al. Probing the integrin-actin linkage using high-resolution protein velocity mapping. Journal of cell science 119, 5204–5214 (2006). [DOI] [PubMed] [Google Scholar]
- 151.Elosegui-Artola A, Trepat X.& Roca-Cusachs P.Control of mechanotransduction by molecular clutch dynamics. Trends in Cell Biology 28, 356–367 (2018). [DOI] [PubMed] [Google Scholar]
- 152.Gardel ML et al. Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed. The Journal of cell biology 183, 999 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Plotnikov SV, Pasapera AM, Sabass B.& Waterman CM Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell 151, 1513–1527, doi: 10.1016/j.cell.2012.11.034 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wu Z, Plotnikov SV, Moalim AY, Waterman CM & Liu J.Two Distinct Actin Networks Mediate Traction Oscillations to Confer Focal Adhesion Mechanosensing. Biophysical Journal 112, 780–794 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ponti A, Machacek M, Gupton S, Waterman-Storer C.& Danuser G.Two distinct actin networks drive the protrusion of migrating cells. Science (New York, N.Y 305, 1782–1786 (2004). [DOI] [PubMed] [Google Scholar]
- 156.Yu M.et al. Force-dependent regulation of talin–KANK1 complex at focal adhesions. Nano letters 19, 5982–5990 (2019). [DOI] [PubMed] [Google Scholar]
- 157.Pasapera AM, Schneider IC, Rericha E, Schlaepfer DD & Waterman CM Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J Cell Biol 188, 877–890, doi: 10.1083/jcb.200906012 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lavelin I.et al. Differential effect of actomyosin relaxation on the dynamic properties of focal adhesion proteins. PLoS One 8, e73549, doi: 10.1371/journal.pone.0073549 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stutchbury B, Atherton P, Tsang R, Wang D-Y & Ballestrem C. J. J. o. c. s. Distinct focal adhesion protein modules control different aspects of mechanotransduction. 130, 1612–1624 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hoffmann J-E, Fermin Y, Stricker RL, Ickstadt K.& Zamir E.Symmetric exchange of multi-protein building blocks between stationary focal adhesions and the cytosol. Elife 3, e02257 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dumbauld DW et al. How vinculin regulates force transmission. Proceedings of the National Academy of Sciences of the United States of America 110, 9788–9793, doi: 10.1073/pnas.1216209110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Case LB, Ditlev JA & Rosen MK Regulation of transmembrane signaling by phase separation. Annu Rev Biophys 48, 465–494 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhu J.et al. GIT/PIX condensates are modular and ideal for distinct compartmentalized cell signaling. Mol Cell 79, 782–796. e786 (2020). [DOI] [PubMed] [Google Scholar]
- 164.Case LB, De Pasquale M, Henry L.& Rosen MK Synergistic phase separation of two pathways promotes integrin clustering and nascent adhesion formation. Elife 11, e72588 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang Y.et al. LIMD1 phase separation contributes to cellular mechanics and durotaxis by regulating focal adhesion dynamics in response to force. Developmental Cell 56, 1313–1325. e1317 (2021). [DOI] [PubMed] [Google Scholar]
- 166.Sun X.et al. Mechanosensing through direct binding of tensed F-actin by LIM domains. Developmental Cell 55, 468–482. e467 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Winkelman JD, Anderson CA, Suarez C, Kovar DR & Gardel ML Evolutionarily diverse LIM domain-containing proteins bind stressed actin filaments through a conserved mechanism. Proceedings of the National Academy of Sciences 117, 25532–25542 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mittag T.& Pappu RV A conceptual framework for understanding phase separation and addressing open questions and challenges. Mol Cell, doi: 10.1016/j.molcel.2022.05.018 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Musacchio A.On the role of phase separation in the biogenesis of membraneless compartments. EMBO J 41, e109952, doi: 10.15252/embj.2021109952 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ramella M, Ribolla LM & de Curtis I.Liquid-Liquid Phase Separation at the Plasma Membrane-Cytosol Interface: Common Players in Adhesion, Motility, and Synaptic Function. J Mol Biol 434, 167228, doi: 10.1016/j.jmb.2021.167228 (2022). [DOI] [PubMed] [Google Scholar]
- 171.Moreno-Layseca P, Icha J, Hamidi H.& Ivaska J.Integrin trafficking in cells and tissues. Nat Cell Biol 21, 122–132, doi: 10.1038/s41556-018-0223-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nolte MA, Nolte-’t Hoen ENM & Margadant C.Integrins Control Vesicular Trafficking; New Tricks for Old Dogs. Trends Biochem Sci 46, 124–137, doi: 10.1016/j.tibs.2020.09.001 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Huet-Calderwood C.et al. Novel ecto-tagged integrins reveal their trafficking in live cells. Nat Commun 8, 570, doi: 10.1038/s41467-017-00646-w (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fourriere L.et al. RAB6 and microtubules restrict protein secretion to focal adhesions. J Cell Biol, doi: 10.1083/jcb.201805002 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Eisler SA et al. A Rho signaling network links microtubules to PKD controlled carrier transport to focal adhesions. Elife 7, doi: 10.7554/eLife.35907 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nader GP, Ezratty EJ & Gundersen GG FAK, talin and PIPKIgamma regulate endocytosed integrin activation to polarize focal adhesion assembly. Nat Cell Biol 18, 491–503, doi: 10.1038/ncb3333 (2016). [DOI] [PubMed] [Google Scholar]
- 177.Stehbens SJ et al. CLASPs link focal-adhesion-associated microtubule capture to localized exocytosis and adhesion site turnover. Nat Cell Biol 16, 561–573, doi: 10.1038/ncb2975 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chen NP, Sun Z.& Fassler R.The Kank family proteins in adhesion dynamics. Curr Opin Cell Biol 54, 130–136, doi: 10.1016/j.ceb.2018.05.015 (2018). [DOI] [PubMed] [Google Scholar]
- 179.Krylyshkina O.et al. Nanometer targeting of microtubules to focal adhesions. J Cell Biol 161, 853–859, doi: 10.1083/jcb.200301102 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Seetharaman S.& Etienne-Manneville S.Microtubules at focal adhesions - a double-edged sword. J Cell Sci 132, doi: 10.1242/jcs.232843 (2019). [DOI] [PubMed] [Google Scholar]
- 181.Ezratty EJ, Bertaux C, Marcantonio EE & Gundersen GG Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J Cell Biol 187, 733–747, doi: 10.1083/jcb.200904054 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ezratty EJ, Partridge MA & Gundersen GG Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat Cell Biol 7, 581–590, doi: 10.1038/ncb1262 (2005). [DOI] [PubMed] [Google Scholar]
- 183.Paul NR, Jacquemet G.& Caswell PT Endocytic Trafficking of Integrins in Cell Migration. Current biology : CB 25, R1092–1105, doi: 10.1016/j.cub.2015.09.049 (2015). [DOI] [PubMed] [Google Scholar]
- 184.Moreno-Layseca P.et al. Cargo-specific recruitment in clathrin- and dynamin-independent endocytosis. Nat Cell Biol 23, 1073–1084, doi: 10.1038/s41556-021-00767-x (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.De Franceschi N.et al. Selective integrin endocytosis is driven by interactions between the integrin alpha-chain and AP2. Nat Struct Mol Biol 23, 172–179, doi: 10.1038/nsmb.3161 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Burckhardt CJ, Minna JD & Danuser G.SH3BP4 promotes neuropilin-1 and alpha5-integrin endocytosis and is inhibited by Akt. Dev Cell 56, 1164–1181 e1112, doi: 10.1016/j.devcel.2021.03.009 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yu CH et al. Integrin-beta3 clusters recruit clathrin-mediated endocytic machinery in the absence of traction force. Nat Commun 6, 8672, doi: 10.1038/ncomms9672 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zuidema A.et al. Mechanisms of integrin alphaVbeta5 clustering in flat clathrin lattices. J Cell Sci 131, doi: 10.1242/jcs.221317 (2018). [DOI] [PubMed] [Google Scholar]
- 189.Lock JG et al. Clathrin-containing adhesion complexes. J Cell Biol 218, 2086–2095, doi: 10.1083/jcb.201811160 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Alfonzo-Mendez MA, Sochacki KA, Strub MP & Taraska JW Dual clathrin and integrin signaling systems regulate growth factor receptor activation. Nat Commun 13, 905, doi: 10.1038/s41467-022-28373-x (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Elkhatib N.et al. Tubular clathrin/AP-2 lattices pinch collagen fibers to support 3D cell migration. Science 356, doi: 10.1126/science.aal4713 (2017). [DOI] [PubMed] [Google Scholar]
- 192.Litschel T.et al. Reconstitution of contractile actomyosin rings in vesicles. 12, 1–10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kelley CF et al. Phosphoinositides regulate force-independent interactions between talin, vinculin, and actin. 9, e56110 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ciobanasu C, Faivre B.& Le Clainche C. J. N. c. Actomyosin-dependent formation of the mechanosensitive talin–vinculin complex reinforces actin anchoring. 5, 1–10 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Xu CS et al. Isotropic 3D electron microscopy reference library of whole cells and tissues. (2020). [Google Scholar]
- 196.Hoffman DP et al. Correlative three-dimensional super-resolution and block-face electron microscopy of whole vitreously frozen cells. 367, eaaz5357 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Elosegui-Artola A.et al. Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nature cell biology 18, 540–548, doi: 10.1038/ncb3336 (2016). [DOI] [PubMed] [Google Scholar]
- 198.Oria R.et al. Force loading explains spatial sensing of ligands by cells. Nature 552, 219–224 (2017). [DOI] [PubMed] [Google Scholar]
- 199.Kiema T.et al. The molecular basis of filamin binding to integrins and competition with talin. Mol Cell 21, 337–347, doi: 10.1016/j.molcel.2006.01.011 (2006). [DOI] [PubMed] [Google Scholar]
- 200.Liu J.et al. Structural mechanism of integrin inactivation by filamin. Nat Struct Mol Biol 22, 383–389, doi: 10.1038/nsmb.2999 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Liu W, Draheim KM, Zhang R, Calderwood DA & Boggon TJ Mechanism for KRIT1 release of ICAP1-mediated suppression of integrin activation. Mol Cell 49, 719–729, doi: 10.1016/j.molcel.2012.12.005 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rantala JK et al. SHARPIN is an endogenous inhibitor of beta1-integrin activation. Nat Cell Biol 13, 1315–1324, doi: 10.1038/ncb2340 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Young LE & Higgs HNJCB Focal adhesions undergo longitudinal splitting into fixed-width units. 28, 2033–2045. e2035 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hu S.et al. Structured illumination microscopy reveals focal adhesions are composed of linear subunits. Cytoskeleton (Hoboken), doi: 10.1002/cm.21223 (2015). [DOI] [PubMed] [Google Scholar]
- 205.Case LB & Waterman CM Integration of actin dynamics and cell adhesion by a three-dimensional, mechanosensitive molecular clutch. Nature cell biology 17, 955 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Te Molder L, de Pereda JM & Sonnenberg A.Regulation of hemidesmosome dynamics and cell signaling by integrin alpha6beta4. J Cell Sci 134, doi: 10.1242/jcs.259004 (2021). [DOI] [PubMed] [Google Scholar]
- 207.Zuidema A, Wang W.& Sonnenberg A.Crosstalk between Cell Adhesion Complexes in Regulation of Mechanotransduction. Bioessays 42, e2000119, doi: 10.1002/bies.202000119 (2020). [DOI] [PubMed] [Google Scholar]
- 208.Jacquemet G.et al. Filopodome Mapping Identifies p130Cas as a Mechanosensitive Regulator of Filopodia Stability. Current biology : CB 29, 202–216 e207, doi: 10.1016/j.cub.2018.11.053 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Miihkinen M.et al. Myosin-X and talin modulate integrin activity at filopodia tips. Cell Rep 36, 109716, doi: 10.1016/j.celrep.2021.109716 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Polacheck WJ & Chen CS Measuring cell-generated forces: a guide to the available tools. Nature Methods 13, 415–423 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chen Y, Pasapera AM, Koretsky AP & Waterman C. M. J. P. o. t. N. A. o. S. Orientation-specific responses to sustained uniaxial stretching in focal adhesion growth and turnover. 110, E2352–E2361 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kusumi A, Tsunoyama TA, Hirosawa KM, Kasai RS & Fujiwara TK Tracking single molecules at work in living cells. Nat Chem Biol 10, 524–532 (2014). [DOI] [PubMed] [Google Scholar]
- 213.Manley S.et al. High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat Methods 5, 155–157 (2008). [DOI] [PubMed] [Google Scholar]
- 214.Colin-York H.et al. Super-resolved traction force microscopy (STFM). 16, 2633–2638 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tan SJ et al. Regulation and dynamics of force transmission at individual cell-matrix adhesion bonds. 6, eaax0317 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Brockman JM et al. Live-cell super-resolved PAINT imaging of piconewton cellular traction forces. Nature methods 17, 1018–1024 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Morimatsu M, Mekhdjian AH, Adhikari AS & Dunn AR Molecular tension sensors report forces generated by single integrin molecules in living cells. Nano letters 13, 3985–3989, doi: 10.1021/nl4005145 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wang X.& Ha T.Defining single molecular forces required to activate integrin and notch signaling. Science (New York, N.Y 340, 991–994, doi: 10.1126/science.1231041 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Fischer LS, Rangarajan S, Sadhanasatish T.& Grashoff C.Molecular Force Measurement with Tension Sensors. Annu Rev Biophys 50, 595–616 (2021). [DOI] [PubMed] [Google Scholar]
- 220.Bu W, Levitskaya Z, Tan SM & Gao YG Emerging evidence for kindlin oligomerization and its role in regulating kindlin function. J Cell Sci 134, doi: 10.1242/jcs.256115 (2021). [DOI] [PubMed] [Google Scholar]
- 221.Kadry YA, Maisuria EM, Huet-Calderwood C.& Calderwood DA Differences in self-association between kindlin-2 and kindlin-3 are associated with differential integrin binding. J Biol Chem 295, 11161–11173, doi: 10.1074/jbc.RA120.013618 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Winograd-Katz SE, Fassler R, Geiger B.& Legate KR The integrin adhesome: from genes and proteins to human disease. Nature reviews. Molecular cell biology 15, 273–288, doi: 10.1038/nrm3769 (2014). [DOI] [PubMed] [Google Scholar]
- 223.Das J, Sharma A, Jindal A, Aggarwal V.& Rawat A.Leukocyte adhesion defect: Where do we stand circa 2019? Genes Dis 7, 107–114, doi: 10.1016/j.gendis.2019.07.012 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Alpha KM, Xu W.& Turner CE Paxillin family of focal adhesion adaptor proteins and regulation of cancer cell invasion. Int Rev Cell Mol Biol 355, 1–52, doi: 10.1016/bs.ircmb.2020.05.003 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sulzmaier FJ, Jean C.& Schlaepfer DD FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer 14, 598–610, doi: 10.1038/nrc3792 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Nurden P, Stritt S, Favier R.& Nurden AT Inherited platelet diseases with normal platelet count: phenotypes, genotypes and diagnostic strategy. Haematologica 106, 337–350, doi: 10.3324/haematol.2020.248153 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Heller KN et al. Human alpha7 Integrin Gene (ITGA7) Delivered by Adeno-Associated Virus Extends Survival of Severely Affected Dystrophin/Utrophin-Deficient Mice. Hum Gene Ther 26, 647–656, doi: 10.1089/hum.2015.062 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Has C.& Fischer J.Inherited epidermolysis bullosa: New diagnostics and new clinical phenotypes. Exp Dermatol 28, 1146–1152, doi: 10.1111/exd.13668 (2019). [DOI] [PubMed] [Google Scholar]
- 229.Sorio C.et al. Mutations of Cystic Fibrosis Transmembrane Conductance Regulator Gene Cause a Monocyte-Selective Adhesion Deficiency. Am J Respir Crit Care Med 193, 1123–1133, doi: 10.1164/rccm.201510-1922OC (2016). [DOI] [PubMed] [Google Scholar]
- 230.Iwamoto DV et al. Structural basis of the filamin A actin-binding domain interaction with F-actin. Nat Struct Mol Biol 25, 918–927, doi: 10.1038/s41594-018-0128-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Henderson NC, Rieder F.& Wynn TA Fibrosis: from mechanisms to medicines. Nature 587, 555–566, doi: 10.1038/s41586-020-2938-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kim KK, Sheppard D.& Chapman HA TGF-beta1 Signaling and Tissue Fibrosis. Cold Spring Harb Perspect Biol 10, doi: 10.1101/cshperspect.a022293 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Dong X.et al. Force interacts with macromolecular structure in activation of TGF-beta. Nature 542, 55–59, doi: 10.1038/nature21035 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.López-Otín C, Blasco MA, Partridge L, Serrano M.& Kroemer GJC The hallmarks of aging. 153, 1194–1217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Jun JI & Lau LF The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol 12, 676–685, doi: 10.1038/ncb2070 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Krizhanovsky V.et al. Senescence of activated stellate cells limits liver fibrosis. Cell 134, 657–667, doi: 10.1016/j.cell.2008.06.049 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Meyer K, Hodwin B, Ramanujam D, Engelhardt S.& Sarikas A.Essential Role for Premature Senescence of Myofibroblasts in Myocardial Fibrosis. Journal of the American College of Cardiology 67, 2018–2028, doi: 10.1016/j.jacc.2016.02.047 (2016). [DOI] [PubMed] [Google Scholar]
- 238.Cho KA et al. Morphological adjustment of senescent cells by modulating caveolin-1 status. 279, 42270–42278 (2004). [DOI] [PubMed] [Google Scholar]
- 239.Rapisarda V.et al. Integrin beta 3 regulates cellular senescence by activating the TGF-β pathway. 18, 2480–2493 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Cheung TM et al. Endothelial cell senescence increases traction forces due to age-associated changes in the glycocalyx and SIRT1. 8, 63–75 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Chala N.et al. Mechanical Fingerprint of Senescence in Endothelial Cells. (2021). [DOI] [PubMed] [Google Scholar]
- 242.Shin EY et al. Integrin-mediated adhesions in regulation of cellular senescence. Sci Adv 6, eaay3909, doi: 10.1126/sciadv.aay3909 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kuo JC, Han X, Hsiao CT, Yates JR 3rd & Waterman CM Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for beta-Pix in negative regulation of focal adhesion maturation. Nature cell biology 13, 383–393 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1
Comparison of consensus adhesome from meta-analysis of enriched IAC proteomes, proximity-dependent adhesome, and super-resolution microscopy-based analysis.
The consensus adhesome list of 60 IAC components40 are sorted based on their physical organization as determined by super-resolution microscopy59,85,87–89, proximity-dependent adhesome bait clustering50, and the putative signaling axes as determined from the meta-adhesome and proximity-dependent adhesome40,50. B1–5: Bait cluster from proximity-dependent adhesome analysis. ISL: Integrin Signaling Layer; FTL: Force Transduction Layer; ARL: Actin Regulatory Layer.