Abstract
Polycyclic aromatic hydrocarbons (PAHs) are a class of environmental contaminants released into the environment from both natural and anthropogenic sources that are associated with carcinogenic, mutagenic, and teratogenic health effects. Many remediation strategies for the treatment of PAH contaminated material, including bioremediation, can lead to the formation of toxic transformation products. Analytical techniques for PAHs and PAH transformation products often require extensive sample preparation including solvent extraction and concentration, chromatographic separation, and mass spectrometry to identify and quantify compounds of interest. Excitation-emission matrix (EEM) fluorescent spectroscopy paired with parallel factor analysis (PARAFAC) is an approach for analyzing PAHs that eliminates the need for extensive sample preparation and separation techniques before analysis. However, this technique has rarely been applied to monitoring PAH biotransformation and formation of PAH metabolites. The objectives of this research were to compare an established targeted analytical method to two-dimensional fluorescent spectroscopy and combined EEM-PARAFAC methods to monitor phenanthrene degradation by a bacterial pure culture, Mycobacterium Strain ELW1, identify and quantify phenanthrene transformation products, and derive kinetic constants for phenanthrene degradation and metabolite formation. Both phenanthrene and its primary transformation product, trans-9,10-dihydroxy-9,10-dihydrophenanthrene, were identified and quantified with the EEM-PARAFAC method. The value of the EEM-PARAFAC method was demonstrated in the superiority of sensitivity and accuracy of quantification to two-dimensional fluorescent spectroscopy. Quantification of targets and derivation of kinetic constants using the EEM-PARAFAC method were validated with an established gas chromatography-mass spectrometry (GC-MS) method. To the authors’ knowledge, this is the first study to use an EEM-PARAFAC method to monitor, identify, and quantify both PAH biodegradation and PAH metabolite formation by a bacterial pure culture.
Graphical Abstract

1.0. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are a class of environmental contaminants released into the environment from both natural and anthropogenic sources, such as forest fires and the use of petroleum products (Patel et al. 2020; Alegbeleye et al. 2017), that are associated with carcinogenic, mutagenic, and teratogenic health effects (Ghosal et al., 2016; Krzyszczak and Czech, 2021). 16 PAHs are listed as priority pollutants by the EPA (US EPA 2014) and although PAH exposure is more commonly associated with inhalation or food ingestion over drinking water (Bansal and Kim, 2015; Patel et al., 2020; Phillips, 1999), the EPA has set a maximum contaminant level (MCL) of 0.2 μg/L for benzo(a)pyrene in drinking water (US EPA 2009). In addition, many regulations exist in the US and other countries for PAH content in industrial soils (Chibwe et al., 2015). Common remediation techniques for PAHs include soil washing, photooxidation, chemical oxidation, and biodegradation (Gan et al. 2009; Sakshi and Haritash 2020; Kumar et al. 2021). PAH transformation products are often produced during chemical or biological remediation processes, with several studies finding these products to be equally, if not more, toxic than their parent compounds (Andersson et al. 2009; Trine et al. 2019; Titaley et al. 2020). Therefore, monitoring both PAHs and their transformation products during and after treatment is of interest in remediation studies.
PAHs typically exist as mixtures in relatively low concentrations in the environment, thus chemical analysis of environmental samples often require extraction, concentration, and separation techniques before identification and quantification of compounds of interest. Several standard methods have been developed by the US-EPA for analysis of PAHs in aqueous matrices such as methods 610, 625, 525, and 8310 that use combinations of extraction, chromatography, and detection techniques (Gitipour et al., 2018). When considering PAH transformation products, more sophisticated analytical techniques are generally required to resolve a complex mixture of PAH transformation products that can have great diversity in structure and chemical properties (Titaley et al., 2020). The time, materials, and costs associated with these techniques has motivated the development of more rapid and less expensive analyses for PAHs and PAH transformation products.
One such analytical technique that forgoes extraction and separation before analysis is fluorescent spectroscopy. Fluorescent spectroscopic analysis can be conducted in 2 dimensions, where fluorescent emissions are measured for one excitation wavelength (λex) and a range of emission wavelengths (λem), or three dimensions, where fluorescent emissions are measured for a range of λex and λem. Excitation-emission matrices (EEMs) are data sets produced by three dimensional scans that are a common tool for environmental monitoring studies, as they are non-destructive, rapidly collectable, and require minimal sample preparation (Yang et al., 2016). While EEMs provide qualitative information of sample composition, decomposition of the fluorescent spectra produced from a bulk sample is often necessary to interpret EEMs (Rutherford et al., 2020).
A tool commonly used to accomplish this decomposition is parallel factor analysis (PARAFAC), a statistical technique that models EEMs as combinations of components with specific excitation and emission spectra (Bro, 1997). Advantages of PARAFAC decomposition include uniqueness, such that pure spectra are obtained from the model (Nahorniak and Booksh, 2006), as well as a second-order advantage, which enables concentrations to be measured even in the presence of unexpected interferences (Gómez and Callao, 2008; Olivieri et al., 2004). As such, components obtained from PARAFAC models can be identified and quantified using external standard calibration. EEM-PARAFAC methods have been used in monitoring studies to investigate the PAH content of oils (Alostaz et al., 2008; Christensen et al., 2005), seawater (Driskill et al., 2018; Ferretto et al., 2014), and freshwater (Yang et al., 2016) samples. PARAFAC components can be identified based on characteristics such as Stokes shift that can be compared with standards or literature values. Targeted chemical analyses such as gas chromatography-mass spectrometry (GC-MS) can also be used to validate sample composition.
Aside from environmental monitoring, EEM-PARAFAC analysis has also been used in kinetic experiments to monitor the degradation of PAHs. Several studies have used EEM-PARAFAC to monitor PAHs undergoing photodegradation, Fenton oxidation, biodegradation, and combined treatment techniques. A summary of these studies is provided in Table 1. Varying success has been reported in identifying PARAFAC components. Often, components were reported as a collection of compounds, such as humic-like substances or amino acid-like substances. This is due in part to the complexity of the compound mixtures investigated in studies included in Table 1. As the number of initial fluorescent compounds increases, so does the possibility of overlapping fluorescent spectra and similar degradation kinetics. Thus, it is more likely that the spectra of compounds will be modeled together as one component that will not match the spectrum of any one compound. Because fluorescent degradation products can be produced in these PAH degradation experiments, such compounds can also be represented in PARAFAC components, although few studies have made these observations. For example, in PAH photodegradation experiments, Seopela et al. identified a single fluoranthene photodegradation product, 2-(3,4)-dihydronaphthalene-1-yl acetic acid, with EEM-PARAFAC analysis, however remaining PARAFAC components were defined as unresolved photoproduct mixtures (Seopela et al., 2021).
Table 1.
Compilation of literature investigating PAH degradation kinetics and using EEM-PARAFAC monitoring methods
| Compounds Studied | Matrix | Treatment | Component Identification Method | Components Identified | Citation |
|---|---|---|---|---|---|
| naphthalene, anthracene, benzo(a)anthracence, benzo(a)pyrene, benzo(ghi)perylene | Water with NOM | Photo-degradation | Literature Comparison, GC-MS Validation | 2-3,4)- dihydronaphthalene-l-yl acetic acid, unresolved photoproduct mixtures | (Seopela et al., 2021) |
| dibenz(a,h)anthracene, benz(a)anthrancence, benzo(a)pyrene, benzo(k)fluoranthene | Water/alcohol/solvent mixture | Photo-degradation | Standard Comparison | dibenz(a,h)anthracene, benz(a)anthrancence, benzo(a)pyrene, benzo(k)fluoranthene | (Bosco et al., 2006) |
| Benzo(a)pyrene, dibenz(a,h)antliracene, benzo(b)fluoranthene, benzo(k)fluoranthene, benz(a)anthracene | Methyl-β - cyclodextrin, acetic/acetate buffer solution | Fenton degradation | Standard Comparison | Benzo(a)pyrene, dibenz(a,h)anthracene, benzo(b)fluoranthene, benzo(k)fluoranthene, benz(a)anthracene | (Carabajal et al., 2017) |
| Diesel | Ultra-pure water/Tween 80 | Electro-Fenton and Electrochemical degradation | Literature Comparison | Fluorene-like, naphthalene/methylnaphthalene-like, BTEX | (Liu et al., 2020) |
| PAH Mixture | Biochar amended sediment | Biodegradation | Literature Comparison | Tyrosine-like, tryptophan- and soluble microbial byproduct-like, fulvic-like, humic-like | (Hung et al., 2022) |
| Crude oil | DI water/seawater Samples | Photo-degradation and biodegradation | GC-FID/GC-MS Validation | Oil-related, Natural DOM | (Zhou et al., 2013) |
| Complex Mixture | Coal mining wastewater | Fenton degradation, biodegradation, ozonation | Literature Comparison, GC-MS Validation | Humic-like, PAHs, organic acids, phenolic compounds, quinones, ketones | (Peng et al., 2018) |
Very few studies have applied an EEM-PARAFAC approach to monitor PAH biodegradation and identify biological transformation products. Those that have investigated biological transformation have been conducted using biostimulation or natural attenuation methods, rather than a bioaugmentation method with known microorganisms.
When cellular material is present in the samples, sample turbidity becomes a challenge in detecting fluorescent emissions. Various solutions have been proposed to account for the effect of sample turbidity on fluorescent emissions. Several mathematical models have been developed to model and correct the effects of turbidity on fluorescent measurements (Chen et al., 2013; Kanick et al., 2012; Wu et al., 1993), however in controlled situations where sample turbidity is consistent across samples, less extensive measures can be taken to account for interreferences. The inclusion of standard scans in data sets used to develop PARAFAC models has been demonstrated to aid in the quantification of target compounds (Elcoroaristizabel et al, 2014; Maggio et al., 2010), as it has been demonstrated that accuracy of quantitation is inversely related to the presence of interfering compounds (Ferretto et al., 2014).
Mycobacterium Sp. Strain ELW1 (ELW1) was the aerobic bacterium used in this investigation. ELW1 was isolated from stream sediment using isobutene as the sole carbon and energy source (Kottegoda et al., 2015). ELW1’s cometabolic potential has been probed for both chlorinated aliphatic hydrocarbons (Krippaehne 2018, Rich 2015) and phenanthrene (Schrlau et al., 2017). Previous work with ELW1 demonstrated the cometabolism of phenanthrene to a mixture of hydroxylated products, as identified by GC-MS analysis, but the full composition of the metabolite mixture was not resolved. The primary metabolite observed in this work was trans-9,10-dihydroxy-9,10-dihydrophenathrene (P1) (Schrlau et al., 2017). The objectives of this research were to compare an established targeted analytical method using solid phase extraction and GC-MS analysis to 2D fluorescent spectroscopy (2D) and EEM-PARAFAC methods to monitor, quantify, and derive kinetic parameters for phenanthrene degradation and P1 formation by ELW1.
2.0. Materials and Methods
2.1. Chemicals
A list of the purity and vendors of phenanthrene, seven hydroxylated phenanthrenes, and three isotope labeled PAHs is provided in Table S1. All solvents used in solid phase extraction and GC-MS analysis: methanol, dichloromethane, acetone, ethyl acetate, acetonitrile, and toluene, were analytical grade purity and purchased from various vendors. Isobutene (99%), 1-octyne (98%) and the derivatizing agent N,O-Bis(trimethylsilyl)trifluoroacetamide (BSTFA) (>97%) were purchased from Sigma-Aldrich (Milwaukee, WI).
2.2. Experimental Set Up
ELW1 was provided by Dr. Michael Hyman from North Carolina State University, and was grown on isobutene as the sole carbon and energy source. Detailed methods on cell culturing are provided in Supplemental Information. Cells were harvested during the late exponential growth phase for use in phenanthrene transformation studies. Resting cell transformation studies were performed in batch bottles prepared in baked Wheaton 500 mL media bottles (nominal volume 720 mL) with solvent washed butyl septa caps. All batch bottles were prepared in a laminar flow hood to maintain aseptic conditions. Active bottles, prepared in triplicate, contained 300 mL of mineral salt media, 90 mg of isobutene grown ELW1 cells, and approximately 1 mg/L phenanthrene added from a concentrated methanol stock. Cells were added to active bottles after the initial liquid samples were taken. Enzyme inhibited bottles were prepared in duplicate as active bottles, but with 45 mg of cells and 1-octyne, a monooxygenase inhibitor, present to block the transformation of phenanthrene. 1-octyne was added from a 1 mM gaseous stock prepared according to (Taylor et al., 2013) at a 3.5% (v/v) headspace concentration. Both active and enzyme inhibited bottles were incubated at 30°C on a 150 RPM shaker table for the 76-hour duration of the experiment. Liquid samples were taken through the cap septa at set times throughout the experiment with a sterile glass syringe. 8 mL of liquid sample was removed at each sampling time, with 2.5 mL analyzed immediately with fluorescent spectroscopy. Cells were not removed from samples prior to fluorescent spectroscopic analysis. The remaining 5.5 mL sample was spiked with a labeled surrogate standard mix (Table S2) and extracted with solid phase extraction (SPE) with a previously published extraction method (Schrlau et al. 2017) using Bond Elut Plexa (60 mg, 3 mL) cartridges (Agilent Technologies, New Castle, DE).
2.3. Targeted Analysis
Extracts analyzed for phenanthrene (PPAH samples) were nitrogen line dried to 225 μL in 300 μL autosampler vials. 75 μL of an internal standard mixture (Table S3) was added to reach a final sample volume of 300 μL. Preparation of hydroxylated PAHs (OHPAHs) samples was carried out with a modified published method (Schrlau et al., 2017) . Briefly, 50 μL of prepared PPAH sample was added to a 300 μL spring insert containing 120 μL of a 5:1 acetonitrile: toluene solvent mixture. This mixture was dried under a nitrogen stream to 20 μL. 30 μL of BSTFA was then added as a derivatizing agent, and the sample was incubated at 60°C for 25 minutes. PPAH and OHPAH samples were analyzed using an Agilent 6890 GC equipped with an Agilent DB-5ms (30m x 0.25 mm x 0.25 μm) capillary column coupled to an Agilent 5977A mass spectrometer detector with electron impact ionization operated in SIM mode. GC-MS instrument parameters are provided in Supplemental Information.
2.4. Fluorescent Spectroscopy
All samples were analyzed with a Varian Cary Eclipse fluorescent spectrophotometer operated in emission (2D) or 3D mode. Samples were scanned in a 4-sided quartz cuvette. 2D scans were conducted with a λex of 250 nm and 270 nm, optimized for phenanthrene and P1 respectively, for a λem range of 300 nm to 450 nm. EEMs were collected for an λex range of 200 nm to 300 nm with a 10 nm increment and an emission wavelength range of 300 nm to 600 nm with a 1 nm increment. Excitation and emission slit widths were each 5 nm, and the scan rate was 600 nm/minute, resulting in a 3D scan time of 5 minutes per sample. It should be noted that the scan time was very short in comparison to the rate of reaction observed, therefore changes in sample composition during scanning were considered negligible. The instrument was zeroed with a deionized water blank in between scans.
2.5. PARAFAC Model Development
PARAFAC models were developed using the drEEM v0.6.2 MATLAB toolbox developed by Murphy et al. (Murphy et al., 2013). The data set used to develop the final PARAFAC models consisted of an experimental data set that included EEMs from active triplicates (n=24) and enzyme inhibited controls (n=16), and a calibration data set that included EEMs for phenanthrene standards (n=18) and P1 standards (n =15). Additional PARAFAC models were developed without the calibration data set to assess the influence of the calibration set on model output. A convergence constraint of 10−6 for relative change of fit was used to develop all models.
2.5.1. Data Pre-Processing
First order Raman scattering was corrected by subtracting a matrix blank spectrum from each sample’s spectrum. First and second order Rayleigh scattering was corrected by excision of scattering within tolerances defined in the drEEM toolbox. Scattering was replaced with missing values (NaN), rather than zeros, as it has been demonstrated that the inclusion of zeros can negatively impact the tri-linearity of the data set (Anderson and Bro 2003). No other adjustments were made to the EEMs. A non-negativity constraint was applied when generating PARAFAC models, as negative values in spectroscopy do not have physical meaning. The data set was inspected for outliers by identifying samples with excessively high leverages and removing them from the data set before it was read into the drEEM toolbox.
2.5.2. Model Component Determination
Several metrics can be used to determine the appropriate number of PARAFAC components for a given data set. First, visual inspection of the components’ excitation and emission loadings can be used to identify components resembling noise rather than fluorescent compounds. Three numerical metrics, sum of squared error (SSE), percent explained, and core consistency, are reported by the drEEM toolbox for each model. While SSE is ideally minimized and the latter metrics are ideally maximized by the proper number of components, analyzing the trends of these metrics as the number of components used increases also provides valuable information. Diminishing returns on percent explained and SSE reduction as components are added to the model can indicate that the appropriate number of components has been exceeded (Stedmon and Markager, 2005). Similarly, a steep decline in core consistency as components are added to the model can also indicate overfitting (Bro and Kiers, 2003). These trends, rather than the values of these metrics exclusively, can inform the appropriate number of components for the model.
2.5.3. Model Validation
The PARAFAC model was validated mathematically with split-half analysis, as it is a common validation tool used for PARAFAC models and the tool of choice for the drEEM toolbox developers (Murphy et al., 2013). Details of the split-half analysis are provided in Supplemental Information. When possible, comparisons between PARAFAC component Stokes shift and spectra and those of standard scans and literature values were used to further validate the model and identify compounds that corresponded to the PARAFAC components.
2.6. Fluorescent Target Quantification
Standards of phenanthrene and P1 were prepared in mineral salt media and scanned as described in section 2.5. ELW1 cells were added to the appropriate concentration corresponding with active or inhibited bottles immediately before scanning each standard to accommodate the influence of turbid media on instrument response. Standard curves with varying concentrations of ELW1 cells were produced from 2D scans as shown in Figure S1 and were used to correlate 2D maximum fluorescent emission (Fmax) measurements with target concentrations. 3D scans of these standards, referred to as the calibration data set, were included with the experimental data set in the development of PARAFAC models. The Fmax of PARAFAC components corresponding to phenanthrene or P1 were used to develop standard curves shown in Figure S1. Estimated method detection limits (MDLs) for 2D and EEM-PARAFAC methods were derived by dividing the standard deviation of the lowest standard by the slope of the calibration curve (Elcoroaristizabal et al., 2014) and are reported in Table S4.
2.7. Kinetic Models
Pseudo-first order models for phenanthrene degradation and P1 formation were fit to quantitated phenanthrene measurements. Cometabolism generally follows pseudo-first order kinetics, as supported by previous PAH cometabolism monitoring studies (Barret et al., 2010; Brimo et al., 2016). Phenanthrene degradation was modeled using the first order kinetic equation CPAH = Coexp(−kPAHt) where CPAH is the concentration of phenanthrene (mg/L) at time t (hour), Co is the initial phenanthrene concentration (mg/L), and kPAH is the first order phenanthrene degradation rate constant (hour−1). Rate of product formation constants was modeled with the following equation: CP1 = CoF(1-exp(-kP1t)) where CP1 is P1 concentration (mg/L) at time t (hours), Co is the initial phenanthrene concentration (mg/L), F is the fraction of phenanthrene transformed into P1 (−), and kP1 is the P1 first order formation rate constant (hour−1). MATLAB’s “fitlm” and “coefCI” functions were used to generate pseudo-first order models and confidence intervals.
3.0. Results and Discussion
3.1. GC-MS
The degradation of phenanthrene and formation of one di-hydroxylated metabolite, P1, and 3 mono-hydroxylated metabolites: 1-, 4-, and 9-hydroxyphenanthrene were quantified with GC-MS, as shown in Figure S2. Phenanthrene degradation and formation of hydroxylated metabolites agreed well with the initial study of phenanthrene degradation by ELW1 (Schrlau et al., 2017). 3-hydroxyphenanthrene and 1,9-dihydroxyphenanthrene, however, were not detected above background in active samples as they were in the former study. This may be due to lower sensitivity of the GC-MS instrument used in the present study, as they were measured in very low concentrations previously (<70 μg/L). Recoveries of phenanthrene and the hydroxylated metabolites, estimated by the recovery of the labelled surrogate compounds, were 43.2 ± 10.1 and 53.7 ± 16.7 percent, respectively. Minor degradation of phenanthrene and accumulation of phenanthrene metabolites was observed in the 1-octyne controls, indicating that near, but not complete, inhibition was achieved. Approximately 58% of the total mass of initial phenanthrene was accounted for with the GC-MS quantified metabolites on a molar basis, demonstrating that the composition of the metabolite mixture was not fully characterized with this method. P1 and mono-hydroxylated metabolites accounted for approximately 96% and 4% of the total metabolite mass, respectively.
3.3. Fluorescent Spectroscopy
EEMs and 2D scans collected from active samples revealed a shift in fluorescent spectra over time, providing qualitative information on reaction progress, as shown in Figure 1. Spectra did not change significantly after 28 hours of incubation, indicating that most of the transformation occurred in the first 28 hours of incubation with the cells. Furthermore, minor changes to spectra after this time indicated the accumulation of a fluorescent metabolite.
Fig. 1.

EEMs and 2D fluorescent scans (λex = 250 nm) from an active bottle after 0 (a, d), 12 (b, e), and 28 (c, f) hours of incubation with phenanthrene
3.3.1. PARAFAC Model Development
EEMs used to generate the PARAFAC models were reduced to a λem range of 300 nm to 450 nm, as the 450 nm to 600 nm range did not include signals above background for experimental samples and standard scans. PARAFAC models with 3 to 7 components were developed with the experimental and calibration data set and compared for over- or underfitting using visual inspection of the component emission spectra, percent explained, core consistency, and SSE. A summary of the latter three metrics is provided in Table S5. A 5-component model was determined to be the most appropriate model based on these criteria, with 98.41% explained and 87.60% core consistency. This model was validated with split-half analysis, details of which are provided in supplemental information.
3.3.2. Component Identification
Comparisons of the modeled component EEMs as well as their Stokes shifts were compared to standard scans of phenanthrene, phenanthrene metabolites, and controls to identify what compound(s) were represented by each component, as shown in Figure 2. Component 1 (C1) was identified to be phenanthrene, and component 5 (C5) was identified as P1. Components 2 (C2) and 3 (C3) originated from the ELW1 cells present in the samples, with C2 matching literature values for the Stokes shift of the amino acid tryptophan (Hu et al., 2021; Hung et al., 2022). Component 4 (C4) was unable to be identified and may represent more than one compound. Comparisons to standard scans of what was identified by GC-MS eliminated mono-hydroxylated phenanthrene products as the source of this component. However, the total composition of the metabolite mixture produced by ELW1 is not resolved, as evidenced by the unknown metabolites identified in the previous study and incomplete mass balance in the present study. These compounds, or other unknowns, may be contributing to C4.
Fig. 2.
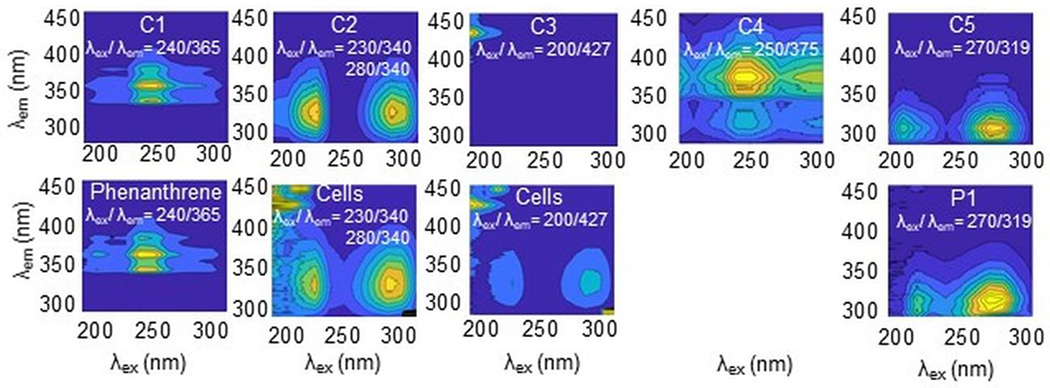
Comparison of PARAFAC components’ EEMs (top row) to standard EEMs (bottom row). Fluorescent intensities of EEMs shown are arbitrary and only reflect trends in intensity as excitation wavelength changes. Text in EEMs represent Stokes shift associated with components and standards
Further investigation of the composition of components was carried out by considering their temporal variation. Fmax values were plotted against the time of sample collection to discern temporal trends in component abundance, shown in Figure 3.
Fig. 3.
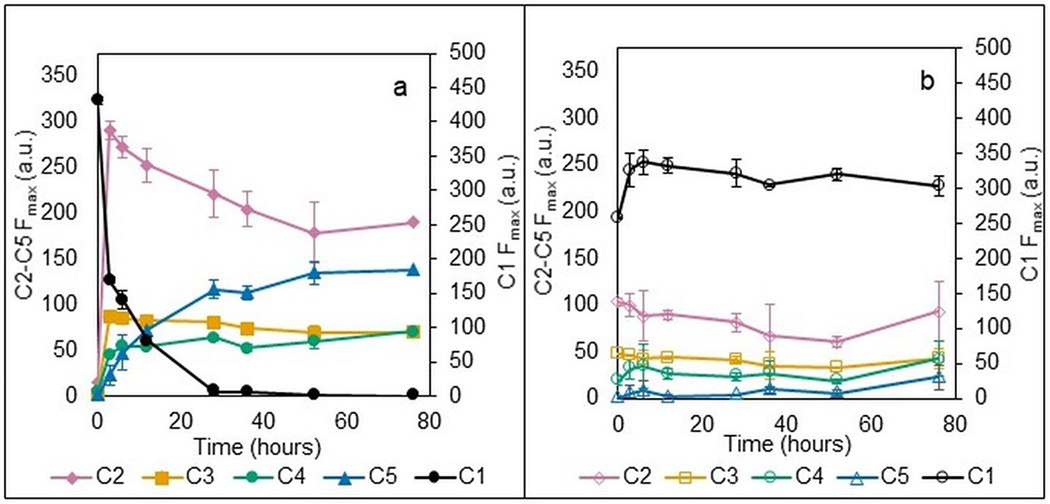
Temporal variation of PARAFAC components for active (a) and inhibited (b) bottles. Error bars represent standard deviation among triplicates in (a) and range between duplicates in (b)
The trends of reduction of C1 and production of the C5 mirror the degradation of phenanthrene and formation of P1 over time. The association of C2 and C3 with the ELW1 cells present in the sample is supported by their presence in active samples with minor temporal variation and inhibited samples with no discernable temporal variation. Additionally, these components were absent in the initial samples of active bottles that were taken prior to cell addition. The same was observed with C4, indicating that C4 may be representative of cellular material as well, rather than exclusively metabolites. C4’s Stokes shift was compared to that of common fluorophores associated with biological material reported in the literature. Fluorophores associated with cellular respiration, NADH and FAD, absorb and emit longer wavelengths of light that fall outside the scan parameters of this study (Gröning et al., 2014; Mayevsky and Rogatsky, 2007). Stokes shifts associated with the fluorescent amino acids tyrosine and phenylalanine (Hu et al., 2021) and fulvic and humic material (Alberts and Takács, 2004; Hung et al., 2022; Peng et al., 2018) do fall within the measured experimental range of λex and λem, but do not match the Stokes shift of C4. Low amounts of C5 in inhibited controls compared to active samples support that these compounds are associated with the biological transformation of phenanthrene by ELW1.
A second PARAFAC model was generated without the calibration data set to test the influence of the calibration data on the components and model fit. As before, 5 components were needed to model the data set, and the spectra of the components were nearly identical to the model generated with the calibration data set included, indicating that the PARAFAC model could extract the pure spectra for phenanthrene and P1 without the inclusion of standards. Details of this model are provided in Supplemental Information.
3.3.3. Target Quantification
An inverse relationship was found for sample turbidity and sensitivity of the analysis, thus the more turbid the sample, the less sensitive the analysis. While a significant improvement in MDL was observed for EEM-PARAFAC compared to 2D fluorescence, the EEM-PARAFAC MDLs were one to two orders of magnitude higher than the GC-MS method (Table S4). The loss of sensitivity with cell addition is likely due to fluorescent quenching from the cellular material, a phenomenon in which fluorescent emissions are reduced due to mechanisms such as absorption by other molecules (Lakowicz et al., 2002). Because of the high turbidity of samples in this study, the inclusion of interfering compounds, in this case cellular material, was critical to achieving accurate quantitation of the target compounds. Linear regression constants and goodness of fit for each standard curve developed are reported in Figure S1.
Phenanthrene and P1 quantitation by EEM-PARAFAC generally matched GC-MS quantitation within one standard deviation, as shown in Figure 4, indicating that the impact of turbidity could be accounted for with the standard curve adjustment. Phenanthrene measurements in the 1-octyne controls matched the GC-MS results within one standard deviation as well, with more consistent measurements observed in EEM-PARAFAC samples compared to the GC-MS samples. P1 in 1-octyne controls was not measured by either fluorescent method, suggesting that concentrations may have been below the limits of detection of the fluorescent methods.
Fig. 4.

Comparison of phenanthrene (a) and trans-9,10-dihydroxy-9,10-dihydrophenathrene (b) quantitation for GC-MS (circle), EEM-PARAFAC (triangle), and 2D fluorescent spectroscopy (squares) methods. Error bars represent standard deviation among triplicates for active bottles (filled markers) and duplicates for inhibited bottles (open markers)
Results from 2D fluorescent scans on active and inhibited samples elucidate the benefits of PARAFAC modeling for fluorophore mixtures. Phenanthrene quantitation was overestimated in the first 12 hours of the experiment to a higher degree than the PARAFAC quantitation for active samples, and appeared to reach an asymptote starting at hour 28. This asymptote is likely the resulting fluorescent signal of the other fluorophores in the mixture, such as the P1, that were in higher concentrations than phenanthrene at that time in the experiment. This is supported by the change in the shape of the fluorescent spectra observed in the 2D scans starting at 28 hours, as seen in Figure S6. 2D quantitation of phenanthrene in the 1-octyne controls generally fell within one standard deviation of GC-MS results, however these measurements were consistently higher than EEM-PARAFAC measurements. Quantitation of P1 was statistically indistinguishable to the quantitation with the EEM-PARAFAC method, and therefore is subject to the same comparison to GC-MS as described previously.
3.4. Kinetics
Phenanthrene transformation and P1 formation both followed first-order kinetics well, as shown in Figure 5. The pseudo-first order rate constants for phenanthrene degradation and P1 formation derived from quantitated GC-MS data and PARAFAC C1 data have no statistically significant difference, demonstrating that the EEM-PARAFAC method provided comparable kinetic information to the GC-MS method. The initial measurement of phenanthrene was omitted from the model, as phenanthrene was not fully dissolved at this time.
Fig. 5.

Comparison of empirical and modeled concentrations of phenanthrene and P1 derived from GC-MS (a), EEM-PARAFAC (b) and 2D fluorescent methods
This result was expected based on the good agreement of phenanthrene quantification shown in Figure 3. Similarly, because of the poor agreement between 2D fluorescent sample phenanthrene quantitation and the other two methods, the model fit of phenanthrene degradation derived from the 2D fluorescent quantitation did not fit empirical measurements well, nor did the rate constant compared to that derived from the alternative methods. The rate of P1 formation is also significantly different than what was derived with the other methods, however, it is faster. This is due to the asymptote reached in 2D quantitated phenanthrene data, which inaccurately indicated that significantly less phenanthrene was transformed compared to what was indicated by EEM-PARAFAC or GC-MS data. Thus, the fraction of phenanthrene transformed that was accounted for as P1 was impacted, leading to the appearance of faster product formation kinetics. This further demonstrates the limitations of techniques such as the 2D fluorescent method that does not include separation techniques before or after analysis. Linear regions used, rate constants and goodness of fit are reported in Figure S7.
4.0. Conclusions
The method developed in this research is a rapid analytical technique that forgoes extensive sample preparation and separation techniques to monitor phenanthrene biodegradation and product formation that is comparable to an established method relying on solid-phase extraction, chromatographic separation, and mass spectrometry. The value of three-dimensional fluorescent spectroscopy paired with parallel factor analysis (EEM-PARAFAC) was demonstrated in the superiority of sensitivity and accuracy in quantification to two-dimensional fluorescent spectroscopy. A five component PARAFAC model appropriately modeled the experimental data with and without additional calibration data, from which four components were identified: phenanthrene, trans-9,10-dihydroxy-9,10-dihydrophenanthrene, one tryptophan-like cellular material, and one general cellular material. The unidentified component likely represents a mixture of compounds that are of cellular material origin, however based on the incomplete mass balance it is possible that unidentified phenanthrene metabolites are also associated with this component. The interference of the cellular material in the samples was accounted for with the use of standards prepared with corresponding cellular interference that were included in the PARAFAC model. While the inclusion of standards in developing the PARAFAC models was not necessary to identify the components, it was necessary quantify the compounds of interest. Individual compounds were able to be identified with the EEM-PARAFAC method due in part to this being a single compound study. It should be noted that the complexity of component identification and quantification reflects the complexity of the initial contaminant(s) present in a study, limiting the application of this method for quantitative applications. Quantification of targets and the development of first order models using the EEM-PARAFAC method were validated with the established GC-MS method. Sensitivity was enhanced with the use of the EEM-PARAFAC method compared to the 2D fluorescent method, however, it was still one to two orders of magnitude higher than the GC-MS method, highlighting the tradeoff between ease and speed of sample analysis and sensitivity of analysis. Greater sensitivity can be achieved in the EEM-PARAFAC method with additional sample preparation steps to remove cells and thus reduce sample turbidity. However, any sample preparation steps can result in losses of compounds of interest due to phenomena such as volatilization or adsorption to materials that can be difficult to account for in quantitative analyses. The advantages of the EEM-PARAFAC method do not lie in its sensitivity, but rather in its ease and rapidity, allowing for real time monitoring of biological PAH transformation with no sample preparation steps required. To the authors’ knowledge, this is the first study to use an EEM-PARAFAC method to monitor, identify, and quantify both PAH biodegradation and PAH metabolite formation by a bacterial pure culture.
Supplementary Material
Highlights.
Analysis of PAHs and PAH transformation products is often time and energy intensive
Excitation emission matrices (EEMs) and parallel factor analysis (PARAFAC) used
EEM-PARAFAC methods were developed for monitoring of PAH biotransformation
Phenanthrene and a transformation product were identified with EEM-PARAFAC
EEM-PARAFAC target quantification and kinetic parameters were validated with GC-MS
5.0. Acknowledgements
The authors wish to thank Dr. Michael Hyman for providing the ELW1 pure culture, Jason Schindler for support in GC-MS sample preparation and analyses, and Dr. Mohammad Azizian for support in fluorescent spectroscopy analyses. Research reported in this publication was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number P42ES016465. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Lewis Semprini contributed to funding acquisition, review and editing, and conceptualization. Juliana Huizenga contributed to conceptualization, data curation and data analysis, visualization, and writing of the original draft.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
6.0 References
- Alberts JJ, and Takács M (2004). Total luminescence spectra of IHSS standard and reference fulvic acids, humic acids and natural organic matter: Comparison of aquatic and terrestrial source terms. Organic Geochemistry, 35(3), 243–256. 10.1016/j.orggeochem.2003.11.007 [DOI] [Google Scholar]
- Alegbeleye OO, Opeolu BO, and Jackson VA (2017). Polycyclic Aromatic Hydrocarbons: A Critical Review of Environmental Occurrence and Bioremediation. Environmental Management, 60(4), 758–783. 10.1007/s00267-017-0896-2 [DOI] [PubMed] [Google Scholar]
- Alostaz M, Biggar K, Donahue R, and Hall G (2008). Petroleum contamination characterization and quantification using fluorescence emission-excitation matrices (EEMs) and parallel factor analysis (PARAFAC). Journal of Environmental Engineering and Science, 7(3), 183–197. 10.1139/S07-049 [DOI] [Google Scholar]
- Andersson E, Rotander A, von Kronhelm T, Berggren A, Ivarsson P, Hollert H, and Engwall M (2009). AhR agonist and genotoxicant bioavailability in a PAH-contaminated soil undergoing biological treatment. Environmental Science and Pollution Research, 16(5), 521–530. 10.1007/sll356-009-0121-9 [DOI] [PubMed] [Google Scholar]
- Bansal V, and Kim K-H (2015). Review of PAH contamination in food products and their health hazards. Environment International, 84, 26–38. 10.1016/j.envint.2015.06.016 [DOI] [PubMed] [Google Scholar]
- Barret M, Carrère H, Delgadillo L, and Patureau D (2010). PAH fate during the anaerobic digestion of contaminated sludge: Do bioavailability and/or cometabolism limit their biodegradation? Water Research, 44(13), 3797–3806. 10.1016/j.watres.2010.04.031 [DOI] [PubMed] [Google Scholar]
- Bosco MV, Callao MP, and Larrechi MS (2006). Simultaneous analysis of the photocatalytic degradation of polycyclic aromatic hydrocarbons using three-dimensional excitation–emission matrix fluorescence and parallel factor analysis. Analytica Chimica Acta, 576(2), 184–191. 10.1016/j.aca.2006.06.005 [DOI] [PubMed] [Google Scholar]
- Anderson CM, and Bro R, (2003). Practical aspects of PARAFAC modeling of fluorescence excitation-emission data. Journal of Chemometrics. 17, 200. 10.1002/cem.790 [DOI] [Google Scholar]
- Brimo K, Gamier P, Sun S, Bertrand-Krajewski J-L, Cébron A, and Ouvrard S, (2016). Using a Bayesian approach to improve and calibrate a dynamic model of polycyclic aromatic hydrocarbons degradation in an industrial contaminated soil. Environmental Pollution, 215, 27–37. 10.1016/j.envpol.2016.04.094 [DOI] [PubMed] [Google Scholar]
- Bro R, (1997). PARAFAC. Tutorial and applications. Chemometrics and Intelligent Laboratory Systems, 23. [Google Scholar]
- Bro R, and Kiers HAL, (2003). A new efficient method for determining the number of components in PARAFAC models. Journal of Chemometrics, 77(5), 274–286. 10.1002/cem.801 [DOI] [Google Scholar]
- Carabajal MD, Arancibia JA, and Escandar GM, (2017). Excitation-emission fluorescence-kinetic data obtained by Fenton degradation. Determination of heavy-polycyclic aromatic hydrocarbons by four-way parallel factor analysis. Talanta, 165, 52–63. 10.1016/j.talanta.2016.12.030 [DOI] [PubMed] [Google Scholar]
- Chen Y, Chen Z-P, Yang J, Jin J-W, Zhang J, and Yu R-Q, (2013). Quantitative Fluorescence Spectroscopy in Turbid Media: A Practical Solution to the Problem of Scattering and Absorption. Analytical Chemistry, 85(4), 2015–2020. 10.1021/ac302815e [DOI] [PubMed] [Google Scholar]
- Chibwe L, Geier MC, Nakamura J, Tanguay RL, Aitken MD, and Simonich SLM, (2015). Aerobic Bioremediation of PAH Contaminated Soil Results in Increased Genotoxicity and Developmental Toxicity. Environmental Science and Technology, 49(23), 13889–13898. 10.1021/acs.est.5b00499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen JH, Hansen AB, Mortensen J, and Andersen O, (2005). Characterization and Matching of Oil Samples Using Fluorescence Spectroscopy and Parallel Factor Analysis. Analytical Chemistry, 77(7), 2210–2217. 10.1021/ac048213k [DOI] [PubMed] [Google Scholar]
- Driskill AK, Alvey J, Dotson AD, and Tomco PL, (2018). Monitoring polycyclic aromatic hydrocarbon (PAH) attenuation in Arctic waters using fluorescence spectroscopy. Cold Regions Science and Technology, 145, 76–85. 10.1016/j.coldregions.2017.09.014 [DOI] [Google Scholar]
- Elcoroaristizabal S, de Juan A, García JA, Durana N, and Alonso L, (2014). Comparison of second-order multivariate methods for screening and determination of PAHs by total fluorescence spectroscopy. Chemometrics and Intelligent Laboratory Systems, 132, 63–74. 10.1016/j.chemolab.2014.01.005 [DOI] [Google Scholar]
- Ferretto N, Tedetti M, Guigue C, Mounier S, Redon R, and Goutx M, (2014). Identification and quantification of known polycyclic aromatic hydrocarbons and pesticides in complex mixtures using fluorescence excitation–emission matrices and parallel factor analysis. Chemosphere, 107, 344–353. 10.1016/j.chemosphere.2013.12.087 [DOI] [PubMed] [Google Scholar]
- Gan S, Lau EV, and Ng HK (2009). Remediation of soils contaminated with polycyclic aromatic hydrocarbons (PAHs). Journal of Hazardous Materials, 772(2–3), 532–549. 10.1016/j.jhazmat.2009.07.l18 [DOI] [PubMed] [Google Scholar]
- Ghosal D, Ghosh S, Dutta TK, and Ahn Y, (2016). Current State of Knowledge in Microbial Degradation of Polycyclic Aromatic Hydrocarbons (PAHs): A Review. Frontiers in Microbiology, 7. 10.3389/fmicb.2016.01369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitipour S, Sorial GA, Ghasemi S, and Bazyari M, (2018). Treatment technologies for PAH-contaminated sites: A critical review. Environmental Monitoring and Assessment, 190(9), 546. 10.1007/sl0661-018-6936-4 [DOI] [PubMed] [Google Scholar]
- Gómez V, and Callao MP (2008). Analytical applications of second-order calibration methods. Analytica Chimica Acta, 627(2), 169–183. 10.1016/j.aca.2008.07.054 [DOI] [PubMed] [Google Scholar]
- Gróning JAD, Kaschabek SR, Schlómann M, and Tischler D, (2014). A mechanistic study on SMOB-ADP1: An NADH:flavin oxidoreductase of the two-component styrene monooxygenase of Acinetobacter baylyi ADP1. Archives of Microbiology, 196(12), 829–845. 10.1007/s00203-014-1022-y [DOI] [PubMed] [Google Scholar]
- Hu Y, Zhang H, and Zhao D, (2021). Transform method in three-dimensional fluorescence spectra for direct reflection of internal molecular properties in rapid water contaminant analysis. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 250, 119376. 10.1016/j.saa.2020.119376 [DOI] [PubMed] [Google Scholar]
- Hung C-M, Chen C-W, Huang C-P, Cheng J-W, and Dong C-D, (2022). Algae-derived metal-free boron-doped biochar as an efficient bioremediation pretreatment for persistent organic pollutants in marine sediments. Journal of Cleaner Production, 336, 130448. 10.1016/j.jclepro.2022.130448 [DOI] [Google Scholar]
- Kanick SC, Robinson DJ, Sterenborg HJCM, and Amelink A, (2012). Extraction of intrinsic fluorescence from single fiber fluorescence measurements on a turbid medium. Optics Letters, 37(5), 948. 10.1364/OL.37.000948 [DOI] [PubMed] [Google Scholar]
- Kottegoda S, Waligora E, and Hyman M, (2015). Metabolism of 2-Methylpropene (Isobutylene) by the Aerobic Bacterium Mycobacterium sp. Strain ELW1. Applied and Environmental Microbiology, 81(6), 1966–1976. 10.1128/AEM.03103-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krippaehne K, (2018). Cometabolism of 1,4-Dioxane and Chlorinated Aliphatic Hydrocarbons by Pure Cultures of Rhodococcus rhodochrous 21198 and Mycobacterium ELW1 and in Groundwater Microcosms Fed Isobutane and Isobutene as Growth Substrates. (Master’s Thesis). Oregon State University. [Google Scholar]
- Krzyszczak A, and Czech B, (2021). Occurrence and toxicity of polycyclic aromatic hydrocarbons derivatives in environmental matrices. Science of The Total Environment, 788, 147738. 10.1016/j.scitotenv.2021.147738 [DOI] [PubMed] [Google Scholar]
- Kumar M, Bolan NS, Hoang SA, Sawarkar AD, Jasemizad T, Gao B, Keerthanan S, Padhye LP, Singh L, Kumar S, Vithanage M, Li Y, Zhang M, Kirkham MB, Vinu A, and Rinklebe J, (2021). Remediation of soils and sediments polluted with polycyclic aromatic hydrocarbons: To immobilize, mobilize, or degrade? Journal of Hazardous Materials, 420, 126534. 10.1016/jjhazmat.2021.126534 [DOI] [PubMed] [Google Scholar]
- Lakowicz JR, Shen Y, D’Auria S, Malicka J, Fang J, Gryczynski Z, and Gryczynski I, (2002). Radiative Decay Engineering. Analytical Biochemistry, 301(2), 261–277. 10.1006/abio.2001.5503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Oturan N, Zhang H, and Oturan MA, (2020). Soil washing in combination with electrochemical advanced oxidation for the remediation of synthetic soil heavily contaminated with diesel. Chemosphere, 249, 126176. 10.1016/j.chemosphere.2020.126176 [DOI] [PubMed] [Google Scholar]
- Maggio RM, Damiani PC, and Olivieri AC (2010). Four-way kinetic-excitation-emission fluorescence data processed by multi-way algorithms. Determination of carbaryl and 1-naphthol in water samples in the presence of fluorescent interferents. Analytica Chimica Acta, 677(2), 97–107. 10.1016/j.aca.2010.07.045 [DOI] [PubMed] [Google Scholar]
- Mayevsky A, and Rogatsky GG, (2007). Mitochondrial function in vivo evaluated by NADH fluorescence: From animal models to human studies. American Journal of Physiology-Cell Physiology, 292(2), C615–C640. 10.1152/ajpcell.00249.2006 [DOI] [PubMed] [Google Scholar]
- Murphy KR, Stedmon CA, Graeber D, and Bro R, (2013). Fluorescence spectroscopy and multi-way techniques. PARAFAC. Analytical Methods, 5(23), 6557. 10.1039/c3ay41160e [DOI] [Google Scholar]
- Nahorniak ML, and Booksh KS, (2006). Excitation-emission matrix fluorescence spectroscopy in conjunction with multiway analysis for PAH detection in complex matrices. The Analyst, 131(12), 1308. 10.1039/b609875d [DOI] [PubMed] [Google Scholar]
- Olivieri AC, Arancibia JA, Muñoz de la Peña A, Durán-Merás I, and Espinosa Mansilla A, (2004). Second-Order Advantage Achieved with Four-Way Fluorescence Excitation–Emission–Kinetic Data Processed by Parallel Factor Analysis and Trilinear Least-Squares. Determination of Methotrexate and Leucovorin in Human Urine. Analytical Chemistry, 76(19), 5657–5666. 10.1021/ac0493065 [DOI] [PubMed] [Google Scholar]
- Patel AB, Shaikh S, Jain KR, Desai C, and Madamwar D, (2020). Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Frontiers in Microbiology, 11. 10.3389/fmicb.2020.562813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, He X, and Pan H, (2018). Spectroscopic study on transformations of dissolved organic matter in coal-to-liquids wastewater under integrated chemical oxidation and biological treatment process. Journal of Environmental Sciences, 70, 206–216. 10.1016/jjes.2018.04.006 [DOI] [PubMed] [Google Scholar]
- Phillips DH, (1999). Polycyclic aromatic hydrocarbons in the diet. Mutation Research Genetic Toxicology and Environmental Mutagenesis, 443(1–2), 139–147. 10.1016/S1383-5742(99)00016-2 [DOI] [PubMed] [Google Scholar]
- Rich S, (2015) Kinetic Analysis of the Aerobic Degradation of Chlorinated Ethenes by the Mycobacterium ELW-1 and Chlorinated Ethanes and Ethenes by Rhodococcus rhodochrous ATCC 21198. (Honor’s Thesis). Oregon State University. [Google Scholar]
- Rutherford JW, Dawson-Elli N, Manicone AM, Korshin GV, Novosselov IV, Seto E, and Posner JD, (2020). Excitation emission matrix fluorescence spectroscopy for combustion generated particulate matter source identification. Atmospheric Environment, 220, 117065. 10.1016/j.atmosenv.2019.117065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakshi, and Haritash AK, (2020). A comprehensive review of metabolic and genomic aspects of PAH-degradation. Archives of Microbiology, 202(8), 2033–2058. 10.1007/s00203-020-01929-5 [DOI] [PubMed] [Google Scholar]
- Schrlau JE, Kramer AL, Chlebowski A, Truong L, Tanguay RL, Simonich SLM, and Semprini L, (2017). Formation of Developmentally Toxic Phenanthrene Metabolite Mixtures by Mycobacterium sp. ELW1. Environmental Science and Technology, 51(15), 8569–8578. 10.1021/acs.est.7b01377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seopela MP, Powers LC, Clark C, Heyes A, and Gonsior M, (2021). Combined fluorescent measurements, parallel factor analysis and GC-mass spectrometry in evaluating the photodegradation of PAHS in freshwater systems. Chemosphere, 269, 129386. 10.1016/j.chemosphere.2020.129386 [DOI] [PubMed] [Google Scholar]
- Stedmon CA, and Markager S, (2005). Resolving the variability in dissolved organic matter fluorescence in a temperate estuary and its catchment using PARAFAC analysis. Limnology and Oceanography, 50(2), 686–697. 10.4319/lo.2005.50.2.0686 [DOI] [Google Scholar]
- Taylor AE, Vajrala N, Giguere AT, Gitelman AI, Arp DJ, Myrold DD, Sayavedra-Soto L, and Bottomley PJ, (2013). Use of Aliphatic n -Alkynes To Discriminate Soil Nitrification Activities of Ammonia-Oxidizing Thaumarchaea and Bacteria. Applied and Environmental Microbiology, 79(21), 6544–6551. 10.1128/AEM.01928-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titaley IA, Simonich SLM, and Larsson M, (2020). Recent Advances in the Study of the Remediation of Polycyclic Aromatic Compound (PAC)-Contaminated Soils: Transformation Products, Toxicity, and Bioavailability Analyses. Environmental Science and Technology Letters, 7(12), 873–882. 10.1021/acs.estlett.0c00677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trine LSD, Davis EL, Roper C, Truong L, Tanguay RL, and Simonich SLM, (2019). Formation of PAH Derivatives and Increased Developmental Toxicity during Steam Enhanced Extraction Remediation of Creosote Contaminated Superfund Soil. Environmental Science and Technology, 53(8), 4460–4469. 10.1021/acs.est.8b07231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- US EPA, 2009. National Primary Drinking Water Regulations. EPA; 816-F-09-004. [Google Scholar]
- US EPA, 2014. Priority Pollutants List. 40 CFR Part 423, Appendix A. [Google Scholar]
- Wu J, Feld MS, and Rava RP, (1993). Analytical model for extracting intrinsic fluorescence in turbid media. Applied Optics, 32(19), 3585. 10.1364/AO.32.003585 [DOI] [PubMed] [Google Scholar]
- Yang R, Zhao N, Xiao X, Yin G, Yu S, Liu J, and Liu W, (2016). Quantifying PAHs in water by three-way fluorescence spectra and second-order calibration methods. Optics Express, 24(14), A1148. 10.1364/OE.24.0A1148 [DOI] [PubMed] [Google Scholar]
- Zhou Z, Liu Z, & Guo L, (2013). Chemical evolution of Macondo crude oil during laboratory degradation as characterized by fluorescence EEMs and hydrocarbon composition. Marine Pollution Bulletin, 66(1–2), 164–175. 10.1016/j.marpolbul.2012.09.028 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


