Abstract
Isonitrile‐containing natural products have garnered attention for their manifold bioactivities but are difficult to detect and isolate due to the chemical lability of the isonitrile functional group. Here, we used the isonitrile‐chlorooxime ligation (INC) in a reactivity‐based screening (RBS) protocol for the detection and isolation of alkaloid and terpene isonitriles in the cyanobacterium Fischerella ambigua and a marine sponge of the order Bubarida, respectively. A trifunctional probe bearing a chlorooxime moiety, a UV active aromatic moiety, and a bromine label facilitated the chemoselective reaction with isonitriles, UV‐Vis spectroscopic detection, and mass spectrometric analysis. The INC‐based RBS allowed for the detection, isolation, and structural elucidation of isonitriles in microgram quantities.
Keywords: Chemoselective, ligation, chlorooximes, isonitriles, natural products, reactivity-based screening
A trifunctional probe consisting of a chlorooxime, a bromine mass tag, and a UV tag facilitated the detection, isolation, and structure elucidation of isonitrile‐containing natural products in microgram quantities in cyanobacteria and a rare marine sponge.
Natural products featuring an isonitrile functional group occur in a wide variety of natural sources including bacteria, fungi, marine sponges, and plants (Figure 1a).[ 1 , 2 , 3 , 4 , 5 , 6 ] Many exhibit potent bioactivity against bacteria, fungi, and parasites.[ 4 , 5 , 6 , 7 ] Natural isonitriles are therefore promising targets and lead structures for drug development. The detection and isolation of isonitrile‐containing natural products is, however, challenging as the isonitrile functional group is not UV‐active and is inherently labile towards hydrolysis under acidic and strongly basic conditions.
Figure 1.
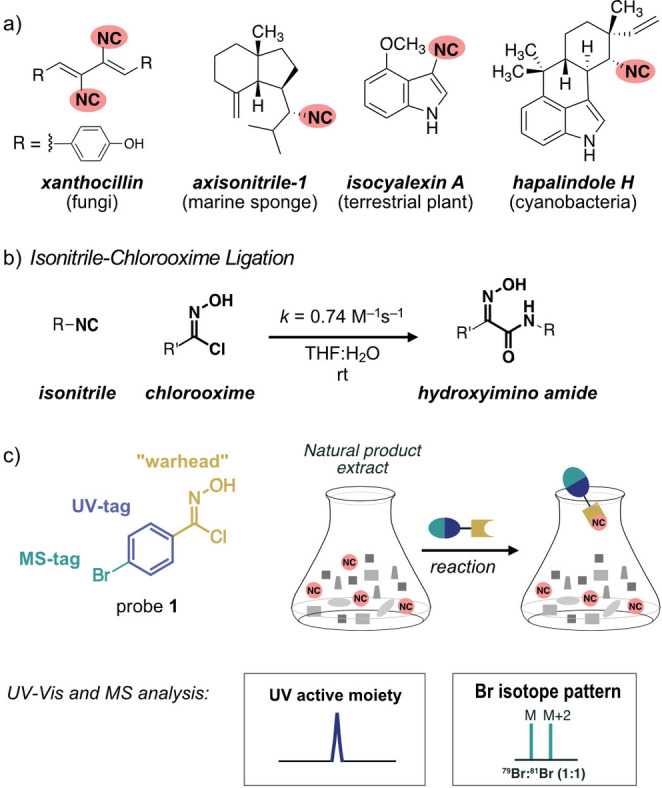
a) Examples of isonitriles and their natural sources. b) Isonitrile‐chlorooxime ligation. c) Reactivity‐based screening (RBS) for isonitriles with probe 1.
Reactivity‐based screening (RBS) – the chemical labeling of a specific functional group in a natural product – is an attractive method to overcome challenges arising from poor detectability, low bioavailability, and chemical instability.[ 8 , 9 ] Ideal RBS reactions are highly chemoselective for the target functional group, form a covalent bond that is stable under isolation conditions, and afford a label that improves detection, for example, by UV‐Vis spectroscopy or mass spectrometry (MS).[ 10 , 11 ] In a recent report, a tetrazine‐based probe was used for the qualitative detection of isonitrile‐containing natural products. [12] This [4+1] tetrazine‐isonitrile ligation provides stable products with tertiary isonitriles. In the case of primary and secondary isonitriles the initially formed 4H‐pyrazol‐4‐imine product undergoes spontaneous hydrolysis and escapes detection [13] if not trapped through reduction with NaBH3CN. [12]
We recently developed the isonitrile‐chlorooxime (INC) ligation (Figure 1b). [14] This highly chemoselective reaction is fast (0.74 M− 1s−1) and allows for the labeling of cell surface glycans in cellulo. [14] The resulting hydroxyimino amide products are stable, regardless of whether primary, secondary, or tertiary isonitriles serve as reaction partners. [15] We, therefore, anticipated that appropriately functionalized chlorooximes could serve as powerful probes for the RBS facilitated detection, isolation, and – ideally also – structural elucidation of isonitriles in complex biological matrices. We envisioned brominated chlorooxime 1 as a suitable probe for RBS for isonitriles (Figure 1c). This probe consists of a) a chlorooxime moiety as a “warhead” for chemoselective reaction with isonitriles, b) a bromine substituent as a mass tag to facilitate MS‐based analysis by providing a distinct 79Br : 81Br (1 : 1) isotope pattern in the labeled natural products, [16] and c) an aromatic moiety for enhanced UV absorption. Probe 1 was readily available in two synthetic steps starting from commercial 4‐bromo benzaldehyde (Scheme S1 in the Supporting Information). [17]
Here, we applied the INC ligation for the detection and identification of isonitrile‐containing alkaloids and terpenes in the cyanobacterium Fischerella ambigua (F. ambigua) and the Bubarida marine sponge. We show that INC is a robust tool for RBS and sufficiently sensitive to allow even for the identification and isolation of isonitrile natural products that occur in minute quantities.
We started our RBS for isonitriles by exploring whether probe 1 detects isonitriles in a crude extract of F. ambigua (Figure 2a). This culturable cyanobacterium is a well‐studied producer of tryptophan‐derived isonitrile‐containing indole alkaloids, including hapalindoles and ambiguines.[ 18 , 19 ] Analysis of a crude methanol extract of cultured strain UTEX 1903 by ultrahigh performance liquid chromatography (UHPLC) coupled with high‐resolution mass spectrometry (HRMS) detected seven known isonitrile indole alkaloids under our laboratory conditions (Figures S1a–S9a).
Figure 2.
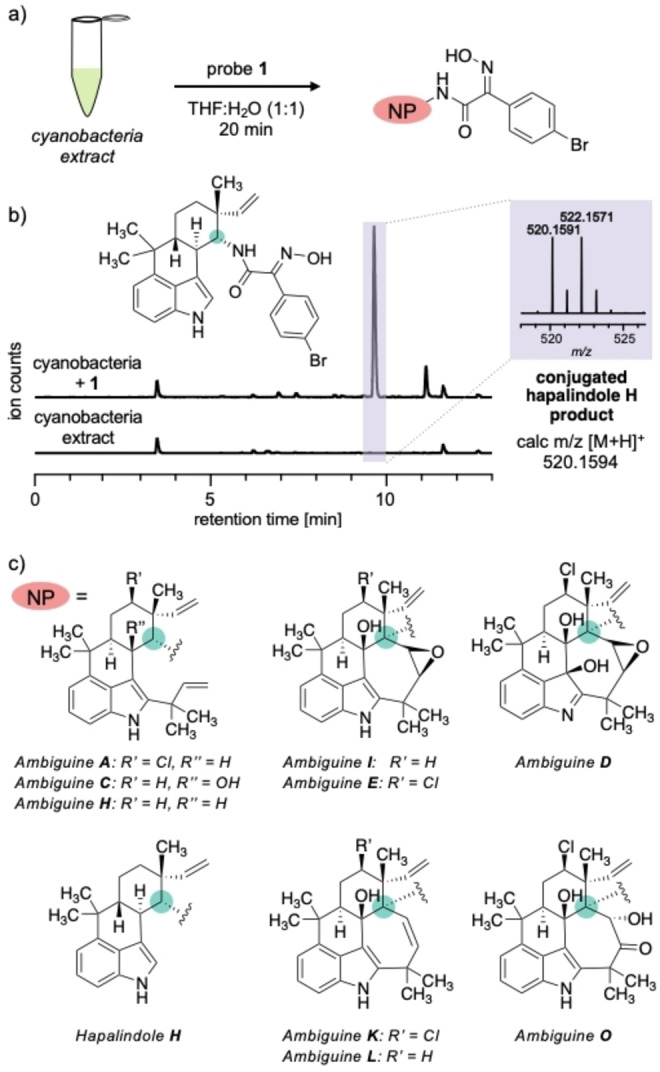
a) Incubation of a crude extract of F. ambigua (strain UTEX 1903) with 1. b) Representative extracted ion chromatogram of the extract with (top) and without (bottom) probe 1, for the mass of conjugated hapalindole H. c) Labeled isonitrile‐containing congeners of natural products (a green dot indicates the location of the isonitrile group and the attachment site of probe 1).
Upon incubation of the dried crude extract with probe 1 in a THF/water (1 : 1) mixture followed by UHPLC‐HRMS analysis, we observed nine signals with a bromine isotope pattern (Figures 2b and S1b–S9b). Controls that contained only probe 1 and only the crude extract, respectively, did not show these signals (Figures S1b–S9b). The signals correspond to the masses of the conjugates between probe 1 and ambiguine A, C, H, I, E, D or O, K, and L and hapalindole H, nine isonitrile indole alkaloids produced by the F. ambigua UTEX 1903 strain (Figure 2c). Thus, conjugation with probe 1 enhanced the detection limit of isonitriles in the natural product extract. The detected natural products contain secondary and tertiary isonitriles. These results highlight the value of probe 1 for the identification of different types of isonitriles through RBS in complex mixtures.
Next, we tested the value of chlorooxime probe 1 for the identification and isolation of isonitrile‐containing natural products in a marine sponge. In contrast to cyanobacteria, which are culturable and therefore allow for increasing the production of natural isonitriles in the laboratory, marine sponges are typically not maintained in the laboratory, least scaled‐up. Additionally, many marine sponge environments are under increased threat from ocean warming, terrestrial pollution, overfishing, and other stressors, which makes it increasingly more challenging to find and collect significant quantities of isonitrile‐producing sponges. Thus, the detection, isolation, and characterization of such low‐abundance sponge natural products require a highly sensitive methodology.
SCUBA collections off the coast of California gave access to 20 g (wet weight) of a marine sponge specimen that is a known producer of isonitrile‐containing terpenes. [20] Extraction with methylene chloride provided ∼60 mg (dry weight) of a crude extract. Gas chromatography−mass spectrometry (GC‐MS) analysis revealed mass‐to‐charge ratios corresponding to putative nitrogenous sesquiterpenes, including isonitrile, isocyanate, formamide, and isothiocyanate congeners as judged by comparison with spectral data from the NIST mass spectral library (Figure S10). [21] Isolation and structural characterization of the isonitriles by conventional means proved, however, challenging due to their low UV activity, high lipophilicity, and volatility. We, therefore, envisioned that labeling the isonitrile‐sesquiterpenes with our chlorooxime probe would facilitate MS and UV analysis‐supported detection and, ideally, chromatographic purification and elucidation of the constitutive structure of the terpene backbone.
Treatment of the Bubarida sponge crude extract with chlorooxime probe 1 in THF and citrate buffer pH 5 (1 : 1) [22] followed by LC‐UV analysis revealed two major signals (dark and light blue) and one minor signal (gray; Figure 3a). [23] The MS spectra associated with these UV signals I, II, and III showed the bromine isotope pattern (Figure 3b). Controls that contained only probe 1 or only the crude extract did not show these signals (Figure 3a) corroborating that these products are derived from labeled isonitriles. [23] The mass spectra corresponding to the UV signals I and II (t=17.5 and 17.9 min) show the same mass‐to‐charge ratio of [M+H]+=447.17 Da suggesting that the isonitrile precursors are constitutional isomers with a C15 terpene‐skeleton (Figure 3a). The UV‐activity of the labeled compounds allowed for isolation of these two major products (Figure 3a, dark and light blue) in a quantity of less than 500 μg by preparative thin‐layer chromatography (TLC) and normal phase HPLC. This amount sufficed for 1D and 2D NMR spectroscopic analysis including 2D nuclear Overhauser effect (NOE) to assign the relative stereochemistry (Tables S1 and S2, Figures S13 and S14). These analyses revealed that LC UV signal II corresponds to sesquiterpene‐probe conjugate 2 and thus the known isonitrile congener (3) of sesquiterpene isothiocyanate epipolasin A (Figure 3b).[ 24 , 25 ] Signal I corresponds to sesquiterpene‐probe conjugate 4 and thus cadinane‐type sesquiterpene 5, which is a constitutional isomer of 3 (Figure 3b). 5 is a known sesquiterpene from the Mediterranean sponge Axinyssa sp. and Axinella cannabina (Figure 3c),[ 26 , 27 ] but has not been reported from this Bubarida sponge.
Figure 3.
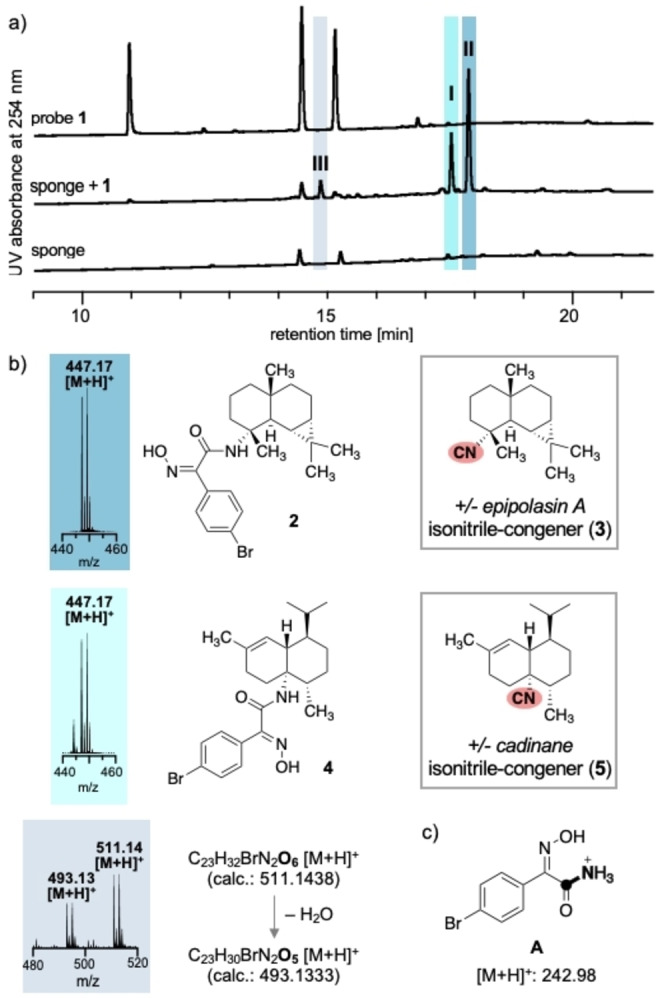
a) Stacked LC‐UV chromatograms of probe 1 (top), sponge crude extract with (middle) and without (bottom) 1. b) MS spectra of the newly formed products as monitored by UV (blue: I and II; gray: III) 2 and 4 and the corresponding isonitrile‐containing epipolasin A (3) and cadinane‐type (5) sesquiterpenoids. c) Fragment A formed by MS/MS from ligation products.
The amount of the compound(s) corresponding to the minor LC UV signal III (t=14.9 min, Figure 3a) did not suffice for isolation. Analysis of the corresponding MS spectrum revealed molecular ions at m/z 493.1314 [M+H]+ and 511.1408 [M+H]+. These masses are in agreement with molecular formulas of oxygenated isonitrile sesquiterpenoids (e. g., [C23H30BrN2 O5 ]+ and [C23H32BrN2 O6 ]+), compounds that are rare [3] and have not yet been observed in the Bubarida sponge. Thus, the chemoselective ligation with probe 1 revealed compounds in a marine sponge that could not be detected with conventional analytical methods.
These findings also corroborate the exquisite chemoselectivity for isonitriles of the INC ligation. Products from reaction of probe 1 with compounds bearing other functional groups, including closely related isocyanates and isothiocyanates that are produced by the Bubarida sponge, [26] were not detected. We confirmed this notable chemoselectivity of chlorooxime reactivity further through competition experiments between isocyanates, isothiocyanates and isonitriles for reaction with probe 1 (Scheme S2, Figures S15 and S16). [28]
Furthermore, MS/MS spectra of each of the three compounds showed upon collision‐induced dissociation a common fragment with a mass‐to‐charge ratio of 242.98 [M+H]+ obs (Figure S17), which corresponds to hydroxyimino amide A (242.9764 [M+H]+ calc, Figure 3c). To ascertain the origin of this MS/MS fragment, we synthesized hydroxyimino amide S3 from probe 1 and 1,1,3,3,‐tetramethyl butyl isocyanide. MS/MS analysis of S3 showed the formation of the same fragment A (Figure S18). Thus, A arises from MS fragmentation of a conjugate between probe 1 and an isonitrile (Scheme S3). The occurrence of MS/MS fragment A is therefore diagnostic for isonitriles and a valuable signature for isonitriles. [29]
In conclusion, reactivity‐based screening by using isonitrile‐chlorooxime ligation is a valuable tool for the labeling, detection, and isolation of isonitrile‐containing natural products at microgram quantities in complex mixtures. This work puts forth a trifunctional probe consisting of a) a chlorooxime for chemoselective reaction with isonitriles in complex mixtures, b) an aromatic moiety for detection by UV‐Vis, and c) a bromine mass tag for detection by mass spectrometry. The probe facilitated the identification, isolation, and structure elucidation of alkaloid and terpene isonitriles in cyanobacteria and marine sponges collected in the field. Our findings open exciting prospects for the identification of isonitrile natural products with as‐yet unknown bioactivities.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgments
H.W., S.S., and R.J.B.S. gratefully acknowledge the Swiss National Science Foundation for funding (200020_18729/1; H.W.) (CRSK‐2_190556; Spark grant S.S.), and (P2EZP3_195643; postdoctoral fellowship R.J.B.S.). B.S.M. acknowledges NIH grant R01‐GM085770. K.W. graciously acknowledges the support from the National Science Foundation's Graduate Research Fellowship Program and the University of California San Diego Marine Sciences Academic Senate Grant. We thank Reiko Cullum (Prof. W. Fenical, Scripps Institution of Oceanography) for access to normal‐phase HPLC analysis and Dr. Constanze Paulus for preliminary experiments with F. ambigua. We thank Prof. Thomas Turner (UCSB) for marine sponge taxonomic analysis.
Schäfer R. J. B., Wilson K., Biedermann M., Moore B. S., Sieber S., Wennemers H., Chem. Eur. J. 2023, 29, e202203277.
Contributor Information
Prof. Bradley S. Moore, Email: bsmoore@ucsd.edu, https://bsmoore.scrippsprofiles.ucsd.edu.
Dr. Simon Sieber, Email: simon.sieber@chem.uzh.ch.
Prof. Helma Wennemers, Email: Helma.Wennemers@org.chem.ethz.ch, https://wennemers.ethz.ch.
References
- 1. Edenborough M. S., Herbert R. B., Nat. Prod. Rep. 1988, 5, 229–245. [DOI] [PubMed] [Google Scholar]
- 2. Garson M. J., Simpson J. S., Nat. Prod. Rep. 2004, 21, 164–179. [DOI] [PubMed] [Google Scholar]
- 3. Emsermann J., Kauhl U., Opatz T., Mar. Drugs 2016, 14, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schnermann M., Shenvi R. A., Nat. Prod. Rep. 2015, 32, 543–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hohlmann R. M., Sherman D. H., Nat. Prod. Rep. 2021, 38, 1567–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Massarotti A., Brunelli F., Aprile S., Giustiniano M., Tron G. C., Chem. Rev. 2021, 121, 10742–10788. [DOI] [PubMed] [Google Scholar]
- 7.For an early example, see: Rothe W., Dtsch. Med. Wochenschr. 1949, 79, 1080–1081. [DOI] [PubMed] [Google Scholar]
- 8. Cox C. L., Tietz J. I., Sokolowski K., Melby J. O., Doroghazi J. R., Mitchell D. A, ACS Chem. Biol. 2014, 9, 2014–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.For a recent review, see: Hughes C. C., Nat. Prod. Rep. 2021, 38, 1684–1705. [DOI] [PubMed] [Google Scholar]
- 10.For examples, see:
- 10a. Ross C., Scherlach K., Kloss F., Hertweck C., Angew. Chem. Int. Ed. 2014, 53, 7794–7798; [DOI] [PubMed] [Google Scholar]
- 10b. Jeon H., Lim C., Lee J. M., Kim S., Chem. Sci. 2015, 6, 2806–2811; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10c. Schnell S. D., Hoff L. V., Panchagnula A., Wurzenberger M. H. H., Klapötke T. M., Sieber S., Linden A., Gademann K., Chem. Sci. 2020, 11, 3042–3047; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10d. Rudolf G. C., Koch M. F., Mandl F. A. M., Sieber S. A., Chem. Eur. J. 2015, 21, 3701–3707. [DOI] [PubMed] [Google Scholar]
- 11.For examples, see:
- 11a. Jiang Q., Zhu Z., Shou P., Teng F., Zhu Y., Zhao H., Yang B., Phytochem. Anal. 2020, 31, 322–332; [DOI] [PubMed] [Google Scholar]
- 11b. Castro-Falcón G., Hahn D., Reimer D., Hughes C. C., ACS Chem. Biol. 2016, 11, 2328–2336; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11c. Maxson T., Tietz J. I., Hudson G. A., Guo X. R., Tai H.-C., Mitchell D. A., J. Am. Chem. Soc. 2016, 138, 15157–15166; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11d. Back D., Shaffer B. T., Loper J. E., Philmus B., J. Nat. Prod. 2022, 85, 105–114; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11e. Castro-Falcón G., Millán-Aguiñaga N., Roullier C., Jensen P. R., Hughes C. C., ACS Chem. Biol. 2018, 13, 3097–3106. [DOI] [PubMed] [Google Scholar]
- 12. Huang Y.-B., Cai W., Del Rio Flores A., Twigg F. F., Zhang W., Anal. Chem. 2020, 92, 599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.
- 13a. Stöckmann H., Neves A. A., Stairs S., Brindle K. M., Leeper F. J., Org. Biomol. Chem. 2011, 9, 7303–7305; [DOI] [PubMed] [Google Scholar]
- 13b. Stairs S., Neves A. A., Stöckmann H., Wainman Y. A., Ireland-Zecchini H., Brindle K. M., Leeper F. J. ChemBioChem 2013, 14, 1063–1067. For a recent report introducing a more stable product, see: [DOI] [PMC free article] [PubMed] [Google Scholar]; Tu J., Svatunek D., Parvez S., Liu A. C., Levandowski B. J., Eckvahl H. J., Peterson R. T., Houk K. N., Franzini R. M., Angew. Chem. Int. Ed. 2019, 58, 9043–9048; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 9141–9146. [Google Scholar]
- 14. Schäfer R. J. B., Monaco M. R., Li M., Tirla A., Rivera-Fuentes P., Wennemers H., J. Am. Chem. Soc. 2019, 141, 18644–18648. [DOI] [PubMed] [Google Scholar]
- 15. Soeta T., Takashita S., Ukaji Y. Sakata Y., Org. Biomol. Chem. 2016, 14, 694–700. [DOI] [PubMed] [Google Scholar]
- 16. Palaniappan K. K., Pitcher A. A., Smart B. P., Spiciarich D. R., Iavarone A. T., Bertozzi C. R., ACS Chem. Biol. 2011, 6, 829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.For a previous use of probe 1, see: Sanders B. C., Friscourt F., Ledin P. A., Mbua N. E., Arumugam S., Guo J., Boltje T. J., Popik V. V., Boons G.-J., J. Am. Chem. Soc. 2011, 133, 949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.
- 18a. Raveh A., Carmeli S., J. Nat. Prod. 2007, 70, 196–201; [DOI] [PubMed] [Google Scholar]
- 18b. Mo S., Krunic A., Chlipala G., Orjala J., J. Nat. Prod. 2009, 72, 894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smitka T. A., Bonjouklian R., Doolin L., Jones N. D., Deeter J. B., Yoshida W. Y., Prinsep M. R., Moore R. E., Patterson G. M. L., J. Org. Chem. 1992, 57, 857–861. [Google Scholar]
- 20. Thompson J. E., Walker R. P., Faulkner D. J., Mariani Found. Paediatr. Neurol. Ser. 1985, 21, 11–21. [Google Scholar]
- 21. https://chemdata.nist.gov.
- 22.Reactions in THF/H2O (1 : 1) gave comparable results, see the Supporting Information for details.
- 23.Of note, conjugation with probe 1 increases the detectability markedly (∼200-fold; Figure S12). Under the chromatography conditions, degradation products of probe 1 emerge, see also Figure S11.
- 24. Tada H., Yasuda F., Chem. Pharm. Bull. 1985, 33, 1941–1945. [Google Scholar]
- 25.
- 25a.R. P. Walker, The Chemical Ecology of Some Sponges and Nudibranchs from San Diego, Dissertation University of California, San Diego, 1981;
- 25b. Thompson J. E., Walker R. P., Wratten S. J., Faulkner D. J., Tetrahedron 1982, 38, 1865–1873. [Google Scholar]
- 26.“Marine Isocyano Compounds”, C. W. J. Chang, P. J. Scheuer in Studiecent. TNO Scheepsbouw Navig. Rep. (Ed.: P. J. Scheuer), Springer, Berlin, 1993. pp. 33–75.
- 27.
- 27a. Ciminiello P., Fattorusso E., Magno S., Mayol L., Experientia 1986, 42, 625–627; [Google Scholar]
- 27b. Capon R. J., MacLeod J. K., Aust. J. Chem. 1988, 41, 979–983; [Google Scholar]
- 27c. Kurnianda V., Hirade H., Jansen R., Tanaka J. J. Asian Nat. Prod. Res. 2022, 24, 39–44. [DOI] [PubMed] [Google Scholar]
- 28.The INC ligation can be performed in water/acetonitrile mixtures, highlighting the chemoselectivity of chlorooximes for isonitriles over nitriles.
- 29.Note, chlorooximes can also react with thiols (ref. [14]). Thus, this signature can be particularly valuable when isonitriles occur in a mixture with thiols.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information


