Graphical abstract
Keywords: COVID-19, Acute respiratory distress syndrome (ARDS), Critical care, Non invasive respiratory support (NIRS), Continuous positive airway pressure (CPAP), High-flow nasal cannula (HFNC)
Abstract
Introduction
Non invasive respiratory support (NIRS) is useful for treating acute respiratory distress syndrome (ARDS) secondary to COVID-19, mainly in mild–moderate stages. Although continuous positive airway pressure (CPAP) seems superior to other NIRS, prolonged periods of use and poor adaptation may contribute to its failure. The combination of CPAP sessions and high-flow nasal cannula (HFNC) breaks could improve comfort and keep respiratory mechanics stable without reducing the benefits of positive airway pressure (PAP). Our study aimed to determine if HFNC + CPAP initiates early lower mortality and endotracheal intubation (ETI) rates.
Methods
Subjects were admitted to the intermediate respiratory care unit (IRCU) of a COVID-19 monographic hospital between January and September 2021. They were divided according to Early HFNC + CPAP (first 24 h, EHC group) and Delayed HFNC + CPAP (after 24 h, DHC group). Laboratory data, NIRS parameters, and the ETI and 30-day mortality rates were collected. A multivariate analysis was performed to identify the risk factors associated with these variables.
Results
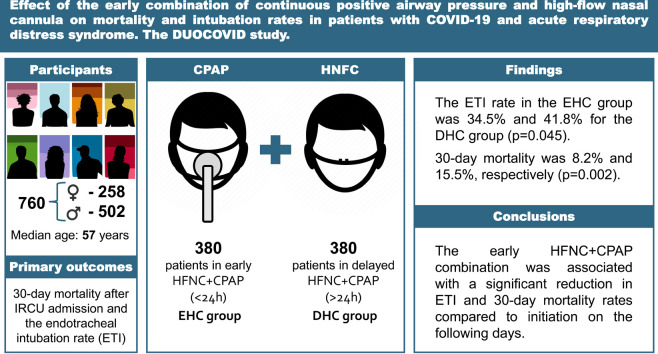
The median age of the 760 included patients was 57 (IQR 47–66), who were mostly male (66.1%). The median Charlson Comorbidity Index was 2 (IQR 1–3) and 46.8% were obese. The median PaO2/FiO2 upon IRCU admission was 95 (IQR 76–126). The ETI rate in the EHC group was 34.5%, with 41.8% for the DHC group (p = 0.045), while 30-day mortality was 8.2% and 15.5%, respectively (p = 0.002).
Conclusions
Particularly in the first 24 h after IRCU admission, the HFNC + CPAP combination was associated with a reduction in the 30-day mortality and ETI rates in patients with ARDS secondary to COVID-19.
Introduction
In December 2019, a series of pneumonia cases of unknown cause emerged in Wuhan (Hubei), China, whose clinical presentation was like viral pneumonia.1 The disease was named COVID-19. In March 2020, the WHO declared a pandemic due to the exponential increase in SARS-CoV-2 infections.2 Since then, healthcare systems around the world have been compromised because about 5% of patients develop critical illness secondary to acute respiratory distress syndrome (ARDS) and have to be admitted to high complexity units for respiratory support.3, 4, 5, 6
Prepandemic studies have considered invasive mechanical ventilation (IMV) to be the first therapeutic option for patients in moderate or severe stages of ARDS.7, 8 However, the “ICU overwhelming” circumstance during the pandemic9, 10 led to a search being made for therapeutic alternatives, such as non invasive respiratory support (NIRS), given the possibility of preventing IMV in some individuals. These therapies are effective in many cases and offer added benefits, such as reducing not only IMV-related complications, including nosocomial infections, myopathy in critically ill patients and delirium,11 but also hospital stays and costs.12 These NIRS cases include high-flow nasal cannula (HFNC) and positive airway pressure (PAP) therapy either with continuous positive airway pressure (CPAP) or non invasive ventilation (NIV).
No consensus has been reached by different scientific societies about the most suitable NIRS for patients with ARDS due to COVID-19.13 A recent study demonstrated reduced tracheal intubation and lower mortality rates in patients with acute respiratory hypoxemic failure (ARHF) treated with CPAP compared to conventional oxygen therapy.14 This could be due to the ability to recruit alveoli and improve respiratory mechanics.15 In contrast, no differences appear between HFNC and conventional oxygen therapy.14 However, in patients who poorly adapt to and/or are intolerant to PAP, increased transpulmonary pressure and the occurrence of patient self-induced lung injury (P-SILI) may increase failure rates16 and result in increased mortality due to delayed IMV.8 For CPAP, prolonged periods of use and delivered pressure may be associated with poorer outcomes.17
Studies in post-extubation patients treated with HFNC + NIV combination have shown a lowering NIRS failure rate. This outcome is attributed to the benefits of positive pressure by allowing breaks with HNFC, which increases patient comfort by maintaining both peripheral oxygen saturation and respiratory rates stable compared to conventional oxygen therapy.18 To date, there is no evidence for the usefulness of this strategy in patients with ARDS.
In this context, we propose that the combined use of therapies, understood as treatment with HFNC plus positive pressure sessions with CPAP, can be a useful tool in the non invasive management of patients with ARDS secondary to SARS-CoV-2. Despite the theoretical benefits, a priori the profile of a patient requiring such treatment has not yet been described, nor has the ideal time to initiate it. For this reason, our main objective was to study the benefits of administering this combined therapy in the first 24 h after admission to intermediate respiratory care units (IRCUs).
Methods
Study design
This single-center observational cohort study was conducted in the IRCU of the “Enfermera Isabel Zendal” Emergency Hospital (HEEIZ, Madrid, Spain) between January 8 and September 28, 2021. This multidisciplinary unit included pulmonologists, anesthesiologists and emergency physicians. The protocol was approved by the local regulatory ethics committee (La Paz University Hospital; Madrid, Spain). It respected the ethical principles of the Declaration of Helsinki. Informed consent was waived given the nature of this study. The manuscript was drafted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement guidelines.19
Participants
It consecutively recruited all the patients aged over 18 years and admitted to the IRCU with confirmed bilateral SARS-CoV-2 pneumonia and AHRF at an arterial oxygen partial pressure/fraction of inspired oxygen (PaO2/FiO2) ratio of ≤300, and who received respiratory support with the HFNC + CPAP combination. The following patients were excluded: those treated exclusively with HFNC or CPAP; had been in the IRCU for less than 24 h; had contraindications for NIRS. The patients were classified into two groups according to the time when HFNC + CPAP began: the Early HFNC + CPAP (EHC) group, formed by those patients who initiated it within the first 24 h after being admitted to the IRCU; the Delayed HFNC + CPAP (DHC) group, formed by those who initiated it at any time after the first 24 h of being admitted to the IRCU.
Procedure in the IRCU
Upon admission to the IRCU, all the patients started HFNC with a V60 Plus® (Philips Respironics, Murrysville, PA, USA) or AIRVO2® (Fisher and Paykel, Auckland, NZ) device. Flow and FiO2 were adjusted for peripheral oxygen saturation (SpO2) > 94% and respiratory rate (RR) < 24 breaths/min. After 1 h of HFNC treatment, arterial blood gases were taken to calculate PaO2/FiO2. Usually, in the patients with PaO2/FiO2 < 100, obesity and/or obstructive sleep apnea (OSA), the treating physician would iniciate a combination with CPAP sessions as soon as possible. CPAP sessions were performed using a V60 Plus® or Trilogy EVO® device (Philips Respironics, Murrysville, PA, USA) for 2 h during morning and afternoon shifts with HFNC breaks, and for at least 8 h at night, to ensure that sessions lasted at least 12 h/day. Pressure between 8 and 12 cm H2O and FiO2 adjusted for SpO2 > 94% were indicated based on previous studies,7, 20, 21 with single-limb circuit, passive leak and antiviral filter, oronasal or full-face mask. In those patients initially treated with HFNC alone, but with a SpO2 < 94%, RR > 24 and/or work of breathing (sternocleidomastoid muscle and abdominal musculature use) in the following hours or days, a combination with CPAP sessions were indicated with the same previously explained protocol. In both cases, parameters were adjusted to use the minimum respiratory support necessary to achieve a target SpO2 of 94% and a RR < 24 bpm, following the IRCU protocol.22
With those patients who clinically deteriorated despite optimizing NIRS or without clinical improvement after 48 h in the IRCU, a joint assessment was performed with the ICU staff to determine whether they were candidates to be admitted for IMV to avoid delaying ETI.
With the patients not admitted to the ICU, HFNC, CPAP or NIV were established as the therapeutic ceiling.
Data collection
Epidemiological, clinical and laboratory data were prospectively collected, and electronic clinical records were prepared. A protected anonymized database was used in compliance with EU data protection laws. All the data were verified by two researchers (JT and PL). A third researcher (JGR) analyzed any differences in the interpretations of the two principal investigators.
Variables and outcomes
The primary outcomes were 30-day mortality after IRCU admission and the ETI rate. The secondary outcomes were days of hospital admission, device tolerance and associated complications.
The following were analyzed: (a) socio-demographic and anthropometric variables: gender, age, obesity (BMI > 30); (b) comorbidities: Charlson Comorbidity Index (CCI), OSA; (c) disease history: date of symptom onset, date of hospital admission, IRCU and initiation of HFNC + CPAP, as well as discharge date, ETI or death in case of any of the above; (d) clinical data: Sequential Organ Failure Assessment scale (SOFA) and Simplified Acute Physiology Score II (SAPS-II) upon admission to the IRCU, RR upon IRCU admission, the ROX index at the baseline and after 24 h, CPAP pressure and FiO2, SpO2/FiO2 at the baseline and after 24, 48 and 72 h, CPAP tolerance and complications.
The analytical variables considered to be predictors of severity in former studies were recorded upon IRCU admission: lymphocyte counts, c-reactive protein, lactate dehydrogenase (LDH), ferritin and D-dimer.
Sample size
The following sample calculation was proposed: confidence level 95%; precision level 3%; proportion of events in the group 30%; expected percentage of losses 5%; total proposed sample of 560 patients.
Statistical analysis
The statistical analysis was carried out with SPSS, version 28.0 (SPSS Inc., Chicago, IL, USA), and the R Software (R-4.1.2, R Foundation, Vienna, Austria).
Baseline characteristics are included according to the two proposed groups for the endpoint analysis. The continuous and categorical variables are presented as the median (IQR) and n (%), respectively. Univariate analysis: Mann–Whitney U, χ 2 or Fisher's exact test was used to compare differences between the EHC and DHC groups according to requirements. Statistical significance was set at p < 0.05. Multivariate analysis: two binary logistic regressions (backward stepwise modeling) were performed to check for confounders. Variables with a p < 0.15 in the univariate analysis or those considered as confounders at the discretion of the researcher were considered. The starting variables in the 30-day mortality model were age, CCI, OSA, obesity, SAPS II at IRCU admission, IROX at IRCU admission, CPAP initial pressure upon IRCU admission, NIRS duration, CPAP tolerance, ferritin, delayed HFNC + CPAP. The starting variables in endotracheal intubation model were age, CCI, OSA, obesity, SAPS II at IRCU admission, IROX at IRCU admission, CPAP initial pressure upon IRCU admission, NIRS duration, CPAP tolerance, ferritin, lymphocytes, delayed HFNC + CPAP and time of symptom onset until IRCU admission. After the backward stepwise modeling, only the relevant variables were presented. The dependent variables were ETI and 30-day mortality. Odds ratio (OR) and 95% confidence interval (CI) were calculated. The 30-day mortality probability between the study groups was analyzed by the Kaplan–Meier method.
Results
Study patients
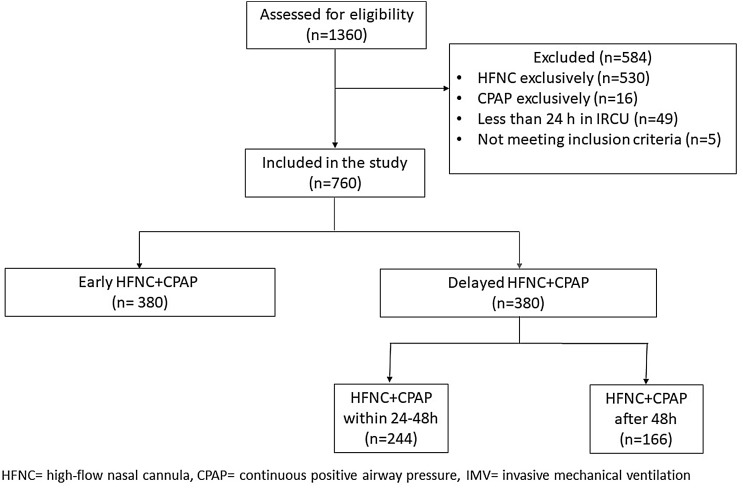
From January 8, 2021, to September 28, 2021, 760 patients aged 19–90 years (258 women [33.9%]) were consecutively hospitalized in the IRCU for ARDS caused by COVID-19 and treated with HFNC + CPAP (Table 1 ). Of them, 716 (94.2%) were candidates for ETI and 380 (50%) patients received the combined therapy in the first 24 h upon IRCU admission (EHC group). An equivalent group (380; DHC group) received it 24 h after admission (Fig. 1 ). The same number of patients in both groups was completely coincidental. Patients in the DHC group were older, with a higher CCI and SAPS II on admission. In contrast, the EHC group were more obese and had a higher OSA diagnosis (Table 1). There were neither significant differences in the RR nor work of breathing between both groups. Respiratory support and treatments were similar in both study groups (Table 2 ).
Table 1.
Baseline characteristics upon IRCU admission.
| Baseline characteristics | Early HFNC + CPAP (n = 380a) | Delayed HFNC + CPAP (n = 380a) | p-Value |
|---|---|---|---|
| Age, median (IQR) | 55.0 (45.0–64.0) | 59.0 (49.0–68.0) | <0.0001 |
| Men, n (%) | 248 (65.3%) | 254 (66.8%) | 0.64 |
| Women, n (%) | 132 (34.7%) | 126 (33.2%) | 0.64 |
| Comorbidities | |||
| Myocardial infarction, n (%) | 13 (3.4%) | 25 (6.6%) | 0.046 |
| Heart failure, n (%) | 7 (1.8%) | 15 (3.9%) | 0.083 |
| Cerebrovascular disease, n (%) | 9 (2.4%) | 10 (2.6%) | 0.81 |
| Chronic pulmonary disease, n (%) | 39 (10.3) | 39 (10.3) | 1 |
| OSA, n (%) | 48 (12.6%) | 28 (7.4%) | 0.016 |
| Oncologic disease, n (%) | 16 (4.2%) | 21 (5.5%) | 0.40 |
| Chronic kidney disease, n (%) | 9 (2.4%) | 9 (2.4%) | 1 |
| Diabetes, n (%) | 63 (16,6%) | 65 (17,1%) | 0.84 |
| Obesity, n (%) | 210 (55.3%) | 146 (38.4%) | <0.0001 |
| Charlson Comorbidity Index, median (IQR) | 1.0 (0.0–3.0) | 2.0 (1.0–3.0) | 0.001 |
| ETI candidate, n (%) | 362 (95.3%) | 354 (93.2%) | 0.21 |
| Time of symptom onset | |||
| Until hospital admission, median (IQR) | 7.0 (5.0–8.0) | 7.0 (5.0–8.0) | 0.46 |
| Until IRCU admission, median (IQR) | 9.0 (8.0–11.0) | 9.0 (7.0–11.0) | 0.48 |
| Laboratory findings | |||
| Lymphocytes (units/μL), median (IQR) | 660.0 (4900–900.0) | 640.0 (4600–870.0) | 0.17 |
| D-dimer (μg/mL), median (IQR) | 620.0 (4100–1000.0) | 620.0 (410.0–1047.5) | 0.95 |
| Lactate dehydrogenase (units/L), median (IQR) | 398.5 (341.3–481.5) | 417.0 (337.3–520.8) | 0.26 |
| C-Reactive Protein (mg/L), median (IQR) | 78.8 (39.9–139.8) | 80.9 (36,125–142.0) | 0.80 |
| Ferritin (ng/mL), median (IQR) | 890.0 (460.5–1348.0) | 972.0 (578.8–1490.0) | 0.052 |
| IRCU admission | |||
| PaO2/FiO2, median (IQR) | 94.0 (75.0–124.5) | 95.0 (76.0–128.0) | 0.78 |
| SpO2/FiO2, median (IQR) | 97.0 (95.0–106.7) | 97.0 (95.0–115.0) | 0.12 |
| Respiratory rate, median (IQR) | 23.0(20.0–27.0) | 22.0 (20.0–26.0) | 0.12 |
| Work of breathing, n (%) | 204 (54.5%) | 188 (50.7%) | 0.29 |
| IROX, median (IQR) | 4.5 (3.8–5.4) | 4.7 (4.0–5.6) | 0.15 |
| SOFA, median (IQR) | 4.0 (3.0–4.0) | 4.0 (3.0–4.0) | 0.30 |
| SAPS-II, median (IQR) | 28.0 (24.0–33.0) | 30.0 (26.0–35.0) | <0.0001 |
HFNC: high-flow nasal cannula, CPAP: continuous positive airway pressure, OSA: obstructive sleep apnea, ETI: endotracheal intubation, IRCU: intermediate respiratory care unit, PaO2/FiO2: arterial oxygen partial pressure/fraction of inspired oxygen, SpO2/FiO2: peripheral oxygen saturation/fraction of inspired oxygen, IROX: ROX index, SOFA: Sequential Organ Failure Assessment scale, SAPS II: Simplified Acute Physiology Score II, IQR: interquartile range.
The fact of having the same number of patients in both groups was a completely coincidental fact.
Fig. 1.
Study flow diagram.
Table 2.
Respiratory support parameters, treatment, and clinical evolution.
| Early HFNC + CPAP | Delayed HFNC + CPAP | p-Value | |
|---|---|---|---|
| Respiratory support parameters | |||
| HFNC FIO2 upon IRCU admission, median (IQR) | 1.0 (0.9–1.0) | 1.0 (0.81–1.0) | 0.065 |
| HFNC flow upon IRCU admission, median (IQR) | 60.0 (60.0–60.0) | 60.0 (60.0–60.0) | 0.43 |
| CPAP initial pressure upon IRCU admission, median (IQR) | 10.0 (8.0–10.0) | 9.0 (8.0–10.0) | 0.04 |
| Other treatment | |||
| Tocilizumab, n (%) | 178 (46.8) | 169 (44.5) | 0.51 |
| Corticosteroids, n (%) | 380 (100) | 379 (99.7) | 0.31 |
| Awake pronation, n (%) | 122 (32.6%) | 118 (31.5%) | 0.73 |
| Clinical evolution | |||
| Respiratory rate at 24 h, median (IQR) | 20 (18–24) | 20 (18–24) | 0.84 |
| SpO2/FiO2 at 24 h, median (IQR) | 116.0 (95.0–136.0) | 112.0 (94.0–131.0) | 0.21 |
| SpO2/FiO2 at 48 h, median (IQR) | 121.0 (96.0–160.0) | 119.0 (95.0–158.0) | 0.42 |
HFNC: high-flow nasal cannula, CPAP: continuous positive airway pressure, IRCU: intermediate respiratory care unit, SpO2/FiO2: peripheral oxygen saturation/fraction of inspired oxygen, IROX: ROX index, IQR: interquartile range.
Outcomes
The univariate analysis (Table 3 ) showed that only 273 patients (38.1%) needed ETI. Early therapy was associated with a significant reduction in 30-day mortality (8.2 vs. 15.5%, p = 0.002), days in the IRCU (6 vs. 8, p < 0.001), days at HEEIZ (18 vs. 22, p < 0.001) and respiratory therapy duration (5 vs. 6, p < 0.001). Additionally, early therapy revealed less probability of ETI (34.5 vs. 41.8%, p = 0.045). An additional benefit of early therapy was found, which was proved by a significant improvement in CPAP tolerance against delayed therapy (84% vs. 71%, p < 0.001).
Table 3.
Outcomes.
| Early HFNC + CPAP | Delayed HFNC + CPAP | p-Value | |
|---|---|---|---|
| Endotracheal intubation, n (%) | 125 (34.5%) | 148 (41.8%) | 0.045 |
| 30-day mortality, n (%) | 31 (8.2%) | 59 (15.5%) | 0.002 |
| CPAP tolerance, n (%) | 310 (84.0%) | 265 (71.4%) | <0.0001 |
| Pneumothorax, n (%) | 7 (1.8%) | 10 (2.6%) | 0.46 |
| Pneumomediastinum, n (%) | 20 (5.3%) | 25 (6.6%) | 0.44 |
| IRCU admission days, median (IQR) | 6.0 (4.0–9.0) | 8.0 (5.0–11.0) | <0.0001 |
| Hospital admission days, median (IQR) | 18.0 (13.0–29.0) | 22.0 (15.0–36.0) | <0.0001 |
| NIRS duration, median (IQR) | 5.0 (3.0–7.0) | 6.0 (4.0–9.0) | <0.0001 |
HFNC: high-flow nasal cannula, CPAP: continuous positive airway pressure, IRCU: intermediate respiratory care unit, IQR: interquartile range.
Despite baseline differences between groups, the multivariate analysis (Table 4 ) showed that delayed therapy had the highest strength of the association with 30-day mortality compared to higher CCI, higher CPAP initial pressure and older age. A shorter stay in the IRCU was presented as a protective factor in both 30-day mortality and ETI. However, only delayed therapy was associated with an increased risk of ETI.
Table 4.
Multivariate analysis.
| OR | CI 95% | p-Value | ||
|---|---|---|---|---|
| Endotracheal intubation | ||||
| Age | 1.02 | 0.99 | 1.05 | 0.09 |
| Charlson Comorbidity Index | 1.21 | 0.99 | 1.48 | 0.054 |
| Delayed HFNC + CPAP | 1.93 | 1.27 | 2.91 | 0.002 |
| CPAP initial pressure | 1.16 | 0.97 | 1.39 | 0.08 |
| NIRS duration | 0.75 | 0.70 | 0.80 | <0.0001 |
| Time of symptom onset until IRCU admission | 0.86 | 0.80 | 0.92 | <0.0001 |
| 30-Day mortality | ||||
| Age | 1.055 | 1.01 | 1.09 | 0.003 |
| Charlson Comorbidity Index | 1.29 | 1.08 | 1.55 | 0.005 |
| Delayed HFNC + CPAP | 2.11 | 1.17 | 3.82 | 0.01 |
| CPAP initial pressure | 1.3 | 1.01 | 1.66 | 0.036 |
| Days of respiratory support | 0.93 | 0.88 | 0.98 | 0.009 |
HFNC: high-flow nasal cannula, CPAP: continuous positive airway pressure, IRCU: intermediate respiratory care unit, OR: odds ratio, CI: confidence interval.
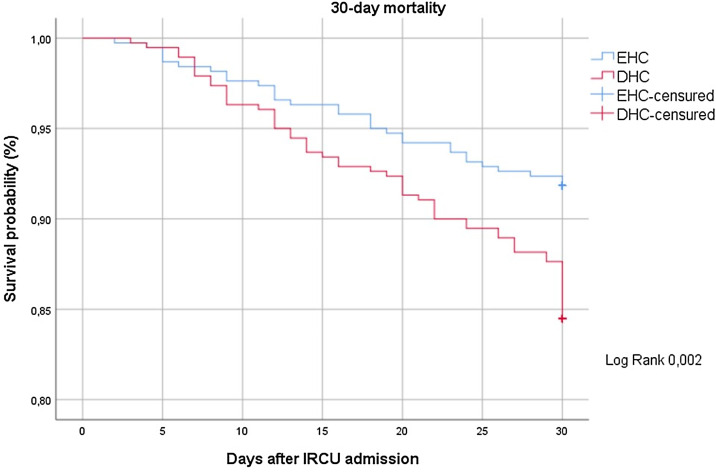
Fig. 2 shows the Kaplan–Meier curve for the probability of 30-day mortality between the study groups.
Fig. 2.
Kaplan–Meier survival probability over 30 days after IRCU admission (EHC: early high-flow nasal cannula plus continuous positive airway pressure, DHC: delayed high-flow nasal cannula plus continuous positive airway pressure).
Discussion
In this cohort study of patients with ARDS due to COVID-19, applying HFNC + CPAP in the first 24 h of NIRS onset was associated with a significant reduction in endotracheal intubation and 30-day mortality compared to delayed onset. The EHC presented a significant reduction in hospital admission days and NIRS duration and an increase in tolerance to CPAP.
Several publications refer to HFNC + CPAP as an alternative for patients with COVID-19 and AHRF,13, 20, 23 but information on its effectiveness is scarce. Based on rotating HFNC with CPAP, the study by Colaianni-Alfonso et al.,24 recently reported an ETI rate of 41.6% and a mortality rate of 20.8%, similar to other published studies that applied different NIRS strategies.25 However, the lower mortality recorded in our series, especially in the EHC group, is remarkable. Studies with a similar sample size to ours, such as Kurtz et al.,26 Wang et al.,27 Bellani et al.21 or Franco et al.,20 describe mortality to be 22%, 63%, 38% and 30%, respectively. A possible explanation may lie in reduced P-SILI development, although in the absence of mechanisms to measure variables such as esophageal pressure, the possible decrease in P-SILI is a hypothesis and never a statement. Weaver et al.28 used a simulation model of patients with distress and NIV to demonstrate that an increase in both the RR and tidal volume (VT) can generate potential lung damage due to increases in pleural pressure, transpulmonary pressure and driving pressure. In the early treated patients, the RR lowered more and SpO2/FiO2 increased more on the days following combined therapy onset, which could serve as a surrogate variable for diminished respiratory drive. Tonelli et al.29 demonstrated that decreased respiratory drive lowers the likelihood of developing P-SILI and predicted the success of NIRS. Finally, in patients with COVID-19 with respiratory failure, Schifino et al. observed a reduction in RR and transesophageal pressure swings, a reliable assessment of respiratory effort, with HFNC compared to conventional oxygen,30 which would support the use of HFNC in CPAP breaks.
When analyzing mortality in patients undergoing ETI for IMV, the EHC group presented a significantly lower mortality rate than the DHC group (31.6% vs. 68.4%; p = 0.01). Perhaps this was once again due to lesser P-SILI development before tracheal intubation, and it probably improved mechanics and response to treatment during IMV. Although between-group differences in NIRS duration could be considered a confounder for mortality by meaning an increased delay in ETI, it was not identified as a risk factor for mortality in the multivariate analysis.
No studies have demonstrated the usefulness of HFNC + CPAP for lowering the ETI rate in patients with de novo AHRF. Thille et al.31 demonstrated reduced reintubation after extubation in subjects treated with HFNC + NIV compared to HFNC alone. This was partly attributed to the combination of the benefits of PAP with diminished work of breathing during NIV rests with HFNC. Despite the different setting, it would seem that the stability of respiratory mechanics in PAP breaks is critical for lowering the risk of ETI in patients undergoing NIRS.
Moreover, CPAP break with HFNC can not only decrease the number of adverse ventilation-related events, but can also be applied for feeding and hydration, physiotherapy sessions, communication with family members, etc. Spoletini et al.18 demonstrated increased comfort and tolerability of the NIV combination with breaks of HFNC compared to breaks of standard oxygen. Significantly fewer adverse events, such as dry nose and mouth, eye irritation and difficulty eating, were observed in the HFNC group. CPAP intolerance in our cohort, understood as the need to discontinue CPAP due to adverse effects (flow or pressure was too strong, breathlessness, coughing, etc.), was almost twice in the DHC despite the median use of more than 5 days in both groups. We consider that early PAP usage in a stable situation can help to get used to the interface with better pressures than upon initiation in response to clinical deterioration.
This study has several limitations. First, due to its observational character, we can only show associations and not causality. In addition, there is no control group because the health care collapse limited the development of more robust clinical studies. Selection bias may limit data interpretation. However, we found that the two groups were homogeneous in the main predictors of severity with a similar disease process, and they received the same treatment and respiratory support, with HFNC + CPAP onset only at different times. To reduce the influence of confounders, we developed a multivariate model to mitigate the effect on the results. Second, its single-center nature limits the results being extrapolated. Additionally, the availability of devices may be a constraint for performing this NIRS strategy in other centers. Third, as no information on vaccination status was collected during the study period, it is impossible to assess the influence on the results. Finally, the treatment protocol in the IRCU does not allow comparisons to be made to CPAP or HFNC in monotherapy. So, it is impossible to reach conclusions about the superiority of therapies. However, our study also has its strengths. First, as far as we know, this is the largest cohort study to date of patients with ARDS by COVID-19 treated with a combination of respiratory therapies. Second, despite the widespread use of HFNC + CPAP in clinical practice and it being mentioned in many publications, our results for the relevant variables, such as mortality or orotracheal intubation, have not yet been reported. Third, our results may help to develop more scientific strength studies to demonstrate the potential usefulness of this strategy in non-COVID ARDS.
Conclusions
In patients with ARDS secondary to COVID-19, the HFNC + CPAP combination in the first 24 h was associated with a significant reduction in ETI and 30-day mortality rates compared to initiation on the following days. Future studies that compare HFNC + CPAP to CPAP or HFNC in monotherapy to treat ARDS would be welcomed.
Authors’ contributions
JT and PL had the idea for and designed the study, had full access to all data in the study and take full responsibility for data integrity and data analysis accuracy. JT, JGR, AN, LJD, JNL and PL drafted the paper. JGR and AN contributed to the statistical analysis. All the authors contributed to data acquisition, data analysis or data interpretation, and reviewed and approved the final version.
JTT, PL, JGR, LJD, JNL and ANL equally contributed.
Funding
This work was supported by University of Castilla-La Mancha intramural fundings.
Conflict of interests
Jose Rafael Teran-Tinedo declares a Madrid Society of Pneumology Young Researchers Grant 2020; funds from Air Liquide Healthcare for registration in the National Congress of the Spanish Society of Pneumology and Thoracic Surgery 2021; funds from Bial for registration in the European Respiratory Congress 2021 and 2022; funds from PharmaMar for secondary researcher in NEPTUNO clinical trial to evaluate utility of plitidepsin in COVID-19 patients. The payments were made to all the researchers; funds from GlaxoSmithKline (GSK) for Secondary researcher in OSCAR clinical trial to evaluate utility of otilimab in COVID-19 patients. The payments were made to all the researchers.
Miguel Lorente-González is a Secondary researcher in NEPTUNO clinical trial to evaluate utility of plitidepsin in COVID-19 patients. PharmaMar payments were made to all the researchers. He also declares payment from Gilead Sciences for a session at his hospital about the use of remdesivir in COVID-19 and registration in the XVII Regional Congress of the Madrilenian Society of Pneumology and Thoracic Surgery (NeumoMadrid) in May 2022.
Annette Zevallos-Villegas is a Secondary researcher in NEPTUNO clinical trial to evaluate utility of plitidepsin in COVID-19 patients. PharmaMar payments were made to all the researchers.
Eduardo Cano-Sanz is a Secondary researcher in NEPTUNO clinical trial to evaluate utility of plitidepsin in COVID-19 patients. PharmaMar payments were made to all the researchers.
Joaquín Hernández-Núñez is a Secondary researcher in NEPTUNO clinical trial to evaluate utility of plitidepsin in COVID-19 patients. PharmaMar payments were made to all the researchers.
Luis Puente Maestu funds from GSK for investigation and educational grants (institution); funds from MSD for sponsored study (no direct money); funds from Palex for investigation grant (institution); funds for Astra sponsored study (no direct money)
María Ángeles Ortega-Fraile is a Secondary researcher in NEPTUNO clinical trial to evaluate utility of plitidepsin in COVID-19 patients. PharmaMar payments were made to all the researchers.
Pedro Landete declares support for educational activities from Linde Healthcare, Bial, Boehringer Ingelheim, Air Liquide, GSK, FAES Farma and Novartis; payment from PharmaMar for expert investigation support; payment from Boehringer Ingelheim for registration for congress, travel and hotel; is on the PharmaMar Advisory board; funding from Phillips/Cardiva formedical writing at his institution.
All other authors have nothing to declare.
Acknowledgments
We want to thank to all the IRCU investigators staff, Daniel Laorden, Soraya Gholamian-Ovejero, Rosalia Navarro-Casado, Pablo Mariscal-Aguilar, Miguel Suarez-Ortiz, Maria Cristina Plaza-Moreno, Berta Gallego-Rodriguez, Mariara Calderon-Alcala, Aylaf Latif-Essa, Manuel Valle-Falcones, Elena Marıa Saiz-Lou, Carmen Rodrıguez-Calle, Juan Martin-Torres, Marta Espuelas Borderia, Ana Sanchez-Azofra, Roberto Vates-Gomez, Fernando García-Prieto, Alba Hernández-Piris, Laura Cotter-Muñoz, Tomás Villén y Miguel Angel Salvador Maya for their contribution to data collection and the IRCU nursing staff for their invaluable collaboration.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jie G.W., Yi N.Z., Hu Y., Hua L.W., Quan O.C., Xing H.J., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 5.Teran-Tinedo J.R., Gonzalez-Rubio J., Najera A., Castany-Faro A., Contreras M.d.l.N., Garcia I.M., et al. Clinical characteristics and respiratory care in hospitalized vaccinated SARS-CoV-2 patients. eClinicalMedicine. 2022;48:101453. doi: 10.1016/j.eclinm.2022.101453. Available from: https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(22)00183-3/fulltext [cited 25.9.22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellani G., Laffey J.G., Pham T., Madotto F., Fan E., Brochard L., et al. Noninvasive ventilation of patients with acute respiratory distress syndrome insights from the LUNG SAFE study. Am J Respir Crit Care Med. 2017;195:67–77. doi: 10.1164/rccm.201606-1306OC. [DOI] [PubMed] [Google Scholar]
- 8.Kangelaris K.N., Ware L.B., Wang C.Y., Janz D.R., Zhuo H., Matthay M.A., et al. Timing of intubation and clinical outcomes in adults with acute respiratory distress syndrome. Crit Care Med. 2016;44:120–129. doi: 10.1097/CCM.0000000000001359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karaca-Mandic P., Sen S., Georgiou A., Zhu Y., Basu A. Association of COVID-19-related hospital use and overall COVID-19 mortality in the USA. J Gen Intern Med. 2020;9:1–3. doi: 10.1007/s11606-020-06084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trentini F., Marziano V., Guzzetta G., Tirani M., Cereda D., Poletti P., et al. Pressure on the health-care system and intensive care utilization during the COVID-19 outbreak in the Lombardy region of Italy: a retrospective observational study in 43,538 hospitalized patients. Am J Epidemiol. 2022;191:137–146. doi: 10.1093/aje/kwab252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stapleton R.D., Wang B.M., Hudson L.D., Rubenfeld G.D., Caldwell E.S., Steinberg K.P. Causes and timing of death in patients with ARDS. Chest. 2005;128:525–532. doi: 10.1378/chest.128.2.525. [DOI] [PubMed] [Google Scholar]
- 12.Nicolini A., Stieglitz S., Bou-Khalil P., Esquinas A. Cost-utility of non-invasive mechanical ventilation: analysis and implications in acute respiratory failure. A brief narrative review. Respir Investig. 2018;56:207–213. doi: 10.1016/j.resinv.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Raoof S., Nava S., Carpati C., Hill N.S. High-flow, noninvasive ventilation and awake (nonintubation) proning in patients with coronavirus disease 2019 with respiratory failure. Chest. 2020;158:1992–2002. doi: 10.1016/j.chest.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perkins G.D., Ji C., Connolly B.A., Couper K., Lall R., Baillie J.K., et al. Effect of noninvasive respiratory strategies on intubation or mortality among patients with acute hypoxemic respiratory failure and COVID-19: the RECOVERY-RS randomized clinical trial. JAMA. 2022;327:546–558. doi: 10.1001/jama.2022.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grieco D.L., Menga L.S., Raggi V., Bongiovanni F., Anzellotti G.M., Tanzarella E.S., et al. Physiological comparison of high-flow nasal cannula and helmet noninvasive ventilation in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2020;201:303–312. doi: 10.1164/rccm.201904-0841OC. [DOI] [PubMed] [Google Scholar]
- 16.Liu J., Duan J., Bai L., Zhou L. Noninvasive ventilation intolerance: characteristics, predictors, and outcomes. Respir Care. 2016;61:277–284. doi: 10.4187/respcare.04220. [DOI] [PubMed] [Google Scholar]
- 17.Jones L., Nightingale R., Burhan H., Jones G., Barber K., Bond H., et al. Is continuous positive airway pressure therapy in COVID-19 associated with an increased rate of pulmonary barotrauma? ERJ Open Res. 2021;7:00886–2020. doi: 10.1183/23120541.00886-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spoletini G., Mega C., Pisani L., Alotaibi M., Khoja A., Price L.L., et al. High-flow nasal therapy vs standard oxygen during breaks off noninvasive ventilation for acute respiratory failure: a pilot randomized controlled trial. J Crit Care. 2018;48:418–425. doi: 10.1016/j.jcrc.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 19.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Franco C., Facciolongo N., Tonelli R., Dongilli R., Vianello A., Pisani L., et al. Feasibility and clinical impact of out-of-ICU noninvasive respiratory support in patients with COVID-19-related pneumonia. Eur Respir J. 2020;56:2002130. doi: 10.1183/13993003.02130-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellani G., Grasselli G., Cecconi M., Antolini L., Borelli M., De Giacomi F., et al. Noninvasive ventilatory support of patients with COVID-19 outside the intensive care units (WARd-COVID) Ann Am Thorac Soc. 2021;18:1020–1026. doi: 10.1513/AnnalsATS.202008-1080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laorden D., Gholamian-Ovejero S., Terán-Tinedo J.R., Lorente-González M., Cano-Sanz E., Ortega-Fraile M.Á., et al. Clinical findings and outcomes from subjects with COVID-19 pneumonia in an intermediate respiratory care unit. Respir Care. 2023;68:67–76. doi: 10.4187/respcare.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winck J.C., Ambrosino N. COVID-19 pandemic and non invasive respiratory management: every Goliath needs a David. An evidence based evaluation of problems. Pulmonology. 2020;26:213–220. doi: 10.1016/j.pulmoe.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colaianni-Alfonso N., Montiel G., Castro-Sayat M., Siroti C., Laura Vega M., Toledo A., et al. Combined noninvasive respiratory support therapies to treat COVID-19. Respir Care. 2021;66:1831–1839. doi: 10.4187/respcare.09162. [DOI] [PubMed] [Google Scholar]
- 25.Weerakkody S., Arina P., Glenister J., Cottrell S., Boscaini-Gilroy G., Singer M., et al. Non-invasive respiratory support in the management of acute COVID-19 pneumonia: considerations for clinical practice and priorities for research. Lancet Respir Med. 2022;10:199–213. doi: 10.1016/S2213-2600(21)00414-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurtz P., Bastos L.S.L., Dantas L.F., Zampieri F.G., Soares M., Hamacher S., et al. Evolving changes in mortality of 13,301 critically ill adult patients with COVID-19 over 8 months. Intensive Care Med. 2021;47:538–548. doi: 10.1007/s00134-021-06388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J.G., Liu B., Percha B., Pan S., Goel N., Mathews K.S., et al. Cardiovascular disease and severe hypoxemia are associated with higher rates of noninvasive respiratory support failure in coronavirus disease 2019 pneumonia. Crit Care Explor. 2021;3:e0355. doi: 10.1097/CCE.0000000000000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weaver L., Das A., Saffaran S., Yehya N., Scott T.E., Chikhani M., et al. High risk of patient self-inflicted lung injury in COVID-19 with frequently encountered spontaneous breathing patterns: a computational modelling study. Ann Intensive Care. 2021;11:109. doi: 10.1186/s13613-021-00904-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tonelli R., Fantini R., Tabbì L., Castaniere I., Pisani L., Pellegrino M.R., et al. Early inspiratory effort assessment by esophageal manometry predicts noninvasive ventilation outcome in de novo respiratory failure. A pilot study. Am J Respir Crit Care Med. 2020;202:558–567. doi: 10.1164/rccm.201912-2512OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schifino G., Vega M.L., Pisani L., Prediletto I., Catalanotti V., Comellini V., et al. Effects of non-invasive respiratory supports on inspiratory effort in moderate–severe COVID-19 patients. A randomized physiological study. Eur J Intern Med. 2022;100:110–118. doi: 10.1016/j.ejim.2022.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thille A.W., Muller G., Gacouin A., Coudroy R., Decavèle M., Sonneville R., et al. Effect of postextubation high-flow nasal oxygen with noninvasive ventilation vs high-flow nasal oxygen alone on reintubation among patients at high risk of extubation failure: a randomized clinical trial. JAMA. 2019;322:1465–1475. doi: 10.1001/jama.2019.14901. [DOI] [PMC free article] [PubMed] [Google Scholar]