Abstract
Background:
The primary aim of this study is to evaluate oncologic outcomes of two popular systemic chemotherapy approaches in patients with for colorectal peritoneal metastases (CPM) undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS-HIPEC).
Methods:
We performed a dual-center retrospective review of consecutive patients who underwent CRS-HIPEC for CPM due to high or intermediate grade CRC. Patients in the total neoadjuvant therapy group (TNT) received 6 months of preoperative chemotherapy. Patients in the “sandwich” chemotherapy group (SAND) received 3 months of preoperative chemotherapy with a maximum of 3 months of postoperative chemotherapy.
Results:
A total of 34 (43%) patients were included in the TNT group and 45 (57%) patients in the SAND group. The median overall survival in the TNT and SAND groups were 77 and 61 months, respectively (p=0.8). Patients in the TNT group had significantly longer RFS than the SAND group (29 vs 12 months, p=0.02). In a multivariable analysis, TNT approach was independently associated with improved RFS.
Conclusion:
In this retrospective study, a TNT approach was associated with improved recurrence-free survival, but not overall survival when compared to a sandwiched approach. Further prospective studies are needed examine these systemic chemotherapeutic approaches in patients with CPM undergoing CRS-HIPEC.
Keywords: Peritoneal Carcinomatosis, colorectal cancer, outcomes, CRS-HIPEC, systemic chemotherapy
Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide with an incidence of over 150,000 cases per year in the United States. Survival is significantly dependent on the presence or absence of metastatic disease1. According to a recent meta-analysis, 13% of patients with metastatic CRC have peritoneal metastases, which confers the worst overall survival when compared to patients with non-peritoneal metastases2. Thus, treatments such as cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS-HIPEC) for select patients have emerged in several studies and randomized trials to improve survival in patients with colorectal peritoneal metastases (CPM)2–8.
Systemic chemotherapy is standard in the management of CRC – either in a total neoadjuvant approach, or with a portion given preoperatively and the remainder as adjuvant therapy1. However, to date, there is limited data regarding the use of perioperative systemic chemotherapy in patients with CPM. However, systemic chemotherapy is standard in the management approach of CPM. According to the Chicago Consensus Working Group, patients with synchronous or metachronous CPM should be given NAC prior to CRS-HIPEC as well as potential adjuvant chemotherapy to achieve a total of 6 months of systemic chemotherapy9. In addition to treating metastatic disease, NAC may also help identify aggressive disease biology, responsiveness to systemic therapy, and potentially exclude patients for whom a complete cytoreduction would not be feasible. However, NAC administration may delay surgery or lead to debilitated functional status in an otherwise suitable operative candidate. Relatedly, adjuvant chemotherapy may be difficult to administer due to the high morbidity associated with CRS-HIPEC.
Several recent studies have shown that administration of either NAC or adjuvant chemotherapy is associated with improved overall survival in patients with CPM compared to surgery alone10–13. However, these treatment approaches may not have the same oncologic benefits in patients undergoing CRS-HIPEC and differences in survival have yet to be studied. Currently, there are no specific guidelines or recommendations as to the duration or timing of NAC in patients undergoing CRS-HIPEC for CPM. We therefore sought to evaluate the impact of two common systemic chemotherapy approaches among these patients: 6 months of preoperative systemic chemotherapy versus 3 months of preoperative systemic chemotherapy followed by postoperative chemotherapy.
Materials and Methods
Data Sources and Definitions
This dual-center retrospective review from Tennessee and New York was approved by the Institutional Review Board of Vanderbilt University Medical Center (IRB #200638) and utilizes a prospectively maintained database of all patients with peritoneal carcinomatosis (PC) who underwent CRS-HIPEC from 2011–2019. The database was used to collect patient demographic information as well as American society of Anesthesiologists (ASA) class and the Eastern Cooperative Oncology Group (ECOG) performance status score. Oncologic data, such as tumor origin and grade, lymphovascular invasion, and perineural invasion, were collected from the final pathology report from the surgical specimen at the time of CRS-HIPEC. Additionally, the peritoneal surface disease severity score (PSDSS), peritoneal carcinomatosis index (PCI), and complete cytoreduction score (CCR) were obtained from the surgeon’s CRS-HIPEC operative note. Patients were included if they were diagnosed with PC due to a primary colorectal tumor that was high or intermediate grade, based on the final pathology report.
Consecutive patients were categorized into two groups based on their systemic chemotherapy regimens. Patients who undergo 6 months of preoperative systemic chemotherapy only, based on the electronic health record, were included in the total neoadjuvant therapy (TNT) group. Patients who underwent 3 months of preoperative systemic chemotherapy followed by a planned maximum of 3 months of postoperative chemotherapy were included in the sandwich (SAND) group. Patients who underwent 3 months of preoperative chemotherapy but could not begin or complete their planned postoperative chemotherapy regimen were included in the SAND group. Patients whose chemotherapy regimen did not fit into these categories or who did not receive systemic chemotherapy at all were excluded from this analysis. The primary outcomes were overall survival (OS) as defined as months from the time of initiation of preoperative systemic chemotherapy and recurrence-free survival (RFS) as defined as months from CRS-HIPEC to evidence of radiographic or pathologic recurrence.
Statistical Analysis
Demographic, oncologic, and systemic treatment regimens based on chemotherapeutic approach were compared as previously defined. Categorical variables are recorded as percentages and compared using Chi-squared test or fisher’s exact test, where applicable. Continuous variables are recorded as means and compared using unpaired t tests. OS and RFS were calculated using the Kaplan-Meier method and groups were compared using the log-rank test. Multiple variable regression analyses of factors associated with OS and RFS, including PCI, complete cytoreduction, high grade disease, and total neoadjuvant chemotherapy were performed using Cox regression analysis. All analyses were performed using IBM Statistical Product and Service Solutions for Mac, Version 27 (IBM Corp., Armonk, N.Y., USA) software package. Statistical significance was set at p=0.05.
Results
We identified a total of 129 consecutive patients with CPM due to high or intermediate grade disease who underwent CRS-HIPEC from 2011–2019. Of these 129 patients, 23 patients underwent adjuvant systemic chemotherapy only and 27 patients did not undergo systemic chemotherapy. Thus, 79 patients were included in the analyses. Overall, 45% were male and the average age was 51.4 years. The median OS and RFS for the entire patient cohort were 60.9 months and 57.4 months, respectively. Of the 79 patients included in the study, 43% (n=34) underwent total neoadjuvant systemic chemotherapy and were included in the TNT group, while 57% (n=45) were in the SAND group. The mean age of patients in the TNT and SAND group were 52.6 years and 49.1 years, respectively (p=0.34). There were no significant differences in demographic characteristics between the groups and both groups exhibited similar comorbidity profiles as evidenced by similar ASA class, ECOG performance status, and Charlson comorbidity index scores. There were no significant differences in treatment modality among the participating centers (Supplementary Table 1; p=0.17). Patients in both groups had similar peritoneal surface disease severity scores (PSDSS; TNT 8.5 vs SAND 8.2; p=0.69), synchronous disease (TNT 32% vs SAND 36%; p=0.81), time from primary resection to development of CPM (TNT 19.3 months vs SAND 17.5 months; p=0.16), lymph node positivity (TNT 83.3% vs SAND 66.7%; p=0.12), and liver metastases (TNT 16.7% vs SAND 17.8%; p=0.98). The TNT group tended towards a higher rate of high-grade disease (64.7% vs 47%; p=0.17) but had overall similar rates of lymphovascular and perineural invasion. Demographic and oncologic characteristics are outlined in Table 1.
Table 1:
Demographics and Oncologic Characteristics
| Variables | TNT % (n) | SAND % (n) | p Value |
|---|---|---|---|
| Total | 43% (34) | 57% (45) | |
| Gender | 0.77 | ||
| Male | 45% (15) | 47% (21) | |
| Female | 55% (19) | 53% (24) | |
| Age (mean ± std) | 52.6 ± 8.3 | 49.1 ± 6.4 | 0.34 |
| Race | 0.67 | ||
| White | 65% (22) | 71% (32) | |
| Black | 24% (8) | 20% (9) | |
| Other | 11% (4) | 9% (4) | |
| BMI (mean ± std) | 29.8 ± 4.2 | 27.2 ± 3.9 | 0.79 |
| ASA Class | 0.82 | ||
| 1 | 0% (0) | 0% (0) | |
| 2 | 14.7% (5) | 20% (9) | |
| 3 | 82.4% (28) | 77.8% (35) | |
| 4 | 2.9% (1) | 2.2% (1) | |
| ECOG Performance Status | 0.31 | ||
| 0 | 38.2% (13) | 44.5% (20) | |
| 1 | 29.4% (10) | 28.9% (13) | |
| 2 | 0% (0) | 4.4% (2) | |
| 3 | 0% (0) | 2.2% (1) | |
| Unknown | 32.4% (11) | 20% (9) | |
| Peritoneal Surface Disease Severity Score (mean ± std) | 8.5 ± 3.3 | 8.2 ± 2.1 | 0.69 |
| Charlson Comorbidity Index (mean ± std) | 6.6 ± 0.8 | 6.7 ± 0.5 | 0.91 |
| Synchronous Disease | 32% (11) | 36% (16) | 0.81 |
| Time between primary resection and diagnosis of CPM (mean ± std) | 19.30 ± 3.8 months | 17.5± 5.2 months | 0.16 |
| Lymph Node Positivity | 83.3% (28) | 66.7% (30) | 0.12 |
| Liver Metastases | 16.7% (6) | 17.8% (8) | 0.98 |
| Tumor Location | 0.98 | ||
| Colon | 94.1% (32) | 93.3% (42) | |
| Rectum | 5.9% (2) | 6.7% (3) | |
| Tumor Grade | 0.17 | ||
| High-grade | 64.7% (22) | 47% (21) | |
| Intermediate Grade | 35.3% (12) | 53% (24) | |
| Lymphovascular Invasion | 41.2% (14) | 42.2% (19) | 0.99 |
| Perineural Invasion | 35.3% (12) | 31.1% (14) | 0.81 |
Patients in both groups had similar PCI scores (TNT 11.4 vs SAND 10.2; p=0.78) and over 90% of patients in each group underwent a complete cytoreduction. All patients were treated with hyperthermic intraperitoneal mitomycin C chemotherapy at the time of CRS-HIPEC. No significant differences in systemic chemotherapy regimens were observed between the two groups, with most patients receiving systemic FOLFOX + bevacizumab (54.4%, n=43). Patients in both groups were similarly likely to undergo an unplanned hospital admission during their neoadjuvant chemotherapy course (TNT 26.5% vs SAND 15.6%; p=0.27). Interestingly, patients in the TNT group underwent CRS-HIPEC on average 7.4 weeks after completion of preoperative systemic chemotherapy compared to 4.1 weeks in the SAND group (p=0.04). Patients in both groups were admitted to the intensive care unit (ICU) at similar rates (TNT 8.8% vs SAND 13.3%; p=0.73) and had similar postoperative hospital length of stay (TNT 8.3 days vs SAND 8.9 days; p=0.34). Notably, only 71.1% (n=32) of patients in the SAND group completed the planned 3 months of postoperative chemotherapy. However, patients in the TNT trended towards a longer duration of total systemic chemotherapy treatment (TNT 5.9 months vs SAND 5.4 months, p=0.08). Patients in the SAND group were more likely to be readmitted to the hospital within 60 postoperative days compared to the TNT group (TNT 14.7% vs SAND 35.5%; p=0.04). There were no mortalities in each group within that time period. Perioperative and treatment characteristics are summarized in Table 2.
Table 2:
Perioperative and Treatment Characteristics
| Variables | TNT % (n) | SAND % (n) | p Value |
|---|---|---|---|
| Peritoneal Carcinomatosis Index (mean ± std) | 11.4 ± 4.9 | 10.2 ± 6.7 | 0.78 |
| Completion of Cytoreduction Score | 0.56 | ||
| 0 | 85.3 % (29) | 75.6% (34) | |
| 1 | 8.8% (3) | 15.5% (7) | |
| 2 | 5.9% (2) | 8.9% (4) | |
| Intra-operative Chemotherapy Regimen | |||
| Mitomycin C | 100% (34) | 100% (45) | 1.0 |
| Neoadjuvant Chemotherapy Regimen 0.88 | |||
| FOLFOX + Bevacizumab | 55.9% (19) | 53.3% (24) | |
| FOLFOX | 23.6% (8) | 31% (14) | |
| FOLFIRI + Bevacizumab | 11.8% (4) | 6.7% (3) | |
| FOLFIRI | 5.9% (2) | 4.5% (2) | |
| CAPE-OX | 2.8% (1) | 4.5% (2) | |
| Unplanned Hospital Admission during neoadjuvant chemotherapy | 26.5% (9) | 15.6% (7) | 0.27 |
| {80Time between Chemotherapy and Surgery (mean ± std) | 7.4 ± 3.2 weeks | 4.1 ± 2.0 weeks | 0.04 |
| ICU Admission | 8.8% (3) | 13.3% (6) | 0.73 |
| Hospital Length of Stay (mean ± std) | 8.3 ± 1.8 days | 8.9 ± 2.1 days | 0.34 |
| Adjuvant Chemotherapy Regimen | - | ||
| FOLFOX + Bevacizumab | - | 62.3% (28) | |
| FOLFOX | - | 22.2% (10) | |
| FOLFIRI + Bevacizumab | - | 11.1% (5) | |
| FOLFIRI | - | 2.2% (1) | |
| CAPE-OX | - | 2.2% (1) | |
| Completion of Adjuvant Chemotherapy Regimen | - | 71.1% (32) | |
| Unplanned Hospital re-admission | 14.7% (5) | 35.5re% (16) | 0.04 |
Patients in the TNT group had a median overall survival of 77.5 months (IQR 52.0 – 91.3 months) compared to 61.1 months (IQR 55.5 – 68.4 months; HR 1.3; 95% CI 0.61–3.1; p=0.81; Figure 1A). TNT patients had a significantly longer RFS compared to patients in the SAND group [TNT (29.6 months; IQR 18.2 – 32.6 months); SAND (12.1 months; IQR 8.1 – 17.8 months); HR 0.52; 95% CI 0.29 – 0.83; p=0.02) Figure 1B]. In a subgroup analysis of patients who received a sandwiched chemotherapy approach, there were no significant differences in overall [Completed: 63.9 months (IQR 53.4 – 67.3 months) vs Incomplete: 59.3 months (IQR 52.2 – 65.2 months); HR 0.78, 95% CI 0.27–2.22; p=0.7] and recurrence-free survival [Completed: 11.9 months (IQR 8.9 – 20.4 months) vs Incomplete: 13.1 months (IQR 9.2 – 21.2 months); HR 0.77, 95% CI 0.34–1.71; p=0.8] among patients who completed their adjuvant chemotherapy course compared to patients who did not (Figure 2A–B).
Figure 1.
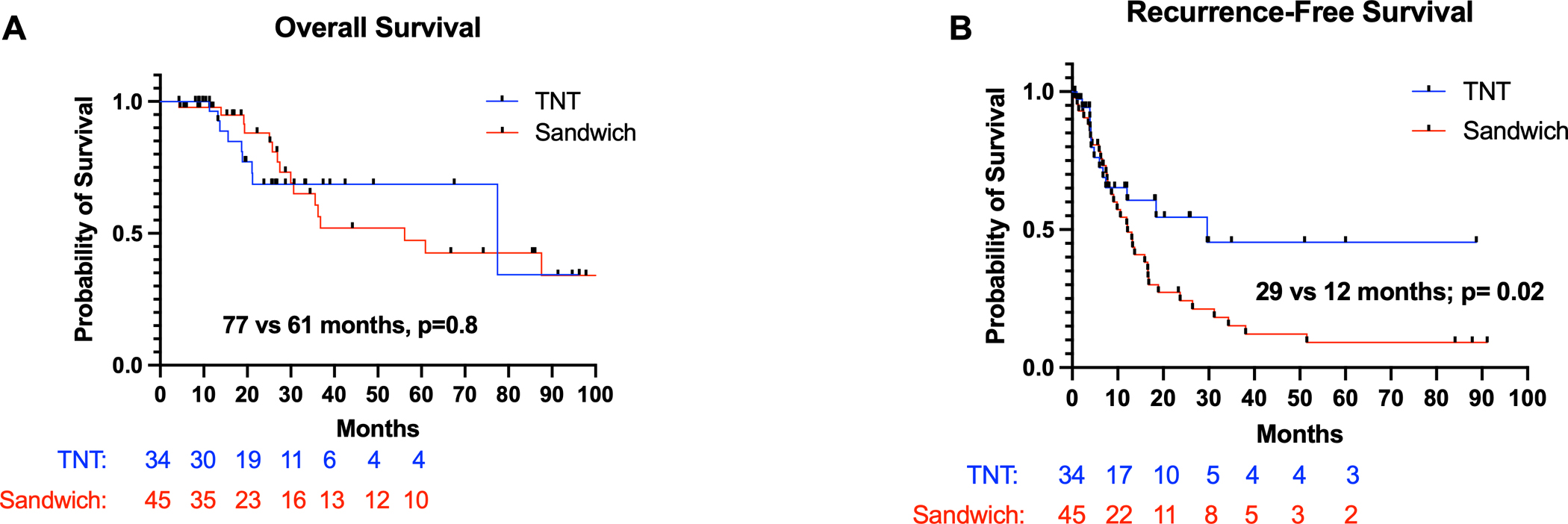
(A) Overall survival and (B) recurrence-free survival for patients with CPM undergoing CRS-HIPEC based on systemic chemotherapy approach
Figure 2.
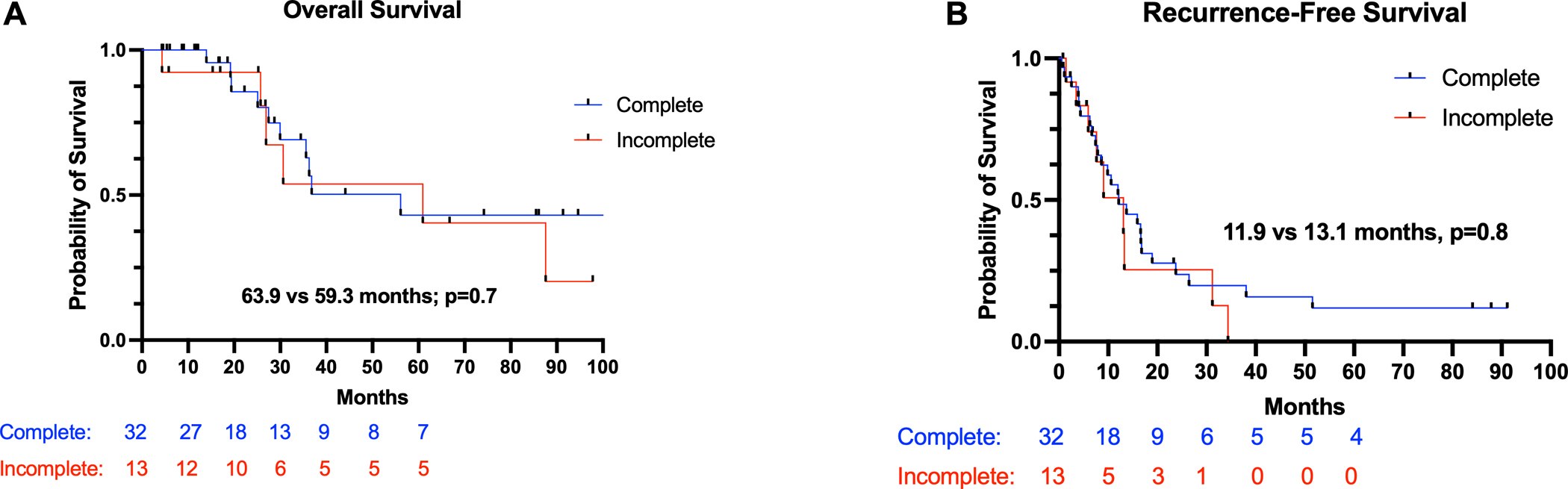
Subgroup analysis demonstrating (A) Overall survival and (B) recurrence-free survival for patients with CPM undergoing CRS-HIPEC who underwent sandwich chemotherapy approach
53% of patients in TNT group experienced cancer recurrence compared to 71% in the SAND group (p=0.04). There were no significant differences in location of tumor recurrence with the most common sites being the liver, peritoneum, or thoracic cavity. Notably, patients in the SAND group were more likely to undergo chemotherapy (75% vs 41.2%; p=0.03) and/or surgery (47% vs 21% (p=0.04) to treat their recurrence compared to patients in the TNT group (Table 3). Furthermore, among patients in the SAND group, patients who did not complete their adjuvant chemotherapy regimen were more likely to undergo chemotherapy (92.3% vs 52.6%; p=0.02) and/or surgery 76.9% vs 10.5%; p=0.002) to treat their recurrence compared to patients in the SAND group who did complete their adjuvant chemotherapy course (Table 4).
Table 3:
Recurrence Patterns and Treatment
| Variables | TNT % (n) | SAND % (n) | p Value |
|---|---|---|---|
| Recurrence | 53% (19) | 71% (32) | 0.04 |
| Recurrence Location | |||
| Liver | 36.8% (7) | 37.5% (12) | 0.98 |
| Peritoneum | 31.6% (6) | 37.5% (12) | 0.77 |
| Pleura/Lung | 26.3% (5) | 15.6% (5) | 0.72 |
| Colon | 15.7% (3) | 12.5% (4) | 0.88 |
| Spine | 5.3% (1) | 9.4% (3) | 0.83 |
| Diaphragm | 5.3% (1) | 0% (0) | - |
| Abdominal Wall | 0% (0) | 3.1% (1) | - |
| Treatment of Recurrence | |||
| Chemotherapy | 42.1% (8) | 75% (24) | 0.03 |
| Chemoradiation | 31.6% (6) | 21.9% (7) | 0.62 |
| Surgery | 21% (4) | 47% (15) | 0.04 |
| None/Unknown | 21% (4) | 6.2% (2) | 0.26 |
Table 4:
Treatment of Recurrence based on Adjuvant Chemotherapy Completion
| Variables | Complete (n=19) | Incomplete (n=13) | p Value |
|---|---|---|---|
| Chemotherapy | 52.6% (10) | 92.4% (12) | 0.02 |
| Chemoradiation | 26.3% (5) | 15.4% (2) | 0.54 |
| Surgery | 10.5% (2) | 76.9% (10) | 0.002 |
| None/Unknown | 10.5% (2) | 0% (0) | 0.45 |
In a Cox multivariable analysis including PCI, complete cytoreduction, high tumor grade, administration of total neoadjuvant chemotherapy, and completion of adjuvant chemotherapy course, PCI and incomplete cytoreduction were independently associated with worse OS and RFS, while completion of adjuvant chemotherapy was not associated with change in oncologic outcomes. Administration of total neoadjuvant chemotherapy was not associated with improved OS (HR 0.96; 95% CI 0.45–1.32; p=0.25) but was independently associated with improved RFS (0.49; 95% CI 0.27–0.72; p=0.01) as summarized in Tables 5–6.
Table 5:
Multiple Variable Cox Regression Analysis for Overall Survival
| Variables | HR | 95% CI | p Value |
|---|---|---|---|
| PCI Score | 1.74 | 1.55 – 2.04 | <0.001 |
| Completion of Cytoreduction Score > 1 | 2.09 | 1.89 – 2.31 | 0.005 |
| High Grade | 1.22 | 0.78 – 1.82 | 0.17 |
| Total Neoadjuvant Chemotherapy | 0.96 | 0.45 – 1.32 | 0.25 |
Table 6:
Multiple Variable Cox Regression Analysis for Recurrence-Free Survival
| Variables | HR | 95% CI | p Value |
|---|---|---|---|
| PCI Score | 1.33 | 1.12 – 1.56 | 0.03 |
| Completion of Cytoreduction Score > 1 | 1.43 | 1.31 – 1.64 | 0.02 |
| High Grade | 0.81 | 0.32 – 1.43 | 0.09 |
| Total Neoadjuvant Chemotherapy | 0.49 | 0.27 – 0.72 | 0.01 |
Discussion
In this multi-institutional retrospective study, we demonstrate that a total neoadjuvant systemic chemotherapy approach, as defined as 6 months of preoperative systemic chemotherapy, is associated with improved recurrence-free survival but not overall survival in patients with CPM undergoing CRS-HIPEC. Despite having similar baseline demographic, performance status scores, oncologic factors, and overall treatment characteristics, patients in the TNT group had significantly improved RFS of 29 months compared to 12 months for patients in the SAND group. While total neoadjuvant chemotherapy was not associated with an overall survival benefit, it was independently associated with improved RFS in a multivariable regression analysis, controlling for PCI score, CCR score, and high tumor grade. To our knowledge, this is the first study to examine the impact of two common systemic chemotherapy approaches in patients with CPM undergoing CRS-HIPEC.
Patients who underwent a total neoadjuvant systemic chemotherapy approach had decreased rate of cancer recurrence compared to patients who underwent a sandwiched systemic chemotherapy approach. However, a TNT approach did not confer an overall survival benefit compared to the SAND approach, which may be due to the significant discrepancies in how recurrences were treated between the two groups. Patients in the TNT group were significantly less likely than their SAND counterparts to undergo chemotherapy or surgery for recurrence. Clinicians are presumably more likely to recommend prolonged chemotherapy or surgery in patients who have not received 6 consecutive months of chemotherapy. Thus, it is possible that whatever survival benefit may be attributed to total neoadjuvant therapy is minimized by the more aggressive treatment of recurrence among patients in the SAND group. This is supported by the fact that patients in the SAND group who did not complete their adjuvant chemotherapy course were more likely to receive chemotherapy or undergo surgery as a treatment for their recurrence compared to patients who did complete their adjuvant chemotherapy course. The lack of overall survival benefit among patients who received 6 months of preoperative chemotherapy may be due to the aggressive treatment of recurrence among patients in the sandwich group. Additionally, patients in the TNT group tended towards a higher rate of high-grade disease compared to patients in the SAND group. Thus, it is possible that patients in the TNT group who had cancer recurrence likely possessed aggressive tumor biology, minimizing a potential overall survival benefit.
Our findings are in line with several studies documenting the survival benefit of systemic chemotherapy among patients undergoing CRS-HIPEC14–16. However, ours is the first study to report differences in oncologic outcomes based on different chemotherapeutic approach in patients with CPM undergoing CRS-HIPEC. The use of NAC for CPM is still a topic of debate, with recent literature providing mixed results17. Several recent retrospective studies of patients with CPM demonstrated that NAC was associated with improved OS compared to patients who underwent only adjuvant therapy14–16. However, another recent large multicenter retrospective study demonstrated that NAC was not associated with improved OS in patients with CPM on multivariate analysis and among patients with poorly differentiated tumors10. Additionally, a retrospective study of 280 consecutive patients demonstrated that timing of systemic chemotherapy did not impact overall survival in patients with CPM undergoing CRS-HIPEC18. These studies do not define the duration of neoadjuvant or adjuvant chemotherapy and do not differentiate patients who may have received both forms of chemotherapy. Thus, it is difficult to determine if or to what extent these systemic chemotherapy approaches may benefit patients with CPM. Nonetheless, NAC can be recommended in patients with CPM to allow for early initiation of systemic chemotherapy and to aid in patient selection by excluding patients who have significant disease progression while on NAC. Furthermore, administration of NAC may improve the chance of complete cytoreduction by potentially decreasing metastatic burden within the peritoneum. Apart from NAC, a recent propensity score-matched cohort study of almost 400 patients showed that adjuvant systemic chemotherapy was associated with improved overall survival compared to active surveillance among patients with synchronous CPM undergoing up-front CRS-HIPEC12. Adjuvant systemic chemotherapy may improve oncologic outcomes by limiting systemic spread of disease and eliminating postoperative micrometastatic peritoneal disease. Furthermore, a recent phase II clinical trial of 79 patients demonstrated that perioperative systemic chemotherapy is safe and can induce radiographic and pathologic response among patients with CPM undergoing CRS-HIPEC11. Thus, the current literature suggests that there is likely role for systemic chemotherapy in addition to CRS-HIPEC, but studies examining the optimal approach and duration are lacking. In this current study, 28.2% of patients who underwent a sandwiched chemotherapy approach did not complete their adjuvant chemotherapy course. However, subgroup analyses within the SAND group demonstrated that completion of adjuvant chemotherapy was not associated improved oncologic outcomes. These findings suggest that the timing of systemic chemotherapy in relation to CRS-HIPEC, rather than just prolonged systemic chemotherapy, may be an important factor for improved oncologic outcomes. Furthermore, patients who underwent a total neoadjuvant chemotherapeutic approach did not suffer potential consequences of prolonged preoperative systemic chemotherapy as evidenced by similar rates of unplanned hospital admissions, postoperative ICU admission, and hospital length of stays compared to patients in the SAND group. Relatedly, patients in the SAND group were more likely to be readmitted in the postoperative period than patients in the TNT group, possibly due to continued adverse effects of systemic chemotherapy.
Several limitations of this study are worth noting. Of most importance is the potential selection bias inherent in this study’s design. This study only included patients who underwent CRS-HIPEC rather than all patients diagnosed with CPM who were then treated with systemic therapy. Patients who remained suitable operative candidates for CRS-HIPEC after successfully completing their neoadjuvant chemotherapy approach may represent a patient population with tumor biology that is distinct from patients who were not deemed operative candidates. Patients who demonstrated significant progression of disease while on NAC are unlikely to undergo CRS-HIPEC. While this is a limitation, it also reflects an advantage of this treatment approach in its ability to exclude unsuitable patients from a morbid surgery Relatedly, it is unclear as to why some patients underwent 3 months or 6 months of systemic chemotherapy prior to CRS-HIPEC. One can argue that patients who demonstrate significant progression of disease on interval surveillance imaging should continue systemic chemotherapy while others may recommend CRS-HIPEC if a complete cytoreduction is still possible. Furthermore, no standard surveillance imaging protocol was followed, and use of diagnostic laparoscopy is variable among providers and institutions. While such factors may not have necessarily impacted the neoadjuvant systemic chemotherapy regimen chosen for these patients, they have may contributed to the inherent selection bias of this study in excluding patients who would not be CRS-HIPEC candidates due to tumor burden. The findings of this paper should be limited to patients with high or intermediate grade disease. While patients with low grade disease may benefit from systemic chemotherapy, any potential RFS in the setting of total neoadjuvant chemotherapy may be less pronounced. Finally, the vast majority of patients in cohort had metachronous CPM. Thus, the findings of this study may not be broadly applicable to patients with synchronous disease, which is typically thought to be a more aggressive form of disease.
A total neoadjuvant systemic chemotherapy approach, as defined as 6 months of preoperative systemic chemotherapy, is associated with improved recurrence-free survival in a multiple variable analysis, but not overall survival compared to a sandwiched chemotherapy approach among patients with colorectal peritoneal metastases undergoing CRS-HIPEC. While recent and current studies have defined a potential role for systemic chemotherapy in this patient setting, this study establishes the foundation for future prospective studies to identify the optimal systemic chemotherapy approach and duration.
Supplementary Material
Synopsis.
Many patients who undergo cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for colorectal peritoneal metastases undergo preoperative systemic chemotherapy. This retrospective study demonstrates that 6 months of preoperative systemic chemotherapy was associated with improved recurrence-free survival compared to a sandwiched approach.
Acknowledgements
We would like to acknowledge our funding source, the National Cancer Institute (T32CA106183).
Footnotes
Disclosures: None
Data Availability:
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1.Colon Cancer. 2019; https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed 03/02/2022, 2022.
- 2.Franko J, Shi Q, Meyers JP, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016;17(12):1709–1719. [DOI] [PubMed] [Google Scholar]
- 3.Elias D, Delperro JR, Sideris L, et al. Treatment of peritoneal carcinomatosis from colorectal cancer: impact of complete cytoreductive surgery and difficulties in conducting randomized trials. Ann Surg Oncol. 2004;11(5):518–521. [DOI] [PubMed] [Google Scholar]
- 4.Elias D, Lefevre JH, Chevalier J, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol. 2009;27(5):681–685. [DOI] [PubMed] [Google Scholar]
- 5.Franko J, Ibrahim Z, Gusani NJ, Holtzman MP, Bartlett DL, Zeh HJ 3rd. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer. 2010;116(16):3756–3762. [DOI] [PubMed] [Google Scholar]
- 6.Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22(16):3284–3292. [DOI] [PubMed] [Google Scholar]
- 7.Mirnezami R, Mehta AM, Chandrakumaran K, et al. Cytoreductive surgery in combination with hyperthermic intraperitoneal chemotherapy improves survival in patients with colorectal peritoneal metastases compared with systemic chemotherapy alone. Br J Cancer. 2014;111(8):1500–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20):3737–3743. [DOI] [PubMed] [Google Scholar]
- 9.Chicago Consensus Working G The Chicago Consensus on peritoneal surface malignancies: Management of colorectal metastases. Cancer. 2020;126(11):2534–2540. [DOI] [PubMed] [Google Scholar]
- 10.Beal EW, Suarez-Kelly LP, Kimbrough CW, et al. Impact of Neoadjuvant Chemotherapy on the Outcomes of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases: A Multi-Institutional Retrospective Review. J Clin Med. 2020;9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rovers KP, Bakkers C, Nienhuijs SW, et al. Perioperative Systemic Therapy vs Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy Alone for Resectable Colorectal Peritoneal Metastases: A Phase 2 Randomized Clinical Trial. JAMA Surg. 2021;156(8):710–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rovers KP, Bakkers C, van Erning FN, et al. Adjuvant Systemic Chemotherapy vs Active Surveillance Following Up-front Resection of Isolated Synchronous Colorectal Peritoneal Metastases. JAMA Oncol. 2020;6(8):e202701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou S, Jiang Y, Liang J, Pei W, Zhou Z. Neoadjuvant chemotherapy followed by hyperthermic intraperitoneal chemotherapy for patients with colorectal peritoneal metastasis: a retrospective study of its safety and efficacy. World J Surg Oncol. 2021;19(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devilee RA, Simkens GA, van Oudheusden TR, et al. Increased Survival of Patients with Synchronous Colorectal Peritoneal Metastases Receiving Preoperative Chemotherapy Before Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann Surg Oncol. 2016;23(9):2841–2848. [DOI] [PubMed] [Google Scholar]
- 15.Passot G, Vaudoyer D, Cotte E, et al. Progression following neoadjuvant systemic chemotherapy may not be a contraindication to a curative approach for colorectal carcinomatosis. Ann Surg. 2012;256(1):125–129. [DOI] [PubMed] [Google Scholar]
- 16.Ceelen W, Van Nieuwenhove Y, Putte DV, Pattyn P. Neoadjuvant chemotherapy with bevacizumab may improve outcome after cytoreduction and hyperthermic intraperitoneal chemoperfusion (HIPEC) for colorectal carcinomatosis. Ann Surg Oncol. 2014;21(9):3023–3028. [DOI] [PubMed] [Google Scholar]
- 17.Waite K, Youssef H. The Role of Neoadjuvant and Adjuvant Systemic Chemotherapy with Cytoreductive Surgery and Heated Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases: A Systematic Review. Ann Surg Oncol. 2017;24(3):705–720. [DOI] [PubMed] [Google Scholar]
- 18.van Eden WJ, Kok NF, Jozwiak K, et al. Timing of Systemic Chemotherapy in Patients With Colorectal Peritoneal Carcinomatosis Treated With Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Dis Colon Rectum. 2017;60(5):477–487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


