Abstract
Objectives
To test whether Bacillus Calmette-Guérin (BCG) vaccination would reduce the incidence of COVID-19 and other respiratory tract infections (RTIs) in older adults with one or more comorbidities.
Methods
Community-dwelling adults aged 60 years or older with one or more underlying comorbidities and no contraindications to BCG vaccination were randomized 1:1 to BCG or placebo vaccination and followed for 6 months. The primary endpoint was a self-reported, test-confirmed COVID-19 incidence. Secondary endpoints included COVID-19 hospital admissions and clinically relevant RTIs (i.e. RTIs including but not limited to COVID-19 requiring medical intervention). COVID-19 and clinically relevant RTI episodes were adjudicated. Incidences were compared using Fine-Gray regression, accounting for competing events.
Results
A total of 6112 participants with a median age of 69 years (interquartile range, 65–74) and median of 2 (interquartile range, 1–3) comorbidities were randomized to BCG (n = 3058) or placebo (n = 3054) vaccination. COVID-19 infections were reported by 129 BCG recipients compared to 115 placebo recipients [hazard ratio (HR), 1.12; 95% CI, 0.87–1.44]. COVID-19-related hospitalization occurred in 18 BCG and 21 placebo recipients (HR, 0.86; 95% CI, 0.46–1.61). During the study period, 13 BCG recipients died compared with 18 placebo recipients (HR, 0.71; 95% CI, 0.35–1.43), of which 11 deaths (35%) were COVID-19-related: six in the placebo group and five in the BCG group.
Clinically relevant RTI was reported by 66 BCG and 72 placebo recipients (HR, 0.92; 95% CI, 0.66–1.28).
Discussion
BCG vaccination does not protect older adults with comorbidities against COVID-19, COVID-19 hospitalization, or clinically relevant RTIs.
Keywords: Bacillus Calmette-Guérin vaccine, BCG vaccine, COVID-19, Randomized controlled trial, Respiratory tract infections
Introduction
Although people of any age can develop COVID-19, older adults are at highest risk of developing severe COVID-19, which is associated with high mortality and morbidity [1]. At the onset of the pandemic, when preventive or curative interventions with demonstrated efficacy were unavailable, strategies to prevent SARS-CoV-2 infection or reduce infection severity in older adults with comorbidities were urgently needed to bridge the gap until effective vaccines and treatments became available.
Bacillus Calmette-Guérin (BCG), a live-attenuated tuberculosis vaccine, has been one of the most used vaccines in the world [2]. Its use has been associated with off-target beneficial effects on the innate immune system, called trained immunity [3]. It has been suggested that BCG vaccine induces protection against various respiratory infections, including those of viral etiology [4]. Protective effect of BCG vaccine has been demonstrated for the respiratory syncytial virus, influenza virus, and herpes simplex virus in animals [5]. In a randomized clinical trial in Greece (ACTIVATE) conducted before the COVID-19 pandemic, BCG vaccination in older adults after hospital discharge was associated with lower incidence of respiratory tract infections during a one-year follow-up period [6]. In addition, murine studies have suggested beneficial effects of BCG on the outcome of experimental infection with SARS-CoV-2 [7].
Therefore, we tested the hypothesis that BCG vaccination would reduce the incidence of COVID-19 and other respiratory tract infections in older adults with one or more comorbidities in a randomized double-blind placebo-controlled multicentre trial.
Methods
Trial design
We conducted a double-blind multicentre randomized placebo-controlled trial with an adaptive primary endpoint in 20 hospitals in the Netherlands.
Ethical considerations
The trial was approved by the Utrecht Institutional Review Board (protocol NL74730.041.20), registered in the European Clinical Trials Database (2020-003470-47), and conducted in compliance with the ethical principles of the Declaration of Helsinki and the Guidelines for Good Clinical Practice. All individuals provided written informed consent prior to any study procedure.
Eligibility
Candidates were recruited at different departments, outpatient clinics, and thrombosis care services; and were approached via the treating physician or through leaflets. Patients could also self-report their interest in the study. The period of inclusion was from 7 September 2020 to 18 December 2020, before the start of vaccination with COVID-19-specific vaccines in the Netherlands on 6 January 2021. Patients were eligible for enrolment in the trial if they were 60 years of age or older and at risk of severe COVID-19, had no contraindications to BCG vaccination, and had no documented COVID-19 infection prior to enrolment. Subjects with previous receipt of BCG were eligible for participation. Full eligibility criteria are provided in Table S1.
Trial procedures
Study participants were randomly assigned in a 1:1 ratio to receive either 0.1 mL of BCG (Danish strain 1331; Statens Serum Institut, Copenhagen, Denmark, all from the same batch) or 0.1 mL saline placebo via intradermal injection in the left upper arm. Concealed allocation was achieved by computer-generated randomization (Castor electronic data capture system) in blocks of varying sizes and stratified by centre and age (60–69, 70–79, and ≥80 years). Participants, caregivers, and study personnel were blinded, except for the medical monitor and the person who randomized participants and prepared placebo or BCG vaccinations; these unblinded persons were not involved in the enrolment or administration of study vaccine. Participants were asked to answer questions about symptoms, SARS-CoV-2 tests, vaccinations, and healthcare visits either via a smartphone application or via regular telephone calls for 6 months (182 days) after study vaccination (see Supplementary Methods). Adherence to completed questionnaires was monitored centrally, and non-adherent participants were contacted. An independent Data Safety Monitoring Board (DSMB) regularly reviewed unblinded data to monitor recruitment, data quality, safety, and progress towards reaching the predefined number of primary endpoints (see Outcomes below). The DSMB could recommend premature termination of the trial if participant safety might be jeopardized, or in case of BCG superiority in preventing at least one of the primary endpoints.
Outcomes
All outcomes were based on participant self-reports, supplemented with data obtained from general practitioners and hospital medical records when considered relevant. The trial was designed with an adaptive primary endpoint. The primary endpoint would either be (a) incidence of self-reported diagnostic test or imaging-confirmed COVID-19 (with at least 67 episodes required) or (b) clinically relevant RTI requiring medical intervention, potentially including COVID-19 episodes (with at least 90 episodes required). Endpoints were determined by adjudication (see Supplementary Methods for definitions).
Secondary endpoints included the incidence of self-reported asymptomatic, mild/moderate, and severe (requiring hospitalization) SARS-CoV-2 infections, as well as pneumonia, medically attended RTIs (irrespective of intervention), RTI-related hospital admissions, and mental, physical, and social functioning (based on validated questionnaires). To understand the impact of COVID-19 on clinically relevant RTI, we added an analysis of clinically relevant RTIs excluding COVID-19. Safety endpoints included 6-month mortality from all causes, adverse events (AEs), and serious adverse events (SAEs). Detailed definitions of primary and secondary endpoints are provided in Table S2.
Statistical analysis
See Supplementary Methods for the sample size calculation.
All analyses were performed from an intention-to-treat perspective, and patients who did not complete follow-up for reasons other than death were censored. The primary endpoint was reported as the cumulative incidence by treatment arm. A competing risk analysis was performed using the Fine-Gray regression model, with death as competing event and variables used for stratified randomization (site of enrolment and age) as covariates. The effect was reported as a sub-distribution hazard ratio with 95% CI. Secondary and explorative endpoints were analyzed using the same Fine-Gray model for time-to-event data, a negative binomial regression model for count data [reported as risk ratio with 95% CI], a Poisson regression with robust standard errors for binary outcome (reported as risk ratio with 95% CI), and linear regression for continuous outcome (reported as difference in mean with 95% CI). For clinically relevant RTIs, we added a priori selected outcome predictors as covariates in the regression models: cardiovascular disease or stroke, chronic pulmonary disease, diabetes mellitus, malignancy, and moderate-to-severe chronic renal disease. An unplanned explorative analysis of SARS-CoV-2-related symptom severity and duration was performed by visually inspecting the mean self-reported symptom severity per day for 30 days after symptom onset or test-positivity, whichever was observed first. Analyses were performed in R version 4.0.3 [8].
Clinical trial registration: NCT04537663, Prevention of Respiratory Tract Infection and COVID-19 Through BCG Vaccination in Vulnerable Older Adults (BCG-PRIME).
Results
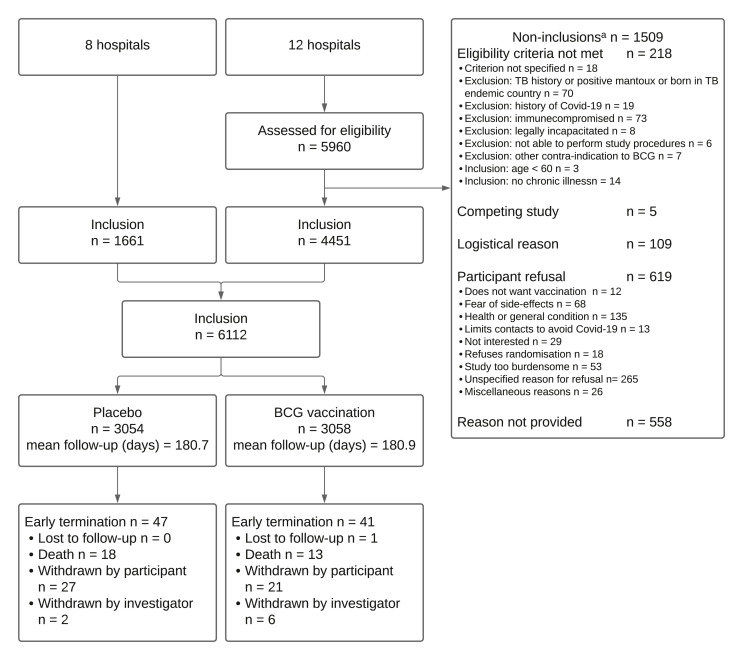
From 7 September to 28 December 2020, 6112 subjects were included and randomized: 3054 in the placebo group and 3058 in the BCG group. In the BCG group, slightly more patients had a history of BCG vaccination compared to the placebo group; otherwise, baseline characteristics were comparable between the groups (Tables 1 and S3 ). Follow-up was terminated prematurely for 88 (1.4%) participants (Fig. 1 ). At the end of study follow-up, 71% of the participants had received first dose of SARS-CoV-2 vaccination (placebo 2123/2962, 71.7% vs. BCG 2121/2979, 71.2%) and 31% were fully vaccinated (placebo 940/2962, 31.7% vs. BCG 902/2979, 30.3%) with no difference in the timing of the first dose between study groups (Fig. S1).
Table 1.
Baseline characteristicsa
| Placebo | BCG | Missing % | |
|---|---|---|---|
| n | 3054 | 3058 | — |
| Female sex (%) | 1150 (37.7) | 1125 (36.8) | 0.0 |
| Age at enrolment, median (IQR) | 69.00 [65.00, 74.00] | 69.00 [65.00, 74.00] | 0.0 |
| Age category (%) 60–69 years 70–79 years 80+ years |
1565 (51.2) 1260 (41.3) 229 (7.5) |
1576 (51.5) 1254 (41.0) 228 (7.5) |
0.0 |
| Country/region of birth (%) The Netherlands Other |
2944 (96.9) 110 (3.1) |
2951 (96.8) 107 (3.2) |
0.4 |
| Body mass index, median (IQR) | 26.53 [24.15, 29.76] | 26.51 [24.07, 29.63] | 0.1 |
| Number of reported comorbidities, median (IQR) | 2 [1–3] | 2 [1–3] | 0.3 |
| Comorbidities (%) Hypertension Cardiovascular disease Post stroke Diabetes mellitus COPD Asthma Other pulmonary disease Moderate-to-severe renal disease Malignancy Dementia |
1622 (53.1) 1769 (58.0) 209 (6.8) 686 (22.5) 511 (16.7) 412 (13.5) 151 (4.9) 240 (7.9) 162 (5.3) 8 (0.3) |
1600 (52.3) 1708 (55.9) 207 (6.8) 656 (21.5) 529 (17.3) 449 (14.7) 166 (5.4) 224 (7.3) 152 (5.0) 4 (0.1) |
0.0 0.1 0.0 0.0 0.0 0.0 0.1 0.0 0.0 0.0 |
| BCG vaccination previously (%) | 413 (14.9) | 495 (17.9) | 9.5 |
| Flu vaccination 2019–2020 season (%) | 2211 (73.3) | 2209 (73.0) | 1.1 |
| Flu vaccination 2020–2021 season (%) | 1578 (51.8) | 1592 (52.1) | 0.2 |
| Pneumococcal vaccination 2019–2020 season (%) | 44 (1.5) | 56 (1.8) | 0.7 |
| Pneumococcal vaccination 2020–2021 season (%)b | 394 (13.0) | 409 (13.4) | 0.4 |
| Other vaccination in past 12 mo (%) | 68 (2.2) | 79 (2.6) | 0.5 |
| Clinical frailty scale (%) 1–3 (Vital) 4–5 (Moderately frail) 6+ (Severely frail) |
2571 (84.3) 421 (13.8) 58 (1.9) |
2588 (84.8) 413 (13.5) 51 (1.7) |
0.2 |
| Dependent (Katz ADL scorec) (%) | 39 (1.3) | 25 (0.8) | 0.4 |
| Dependent (Lawton & Brody iADL scorec) (%) | 270 (8.9) | 281 (9.2) | 0.4 |
| EQ-5D-3L quality of life scorec, median (IQR) |
0.93 [0.81, 0.93] | 0.93 [0.81, 0.93] | 0.4 |
| EQ VASc score, median (IQR) | 80.00 [70.00, 89.25] | 80.00 [70.00, 90.00] | 0.5 |
ADL, activities of daily living; BCG, Bacillus Calmette-Guérin; iADL, instrumental activities of daily living; IQR, interquartile range; VAS, visual analog scale.
For the complete baseline characteristics and medication use at enrolment, please see Table S3.
Routine vaccination of adults over 60 years of age with 23-valent pneumococcal polysaccharide vaccine started in autumn of 2020. The target group for the 2020–2021 season was 73–79 years old.
See Table S2 for definition.
Fig. 1.
Flowchart.
∗ A total of 1509 non-inclusions were recorded in 12 hospitals. These hospitals together enrolled 4451 participants in the study. Other participating hospitals did not keep a record of screened patients.
Most participants (68%) used the mobile application to fill in the (bi)weekly questionnaires with no difference between the BCG and placebo group. In total 90 649 of 91 680 planned follow-up questionnaires (98.9%) were completed and 5900 (96.5%) participants completed all questionnaires (see Supplementary Results).
Clinical outcomes
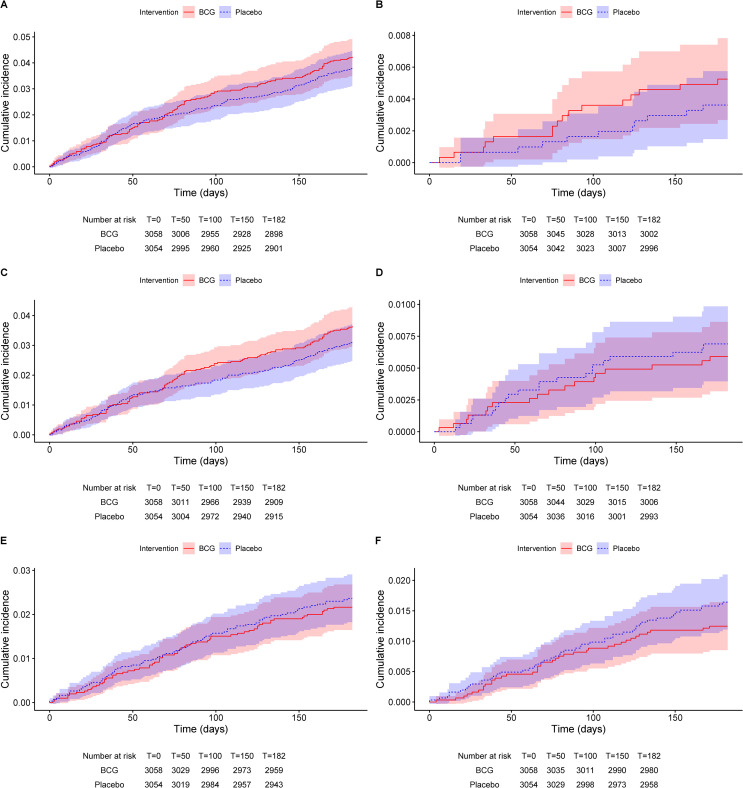
The target number of primary endpoints was met for SARS-CoV-2 infection on 5 January 2021. Based on the lack of a difference between BCG and placebo (Table S4), the DSMB recommended continuation of collecting endpoints as planned. At the end of the follow-up period, there were no statistically significant differences in any of the primary or secondary endpoints (Tables 2 , S2, and S5–S7 and Fig. 2 ). Hospital admission occurred in 39 of 270 (14%) episodes of SARS-CoV-2 infection. SARS-CoV-2-related symptom severity and duration was comparable between the placebo and BCG groups (Fig. S2).
Table 2.
Primary and secondary outcomes
| Endpoint | Intervention | Number of subjects | FU timea | Number of events | Cumulative incidenceb | SDHR |
|---|---|---|---|---|---|---|
| COVID-19 | Placebo | 3054 | 1479.4 | 115 | 0.038 (0.031–0.045) | [ref] |
| BCG | 3058 | 1480.8 | 129 | 0.042 (0.036–0.050) | 1.12 (0.87–1.44) | |
| Documented SARS-CoV-2 infection | Placebo | 3054 | 1477.3 | 126 | 0.041 (0.035–0.049) | [ref] |
| BCG | 3058 | 1476.9 | 144 | 0.047 (0.040–0.055) | 1.15 (0.90–1.45) | |
| Asymptomatic SARS-CoV-2 infection | Placebo | 3054 | 1508.0 | 11 | 0.004 (0.002–0.006) | [ref] |
| BCG | 3058 | 1510.7 | 16c | 0.005 (0.003–0.008) | 1.46 (0.68–3.14) | |
| Mild-moderate SARS-CoV-2 infection | Placebo | 3054 | 1485.1 | 94 | 0.031 (0.025–0.037) | [ref] |
| BCG | 3058 | 1485.0 | 111c | 0.036 (0.030–0.044) | 1.18 (0.90–1.55) | |
| COVID-19-related admissions | Placebo | 3054 | 1505.7 | 21 | 0.007 (0.004–0.010) | [ref] |
| BCG | 3058 | 1510.9 | 18 | 0.006 (0.004–0.009) | 0.86 (0.46–1.61) | |
| Clinically relevant RTI | Placebo | 3054 | 1491.7 | 72d | 0.024 (0.019–0.030) | [ref] |
| BCG | 3058 | 1497.4 | 66e | 0.022 (0.017–0.027) | 0.89 (0.63–1.24) | |
| Medically attended RTI | Placebo | 3054 | 1492.8 | 68 | 0.022 (0.018–0.028) | [ref] |
| BCG | 3058 | 1496.1 | 68 | 0.022 (0.018–0.028) | 1.00 (0.72–1.41) | |
| RTI-related hospital admission | Placebo | 3054 | 1503.6 | 29 | 0.010 (0.007–0.014) | [ref] |
| BCG | 3058 | 1508.1 | 26 | 0.009 (0.006 - 0.012) | 0.90 (0.53 - 1.53) | |
| Clinically relevant RTI not including COVID-19 | Placebo | 3054 | 1497.4 | 50 | 0.016 (0.012–0.021) | [ref] |
| BCG | 3058 | 1503.6 | 38 | 0.012 (0.009–0.017) | 0.76 (0.50–1.16) | |
| RTI-like symptomsf | Placebo | 3054 | 1367.0 | 440 | 0.144 (0.132–0.157) | [ref] |
| BCG | 3058 | 1364.6 | 465 | 0.152 (0.140–0.165) | 1.06 (0.93–1.21) | |
| Pneumoniag | Placebo | 3054 | 1505.9 | 24 | 0.008 (0.005–0.012) | [ref] |
| BCG | 3058 | 1509.3 | 18 | 0.006 (0.004–0.009) | 0.75 (0.41–1.39) |
BCG, Bacillus Calmette-Guérin; FU, follow-up; RTI, respiratory tract infection; SDHR, sub-distribution hazard ratio; [ref], reference category.
Follow-up time (person-years). This includes time until the first event, occurrence of a competing event, loss to follow-up, or day 182, whichever occurred first.
From the cumulative incidence function, taking into account competing events and censoring. The value represents a proportion.
One participant had two episodes, the first mild-moderate and the second asymptomatic.
Five participants had two episodes, one had three episodes and other one had four episodes.
Two participants had two episodes and one had three episodes.
Based on self-reported symptoms.
Self-reported, medically diagnosed, not including documented COVID-19.
Fig. 2.
Cumulative incidence function of primary and secondary endpoints.
(A) COVID-19, (B) documented asymptomatic SARS-CoV-2 infection, (C) mild-moderate SARS-CoV-2 infection, (D) COVID-19-related hospitalization, (E) clinically relevant RTI, and (F) clinically relevant RTI excluding COVID-19.
In an unplanned sensitivity analysis of the primary endpoint, we censored participants at the time of their first SARS-CoV-2 vaccination. Twenty-one COVID-19 infections (placebo 10 and BCG 11) and one COVID-19-related hospitalization (BCG) occurred after the first SARS-CoV-2 vaccination, yielding an adjusted sub-distribution hazard ratio of 1.13 (95% CI, 0.87–1.47) for COVID-19 and 0.81 (95% CI, 0.43–1.54) for COVID-19-related hospitalizations.
Safety outcomes
Safety reports included 1749 non-serious AEs in 1218 placebo recipients and 2964 non-serious AEs in 2050 BCG recipients, as well as 249 SAEs in 217 placebo recipients and 300 SAEs in 256 BCG recipients (Tables 3 , S8, and S9).
Table 3.
Safety endpoints
| Endpoint | Intervention | Number of subjects | FU timea | Number of events | Cumulative incidence | SDHR |
|---|---|---|---|---|---|---|
| First non-serious adverse event | Placebo | 3054 | 1121.0 | 1213 | 0.399 (0.382–0.416) | [ref] |
| BCG | 3058 | 641.5 | 2047 | 0.671 (0.654–0.687) | 2.51 (2.35–2.69) | |
| First serious adverse event | Placebo | 3054 | 1453.1 | 216 | 0.071 (0.062–0.080) | [ref] |
| BCG | 3058 | 1447.0 | 251 | 0.082 (0.073–0.092) | 1.17 (0.97–1.40) | |
| Death | Placebo | 3054 | 1510.6 | 18 | 0.006 (0.004–0.009) | [ref] |
| BCG | 3058 | 1514.7 | 13 | 0.004 (0.002–0.007) | 0.71 (0.35–1.43) | |
| First cardiac arrhythmia event | Placebo | 3054 | 1500.4 | 35 | 0.011 (0.008–0.016) | [ref] |
| BCG | 3058 | 1502.5 | 45 | 0.015 (0.011–0.020) | 1.29 (0.83–2.01) | |
| First coronary artery disorder event | Placebo | 3054 | 1508.1 | 9 | 0.003 (0.001–0.005) | [ref] |
| BCG | 3058 | 1509.1 | 22 | 0.007 (0.005–0.011) | 2.44 (1.12–5.32) |
BCG, Bacillus Calmette-Guérin; FU, follow-up; SDHR: sub-distribution hazard ratio; [ref], reference category.
Follow-up time (person-years). This includes the time until the first event, occurrence of a competing event, loss to follow-up, or day 182, whichever occurred first.
Injection site AEs were most common, with local pain or tenderness within 4 weeks reported by 246 participants in the placebo group and 2137 in the BCG group. Most injection-site reactions were graded as mild or moderate and resolved within 12 weeks. However, 101 participants in the BCG group and one in the placebo group reported severe local site reactions. Participants in the BCG group with BCG vaccination prior to the trial had a higher incidence and longer duration of local side effects (Fig. S3).
As the DSMB noted more cardiac-related SAEs among BCG recipients, an unplanned analysis of these events was requested. The incidence of first cardiac arrhythmia SAEs was comparable between both study groups, but first coronary artery SAEs occurred more frequently in the BCG group than in the placebo group (Table 3 and Fig. S4). Four of 22 (18%) patients with a coronary artery SAE also had infection or inflammation-related (S)AEs prior to the event, as compared to one of nine (11%) patients in the placebo group. Due to the lack of a clear association between cardiac events and preceding infection or inflammation episodes, the DSMB concluded that the higher incidence of cardiac SAEs in the BCG group was unlikely to be mediated by BCG but it advised further monitoring for the occurrence of cardiac SAEs in study participants.
All-cause 6-month mortality did not differ significantly between the two groups (Table 3). Eleven of the 31 (35%) deaths were related to COVID-19: six in the placebo group and five in the BCG group.
Discussion
In this multicentre double-blind randomized placebo-controlled trial, BCG vaccination did not reduce the incidence or severity of COVID-19 or other RTIs in older adults with comorbidities. This study reports the largest prospective evaluation of BCG vaccination in older adults with comorbidities, and reveals that in this age group, BCG vaccination frequently leads to prolonged local site reactions (mild/moderate), and an increased incidence of cardiovascular SAEs, in particular coronary artery events, which appeared unlikely to be mediated by BCG vaccination. These observations warrant cautious use of BCG in older adults with comorbidities.
There is extensive in vitro and experimental evidence that BCG vaccination activates the innate immune system in a non-specific manner, thereby boosting the immunity for months [9]. Likewise, several smaller studies have found a protective effect of BCG on the incidence of respiratory infections in adults [6,10,11]. The ACTIVATE study was the first randomized controlled trial in which BCG vaccination, given to 100 subjects at the time of hospital discharge, was associated with 45% reduction in the incidence of infections during a 12-month follow-up that ended before the COVID-19 pandemic began. Most infections were RTIs and most likely of viral origin [6]. In the Netherlands, two other randomized placebo-controlled trials, one in healthcare workers (BCG-CORONA, N = 1511) and the other in relatively healthy adults over 60 years of age (BCG-ELDERLY, N = 2014), failed to demonstrate the impact of BCG on the incidence of COVID-19 [12,13]. However, severe COVID-19 events were rare in both trials, limiting the possibility to exclude clinically relevant benefits. The results of the current trial confirm the higher risk of developing severe COVID-19 in this population, and also confirm the results of other trials that BCG vaccination does not offer protection against COVID-19 or clinically relevant RTIs caused by other pathogens.
A study with multiple BCG vaccinations vs. placebo (three vaccinations over a 2-year time period preceding the pandemic) in patients with type 1 diabetes showed lower cumulative incidence of COVID-19 in the BCG-treated (1%) vs. the placebo-treated (12.5%) groups [14]. Although promising, this was a small study of 144 participants and thus requires replication in a larger trial. Considering the frequency and timing of repeated BCG vaccinations, this intervention is also inappropriate for an epidemic setting.
In the Greek ACTIVATE-2 trial, 301 individuals aged 50 years or older with underlying comorbidities were randomly assigned to BCG or placebo group. After 6 months, COVID-19 was observed in seven of 92 BCG and 20 of 98 placebo recipients with at least 6 months of follow-up (odds ratio, 0.32; 95% CI, 0.13–0.79) [15]. The reason for the discrepancy between the Greek and the three Dutch trials is unclear. A difference between the Greek and Dutch populations is that in ACTIVATE-2, all participants had prior exposure to BCG and underwent re-vaccination during the trial, whereas the majority of participants in the Dutch trials were BCG-naïve. However, sub-group analysis of the 908 BCG-PRIME participants with previous exposure to BCG did not reveal a differential effect of BCG. Genetic or environmental differences between the populations cannot be excluded, and the influence of geography on BCG has been documented in children [16,17]. ACTIVATE-2 results could be biased due to a high loss-to-follow-up, with 111 of 301 participants lost to follow-up between 3 months and 6 months.
This trial has several limitations. First, the injection site reactions may have unblinded many of the BCG recipients. However, we used objective criteria for the primary and most of the secondary endpoints and adjudication of the primary endpoints was blinded for treatment allocation, thereby avoiding information bias.
A second limitation is that SARS-CoV-2 infections was based on self-reported PCR or antigen test results. We confirmed reported positive tests by phone, thus avoiding false-positive reports. However, we may have missed positive tests. We do not expect this to be different for BCG and placebo participants. Therefore, the relative effect estimate is considered unbiased. Additionally, we may have missed asymptomatic and mild infections by not using serological data [12]. General practitioner visits for RTIs and hospitalizations for any reason are always confirmed in medical records.
Furthermore, the SARS-CoV-2 vaccination programme in the Netherlands started during the follow-up phase. At the end of follow-up, 71% of the study population had received first dose and 31% of the population was fully vaccinated. Yet, vaccination occurred at equal pace in both study groups. Although vaccination most likely reduced the incidence of COVID-19, there is no evidence that this influenced the conclusion of the study, as demonstrated by the sensitivity analysis. Although there is some evidence that BCG vaccination leads to higher antibody titres after SARS-CoV-2 vaccination, which may enhance the effect of BCG in preventing COVID-19 [18], less number of COVID-19 episodes after SARS-CoV-2 vaccination in our study precludes demonstration of such an effect.
Finally, for the hazard ratio of BCG for COVID-19-related hospitalizations, the lower bound of the CI was 0.46, which, therefore, included a meaningful reduction. With 39 episodes of COVID-19-related hospitalizations, this study cannot completely exclude a clinically relevant reduction in severe COVID-19 incidence through BCG vaccination, and future meta-analyses may answer this question. However, the lack of effect on symptom severity and duration of symptoms does not suggest beneficial effects on the course of SARS-CoV-2 infections.
Strengths of the BCG-PRIME study include its sample size and completeness of follow-up in a study population at higher risk of developing severe COVID-19 than enrolled in previous studies [[12], [13], [15]]. Yet, despite a median age of 69 years and a median of two comorbidities, 85% of the participants were considered important based on clinical frailty scores. Subjects with lower frailty scores may be at higher risk of severe COVID-19, but based on our findings, beneficial effects of BCG vaccination seem unlikely in this population.
In conclusion, BCG vaccination did not protect against COVID-19 infection and did not reduce the incidence of clinically relevant RTIs in a large cohort of older adults with comorbidities from the Netherlands.
Author contributions
E.L.K. is the lead and corresponding author. J.S.v.d.M., C.v.N., J.J.H., M.A.A.J.v.d.B., M.G.N., F.R.R., M.J.M.B., and C.H.v.W. conceptualized the study. J.S.v.d.M., J.J.O., J.J.H., J.H.H.v.d.W., F.R.R., M.J.M.B., and C.H.v.W. developed methodology. J.S.v.d.M. was responsible for the software. C.H.v.W. performed the formal analysis. E.L.K., K.F., J.S.v.d.M., J.J.O., J.J.H., M.P.G., and C.H.v.W. performed the investigation. J.J.O., C.v.N., M.A.A.J.v.d.B., M.J.M.B., and C.H.v.W. were responsible for the resources. E.L.K., K.F., and C.H.v.W. were responsible for data curation. E.L.K., M.J.M.B., and C.H.v.W. wrote the original draft of the manuscript. E.L.K., K.F., J.S.v.d.M., C.v.N., M.P.G., J.H.H.v.d.W., M.G.N., F.R.R., M.J.M.B., and C.H.v.W. reviewed and edited the manuscript. E.L.K. and C.H.v.W. were responsible for visualization. J.S.v.d.M., J.J.O., M.P.G., M.A.A.J.v.d.B., J.H.H.v.d.W., M.G.N., F.R.R., M.J.M.B., and C.H.v.W. were responsible for supervision. E.L.K., K.F., J.S.v.d.M., J.J.H., F.R.R., and C.H.v.W. were responsible for project administration. J.J.H., J.H.H.v.d.W., M.A.A.J.v.d.B., F.R.R., M.G.N., M.J.M.B., and C.H.v.W. were responsible for funding acquisition.
Transparency declaration
M.G.N. is a scientific founder and member of scientific advisory board of TTxD and Lemba.
Other authors report no conflict of interests. This study was funded by the Dutch public funding agency ZonMw, grant number 10430072010001. M.G.N. was supported by an ERC Advanced Grant (#833247).
Acknowledgements
We thank the participants, study personnel, the DSMB (Prof. Heiman F. L. Wertheim, Department of Medical Microbiology, Radboud University Medical Center; Prof. Ewout W. Steyerberg, Department of Biomedical Data Sciences, Leiden University Medical Centre; Patrick M.M. Bossuyt, Department of Epidemiology & Data Science, Amsterdam UMC, Miquel Ekkelenkamp, University of Amsterdam), the medical monitor (Rob R. Bohte, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht) and the independent doctor (Miquel Ekkelenkamp, department of Medical Microbiology, University Medical Center Utrecht) for their time investment and dedication towards the study.
Editor: L. Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2023.01.019.
Contributor Information
BCG-PRIME study group:
Astrid Aardenburg-van Huisstede, Heidi S.M. Ammerlaan, Willem G. Boersma, Marc J.M. Bonten, Maurice A.A.J. van den Bosch, Kees Brinkman, Patricia C.J. Bruijning-Verhagen, Reinout van Crevel, Corine Delsing, Thijs ten Doesschaten, Anton S.M. Dofferhoff, Ruud Duijkers, Konstantin Fohse, Martin P. Grobusch, Rolf H.H. Groenwold, Corine de Haas, Robert-Jan Hassing, Marieke L.A. de Hoog, Jacobien J. Hoogerwerf, Susanne M. Huijts, Astrid van Hylckama-Vlieg, Eefje Jong, Hanna K. de Jong, Martijn Knap, Eva L. Koekenbier, Michael Koenders, Ilse J.E. Kouijzer, Henk Kramer, Roel van de Laar, Arief Lalmohamed, Karel-Jan D.F. Lensen, Willem M. Lijfering, Josephine S. van de Maat, Fabienne Magdelijns, Bob Meek, Rutger A. Middelburg, Hazra S. Moeniralam, Simon P. Mooijaart, Barbara C. van Munster, Mihai G. Netea, Cees van Nieuwkoop, Jaap ten Oever, Jan Jelrik Oosterheert, Marc Padros Goossens, Vincent Peters, Douwe F. Postma, Niels Pouw, Herre J. Reesink, Marieke J.A. de Regt, Anneli C.J. van der Reijden, Frits R. Rosendaal, R. Schaakxs, Kitty Slieker, Robbert J. Slingerland, Nicolette L.J. van Sluis, Coen D.A. Stehouwer, Frank van de Veerdonk, Annelies Verbon, C.H. (Henri) van Werkhoven, and Janneke H.H. van de Wijgert
Appendix B.
The BCG-PRIME study group.
Astrid Aardenburg-van Huisstede (Noordwest Ziekenhuis, Alkmaar), Heidi S.M. Ammerlaan (Catharina Ziekenhuis, Eindhoven), Willem G. Boersma (Noordwest Ziekenhuis, Alkmaar), Marc J.M. Bonten (University Medical Center Utrecht, Utrecht), Maurice A.A.J. van den Bosch (OLVG, Amsterdam), Kees Brinkman (OLVG, Amsterdam), Patricia C.J. Bruijning-Verhagen (University Medical Center Utrecht, Utrecht), Reinout van Crevel (Radboud University Medical Center, Nijmegen), Corine Delsing (Medisch Spectrum Twente, Enschede), Thijs ten Doesschaten (University Medical Center Utrecht, Utrecht), Anton S. M. Dofferhoff (Canisius-Wilhelmina Hospital, Nijmegen), Ruud Duijkers (Noordwest Ziekenhuis, Alkmaar), Konstantin Fohse (Radboud University Medical Center, Nijmegen), Martin P. Grobusch (Amsterdam UMC, Amsterdam), Rolf H.H. Groenwold (Leiden University Medical Center, Leiden), Corine de Haas (University Medical Center Utrecht, Utrecht), Robert-Jan Hassing (Rijnstate hospital, Arnhem), Marieke L.A. de Hoog (University Medical Center Utrecht, Utrecht), Jacobien J. Hoogerwerf (Radboud University Medical Center, Nijmegen), Susanne M. Huijts (Erasmus Medical Center, Rotterdam), Astrid van Hylckama-Vlieg (Leiden University Medical Center, Leiden), Eefje Jong (Meander Medisch Centrum, Amersfoort), Hanna K. de Jong (University Medical Center Amsterdam, Amsterdam), Martijn Knap (University Medical Center Amsterdam, Amsterdam), Eva L. Koekenbier (University Medical Center Utrecht, Utrecht), Michael Koenders (Santeon Bureau of Research & Innovation, Utrecht, the Netherlands), Ilse J.E. Kouijzer (Radboud University Medical Center, Nijmegen), Henk Kramer (Martini Hospital, Groningen), Roel van de Laar (Ikazia Hospital, Rotterdam), Arief Lalmohamed (University Medical Center Utrecht, Utrecht), Karel-Jan D.F. Lensen (University Medical Center Groningen, Groningen), Willem M. Lijfering (Leiden University Medical Center, Leiden), Josephine S. van de Maat (Radboud University Medical Center, Nijmegen), Fabienne Magdelijns (University Medical Center Maastricht, Maastricht), Bob Meek (St. Antonius Hospital, Nieuwegein), Rutger A. Middelburg (Leiden University Medical Center, Leiden), Hazra S. Moeniralam (St. Antonius Hospital, Nieuwegein), Simon P. Mooijaart (Leiden University Medical Center, Leiden), Barbara C. van Munster (University Medical Center Groningen, Groningen), Mihai G. Netea (Radboud University Medical Center, Nijmegen), Cees van Nieuwkoop (Haga Teaching Hospital, The Hague), Jaap ten Oever (Radboud University Medical Center, Nijmegen), Jan Jelrik Oosterheert (University Medical Center Utrecht), Marc Padros Goossens (University Medical Center Utrecht, Utrecht), Vincent Peters (Catharina Ziekenhuis, Eindhoven), Douwe F. Postma (University Medical Center Groningen, Groningen), Niels Pouw (Radboud University Medical Center, Nijmegen), Herre J. Reesink (OLVG, Amsterdam), Marieke J.A. de Regt (OLVG, Amsterdam), Anneli C.J. van der Reijden (Anticoagulation Clinic Leiden, Leiden), Frits R. Rosendaal (Leiden University Medical Center, Leiden), R. Schaakxs (University Medical Center Utrecht, Utrecht), Kitty Slieker (Bernhoven Hospital, Uden), Robbert J. Slingerland (Maasstad Hospital, Rotterdam), Nicolette L.J. van Sluis (University Medical Center Utrecht, Utrecht), Coen D.A. Stehouwer (University Medical Center Maastricht, Maastricht), Frank van de Veerdonk (Radboud University Medical Center, Nijmegen), Annelies Verbon (Erasmus Medical Center, Rotterdam), C.H. (Henri) van Werkhoven (University Medical Center Utrecht, Utrecht), Janneke H.H. van de Wijgert (University Medical Center Utrecht, Utrecht).
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Blomaard L.C., van der Linden C.M.J., van der Bol J.M., Jansen S.W.M., Polinder-Bos H.A., Willems H.C., et al. Frailty is associated with in-hospital mortality in older hospitalized COVID-19 patients in The Netherlands: the COVID-OLD study. Age Ageing. 2021;50:631–640. doi: 10.1093/ageing/afab018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moorlag S.J.C.F.M., Arts R.J.W., van Crevel R., Netea M.G. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect. 2019;25:1473–1478. doi: 10.1016/j.cmi.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 3.Benn C.S., Netea M.G., Selin L.K., Aaby P. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends Immunol. 2013;34:431–439. doi: 10.1016/j.it.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Taks E.J.M., Moorlag S.J.C.F.M., Netea M.G., van der Meer J.W.M. Shifting the immune memory paradigm: trained immunity in viral infections. Annu Rev Virol. 2022;9:469–489. doi: 10.1146/annurev-virology-091919-072546. [DOI] [PubMed] [Google Scholar]
- 5.O'Neill L.A.J., Netea M.G. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20:335–337. doi: 10.1038/s41577-020-0337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giamarellos-Bourboulis E.J., Tsilika M., Moorlag S., Antonakos N., Kotsaki A., Domínguez-Andrés J., et al. Activate: randomized clinical trial of BCG vaccination against infection in the elderly. Cell. 2020;183:315–323.e9. doi: 10.1016/j.cell.2020.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hilligan K.L., Namasivayam S., Clancy C.S., O'Mard D., Oland S.D., Robertson S.J., et al. Intravenous administration of BCG protects mice against lethal SARS-CoV-2 challenge. J Exp Med. 2022;219 doi: 10.1084/jem.20211862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.R: a language and environment for statistical computing. R Foundation for Statistical Computing; 2020. https://www.R-project.org/ [Google Scholar]
- 9.Netea M.G., Domínguez-Andrés J., Barreiro L.B., Chavakis T., Divangahi M., Fuchs E., et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20:375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nemes E., Geldenhuys H., Rozot V., Rutkowski K.T., Ratangee F., Bilek N., et al. Prevention of M. tuberculosis Infection with H4:IC31 vaccine or BCG revaccination. N Engl J Med. 2018;379:138–149. doi: 10.1056/NEJMoa1714021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wardhana D.E., Sultana A., Mandang V.V., Jim E. The efficacy of Bacillus Calmette-Guerin vaccinations for the prevention of acute upper respiratory tract infection in the elderly. Acta Med Indones. 2011;43:185–190. PMID: 21979284. [PubMed] [Google Scholar]
- 12.Ten Doesschate T., van der Vaart T.W., Debisarun P.A., Taks E., Moorlag S.J.C.F.M., Paternotte N., et al. Bacillus Calmette-Guérin vaccine to reduce healthcare worker absenteeism in COVID-19 pandemic, a randomized controlled trial. Clin Microbiol Infect. 2022;28:1278–1285. doi: 10.1016/j.cmi.2022.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moorlag S.J.C.F.M., Taks E., Ten Doesschate T., van der Vaart T.W., Janssen A.B., Müller L., et al. Efficacy of BCG vaccination against respiratory tract infections in older adults during the coronavirus disease 2019 pandemic. Clin Infect Dis. 2022;75:e938–e946. doi: 10.1093/cid/ciac182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faustman D.L., Lee A., Hostetter E.R., Aristarkhova A., Ng N.C., Shpilsky G.F., et al. Multiple BCG vaccinations for the prevention of COVID-19 and other infectious diseases in type 1 diabetes. Cell Rep Med. 2022;3:100728. doi: 10.1016/j.xcrm.2022.100728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsilika M., Taks E., Dolianitis K., Kotsaki A., Leventogiannis K., Damoulari C., et al. ACTIVATE-2: a double-blind randomized trial of BCG vaccination against COVID-19 in individuals at risk. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.873067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mangtani P., Abubakar I., Ariti C., Beynon R., Pimpin L., Fine P.E., et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis. 2014;58:470–480. doi: 10.1093/cid/cit790. [DOI] [PubMed] [Google Scholar]
- 17.Wilson M.E., Fineberg H.V., Colditz G.A. Geographic latitude and the efficacy of Bacillus Calmette-Guérin vaccine. Clin Infect Dis. 1995;20:982–991. doi: 10.1093/clinids/20.4.982. [DOI] [PubMed] [Google Scholar]
- 18.Ramos-Martinez E., Falfán-Valencia R., Pérez-Rubio G., Andrade W.A., Rojas-Serrano J., Ambrocio-Ortiz E., et al. Effect of BCG revaccination on occupationally exposed medical personnel vaccinated against SARS-CoV-2. Cells. 2021;10:3179. doi: 10.3390/cells10113179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.