Abstract
Coronavirus disease 2019 is caused by severe acute respiratory syndrome coronavirus 2 and is associated with pronounced hematopathologic findings. Peripheral blood features are heterogeneous and very often include neutrophilia, lymphopenia, myeloid left shift, abnormally segmented neutrophils, atypical lymphocytes/plasmacytoid lymphocytes, and atypical monocytes. Bone marrow biopsies and aspirates are often notable for histiocytosis and hemophagocytosis, whereas secondary lymphoid organs may exhibit lymphocyte depletion, pronounced plasmacytoid infiltrates, and hemophagocytosis. These changes are reflective of profound innate and adaptive immune dysregulation, and ongoing research efforts continue to identify clinically applicable biomarkers of disease severity and outcome.
Keywords: COVID-19, SARS-CoV-2, Neutrophilia, Lymphopenia, Dysgranulopoiesis, Atypical lymphocytes, Lymphadenopathy, Hemophagocytic lymphohistiocytosis
-
•
Coronavirus disease 2019 (COVID-19) disease is accompanied by an array of hematologic alterations.
-
•
Peripheral blood abnormalities are present in most COVID-19 patients but are heterogeneous. They very often include neutrophilia, lymphopenia, myeloid left shift, abnormally segmented neutrophils, atypical lymphocytes/plasmacytoid lymphocytes, and atypical monocytes.
-
•
Bone marrow biopsies and aspirates may show left-shifted maturation, occasional erythroid dysplasia, and evidence of hemophagocytic lymphohistiocytosis.
-
•
Secondary lymphoid organs often show lost/hypoplastic germinal centers, altered lymphocyte compositions, sinus histiocytosis/hemophagocytosis, and plasmacytoid proliferations.
Overview
Individuals with coronavirus disease 2019 (COVID-19), the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), exhibit a range of pulmonary, cerebral, myocardial, hepatic, and renal dysfunctions. Large-scale autopsy studies have documented multi-organ pathology findings1 , 2 with substantial remodeling in lung epithelial, immune, and stromal compartments and evidence of multiple paths of failed tissue regeneration.3 SARS-CoV-2 infection is also associated with pronounced hematologic alterations. During acute infection, peripheral blood abnormalities are present that occur in a complex network of innate and adaptive immune dysregulation,4 some of which can serve as clinical biomarkers of disease severity and outcome.5 , 6 Secondary lymphoid organs often show lymphocyte depletion and occasionally hemophagocytosis.7 , 8 Hemophagocytic lymphohistiocytosis (HLH) is also common in bone marrow biopsies and aspirates.9, 10, 11 We herein review currently well-established and clinically relevant hematopathologic findings in patients with COVID-19 and discuss their diagnostic and prognostic relevance. Although COVID-19 is also associated with pronounced hemostasis-/thrombosis-related changes, a review of this comprehensive topic is beyond the scope of this article and can be found elsewhere.12
Peripheral blood features
Quantitative Changes
Peripheral blood key features are summarized in Box 1 . Initial studies reported that complete blood counts (CBCs) and white blood cell differential counts from patients admitted with COVID-19 were notable for leukocytosis, absolute neutrophilia, and lymphopenia, especially in the intensive care unit (ICU) compared with non-ICU patients.13, 14, 15 Early meta-analyses confirmed that lymphopenia is frequent in acute COVID-19,16 , 17 and that patients with severe and fatal disease had significantly increased white blood counts (WBCs) and absolute neutrophil counts (ANCs), decreased absolute lymphocyte counts (ALCs), platelet and eosinophil counts, and decreased hemoglobin compared with those with non-severe disease and survivors.17 In subsequent single-institution studies, lymphopenia, neutrophilia, and thrombocytopenia remained prominent features,18, 19, 20, 21 while eosinopenia and monocytosis occurred at highly varying frequencies.19, 20, 21
Box 1. Peripheral blood key features.
| Frequent quantitative changes | WBC↕ Neutrophils ↑ Lymphocytes ↓ Eosinophils may be↓ Basophils may be ↓ Monocytes may be ↓ Hemoglobin ↕ Platelets ↕ |
| Frequent qualitative changes |
|
| Association with severe disease including death |
|
a Most significant morphologic findings in COVID-19 patients when compared with patients without COVID-19.
When directly compared with SARS-CoV-2 negative patients, WBCs in SARS-CoV-2-positive patients varied widely but were generally lower.22, 23, 24, 25 Similarly, lymphopenia was frequent, and ALCs tended to be lower when compared with SARS-CoV-2-negative patients, but often failed to reach statistical significance.22, 23, 24, 25, 26 Neutrophilia was frequent, and ANCs were not significantly different from negative patients in some,22, 23, 24 but significantly lower in other studies.25 In some studies, significantly lower absolute eosinophil,24, 25, 26 basophil,25 and monocyte counts23 , 25 were present in positive patients. Hemoglobin values appeared to be highly variable, and findings included anemia in a proportion of SARS-CoV-2-positive patients,22 , 24 , 26 , 27 but also increased hemoglobin in other populations.23 , 25
Qualitative Changes
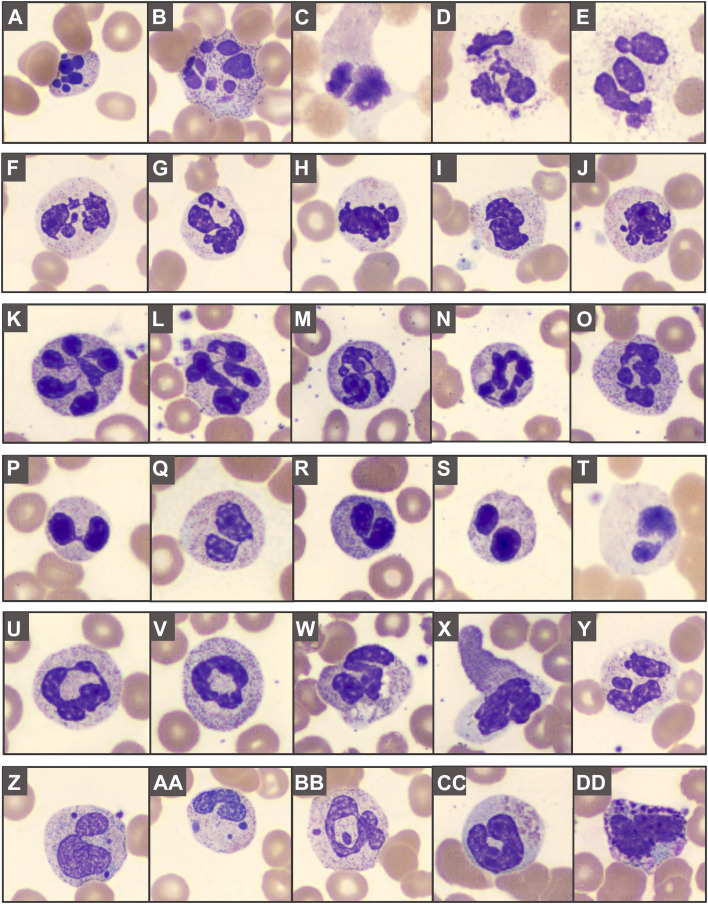
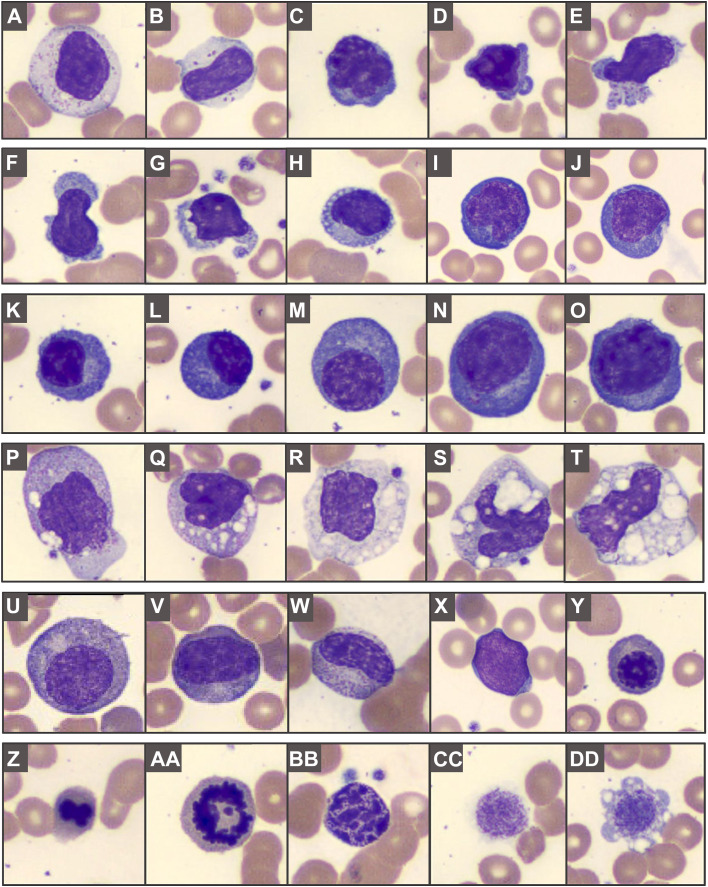
Qualitative (morphologic) changes affecting all cell lineages are found in blood smears from up to 100% of patients with COVID-19 (see Box 1), including circulating apoptotic cells.6 One of the most striking findings is dysgranulopoiesis (Fig. 1 ). Neutrophils demonstrate a range of abnormal nuclear shapes, such as pseudo-Pelger–Huët morphology, nuclear hyposegmentation or hypersegmentation, and ring-shaped nuclei.18, 19, 20 , 23 , 24 , 26, 27, 28 Abnormal granulation patterns are frequent.18, 19, 20 , 22, 23, 24 , 26 , 27 , 29 Several studies reported apoptotic, pyknotic, disintegrating, or smudged neutrophils,18 , 21 , 22 , 24 , 26 potentially reflecting neutrophil extracellular traps (NET), enzymatic webs released by activated neutrophils.30 In addition, left-shifted myeloid cells can be found, occasionally with leukoerythroblastosis (Fig. 2 ).18 , 22, 23, 24 , 26 , 27 , 31 Pronounced morphologic changes are also present within the lymphoid lineage (see Fig. 2), and several types of atypical and/or enlarged lymphocytes have been observed.6 , 20 , 23 , 24 , 26 , 27 These include plasmacytoid lymphocytes, occasionally with bizarre-shaped nuclei and pseudopods,20 , 23 , 24 , 26 , 27 large granular lymphocytes (LGLs),20 , 23 atypical lymphocytes,23 and lymphocytes with pronounced cytoplasmic vacuoles.23 , 26 Some studies reported a small proportion of circulating plasma cells or Mott cells.22 , 26 Vacuolization, including numerous large coalescing vacuoles, has been seen in monocytes (see Fig. 2),23 , 24 , 26 along with blue-green cytoplasmic inclusions.29 Vacuolated eosinophils have been noted,19 , 23 as well as increased large or giant platelets,18 , 19 , 21 , 22 occasionally with pseudopodia formations.18
Fig. 1.
Abnormal granulopoiesis in peripheral blood smears: (A–E) apoptotic/pyknotic, disintegrating, and smudged neutrophils; (F–J) abnormally segmented nuclei, including (K–O) hypersegmentation, (P–T) pseudo-Pelger–Huët morphology, and (U, V) ring forms. Abnormal granulation can feature (W–Y) toxic granules, cytoplasmic vacuolization, patchy hypogranularity, and (Z–BB) Döhle body-like inclusions or apparent nuclear fragments. Abnormally granulated eosinophils (CC) and basophils (DD) can be seen.
Fig. 2.
Atypical lymphocytes and other COVID-19-related blood findings: (A, B) large granular lymphocytes; (C–O) atypical lymphocytes can show a spectrum of features, with nuclear irregularities, variably coarse chromatin, deeply basophilic cytoplasm, occasionally with cytoplasmic blebs, granules or vacuolization; (K–O) plasma cells or plasmacytoid forms can include irregular enlarged forms. (P–T) Monocytes can show coarser granules or varying degrees of prominent coalescing vacuolization. (U–Y) Left-shifted myeloid cells and leukoerythroblastosis; (Z–AA) budding erythroid nucleus and mitotic figure, (BB) pyknotic cell, and (CC–DD) giant platelets.
When directly compared with patients without COVID-19, the most significant morphologic findings included left-shifted myeloid cells, smudged neutrophils, neutrophil vacuolation, pseudo-Pelger–Huët anomaly, non-segmented and ring neutrophils, atypical lymphocytes, plasma cells/ plasmacytic cells, pyknotic cells, and large/ giant platelets (see Box 1).22 , 24
Morphology Scores
Several morphology scores have been developed. Using abnormalities in granulocytes, lymphocytes, monocytes, maturational left shift, and pyknotic cells, with a scoring scale of zero to five, no patient with COVID-19 had score zero, whereas 27%, 42%, and 26% had score one, two, and three, respectively, with a score range of zero to two in non-COVID-19 blood smears.26 Another study scored WBC morphology using a four-point scale (0: absent; 1: present in ≤10% of cell lineage; 2: present in 11% to 25%; 3: present in >25%).23 When directly compared with negative ICU patients, positive patients showed significantly fewer monocytes with abnormal vacuolization, whereas the presence of atypical lymphocytes, especially plasmacytoid forms, was more predictive of COVID-19 infection.
Association with Disease Severity and Outcome
Several parameters and ratios between blood cells have been associated with disease severity and outcome, including death23, 24, 25, 26 , 32, 33, 34 (see Box 1). When applying the score by Gabr and colleagues, patients with higher scores had generally unfavorable outcomes.26 Myeloid left shift was associated with requiring ICU admission, whereas atypical lymphocytes and monocytes with large coalescent cytoplasmic vacuoles were associated with non-ICU patients in one study.23 In contrast, Gabr and colleagues found significantly more atypical monocytes in ICU patients,26 whereas Pezeshki and colleagues did not identify any statistically significant associations between blood findings and clinical course.21 Importantly, several non-hematologic factors (eg, age, gender), and other laboratory parameters (including ferritin, C-reactive protein, interleukin-6) are often associated with death, and machine learning-based algorithms have been employed for diagnosis and prediction of care needs and outcome.35, 36, 37
Bone Marrow Features
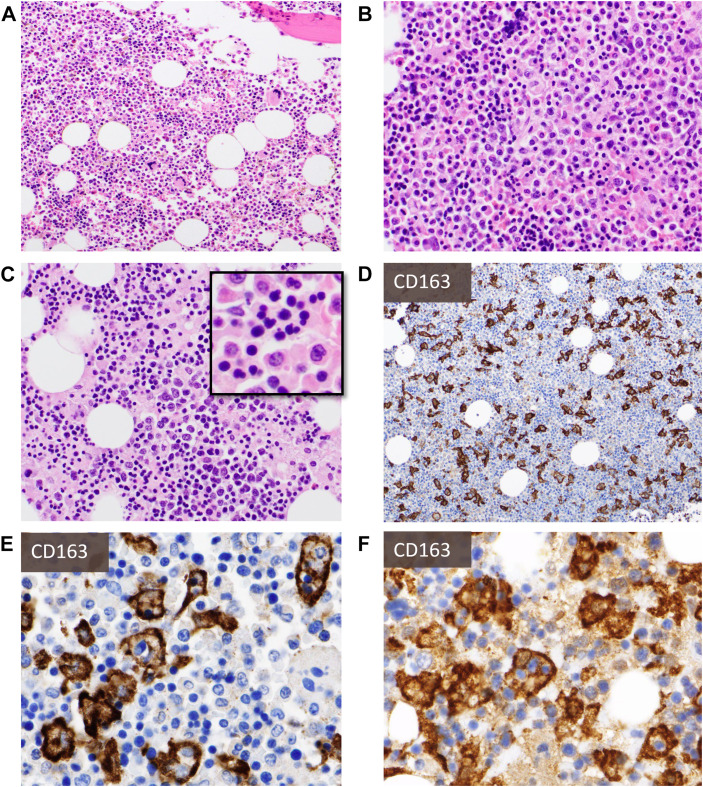
Examination of bone marrow aspirations and biopsies from individuals with COVID-19 showed varying cellularity (normocellular to hypercellular), mostly maturing trilineage hematopoiesis with occasional myeloid left shift, occasional dyserythropoiesis, increased pleomorphic megakaryocytes with focal clustering, increased polyclonal plasma cells, lymphocytosis, and varying degrees of histiocytosis and hemophagocytosis8, 9, 10, 11 , 38 , 39 (Fig. 3 ). The latter is an important finding as it provides histopathologic evidence of secondary development of HLH, a life-threatening inflammatory syndrome associated with significant mortality. Based on these findings, it has been suggested to promptly perform bone marrow aspiration and biopsy in COVID-19 patients with suspected HLH to guide appropriate treatment.11
Fig. 3.
Bone marrow biopsy findings: H&E sections (A–C, from four different decedents) show varying cellularity with frequent hypercellularity, maturing trilineage hematopoiesis with frequent myeloid left shift (A, B), and occasional decreased M:E ratio (C). There may be pronounced dyserythropoiesis (C-inlay) and varying degrees of histiocytosis and hemophagocytosis that may not be readily appreciated on H&E sections but are unearthed with immunohistochemistry for histiocytic markers (eg, CD163) (D, E). Autopsy cases kindly provided by Dr Olga Pozdnyakova.
Spleen and lymph node features
Acute Infection
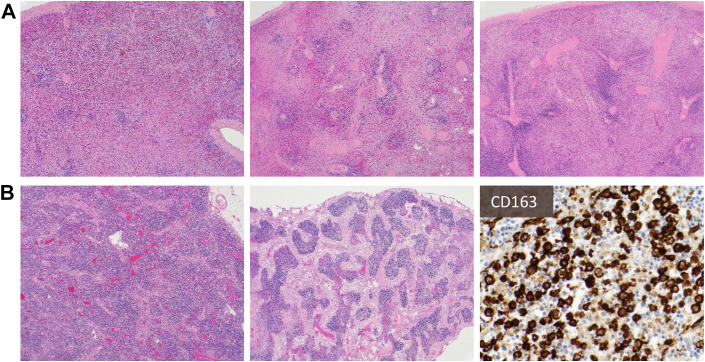
The key features are summarized in Box 2 and Fig. 4 . Several autopsy studies have described splenic white pulp atrophy or depletion, absence of marginal zones, increased red-to-white pulp ratio, and hemophagocytosis in a subset of COVID-19 decedents.7 , 8 In addition, varying degrees of lymphoplasmacytic infiltrates in the red pulp and immunoblasts in the white pulp were noted. Other occasional findings included extensive red pulp necrosis, red pulp congestion, and frank hemorrhage.40
Box 2. Key features in spleen and lymph nodes.
| Spleen |
|
|
| Lymph nodes |
|
Fig. 4.
Common findings in spleen (A) and lymph node (B): Spleens show diminished white pulp and increased red-to-white pulp ratio, with areas of hemorrhage (A). On low magnification, lymph nodes show attenuation of follicular architecture (B, left) and varying degrees of sinus histiocytosis (B, middle), with some cases also showing prominent hemophagocytosis (B, right, highlighted by CD163).
Similarly, lymphocyte depletion or absence of germinal centers and hemophagocytosis (see Fig. 4) are frequent in lymph nodes (approximately 20% of patients).8 When present, germinal centers may be hypoplastic, whereas sinus histiocytosis and increased immunoblasts and plasmablasts are frequently noted in sinuses and within the paracortex.7 , 8 , 40 Other features may include vascular congestion with vascular transformation of sinuses and hemorrhage.40 Immunohistochemistry (IHC)-based studies further highlighted a defect of germinal center structure, with T-follicular helper (TFH) cells and germinal center formation largely absent in draining hilar lymph nodes, which correlated with reduced Immunoglobulin M (IgM) and Immunoglobulin G (IgG) levels compared with convalescent COVID-19 patients.41 Another study demonstrated decreased T lymphocytes in most examined lymph nodes, with a disproportionate decrease of CD8+ T cells, and relative preservation of B lymphocytes.40
A comprehensive analysis of lymphoid architecture and lymphocyte populations of thoracic lymph nodes and spleens from patients with early and late COVID-19 revealed the absence of lymph node and splenic germinal centers and depletion of Bcl-6-expressing B cells, but the preservation of activation-induced cytidine deaminase-positive B cells.42 In addition, Bcl-6+ TFH-cell generation and differentiation were defective, whereas abundant T-helper 1 (TH1) cells and aberrant TNF-alpha production were seen. Interestingly, extramedullary megakaryocytes and clusters of erythroid precursors were noted in several studies,38 , 40 and their role in COVID-19-related hemostatic and thrombotic alterations is an area of active research.
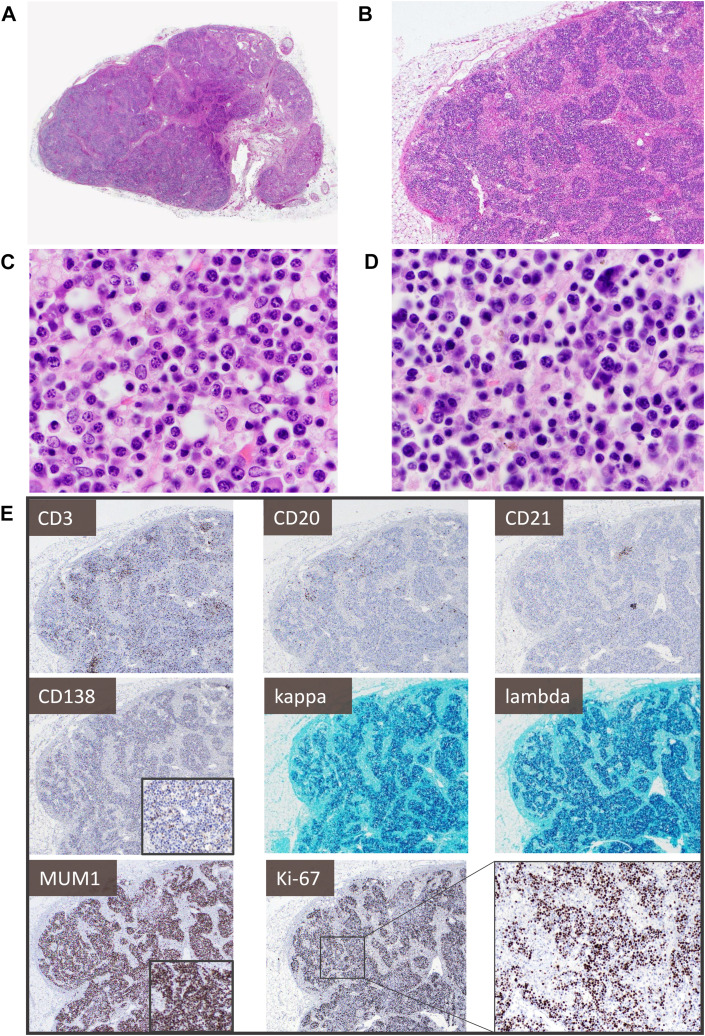
Fig. 5 highlights a lymph node showing lymphocyte depletion and prominent plasmacytosis with an increased proliferation rate. Despite being polyclonal, plasma cells and plasmacytoid forms can be markedly enlarged, include hyperchromatic and binucleated forms, and show a plasmablast-like immunophenotype (MUM1+, CD138 variable/ negative). These features can pose a diagnostic challenge, especially in patients with pre-existing hematologic conditions, and extensive ancillary studies may be warranted to exclude a neoplastic process (Fig. 6 ). Similarly, careful exclusion of an underlying malignancy, or autoimmune or infectious disease, is advised in the workup of mediastinal lymphadenopathy, which has been noted in up to 66% of patients with COVID-19 and is frequently associated with inferior outcomes.43 , 44
Fig. 5.
Lymph node showing lymphocyte depletion and prominent polyclonal plasmacytosis with increased proliferation in acute infection: (A, B) show an architecturally intact lymph node with patent subcapsular and medullary sinuses, many with reactive histiocytosis. There are almost no apparent follicles and lymphocytes are diminished, with the parenchyma (C, D) mostly replaced by a population of small to medium-sized plasma cells and plasmacytoid forms with occasional markedly enlarged, hyperchromatic nuclei or binucleated forms, as well as ones with more dispersed chromatin and prominent nucleoli. (E) CD3+ T cells and CD20+ B cells are markedly reduced, with only rare CD21+ follicular dendritic cell meshworks. The plasma cell population shows dim-to-negative CD138, uniform MUM1 expression, and considerably increased Ki-67 proliferation (approximately 50%) but polytypic kappa and lambda light chain expression.
Fig. 6.
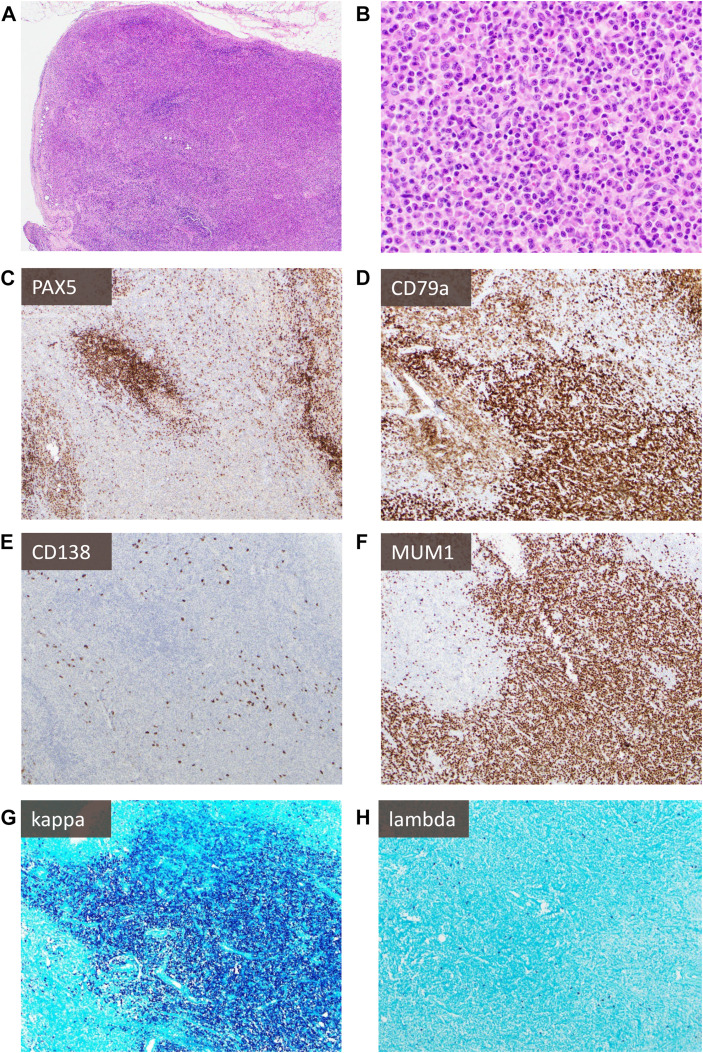
Kappa-predominant plasmacytoid proliferation in a patient with a history of lymphoma: A patient with stage IV diffuse large B-cell lymphoma developed new FDG-avid lymphadenopathy concerning recurrent lymphoma (note: case has previously been published,55 new images were taken for this publication with permission). H&E sections (A, B) show an interfollicular expansion by numerous small lymphocytes, epithelioid histiocytes, and focal sheets of plasmacytoid cells variably positive for PAX5 (C), positive for CD79a (D), negative for CD138 (E), and positive for MUM1 (F) with marked excess kappa (G) light chain expression compared with lambda (H). However, molecular studies showed polyclonal immunoglobulin heavy chain gene (IGH) rearrangements, absence of clonal aberrancies by cytogenetics, and lymphadenopathy resolved over time without evidence of lymphoma recurrence.
COVID-19 Post-Vaccination Lymphadenopathy
There is a growing body of evidence highlighting the diagnostic dilemmas related to COVID-19 post-vaccination lymphadenopathy. This often affects axillary lymph nodes at the ipsilateral injection site and occurs at an overall rate of 14%, but varies widely in published cohort studies and clinical trials.45 Although ultimately there is spontaneous resolution, the reported duration varies, and lymphadenopathy post-COVID-19 vaccination can persist for weeks45 , 46 or even months.47 To avoid unnecessary workup, especially in women undergoing routine breast cancer screening and patients with a pre-existing malignancy, recommendations for imaging and imaging-based additional workup have been developed.48 In individuals who underwent a biopsy for further workup, cytologic smears and histologic sections have generally shown reactive features with follicular hyperplasia, prominent germinal centers, and paracortical expansion49, 50, 51 (Fig. 7 ). However, rare cases of lymphoma have been described, highlighting that malignancy should remain an important differential diagnostic consideration.52 , 53
Fig. 7.
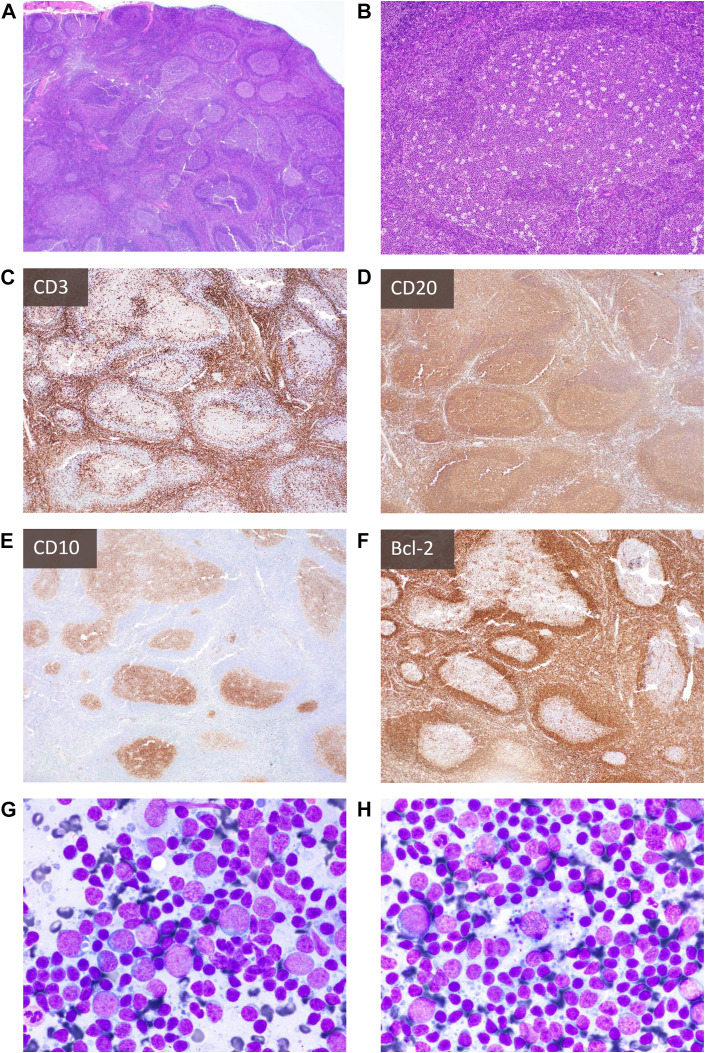
Histologic and cytologic findings of COVID-19 vaccine-related lymphadenopathy: An individual in their twenties suddenly developed axillary, cervical, and supraclavicular lymphadenopathy (up to 2 cm in greatest dimension) 1 week after receiving the second mRNA COVID-19 vaccine in the deltoid muscle. H&E sections (A, B) and IHC for CD3 (C), CD20 (D), CD10 (E), and Bcl-2 (F) of lymph nodes (ipsilateral to vaccine site) show marked reactive lymphoid hyperplasia. Aspirate smears (G, H) show a polymorphous mixture of small and large lymphocytes, occasional admixed inflammatory cells, tingible-body macrophages, and lymphoglandular bodies (cytoplasmic fragments). Case and images kindly provided by Dr Amy Duffield.
Ancillary Testing: Flow Cytometry
A plethora of cytometry-based studies has explored COVID-19-associated immune-subset alterations. Although currently not clinically used, this has provided meaningful insights into disease pathogenesis, severity, and outcome.54 Flow cytometry testing has been employed in various patient cohorts and settings, and the available knowledge continues to rapidly evolve. A comprehensive and up-to-date review is, therefore, beyond the scope of this article.
Differential diagnosis
The differential diagnosis of COVID-19 generally includes infection with another pathogen, co-infection, and other inflammatory conditions. The findings of left-shifted myeloid lineage cells, leukoerythroblastosis, and enlarged platelets point to early release of immature forms from the bone marrow, reflecting a stress response that may be seen in several infectious and inflammatory conditions. Ultimately, microbiology and laboratory studies are required for further workup.
Lymphadenopathy or splenomegaly in the setting of COVID-19 infection, recovery, or vaccination is likely a reactive feature. However, underlying malignancy, autoimmune conditions, or infectious (particularly viral) lymphadenopathies cannot be completely excluded, and clinical correlation and ancillary studies are needed.
Summary
The inflammatory response to COVID-19 infection is reflected in prominent hematopathologic alterations. Peripheral blood findings are heterogeneous, but several alterations have emerged as markers for disease severity and outcome. Bone marrow biopsies and aspirates may show varying degrees of histiocytosis and hemophagocytosis, whereas myeloid left shift is frequent, and erythroid dysplasia might be present. Lymph node and spleen germinal centers are often hypoplastic or absent, and lymphocyte compositions may be altered. In addition, the presence of plasmacytoid proliferations with plasmablast-like immunophenotypes might require extensive workup to exclude malignancy. As knowledge continues to evolve, more specific diagnostic or predictive biomarkers that are amenable for clinical diagnostic testing will likely appear.
Clinics care points
Key points.
-
•
Peripheral blood abnormalities can be heterogeneous and dynamic, depending on the disease course and disease severity (see Box 1).
-
•
Serial CBCs with comprehensive morphologic analysis might predict the disease course and clinical severity in newly-diagnosed hospitalized COVID-19 patients.
-
•
A clinical suspicion of HLH may necessitate bone marrow aspiration and biopsy in COVID-19 patients, and IHC for histiocyte markers may be helpful to visualize hemophagocytosis.
-
•
Secondary lymphatic organs may show pronounced atypical features and must be distinguished from other viral lymphadenopathies and malignancies.
Disclosure
The authors have nothing to disclose. Written permission was obtained for select cases.
References
- 1.Hooper J.E., Padera R.F., Dolhnikoff M., et al. A Postmortem Portrait of the Coronavirus Disease 2019 (COVID-19) Pandemic: A Large Multi-institutional Autopsy Survey Study. Arch Pathol Lab Med. 2021;145(5):529–535. doi: 10.5858/arpa.2020-0786-SA. [DOI] [PubMed] [Google Scholar]
- 2.von Stillfried S., Bülow R.D., Röhrig R., et al. German Registry of COVID-19 Autopsies (DeRegCOVID) DeRegCOVID Collaborators First report from the German COVID-19 autopsy registry. Lancet Reg Health Eur. 2022;15:100330. doi: 10.1016/j.lanepe.2022.100330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delorey T.M., Ziegler C.G.K., Heimberg G., et al. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature. 2021;595(7865):107–113. doi: 10.1038/s41586-021-03570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahern D.J., Ai Z., Ainsworth M., et al. A blood atlas of COVID-19 defines hallmarks of disease severity and specificity. Cell. 2022;185(5):916–938.e58. doi: 10.1016/j.cell.2022.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terpos E., Ntanasis-Stathopoulos I., Elalamy I., et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;95(7):834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell R., Zini G., d’Onofrio G., et al. The hematology laboratory’s response to the COVID-19 pandemic: A scoping review. Int J Lab Hematol. 2021;43(2):148–159. doi: 10.1111/ijlh.13381. [DOI] [PubMed] [Google Scholar]
- 7.Satturwar S., Fowkes M., Farver C., et al. Postmortem Findings Associated With SARS-CoV-2: Systematic Review and Meta-analysis. Am J Surg Pathol. 2021;45(5):587–603. doi: 10.1097/PAS.0000000000001650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammoud H., Bendari A., Bendari T., et al. Histopathological Findings in COVID-19 Cases: A Systematic Review. Cureus. 2022;14(6):e25573. doi: 10.7759/cureus.25573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris C.K., Hung Y.P., Nielsen G.P., et al. Bone Marrow and Peripheral Blood Findings in Patients Infected by SARS-CoV-2. Am J Clin Pathol. 2021;155(5):627–637. doi: 10.1093/ajcp/aqaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dandu H., Yadav G., Malhotra H.S., et al. Hemophagocytic histiocytosis in severe SARS-CoV-2 infection: A bone marrow study. Int J Lab Hematol. 2021;43(6):1291–1301. doi: 10.1111/ijlh.13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ioannou M., Zacharouli K., Doukas S.G., et al. Hemophagocytic lymphohistiocytosis diagnosed by bone marrow trephine biopsy in living post-COVID-19 patients: case report and mini-review. J Mol Histol. 2022;53(4):753–762. doi: 10.1007/s10735-022-10088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conway E.M., Mackman N., Warren R.Q., et al. Understanding COVID-19-associated coagulopathy. Nat Rev Immunol. 2022;22(10):639–649. doi: 10.1038/s41577-022-00762-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond Engl. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan W jie, Ni Z., Hu Y., et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan B.E., Chong V.C.L., Chan S.S.W., et al. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020;95(6):E131–E134. doi: 10.1002/ajh.25774. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutiérrez-Ocampo E., et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henry B.M., de Oliveira M.H.S., Benoit S., et al. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med CCLM. 2020;58(7):1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 18.Zini G., Bellesi S., Ramundo F., et al. Morphological anomalies of circulating blood cells in COVID-19. Am J Hematol. 2020;95(7):870–872. doi: 10.1002/ajh.25824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahnach M., Ousti F., Nejjari S., et al. Peripheral Blood Smear Findings in COVID-19. Turk J Haematol Off J Turk Soc Haematol. 2020;37(4):310–312. doi: 10.4274/tjh.galenos.2020.2020.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schapkaitz E., De Jager T., Levy B. The characteristic peripheral blood morphological features of hospitalized patients infected with COVID-19. Int J Lab Hematol. 2021;43(3):e130–e134. doi: 10.1111/ijlh.13417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pezeshki A., Vaezi A., Nematollahi P. Blood cell morphology and COVID-19 clinical course, severity, and outcome. J Hematop. 2021;14(3):221–228. doi: 10.1007/s12308-021-00459-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadigh S., Massoth L.R., Christensen B.B., et al. Peripheral blood morphologic findings in patients with COVID-19. Int J Lab Hematol. 2020;42(6):e248–e251. doi: 10.1111/ijlh.13300. [DOI] [PubMed] [Google Scholar]
- 23.Pozdnyakova O., Connell N.T., Battinelli E.M., et al. Clinical Significance of CBC and WBC Morphology in the Diagnosis and Clinical Course of COVID-19 Infection. Am J Clin Pathol. 2021;155(3):364–375. doi: 10.1093/ajcp/aqaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain S., Meena R., Kumar V., et al. Comparison of hematologic abnormalities between hospitalized coronavirus disease 2019 positive and negative patients with correlation to disease severity and outcome. J Med Virol. 2022;94(8):3757–3767. doi: 10.1002/jmv.27793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chandler C.M., Reid M.C., Cherian S., et al. Comparison of Blood Counts and Markers of Inflammation and Coagulation in Patients With and Without COVID-19 Presenting to the Emergency Department in Seattle, WA. Am J Clin Pathol. 2021;156(2):185–197. doi: 10.1093/ajcp/aqab052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gabr H., Bastawy S., Abdel Aal A.A., et al. Changes in peripheral blood cellular morphology as diagnostic markers for COVID-19 infection. Int J Lab Hematol. 2022;44(3):454–460. doi: 10.1111/ijlh.13799. [DOI] [PubMed] [Google Scholar]
- 27.Nazarullah A., Liang C., Villarreal A., et al. Peripheral Blood Examination Findings in SARS-CoV-2 Infection. Am J Clin Pathol. 2020;154(3):319–329. doi: 10.1093/ajcp/aqaa108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh A., Sood N., Narang V., et al. Morphology of COVID-19–affected cells in peripheral blood film. BMJ Case Rep. 2020;13(5):e236117. doi: 10.1136/bcr-2020-236117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cantu M.D., Towne W.S., Emmons F.N., et al. Clinical significance of blue-green neutrophil and monocyte cytoplasmic inclusions in SARS-CoV-2 positive critically ill patients. Br J Haematol. 2020;190(2):e89–e92. doi: 10.1111/bjh.16882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ackermann M., Anders H.J., Bilyy R., et al. Patients with COVID-19: in the dark-NETs of neutrophils. Cell Death Differ. 2021;28(11):3125–3139. doi: 10.1038/s41418-021-00805-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitra A., Dwyre D.M., Schivo M., et al. Leukoerythroblastic reaction in a patient with COVID-19 infection. Am J Hematol. 2020;95(8):999–1000. doi: 10.1002/ajh.25793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin Chim Acta Int J Clin Chem. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azghar A., Bensalah M., Berhili A., et al. Value of hematological parameters for predicting patients with severe coronavirus disease 2019: a real-world cohort from Morocco. J Int Med Res. 2022;50(7) doi: 10.1177/03000605221109381. 3000605221109381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan A.S., Rout A. Use of Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios in COVID-19. J Clin Med Res. 2020;12(7):448–453. doi: 10.14740/jocmr4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adamidi E.S., Mitsis K., Nikita K.S. Artificial intelligence in clinical care amidst COVID-19 pandemic: A systematic review. Comput Struct Biotechnol J. 2021;19:2833–2850. doi: 10.1016/j.csbj.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magunia H., Lederer S., Verbuecheln R., et al. Machine learning identifies ICU outcome predictors in a multicenter COVID-19 cohort. Crit Care Lond Engl. 2021;25(1):295. doi: 10.1186/s13054-021-03720-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Famiglini L., Campagner A., Carobene A., et al. A robust and parsimonious machine learning method to predict ICU admission of COVID-19 patients. Med Biol Eng Comput. 2022:1–13. doi: 10.1007/s11517-022-02543-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rapkiewicz A.V., Mai X., Carsons S.E., et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine. 2020;24:100434. doi: 10.1016/j.eclinm.2020.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prieto-Pérez L., Fortes J., Soto C., et al. Histiocytic hyperplasia with hemophagocytosis and acute alveolar damage in COVID-19 infection. Mod Pathol. 2020;33(11):2139–2146. doi: 10.1038/s41379-020-0613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bryce C., Grimes Z., Pujadas E., et al. Pathophysiology of SARS-CoV-2: the Mount Sinai COVID-19 autopsy experience. Mod Pathol. 2021;34(8):1456–1467. doi: 10.1038/s41379-021-00793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duan Y., Xia M., Ren L., et al. Deficiency of Tfh Cells and Germinal Center in Deceased COVID-19 Patients. Curr Med Sci. 2020;40(4):618–624. doi: 10.1007/s11596-020-2225-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaneko N., Kuo H.H., Boucau J., et al. Loss of Bcl-6-Expressing T Follicular Helper Cells and Germinal Centers in COVID-19. Cell. 2020;183(1):143–157.e13. doi: 10.1016/j.cell.2020.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee J.E., Jeong W.G., Nam B.D., et al. Impact of Mediastinal Lymphadenopathy on the Severity of COVID-19 Pneumonia: A Nationwide Multicenter Cohort Study. J Korean Med Sci. 2022;37(22):e78. doi: 10.3346/jkms.2022.37.e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khatri G., Priya null, Saleem M.B., et al. Mediastinal lymphadenopathy: A serious complication in COVID-19 patients. Ann Med Surg (Lond) 2022;79:104039. doi: 10.1016/j.amsu.2022.104039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bshesh K., Khan W., Vattoth A.L., et al. Lymphadenopathy post-COVID-19 vaccination with increased FDG uptake may be falsely attributed to oncological disorders: A systematic review. J Med Virol. 2022;94(5):1833–1845. doi: 10.1002/jmv.27599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garreffa E., Hamad A., O’Sullivan C.C., et al. Regional lymphadenopathy following COVID-19 vaccination: Literature review and considerations for patient management in breast cancer care. Eur J Cancer Oxf Engl. 2021;159:38–51. doi: 10.1016/j.ejca.2021.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu Q., Jiang W., Chen N., et al. Misdiagnosis of Reactive Lymphadenopathy Remotely After COVID-19 Vaccination: A Case Report and Literature Review. Front Immunol. 2022;13:875637. doi: 10.3389/fimmu.2022.875637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schiaffino S., Pinker K., Magni V., et al. Axillary lymphadenopathy at the time of COVID-19 vaccination: ten recommendations from the European Society of Breast Imaging (EUSOBI) Insights Imaging. 2021;12(1):119. doi: 10.1186/s13244-021-01062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.García-Molina F., Cegarra-Navarro M.F., Andrade-Gonzales R.J., et al. Cytologic and histologic features of COVID-19 post-vaccination lymphadenopathy. CytoJournal. 2021;18:34. doi: 10.25259/Cytojournal_21_2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan N.J.H., Tay K.X.J., Wong S.B.J., et al. COVID-19 post-vaccination lymphadenopathy: Report of cytological findings from fine needle aspiration biopsy. Diagn Cytopathol. 2021;49(12):E467–E470. doi: 10.1002/dc.24863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hagen C., Nowack M., Messerli M., et al. Fine needle aspiration in COVID-19 vaccine-associated lymphadenopathy. Swiss Med Wkly. 2021;151:w20557. doi: 10.4414/smw.2021.20557. [DOI] [PubMed] [Google Scholar]
- 52.Sekizawa A., Hashimoto K., Kobayashi S., et al. Rapid progression of marginal zone B-cell lymphoma after COVID-19 vaccination (BNT162b2): A case report. Front Med. 2022;9:963393. doi: 10.3389/fmed.2022.963393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mizutani M., Mitsui H., Amano T., et al. Two cases of axillary lymphadenopathy diagnosed as diffuse large B-cell lymphoma developed shortly after BNT162b2 COVID-19 vaccination. J Eur Acad Dermatol Venereol JEADV. 2022;36(8):e613–e615. doi: 10.1111/jdv.18136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chattopadhyay P.K., Filby A., Jellison E.R., et al. A cytometrist’s guide to coordinating and performing COVID-19 research. Cytometry. 2021;99(1):11–18. doi: 10.1002/cyto.a.24210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Evans M.G., Crymes A., Crombie J.L., et al. Monotypic plasmacytoid cells mimicking lymph node malignancy in the setting of COVID-19 recovery. Am J Hematol. 2022;97(5):666–667. doi: 10.1002/ajh.26408. [DOI] [PMC free article] [PubMed] [Google Scholar]