Abstract
In vivo gene therapy is rapidly emerging as a new therapeutic paradigm for monogenic disorders. For almost three decades, hemophilia A (HA) and hemophilia B (HB) have served as model disorders for the development of gene therapy This effort is soon to bear fruit with completed pivotal adeno-associated viral (AAV) vector gene addition trials reporting encouraging results and regulatory approval widely anticipated in the near future for the current generation of HA and HB AAV vectors. Here we review the clinical development of AAV gene therapy for HA and HB and examine outstanding questions that have recently emerged from AAV clinical trials for hemophilia and other monogenic disorders.
Keywords: hemophilia A, hemophilia B, factor VIII, factor IX, AAV, gene therapy
INTRODUCTION
Congenital hemophilia A (HA) and hemophilia B (HB) are X-linked bleeding disorders due to deficiencies in coagulation factor VIII (FVIII) or factor IX (FIX), respectively. The bleeding in hemophilia is characterized by a preponderance of joint and muscle bleeding, though bleeding into closed spaces can be fatal. The severity of the bleeding phenotype is predicted by factor activity. Patients with severe disease have < 1% of normal factor activity and frequent spontaneous bleeds, while patients with moderate disease have 1–5% of normal activity and bleed following minor trauma and sometimes spontaneously, and patients with mild disease have 5–40% of normal activity and typically only bleed with trauma (1).
Prior to modern blood banking, hemophilia was a lethal pediatric disease (2); however, continuous advances in hemophilia care have markedly improved outcomes such that hemophilia patients are now anticipated to have a normal life expectancy in resource-rich nations. Importantly, current costs of hemophilia therapeutics preclude routine access to care in 80% of the world’s hemophilia population (3). The current standard of care is recurrent infusion of FVIII or FIX concentrate to improve hemostasis, administered in response to bleeding or prophylactically to prevent hemorrhage. More recently, a bispecific antibody that mimics FVIII function, emicizumab, has been approved for HA, and other novel approaches for HA and HB are in various phases of clinical development (1). Emicizumab is delivered subcutaneously and is now used in the United States for prophylaxis in the majority of HA patients with and without alloinhibitory antibodies to FVIII (4, 5). Most people with hemophilia born before the 1980s acquired iatrogenic HIV HBV and/or HCV infections secondary to contaminated plasma-derived factor concentrates (6), a risk that is currently eliminated by the use of highly effective virucidal procedures and recombinant proteins. Prophylactic therapy for hemophilia is based on the association between factor level and bleeding phenotype with the goal of converting the severe phenotype (<1% normal) to the moderate or mild range (7). Practically, this requires intravenous infusions of FVIII or FIX concentrate several times weekly to maintain factor activity troughs >1% of normal, though this frequency has decreased with the advent of extended-half-life factors (7, 8). Recently, a bispecific antibody that mimics FVIII function, emicizumab, has been approved for HA patients, and other novel approaches for HA and HB are in various phases of clinical development (1). Emicizumab is administered subcutaneously and is now in widespread use for HA.
HEMOPHILIA GENE THERAPY STRATEGIES IN CURRENT CLINICAL DEVELOPMENT
Gene therapy for severe HA and HB proffers an idealized prophylactic regimen in which a single administration of the gene delivery product provides sustained long-term factor levels sufficient to ameloriate bleeding. Although multiple hemophilia gene therapy strategies have been studied over the last three decades (9), the most advanced employ hepatocyte-directed systemically administered recombinant adeno-associated viral (AAV) vectors encapsidating a codon-optimized F8 or a F9 variant transgene under the control of a liver-specific promoter (Table 1). HA and HB AAV vectors in clinical development are distinguished by outcomes, including level of transgene-derived factor activity, durability of expression, heterogeneity between recipients, and annualized bleeding rate (ABR). To date, only adult men with endogenous factor levels ≤2% and without advanced liver disease have received gene therapy. While there has been repeated proof-of-concept success in HA and HB gene therapy, the goal of stable factor expression adequate to eliminate or nearly eliminate bleeding in all patients has not yet been achieved.
Table 1.
Ongoing hemophilia A and hemophilia B AAV gene therapy trials
| Sponsor | ClinicalTrials identifier |
Manufactoring platform |
Transgene | AAV Serotype |
Dose (× 1011 vg/kg) |
AAV antibody criteria |
Phase | Reference |
|---|---|---|---|---|---|---|---|---|
| Hemophilia B | ||||||||
| Pfizer/Spark |
NCT02484092
NCT03307980 |
HEK293 | ssFIX-R338L | SPK100 | 5 | ≤1:5 | I/II, III | 27 |
| CSL/uniQure |
NCT02396342
NCT03489291 NCT03569891 |
Sl9 | ssFIX-R338L | AAV5 | 200 | None | III | 101 |
| Freeline | NCT03369444 | HEK293 | scFIX-R338L | AAVS3 | 7.5–9.5 | Negative | I/II | 59 |
| Hemophilia A | ||||||||
| BioMarin |
NCT02576795
NCT03392974 NCT03370913 |
Sl9 | ssFVIII-SQ | AAV5 | 600 | Negative | I/II, III | 57, 61 |
| Pfizer/Sangamo | NCT03061201 | Sl9 | ssFVIII-SQ | AAV6 | 300 | Negative | I/II, III | 60 |
| Spark |
NCT03003533
NCT03432520 |
HEK293 | ssFVIII-SQ | LK03 | 5–20 | ≤1:5 | I/II | 56 |
| UCL/St.Jude | NCT03001830 | HEK293 | ssFVIII-V3 | AAV8 | 6–60 | Negative | I/II | 21 |
| Bayer/Ultragenyx | NCT03588299 | HEK293 | ssFVIII-SQ | AAVhu37 | 50–200 | Negative | I/II | 102 |
| Takeda | NCT03370172 | HEK293 | ssFVIII-SQ | AAV8 | 20–120 | <1:5 | I/II | 102 |
Abbreviations: AAV, adeno-associated viral; UCL, University College London; sc, self-complementary; ss, single stranded; vg, vector genomes.
Wild-type AAV is a nonpathogenic, replicative-deficient DNA parvovirus (10). AAV vectors are composed of an icosahedral protein capsid surrounding the genetic payload flanked by an inverted terminal repeat at each end. The inverted terminal repeats are the only component of the expression cassette of viral origin and are essential for packaging the vector genome into the capsid and for episomal DNA formation after transduction. Recombinant AAV vectors are manufactured with methods using either transient transfection of human HEK293 cells or baculovirus infection of Sf9 insect cells (11). Successful transduction of target tissue follows an incompletely understood, multistep pathway from capsid recognition by cell surface receptors to the formation of a circularized episomal vector genome in the nucleus (10). Though AAV vector genomes are predominantly non-integrating, stringent examinations demonstrate rare integration events (12, 13).
The vector serotype is defined by the proteins comprising the capsid, which impacts vector tissue tropism, host immune response to the vector, and manufacturing considerations (10, 14). Both naturally occurring AAV serotypes and bioengineered capsids are being evaluated for hemophilia gene therapy (Table 1). The liver-specific promoters employed for the current gene therapy products are iterations of the human α-1-antitrypsin (hAAT) promoter with enhancer elements from ApoE or a modified transthyretin promoter (15-17).
Because full-length F8 cDNA (7 kb) exceeds the packing capacity of AAV vectors (~4.7 kb), AAV-based gene therapy approaches for HA use B-domain deleted FVIII variants. While the B-domain comprises 40% of F8 cDNA, it is not necessary for clotting activity (18). Most studies utilize the FVIII-SQ variant (19, 20), which is also used for commercial recombinant FVIII products. A single trial is evaluating novel B-domain deleted FVIII variant (FVIII-V3) that has additional glycosylation sites added to the residual B-domain replacement linker to improve protein secretion (21).
The F9 cDNA (1.6 kb) is easily packaged within an AAV vector. Early studies of AAV-based gene therapy for HB utilized wild-type FIX (22-25). However, current AAV approaches for HB use the FIX-R338L (FIX-Padua) variant, which is a naturally occurring missense mutation that results in approximately eightfold-enhanced FIX activity relative to wild-type FIX (26-29). The R338L substitution results in an enhanced interaction between activated FIX-R338L and activated FVIII, but it does not change FIX-R338L activation or inactivation compared to wild-type FIX (30).
ROLE OF ANTI-AAV ANTIBODIES
Preexisting AAV neutralizing antibodies can limit target tissue transduction and therefore therapeutic efficacy, though the clinical implications of preexisting humoral AAV immunity are incompletely understood (31). AAV antibodies are quantified as total AAV binding antibodies or specifically AAV neutralizing antibodies (NAbs), with a high degree of concordance reported in some studies (32, 33). However, NAb assays are not standardized and yield heterogeneous results depending on the methodology, complicating the generalizability of individual study observations. The reported NAb titer is the serum dilution needed to limit vector transduction by 50%.
Anti-AAV NAbs develop following natural infection with wild-type AAV or after administration of systemic recombinant AAV vectors; however, the AAV NAb titers that develop post vector administration are typically at least a log-fold higher than those that develop following environmental exposure. Environmental exposure results in an approximate 30% NAb seroprevalence, though this varies widely with geography, assay employed, and serotype (34-36). Because AAV serotype 2 (AAV2) is endemic, humans generally have the highest seroprevalence of NAbs to AAV2, which classically develop during childhood (36). Small-cohort analysis of the first subjects to receive a systemic AAV vector demonstrated persistent, multi-serotype cross-reactive AAV NAbs for up to 15 years post vector (37); these results are consistent with NAb persistence after natural infection in humans and systemic AAV-vector administration in preclinical animal models (38-41). Importantly, no clinical data support an effective strategy to overcome AAV NAb titers of the magnitude that develop post systemic AAV administration. Thus, current data indicate that repeated administration of AAV vector (of the same or alternative serotype) would not have efficacy.
The efficacy limitations imposed by preexisting NAbs were identified in the first-in-human study of systemically administrated AAV vector, which was conducted in men with HB. Here, higher FIX transgene levels were observed in a subject with a NAb titer <1:5 compared to a subject with a titer of 1:17 (24). Based on these limited data, subsequent hemophilia gene therapy mostly excluded patients with NAb titer >1:5 (Table 1). However, even low-titer NAbs likely impact efficacy. We observed, in the first successful trial evaluating FIX-R338L for HB, that the only recipient with a measurable NAb titer (1:1) had lower FIX activity levels than the seven other recipients with fully sustained transgene expression, who had negative titers (<1:1) (27).
Recently, the role of NAbs in limiting efficacy has been questioned by the clinical development of a systemic AAV5 vector for HB (AMT-061, now etranocogene dezaparvovec). In the initial phase I/IIa study, participants with positive AAV5 NAbs were excluded (25). However, retrospective analysis with a more sensitive luciferase-based NAb assay revealed that 3 of the 10 recipients had detectable AAV5 NAbs (range 1:21–1:340) prior to vector administration without resultant clear differences in transgene expression (33). Preclinical studies in nonhuman primates with NAb titers up to 1:1,000 similarly did not demonstrate decreased transgene expression. Thus, in the subsequent phase IIb and III studies, NAb titers were not an exclusion criterion (28). Published phase IIb data demonstrated FIX activity levels of 30–60% in 3 recipients with luciferase-based NAb titers between 1:20 and 1:50 (28). Similar FIX activity levels have been reported in 54 subjects in the phase III study; however, the subject with the highest NAb titer (>1:3,000) did not respond (42). Rather than demonstrating that preexisting NAbs are irrelevant for systemic AAV efficacy, these results suggest that the threshold that distinguishes between a treatable and refractory NAb titer depends on characteristics of the NAb assay, the vector administered, and the vector dose. Indeed, AMT-061 is administered at a forty-fold higher vector dose [2 × 1013 vector genomes per kilogram (vg/kg)] than AAV vectors studied with NAb titer >1:5 as an exclusion criterion (e.g., 5 × 1011 vg/kg); this is consistent with animal studies that demonstrated a nonlinear relationship between the vector dose and the threshold NAb titer necessary to preclude transgene expression (27, 43). The regulatory approval of AAV vectors for hemophilia will likely include an anti-AAV antibody cutoff based on inclusion criteria of the relevant pivotal study.
Because preexisting anti-AAV antibodies are currently highly likely to prevent vector readministration and restrict the number of patients eligible for AAV gene therapy, work is ongoing to develop methodologies to eradicate or avoid preexisting antibodies as well as prevent formation after vector administration. Thus far, no animal data have demonstrated the ability to overcome the magnitude of NAb titers observed post systemic AAV vector (i.e., >1:1,000) (44-46). Further, to date, there has been no successful systemic AAV readministration in humans. Given the current state of clinical development of AAV for hemophilia and marked activity with potential for improvements in the short term, the current lack of a proven strategy for vector readministration is among the most salient considerations for clinicians and hemophilia patients when deciding on a gene therapy product. Patients should be counseled so that they are fully aware that the current state of clinical development likely only permits one lifetime systemic AAV vector administration; this is important to prevent “buyer’s remorse” should one vector ultimately be inferior to another and underscores the importance of comprehensive understanding of all available information of each vector prior to administration to ensure a well-informed decision.
SHORT-TERM SAFETY CONSIDERATIONS OF AAV THERAPEUTICS
Thus far, there have been no major safety concerns across the 300-fold range of systemic AAV vector doses (2 × 1011 to 6 × 1013 vg/kg) evaluated in hemophilia patients, though there are well-described asymptomatic hepatotoxicities and immune responses. However, AAV gene therapy trials studying higher vector doses than those employed in hemophilia have recently unveiled major safety concerns observed with vector doses >1 × 1014 vg/kg and suggest dose-limiting toxicities of systemic AAV vector administration (47-50) (reviewed in Supplemental Table 1). While dose-limiting toxicities are not a novel concept in drug development, there is now evidence to suggest that this general concept applies to systemic AAV vectors.
Emerging Short-Term Toxicities of AAV
The direct relevance of AAV toxicities observed in other disease cohorts to hemophilia gene therapy is undefined. At a minimum, these emerging observations provide a rationale to use the lowest therapeutic vector dose and support the need for treating physicians to understand AAV as a therapeutic class (Supplemental Table 1). For example, thrombotic microangiopathy (TMA) within 2 weeks post vector with evidence of complement activation, consistent with an atypical hemolytic uremic syndrome, has been observed in some neuromuscular trials evaluating systemic AAV vector doses >1.1 × 1014 vg/kg (47, 48, 51). While there are few publications, reported data outline end-organ TMA toxicities, including malignant hypertension, renal failure requiring hemodialysis, and responsiveness to eculizumab (47, 48, 50, 51). However, this management has not been universally effective, with outcomes including mortality related to TMA complications in at least one patient (52).
In addition to TMA, the possibility for short- and long-term dorsal root ganglia (DRG) toxicity post AAV vector was recently identified in a meta-analysis of 256 nonhuman primates that received 33 different vectors with variable serotypes, expression cassettes, and administration routes (intrathecal, intracisternal magna, and systemic) (53). Some animals demonstrated mild histological evidence of DRG toxicity, while clinical symptomatology and severe histology findings were found in only two animals (53). The etiology of this largely histological finding is unknown but is hypothesized to be related to an unfolded protein response in transduced DRG cells (54). Clinical confirmation of this toxicity has thus far been limited to a single trial participant who received an intrathecally delivered AAV vector for amyotrophic lateral sclerosis; symptoms were reduced with glucocorticoids, but not ameliorated at the time of publication (55). It is unclear if DRG toxicity is dependent on the AAV capsid, dose, cassette, or administration route versus a possible AAV vector platform toxicity.
Hepatotoxicity and Immunological Response to AAV
All AAV serotypes efficiently traffic to the liver, irrespective of target cell. Hepatoxicity is therefore a primary safety consideration of systemic AAV delivery. All AAV gene therapy trials for hemophilia have demonstrated asymptomatic hepatocellular toxicity, albeit with variable frequency and courses depending on the employed vector and dose (23-28, 56-59). This hepatocellular pattern of injury is characterized by transaminase elevation, in which the alanine aminotransferase elevation exceeds aspartate aminotransferase increase without associated increases in bilirubin or γ-glutamyl transferase. In most but not all cases (25, 56-58), this is accompanied by a decline in factor activity that may or may not be responsive to immunomodulation (23, 24, 26, 27, 56, 59, 60). Post administration, a single AAV vector currently in a pivotal trial for HA resulted in multimonth transaminase elevation of unclear etiology that was not associated with a decline in factor activity or evidence of responsiveness to immunomodulation (57, 58, 61). The majority of participants (n = 115 of 134) received immunomodulation for transaminase elevation without evidence of efficacy or identified treating pathology (57, 58, 61).
However, most transaminase elevations observed in hemophilia trials occurred in the setting of a cytotoxic immune response to the AAV capsid (23, 24, 26, 27, 56, 59). This AAV “capsid immune response” is hypothesized to result from capsid-specific cytotoxic CD8+ T cell recognition of AAV-capsid peptides presented on major histocompatibility complex I molecules of the transduced hepatocytes and the resultant clearance (62); if enough transduced hepatocytes were to be targeted, this theoretically poses a safety concern. However, thus far, the AAV-capsid cellular immune response has largely been an efficacy consideration for hemophilia gene therapy, accounting for loss of some or all transgene expression in multiple trials (23, 24, 26, 27, 56, 59). The capsid immune response is characterized by a rise in transaminases, decline in factor activity, and often but not always peripheral blood mononuclear cells that react to AAV-capsid peptides in an interferon-γ anticapsid enzyme linked immune absorbent spot (ELISpot) assay. Notably, the ELISpot assay has had variable predictive value across clinical trials and, being technically intensive, is unlikely to be translatable post licensure. Nonetheless, even modest transaminase elevations and a decline in factor level have proven reasonably sensitive to support a capsid immune response. Capsid immune responses are typically initially observed within 12 weeks after vector administration.
Once there is evidence of a capsid immune response, management with immunomodulation is necessary to maintain transgene expression. Various strategies have been pursued, with glucocorticoids predominating (23, 25, 27, 56, 58-60). Importantly, immunomodulation is not universally successful and is likely influenced by both vector-related and recipient-related elements (26, 56). Rationally designed immunomodulation regimens have thus far been elusive. Lastly, the duration of immunomodulation is highly variable, anywhere from several weeks to >1 year, with resultant safety concerns. In order to best assess the risk/benefit profile of immune suppression, it is essential to understand what is being treated and to have confirmation that the intervention is both necessary and effective.
While liver failure has not been observed after AAV gene therapy for hemophilia, it has been observed with AAV vectors targeting two other genetic diseases. Specifically, onasemnogene abeparvovec (Zolgensma®) administered at doses of 1.1 × 1014 vg/kg for spinal muscular atrophy (SMA) was approved with a boxed safety warning for hepatotoxicity (63). Post licensure, two children presented 6–8 weeks post infusion with acute liver failure that was responsive to glucocorticoid intervention (49). While the exact etiology is unclear, an AAV-capsid cellular immune response is strongly supported by the timing of presentation post vector, liver biopsy specimens with CD8+ T cell infiltration, and steroid responsiveness. Last, unlike the hepatocellular toxicity observed in hemophilia trials and with Zolgensma, a study in X-linked myotubular myopathy (XLMTM), using the highest vector doses thus far employed in humans for AAV-mediated gene transfer, observed hepatobiliary toxicity that progressed to liver failure and death in four trial participants (n = 3 of 17 in the 3.5 × 1014 vg/kg cohort; 1 of 7 in the 1.1 × 1014 vg/kg cohort) (50, 64). The hepatobiliary pattern of injury and undefined natural history of the XLMTM, which may include underlying liver disease (47, 50, 65), are not consistent with a capsid immune response. The relevance of these severe hepatoxicities to hemophilia gene therapy is unclear, but their occurrence nonetheless supports using the lowest possible AAV vector dose and understanding the full complement of potential AAV toxicities before prescribing AAV gene therapy.
LONG-TERM SAFETY CONSIDERATIONS OF AAV THERAPEUTICS
The major hypothesized long-term safety concerns of systemic AAV vector administration remain the risks of liver and target organ toxicity as well as genotoxicity. While AAV is predominantly non-integrating, sequencing data in animals and humans post environmental AAV exposure or vector administration demonstrate low-frequency AAV integration events with a proclivity for sites of active transcription (13, 66-69). Systemic administration of AAV vector to neonatal mice demonstrated that AAV integration at the Rian locus (which does not have a direct human ortholog) resulted in clonality and near complete hepatocellular carcinoma (HCC) penetrance (69). A subsequent study determined that the HCC risk correlated with vector dose and degree of cellular division, and was abrogated by using a hepatocyte-specific promoter, but was enhanced with a non-hepatocyte-specific promoter (67). Evidence of AAV integration and clonality post systemic AAV vector was also recently demonstrated in a large-animal HA model. Importantly, however, the same animals were followed for 10 years without evidence of tumorigenesis; nonetheless, this study provides the first large-animal data to highlight the risk of AAV-mediated integration (13).
Understanding the theoretical risk of HCC post AAV in hemophilia patients is confounded by the fact that almost all severe hemophilia patients >45 years old iatrogenically acquired HBV and/or HCV, resulting in an enhanced HCC incidence relative to the general population (6). AAV clinical trial data in hemophilia and other disease cohorts are reassuring, albeit limited in cohort size and longitudinal follow-up. Within the hemophilia gene therapy recipient population, a single participant with prior longstanding HCV infection developed HCC 1 year post vector. Recent sequencing analysis of the tumor tissue identified expected low-frequency random AAV integration without clonality and the presence of mutations otherwise found in HCV-related HCC, suggesting that AAV vector infusion did not contribute to the patient’s HCC (70). Beyond this, there have been no reports of HCC among the hundreds of hemophilia gene therapy trial participants, even in 15-year follow-up data on the first participants (HB patients) to receive a systemic AAV vector (37). In summary, there is thus far no clinical evidence to directly support AAV vector infusion as a risk factor for HCC development. However, this theoretical risk would only be anticipated to develop decades post vector administration. Given the multiple unknowns about the long-term risk of oncogenesis after AAV vector administration as well as increased risk of HCC in the hemophilia population, the perspective of the authors is that hemophilia patients who receive an AAV gene therapy should be monitored with serum α-fetoprotein levels, liver function studies, and liver ultrasounds for multiple decades post vector to surveil for HCC and long-term liver toxicities. Ultimately, cohorts outside the adult hemophilia population draw closer parallels to the risk factors identified in mice for HCC development post AAV administration. Specifically, risk factors in infants—with the rapid growth of the liver and resultant high rates of cellular division that receive high systemic AAV vector doses with a ubiquitous promoter (such as Zolgensma given to infants with SMA)—more closely mimic the genotoxicity risk factors identified in murine models; thus, these infants are an important cohort to carefully follow to answer questions about in-human risk of AAV genotoxicity and HCC after systemic AAV vector administration.
SHORT- AND LONG-TERM SAFETY CONSIDERATIONS OF THE TRANSGENE-DERIVED PROTEIN
The major safety considerations of the transgene-derived protein include (a) an immunological response with or without cross reactivity to self-antigens and (b) prothrombotic risk. Prothrombotic risk is salient when considering the heterogenous expression of FVIII and FIX observed in both HA and HB gene therapy trials to date. Supraphysiologic levels of FVIII and FIX activity are established independent risk factors for venous thrombosis (71-73). Epidemiological data outline that the venous thrombosis risk of supraphysiologic FVIII activity [odds ratio (OR) 8.8–21.3] is higher than that of FIX (venous thrombosis OR 1.8–4.0), although not desirable in either case (73). Indeed, one HB study subject who expressed supraphysiologic FIX activity levels (200–520%) developed a thrombosis in his arteriovenous fistula and was treated with anticoagulation (59). Similarly, there was a recent hold on a phase III AAV gene therapy trial sponsored by Pfizer because of supraphysiologic FVIII expression and venous thrombosis in one subject (74). For these reasons, the therapeutic window for HA or HB gene therapy likely ends at the upper limit of normal factor levels.
In addition to supraphysiologic expression, a prothrombotic risk could theoretically be imparted by an enhanced hemostatic function variant protein, such as FIX-R338L, which is employed in all currently enrolling HB gene therapy efforts. Importantly, FIX-R338L is regulated the same way as wild-type FIX, which is consistent with preclinical data that demonstrate thrombosis risk correlated with FIX activity, independent of wild-type versus FIX-R338L expression; combined, these results support the conclusion that FIX-R338L is not inherently prothrombotic (30, 75).
Additionally, while multiple immunological responses to the expressed transgene are possible, thus far no trial participants have developed NAbs to the transgene-derived protein; it is important to note that current enrollment criteria for hemophilia gene therapy trials stringently exclude patients who are the most likely to develop an inhibitor (e.g., patients with a current or prior history of inhibitor or who have <50–150 factor exposures). However, small- and large-animal data suggest that hepatocyte expression of a transgene induces transgene tolerance; this has been demonstrated for a variety of proteins, including FVIII, FIX, and FIX-R338L (75-77). Furthermore, available preclinical data suggest that liver-directed gene transfer may induce transgene-product immune tolerance. A sustained immune response to the transgene protein after liver-directed gene therapy is therefore unlikely. Indeed, future expanded indications for gene therapy may include tolerance induction in HA patients with preexisting FVIII inhibitors (78). Consensus recommendations from a “state of the science of FVIII inhibitors” workshop sponsored by the National Heart, Lung, and Blood Institute explicitly supported pursuing gene therapy for FVIII tolerance induction for HA (79). However, antibodies against the transgene protein have recently been observed after AAV muscle-directed gene therapy in subjects with Duchenne muscular dystrophy (50); these antibodies were hypothesized to be cross reactive to self-antigens, highlighting some of the continued unknowns of AAV gene therapy for monogenetic disorders.
AVAILABLE EFFICACY DATA ON HEMOPHILIA GENE THERAPY
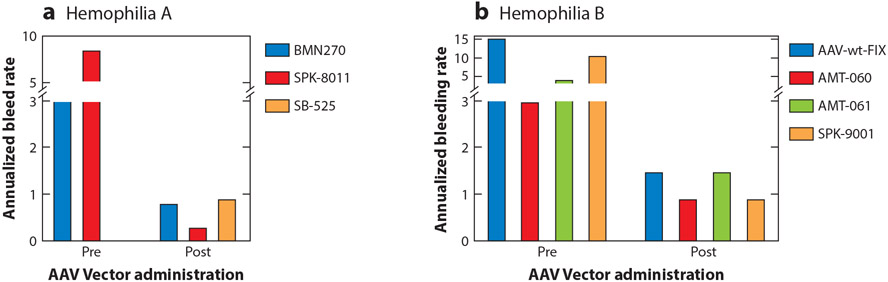
Among clinical trials with sustained transgene expression, phenotypic improvement has been demonstrated in most trial participants with sustained transgene expression (Figure 1); the potential for superior efficacy of gene therapy relative to current hemophilia care is not in dispute. Further, despite heterogeneous levels of factor expression, there are relatively homogeneous short-term reductions in annualized bleeding rate (ABR). However, individual vectors, particularly HA vectors, are likely to distinguish themselves based on expression durability and long-term efficacy.
Figure 1.
Annualized bleeding rate (ABR) for participants in (a) hemophilia A and (b) hemophilia B AAV gene therapy clinical trials before and after AAV vector administration. Data were extracted as follows: BMN270 (valoctocogene roxaparvovec) represents mean ABR (n = 134 participants) (58); SPK-8011 represents mean ABR of participants who maintained expression outside an AAV capsid immune response and were followed >1 year (n = 15) (56); SB-525 (giroctocogene fitelparvovec) represents mean ABR for participants following vector administration only (n = 4) (60); AAV-wt-FIX (scAAV2/8-LP1-hFIXco) represents median reported ABR (n = 10 participants) (22, 23); AMT-060 represents mean ABR (n = 10 participants) (25); AMT-061 (etranacogene dezaparvovec) represents mean ABR of all bleeding events (n = 54 participants) (103); SPK-9001 (fidanacogene elaparvovec) represents mean ABR (n = 10 participants) (27).
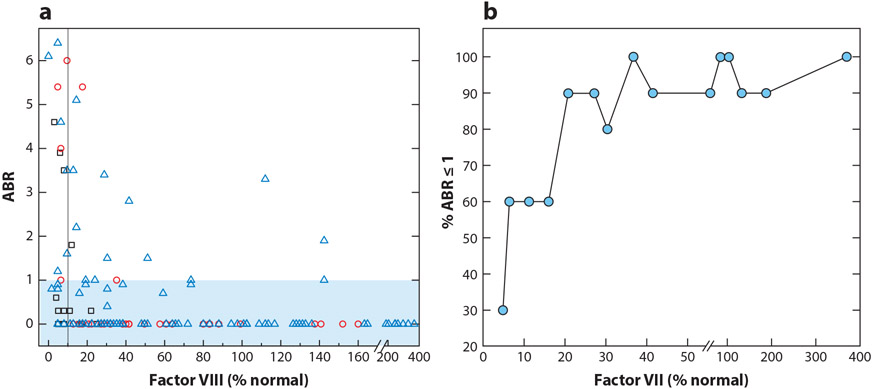
While epidemiological studies provide a strong rationale to avoid supratherapeutic expression and resultant safety concerns (71-73), the precise minimally desired factor activity remains unclear and likely varies with patient characteristics such as activity level and joint health. The targeted minimally desired factor activity may be modeled from existing HA natural history data that demonstrate that one-stage FVIII activity ≥12% confers an ABR <1. Additionally, emicizumab prophylaxis confers an ABR <1 in >50% of trial participants and has an estimated in vivo FVIII hemostatic equivalency of 10–30%; these findings and hemophilia natural history data generally support a similar targeted minimum expression (5, 80). Indeed, these approximations are thus far supported by analysis of cumulative available gene therapy data in which sustained FVIII activity >10–20% by one-stage assay reliably permitted an ABR <1 in most recipients (Figure 2) (56, 58).
Figure 2.
Relationship between annualized bleeding rate (ABR) and factor VIII (FVIII) activity. (a) The ABR and FVIII activity for all gene therapy recipients of 0.5–2 × 1012 vg/kg of SPK-8011 (black squares), 4–6 × 1013 vg/kg AAV5-FVIII phase I/II (red circles), and 6 × 1013 vg/kg AAV5-FVIII phase III (blue triangles). Plotted FVIII activity levels are determined by one-stage assay or converted from a chromogenic assay results using a correction factor of 1.6 (56, 58, 61, 104). The black vertical line represents 10% of normal FVIII level. The blue shaded box represents recipients with an ABR ≤1. (b) Percentage of gene therapy recipients with an ABR ≤1 as a function of their FVIII activity determined by one-state assay. Ascending ABR data from gene therapy recipients were binned into groups of 10 and then plotted as a function of the maximum FVIII level in the binned group.
However, depending on the AAV vector, observed transgene expression varies 10–100-fold (23, 25, 27, 56, 58-60). Therefore, it is not possible to target individual patient factor threshold needs. Further, studies in a clonal population of mice chimeric with human hepatocytes demonstrated up to sevenfold variability in transduction efficiency alone (81). These data are consistent with the presence of multiple biological variables impacting each step between vector infusion and steady-state transgene expression (10) and underscore the reality that the field will likely have to tolerate interpatient variability of transgene expression in the near future.
Complicating our ability to clearly define the therapeutic range of targeted and acceptable transgene expression is the degree of confidence in the correlation of measured transgene FIX or FVIII activity with in vivo hemostatic function. Specifically, measurement of transgene-derived FVIII-SQ and FIX-R338L by one-stage (OSA) or chromogenic assay (CSA) varies. FVIII:C measures approximately 1.6-fold higher by OSA than by CSA in humans (56, 60, 61) and mice (82), an observation not seen with two decades of recombinant FVIII-SQ protein clinical use. Analogously, FIX-R338L OSA determined FIX activity measures higher than FIX activity determined by CSA. However, unlike with transgene-derived FVIII-SQ, the relative assay discrepancy is maintained with recombinant FIX-R388L and is overall consistent with our understanding of FIX-R338L enzymatic function (30, 83).
DURABILITY OF TRANSGENE EXPRESSION
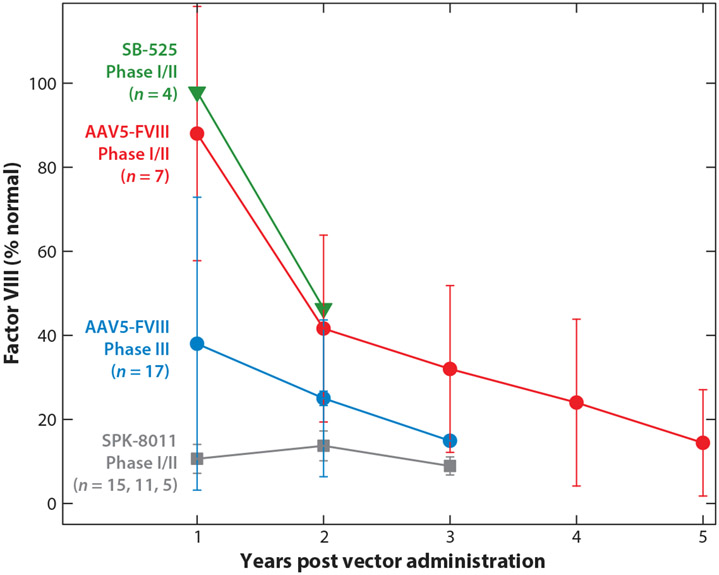
Despite relatively homogeneous excellent short-term clinical benefit in the setting of successful, but variable, factor expression, there is heterogeneity between vectors with regard to long-term stable expression (Figure 3). Large-animal data demonstrated stable or no decrease in either FIX or FVIII expression for up to 8 years post vector infusion (84). Available HB clinical trial data thus far mimic what observed in large-animal data; published data up to 3 years and abstract data up to 8 years demonstrate durable and stable FIX expression (22, 85). The data on durability of expression for HA are conflicting (56-58, 86, 87).
Figure 3.
Multiyear factor VIII (FVIII) activity after AAV gene therapy. Data were extracted from publicly available publications or abstracts (56-58, 86-88). Points represent median FVIII levels (except for SB-525, where only mean values were available), and error bars are the interquartile range. Data are from cohorts that received AAV vectors doses of 3 × 1013, 6 × 1013, and 0.5–2 × 1012 vg/kg of SB-525, AAV5-FVIII, and SPK-8011, respectively. Two of the participants who received SPK-8011 lost all FVIII expression due to a capsid cellular immune response within 3 months post vector administration and are not included. Plotted SPK-8011 participant data represent n = 15 at year 1, n = 11 at year 2, and n = 5 at year 3, reflecting duration of available follow-up data. FVIII activity was determined by one-stage assay or converted from a chromogenic assay result using a correction factor of 1.6 (56, 61, 104) for AAV5-FVIII results.
Specifically, among the current vectors in phase III trials for HA, both have demonstrated a loss of nearly half of the FVIII levels from year 1 to year 2. Among these vectors, valoctocogene roxaparvovec (AAV5-FVIII or BMN-270) has now demonstrated continuously decreased FVIII levels out to 6 years follow-up in phase I/II trial participants (n = 7) (57, 88). Similar results have been observed in a larger cohort of phase III trial participants (n = 17), demonstrating loss of 40% of expression from year 1 to year 2 and continued decrease in FVIII activity out to currently available 3 year follow-up data (Figure 3) (58, 86, 87). Interestingly, the loss in FVIII levels observed with valoctocogene roxaparvovec is closely mirrored by phase I/II data on participants (n = 4) who received SB-525 (now giroctocogene fitelparvovec) and followed for 2 years (Figure 3); this vector is in a pivotal trial that is on hold (60, 74). In contrast to observations with valoctocogene roxaparvovec and giroctocogene fitelparvovec, participants who received SPK-8011 and maintained FVIII expression outside a capsid immune response (n = 5) demonstrated apparently stable FVIII levels for up to 3 years post vector (56) (Figure 3). While the long-term FVIII pharmacokinetics of giroctocogene fitelparvovec (SB-525) and valoctocogene roxaparvovec (BMN27) appear similar, they markedly differ from observations with SPK-8011, which provide proof of principle that approximately stable multi-year FVIII expression is possible with current HA AAV-mediated gene addition strategies.
The etiology for the differences in durability of expression observed in HA clinical trials is unclear. Given that current strategies target maintenance of an episomal transgene, a degree of decline in expression is anticipated due to cellular division and loss, albeit gradual, as evidenced by current HB efforts. The aggregate data presented in Figure 3 may suggest that high levels of transgene FVIII are not sustainable; however, careful review of individual recipient FVIII levels after valoctocogene roxaparvovec does not suggest a clear relationship between FVIII level and FVIII decline (89). This needs additional study. Hypothesized mechanisms of the decline in FVIII expression observed for the two HA vectors currently in phase III trials include (a) transduced cell loss due to direct vector or FVIII toxicity, (b) gene silencing, (c) an undetected immune response to the AAV capsid or expressed transgene, or (d) properties of the individual vector that preclude formation of stable concatemerized episomal vector expression cassette DNA. The hypothesis that loss of transduced hepatocytes is due to FVIII unfolded protein response is supported by prior observations of this phenomenon in recombinant FVIII mammalian expression systems and in supratherapeutic expression of FVIII post liver-directed AAV-mediated gene transfer in mouse models (90-92). However, there is no evidence of an unfolded protein response in liver biopsies from HA subjects, though sampling and/or timing considerations limit interpretation (93). How the manufacturing platforms used for the different AAV products contribute to the declining transgene FVIII levels is also unknown. Given what we know about the development of persistent, high-titer, multiserotype, cross-reactive AAV NAbs post AAV vector infusion and our current inability to overcome high-titer AAV NAbs, the stability of the FVIII levels is a critical consideration in decisions about clinical trial enrollment or licensed AAV product selection. Potential recipients will need to fully understand that they can receive AAV gene therapy only once, and the loss of transgene expression of FVIII must be considered in their risk/benefit calculus.
FUTURE DIRECTIONS
Comprehensive consideration of next-generation gene therapy approaches for HA and HB are outside the scope of this review. A multitude of novel approaches are poised to build on current progress. These include next-generation AAV vectors using FVIII variants to improve secretion or hemostatic function as well as AAV vectors with lipid nanoparticles used for gene editing (94-96). Though still in preclinical development, some of these approaches are anticipated in the clinic shortly. In addition, systemic delivery to target the liver as well as ex vivo transduction of hematopoietic stem cells using lentiviral vectors are being pursued in either preclinical or first-in-human clinical trials (97-100).
Current efforts exclude the entire pediatric population, as well as patients who lack routine access to treatment (a majority of the world’s hemophilia population). These are the populations for which gene therapy may have the greatest impact.
CONCLUSION
Demonstrated progress in AAV-based gene addition for hemophilia and other monogenic disorders establishes a new therapeutic paradigm. Understanding of the AAV platform as well as a comprehensive knowledge of information thus far available for an individual vector is necessary to enable true informed consent to treatment with either an investigational or licensed vector. The hemophilia community eagerly awaits the current generation of licensed AAV vectors for HA and HB, which are anticipated in the clinic shortly. Importantly, despite repeated proof-of-concept success in current hemophilia gene therapy, stable, durable FVIII or FIX expression able to ameliorate bleeding in all patients is an unrealized hope. This defines the development goals of the next generation of gene-based therapies for hemophilia.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute through K08 HL146991 (L.A.G.) and NIH K08 HL140078 (B.J.S.-J.).
Footnotes
DISCLOSURE STATEMENT
L.A.G. is on the scientific advisory board of STRM.Bio and a data safety monitoring committee for Avrobio. She has been a consultant for Intellia, Biomarin, Pfizer, Spark, and Bayer and receives licensing fees from AskBio Therapeutics. B.J.S.-J. is on the scientific advisory board of GeneVentiv; has been a consultant for Genentech, Frontera, and Cabaletta; and has received royalty payments from Cabaletta.
LITERATURE CITED
- 1.Fassel H, McGuinn C. 2021. Haemophilia: factoring in new therapies. Br. J. Haematol 194(5):835–50 [DOI] [PubMed] [Google Scholar]
- 2.Birch CL. 1937. Hemophilia: Clinical and Genetic Aspects. Champaign: Univ. Ill. [Google Scholar]
- 3.Skinner MW. 2012. WFH: closing the global gap—achieving optimal care. Haemophilia 18(4):1–12 [DOI] [PubMed] [Google Scholar]
- 4.Oldenburg J, Mahlangu JN, Kim B, et al. 2017. Emicizumab prophylaxis in hemophilia A with inhibitors. N. Engl. J. Med 377(9):809–18 [DOI] [PubMed] [Google Scholar]
- 5.Mahlangu J, Oldenburg J, Paz-Priel I, et al. 2018. Emicizumab prophylaxis in patients who have hemophilia A without inhibitors. N. Engl. J. Med 379(9):811–22 [DOI] [PubMed] [Google Scholar]
- 6.Mazepa MA, Monahan PE, Baker JR, et al. 2016. Men with severe hemophilia in the United States: birth cohort analysis of a large national database. Blood 127(24):3073–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. 2007. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N. Engl. J. Med 357(6):535–44 [DOI] [PubMed] [Google Scholar]
- 8.Nilsson I, Berntorp E, Löfqvist T, Pettersson H. 1992. Twenty-five years’ experience of prophylactic treatment in severe haemophilia A and B. J. Intern. Med 232(1):25–32 [DOI] [PubMed] [Google Scholar]
- 9.Arruda VR, Doshi BS, Samelson-Jones BJ. 2017. Novel approaches to hemophilia therapy: successes and challenges. Blood 130(21):2251–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li C, Samulski RJ. 2020. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet 21 (4):255–72 [DOI] [PubMed] [Google Scholar]
- 11.Rumachik NG, Malaker SA, Poweleit N, et al. 2020. Methods matter: standard production platforms for recombinant AAV produce chemically and functionally distinct vectors. Mol. Ther. Methods Clin. Dev 18:98–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nowrouzi A, Penaud-Budloo M, Kaeppel C, et al. 2012. Integration frequency and intermolecular recombination of rAAV vectors in non-human primate skeletal muscle and liver. Mol. Ther 20(6):1177–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen GN, Everett JK, Kafle S, et al. 2021. A long-term study of AAV gene therapy in dogs with hemophilia A identifies clonal expansions of transduced liver cells. Nat. Biotechnol 39(1):47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D, Tai PWL, Gao G. 2019. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug. Discov 18(5):358–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okuyama T, Huber RM, Bowling W, et al. 1996. Liver-directed gene therapy: a retroviral vector with a complete LTR and the ApoE enhancer-α 1-antitrypsin promoter dramatically increases expression of human α 1-antitrypsin in vivo. Hum. Gene Ther 7(5):637–45 [DOI] [PubMed] [Google Scholar]
- 16.Rettinger SD, Kennedy SC, Wu X, et al. 1994. Liver-directed gene therapy: quantitative evaluation of promoter elements by using in vivo retroviral transduction. PNAS 91(4):1460–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costa RH, Grayson DR. 1991. Site-directed mutagenesis of hepatocyte nuclear factor (HNF) binding sites in the mouse transthyretin (TTR) promoter reveal synergistic interactions with its enhancer region. Nucleic Acids Res. 19(15):4139–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brinkhous KM, Sandberg H, Garris JB, et al. 1985. Purified human factor VIII procoagulant protein: comparative hemostatic response after infusions into hemophilic and von Willebrand disease dogs. PNAS 82(24):8752–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lind P, Larsson K, Spira J, et al. 1995. Novel forms of B-domain deleted recombinant factor VIII molecules. Construction and biochemical characterization. Eur. J. Biochem 232:19–27 [DOI] [PubMed] [Google Scholar]
- 20.Pittman DD, Alderman EM, Tomkinson KN, et al. 1993. Biochemical, immunological, and in vivo functional characterization of B-domain-deleted FVIII. Blood 81(11):2925–35 [PubMed] [Google Scholar]
- 21.McIntosh J, Lenting PJ, Rosales C, et al. 2013. Therapeutic levels of FVIII following a single peripheral vein administration of rAAV vector encoding a novel human factor VIII variant. Blood 121(17):3335–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nathwani AC, Reiss UM, Tuddenham EG, et al. 2014. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med 371(21):1994–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nathwani AC, Tuddenham EG, Rangarajan S, et al. 2011. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med 365(25):2357–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manno CS, Pierce GF, Arruda VR, et al. 2006. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 12(3):342–47 [DOI] [PubMed] [Google Scholar]
- 25.Miesbach W, Meijer K, Coppens M, et al. 2018. Gene therapy with adeno-associated virus vector 5-human factor IX in adults with hemophilia B. Blood 131(9):1022–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konkle BA, Walsh CE, Escobar MA, et al. 2021. BAX 335 hemophilia B gene therapy clinical trial results: potential impact of CpG sequences on gene expression. Blood 137(6):763–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.George LA, Sullivan SK, Giermasz A, et al. 2017. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N. Engl. J. Med 377(23):2215–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Von Drygalski A, Giermasz A, Castaman G, et al. 2019. Etranacogene dezaparvovec (AMT-061 phase 2b): normal/near normal FIX activity and bleed cessation in hemophilia B. Blood Adv. 3(21):3241–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simioni P, Tormene D, Tognin G, et al. 2009. X-linked thrombophilia with a mutant factor IX (factor IX Padua). N. Engl. J. Med 361(17):1671–75 [DOI] [PubMed] [Google Scholar]
- 30.Samelson-Jones BJ, Finn JD, George LA, et al. 2019. Hyperactivity of factor IX Padua (R338L) depends on factor VIIIa cofactor activity. JCI Insight 4(14):e128683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shirley JL, de Jong YP, Terhorst C, Herzog RW. 2020. Immune responses to viral gene therapy vectors. Mol. Ther 28(3):709–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falese L, Sandza K, Yates B, et al. 2017. Strategy to detect pre-existing immunity to AAV gene therapy. Gene Ther. 24(12):768–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Majowicz A, Nijmeijer B, Lampen MH, et al. 2019. Therapeutic hFIX activity achieved after single AAV5-hFIX treatment in hemophilia B patients and NHPs with pre-existing anti-AAV5 NABs. Mol. Ther. Methods Clin. Dev 14:27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klamroth R, Hayes G, Andreeva T, et al. 2022. Global seroprevalence of pre-existing immunity against AAV5 and other AAV serotypes in people with hemophilia A. Hum. Gene Ther 33(7–8):32–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calcedo R, Vandenberghe LH, Gao G, et al. 2009. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis 199(3):381–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrington EA, Sloan JL, Manoli I, et al. 2016. Neutralizing antibodies against adeno-associated viral capsids in patients with mut methylmalonic acidemia. Hum. Gene Ther 27(5):345–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.George LA, Ragni MV, Rasko JEJ, et al. 2020. Long-term follow-up of the first in human intravascular delivery of AAV for gene transfer: AAV2-hFIX16 for severe hemophilia B. Mol. Ther [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calcedo R, Wilson JM. 2016. AAV natural infection induces broad cross-neutralizing antibody responses to multiple AAV serotypes in chimpanzees. Hum. Gene Ther. Clin. Dev 27(2):79–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aronson SJ, Veron P, Collaud F, et al. 2019. Prevalence and relevance of pre-existing anti-adeno-associated virus immunity in the context of gene therapy for Crigler-Najjar syndrome. Hum. Gene Ther 30(10):1297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L, Bell P, Somanathan S, et al. 2015. Comparative study of liver gene transfer with AAV vectors based on natural and engineered AAV capsids. Mol. Ther 23(12)1877–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mingozzi F, Chen Y, Murphy SL, et al. 2012. Pharmacological modulation of humoral immunity in a nonhuman primate model of AAV gene transfer for hemophilia B. Mol. Ther 20(7):1410–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pipe SW, Recht M, Key NS, et al. 2020. First data from the phase 3 HOPE-B gene therapy trial: efficacy and safety of etranacogene dezaparvovec (AAV5-Padua hFIX variant; AMT-061) in adults with severe or moderate-severe hemophilia B treated irrespective of pre-existing anti-capsid neutralizing antibodies. Blood 136:LBA–6 [Google Scholar]
- 43.Wang L, Calcedo R, Bell P, et al. 2011. Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors. Hum. Gene Ther 22(11):1389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leborgne C, Barbon E, Alexander JM, et al. 2020. IgG-cleaving endopeptidase enables in vivo gene therapy in the presence of anti-AAV neutralizing antibodies. Nat. Med 26(7)1096–101 [DOI] [PubMed] [Google Scholar]
- 45.Majowicz A, Salas D, Zabaleta N, et al. 2017. Successful repeated hepatic gene delivery in mice and non-human primates achieved by sequential administration of AAV5ch and AAV1. Mol. Ther 25(8):1831–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paulk NK, Pekrun K, Zhu E, et al. 2018. Bioengineered AAV capsids with combined high human liver transduction in vivo and unique humoral seroreactivity. Mol. Ther 26(1)289–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mullard A 2021. Gene therapy community grapples with toxicity issues, as pipeline matures. Nat. Rev. Drug. Discov 20(11):804–5 [DOI] [PubMed] [Google Scholar]
- 48.Chand DH, Zaidman C, Arya K, et al. 2021. Thrombotic microangiopathy following onasemnogene abeparvovec for spinal muscular atrophy: a case series. J. Pediatr 231:265–68 [DOI] [PubMed] [Google Scholar]
- 49.Feldman AG, Parsons JA, Dutmer CM, et al. 2020. Subacute liver failure following gene replacement therapy for spinal muscular atrophy type 1. J. Pediatr 225:252–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.FDA Cellular, Tissue, and Gene Therapies Advisory Committee. 2021. Toxicity risks of adeno-associated virus (AAV) vectors for gene therapy (GT). Briefing doc., CTGTAC Meet. 70. https://www.fda.gov/media/151599/download [Google Scholar]
- 51.Rocket Pharm. 2021. Rocket Pharmaceuticals announces positive updates from phase 1 clinical trial of RP-A501 in Danon disease. News release, Nov. 15. https://ir.rocketpharma.com/news-releases/news-release-details/rocket-pharmaceuticals-announces-positive-updates-phase-1 [Google Scholar]
- 52.Guillou J, de Pellegars A, Porcheret F, et al. 2022. Fatal thrombotic microangiopathy case following adeno-associated viral SMN gene therapy. Blood Adv. 6:4266–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hordeaux J, Buza EL, Dyer C, et al. 2020. Adeno-associated virus-induced dorsal root ganglion pathology. Hum. Gene Ther 31(15–16):808–18 [DOI] [PubMed] [Google Scholar]
- 54.Hordeaux J, Buza EL, Jeffrey B, et al. 2020. MicroRNA-mediated inhibition of transgene expression reduces dorsal root ganglion toxicity by AAV vectors in primates. Sci. Transl. Med 12(569) aba9188. [DOI] [PubMed] [Google Scholar]
- 55.Mueller C, Berry JD, McKenna-Yasek DM, et al. 2020. SOD1 suppression with adeno-associated virus and microRNA in familial ALS. N. Engl. J. Med 383(2):151–58 [DOI] [PubMed] [Google Scholar]
- 56.George LA, Monahan PE, Eyster ME, et al. 2021. Multiyear factor VIII expression after AAV gene transfer for hemophilia A. N. Engl. J. Med 385(21):1961–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pasi KJ, Rangarajan S, Mitchell N, et al. 2020. Multiyear follow-up of AAV5-hFVIII-SQ gene therapy for hemophilia A. N. Engl. J. Med 382(1):29–40 [DOI] [PubMed] [Google Scholar]
- 58.Ozelo MC, Mahlangu J, Pasi KJ, et al. 2022. Valoctocogene roxaparvovec gene therapy for hemophilia A. N. Engl. J. Med 386(11):1013–25 [DOI] [PubMed] [Google Scholar]
- 59.Chowdary P, Shapiro S, Makris M, et al. 2020. A novel adeno associated virus (AAV) gene therapy (FLT180a) achieves normal FIX activity levels in severe hemophilia B (HB) patients (B-AMAZE Study). Res. Pract. Thromb. Haemost 4(Suppl. 2):17 [Google Scholar]
- 60.Visweshwar N, Harrington TJ, Leavitt AD, et al. 2021. Updated results of the Alta Study, a phase 1/2 study of giroctocogene fitelparvovec (PF-07055480/SB-525) gene therapy in adults with severe hemophilia A. Blood 138:564 [Google Scholar]
- 61.Rangarajan S, Walsh L, Lester W, et al. 2017. AAV5-factor VIII gene transfer in severe hemophilia A. N. Engl. J. Med 377(26):2519–30 [DOI] [PubMed] [Google Scholar]
- 62.Mingozzi F, Maus MV, Hui DJ, et al. 2007. CD8+ T-cell responses to adeno-associated virus capsid in humans. Nat. Med 13(4):419–22 [DOI] [PubMed] [Google Scholar]
- 63.FDA. 2021. Highlights of prescribing information, Zolgensma 2019. https://www.fda.gov/media/126109/download
- 64.Shieh PB, Bonnemann CG, Muller-Felber W, et al. 2020. Re: “Moving forward after two deaths in a gene therapy trial of myotubular myopathy” by Wilson and Flotte. Hum. Gene Ther 31(15–16):787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neese JM, Yum S, Matesanz S, et al. 2021. Intracranial hemorrhage secondary to vitamin K deficiency in X-linked myotubular myopathy. Neuromuscul. Disord 31(7):651–55 [DOI] [PubMed] [Google Scholar]
- 66.Nault JC, Datta S, Imbeaud S, et al. 2015. Recurrent AAV2-related insertional mutagenesis in human hepatocellular carcinomas. Nat. Genet 47(10):1187–93 [DOI] [PubMed] [Google Scholar]
- 67.Chandler RJ, LaFave MC, Varshney GK, et al. 2015. Vector design influences hepatic genotoxicity after adeno-associated virus gene therapy. J. Clin. Investig 125(2):870–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakai H, Montini E, Fuess S, et al. 2003. AAV serotype 2 vectors preferentially integrate into active genes in mice. Nat. Genet 34(3):297–302 [DOI] [PubMed] [Google Scholar]
- 69.Donsante A, Miller DG, Li Y, et al. 2007. AAV vector integration sites in mouse hepatocellular carcinoma. Science 317(5837):477. [DOI] [PubMed] [Google Scholar]
- 70.Schmidt MRFG, Coppens M, Thomsen H, et al. 2021. Liver safety case report from the phase 3 HOPE-B gene therapy trial in adults with hemophilia B. Res. Pract. Thromb. Haemost 5(Suppl. 2):OC 67.4 (Abstr.) [Google Scholar]
- 71.Koster T, Blann AD, Briet E, et al. 1995. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet 345(8943):152–55 [DOI] [PubMed] [Google Scholar]
- 72.Kyrle PA, Minar E, Hirschl M, et al. 2000. High plasma levels of factor VIII and the risk of recurrent venous thromboembolism. N. Engl. J. Med 343(7):457–62 [DOI] [PubMed] [Google Scholar]
- 73.Rietveld IM, Lijfering WM, le Cessie S, et al. 2019. High levels of coagulation factors and venous thrombosis risk: strongest association for factor VIII and von Willebrand factor. J. Thromb. Haemost 17(1):99–109 [DOI] [PubMed] [Google Scholar]
- 74.Pfizer. 2021. Pfizer and Sangamo announce updated phase 1/2 results showing sustained bleeding control in highest dose cohort through two years following hemophilia A gene therapy. Presss Release, Dec. 14. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-sangamo-announce-updated-phase-12-results-1 [Google Scholar]
- 75.Crudele JM, Finn JD, Siner JI, et al. 2015. AAV liver expression of FIX-Padua prevents and eradicates FIX inhibitor without increasing thrombogenicity in hemophilia B dogs and mice. Blood 125(10):1553–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Finn JD, Ozelo MC, Sabatino DE, et al. 2010. Eradication of neutralizing antibodies to factor VIII in canine hemophilia A after liver gene therapy. Blood 116(26):5842–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mingozzi F, Hasbrouck NC, Basner-Tschakarjan E, et al. 2007. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood 110(7):2334–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Samelson-Jones BJ, Arruda VR. 2020. Translational potential of immune tolerance induction by AAV liver-directed factor VIII gene therapy hemophilia A. Front. Immunol 11:618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ragni MV, George LA. 2019. The national blueprint for future factor VIII inhibitor clinical trials: NHLBI State of the Science (SOS) Workshop on factor VIII inhibitors. Haemophilia 25(4):581–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ferriere S, Peyron I, Christophe OD, et al. 2020. A hemophilia A mouse model for the in vivo assessment of emicizumab function. Blood 136(6):740–48 [DOI] [PubMed] [Google Scholar]
- 81.Zou C, Vercauteren KOA, Michailidis E, et al. 2020. Experimental variables that affect human hepatocyte AAV transduction in liver chimeric mice. Mol. Ther. Methods Clin. Dev 18:189–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sternberg AR, Davidson RJ, Samelson-Jones BJ, George L. 2021. In vitro and in vivo models to understand one-stage and chromogenic factor VIII activity assay discrepancy of hepatocyte-derived factor VIII. Res. Pract. Thromb. Haemost 5(Suppl. 1):OC 75.3 (Abstr.) [Google Scholar]
- 83.Robinson MM, George LA, Carr ME, et al. 2021. Factor IX assay discrepancies in the setting of liver gene therapy using a hyperfunctional variant factor IX-Padua. J. Thromb. Haemost 19(5):1212–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Niemeyer GP, Herzog RW, Mount J, et al. 2009. Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy. Blood 113(4):797–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nathwani AC, Reiss U, Tuddenham E, et al. 2019. Adeno-associated mediated gene transfer for hemophilia B: 8 year follow up and impact of removing “empty viral particles” on safety and efficacy of gene transfer. Blood 132(Suppl. 1):491 [Google Scholar]
- 86.Lopes TJdS, Rios R, Nogueira T. 2022. FP-01.03 (1160079) HemB-Class: A machine learning framework to predict the clinical severity of Haemophilia B caused by point-mutations. Haemophilia 28:1154168 (Abstr.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.BioMarin Pharm. 2022. BioMarin announces stable and durable annualized bleed control in the largest phase 3 gene therapy study in adults with severe hemophilia A; 134-participant study met all primary and secondary efficacy endpoints at two year analysis. News Release, Jan. 9. https://investors.biomarin.com/2022-01-09-BioMarin-Announces-Stable-and-Durable-Annualized-Bleed-Control-in-the-Largest-Phase-3-Gene-Therapy-Study-in-Adults-with-Severe-Hemophilia-A-134-Participant-Study-Met-All-Primary-and-Secondary-Efficacy-Endpoints-at-Two-Year-Analysis [Google Scholar]
- 88.Pasi KJ, Rangarajan S, Robinson TM, et al. 2021. Hemostatic response is maintained for up to 5 years following treatment with valoctocogene roxaparvovec, an AAV5-hFVIII-SQ gene therapy for severe hemophilia A. Res. Pract. Thromb Haemost 5(Suppl. 2):OC 67.1 (Abstr.) [Google Scholar]
- 89.George LA. 2021. Hemophilia gene therapy: ushering in a new treatment paradigm? Hematol. Am. Soc. Hematol. Educ. Progr 2021(1):226–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lange AM, Altynova ES, Nguyen GN, Sabatino DE. 2016. Overexpression of factor VIII after AAV delivery is transiently associated with cellular stress in hemophilia A mice. Mol. Ther. Methods Clin. Dev 3:16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zolotukhin I, Markusic DM, Palaschak B, et al. 2016. Potential for cellular stress response to hepatic factor VIII expression from AAV vector. Mol. Ther. Methods Clin. Dev 3:16063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Poothong J, Pottekat A, Siirin M, et al. 2020. Factor VIII exhibits chaperone-dependent and glucose-regulated reversible amyloid formation in the endoplasmic reticulum. Blood 135(21):1899–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fong S, Yates B, Sihn CR, et al. 2022. Interindividual variability in transgene mRNA and protein production following adeno-associated virus gene therapy for hemophilia A. Nat. Med 28(4):789–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang HM-EC, Bialek P, Wang C, Gong G, Hartfort S, et al. 2019. CRISPR/Cas9-mediated targeted insertion of human F9 achieves therapeutic circulating protein levels in mice and non-human primates. Mol. Ther 27(4 Suppl. 1):7 (Abstr.) [Google Scholar]
- 95.Samelson-Jones BJ, Arruda VR. 2019. Protein-engineered coagulation factors for hemophilia gene therapy. Mol. Ther. Methods Clin. Dev 12:184–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wilhelm AR, Parsons NA, Samelson-Jones BJ, et al. 2021. Activated protein C has a regulatory role in factor VIII function. Blood 137(18):2532–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Du LM, Nurden P, Nurden AT, et al. 2013. Platelet-targeted gene therapy with human factor VIII establishes haemostasis in dogs with haemophilia A. Nat. Commun 4:2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shi Q, Wilcox DA, Fahs SA, et al. 2006. Factor VIII ectopically targeted to platelets is therapeutic in hemophilia A with high-titer inhibitory antibodies. J. Clin. Investig 116(7):1974–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Greene TK, Wang C, Hirsch JD, et al. 2010. In vivo efficacy of platelet-delivered, high specific activity factor VIII variants. Blood 116(26):6114–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Follenzi A, Benten D, Novikoff P, et al. 2008. Transplanted endothelial cells repopulate the liver endothelium and correct the phenotype of hemophilia A mice. J. Clin. Investig 118(3):935–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pipe SW, Leebeek FW, Recht M, et al. 2021. 52 Week efficacy and safety of etranacogene dezaparvovec in adults with severe or moderate-severe hemophilia B: data from the phase 3 HOPE-B gene therapy trial. Res. Pract. Thromb. Haemost 5(Suppl. 2):PB0653 (Abstr.) [Google Scholar]
- 102.Pipe SW, Hay CRM, Sheehan J, et al. 2020. First-in-human gene therapy study of AAVhu37 capsid vector technology in severe hemophilia A: safety and FVIII activity results. Res. Pract. Thromb. Haemost 4(Suppl. 1):OC 09.4 (Abstr.) [Google Scholar]
- 103.uniQure. 2021. uniQure and CSL Behring announce primary endpoint achieved in HOPE-B pivotal trial of etranacogene dezaparvovec gene therapy in patients with hemophilia B. News Release, Dec. 9. https://www.globenewswire.com/news-release/2021/12/09/2349067/0/en/uniQure-and-CSL-Behring-Announce-Primary-Endpoint-Achieved-in-HOPE-B-Pivotal-Trial-of-Etranacogene-Dezaparvovec-Gene-Therapy-in-Patients-with-Hemophilia-B.html [Google Scholar]
- 104.Rosen S, Tiefenbacher S, Robinson M, et al. 2020. Activity of transgene-produced B-domain-deleted factor VIII in human plasma following AAV5 gene therapy. Blood 136(22):2524–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.