Abstract
Introduction
Cerebral projections of nociceptive stimuli are of great interest as targets for neuromodulation in chronic pain. To study cerebral networks involved in processing noxious stimuli, researchers often rely on thermo-nociception to induce pain. However, various limitations exist in many pain-inducing techniques, such as not accounting for individual variations in pain and trial structure predictability.
Methods
We propose an improved and reliable psychometric experimental method to evaluate human nociceptive processing to overcome some of these limitations. The developed testing paradigm leverages a custom-built, open-source, thermoelectric device (TED). The device construction and hardware are described. A maximum-likelihood adaptive algorithm is integrated into the TED software, facilitating individual psychometric functions representative of both hot and cold pain perception. In addition to testing only hot or cold thresholds, the TED may also be used to induce the thermal grill illusion (TGI), where the bars are set to alternating warm and cool temperatures.
Results
Here, we validated the TED’s capability to adjust between different temperatures and showed that the device quickly and automatically changes temperature without any experimenter input. We also validated the device and integrated psychometric pain task in 21 healthy human subjects. Hot and cold pain thresholds (HPT, CPT) were determined in human subjects with <1°C of variation. Thresholds were anticorrelated, meaning a volunteer with a low CPT likely had a high HPT. We also showed how the TED can be used to induce the TGI.
Conclusion
The TED can induce thermo-nociception and provide probabilistic measures of hot and cold pain thresholds. Based on the findings presented, we discuss how the TED could be used to study thermo-nociceptive cerebral projections if paired with intracranial electrode monitoring.
Keywords: Nociception, pain, psychophysical, sensory system, hot/cold pain
1. Introduction
In 2016, an estimated 28.4% of people in the United States (U.S.) had chronic pain [1–3], making it one of the country’s most significant public health problems [4]. Total U.S. healthcare expenses attributable to chronic pain are $560 to $635 billion annually [5]. In some cases, current pharmacological pain treatments may do more harm than good, as evident by the opioid crisis [6]. Alternative pain treatments are needed.
Alternatives to opioids are drugs targeting transient receptor potential (TRP) channels and their projections, as these channels transduce physiological sensations. TRP channels are also recruited under pathophysiological chronic pain conditions and may modulate neurotransmitter release in the spinal cord [7–10]. To study the cerebral projections of nociception, researchers often rely on thermo-nociceptive techniques [11–14]. However, many pain-inducing methods use a fixed stimulus intensity [15–22] and don’t account for subject variations. Short thermal stimuli pulses are often presented within a set time and repeated at long intervals [23], leading to anticipation and predictability. Pain anticipation correlates with greater placebo-induced pain relief and reductions in pain region neural activity [24], which influences the descending pain modulatory system and disrupts psychophysical responses and neural analyses of nociceptive transmission. Studies that varied the inter-stimulus interval have shown that this is a sufficient method to avoid expectation and habituation [25].
To overcome these limitations, we propose a psychometric testing paradigm that leverages a custom-built, open-source, thermoelectric device (TED) facilitating pain threshold estimation with <1°C standard deviation. Adaptive temperature stimuli accounts for individual differences in perception. While this takes more time than prescribing one extreme value, the TED hardware enables the interface to quickly adapt between cold and hot temperatures known to activate transient receptor potential (TRP) channels (0–50°C) [26]. Minimizing adjustment time decreases the task time to keep the test subject focused. The related methodological section describes additional features of the device.
The device and programming were designed for research purposes; however, the TED may also be utilized in quantitative clinical sensory testing [27,28]. The device interface is comprised of multiple independently controlled bars. During the psychometric pain task, all the bars are set to be all one temperature—either hot or cold. The TED can be used to determine hot or cold pain thresholds (HPT, CPT, respectively). We performed both in this validation study and show individual probabilistic pain curves. In addition to testing only hot or cold thresholds, the TED may also be used to induce the thermal grill illusion (TGI).
The TGI, first discovered by Thunberg in 1896, consists of closely spaced alternating hot and cold stimuli—themselves nonpainful—perceived as painful when felt simultaneous [29–32]. Subjects perceive burning pain from the alternating surface without enduring physical harm [30,33]. Many groups have used the TGI to study pain; therefore, we incorporated a TGI setting into our graphical user interface (GUI). The TED is versatile and incorporates various methods to study pain.
The novel TED and programmed experimental protocols establish a new way to study pain in humans. The device and two pain tests were validated in healthy volunteers. Based on the findings presented, we discuss how the TED can facilitate studying pain in patients undergoing intracranial electrode monitoring.
2. Methods
Many currently available thermoelectric devices have several limitations, including not accounting for individual pain perception and predictable trial structures. We designed and built a novel device improving upon improve these limitations. We describe the methodology of designing, building, and testing the device. We also discuss the steps involved with the psychophysical pain task. Lastly, we present results from 21 healthy human subjects who participated in the psychophysical pain task. Each bar on the surface of the TED is independently controlled making it relatively straightforward to control each bar’s temperature. We demonstrate this feature by validating the device’s capability to elicit the TGI. While not shown in this work, the device is fully compatible with various encephalographic recording systems, including stereoelectroencephalography (SEEG). All studies were performed following approved Institutional Review Board protocol (#00122256) and carried out in accordance with The Code of Ethics of the World Medical Association.
2.1. Construction of the thermoelectric device (TED)
The construction of the TED included both hardware and software components.
The device layout is similar to previously published thermoelectric devices [34–37]. Six copper bars, each 9.8 cm long and 1.0 cm wide, were spaced 0.15 cm apart on the device surface. This layout provides sufficient coverage of the average human palm. Copper was chosen as the surface material due to its excellent thermal conductivity. The copper bars were thermally and electrically isolated using plastic separators and each copper bar was connected to a separate Peltier device. The bars were isolated from the other electronics to ensure low noise interference (Figure 1A, Component II). Multiple Peltier devices (Laird Thermal Systems, Inc., 6.2 × 0.95 cm, 20.6 W) were connected to each bar, allowing individual control of each element (Figure 1A, Component II). A large aluminum heatsink (Wakefield-Vette, 22.9 × 18.8 × 8.0 cm) was incorporated to dissipate heat and allow accurate temperature control (Figure 1C, Component IX). A high-current motor driver shield (Pololu VNH5019) supplies current to change the Peltier device temperature (Figure 1A, Component I). The real-time temperature of the copper bars is measured using a digital integrated circuit sensor (Maxim DS18B20). Six sensors were installed (one for each bar) to ensure that the desired temperature was maintained. An ATmega328P microcontroller (Arduino Uno Rev3) running a proportional-integral-derivative (PID) control loop adjusts the bar temperatures to match a user-defined set point by changing a pulse-width modulated (PWM) output from the motor drivers to the Peltier devices. The temperature of each bar can be controlled independently. Temperatures are held within 0.5 deg Celsius of the set point. Temperatures and setpoints are transmitted in real-time via USB between the Arduino and a MATLAB client on a separate experimenter computer. A GUI, developed in LabVIEW, is then used to display these temperatures and control the setpoints based on the experimental task. Capacitive touch sensors (SparkFun AT42QT1011) and load cells (Degraw HX711) were installed within the 3D printed plastic support structure to indicate when a subject’s hand is on the bars (Figure 1A, Component IV). The trial timer starts once the capacitive sensors and load cell force exceeds two pounds. The trial aborts if force drops below two pounds. The experimenter can use the load cell force output to inform the test subject if they need to alter the amount of pressure applied to the device. The load cell force throughout the task is automatically saved. The overall device structure and integrated components facilitated several key improvements for studying pain.
Figure 1.
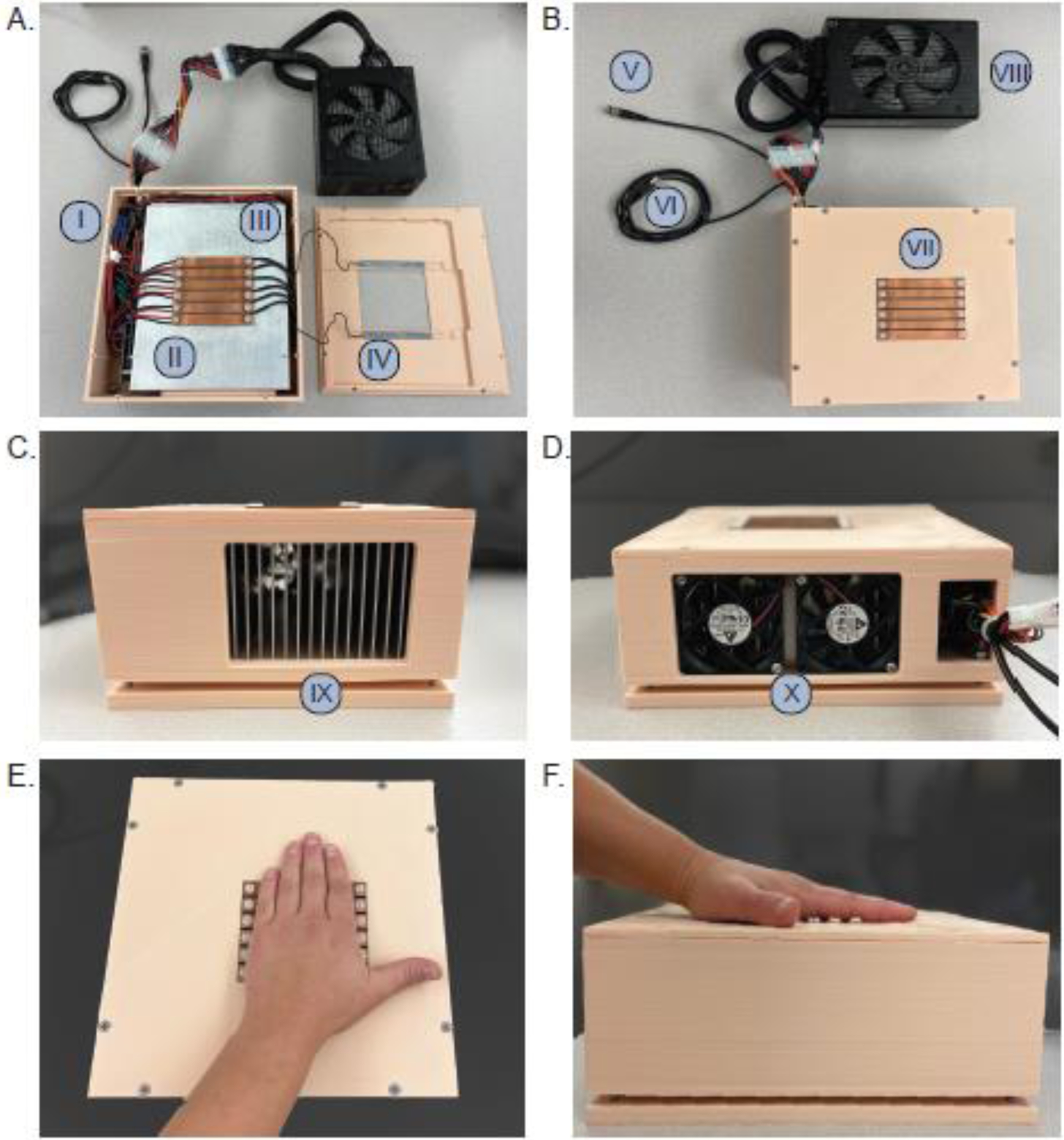
A. Internal components of the device, including an: I) Arduino Uno and three dual VNH5019 motor driver shields, II) six Peltier devices placed under each copper bar, III) six DS18B20 digital thermometers, and IV) capacitive touch sensors and load cells (2). B. External outputs and device surface, including: V) capacitive touch output, VI) USB for software communication, VII) copper bars that change temperature, and the VIII) power supply. C. The side of the device demonstrating the IX) heat sink and in D., the X) fans. E. demonstrates an axial view of hand placement. F. shows the sagittal view of hand placement.
2.2. Evaluation of the adjustment time for the thermoelectric surface between different temperatures
We assessed the time it took the TED to adjust between different temperatures to compare it to currently available thermoelectric devices. A separate MATLAB script was written to iterate through temperatures between 5 and 50°C with 5°C increments. The adjustment time was recorded when all six interface bars reached the target temperature from the starting temperature. The commanded temperature and setpoints were recorded in real-time via USB between the Arduino and the MATLAB client on a separate experimenter computer. An infrared camera (Teledyne FLIR ONE Pro) that captured the real-time temperature of the bars was used to confirm the MATLAB script was correctly iterating through the commanded temperatures and saving adjustment times. The temperature was considered reached, and the adjustment time was recorded when the average temperature of all bars was less than 0.50°C from the target temperature for at least 5 seconds. Once the temperature was reached, the bars returned to the starting temperature and the new target temperature increased by 5°C. Each path, or start-to-target temperature, was tested three times to establish a mean. The mean across trials for each path was plotted in MATLAB using a heatmap (Figure 2).
Figure 2.
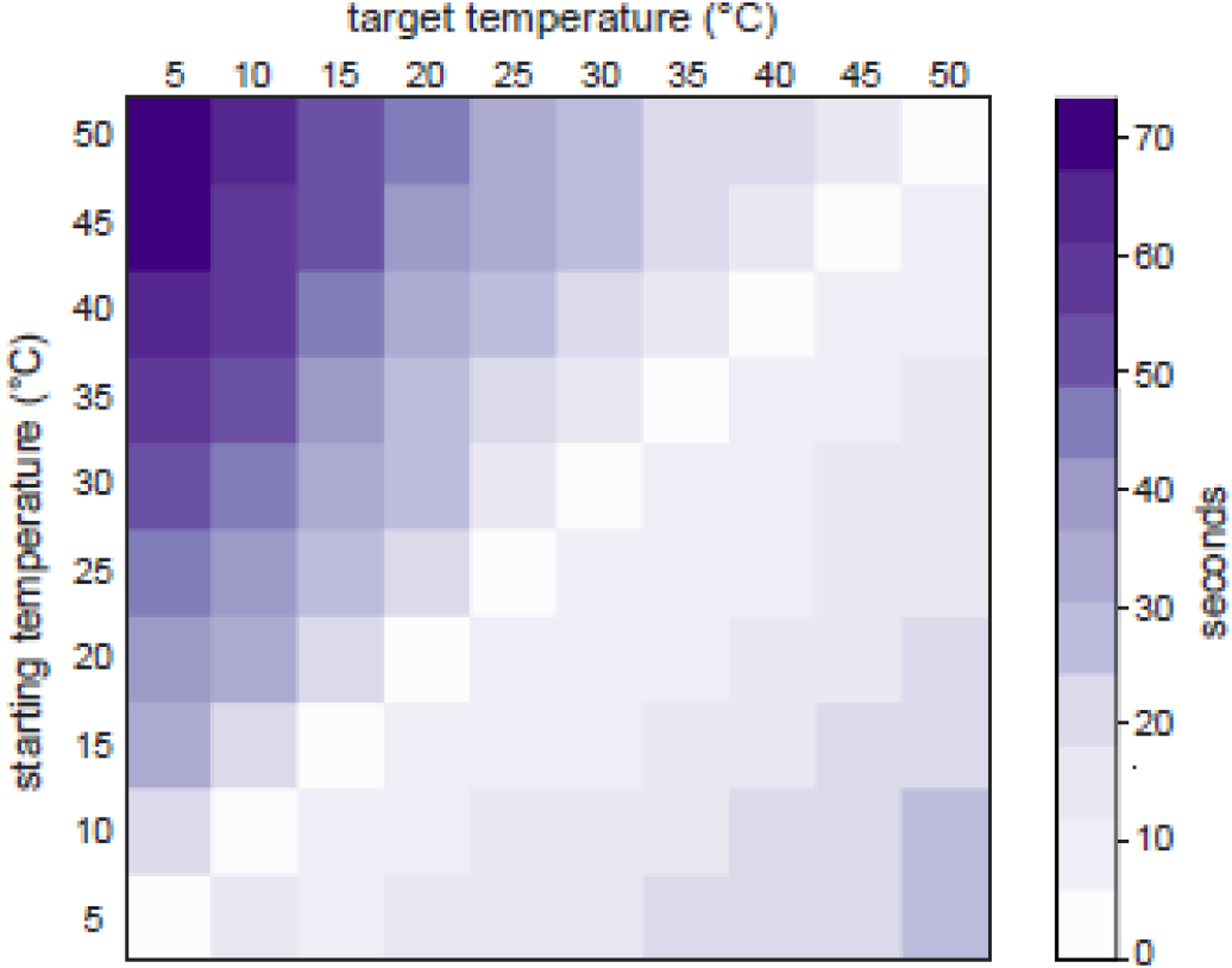
Average time for the interface to adjust from a starting temperature to a target temperature. Temperatures between 5 and 50°C were tested. Lighter shades of purple represent faster adjustment times while darker shades represent longer adjustment times. Temperature adjustment was tested three times and the average time is shown.
2.3. A new psychophysical pain task based on a maximum-likelihood adaptive procedure
The main limitation we sought to improve in the pain task development was accounting for individual differences in pain perception. We did not want to assume that the same temperature would cause pain in all subjects. Instead, we wanted to account for individual variation in pain thresholds across subjects. Individual pain thresholds in subjects can be determined using a staircase algorithm (i.e., ramping up the temperature stepwise) [35,38–40]. This method can be tedious, as many trials are required to determine the pain threshold. Staircase algorithms are susceptible to more variation between subjects, as one must verbally or physically respond that their pain threshold has passed [41]. To overcome this limitation, we integrated a maximum-likelihood adaptive algorithm (QUEST Psychophysics) to describe the relationship between the temperature stimulus and the probability that the stimulus was painful [42,43].
The maximum-likelihood adaptive algorithm was used to parameterize a Weibull psychometric function representative of the subject’s tested pain perception (i.e., either HPT or CPT in our task). The procedure incorporates the test subject’s response after each trial. Each trial consists of the test subject placing their hand on the device while it is set to a specific temperature (Supplementary Video 1). The response to each trial was incorporated to update the Weibull probability density function until the number of desired trials are complete or a minimum standard deviation of the estimated threshold is reached. The psychophysical experimental pain task utilizing QUEST was programmed in MATLAB and LabVIEW.
The main advantage of using the probabilistic method built-in to QUEST is that it is more accurate than other threshold-determining methods [42]. It also enables parsing of trials that were most likely painful from those that were not on an individual basis since the task is uniquely maximized for each test individual. The overall task objective is to determine a test subject’s hot and cold pain thresholds (HPT, CPT)—the temperatures at which the bars are uniform and painful. However, using a probabilistic tool also enables the derivation of probability function shape parameters that contribute to perception of pain, such as the slope, which represents pain sensitivity.
Subjects who participated in the task were blinded to the stimulus parameters to limit experimental biases. The maximum-likelihood adaptive algorithm suggested a new test temperature each trial, but the subject did not know whether the temperature increased, decreased, or stayed the same before playing their hand on the device for the next trial. The subject interacted primarily with the touchscreen, which instructed them when to place their hand on the device. The trial structure was automated to help reduce interaction between the experimenter and test subject. Using a maximum-likelihood adaptive procedure enabled the derivation of individualized probability density functions subjects’ pain perception.
2.4. Device setup and psychometric pain task structure
A few simple steps were required to start the experiment. The power supply (Figure 1, VIII) was connected to a standard wall outlet. The capacitive touch and USB output (Figure 1, Component V) were plugged into a desktop computer. The desktop computer was connected to a monitor that the experimenter used to run the software, and a second touchscreen monitor was placed within reach for the test subject. The touchscreen monitor was placed within reach of the test subject so they could readily respond to questions about the sensation of the TED. For all our experiments, a touchscreen was available. However, if a touchscreen is not available, one could have the test subject use a mouse to respond to the survey questions within the task about the sensation experienced.
After plugging in the necessary components, the experimenter opened the GUI and selected either HPT or CPT in the drop-down experimentation options. Several parameters can be altered after this step, but we have determined the values that worked well for the pain task described and include them as default initial options.
Several parameters are adjustable within the TED software before starting the task. Our study tested different temperatures until the predicted threshold standard deviation was <1°C, and the subject completed 20 trials. An a priori power analysis revealed that a minimum trial count of 16 would be adequate to differentiate painful heat from innocuous heat. We rounded up to account for noisy trials or those with errors. However, the experimenter may adjust the trial count or the minimum required standard deviation. The experimenter may also randomly integrate catch trials into the experiment. Catch trials involve exposing the test subject to 1°C above (HPT) or below (CPT) the current estimated pain threshold. If a catch trial occurred at the beginning of the task, the catch temperature was likely nonpainful since the intensity is initially low and gradually increases towards the true pain threshold throughout the task. However, if the catch trial occurred near the end of the task, then it would likely be painful since the tested temperatures oscillate near the true pain threshold. We have found adequate space sampling in that psychometric data could be parameterized (Figure 4B and D) by incorporating a catch trial rate of 0.10. Again, the catch trial rate can be adjusted if preferred. Other easily adjustable parameters include those involving timing. The experimenter may adjust the length of time the user puts their hand on the device, the inter-trial interval (i.e., the minimum required time between trials), and the length of time the subject is given to respond to the questions regarding the sensation. In that case that the experimenter wishes not to perform the described psychophysical pain task, they may select “Manual” in the drop-down experiment options instead of “Cold” for testing CPT or “Hot” for testing the HPT.
Figure 4.
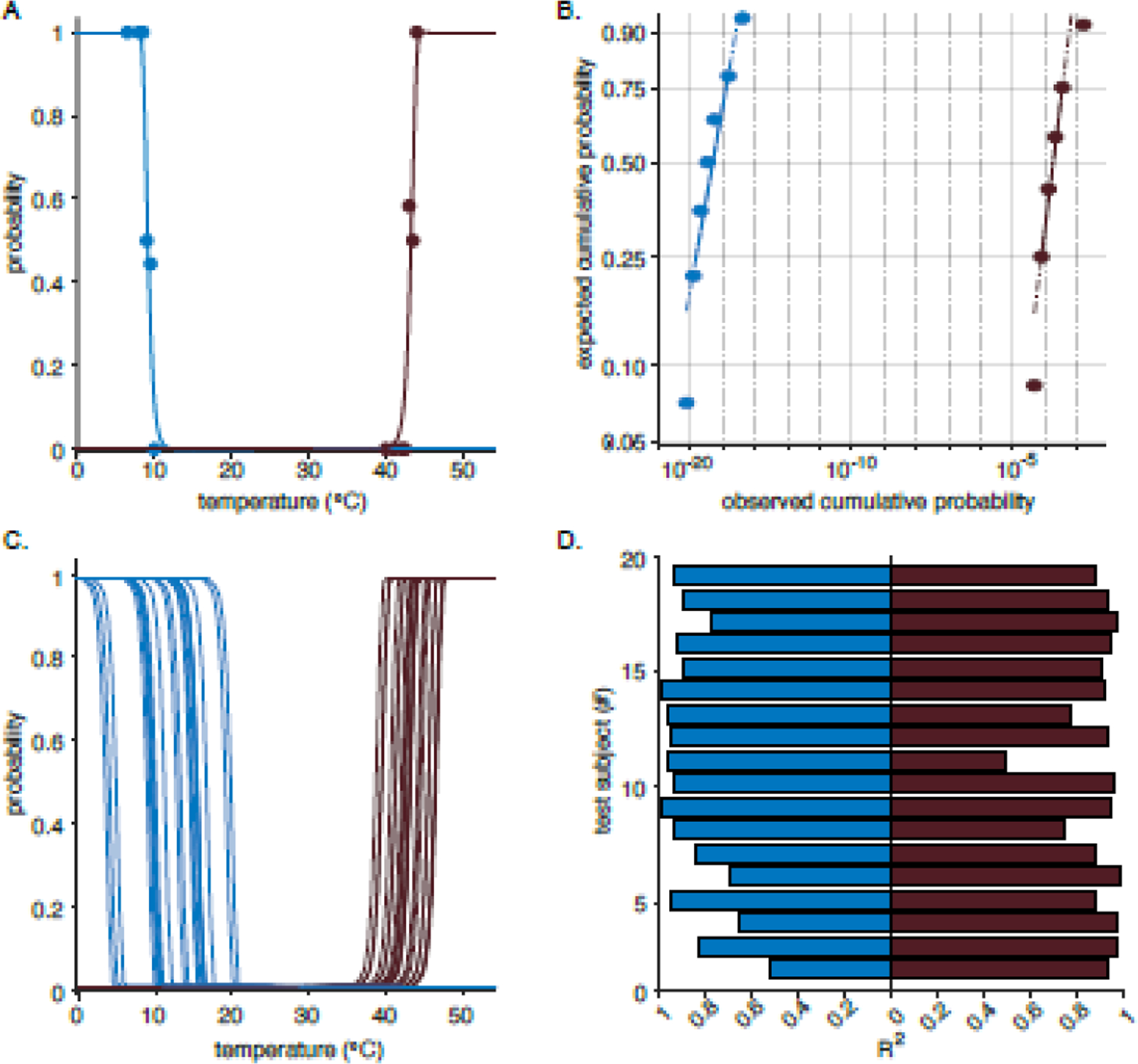
Psychometric curves for hot and cold pain. A. Fitted psychometric curve from one patient. Blue for all subplots represents cold pain perception while the purple is hot pain. The filled circles are the subjects’ proportional responses to pain at various temperatures. The psychometric curve gives an estimated probability for an individual experiencing pain at specific temperature. C. Fitted psychometric curves for all patients. B. Weibull probability plots for cold and hot (C) pain for one test subject. The log-log probability plot compares the distribution of the temperatures to the Weibull distribution. The solid reference line connects the first and third quartiles of the data. The dashed line extends the solid line to the end of the data. Since the data points appear along the reference lines, the sample data is assumed to have a Weibull distribution and it is appropriate to use the Weibull distribution to parameterize the collected data. The Lilliefors test was used to confirm this visual comparison. D. Adjusted R2 of all subjects’ CPT and HPT fits.
Our study used the described default parameters for the initial trial stimulus. All settings were communicated to the device programmatically and adjusted to the initial test intensity. All initial test intensities were determined using previously established pain thresholds and parameters [7,35,44]. After the experimenter started the task, they did not need to adjust anything else, as the copper bar interface automatically adjusted to the stimulus temperature. Once the interface reached the desired temperature, the touchscreen display that the test subject sees is automatically updated to indicate to the test subject that they should place their hand on the device (Figure 3, Step 1–2). Once the test subject placed their hand on the device (determined by the capacitive touch sensor), the timer on the touchscreen started counting down from 10 seconds (Figure 3, Step 2–3). After the trial was complete, the touchscreen automatically updated and asked the subject, “Was that painful?” The subject selected “Yes” or “No” (Figure 3, Step 3). However, the precise text of the questions are adjustable within the code running the GUI. The touchscreen was updated again to three different sliding bar scales from 0 to 10 (Figure 3, Step 4). The subject rated the pain, hot, and cold.
Figure 3.
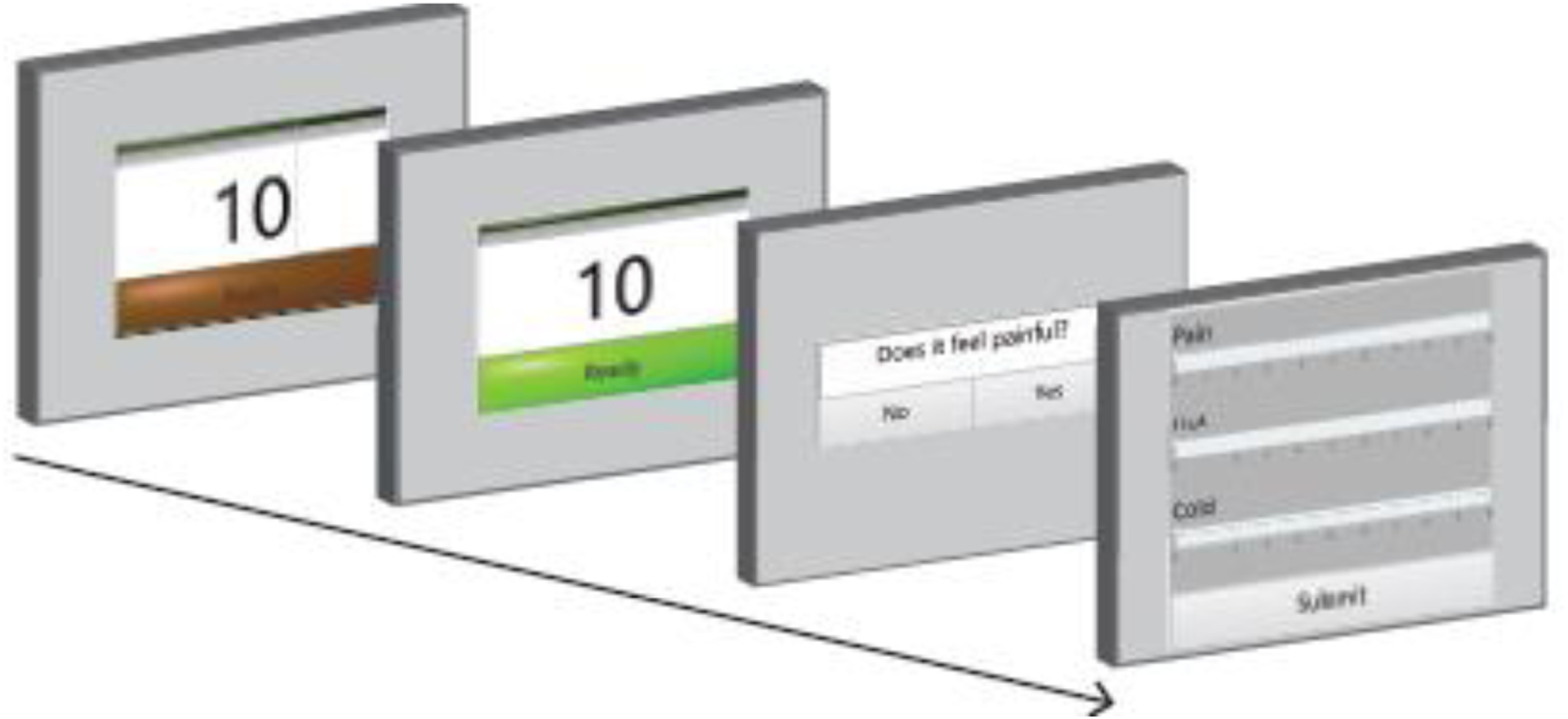
Touch screen display viewed by test subject. The test subject first sees back-left screen. The dark green color surrounding “Ready” means that the interface bars are adjusting to the target temperature and the subject should not yet put their hand on the bars. One screen forward, the “Ready” bar changes to neon green indicating that the bars are at the target temperature, and the patient may put their hand on the interface. Once their hand is detected by the capacitive touch sensor, the timer on the screen counts down to 0 and then the display changes to the next screen. The test subject must touch the display screen to answer a question regarding the sensation they just experienced. After answering the question displayed, the screen changes to front-most image, where the test subject rates the prior sensation based on pain, hot, and cold on a sliding scale between 0–10. The test subject slides the cursor within each white horizontal bar to the desired numerical rating.
The maximum-likelihood adaptive algorithm used the answer to each yes-no question to update the psychometric function and test temperature for the next trial. After submitting their answer regarding the sensation, the touchscreen returned to the starting screen, as the temperature on the bars adjusted from the previously tested temperature to the new test temperature (Figure 3, Step 1). The updated test intensity was communicated to the TED programmatically. When the “Ready” bar turned neon green again, the interface was ready for another trial. The task proceeded until the minimum threshold requirements were met (pain threshold standard deviation is <1°C, and the subject completed 20 trials). For our validation studies, the experimenter first performed the psychometric pain task involving at least 20 hot and 20 cold trials.
2.5. Validation of the TED in healthy human subjects
Twenty-one healthy human subjects (18–65 years old) participated in the psychophysical pain task experiment. The sample size was determined from an a priori power analysis with an assumed effect size (f = 0.625), type I error probability (α = 0.05), and power threshold (0.80). All experiments were approved by the University of Utah Institutional Review Board. Individuals were recruited on and around the University of Utah campus. The screening included a questionnaire to exclude people with a neurological injury or neuropathy history. Individuals were checked for cutaneous lesions on their dominant hand before placing the hand on the TED. The pain task was performed as outlined in the Test subjects’ interaction with the guided-user interface (GUI) section. The experiments all took place in an air temperature-controlled room at 20°.
All participants completed at least 20 hot and 20 cold trials to determine their HPT and CPT. The standard deviation of each threshold was <1°C after 20 trials. The psychometric function of each subject’s pain threshold was plotted and parameterized in MATLAB. Given that a four-variable Weibull function was used for the parameterization, at least four different temperatures needed to be tested for parameterization. The probability plots were used to evaluate if the Weibull parameterization adequately represented the psychometric data. The logarithm of the probability of pain at a given temperature was plotted with reference data following a Weibull distribution. The temperature data was inspected to determine if the data points of the plot appeared along the reference data. If the data has a distribution other than a Weibull, it does not follow the reference line and instead has a curve. The visual comparison of distributions was quantified using the Lilliefors test. The Lilliefors test was used to test if the logarithm of the subject data had an extreme value distribution. If this null hypothesis could not be rejected, then the subject data would be assumed to follow a Weibull distribution. Evaluating the distribution of the gathered psychometric data was an important step in verifying the validity of parameterizing the data using the Weibull function.
2.6. Utilization of the TED in performing the thermal grill illusion (TGI)
All subjects from the TED validation study also participated in a TGI task. One may use set temperatures for the TGI or incorporate individual temperature pain thresholds. To account for variation across subjects, we opted to test subjects’ HPT and CPT and then use those thresholds for an individualized TGI. The TGI task was similar to the psychometric temperature threshold experiments. Subjects first completed the HPT and CPT task, then nonpainful cool temperatures (1°C + CPT) and nonpainful warm temperatures (HPT − 1°C) were used for the TGI task. Since the cool and warm temperatures used in the illusion should be innocuous on their own and thresholds were determined <1°C of certainty, the thresholds were padded with 1°C to establish the innocuous cool and warm settings. Subjects completed 20 trials each at three different temperature settings—cool, warm, and alternating. All sixty trials were randomized. After every trial, the program generated a question on the touchscreen asking the subject if they experienced pain. The subject then rated pain, cold, and hot on a scale from 0 to 10. The ratings were compared between stimuli instead of determining a psychometric function.
As previously described, not all subjects thought the illusion was painful and were classified as non-responders [34,39]. Subjects were defined as responders if they rated pain experienced by the TGI stimulus as significantly different than the cool or warm stimuli, determined using a Tukey-Kramer post hoc pairwise comparison (α = 0.05). The responders’ median VAS response to each temperature setting was normalized by the average median responses to all temperature settings. Normalization accounted for the subjective nature of quantifying pain among individuals.
Within the responders, a Kruskal-Wallis H test determined if there was a statistically significant difference in how the test subject rated Pain, Hot, and Cold between the three different experimental conditions: TGI, Cool, and Warm. A Tukey-Kramer post hoc pairwise comparison on the mean ranks of the rating group (Pain, Hot, or Cold) was used to test for a significant difference between each rating group.
3. Results
3.1. The TED quickly adjusts to different temperatures
We evaluated the time it took for the system to adjust to selected temperatures. Figure 2 shows the average time for each temperature path (start-to-target temperature). The longest adjustment times were when the starting temperature was high with a low target temperature—e.g., going from 50°C to 5°C took the longest time, 73 seconds—while the shortest occurred during heating (e.g., 5°C to 50°C path took 26 seconds).
The recorded adjustment times demonstrate the technical capability of the device. We chose initial test thresholds and probable ranges for the CPT and HPT. It would be improbable that you would see a “cold” (i.e., <30°C during HPT) or “hot” (>30°C during CPT) temperature during pain threshold testing of the opposite temperature category. Instead, it is most likely that the temperatures tested during HPT would stay between 40°C and 50°C and adjust with a much smaller increment than 5°C, resulting in an average adjustment time of <10 seconds.
3.2. QUEST-determined hot and cold pain thresholds are determined with <1°C of variation
Twenty-one healthy human volunteers participated in the TED pain task. Nineteen subjects had enough tested temperatures to fit the four-parameter Weibull function used by QUEST. An example of the psychometric curves generated for one test subject are shown in Figure 4A. All subjects’ psychometric curves are shown in Figure 4C. CPTs appeared to be more variable than the HPTs, so Levene’s test for variance equality was computed. A Levene’s test indicated unequal variances between the CPTs and the HPTs (F = 8.59, p = 0.00545), suggesting that inter-individual CPTs are more variable than HPTs. The adjusted R2 of the psychometric fits are shown in Figure 4D. All CPT fits had R2 values of >0.50 and all HPT R2 were >0.40. The p-values for all fits were <0.05.
Corresponding log-log probability plots were generated for all subjects to verify that the data distribution fit a Weibull distribution. The data is deemed to follow a Weibull distribution if it follows a reference line on the probability plot. While the data appeared to follow the reference line, a Lilliefors test was also used to test the hypothesis that the log of the temperature data followed an extreme value distribution. The Lilliefors test confirmed that none of the data distributions were significantly non-normal. An example of the probability plot for one test subject is shown in Figure 4B.
3.3. The thermal grill illusion is perceived as painful in a subset of healthy human subjects
Eleven out of the 21 healthy human subjects who participated in the TGI task were classified as responders based on our stringent inclusion criteria (52%). Within the responders, pain, hot, and cold were rated significantly different in the three different experimental conditions, which were TGI, Cool, and Warm (pain: 𝒳2 = 13.09, p < 0.002; hot: 𝒳2 = 26.53, p << 0.001; cold: 𝒳2 = 23.94, p << 0.001). The pairwise comparison between the mean ranks of the VAS rating groups (pain, hot, and cold) shown in Figure 5 demonstrated significant differences. The pain was rated higher for the TGI than for the hot and cold settings. There was no difference in how test subjects rated hot between the TGI and warm settings. Similarly, no difference was present for cold ratings between the TGI and cool settings.
Figure 5.
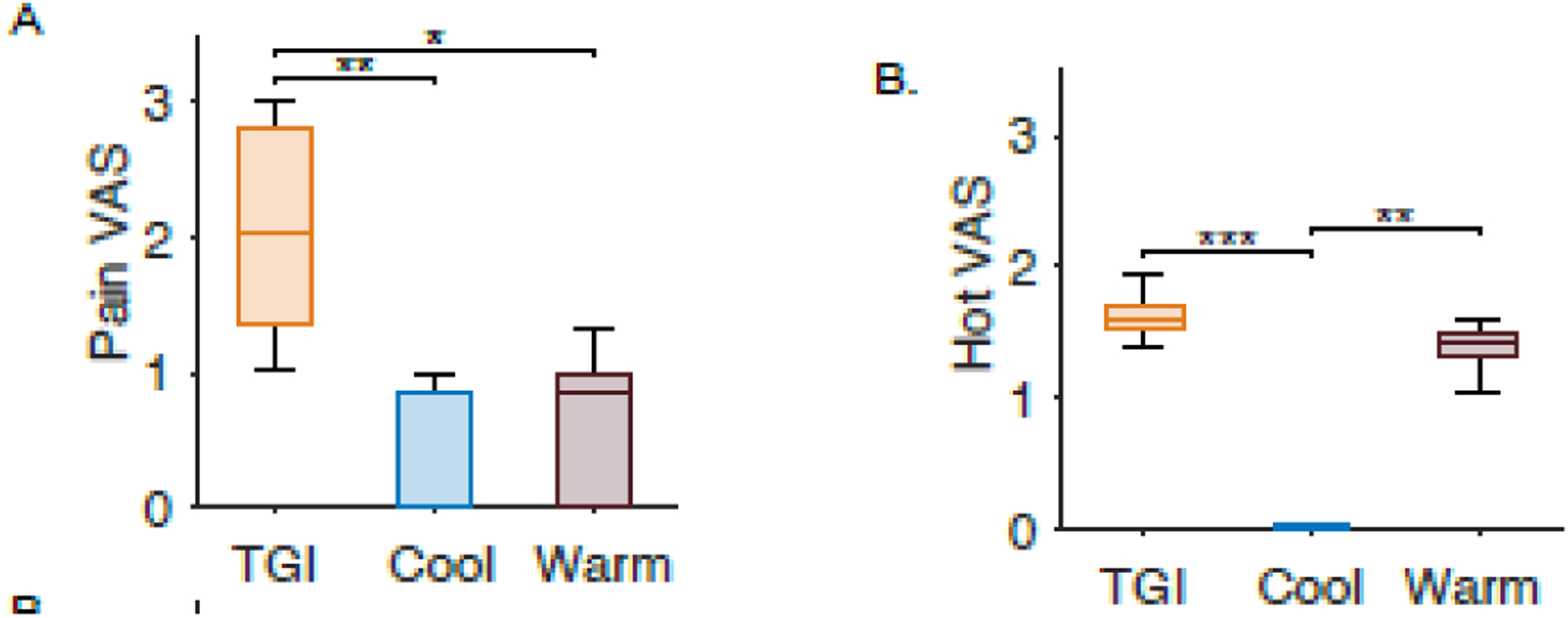
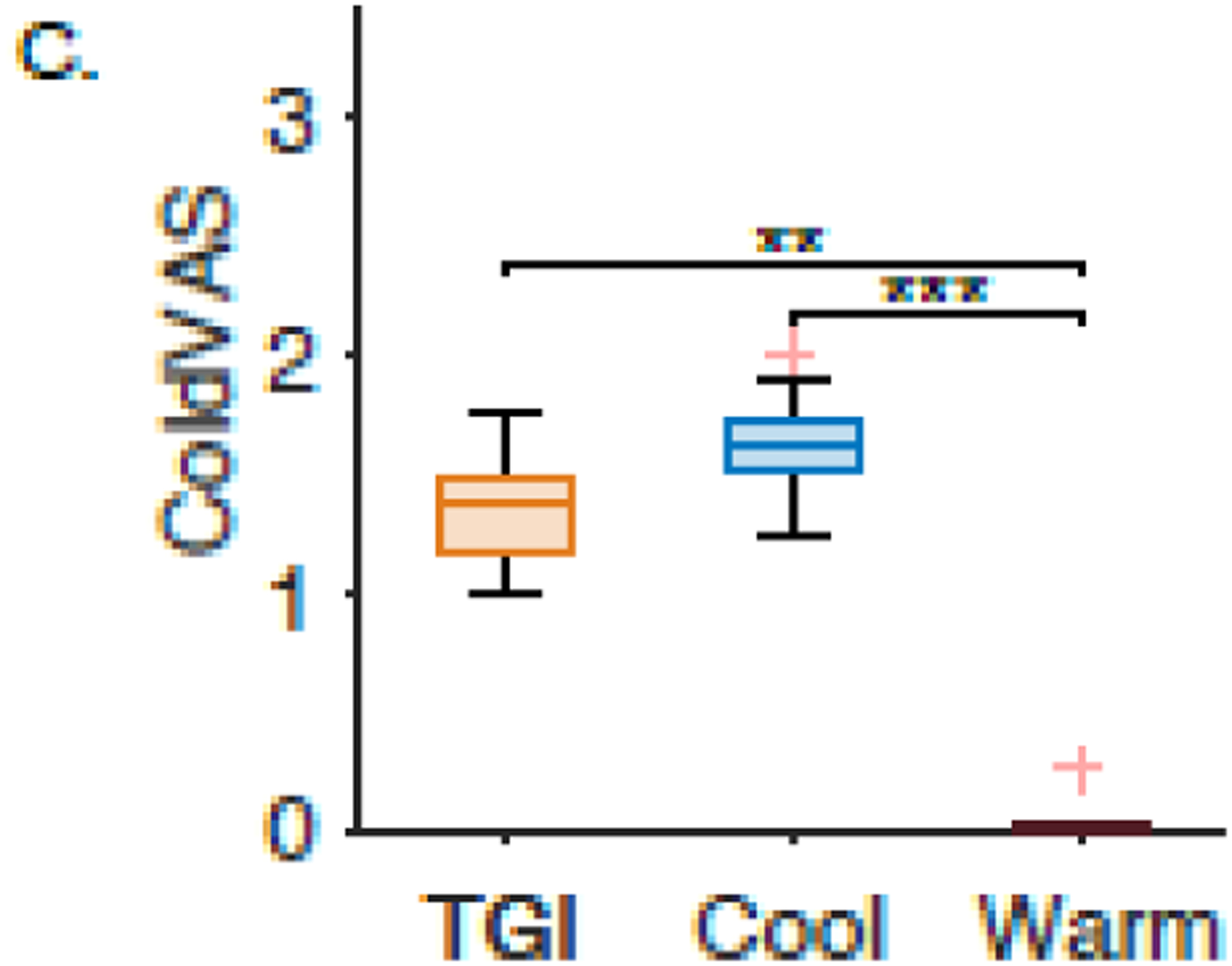
Normalized visual analogue scale (VAS) ratings to three different interface settings for healthy volunteers. Warm (purple) represents when the interface was set to one degree below the patients’ hot pain threshold. Cool (blue) was when the interface was set to one degree above the patients’ cold pain threshold. The TGI trials are the cool and warm bars interlaced (orange). The subplots represent the subjects’ response to A. “Pain,” B. “Hot,” and C. “Cold” on a visual analog scale. One asterisk represents p<0.05, two asterisks represent p<0.005, and three asterisks represent p<0.0005.
4. Discussion
We present a novel TED with an integrated experimental protocol to study thermal pain. We describe the hardware used to build the device and showed that the TED is a unique approach to study pain processing in human subjects. The pain task and developed software were also described. The pain task was validated in healthy volunteers. We accounted for individual differences in pain thresholds across subjects. The thresholds determined by the psychometric functions were generated with <0.5°C error and were similar to thresholds described using similar methods. In addition to the thresholds we report, the psychometric function represents a probabilistic relationship between temperature and pain for all subjects. We show that thermo-nociception follows a Weibull distribution, similar to how other perceptions are modeled. Lastly, we showed the versatility of the TED by showing how pain, hot, and cold are rated differently under TGI, warm, and cool experimental conditions.
Similar devices using Peltier-based mechanisms tend to use a continuous staircase method to change temperature [34,35,39,45]. The temperature is ramped up for HPT testing or down for CPT testing. Temperature changes at a rate of 1–3°C per second until the test subject indicates that they are experiencing pain [31,33,46]. The staircase method with a typical starting temperature of 30°C takes 15–45 seconds to reach a common HPT of 45°C. According to our results, the TED takes <20 seconds to adjust from 30°C to 45°C. The timing of the TED is comparable to other experimental thermoelectric devices. Anecdotally, completing 20 trials of HPT or CPT takes the test subjects approximately ten minutes to complete. This time includes the inter-trial interval where the subject answers questions about the sensation experienced in the trial. We acquire this amount of data by keeping the interface set near the temperature from the previous trial until the QUEST algorithm updates to the next trial temperature. The time to complete the trials was manageable for the test subjects.
The CPT and HPT were determined for each test subject and utilized for performing the TGI. Using individual pain thresholds facilitated an optimal temperature difference between the cool and warm bars for the illusion, as the greatest difference between the cool and warm bars causes the most intense pain response [34,39,47]. We hypothesized that our TGI responder rate would be less than previous reports (~60%, [39,48]), as our responder criteria required a statistical difference in TGI pain ratings. We also hypothesized that when all the bars on the TED were 1°C below the HPT (warm setting) or 1°C above the CPT (cool), the reported pain would be less than when the warm and cool bars were alternating (TGI setting). If the alternating temperature caused pain, it would verify that the TED could perform the TGI. Our results showed that the TGI was rated more painful than just the cool or warm bars on their own. The TGI has also been described as paradoxical burning or a cold burn [30,38,39]. This cold-burning phenomenon was also present in our findings, as indicated by the cold VAS and hot VAS ratings for the TGI. The pain ratings demonstrate how experimenters can also use the TED to perform the TGI.
The basis of the TED psychometric pain task is determining HPT and CPT using QUEST. Most healthy human volunteers who participated in the validation study (19/21 subjects) had parameterizable psychometric functions of their HPT and CPT. The two subjects did not reach the minimum allowed temperature of 5°C and did not experience pain. Therefore, they did not complete enough “painful” trials to construct an individualized psychometric function confidently. These subjects could have had a high pain tolerance, not understood the task instructions, or their skin adapted to the temperature. The remaining test subjects’ psychometric curves were evaluated with a Lilliefors test to confirm that it was appropriate to use a Weibull distribution to parameterize the collected data. Based on the adjusted R2 values, parameterization of the psychometric curves yielded fits that were acceptable representations of individuals’ pain perception.
Test subjects’ psychometric curves showed a broader range in CPTs than for HPTs. There was a 16°C difference between the lowest and highest CPT and an 8°C difference between the lowest and highest HPT. We quantified this observation using Levene’s test to evaluate if the CPTs had an equal variance to the HPT and found that the variance was unequal between the two groups. This variance may represent the differing peripheral processing mechanisms of cold and hot nociceptive processing [7,8,26]. It is also possible that the increased variation in CPTs is due to cold being harder to quantify as pain. If this is the case, it would make sense for clinicians using the cold pressor test to consider testing hot pain instead. However, these results were obtained in subjects without pathological pain, so further investigation in additional populations is warranted.
We plan to use TED to investigate cerebral pain processing in future studies. Prior work shows that hot and cold pain can be conveyed to the brain separately from the peripheral nervous system. Thermal pain is likely the result of converging nociceptive signals within the brain [13,45,49]. Painful experiences within the brain are thought to derive from a “dynamic pain connectome” consisting of sensory-discriminative, affective-motivational, and cognitive-evaluative dimensions [50–53]. The normal adaptive relationship between nociception and experiential pain within these networks becomes disrupted in chronic pain [54].
Various other tools currently exist to quantify pain networks in the human brain. Studies using non-invasive functional imaging and surface electroencephalography (EEG) show the thalamus, posterior and anterior insula, somatosensory cortex, anterior cingulate cortex, and the periaqueductal gray matter exhibit neurological patterns of pain processing [14,22,55–59]. SEEG is another invasive approach which records neural electrical activity using penetrating intracranial electrodes. This methodology offers both surface and deep cortical recordings [60]. SEEG is most commonly performed in patients with drug-resistant epilepsy undergoing intracranial monitoring to identify potential seizure onset zones. The procedure is minimally invasive as a neurosurgeon stereotactically places electrodes into numerous brain structures—many of which are part of the pain processing network [61]. Patients are clinically observed in the hospital for 1–3 weeks after placement of SEEG electrodes. The patient is asked to participate in institutionally approved research studies. Performing the TED pain task with the SEEG patient population facilitates investigation into pain processing within the human brain [21,51,62–64]. Since the TED is portable and easily fits on a small table, it can easily be brought into a hospital room for experimentation. Our unique method of inducing and quantifying pain, paired with intracranial electrodes, will enable mechanistic and causal investigation of cerebral pain processing. The TED, developed software, algorithms, and methods developed for our experiments are available upon request.
Supplementary Material
Highlight.
A custom-built, novel thermoelectric device with an integrated psychophysical pain task is validated in healthy human subjects. Experimental data show the device’s utility in bringing together psychometrics and human nociceptive processing.
Acknowledgments
Thank you to the volunteers who participated in our study. Thank you to the National Institute of Neurological Disorders and Stroke (K23 NS114178) for supporting JDR, and the National Institute of Neurological Disorders and Stroke T32 NS115723 for supporting RMC. JDR has received consulting fees from ClearPoint, NeuroPace, and Corlieve Therapeutics, but companies were not involved in this project or manuscript preparation.
Funding
JDR was supported by the National Institute of Neurological Disorders and Stroke (K23 NS114178). RMC is supported by the National Institute of Neurological Disorders and Stroke T32 NS115723.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
JDR has received consulting fees from ClearPoint, NeuroPace, and Corlieve Therapeutics. Other authors have no relevant conflicts of interest and no competing interests to declare.
Declaration of Competing Interest
We have no competing interests. Disclosures are mentioned in the manuscript.
CRediT authorship contribution statement
The authors contributed in the following ways: RC, TD, and JR Conceptualization; RC, TD Data curation; RC Formal analysis; RC and JR Funding acquisition; RC, TD, ES, JR Investigation; RC, TD, ES, JR Methodology; RC, TD, ES, JR Project administration; RC and TD Software; SR, TD, JR Supervision; RS, TD Validation; RC, TD, ES, JR Visualization; RC Roles/Writing - original draft; RC, TD, ES, SR, JR Writing - review & editing.
Data Transparency:
Data is available by requesting it from the corresponding author.
References
- 1.Dahlhamer J; Lucas J; Zelaya C; Nahin R; Mackey S; DeBar L; Kerns R; von Korff M; Porter L; Helmick C Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults - United States, 2016. MMWR Morb Mortal Wkly Rep 2018, 67, 1001–1006, doi: 10.15585/mmwr.mm6736a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pitcher MH; von Korff M; Bushnell MC; Porter L Prevalence and Profile of High-Impact Chronic Pain in the United States. J Pain 2019, 20, 146–160, doi: 10.1016/j.jpain.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yong RJ; Mullins PM; Bhattacharyya N Prevalence of Chronic Pain among Adults in the United States. Pain 2022, 163, e328–e332, doi: 10.1097/j.pain.0000000000002291. [DOI] [PubMed] [Google Scholar]
- 4.Loeser JD Relieving Pain in America; The National Academies Press: Washington, D.C., 2012; Vol. 28; ISBN 9780309214841. [Google Scholar]
- 5.Gaskin DJ; Richard P The Economic Costs of Pain in the United States. Journal of Pain 2012, doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Jones MR; Viswanath O; Peck J; Kaye AD; Gill JS; Simopoulos TT A Brief History of the Opioid Epidemic and Strategies for Pain Medicine. Pain Ther 2018, doi: 10.1007/s40122-018-0097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.González-Ramírez R; Chen Y; Liedtke WB; Morales-Lázaro SL TRP Channels and Pain; CRC Press/Taylor & Francis, 2017; ISBN 9781315152837. [PubMed] [Google Scholar]
- 8.Levine JD; Alessandri-Haber N TRP Channels: Targets for the Relief of Pain. Biochim Biophys Acta Mol Basis Dis 2007, 1772, 989–1003, doi: 10.1016/j.bbadis.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Koivisto AP; Belvisi MG; Gaudet R; Szallasi A Advances in TRP Channel Drug Discovery: From Target Validation to Clinical Studies. Nat Rev Drug Discov 2021, doi: 10.1038/s41573-021-00268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weyer AD; Lehto SG Development of TRPM8 Antagonists to Treat Chronic Pain and Migraine. Pharmaceuticals 2017, 10, 1–9, doi: 10.3390/ph10020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liberati G; Algoet M; Santos SF; Ribeiro-Vaz JG; Raftopoulos C; Mouraux A Tonic Thermonociceptive Stimulation Selectively Modulates Ongoing Neural Oscillations in the Human Posterior Insula: Evidence from Intracerebral EEG. Neuroimage 2019, doi: 10.1016/j.neuroimage.2018.11.059. [DOI] [PubMed] [Google Scholar]
- 12.Wong F; Vierck CJ; Iii JLR; King C; Mauderli AP A New Thermal Stimulation Method for Human Psychophysical Studies: Pain Intensity Clamping. 2010, doi: 10.1016/j.jneumeth.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liberati G; Mulders D; Algoet M; van den Broeke EN; Santos SF; Ribeiro Vaz JG; Raftopoulos C; Mouraux A Insular Responses to Transient Painful and Non-Painful Thermal and Mechanical Spinothalamic Stimuli Recorded Using Intracerebral EEG. Sci Rep 2020, 10, 1–15, doi: 10.1038/s41598-020-79371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen CC; Chang C; Hsu YH; Peng YJ; Lee HS; Huang GS FMRI Indicates Cortical Activation through TRPV1 Modulation during Acute Gouty Attacks. Sci Rep 2019, 9, 1–11, doi: 10.1038/s41598-019-48656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Larrea L; Frot M; Valeriani M Brain Generators of Laser-Evoked Potentials: From Dipoles to Functional Significance. Neurophysiologie Clinique/Clinical Neurophysiology 2003, 33, 279–292, doi: 10.1016/j.neucli.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Chen Z; Ohara S; Cao J; Vialatte F; Lenz FA; Cichocki A Statistical Modeling and Analysis of Laser-Evoked Potentials of Electrocorticogram Recordings from Awake Humans. Comput Intell Neurosci 2007, 24, doi: 10.1155/2007/10479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorenz J; Garcia-Larrea L Contribution of Attentional and Cognitive Factors to Laser Evoked Brain Potentials. Neurophysiologie Clinique/Clinical Neurophysiology 2003, 33, 293–301, doi: 10.1016/j.neucli.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Arendt-Nielsen L; Chen ACN Lasers and Other Thermal Stimulators for Activation of Skin Nociceptors in Humans. Neurophysiologie Clinique 2003, 33, 259–268, doi: 10.1016/j.neucli.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Markman T; Liu CC; Chien JH; Crone NE; Zhang J; Lenz FA EEG Analysis Reveals Widespread Directed Functional Interactions Related to a Painful Cutaneous Laser Stimulus. J Neurophysiol 2013, 110, 2440–2449, doi: 10.1152/jn.00246.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohara S; Crone NE; Weiss N; Lenz FA Attention to a Painful Cutaneous Laser Stimulus Modulates Electrocorticographic Event-Related Desynchronization in Humans. Clinical Neurophysiology 2004, doi: 10.1016/j.clinph.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 21.Kim JH; Chien JH; Liu CC; Lenz FA Painful Cutaneous Laser Stimuli Induce Event-Related Gamma-Band Activity in the Lateral Thalamus of Humans. J Neurophysiol 2015, 113, 1564–1573, doi: 10.1152/jn.00778.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu C-CC; Ohara S; Franaszczuk PJ; Lenz FA; Crone NE; Lenz FA Attention to Painful Cutaneous Laser Stimuli Evokes Directed Functional Interactions between Human Sensory and Modulatory Pain-Related Cortical Areas. Pain 2011, 152, doi: 10.1016/j.pain.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arendt-Nielsen L; Yarnitsky D Experimental and Clinical Applications of Quantitative Sensory Testing Applied to Skin, Muscles and Viscera. J Pain 2009, 10, 556–572, doi: 10.1016/j.jpain.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Wager TD; Casey K; Kosslyn SM Placebo-Induced Changes in FMRI in the Anticipation and Experience of Pain Placebo Effects on Social and Physical Pain View Project Neural Signature of Fear Overgeneralization in Trauma Exposed Adults View Project., doi: 10.1126/science.1093065. [DOI] [Google Scholar]
- 25.Iannetti GD; Hughes NP; Lee MC; Mouraux A Determinants of Laser-Evoked EEG Responses: Pain Perception or Stimulus Saliency? J Neurophysiol 2008, 100, 815–828, doi: 10.1152/jn.00097.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tominaga M The Role of TRP Channels in Thermosensation; CRC Press/Taylor & Francis, 2007; ISBN 0849340489. [PubMed] [Google Scholar]
- 27.Tilley P; Bisset L The Reliability and Validity of Using Ice to Measure Cold Pain Threshold. 2017, doi: 10.1155/2017/7640649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heldestad V; Linder J; Sellersjö L; Nordh E Reproducibility and Influence of Test Modality Order on Thermal Perception and Thermal Pain Thresholds in Quantitative Sensory Testing. Clinical Neurophysiology 2010, 121, 1878–1885, doi: 10.1016/j.clinph.2010.03.055. [DOI] [PubMed] [Google Scholar]
- 29.Craig A; Bushnell M The Thermal Grill Illusion: Unmasking the Burn of Cold Pain. Science (1979) 1994, 265, 252–255, doi: 10.1126/science.8023144. [DOI] [PubMed] [Google Scholar]
- 30.Bach P; Becker S; Kleinböhl D; Hölzl R The Thermal Grill Illusion and What Is Painful about It. Neurosci Lett 2011, 505, 31–35, doi: 10.1016/j.neulet.2011.09.061. [DOI] [PubMed] [Google Scholar]
- 31.Leung A; Shukla S; Li E; Duann JR; Yaksh T Supraspinal Characterization of the Thermal Grill Illusion with FMRI. Mol Pain 2014, 10, doi: 10.1186/1744-8069-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patwardhan S; Kawazoe A; Kerr D; Nakatani M; Visell Y Dynamics and Perception in the Thermal Grill Illusion. IEEE Trans Haptics 2019, doi: 10.1109/TOH.2019.2904226. [DOI] [PubMed] [Google Scholar]
- 33.Leung AY; Wallace MS; Schulteis G; Yaksh TL Qualitative and Quantitative Characterization of the Thermal Grill. Pain 2005, 116, 26–32, doi: 10.1016/j.pain.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 34.Adam F; Alfonsi P; Kern D; Bouhassira D Relationships between the Paradoxical Painful and Nonpainful Sensations Induced by a Thermal Grill. Pain 2014, 155, 2612–2617, doi: 10.1016/j.pain.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 35.Yarnitsky D; Sprecher E; Zaslansky R; Hemli JA Heat Pain Thresholds: Normative Data and Repeatability. Pain 1995, 60, 329–332, doi: 10.1016/0304-3959(94)00132-X. [DOI] [PubMed] [Google Scholar]
- 36.Kyle BN; Mcneil DW Autonomic Arousal and Experimentally Induced Pain: A Critical Review of the Literature; Vol. 19;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Starr CJ; Sawaki L; Wittenberg GF; Burdette JH; Oshiro Y; Quevedo AS; Coghill RC Roles of the Insular Cortex in the Modulation of Pain: Insights from Brain Lesions. Journal of Neuroscience 2009, 29, 2684–2694, doi: 10.1523/JNEUROSCI.5173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X; Petrini L; Wang L; Defrin R; Arendt-Nielsen L The Importance of Stimulus Parameters for the Experience of the Thermal Grill Illusion. Neurophysiologie Clinique 2009, 39, 275–282, doi: 10.1016/j.neucli.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Bouhassira D; Kern D; Rouaud J; Pelle-Lancien E; Morain F Investigation of the Paradoxical Painful Sensation (‘illusion of Pain’) Produced by a Thermal Grill. Pain 2005, doi: 10.1016/j.pain.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 40.Mischkowski D; Palacios-Barrios EE; Banker L; Dildine TC; Atlas LY Pain or Nociception? Subjective Experience Mediates the Effects of Acute Noxious Heat on Autonomic Responses. Pain 2018, doi: 10.1097/j.pain.0000000000001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taesler P; Rose M Psychophysically-Anchored, Robust Thresholding in Studying Pain-Related Lateralization of Oscillatory Prestimulus Activity. J Vis Exp 2017, doi: 10.3791/55228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watson AB; Pelli DG QUEST: A Bayesian Adaptive Psychometric Method; 1983; Vol. 33;. [DOI] [PubMed] [Google Scholar]
- 43.Watson AB QUEST: A General Multidimensional Bayesian Adaptive Psychometric Method. J Vis 2017, 17, 1–27, doi: 10.1167/17.3.10. [DOI] [PubMed] [Google Scholar]
- 44.Liu C-C; Moosa S; Quigg M; Elias WJ Anterior Insula Stimulation Increases Pain Threshold in Humans: A Pilot Study. J Neurosurg 2021, 1–6, doi: 10.3171/2020.10.jns203323. [DOI] [PubMed] [Google Scholar]
- 45.Horing B; Kugel H; Brenner V; Zipfel S; Enck P Perception and Pain Thresholds for Cutaneous Heat and Cold, and Rectal Distension: Associations and Disassociations. Neurogastroenterology & Motility 2013, 25, e791–e802, doi: 10.1111/nmo.12207. [DOI] [PubMed] [Google Scholar]
- 46.Bekrater-Bodmann R; Chung BY; Richter I; Wicking M; Foell J; Mancke F; Schmahl C; Flor H Deficits in Pain Perception in Borderline Personality Disorder: Results from the Thermal Grill Illusion. Pain 2015, 156, 2084–2092, doi: 10.1097/j.pain.0000000000000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fardo F; Beck B; Allen M; Finnerup NB Beyond Labeled Lines: A Population Coding Account of the Thermal Grill Illusion. Neurosci Biobehav Rev 2019. [DOI] [PubMed] [Google Scholar]
- 48.Kern D; Pelle-lancien E; Luce V; Bouhassira D Pharmacological Dissection of the Paradoxical Pain Induced by a Thermal Grill. Pain 2008, 135, 291–299, doi: 10.1016/j.pain.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Green BG; Akirav C Individual Differences in Temperature Perception: Evidence of Common Processing of Sensation Intensity of Warmth and Cold. Somatosens Mot Res 2007, 24, 71–84, doi: 10.1080/08990220701388117. [DOI] [PubMed] [Google Scholar]
- 50.Kucyi A; Davis KD The Dynamic Pain Connectome. Trends Neurosci 2015, 38, 86–95, doi: 10.1016/J.TINS.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Bastuji H; Frot M; Perchet C; Magnin M; Garcia-Larrea L Pain Networks from the inside: Spatiotemporal Analysis of Brain Responses Leading from Nociception to Conscious Perception. Hum Brain Mapp 2016, doi: 10.1002/hbm.23310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu CC; Franaszczuk P; Crone NE; Jouny C; Lenz FA Studies of Properties of “Pain Networks” as Predictors of Targets of Stimulation for Treatment of Pain. Front Integr Neurosci 2011, doi: 10.3389/fnint.2011.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohara S; Crone NE; Weiss N; Lenz FA Analysis of Synchrony Demonstrates ‘Pain Networks’ Defined by Rapidly Switching, Task-Specific, Functional Connectivity between Pain-Related Cortical Structures. Pain 2006, 123, 244–253, doi: 10.1016/j.pain.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 54.Tan LL; Kuner R Neocortical Circuits in Pain and Pain Relief. Nat Rev Neurosci 2021, 1–14, doi: 10.1038/s41583-021-00468-2. [DOI] [PubMed] [Google Scholar]
- 55.Wager TD; Atlas LY; Lindquist MA; Roy M; Woo C-W; Kross E An FMRI-Based Neurologic Signature of Physical Pain. New England Journal of Medicine 2013, 368, 1388–1397, doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yen C-T; Lu P-L Thalamus and Pain. Acta Anaesthesiologica Taiwanica 2013, 51, 73–80, doi: 10.1016/j.aat.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 57.Xu A; Larsen B; Baller EB; Cobb Scott J; Sharma V; Adebimpe A; Basbaum AI; Dworkin RH; Edwards RR; Woolf CJ; et al. Convergent Neural Representations of Experimentally-Induced Acute Pain in Healthy Volunteers: A Large-Scale FMRI Meta-Analysis. Neurosci Biobehav Rev 2020, doi: 10.1016/j.neubiorev.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moisset X; Bouhassira D Brain Imaging of Neuropathic Pain. Neuroimage 2007, doi: 10.1016/j.neuroimage.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 59.Stern J; Jeanmonod D; Sarnthein J Persistent EEG Overactivation in the Cortical Pain Matrix of Neurogenic Pain Patients. Neuroimage 2006, 31, 721–731, doi: 10.1016/j.neuroimage.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 60.Parvizi J; Kastner S Human Intracranial EEG: Promises and Limitations. Nat Neurosci 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Caston RM; Smith EH; Davis TS; Rolston JD The Cerebral Localization of Pain: Anatomical and Functional Considerations for Targeted Electrical Therapies. J Clin Med 2020, doi: 10.3390/jcm9061945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frot M; Faillenot I; Mauguiè Re F Processing of Nociceptive Input From Posterior to Anterior Insula in Humans., doi: 10.1002/hbm.22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baumgärtner U; Vogel H; Ohara S; Treede RD; Lenz F Dipole Source Analyses of Laser Evoked Potentials Obtained from Subdural Grid Recordings from Primary Somatic Sensory Cortex. J Neurophysiol 2011, 106, 722–730, doi: 10.1152/jn.00135.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chien JH; Liu CC; Kim JH; Markman TM; Lenz FA Painful Cutaneous Laser Stimuli Induce Event-Related Oscillatory EEG Activities That Are Different from Those Induced by Nonpainful Electrical Stimuli. J Neurophysiol 2014, 112, 824–833, doi: 10.1152/jn.00209.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available by requesting it from the corresponding author.


